Abstract
Objectives
This study aimed to assess kinetics and predictive variables of humoral immune response to mRNA SARS-CoV-2 vaccine administration.
Methods
We collected blood samples before (T0) and 15, 90, and 180 days after vaccination (T1, T2, and T3, respectively). The Quant SARS-CoV-2 Immunoglobulin (IgG) II Chemiluminescent Microparticle Immunoassay was used to determine anti-spike IgG.
Results
In almost 3000 healthcare-collected blood samples at the three time points, we found the following: at 15 days postvaccination, 97.6% of subjects presented a robust IgG anti-spike response (>4160 AU/ml); then, at three and six months, it decreased in median 6.5-fold to 35.0% and 3.0-fold to 3.3%, respectively. A linear mixed-effects model supported that female gender, younger age groups, and being seropositive prevaccination maintained higher antibody titers. Curves became tighter with time progression, although titers from seropositive subjects decrease at a slower rate than seronegative ones.
Conclusion
These findings strengthen the case for a steep decrease of anti-SARS-CoV-2 antibodies up to six months, suggesting that serological evaluation might guide the need for periodic booster vaccinations in specific groups prone to lower antibody titers.
Keywords: SARS-CoV-2, mRNA vaccine, Humoral immunity, Real-world data, 6 months
Introduction
In late 2019, SARS-CoV-2 triggered a new pandemic. Vaccines started to be urgently developed, and the United States Food and Drug Administration authorized their use in an emergency context on December 11, 2020, after demonstrating 95% efficacy (United States Food and Drug Administration, 2020). In Portugal, healthcare workers (HCWs) received the first doses of the BNT162b2 mRNA COVID-19 vaccine (Pfizer/BioNTech) by the end of December 2021. Vaccine efficacy against COVID-19 was 91.3% through six months of follow-up in subjects without evidence of previous SARS-CoV-2, thus reflecting a gradual decline in vaccine efficacy (Thomas et al., 2021).
By this time, numerous studies of Immunoglobulin G (IgG) humoral immunity were being carried out to understand the kinetics of antibodies (Lo Sasso et al., 2021; Oliveira-Silva et al., 2022; Salvagno et al., 2021; Tré-Hardy et al., 2021) better. Nevertheless, the long-term duration of humoral immunity from the SARS-CoV-2 vaccine remains unclear because of the lack of data from large, real-world studies. Bayart et al. (2021) observed a waning of IgG antibodies over time, although at 180 days after vaccination, subjects still had detectable anti-Spike antibodies. As reported elsewhere, after the first contact with the virus, B cells produce antibodies that decrease over months, particularly in older patients, men, and immunosuppressed subjects (Chavarot et al., 2021; Geisen et al., 2021; Levin et al., 2021). Concordantly, previously infected individuals maintained higher IgG titers over three-month studies (Lau et al., 2021; Tré-Hardy et al., 2021).
Considering that antibody titers might be a good biomarker for the protective efficacy of antibodies and successful humoral immune responses after SARS-CoV-2 exposure or vaccination, it is considered that SARS-CoV-2 IgG kinetics concedes relevant information concerning the immune status and a proxy for immunization status (Bayart et al., 2021; Levin et al., 2021). In this study, we report humoral immunity data of the first six months of follow-up after vaccination from a large cohort, which emphasizes the decline of IgG antibodies.
Material and Methods
HCWs from the Centro Hospitalar e Universitário de Coimbra were vaccinated in late December 2020 with BNT162b2 mRNA and included in a prospective cohort to evaluate SARS-CoV-2 IgG serological kinetics. Subjects were tested for anti-spike IgG antibody before the first dose (T0) and then 15 days (T1), 3 months (T2), and 6 months (T3) after completion of the second dose. HCWs with a previous diagnosis of SARS-CoV-2 were excluded from the first phase of vaccination. Only subjects with complete serological data at all time points were included in analyses (n = 2968). In this population, most HCWs were naïve (seronegative for SARS-CoV-2 IgG before vaccination), whereas 63 were seropositive (because of eventual asymptomatic past contact with the virus). This study was approved by the hospital's Ethics Committee (OBS.SF.106-2021), and deferred consent was obtained under stringent application of ethical and legal procedures for data collection, such as protection of confidentiality of the personal data and mitigation of risks to privacy.
Blood was collected from each participant at every time point and processed to serum within four hours. A chemiluminescent microparticle immunoassay, SARS-CoV-2 IgG II Quant, was used to determine the IgG anti-spike, receptor-binding domain (RBD) S1 subunit of SARS-CoV-2 on Alinity i (Abbott Laboratories). The cutoff and upper detection limits of the Abbott S-RBD IgG test were 50 and 80 000 AU/ml, respectively, whereas sensitivity and specificity were 99.37% and 99.55%. As per manufacturer recommendations, antibody titers above 50 AU/ml were considered reactive. We used IgG antibody titers > 4160 AU/ml as an indicator of strong neutralizing activity, as previously reported (Ebinger et al., 2021). All measurements were undertaken following appropriate quality control procedures and performed daily for routine clinical assessment of SARS-CoV-2 IgG.
Statistical analysis
Departure from normality was tested using the Shapiro-Wilk test, and data were presented as median and interquartile range (IQR). For longitudinal comparison of SARS-CoV-2 IgG titers between time points (T0, T1, T2, and T3), the Friedman's followed by Wilcoxon tests were used, with Bonferroni correction for multicomparison. To assess differences among independent variables (gender, age groups, and reactive titers in T0) at each time point, the Mann-Whitney or Kruskal-Wallis tests were applied.
We modeled the decrease after vaccination (over T1, T2, and T3) using a linear regression model with mixed-effects. Our data were grouped by subject. This model is appropriate for longitudinal data and extends the linear model by adding random effects that can be seen in terms of additional error, accommodating correlation between observations from the same individual. Fixed effect covariates included gender, age group (18–30, 30–40, 40–50, 50–60, and >60 years), humoral status before vaccination (T0 above or below 50 AU/ml), and time (in months). Interactions with time were also included. After log10 transformation of IgG titers, models were fitted with population-level fixed effects and individual-level random effects (Worker ID) for intercept and slope. Models with random effects only for intercept were also fitted. We started by fitting the null model, only including the outcome variable and individual-level random effects. The model presented is the model that fits our data better. Model comparisons were conducted using the difference in Akaike information criterion (AIC) above 4 (Burnham et al., 2011) as significant and fitted using maximum likelihood. We estimated the marginalized R2, the proportion of variance explained by the fixed effects (Nakagawa and Schielzeth, 2013), and the conditional R2 of the model, that is, the proportion of variance explained by both the fixed and random factors (Nakagawa and Schielzeth, 2013). Statistical analyses were conducted using R Software version 4.0.05 (The R Foundation for Statistical Computing, Vienna, Austria), and a linear model with mixed-effects was fitted using the lmer function (lme4 package).
Results
Data were collected between December 2020 and August 2021. Close to 3000 subjects participated in this study, with median age of 45 years (IQR 36–55) (77.5% female). All had full data on IgG titers at the time points T0, T1, T2, and T3. Before vaccination, most participants were naïve (median = 6.8, IQR = 6.8–6.8 AU/ml), although 2.1% (n = 63) of the subjects had IgG anti-SARS-CoV-2 above cutoff (>50 AU/ml) but below 4160 AU/ml.
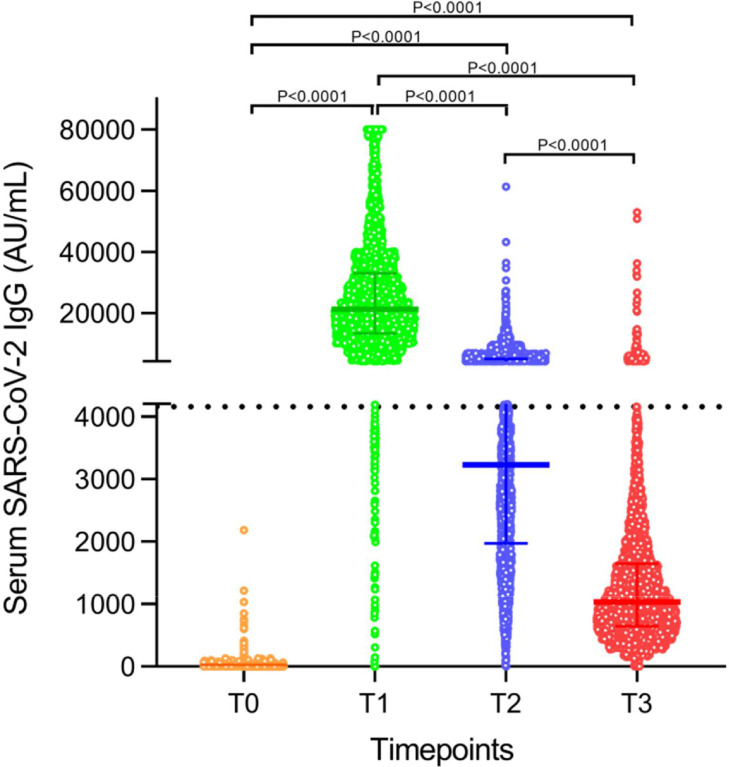
After vaccination, test reactivity (>50 AU/ml) was maintained throughout the study in 99.9%, 99.8%, and 99.7% of the population at T1, T2, and T3 time points, respectively. Fifteen days after vaccination (median IgG = 21.3 × 103, IQR = 13.3 × 103 − 33.0 × 103 AU/ml), 97.6% subjects presented a robust humoral response (>4160 AU/ml), whereas at three months (median = 3.2 × 103, IQR = 2.0 × 103 − 5.1 × 103 AU/ml) it decreased in median 6.5-fold × to 35.0% and then by 3.0-fold to 3.3% at six months (median = 1.0 × 103, IQR = 0.64 × 103 − 1.6 × 103 AU/ml). The Friedman's test [c2(3) = 8652.4, P < 0.0001] revealed a statistically significant difference in SARS-CoV-2 IgG throughout the follow-up, further confirmed by Wilcoxon between time points (P < 0.0001) (Figure 1 ). The comparison between strata of the independent variables, gender, age group, and IgG reactivity before vaccination is listed in Table 1 .
Figure 1.
Serum SARS-CoV-2 IgG throughout the study follow-up, depicting the kinetics of antibodies. Data are presented as median and interquartile range. AU = arbitrary units.
Table 1.
Serological levels SARS-CoV-2 Immunoglobulin G (IgG), overall, and by strata of gender, age group, and IgG reactivity, before and after COVID-19 mRNA vaccination.
| Prevaccination | 15 days postvaccination | 90 days postvaccination | 180 days postvaccination | ||
|---|---|---|---|---|---|
| N (%) | T0 (× 101) | T1 (× 103) | T2 (× 103) | T3 (× 103) | |
| IgG titers, AU/ml* | 2968 (100) | 6.8 [6.8, 6.8] | 21.3 [13.3, 33.0] | 3.2 [2.0, 5.1] | 1.0 [0.64, 1.6] |
| Gender Male Female |
667 (22.5) 2301 (77.5) |
6.8 [6.8, 6.8] 6.8 [6.8, 6.8] |
18.7 [11.7, 29.3] 22.1 [14.0, 34.0] |
2.9 [1.7, 4.8] 3.3 [2.0, 5.2] |
0.97 [0.58, 1.6] 1.1 [0.66, 1.7] |
| P-value *** | 0.655 | <0.0001 | <0.0001 | 0.004 | |
| Age group, years 20–30 30–40 40–50 50–60 >60 |
416 (14.0) 694 (23.4) 802 (27.0) 787 (26.5) 267 (9.0) |
6.8 [6.8, 6.8] 6.8 [6.8, 6.8] 6.8 [6.8, 6.8] 6.8 [6.8, 6.8] 6.8 [6.8, 6.8] |
26.9 [17.9, 38.9] 22.6 [16.0, 34.0] 20.2 [12.5, 31.9] 19.9 [11.8, 31.6] 16.8 [10.5, 28.8] |
4.3 [2.9, 6.3] 3.4 [2.3, 5.3] 2.8 [1.7, 4.5] 3.0 [1.7, 5.0] 2.8 [1.7, 4.2] |
1.5 [0.96, 2.1] 1.1 [0.73, 1.7] 0.91 [0.56, 1.5] 0.95 [0.58, 1.6] 0.89 [0.59, 1.6] |
| P-value ** | 0.529 | <0.0001 a | <0.0001 b | <0.0001 c | |
| IgG reactivity at T0, AU/ml <50 ≥50 |
2905 (97.9) 63 (2.1) |
6.8 [6.8, 6.8] 134.4 [90.4, 322.8] |
21.3 [13.3, 32.6] 32.5 [1.7, 4.2] |
3.2 [1.9, 5.0] 7.7 [3.4, 12.4] |
1.0 [0.64, 1.6] 3.1 [1.4, 5.4] |
| P-value *** | <0.0001 | <0.0001 | <0.0001 | <0.0001 |
Data are presented as median and interquartile range.
*P-value <0.0001 for all comparisons between time points (Wilcoxon test).
**Kruskal-Wallis test, followed by Mann-Whitney tests. aP < 0.0001 for all comparisons except 40–50 vs 50–60 (P = 0.288), 40–50 vs >60 (P = 0.002) and 50–60 vs >60 (P = 0.027) (Mann-Whitney tests). bP< 0.0001 for all comparisons except 40–50 vs 50–60 (P = 0.238), 40–50 vs >60 (P = 0.745) and 50–60 vs >60 (P = 0.262) (Mann-Whitney tests). cP < 0.0001 for all comparisons except 40–50 vs 50–60 (P = 0.199), 40–50 vs >60 (P = 0.804) and 50–60 vs >60 (P = 0.535) (Mann-Whitney tests).
***Mann-Whitney test.
AU = arbitrary units.
Regarding the mixed-effects model, the final model included random effects for the intercept and slope. The marginalized and the conditional R2 were 0.71 and 0.91, respectively.
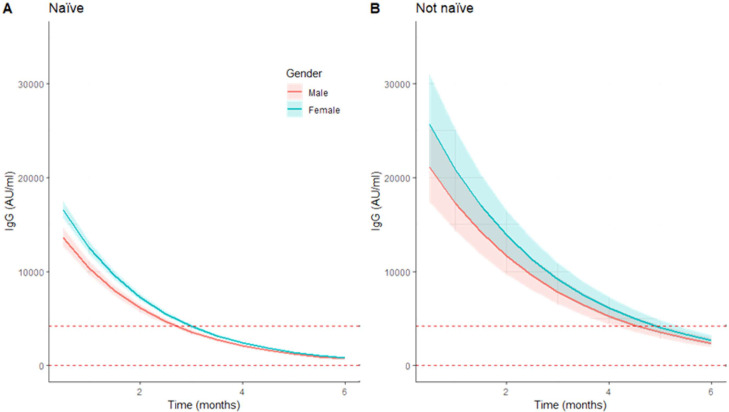
Figure 2.
Predicted trajectories of immunoglobulin G (IgG) levels over six months by naïve status at T0 between gender. Predicted trajectories of IgG levels, after base 10 exponentiation of predicted values and 95% confidence interval limits. Figures represent the predictions for naïve HCWs (A) and for HCWs with previous titers >50 AU/ml (B). Median age was used on these estimates. The dotted horizontal lines represent 4160 AU/ml and 50 AU/ml levels. HCWs = healthcare workers; AU = arbitrary units.
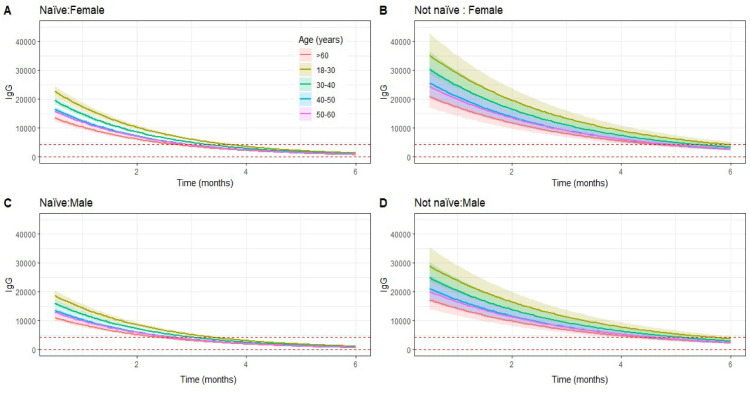
Figure 3.
Predicted trajectories of immunoglobulin G (IgG) levels over six months by gender and naïve status at T0 between age groups. On the top (A and B) are represented the predictions for female gender and on the bottom (C and D) the ones for male HCWs. Also, on the left (A and C) are represented the predictions naïve HCWs and on the right (B and D) for HCWs with previous titers >50 AU/ml. The dotted horizontal lines represent 4160 AU/ml and 50 AU/ml levels. HCWs = healthcare workers; AU = arbitrary units.
Female gender, previous reactive titers, and younger age group each contributed to higher antibody levels at the first time point after vaccination (Table 2 ). We verified that every month, the log-transformed IgG levels decreased 0.230 times (P < 0.001). Interaction of gender and age with time was strongly correlated with the variate time and was excluded. (Table 3 ). The variable interaction of time and IgG titers for seropositive participants was significant (P = 0.0002). Therefore, antibody levels from participants seropositive at T0 showed higher values after vaccination and decreased at a slower rate (−0.23 vs −0.168, P < 0.001), suggesting that at six months after vaccination, the IgG levels remain divergent.
Table 2.
Results from linear mixed effects model for log10-transformed Immunoglobulin gG (IgG) antibody titers. Reported are the estimated fixed effects along with their standard error and P-values.
| Dependent variable: IgG levels (AU/ml) log10-transformed | |||
|---|---|---|---|
| Variables | Value | Std. Error | P-value |
| Intercept | 4.51 | 0.0193 | <0.001 |
| Month | −0.233 | 0.0009 | <0.001 |
| Age (years, >60 as reference) | |||
| 18–30 | 0.219 | 0.0231 | <0.001 |
| 30–40 | 0.136 | 0.0205 | <0.001 |
| 40–50 | 0.056 | 0.0199 | 0.0053 |
| 50–60 | 0.050 | 0.0199 | 0.0120 |
| Gender (female) | 0.070 | 0.0135 | <0.001 |
| T0 (titers >50 AU/ml) | 0.155 | 0.0422 | 0.0002 |
| Interactions | |||
| Months xT0 (titers >50 AU/ml) |
0.062 | 0.0062 | <0.001 |
AIC = 1849.178; Marginalized R2 = 0.71; Conditional R2 = 0.91.
AU = arbitrary units.
Table 3.
Estimations of intercept and decrease rates based on the linear mixed effects model for log10-transformed Immunoglobulin gG (IgG) antibody titers, for each group of covariates included.
| Intercept |
|||||
|---|---|---|---|---|---|
| Age group (years) | |||||
| Female | 18–30 | 30–40 | 40–50 | 50–60 | >60 |
| Naïve | 4.994 | 4.911 | 4.831 | 4.825 | 4.775 |
| Not naïve | 5.180 | 5.097 | 5.017 | 5.011 | 4.961 |
| Male | |||||
| Naïve | 4.194 | 4.211 | 4.131 | 4.125 | 4.075 |
| Not naïve | 4.480 | 4.397 | 4.316 | 4.311 | 4.261 |
Discussion
This real-world study of COVID-19 humoral response after BNT162b2 vaccination demonstrated a significant decline in anti-spike IgG titers six months after vaccination. Despite an early increase at 15 days after completing the second dose, the IgG levels decreased significantly at both three-month and six-month time points. Our findings agree with data reported by others (Gaebler et al., 2021; Naaber et al., 2021; Salvagno et al., 2022; Bilich et al., 2021).
A recently published randomized placebo-controlled clinical trial, following up over 40,000 subjects vaccinated with BNT162b2 for COVID-19, described that effectiveness peaked at 96.2% during the first two months after the second dose and declined to 83.7% in the four to six months after immunization, marking an average decline of 6% every two months (Tartof et al., 2021; Thomas et al., 2021).
After vaccination with the BNT162b2 vaccine, anti-SARS-CoV-2 IgG kinetics peak around 4 to 30 days, followed by a substantial reduction over time, with significantly lower levels at six months (Levin et al., 2021; Naaber et al., 2021). Here, in a large cohort of HCWs, we observed that although postvaccination IgG titers were reactive (>50 AU/ml) for over 99.5% of the population at T1, T2, and T3 time points, when we used the cutoff indicating a robust humoral response (>4160 AU/ml), the frequency of participants declined by 6.5-fold from 97.6% after 15 days to 35.0% at 3 months, and then by 3.0-fold to 3.3% at 6 months. Similar studies with a reduced number of participants yielded common findings (Bayart et al., 2021; Levin et al., 2021). Seemingly, the decrease in IgG levels throughout postvaccination follow-up occurs in parallel with neutralization titers (Terpos et al., 2021). In our study, the significant decrease in titers was independent of gender, age, or IgG reactivity before vaccination, which agrees with previous data (Dan et al., 2021). The lack of proportionality between the decline in mRNA vaccine effectiveness and the decrease in humoral immune response kinetics over time suggests that, during postvaccination follow-up, the protection might have become dependent on immunological mechanisms other than humoral. Notably, declines in effectiveness of the COVID-19 vaccine have also been attributed to the widespread dissemination of the Delta variant (Bayart et al., 2021).
The efficacy of humoral immunity alone against SARS-CoV-2 has been questioned, and the relevance of T cell memory evaluated. Studies investigating antibody and T cell responses in matched samples of convalescent patients revealed decreasing spike-specific and stable nucleocapsid-specific antibody responses. In contrast, functional T cell responses remained robust, increasing in both frequency and intensity (Bilich et al., 2021). Circulating antibody titers were shown to be not predictive of T cell response to SARS-CoV-2 (Dan et al., 2021). Notably, whereas IgG antibodies decreased significantly over time, the number of RBD-specific memory B cells remained unchanged six months after infection (Gaebler et al., 2021). Despite a slight decrease in association with age, memory B cells seem to be efficiently primed by mRNA vaccination and detectable after the second vaccine dose, which concedes memory B cells a role in mounting recall responses to SARS-CoV-2 (Goel et al., 2021).
Taken together, our and others’ findings suggest that serological tests for SARS-CoV-2 might not reflect the immune memory response in terms of robustness and durability, highlighting the need to determine cellular responses in addition to serologies (Cromer et al., 2021; Tretyn et al., 2021).
We found significant differences in antibody titers between naïve versus seropositive subjects before vaccination and in each of the subsequent time points. There was a trend to decrease in absolute difference as a time to depart from the antibody peak. Those with reactive titers before vaccination remained with higher levels at six-month follow-up. Indeed, the mixed-effects linear model revealed that being seropositive at T0 contributes to higher antibody levels, at the peak and during the observed period. These results agree with previous studies showing that baseline seropositives have a longer estimated half-life and less accentuated decline in SARS-CoV-2 IgG titers (Bayart et al., 2021; Salvagno et al., 2021; Zhong et al., 2021).
Age was inversely related to the immune response at all time points of the postvaccination follow-up. We observed that median IgG antibody levels decreased over six months in all age groups, although the difference among age groups decreased over time. Nonetheless, in the mixed-effects model, age remained a significant independent factor to predict antibody levels. These results align with other reports that observed a negative correlation between age and antibody levels (Naaber et al., 2021; Salvagno et al., 2021) and with neutralizing antibodies (Salvagno et al., 2021). Given that our study is from a working population, subjects aged over 68 years were not included. Nonetheless, evidence suggests there is a lower humoral response at six months after the vaccine for patients above 60 (Tretyn et al., 2021) and over 65 years old (Levin et al., 2021).
Gender remained a significant factor throughout all time points analyzed, with female HCWs presenting higher titers than their male counterparts after vaccination. The linear model with mixed-effects showed that the female gender contributed independently toward higher antibody levels, albeit this effect seems to decrease through time. These observations agree with previous reports (Levin et al., 2021; Salvagno et al., 2021).
This study included a cohort of HCWs, a professional group exposed to the occupational risk of COVID-19. Eligible participants were active workers, younger than 67 years of age, without substantial co-morbidities, with only limited generalizability to the older population and adults with serious co-morbidities. Although initially designed to include only naïve subjects, a small number of participants were found to be seropositive. Therefore, findings comparing titers from naïve versus seropositive participants should be interpreted cautiously, despite their being in line with other larger studies (Bayart et al., 2021; Ebinger et al., 2021). Here, we focused on the serological evaluation of immunological response to the COVID-19 mRNA vaccine, even though the immune response to the vaccine is multifaceted and involves neutralizing antibodies and T memory cells beyond IgG antibodies in postvaccination protection (Krause et al., 2021).
Notwithstanding those limitations, we present serological data from a cohort with large sample size and a longer follow-up period compared with others in the literature. Moreover, we used a mixed-effects model suitable for longitudinal datasets where multiple correlated measurements were taken from each subject, allowing more accurate and precise estimates of population heterogeneity (Bottino et al., 2021).
Data presented here provide further evidence for the eventual requirement of SARS-CoV-2 IgG serology-guided booster vaccinations. Despite being controversial, this strategy has been adopted by some countries for older subjects and immunocompromised patients with over six months of postvaccination follow-up time (Bar-On et al., 2021; Krause et al., 2021).
Although we present data of IgG antibodies decline over time, which could be expected, provided that not all vaccine-induced plasmablasts commit or are maintained as long-lived memory plasma cells (Naaber et al., 2021), it is also well established that vaccine efficacy remains high after six months (Thomas et al., 2021). Thus, even if humoral immunity appears to wane, it does not necessarily mean a reduction in efficacy (Krause et al., 2021).
Conclusion
The decline of specific anti-SARS-CoV-2 IgG antibodies over time through six months postvaccination suggests waning of humoral immunity and impaired capacity to fight the virus and supports the need to re-activate IgG production. This is a cohort study planned for a one-year follow-up, which will permit the sharpening of the antibodies’ kinetics model. Accurately evaluated antibody response, together with cellular immunity status and other covariates, including age and gender, may add to clinical reasoning to support the individualization of the immunization plan. This work further contributes to delineating the pattern of the immune response to the COVID-19 mRNA vaccine, fostering additional research to determine the titers needed for protection.
Acknowledgments
Conflicts of interest
Authors disclose support from Abbott Laboratories to participate in meetings (TR, CL, RR, LA, FR). The remaining authors do not have any conflicts of interest to declare.
Funding
No funding.
Acknowledgment
The authors acknowledge the willingness and effort of nurses and laboratory personnel from the Occupational Health and Clinical Pathology Departments to handle phone contacts, blood sampling, and laboratory analysis whenever solicited.
Authors contributions
JOS, TReis, CL, and RB contributed to conceptualization, methodology, investigation, writing the original draft, and final review and editing of the manuscript. RR contributed to conceptualization, methodology, investigation, writing the original draft, supervision, and final review and editing of the manuscript. GM contributed to data curation and to final review and editing of the manuscript. VP, TRodrigues, AA, VP, contributed to resources, investigation, and final review and editing of the manuscript. LA, FR, and IA contributed to the conceptualization, supervision, and final review and editing of the manuscript.
References
- Bar-On YM, Goldberg Y, Mandel M, et al. Protection of BNT162b2 vaccine booster against COVID-19 in Israel. N Engl J Med. 2021;385:1393–1400. doi: 10.1056/NEJMoa2114255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bayart JL, Douxfils J, Gillot C, David C, et al. Waning of IgG, total and neutralizing antibodies 6 months post-vaccination with BNT162b2 in healthcare workers. Vaccines. 2021;9:1–12. doi: 10.3390/vaccines9101092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bilich T, Nelde A, Heitmann JS, et al. T cell and antibody kinetics delineate SARS-CoV-2 peptides mediating long-term immune responses in COVID-19 convalescent individuals. Sci Transl Med. 2021;13:eabf7517. doi: 10.1126/scitranslmed.abf7517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bottino D, Hather G, Yuan L, et al. Using mixed-effects modeling to estimate decay kinetics of response to SARS-CoV-2 infection. Antib Ther. 2021;4:144–148. doi: 10.1093/abt/tbab013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burnham KP, Anderson DR, Huyvaert KP. AIC model selection and multimodel inference in behavioral ecology: some background, observations, and comparisons. Behav Ecol Sociobiol. 2011;65:23–35. [Google Scholar]
- Chavarot N, Ouedrani A, Marion O, et al. Poor anti-SARS-CoV-2 humoral and T-cell responses after 2 injections of mRNA vaccine in kidney transplant recipients treated with Belatacept. Transplantation. 2021;105:e94–e95. doi: 10.1097/TP.0000000000003784. [DOI] [PubMed] [Google Scholar]
- Cromer D, Juno JA, Khoury D, et al. Prospects for durable immune control of SARS-CoV-2 and prevention of reinfection. Nat Rev Immunol. 2021;21:395–404. doi: 10.1038/s41577-021-00550-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dan JM, Mateus J, Kato Y, et al. Immunological memory to SARS-CoV-2 assessed for up to 8 months after infection. Science. 2021;371:eabf4063. doi: 10.1126/science.abf4063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ebinger JE, Fert-Bober J, Printsev I, et al. Antibody responses to the BNT162b2 mRNA vaccine in individuals previously infected with SARS-CoV-2. Nat Med. 2021;27:981–984. doi: 10.1038/s41591-021-01325-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gaebler C, Wang Z, Lorenzi J, et al. Evolution of antibody immunity to SARS-CoV-2. Nature. 2021;591:639–644. doi: 10.1038/s41586-021-03207-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geisen UM, Wang Z, Lorenzi JCC, et al. Immunogenicity and safety of anti-SARS-CoV-2 mRNA vaccines in patients with chronic inflammatory conditions and immunosuppressive therapy in a monocentric cohort. Ann Rheum Dis. 2021;80:1306–1311. doi: 10.1136/annrheumdis-2021-220272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goel RR, Apostolidis SA, Painter MM, et al. Distinct antibody and memory B cell responses in SARS-CoV-2 naïve and recovered individuals following mRNA vaccination. Sci Immunol. 2021;6:eabi6950. doi: 10.1126/sciimmunol.abi6950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krause PR, Fleming TR, Peto R, et al. Considerations in boosting COVID-19 vaccine immune responses. Lancet. 2021;398:1377–1380. doi: 10.1016/S0140-6736(21)02046-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lau Phua SK, Liang YL, C S, et al. Robust SARS-CoV-2 antibody responses in Asian COVID-naïve subjects 180 days after two doses of BNT162b2 mRNA COVID-19 vaccine. Vaccines. 2021;9:1241. doi: 10.3390/vaccines9111241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levin EG, Lustig Y, Cohen C, et al. Waning immune humoral response to BNT162b2 Covid-19 vaccine over 6 months. N Engl J Med. 2021;385:e84. doi: 10.1056/NEJMoa2114583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lo Sasso B, Giglio RV, Vidali M, et al. Evaluation of anti-SARS-Cov-2 S-RBD IgG antibodies after COVID-19 mRNA BNT162b2 vaccine. Diagnostics (Basel) 2021;11:1135. doi: 10.3390/diagnostics11071135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Naaber P, Tserel L, Kangro K, et al. Dynamics of antibody response to BNT162b2 vaccine after six months : a longitudinal prospective study. Lancet Reg Health Eur. 2021;10 doi: 10.1016/j.lanepe.2021.100208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakagawa S, Schielzeth H. A general and simple method for obtaining R 2 from generalized linear mixed-effects models. Methods Ecol Evol. 2013;4:133–142. [Google Scholar]
- Oliveira-Silva J, Reis T, Lopes C, Batista-Silva R, et al. Humoral response to the SARS-CoV-2 BNT162b2 mRNA vaccine: real-world data from a large cohort of healthcare workers. Vaccine. 2022;40:650–655. doi: 10.1016/j.vaccine.2021.12.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salvagno GL, Henry BM, Pighi L, De Nitto S, Gianfilippi GL, Lippi G. Three-month analysis of total humoral response to Pfizer BNT162b2 mRNA COVID-19 vaccination in healthcare workers. J Infect. 2021;83:e4–e5. doi: 10.1016/j.jinf.2021.06.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salvagno GL, Henry BM, Pighi L, De Nitto S, Lippi G. Total anti-SARS-CoV-2 antibodies measured 6 months after Pfizer-BioNTech COVID-19 vaccination in healthcare workers. J Med Biochem. 2022;41:199–203. doi: 10.5937/jomb0-33999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tartof SY, Slezak JM., Fischer H., et al. Effectiveness of mRNA BNT162b2 COVID-19 vaccine up to 6 months in a large integrated health system in the USA: a retrospective cohort study. Lancet. 2021;398:1407–1416. doi: 10.1016/S0140-6736(21)02183-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Terpos E, Karalis V, Ntanasis-Stathopoulos, et al. Robust neutralizing antibody responses 6 months post vaccination with BNT162b2: a prospective study in 308 healthy individuals. Life (Basel) 2021;11:1077. doi: 10.3390/life11101077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomas SJ, Moreira ED, Jr., Kitchin N, et al. Safety and efficacy of the BNT162b2 mRNA COVID-19 vaccine through 6 months. N Engl J Med. 2021;385:1761–1773. doi: 10.1056/NEJMoa2110345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tré-Hardy M, Cupaiolo R, Wilmet A, et al. Waning antibodies in SARS-CoV-2 naïve vaccinees: results of a three-month interim analysis of ongoing immunogenicity and efficacy surveillance of the mRNA-1273 vaccine in healthcare workers. J Infect. 2021;83:381–412. doi: 10.1016/j.jinf.2021.06.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tretyn A, Szczepanek J, Skorupa M, et al. Differences in the concentration of anti-SARS-CoV-2 IgG antibodies post-COVID-19 recovery or post-vaccination. Cells. 2021;10:1952. doi: 10.3390/cells10081952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- US FDA; Silver Spring: 2020. Vaccines and related biological products advisory committee meeting, December 17, 2020. FDA Briefing Document Moderna COVID-19 Vaccine; pp. 1–54. United States Food and Drug Administration. [Google Scholar]
- Zhong D, Xiao S, Debes AK, et al. Durability of antibody levels after vaccination with mRNA SARS-CoV-2 vaccine in individuals with or without prior infection. JAMA. 2021;326:2524–2526. doi: 10.1001/jama.2021.19996. [DOI] [PMC free article] [PubMed] [Google Scholar]