Abstract
Aim
To determine the prevalence and associated factors of vitamin D deficiency in COVID-19 survivors and the relationship between vitamin D status and physical performance.
Methods
Vitamin D status was assessed in a sample of patients who had recovered from COVID-19 and were admitted to a post-acute outpatient service at the Fondazione Policlinico Universitario Agostino Gemelli IRCCS (Rome, Italy). Participants were offered comprehensive medical assessment, including physical performance and muscle strength tests. Self-rated health was assessed. Vitamin D deficiency was defined as a serum concentration of 25-OH vitamin D < 20 ng/mL.
Results
Mean age of 681 participants was 53.4 ± 15.2 years and 49% were women. Vitamin D deficiency was detected in 35.6% of the whole study population, and in 40.2% of those 65 and older. Vitamin D deficiency was associated with diabetes, higher body mass index, and COVID-19 severity, and showed a seasonal pattern with a peak in autumn/winter. Participants with vitamin D deficiency performed poorer on the six-minute walking test, with the lowest performance observed in those 65 and older. No significant associations with any other parameters were found.
Conclusion
Our findings indicate that vitamin D deficiency is frequent in COVID-19 survivors, especially in older adults. Low vitamin D levels are associated with poor physical performance, in particular in old age.
Keywords: Aging, Six-minute walking test, Physical performance, Exercise tolerance, Geriatrics
1. Introduction
Low vitamin D status is as a common condition worldwide (Amrein et al., 2020, Cashman et al., 2016). The assessment of vitamin D status and the use of vitamin D supplements have increased substantially in recent years, though intervention trials testing vitamin D supplementation failed to show clear improvements in clinically relevant outcomes (Amrein et al., 2020, Bouillon et al., 2022). Inadequate dietary intake, insufficient sunlight exposure, and physical inactivity are among the key factors associated with low vitamin D levels. Older adults are at greater risk of vitamin D deficiency due to several age-related factors (e.g., reduced skin production and kidney conversion of vitamin D precursors) (Remelli et al., 2019). Low serum concentrations of vitamin D are associated with a number of negative health-related outcomes (Theodoratou et al., 2014), including increased risk of falls and fractures, impaired immune response, and lower muscle strength (Zhang and Naughton, 2010). Conversely, adequate vitamin D levels may support the beneficial effects of regular physical activity on physical performance in healthy older adults (Koundourakis et al., 2016). In addition, normal vitamin D levels are associated with reduced risk of cardiovascular disease, hypertension, type 2 diabetes, obesity, and metabolic syndrome (Theodoratou et al., 2014).
The active form of vitamin D (1,25-dihydroxyvitamin D) is involved in different pathways of the innate and adaptive immune system (Charoenngam and Holick, 2020). Persons with vitamin D deficiency tend to be more vulnerable to infectious diseases. Notably, vitamin D deficiency has been associated with increased risk of SARS-CoV-2 infection and worse clinical outcomes of COVID-19 (Baktash et al., 2021). However, whether poor vitamin D status per se or factors associated with vitamin D deficiency, such as advanced age and comorbidities, is related to COVID-19 severity is still a matter of debate (Rubin, 2021).
COVID-19 survivors often report persistent fatigue, joint pain, and myalgia (Carfì et al., 2020). Long-lasting weakness and muscle ache heavily impact quality of life (Glaser and Kiecolt-Glaser, 1987). Sequalae of acute COVID-19 are reported in all age groups. The prevalence of fatigue and joint and muscle pain in young adults is about 30–40%, and reaches 50–60% in those 50 and older (Lombardo et al., 2021). However, little is known on the possible association between vitamin D status and symptom persistence in COVID-19 survivors.
The aim of the present study was to determine the prevalence of low serum vitamin D levels across ages in a sample of COVID-19 survivors attending a dedicated outpatient service. The association of vitamin D status with self-rated health and physical performance was also investigated, with particular focus on older adults.
2. Materials and methods
The Gemelli Against COVID-19 Post-Acute Care (GAC19-PAC) project is an ongoing initiative developed by the Department of Geriatrics and Orthopedics of the Università Cattolica del Sacro Cuore (Rome, Italy) to investigate long-term consequences of COVID-19 and their impact on overall health, physical/cognitive performance, and quality of life. In April 2020, the Fondazione Policlinico Universitario Agostino Gemelli IRCCS (Rome, Italy) established a post-acute outpatient service for people who recovered from COVID-19. Details on the post-acute outpatient service and patient evaluation are reported elsewhere (Gemelli Against COVID-19 Post-Acute Care Study Group, 2020).
2.1. Study sample
The study population included adults admitted to the post-COVID-19 outpatient service between April 2020 and March 2021. On admission, all patients met the World Health Organization criteria for quarantine discontinuation: a period of 10 days after symptom onset plus at least three additional days without symptoms, for symptomatic patients, followed by at least one negative SARS-CoV-2 swab; or 10 days after positive test for SARS-CoV-2 for asymptomatic cases and at least one negative SARS-CoV-2 test (World Health Organization, 2020).
2.2. Data collection
Patients were offered a comprehensive medical assessment. A multidisciplinary approach was adopted to evaluate the long-term consequences of SARS-CoV-2 infection (Amin, 2021, Tajbakhsh et al., 2021, Wang et al., 2020, Aghagoli et al., 2021). Clinical parameters, medical history, current medications, lifestyle habits including physical activity and diet, and anthropometric measures were collected in a structured electronic database. Body weight was measured through an analog medical scale. Body height was measured using a standard stadiometer. Body mass index (BMI) was calculated as weight (kg) divided by the square of height (m). Regular participation in physical activity was operationalized as the engagement in leisure-time physical activity at least twice weekly during the past year.
COVID-19 severity was categorized as follows: (a) no hospitalization; (b) hospitalization with no oxygen supplementation; (c) hospitalization with low-flow oxygen supplementation; (d) hospitalization with noninvasive ventilation (NIV) or intensive care unit (ICU) admission with invasive ventilation.
Since vitamin D levels may vary across seasons due to differences in sun exposure, the timing of vitamin D assessment was categorized in two half-year periods (i.e., spring/summer and fall/winter) defined by equinox-to-equinox interval periods (Kasahara et al., 2013).
2.3. Serum vitamin D levels and standard blood biochemistry
Blood samples were collected by venipuncture after overnight fasting. Total serum 25-OH vitamin D levels were determined using a fully automated immunoassay (ADVIA Centaur Vitamin D Total Assay, Siemens Healthcare Diagnostics, Malvern, PA). Vitamin D deficiency was defined as a serum concentration of 25-OH vitamin D lower than 20 ng/mL (Holick, 2007, de Smet et al., 2021). Severe vitamin D was defined as serum 25-OH vitamin D levels lower than 12 ng/mL.
Plasma albumin, C-reactive protein (CRP), and hemoglobin levels were measured using standard biochemistry methods on fully automated testing systems.
2.4. Assessment of muscle strength and physical performance
Upper extremity muscle strength was measured by handgrip strength testing using a North Coast hand-held hydraulic dynamometer (North Coast Medical, Inc, Morgan Hill, CA) (Landi et al., 2020). The participant was seated on a chair with shoulder in a neutral position, the elbow near the trunk and flexed at 90°, and the wrist in a neutral position (thumbs up) (Landi et al., 2020). After one familiarization trial, muscle strength was measured in both hands and the higher value (kg) was used for the analysis.
Physical performance was evaluated by the one-min sit-to-stand test (1STST) and the six-minute walking test (6MWT). Both tests are commonly used to assess exercise-induced respiratory impairment in patients with respiratory diseases (Briand et al., 2018). For the 1STST, participants were asked to stand up from a chair and sit down with their arms folded across the chest for one minute as quickly as possible. A standard armless chair (43–47 cm in height) was used. The back of the chair was stabilized against a wall to ensure safety and stability. The number of times the patient completed the sit-to-stand movements was recorded; higher numbers reflect better performance (Bohannon and Crouch, 2019). The 6MWT was performed on a 20-m long track and the distance covered (m) was recorded; a longer distance covered indicates better performance (Guyatt et al., 1985).
2.5. Self-rated health
A visual analog scale (VAS) was used to obtain a quick evaluation of self-rated health, on a scale from 0 to 100, with 0 corresponding to the worst imaginable health and 100 indicating the best imaginable health (de Boer et al., 2004).
2.6. Statistical analyses
Continuous variables are expressed as mean ± standard deviation (SD), categorical variables are reported as frequencies by absolute value and percentage (%). Descriptive statistics were used to describe the clinical characteristics of the study population according to serum vitamin D levels. Differences in proportions and means of covariates between participants with and without vitamin D deficiency were assessed using Fisher’s Exact Test and t test statistics, respectively.
Cox regression with robust variance was used to assess the association between clinical and functional characteristics and vitamin D status. Candidate variables to be included in the Cox model were selected on the basis of biological and clinical plausibility. To identify factors independently associated with vitamin D deficiency, we first estimated the crude prevalence rate ratio (PR) and 95% confidence interval (CI). A multivariable Cox model was then computed including all variables that were associated with the outcome at an alpha level of 0.05, after adjustment for age and sex.
Analysis of covariance (ANCOVA) was also used to examine the association of vitamin D status with physical performance, muscle strength, and self-rated health, after adjustment for potential confounding variables. Variables considered as covariates were chosen according to their clinical significance and/or their significant difference at univariate analyses between participants with and without vitamin D deficiency. Interactions of covariates on the relationship between physical performance and muscle strength measures and vitamin D deficiency were tested by adding the interaction term into the adjusted models. No significant interactions were reported.
All analyses were performed using the SPSS software (version 11.0, SPSS Inc., Chicago, IL).
3. Results
Between April 2020 and March 2021, 742 patients were admitted to the post-COVID-19 outpatient service. The present investigation included 681 participants (mean age 53.4 ± 15.2 years; range 18–86, 49% women) for whom all variables of interest were available. The main characteristics of the study population according to vitamin D status are summarized in Table 1.
Table 1.
Characteristics of study participants according to vitamin D status.a.
| Characteristics | Whole sample (n = 681) | Serum vitamin D |
p | |
|---|---|---|---|---|
| Normal (n = 452) | Deficient (n = 229) | |||
| General and clinical characteristics | ||||
| Age (years) | 53.4 ± 15.2 | 52.2 ± 15.0 | 55.8 ± 15.3 | 0.004 |
| Sex (female) | 335 (49) | 233 (52) | 102 (44) | 0.05 |
| Education (years) | 14.5 ± 4.4 | 14.8 ± 3.8 | 13.9 ± 4.4 | 0.009 |
| Physically active | 374 (56) | 266 (60) | 108 (48) | 0.003 |
| Hypertension | 203 (29) | 121 (26) | 82 (36) | 0.01 |
| Heart failure | 17 (3) | 8 (2) | 9 (4) | 0.07 |
| Diabetes | 51 (7) | 20 (4) | 31 (13) | < 0.001 |
| Renal failure | 21 (3) | 8 (2) | 13 (5) | 0.007 |
| COPD | 54 (8) | 28 (6) | 26 (11) | 0.01 |
| Cancer | 15 (2) | 9 (2) | 6 (3) | 0.39 |
| BMI (kg/m2) | 25.7 ± 4.4 | 25.2 ± 4.3 | 26.7 ± 4.4 | < 0.001 |
| Severity of acute COVID-19 | ||||
| No hospitalization | 285 (41) | 210 (47) | 73 (32) | < 0.001 |
| Hospitalization - no O2 supplementation | 114 (17) | 71 (16) | 42 (19) | |
| Hospitalization - low-flow O2 supplementation | 176 (26) | 112 (25) | 65 (28) | |
| Hospitalization - NIV or IV | 106 (16) | 59 (13) | 49 (21) | |
| Length of hospital stay (days) | 16.8 ± 13.9 | 16.9 ± 14.6 | 16.6 ± 12.7 | 0.85 |
| Blood parameters | ||||
| Albumin (g/L) | 42.7 ± 3.2 | 43.1 ± 3.0 | 42.1 ± 3.5 | < 0.001 |
| Hemoglobin (g/dL) | 13.8 ± 1.4 | 13.8 ± 1.4 | 13.8 ± 1.5 | 0.52 |
| 25-OH vitamin D (ng/mL) | 25.6 ± 12.2 | 31.1 ± 11.2 | 14.8 ± 4.6 | < 0.001 |
| CRP (mg/L) | 2.4 ± 5.4 | 2.0 ± 4.4 | 3.3 ± 7.0 | < 0.001 |
BMI: body mass index; COPD: chronic obstructive pulmonary disease; CRP: C-reactive protein; IV: invasive ventilation; NIV: non-invasive ventilation.
Data are given as number (percentage) for sex, physical activity, diseases, and categories of COVID-19 severity; for all other variables, means ± standard deviations are reported.
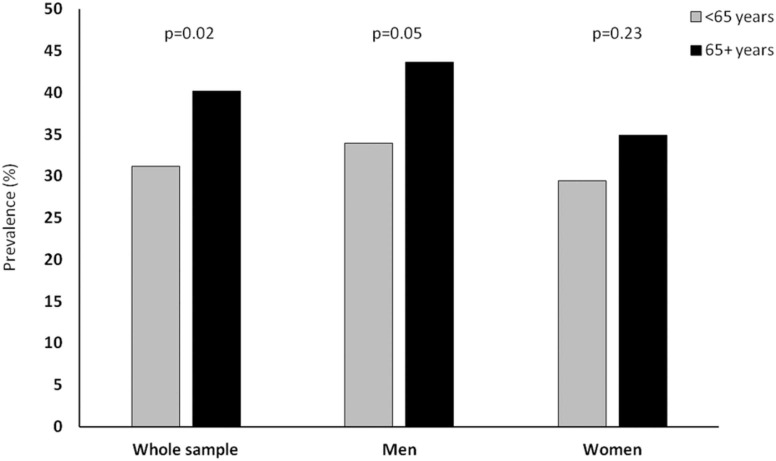
Vitamin D deficiency was detected in 35.6% of participants, with a higher prevalence in men than in women (36.7% vs. 30.4%, respectively; p = 0.05). Vitamin D deficiency was present in 29.6% of participants younger than 45, 33.5% of those between 45% and 65%, and 40.2% of people 65 and older. As in the whole sample, in older adults, the prevalence of vitamin D deficiency was higher in men than in women (43.6% vs. 33.9%, p = 0.05, Fig. 1).
Fig. 1.
Prevalence of vitamin D deficiency according to age groups and sex.
In participants 65 and older, mean 25-OH vitamin D levels were 20.5 ng/mL during autumn/winter and 26.1 ng/mL in spring/summer. A similar seasonal pattern was observed in those younger than 65 (24.2 ng/mL in autumn/winter and 27.5 ng/mL in spring/summer). The prevalence of vitamin D deficiency also showed a seasonal distribution, with a peak in autumn/winter (52.8% in older adults and 36.6% in younger participants) and through in spring/summer (34.8% in older adults and 27.6% in younger participants). The prevalence of severe vitamin D deficiency was 6.9%, and was higher in people 65 and older relative to younger adults (10.9% vs. 5.6%; p = 0.02). No differences were found between men and women.
The average time elapsed from COVID-19 diagnosis to admission to the outpatient service was similar between participants with and without vitamin D deficiency (95.8 ± 52.7 days vs. 85.3 ± 44.8 days, respectively; p = 0.1). Compared with those with no vitamin D deficiency, participant with low serum vitamin D levels were older, had higher prevalence of hypertension, diabetes, renal failure, and chronic obstructive pulmonary disease (COPD). Participants with normal vitamin D levels were more physically active and had higher education, while those with vitamin D deficiency had higher BMI and circulating CRP levels. The prevalence of vitamin D deficiency was higher in participants who had been hospitalized, particularly among those who had received supplemental oxygen. The length of hospital stay was similar between participants with and without vitamin D deficiency.
Cox regression models were used to assess the association between specific characteristics of the study population and vitamin D status. Results of unadjusted and adjusted models are shown in Table 2.
Table 2.
Unadjusted and adjusted association between specific participant characteristics and vitamin D deficiency.
| Characteristics | Unadjusted model | Adjusted model |
|---|---|---|
| OR (95% CI) | OR (95% CI) | |
| Age (years) | 1.01 (1.00–1.02) | 1.00 (0.99–1.02) |
| Sex (female) | 1.32 (0.96–1.82) | 1.10 (0.75–1.60) |
| Education | 0.94 (0.91–0.98) | 0.98 (0.93–1.02) |
| Physically active | 0.62 (0.45–0.86) | 0.84 (0.58–1.21) |
| Hypertension | 1.51 (1.07–2.12) | 0.85 (0.54–1.34) |
| Diabetes | 3.36 (1.87–6.04) | 2.02 (1.04–3.91) |
| Renal failure | 3.32 (1.35–8.14) | 1.53 (0.53–4.25) |
| COPD | 1.93 (1.10–3.37) | 1.43 (0.73–2.82) |
| BMI | 1.08 (1.04–1.12) | 1.06 (1.01–1.10) |
| CRP | 1.04 (1.01–1.08) | 1.03 (0.99–1.06) |
| Severity of COVID-19 | ||
| No hospitalization | 1.0 (Reference) | 1.0 (Reference) |
| Hospitalization − no O2 supplementation | 1.70 (1.07–2.70) | 1.64 (0.94–2.85) |
| Hospitalization − low-flow O2 supplementation | 1.65 (1.10–2.47) | 1.62 (0.96–2.73) |
| Hospitalization − NIV or IV | 2.36 (1.49–3.75) | 2.25 (1.27–3.98) |
| Autumn/winter season | 1.56 (1.13–2.15) | 2.28 (1.53–3–38) |
BMI: body mass index; COPD: chronic obstructive pulmonary disease; CI: confidence interval; CRP: C-reactive protein; IV: invasive ventilation; NIV: non-invasive ventilation; OR: odds ratio.
In the unadjusted model, a lower risk of vitamin D deficiency was found in participants with higher education (PR 0.94; 95% CI 0.91–0.98) and in those who were physically active (PR 0.62; 95% CI 0.45–0.86). Vitamin D deficiency was associated with hypertension, diabetes, renal failure, COPD, higher BMI and CRP levels, and with hospitalization during acute COVID-19. After multivariable adjustment, the likelihood of being vitamin D deficient was still significantly higher in participants with diabetes (PR 2.02; 95% CI 1.04–3.91), higher BMI (PR 1.06; 95% CI 1.01–1.10), and in those who had been hospitalized and required supplemental oxygen (PR 2.25; 95% CI 1.27–3.98). Seasonality was significantly associated with vitamin D status, with higher risk of vitamin D deficiency associated with assessment in autumn/winter both in unadjusted and adjusted models.
Unadjusted and adjusted ANCOVA models were built to evaluate the association between vitamin D status and physical performance and muscle strength measures as well as self-rated health. In the whole sample, vitamin D deficiency was associated with lower performance on the 6MWT in both men and women ( Table 3). No significant associations with any other parameters were found. In age-stratified analysis, vitamin D deficiency was associated with lower performance on the 6MWT in both younger and older participants ( Table 4). Older adults with normal vitamin D levels walked on average 50 m more than those with vitamin D deficiency (475.0 m vs. 421.9 m, respectively; p < 0.01). No other significant associations were determined in either age group.
Table 3.
Unadjusted and adjusteda means of physical performance and muscle strength measures and self-rated health in men and women according to vitamin D status.
| Unadjusted |
Adjusteda |
|||||||
|---|---|---|---|---|---|---|---|---|
| Normal vitamin D (n = 452) | Vitamin D deficiency (n = 229) | p | Normal vitamin D (n = 452) | Vitamin D deficiency (n = 229) | p | |||
| Physical performance measures | ||||||||
| Men | ||||||||
| Six-minute walking test (m) | 563.2 (5.80) | 521.4 (10.3) | < 0.001 | 558.2 (5.52) | 531.3 (7.62) | < 0.001 | ||
| One-minute chair stand test (no. of repetitions) | 27.6 (0.62) | 24.8 (1.05) | 0.01 | 26.9 (0.65) | 25.7 (0.87) | 0.29 | ||
| Women | ||||||||
| Six-minute walking test (m) | 531.3 (6.33) | 493.7 (12.9) | < 0.001 | 524.7 (5.65) | 509.3 (9.21) | 0.01 | ||
| One-minute chair stand test (no. of repetitions) | 26.0 (0.66) | 24.5 (0.99) | 0.21 | 25.7 (0.60) | 24.8 (0.92) | 0.42 | ||
| Handgrip strength (kg) | ||||||||
| Men | 35.8 (0.76) | 34.9 (1.05) | 0.49 | 35.4 (0.73) | 35.9 (0.99) | 0.66 | ||
| Women | 21.8 (0.49) | 21.2 (0.67) | 0.47 | 21.5 (0.46) | 21.7 (0.74) | 0.79 | ||
| Self-rated health (VAS) | ||||||||
| Men | 74.6 (1.02) | 71.2 (1.46) | 0.05 | 74.0 (1.07) | 71.9 (1.45) | 0.27 | ||
| Women | 66.1 (1.38) | 67.3 (1.83) | 0.63 | 66.6 (1.31) | 67.7 (2.02) | 0.65 | ||
Adjusted for age, education, physical activity, hypertension, chronic obstructive pulmonary disease, diabetes, renal failure, body mass index, albumin, C-reactive protein, and acute COVID-19 severity. VAS: visual analog scale.
Table 4.
Unadjusted and adjusteda means of physical performance and muscle strength measures and self-rated health in participants younger than 65 and 65 and older according to vitamin D status.
| Unadjusted |
Adjusteda |
|||||||
|---|---|---|---|---|---|---|---|---|
| Normal vitamin D (n = 452) | Vitamin D deficiency (n = 229) | p | Normal vitamin D (n = 452) | Vitamin D deficiency (n = 229) | p | |||
| Physical performance measures | ||||||||
| < 65 years | ||||||||
| Six-minute walking test (m) | 567.7 (3.90) | 542.5 (7.5) | < 0.001 | 563.0 (4.28) | 549.3 (6.53) | 0.05 | ||
| One-minute chair stand test (no. of repetitions) | 28.1 (0.45) | 25.6 (0.77) | 0.05 | 27.8 (0.48) | 26.6 (0.71) | 0.10 | ||
| ≥ 65 years | ||||||||
| Six-minute walking test (m) | 475.0 (10.7) | 421.9 (16.5) | < 0.01 | 470.6 (12.2) | 422.8 (16.7) | 0.02 | ||
| One-minute chair stand test (no. of repetitions) | 21.7 (1.16) | 19.5 (1.51) | 0.21 | 20.9 (1.32) | 19.9 (1.59) | 0.66 | ||
| Handgrip strength (kg) | ||||||||
| < 65 years | 29.6 (0.67) | 30.5 (0.99) | 0.48 | 29.7 (0.64) | 30.4 (1.01) | 0.53 | ||
| ≥ 65 years | 24.9 (0.93) | 24.2 (1.28) | 0.64 | 24.9 (1.04) | 23.7 (1.30) | 0.51 | ||
| Self-rated health (VAS) | ||||||||
| < 65 years | 70.7 (1.02) | 70.1 (1.39) | 0.70 | 71.3 (0.99) | 69.9 (1.50) | 0.46 | ||
| ≥ 65 years | 68.8 (1.68) | 67.9 (2.03) | 0.75 | 67.7 (1.78) | 68.2 (2.21) | 0.89 | ||
Adjusted for sex, education, physical activity, hypertension, chronic obstructive pulmonary disease, diabetes, renal failure, body mass index, albumin, C-reactive protein, and acute COVID-19 severity. VAS: visual analog scale.
4. DISCUSSION
In the present study, we investigated the prevalence of vitamin D deficiency and its association with clinical and functional parameters in a well-characterized sample of COVID-19 survivors. The overall prevalence of vitamin D deficiency was 35.6%, and reached 43.6% in men 65 and older. These findings are in keeping with those obtained in a post-COVID-19 outpatient clinic in Dublin, where vitamin D deficiency was found in approximately one-third of participants and more frequently in men (Townsend et al., 2021). A recent systematic review reported a wide range of prevalence of low vitamin D status (18–75%) in Southern European adult population, mainly because of high heterogeneity across studies (Manios et al., 2018). In the Italian population, the prevalence of vitamin D deficiency was estimated to be 35% in adults and 36% in those 65 and older aged (Manios et al., 2018). The prevalence of severe vitamin D deficiency (<10 ng/mL) was estimated to be around 16% across all age groups. It is noteworthy that, in post-COVID-19 patients, the prevalence of vitamin D deficiency shows a sex distribution different from the general population of the same geographic areas, in which lower vitamin D levels were found among adolescent girls, adult and older women compared with their male counterparts (Manios et al., 2018).
Findings on the association between serum vitamin D levels and COVID-19 are mixed (Baktash et al., 2021, Bassatne et al., 2021, Teshome et al., 2021). In older adults hospitalized with symptoms consistent with COVID-19, serum vitamin D levels were found to be lower in those who eventually tested positive for SARS-CoV-2 infection (Rubin, 2021). Moreover, an Israeli population-based study reported an association between low serum vitamin D levels and increased risk of COVID-19 and hospitalization (Merzon et al., 2020). A similar finding was reported in a retrospective cohort study of 489 patients at a medical center in Chicago (Meltzer et al., 2020). However, a large study in UK Biobank participants did not support a relationship between vitamin D status and susceptibility to SARS-CoV-2 infection (Hastie et al., 2020). Finally, in a cohort study of 18,148 individuals tested for SARS-CoV-2 seropositivity as part of a health screening program, 24.8% had a serum vitamin D level lower than 20 ng/mL (Li et al., 2021). However, SARS-CoV-2 seropositivity was not independently associated with vitamin D deficiency. As for COVID-19 severity, low serum vitamin D levels have been associated with worse clinical outcomes, including more severe lung involvement (Sulli et al., 2021), need of NIV, ICU admission, and mortality (Baktash et al., 2021).
In the present investigation, vitamin D deficiency was associated with diabetes, higher BMI, and COVID-19 severity. Low vitamin D levels are frequently observed in people with obesity, type 2 diabetes, and inflammatory conditions (Cheng et al., 2010, Lips et al., 2017, Pereira-Santos et al., Apr. 2015, Wöbke et al., 2014). In particular, an association between higher BMI and low vitamin D levels has been described and attributed to volumetric dilution in greater body structures (including serum, fat tissue, liver, muscle) Drincic et al., 2012). Noticeably, obesity and diabetes are among the strongest risk factors for severe COVID-19 (Kompaniyets et al., 2021, Mohammad et al., 2021). However, the design of our study does not allow causal relationships to be established between vitamin D status, its associated factors, and COVID-19 severity.
An association was found between vitamin D deficiency and lower physical performance in both younger and older COVID-19 survivors. In particular, participants 65 and older with vitamin D deficiency walked approximately 50 m less on the 6MWT than their peers with normal serum vitamin D levels. Consistent with our findings, a previous study showed that COVID-19 survivors with vitamin D deficiency had reduced exercise tolerance assessed using the Borg scale (Townsend et al., 2021). Although no causation can be established between vitamin D deficiency and poor physical performance in people who have recovered from COVID-19, vitamin D is known to influence cardiac and respiratory function, muscle health, and inflammation (Polly and Tan, 2014, Boxer et al., 2008). Indeed, vitamin D deficiency has been associated with high CRP levels and lower 6MWT performance in patients with heart failure (Boxer et al., 2008). Furthermore, low serum vitamin D levels have been linked with poor physical performance in older community-dwellers (Toffanello et al., 2012, Annweiler et al., Dec. 2017). While an association between vitamin D status and physical performance has consistently been found in older adults across different age decades and clinical conditions, a wide range of distances on the 6MWT has been reported, mainly due to heterogeneity of study populations (Annweiler et al., 2017). In a sample of frail older adults with heart failure (mean vitamin D levels, 26.7 ng/mL), the average distance covered on the 6MWT was 309 m (Boxer et al., 2008), while in older community-dwellers with low serum vitamin D levels (<20 ng/mL) from the Progetto Veneto Anziani (Pro.V.A.), the average 6MWT performance was 283.5 m in women and 345.6 m in men, respectively (Toffanello et al., 2012). In Icelandic older adults living in the community, serum vitamin D levels (mean value, 67 nmol/L) were associated with walking performance on the 6MWT (459 m in women and 466 m in men) (Geirsdottir et al., 2016). However, the association was not independent and was mostly explained by confounding factors, such as BMI and self-reported physical activity (Geirsdottir et al., 2016). Our findings support the existence of an association between vitamin D status and physical performance in older adults that may be independent of pre-existing conditions, including previous SARS-CoV-2 infection or long COVID. The effects of vitamin D on physical performance may be mediated by vitamin D receptors that are expressed throughout the cardiovascular system and the skeletal muscle as well as by pleiotropic actions of vitamin D on metabolic and inflammatory pathways (Bouillon et al., 2014, Bouillon et al., 2019, Rodriguez et al., 2018, Gardner et al., 2013).
Some methodological issues should be taken into account in the interpretation of results. Limitations of the study include the lack of information on vitamin D status before COVID-19. Furthermore, this was a single-center study with a relatively large number of COVID-19 survivors, but without a control group of participants who had recovered from other acute conditions. Community-acquired pneumonia and other viral diseases have also been associated with high prevalence of vitamin D deficiency (Siddiqui et al., 2020, Zhou et al., 2019), suggesting that our findings may be not exclusive to COVID-19. However, the clinical characteristics of participants make it possible to exclude that acute illnesses were present at the time of evaluation. The observational nature of our study prevents establishing a cause-effect relationship between vitamin D deficiency and poor physical performance because reverse causation of residual confounding cannot be excluded. Indeed, diabetes, obesity, and elevated CRP levels are all associated with vitamin D deficiency and well-established risk factors for severe COVID-19 (Luan et al., 2021, Kompaniyets et al., 2021). Moreover, vitamin D status is a general marker of good health. Information regarding the intake of macro- and micronutrients (including vitamin D and protein) or the use of vitamin D supplements was not available for most study participants. Hence, influence of diet and/or supplementation on vitamin D status and physical performance could not be evaluated.
In conclusion, we provide an appraisal of vitamin D status in a large sample of well-characterized COVID-19 survivors. We found a high prevalence of vitamin D deficiency, especially in older men. Low vitamin D levels were more common in participants who had been hospitalized and required supplemental oxygen during acute COVID-19. Moreover, vitamin D deficiency was associated with lower performance on the 6MWT, with the shorter distance walked by older participants. Future studies are warranted to clearly establish the association between vitamin D status and acute and post-acute COVID-19 and to assess whether vitamin D supplementation may improve physical performance in COVID-19 survivors.
The Gemelli Against COVID-19 Post-Acute Care Study Group is composed as follows:
Steering committee: Landi Francesco, Gremese Elisa.
Coordination: Bernabei Roberto, Fantoni Massimo, Gasbarrini Antonio.
Field investigators:
-
–
Gastroenterology team: Porcari Serena, Settanni Carlo Romano
-
–
Geriatric team: Benvenuto Francesca, Bramato Giulia, Brandi Vincenzo, Carfì Angelo, Ciciarello Francesca, Fabrizi Sofia, Galluzzo Vincenzo, Lo Monaco Maria Rita, Martone Anna Maria, Marzetti Emanuele, Napolitano Carmen, Pagano Francesco Cosimo, Pais Cristina, Rocchi Sara, Rota Elisabetta, Salerno Andrea, Tosato Matteo, Tritto Marcello, Zazzara Maria Beatrice, Calvani Riccardo, Catalano Lucio, Picca Anna, Savera Giulia, Damiano Francesco Paolo, Rocconi Alessandra, Galliani Alessandro, Spaziani Giovanni, Tupputi Salvatore, Cocchi Camilla, Pirone Flavia
-
–
Infectious Disease team: Cauda Roberto, Tamburrini Enrica, Borghetti A, Di Gianbenedetto Simona, Murri Rita, Cingolani Antonella, Ventura Giulio, Taddei Eleonora, Moschese Davide, Ciccullo Arturo, Dusina Alex
-
–
Internal Medicine team: Stella Leonardo, Addolorato Giovanni, Franceschi Francesco, Mingrone Gertrude, Zocco Maria Assunta
-
–
Microbiology team: Sanguinetti Maurizio, Cattani Paola, Marchetti Simona, Posteraro Brunella, Sali Michela
-
–
Neurology team: Bizzarro Alessandra, Lauria Alessandra
-
–
Ophthalmology team: Rizzo Stanislao, Savastano Maria Cristina, Gambini G, Cozzupoli Maria Grazie, Culiersi Carola
-
–
Otolaryngology team: Passali Giulio Cesare, Paludetti Gaetano, Galli Jacopo, Crudo Fabrizio, Di Cintio Giovanni, Longobardi Ylenia, Tricarico Laura, Santantonio Maria Teresa
-
–
Pediatric team: Buonsenso Danilo, Valentini Piero, Pata Davide, Sinatti Dario, De Rose Cristina
-
–
Pneumology team: Richeldi Luca, Lombardi Francesco, Calabrese Angelo, Leone Paolo Maria, Calvello Maria Rosaria, Intini Enrica, Montemurro Giuliano
-
–
Psychiatric team: Sani Gabriele, Janiri Delfina, Simonetti Alessio, Giuseppin Giulia, Molinaro Marzia, Modica Marco
-
–
Radiology team: Natale Luigi, Larici Anna Rita, Marano Riccardo
-
–
Rheumatology team: Paglionico Annamaria, Petricca Luca, Gigante Luca, Natalello Gerlando, Fedele Anna Laura, Lizzio Marco Maria, Tolusso Barbara, Di Mario Clara, Alivernini Stefano
-
–
Vascular team: Santoliquido Angelo, Santoro Luca, Di Giorgio Angela, Nesci Antonio, Popolla Valentina
Ethics approval and consent to participate
The study was approved by the Ethics Committee of the Università Cattolica del Sacro Cuore (Rome, Italy).
Funding
This study was supported by Fondazione Memmo, Fondazione Angelini, Danone, Nutricia, Ministero della Salute - Ricerca Corrente 2022.
Acknowledgments
The Gemelli Against COVID-19 Post-Acute Care team thanks La Torre R, Brisetti S, Fella L, Sofo MT, and all the nurse staff of the “Post-COVID Day Hospital Unit” for their extraordinary dedication and expertise in treating COVID-19 patients.
Consent for publication
The author gives consent for publication of this paper.
Competing interests
None of the members of the Gemelli Against COVID-19 Post-Acute Care Study Group has any conflict of interest.
Data availability
The authors do not have permission to share data.
References
- Aghagoli G., Gallo Marin B., Katchur N.J., Chaves-Sell F., Asaad W.F., Murphy S.A. Neurological involvement in COVID-19 and potential mechanisms: a review. Neurocrit. Care. 2021;34(3):1062–1071. doi: 10.1007/s12028-020-01049-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amin M. COVID-19 and the liver: overview. Eur. J. Gastroenterol. Hepatol. 2021;33(3):309–311. doi: 10.1097/MEG.0000000000001808. 10.1097/MEG.0000000000001808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amrein K., Scherkl M., Hoffmann M., Neuwersch-Sommeregger S., Köstenberger M., Tmava Berisha A., Martucci G., Pilz S., Malle O. Vitamin D deficiency 2.0: an update on the current status worldwide. Eur. J. Clin. Nutr. 2020;74(11):1498–1513. doi: 10.1038/s41430-020-0558-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Annweiler C., Henni S., Walrand S., Montero-Odasso M., Duque G., Duval G.T. Vitamin D and walking speed in older adults: systematic review and meta-analysis. Maturitas. 2017;106:8–25. doi: 10.1016/j.maturitas.2017.07.012. [DOI] [PubMed] [Google Scholar]
- Baktash V., Hosack T., Patel N., Shah S., Kandiah P., Van den Abbeele K., Mandal A., Missouris C.G. Vitamin D status and outcomes for hospitalised older patients with COVID-19. Postgrad. Med. J. 2021;97(1149):442–447. doi: 10.1136/postgradmedj-2020-138712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bassatne A., Basbous M., Chakhtoura M., el Zein O., Rahme M., El-Hajj Fuleihan G. The link between COVID-19 and VItamin D (VIVID): a systematic review and meta-analysis. Metabolism. 2021;119 doi: 10.1016/j.metabol.2021.154753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bohannon R.W., Crouch R. 1-minute sit-to-stand test: systematic review of procedures, performance, and clinimetric properties. J. Cardiopulm. Rehabil. Prev. 2019;39(1):2–8. doi: 10.1097/HCR.0000000000000336. [DOI] [PubMed] [Google Scholar]
- Bouillon R., Carmeliet G., Lieben L., Watanabe M., Perino A., Auwerx J., Schoonjans K., Verstuyf A. Vitamin D and energy homeostasis: of mice and men. Nat. Rev. Endocrinol. 2014;10(2):79–87. doi: 10.1038/nrendo.2013.226. [DOI] [PubMed] [Google Scholar]
- Bouillon R., Marcocci C., Carmeliet G., Bikle D., White J.H., Dawson-Hughes B., Lips P., Munns C.F., Lazaretti-Castro M., Giustina A., Bilezikian J. Skeletal and extraskeletal actions of vitamin D: current evidence and outstanding questions. Endocr. Rev. 2019;40(4):1109–1151. doi: 10.1210/er.2018-00126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bouillon R., Manousaki D., Rosen C., Trajanoska K., Rivadeneira F., Richards J.B. The health effects of vitamin D supplementation: evidence from human studies. Nat. Rev. Endocrinol. 2022;18(2):96–110. doi: 10.1038/s41574-021-00593-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boxer R.S., Dauser D.A., Walsh S.J., Hager W.D., Kenny A.M. The association between vitamin D and inflammation with the 6-minute walk and frailty in patients with heart failure. J. Am. Geriatr. Soc. 2008;56(3):454–461. doi: 10.1111/j.1532-5415.2007.01601.x. [DOI] [PubMed] [Google Scholar]
- Briand J., Behal H., Chenivesse C., Wémeau-Stervinou L., Wallaert B. The 1-minute sit-to-stand test to detect exercise-induced oxygen desaturation in patients with interstitial lung disease. Ther. Adv. Respir. Dis. 2018;12 doi: 10.1177/1753466618793028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carfì A., Bernabei R., Landi F., Gemelli Against COVID-19 post-acute care study group, “persistent symptoms in patients after acute COVID-19. JAMA. 2020;324(6):603–605. doi: 10.1001/jama.2020.12603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cashman K.D., Dowling K.G., Škrabáková Z., Gonzalez-Gross M., Valtueña J., De Henauw S., Moreno L., Damsgaard C.T., Michaelsen K.F., Mølgaard C., Jorde R., Grimnes G., Moschonis G., Mavrogianni C., Manios Y., Thamm M., Mensink G.B., Rabenberg M., Busch M.A., Cox L., Meadows S., Goldberg G., Prentice A., Dekker J.M., Nijpels G., Pilz S., Swart K.M., van Schoor N.M., Lips P., Eiriksdottir G., Gudnason V., Cotch M.F., Koskinen S., Lamberg-Allardt C., Durazo-Arvizu R.A., Sempos C.T., Kiely M. Vitamin D deficiency in Europe: pandemic? Am. J. Clin. Nutr. 2016;103(4):1033–1044. doi: 10.3945/ajcn.115.120873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Charoenngam N., Holick M.F. Immunologic effects of vitamin D on human health and disease. Nutrients. 2020;12(7) doi: 10.3390/nu12072097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheng S., Massaro J.M., Fox C.S., Larson M.G., Keyes M.J., McCabe E.L., Robins S.J., O’Donnell C.J., Hoffmann U., Jacques P.F., Booth S.L., Vasan R.S., Wolf M., Wang T.J. Adiposity, cardiometabolic risk, and vitamin D status: the Framingham heart study. Diabetes. 2010;59(1):242–248. doi: 10.2337/db09-1011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drincic A.T., Armas L.A.G., van Diest E.E., Heaney R.P. Volumetric dilution, rather than sequestration best explains the low vitamin D status of obesity. Obesity. 2012;20(7):1444–1448. doi: 10.1038/oby.2011.404. [DOI] [PubMed] [Google Scholar]
- Gardner D.G., Chen S., Glenn D.J. Vitamin D and the heart. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2013;305(9):R969–R977. doi: 10.1152/ajpregu.00322.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geirsdottir O.G., Ramel A., Chang M., Briem K., Jonsson P.V., Thorsdottir I. Vitamin D and associations with walking ability in community- dwelling elderly adults. J. Food Nutr. Disord. 2016;5 doi: 10.4172/2324-9323.1000192. [DOI] [Google Scholar]
- Gemelli Against COVID-19 Post-Acute Care Study Group Post-COVID-19 global health strategies: the need for an interdisciplinary approach. Aging Clin. Exp. Res. 2020;32(8):1613–1620. doi: 10.1007/s40520-020-01616-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glaser R., Kiecolt-Glaser J. Stress-associated depression in cellular immunity: implications for acquired immune deficiency syndrome (AIDS) Brain Behav. Immun. 1987;1(2):107–112. doi: 10.1016/0889-1591(87)90013-4. [DOI] [PubMed] [Google Scholar]
- Guyatt G.H., et al. The 6-minute walk: a new measure of exercise capacity in patients with chronic heart failure. Can. Med. Assoc. J. 1985;132(8):919–923. [PMC free article] [PubMed] [Google Scholar]
- Hastie C.E., Mackay D.F., Ho F., Celis-Morales C.A., Katikireddi S.V., Niedzwiedz C.L., Jani B.D., Welsh P., Mair F.S., Gray S.R., O’Donnell C.A., Gill J.M., Sattar N., Pell J.P. Vitamin D concentrations and COVID-19 infection in UK Biobank Diabetes. Metab. Syndr. 2020;14(4):561–565. doi: 10.1016/j.dsx.2020.04.050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holick M.F. Vitamin D deficiency. N. Engl. J. Med. 2007;357(3):266–281. doi: 10.1056/NEJMra070553. (Jul.) [DOI] [PubMed] [Google Scholar]
- Kasahara A.K., Singh R.J., Noymer A. Vitamin D (25OHD) serum seasonality in the United States. PLOS One. 2013;8(6) doi: 10.1371/journal.pone.0065785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kompaniyets L., Pennington A.F., Goodman A.B., Rosenblum H.G., Belay B., Ko J.Y., Chevinsky J.R., Schieber L.Z., Summers A.D., Lavery A.M., Preston L.E., Danielson M.L., Cui Z., Namulanda G., Yusuf H., Mac Kenzie W.R., Wong K.K., Baggs J., Boehmer T.K., Gundlapalli A.V. Underlying medical conditions and severe illness among 540,667 adults hospitalized with COVID-19, March 2020-March 2021. Prev. Chronic Dis. 2021;18 doi: 10.5888/pcd18.210123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koundourakis N.E., Avgoustinaki P.D., Malliaraki N., Margioris A.N. Muscular effects of vitamin D in young athletes and non-athletes and in the elderly. Hormones. 2016;15(4):471–488. doi: 10.14310/horm.2002.1705. [DOI] [PubMed] [Google Scholar]
- Landi F., Calvani R., Martone A.M., Salini S., Zazzara M.B., Candeloro M., Coelho-Junior H.J., Tosato M., Picca A., Marzetti E. Normative values of muscle strength across ages in a ‘real world’ population: results from the longevity check-up 7+ project. J. Cachexia Sarcopenia Muscle. 2020;11(6):1562–1569. doi: 10.1002/jcsm.12610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Y., Tong C.H., Bare L.A., Devlin J.J. Assessment of the association of vitamin D level with SARS-CoV-2 seropositivity among working-age adults. JAMA Netw. Open. 2021;4(5) doi: 10.1001/jamanetworkopen.2021.11634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lips P., Eekhoff M., van Schoor N., Oosterwerff M., de Jongh R., Krul-Poel Y., Simsek S. Vitamin D and type 2 diabetes. J. Steroid Biochem. Mol. Biol. 2017;173:280–285. doi: 10.1016/j.jsbmb.2016.11.021. [DOI] [PubMed] [Google Scholar]
- Lombardo M., Foppiani A., Peretti G.M., Mangiavini L., Battezzati A., Bertoli S., Martinelli Boneschi F., Zuccotti G.V. Long-term coronavirus disease 2019 complications in inpatients and outpatients: a one-year follow-up cohort study. Open Forum Infect. Dis. 2021;8(8):384. doi: 10.1093/ofid/ofab384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luan Y.-Y., Yin C.-H., Yao Y.-M. Update advances on C-reactive protein in COVID-19 and other viral infections. Front. Immunol. 2021;12 doi: 10.3389/fimmu.2021.720363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manios Y., Moschonis G., Lambrinou C.P., Tsoutsoulopoulou K., Binou P., Karachaliou A., Breidenassel C., Gonzalez-Gross M., Kiely M., Cashman K.D. A systematic review of vitamin D status in southern European countries. Eur. J. Nutr. 2018;57(6):2001–2036. doi: 10.1007/s00394-017-1564-2. [DOI] [PubMed] [Google Scholar]
- Meltzer D.O., Best T.J., Zhang H., Vokes T., Arora V., Solway J. Association of vitamin D status and other clinical characteristics with COVID-19 test results. JAMA Netw. Open. 2020;3(9) doi: 10.1001/jamanetworkopen.2020.19722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Merzon E., Tworowski D., Gorohovski A., Vinker S., Golan Cohen A., Green I., Frenkel-Morgenstern M. Low plasma 25(OH) vitamin D level is associated with increased risk of COVID-19 infection: an Israeli population-based study. FEBS J. 2020;287(17):3693–3702. doi: 10.1111/febs.15495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mohammad S., Aziz R., Al Mahri S., Malik S.S., Haji E., Khan A.H., Khatlani T.S., Bouchama A. Obesity and COVID-19: what makes obese host so vulnerable? Immun. Ageing. 2021;18(1):1. doi: 10.1186/s12979-020-00212-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pereira-Santos M., Costa P.R.F., Assis A.M.O., Santos C.A.S.T., Santos D.B. Obesity and vitamin D deficiency: a systematic review and meta-analysis. Obes. Rev. 2015;16(4):341–349. doi: 10.1111/obr.12239. [DOI] [PubMed] [Google Scholar]
- Polly P., Tan T.C. The role of vitamin D in skeletal and cardiac muscle function. Front. Physiol. 2014;5:145. doi: 10.3389/fphys.2014.00145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Remelli F., Vitali A., Zurlo A., Volpato S. Vitamin D deficiency and sarcopenia in older persons. Nutrients. 2019;11(12) doi: 10.3390/nu11122861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodriguez A.J., Mousa A., Ebeling P.R., Scott D., de Courten B. Effects of vitamin D supplementation on inflammatory markers in heart failure: a systematic review and meta-analysis of randomized controlled trials. Sci. Rep. 2018;8(1):1169. doi: 10.1038/s41598-018-19708-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rubin R. Sorting out whether vitamin D deficiency raises COVID-19 risk. JAMA. 2021;325(4):329–330. doi: 10.1001/jama.2020.24127. [DOI] [PubMed] [Google Scholar]
- Siddiqui M., Manansala J.S., Abdulrahman H.A., Nasrallah G.K., Smatti M.K., Younes N., Althani A.A., Yassine H.M. Immune modulatory effects of vitamin D on viral Infections. Nutrients. 2020;12(9) doi: 10.3390/nu12092879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Boer A., van Lanschot J.J., Stalmeier P.F., van Sandick J.W., Hulscher J.B., de Haes J.C., SprangersBoer M.A. Is a single-item visual analogue scale as valid, reliable and responsive as multi-item scales in measuring quality of life? Qual. Life Res. 2004;13(2):311–320. doi: 10.1023/B:QURE.0000018499.64574.1f. [DOI] [PubMed] [Google Scholar]
- de Smet D., de Smet K., Herroelen P., Gryspeerdt S., Martens G.A. Serum 25(OH)D level on hospital admission associated with COVID-19 stage and mortality. Am. J. Clin. Pathol. 2021;155(3):381–388. doi: 10.1093/ajcp/aqaa252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sulli A., Gotelli E., Casabella A., Paolino S., Pizzorni C., Alessandri E., Grosso M., Ferone D., Smith V., Cutolo M. Vitamin D and lung outcomes in elderly COVID-19 patients. Nutrients. 2021;13(3) doi: 10.3390/nu13030717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tajbakhsh A., Gheibi Hayat S.M., Taghizadeh H., Akbari A., Inabadi M., Savardashtaki A., Johnston T.P., Sahebkar A. COVID-19 and cardiac injury: clinical manifestations, biomarkers, mechanisms, diagnosis, treatment, and follow up. Expert Rev. Anti Infect. Ther. 2021;19(3):345–357. doi: 10.1080/14787210.2020.1822737. [DOI] [PubMed] [Google Scholar]
- Teshome A., Adane A., Girma B., Mekonnen Z.A. The impact of vitamin D level on COVID-19 infection: systematic review and meta-analysis Front. Front. Public Health. 2021;9 doi: 10.3389/fpubh.2021.624559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Theodoratou E., Tzoulaki I., Zgaga L., Ioannidis J.P.A. Vitamin D and multiple health outcomes: umbrella review of systematic reviews and meta-analyses of observational studies and randomised trials. BMJ. 2014;348 doi: 10.1136/bmj.g2035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Toffanello E.D., Perissinotto E., Sergi G., Zambon S., Musacchio E., Maggi S., Coin A., Sartori L., Corti M.C., Baggio G., Crepaldi G., Manzato E. Vitamin D and physical performance in elderly subjects: the Pro.V.A study. PLOS One. 2012;7(4) doi: 10.1371/journal.pone.0034950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Townsend L., Dyer A.H., McCluskey P., O’Brien K., Dowds J., Laird E., Bannan C., Bourke N.M., Ní Cheallaigh C., Byrne D.G., Kenny R.A. Investigating the relationship between vitamin D and persistent symptoms following SARS-CoV-2 infection. Nutrients. 2021;13(7) doi: 10.3390/nu13072430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang F., Kream R.M., Stefano G.B. Long-term respiratory and neurological sequelae of COVID-19. Med Sci. Monit. 2020;26 doi: 10.12659/MSM.928996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wöbke T.K., Sorg B.L., Steinhilber D. Vitamin D in inflammatory diseases. Front. Physiol. 2014;5 doi: 10.3389/fphys.2014.00244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang R., Naughton D.P. Vitamin D in health and disease: current perspectives. Nutr. J. 2010;9:65. doi: 10.1186/1475-2891-9-65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou Y.-F., Luo B.-A., Qin L.-L. The association between vitamin D deficiency and community-acquired pneumonia: a meta-analysis of observational studies. Medicine. 2019;98(38) doi: 10.1097/MD.0000000000017252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- World Health Organization, 2020. Criteria for releasing COVID-19 patients from isolation. https://www.who.int/news-room/commentaries/detail/criteria-for-releasing-covid-19-patients-from-isolation. Accessed on 5 January 2022.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The authors do not have permission to share data.