Abstract
Introduction:
Hydrogen peroxide is a strong oxidant that possesses an antimicrobial activity. It has been successfully used in surface/room decontamination processes either under the form of hydrogen peroxide vapor (HPV) or of vaporized hydrogen peroxide (VHP). Aerosolized hydrogen peroxide (aHP) offers a third alternative. The technology relies on the dispersion of aerosols of a hydrogen peroxide solution often complemented with silver cations. aHP provides an inexpensive and safe approach to treat contaminated rooms but sometimes fails to achieve the 6-log10 reduction limit in the number of viable microorganisms.
Methods:
Here, we used a venturi-based aHP generator that generates 4 mm in size aerosols from a 12% plasma-activated hydrogen peroxide solution free of silver cations.
Results & Discussion:
We could successfully and constantly inactivate bacterial growth from biological indicators containing at least 106 spores of Geobacillus stearothermophilus placed on stainless steel discs wrapped in Tyvek pouches. We could also show that the biological indicators placed at various locations in a class II biosafety cabinet were equally inactivated, showing that hydrogen peroxide aerosols migrate through HEPA filters.
Conclusions:
Considering that our method for aerosol generation is simple, reproducible, and highly effective at inactivating spores, our approach is expected to serve as a relatively cost effective alternative method for disinfecting potentially contaminated rooms or surfaces.
Keywords: aerosolized hydrogen peroxide, aHP, room decontamination, spore inactivation, fogging
Fumigation is a widely used method to disinfect large volumes potentially contaminated with microorganisms. Traditional gaseous fumigants such as formaldehyde, ethylene oxide, or chlorine dioxide show high efficiency and reliability but are potentially harmful to users. At best, they act as a respiratory and mucosal irritant, while at worst they are highly toxic, even at low exposure levels.1 Indeed, formaldehyde is a potent toxin (Occupational Safety and Health Administration [OSHA] Permissible Exposure Limit [PEL] = 0.75 ppm; OSHA Short-Term Exposure Limit [STEL] = 2 ppm; revised Immediately Dangerous to Life or Health [IDLH] = 20 ppm) and a carcinogen,2 whereas chlorine dioxide gas is toxic3 (OSHA PEL = 0.1 ppm; OSHA STEL = 0.3 ppm; revised IDLH = 5 ppm) and explosive at concentrations of more than 9% in air.4
Hydrogen peroxide is a powerful oxidant that proved to be efficient as a sterilant and disinfectant5 (OSHA PEL = 1 ppm; OSHA STEL = none; revised IDHL = 75 ppm). It is colorless, odorless, and relatively safe since it readily decomposes to form water and oxygen. Numerous studies have demonstrated the efficiency of hydrogen peroxide surface decontamination using hydrogen peroxide vapors (HPV), vaporized hydrogen peroxide (VHP), or aerosolized hydrogen peroxide (aHP), the latter being sometimes referred to as dry mist hydrogen peroxide (DMHP).1,6 -9 These approaches have been proven successful with bacteria,10 -13 spores of bacteria5,14 -16 and fungi,17 protozoa and their cysts, viruses,8 and even prions.18 VHP19 and HPV11 were shown to kill Mycobacterium tuberculosis, whereas the genuine efficiency of aHP in inactivating the same bacteria12,20 was the subject of lively discussion21,22 stressing the fact that hydrogen peroxide vapor and hydrogen peroxide aerosol decontamination might not be directly compared. Indeed, in studies looking at the efficacy of hydrogen peroxide against spores of Clostridium difficile, HPV or VHP systems resulted in a more than 6-log10 reduction in the number of viable spores,13 whereas the aHP approach was 1- to 2-log10 less efficient.23,24 Withstanding important differences in experimental setting and methodology, these data pointed to the fact that hydrogen peroxide aerosols were less efficient than hydrogen peroxide vapors.22 Substantial differences in the microbiological impact of the 2 systems were also observed when targeting environmental C difficile contaminations.25,26 There are a few major differences between the vapor (HPV or VHP) and the aerosol (aHP) approaches. First, the concentration of products used was quite different. Vapors are generated from a 30% to 35% H2O2 solution,27 whereas in aerosols, the active solution is made from a 5% to 6% H2O2 solution containing less than 50 ppm of silver ions.28 Second, HPV or VHP generators produce a more or less homogeneous condensing or noncondensing vapor, whereas aerosol generators produce a nonhomogeneous dry mist of particles ranging from 0.5 to 12 μm in size.29,30 To achieve an effective kill, the required sporicidal concentrations with hydrogen peroxide vapors range from 150 to 750 ppm (≈0.2 to 1 mg/L) at room temperature,31 but higher concentrations are routinely used.32 When aerosolized hydrogen peroxide systems were used, the final concentration of decontaminants was much lower, between 2 and 160 ppm depending on the experimental system.20,28,33 Weak concentrations in active compound might account for the poor sporicidal effect of hydrogen peroxide aerosols.34 Based on these observations, Otter et al34 proposed that hydrogen peroxide vapor-based protocols should be considered as fumigation processes, whereas the use of hydrogen peroxide aerosols should be seen as a fogging application, whose decontaminating (sporicidal) potential still needs to be set straight.
For room or surface decontamination, hydrogen peroxide is generally delivered as HPV or as VHP. In the first system, the HPV generator is vaporizing from a 35% solution of hydrogen peroxide until the air is saturated and the hydrogen peroxide begins to condense on surfaces.11 In the latter system, the VHP generator is vaporizing a 35% solution without condensing.19 Both approaches proved to be successful in inactivating hard to kill bacteria such as Mycobacterium tuberculosis or spores.5,35 Since it is a powerful oxidant, there is the risk that exposing sensitive instruments to condensed hydrogen peroxide may result in increased stress or corrosion on certain materials.11,36,37 This, together with quite the high cost of purchasing and maintaining VHP and HPV generators, prompted us to consider the aHP method to decontaminate fully equipped medical or research settings.
Classical aHP combines pressure-generated, small, charged aerosols generated from a 5% to 10% hydrogen peroxide solution with <50 ppm silver cations.38 The 2 agents synergize for increased effect on microorganisms.39 aHP proved to be quite efficient in the decontamination of hospital rooms but showed more contrasted results in the inactivation of vegetative bacteria or M tuberculosis by sometimes failing to reach a consistent 6-log10 reduction.12,23,24,26,29,40,41
The objective of this study was to measure the effectiveness of a new generation of aHP generators using plasma-activated hydrogen peroxide (containing no silver ions) in producing a consistent high-level (at least 6-log10) inactivation of bacterial spores in a laboratory setting. Plasma activation generates an antimicrobial H2O2 solution that consists of superoxidized water containing various ions, peroxides, superoxide molecules, and oxygen derivatives. The ionic charge of the solution disrupts the cellular membrane permeability, inhibiting the enzymatic activities and denaturing cellular proteins. It also opens up transport mechanisms across the cellular wall, enabling the rapid permeation of peroxide into the cell. Over time, plasma-activated H2O2 reverts to water and oxygen, leaving no residues.
Materials and Methods
Description of the aHP Generator and of the Plasma-Activated Hydrogen Peroxide Solution
The aHP generator was purchased from Saniswiss (Saniswiss SA, Geneva, Switzerland) (Figure 1A,B). It generates aerosols (made of microparticles of approximately 4 μm in size) by a venturi system, creating powerful cyclones dispersing the hydrogen peroxide solution. The venturi system enhances the ionization of the plasma-activated peroxide solution.
Figure 1.
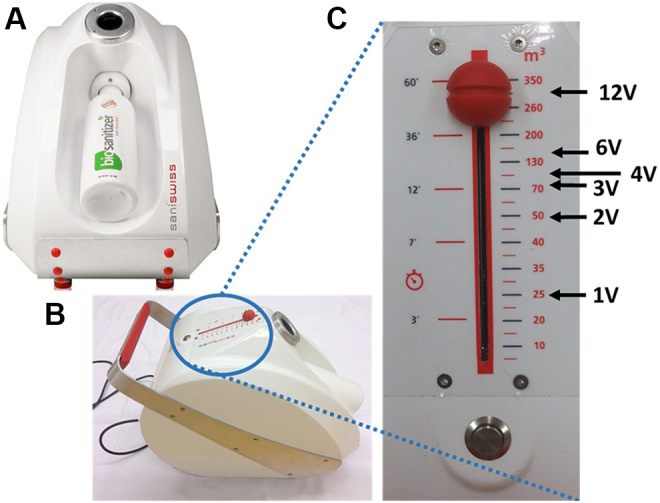
Description of the aerosolized hydrogen peroxide (aHP) generator used in the study. (A) Front view. The aHP generator can accommodate a 1-L bottle of 12% plasma-activated hydrogen peroxide that is sufficient to produce enough aHP to treat a 350-m3 hospital room (2.86 mL of plasma-activated H2O2 for 1 m3). (B) Top lateral view showing the control panel with the on/off switch button. (C) Operating the control cursor. The cursor allows the setup of either the time of injection or the theoretical room volume that could be treated within that time of injection. Decontamination assays were performed in a 25-m3 room; thus, the 25-m3 mark on the control panel was set up as the basic amount necessary to treat our 25-m3 test room (1 room volume = 1 V). To double the amount of hydrogen peroxide produced, one needed to set up the aHP generator to 50 m3 (2 V) or to increase 3-fold the quantity of H2O2 the cursor is set to at 150 m3 (3 V), and so on.
The 12% plasma-activated hydrogen peroxide solution was also purchased from Saniswiss. To generate it, the hydrogen peroxide solution is subjected to a pulsed discharge process. The plasma is formed across 2 electrodes; one grounds the water. In short, during processing, water is bombarded with ultra-short, high-voltage electrical pulses. The plasma is formed by an avalanche of electrons reacting with the air and water surface. The high electron impact on the water surface enables the breakdown of water and hydrogen peroxide molecules, generating highly reactive antimicrobial molecules consisting of superoxidized water containing various ions, peroxides, and superoxides.
The aHP generator from Saniswiss is calibrated for the disinfection of a 350-m3 (eg, hospital) room by aerolizing 1 L of plasma-activated hydrogen peroxide in 60 minutes. Thus, treating our 25-m3 test lab would require 71.4 mL of plasma-activated H2O2 aerosolized in 4 minutes, 18 seconds. The control panel allows the set up of either the time of injection or the theoretical equivalent room volume that could be treated within that time of injection (Figure 1C). For the study, as an implicit rule, one decided to consider the 25-m3 mark on the control panel as the basic amount necessary to treat our 25-m3 test room (1 room volume = 1 V). To double the amount of hydrogen peroxide produced, one needed to set up the generator to 50 m3 (2 V) or to increase 6-fold the quantity of H2O2 to 150 m3 (6 V). The time required to generate 1 V of aHP is 4 minutes, 18 seconds (4′18′′); 2 V: 8′36′′; 3 V: 12′54′′; 4 V: 17′12′′; 6 V: 25′48′′; and 12 V: 51′36′′.
Hydrogen Peroxide Biological Indicators
Geobacillus stearothermophilus biological indicators were obtained from MesaLabs (Mesa Laboratories, Lakewood, Colorado). They consist of “Apex” stainless steel discs spotted with a minimum of 1.0 × 106 spores per carrier. Each individual disc is packaged in Tyvek material. “Apex” biological indicators are normally designed for H2O2 vapor sterilization but responded successfully to aerosolized hydrogen peroxide (ie, the peroxide penetrated through the Tyvek membrane and spores were killed). After exposure, the pouches were opened and the discs transferred to a tube containing 3.8 mL of “Releasat” soybean casein digest culture medium (MesaLabs). Tubes were incubated in a heating block (Grant instrument, QBD series, Cambridge, UK) at 56°C. Depending on the assays, spores were incubated for 4 hours up to 6 days before observing the color of the growth medium. A yellow color shows bacterial growth (ie, no inactivation). A blue color indicates absence of growth (spore inactivation).
Monitoring of Hydrogen Peroxide Levels, Temperature, and Relative Humidity
Continuous monitoring of the amount of hydrogen peroxide present in the room was performed using an H2O2 probe (PortaSens II; Analytical Technology, Collegeville, Pennsylvania). Low levels of H2O2 were measured with hydrogen peroxide 0.1/a Dräger tubes (range, 0.1-3 ppm). H2O2 levels were expressed in parts per million (ppm). Temperature (in degree Celsius) and percentage of relative humidity (% rh) were registered using a rh and °C data logger sensor from Lascar Electronics (model Easy log EL-USB-2, Lascar Electronics, Whiteparish, UK).
Room Decontamination with aHP
The test room is a 25-m3 lab (4.1 × 1.9 × 3.2 m) formerly used as a dark room. There is 1 access door and no windows. The room could be completely sealed during operations. Tyvek pouches containing carriers with 106 spores were placed at different locations within the room (or inside the biosafety cabinet). The pouches were taped to the walls or put in Petri dishes left on flat surfaces (floor, tables, working surface in biosafety cabinet). For decontamination of the biosafety cabinet, Tyvek pouches were also put in plastic Petri dishes. The Petri dishes were then taped to the grid protecting the HEPA filter, the open side facing the filter. That way, the aHP reaching the Tyvek pouch placed in the Petri dish could only arrive through the filter. The generator was set to a given volume (from 1 to 12 V; see above: description of the generator), enabling the desired amount of hydrogen peroxide to be delivered into the room. Each H2O2 aerosolization episode was followed by a contact period varying between experiments from 15 minutes up to 4 hours. One decontamination cycle was composed of an H2O2 aerosolization episode followed by a given contact period.
Results
Inactivation of G stearothermophilus Spores Exposed to Aerosolized Plasma-Activated Hydrogen Peroxide
To test the efficiency of plasma-activated hydrogen peroxide (H2O2), we conducted a series of assays to optimize the amount of H2O2 needed to get at least a 6-log10 reduction in the viability of G stearothermophilus spores. Those assays were set up to assess 2 parameters: (1) the hydrogen peroxide concentration of the plasma-activated H2O2 solution and (2) the amount of aerosolized hydrogen peroxide needed to inactivate spores.
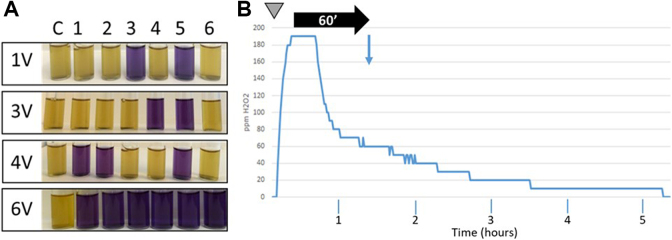
Thus, in the first experiments, a constant volume of hydrogen peroxide (ie, the amount of product normally required to disinfect a 25-m3 room) was aerosolized using various increasing concentrations, 1.5%, 3%, 6%, and 12%. Tyvek pouches containing G stearothermophilus spores were placed in the 4 corners as well as in the center of the room and behind the aHP generator (on the opposite side of the nozzle). Only partial inhibition of spore growth (between 4-log10 and 5-log10 depending on the concentration of the hydrogen peroxide solution) could be observed (data not shown). In the next step, the concentration of H2O2 was kept constant (ie, 12%), but the volume of solution aerosolized was gradually increased, starting from the amount normally required to disinfect a 25-m3 room (1 V) up to 3, 4, and 6 times the initial amount (3 V, 4 V, and 6 V, respectively). No H2O2 condensation was observed at any of these conditions. Figure 2A shows that a consistent and reproducible 6-log10 decrease in the number of viable spores was obtained with amounts of hydrogen peroxide 6 times higher than the initial volume used in the preliminary studies (6 V = 428.4 mL of 12% plasma-activated hydrogen peroxide; 25′48′′ of aerosolization time). Figure 2B shows that during the aerosolization episode, the amount of hydrogen peroxide in the room increased rapidly to reach approximately 190 ppm. A plateau was then maintained during approximately 15 minutes, before the hydrogen peroxide started to gradually decompose. Sixty minutes after the end of the aerosolization episode, 70% to 80% of the H2O2 aerosols were degraded. Based on these observations, we set up a basic decontamination protocol for our test lab using the following parameters: 12% plasma-activated hydrogen peroxide, generator set to 6 V (Figure 1C), 60 minutes of contact time, retrieval of the Tyvek pouches containing the spores, inoculation of growth medium, and incubation for 6 days at 56°C. To retrieve biological indicators after the 60 minutes of contact time, staff wore a full suit equivalent to a Tyvek type 400, nitrile gloves, and a respiratory mask equipped with a type AXB2 gas cartridge (Dräger, Houston, Texas).
Figure 2.
Aerosolized hydrogen peroxide (aHP) inactivates spores from Geobacillus stearothermophilus. (A) Spore inactivation assays. Spores were exposed to various increasing amounts of plasma-activated hydrogen peroxide. The contact time was 60 minutes. Spores were incubated 6 days at 56°C. 1 V = amount of H2O2 theoretically required to treat a 25-m3 room; 3 V, 4 V, and 6 V = 3, 4, and 6 times the basic amount of H2O2, respectively. Yellow: bacterial growth (no inactivation). Blue: absence of growth (spore inactivation). Sample C: control (untreated); samples 1 to 6: spores exposed to aHP. (B) Monitoring of H2O2 levels for a 6-V aerosolization cycle. x axis: time; y axis: amounts of hydrogen peroxide in ppm. Gray inverted triangle: start of the aerosolization cycle; aerosolization time: 25′48′′; horizontal black arrow: contact time (60 minutes). Vertical blue arrow: collection of Tyvek pouches and start of spore growth control.
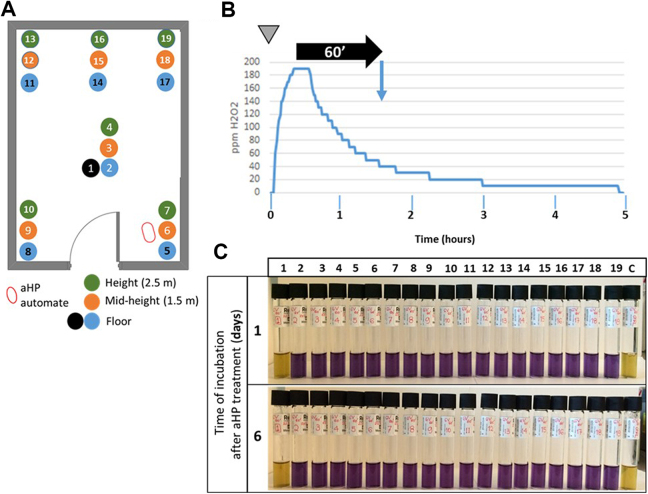
The next step was to assess the distribution of the H2O2 aerosol within the room (Figure 3A). The Tyvek pouches containing 106 spores were placed at different locations and various heights in the laboratory and exposed to H2O2. Figure 3C shows that the inactivation pattern was uniform (horizontally and vertically) within the entire room.
Figure 3.
Efficient distribution of aerosolized hydrogen peroxide (aHP) within the room space. (A) Location of Geobacillus stearothermophilus spores in the test laboratory. Blue and black dots: spores placed at the floor level; orange dots: spores placed at mid-height (1.5 m from floor level); green dots: spores placed at 2.8 m from floor level. Sample 1: negative control (the Tyvek pouch containing spores is placed in a plate sealed in with parafilm). Samples 2 to 19: spores exposed to aHP. (B) Spraying protocol. At time zero (inverted gray triangle), 6 V of plasma-activated H2O2 was aerosolized. The injection was followed by a contact time of 60 minutes (horizontal black arrow), after which the spores were collected and put into culture (blue vertical arrow). H2O2 levels (in ppm) were continuously recorded. (C) Growth results after aHP treatment. Samples 1 and c are nonexposed and nontreated, respectively. In those samples, the yellow color of the medium indicates that growth occurred. Samples 2 to 19 were treated with aHP. The blue color of the medium indicates no bacterial growth. Samples were checked 24 hours and 6 days after initiating the culture.
Decontamination of Biosafety Cabinets
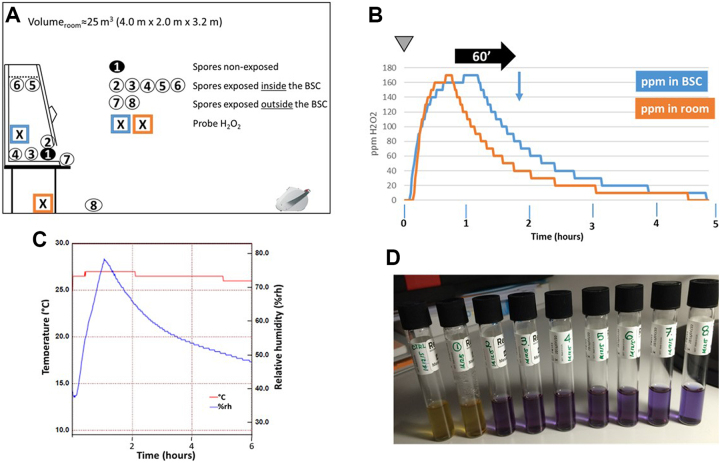
One advantage when performing room decontaminations is the possibility to decontaminate biosafety cabinets at the same time. The issue with aHP is the possibility that aerosolized particles are retained by the HEPA filter, resulting, at the best, in only a partial decontamination of the cabinet chamber. To challenge this problem, we placed carriers containing spores of G stearothermophilus at various locations outside and inside the biosafety cabinet (Figure 4A). In particular, 2 samples (No. 5 and No. 6) were placed in Petri dishes that were tightly located against the grid protecting the HEPA filter in the biosafety cabinet chamber. To get a full picture, H2O2 concentrations were monitored during the decontamination process inside and outside the cabinet chamber. Likewise, relative humidity (% rh) and temperature (°C) data were collected within the room during the cycle. A complete room decontamination process was initiated with the biosafety cabinet (BSC) operating normally. As shown in Figure 4B, H2O2 accumulated inside the biosafety cabinet chamber, indicating that the hydrogen peroxide was sucked up from the room, moved along the air circulation ducts, and blown through the HEPA filter into the cabinet chamber. We also observed that H2O2 started to accumulate in the BSC chamber with a slight delay compared to the rise of H2O2 within the room (Figure 4B). Then, the increase in peroxide concentrations inside and outside the cabinet followed a similar curve to reach the plateau phase. In the decrease phase, the H2O2 levels measured outside the BSC diminished with a slower rate than the levels measured inside the cabinet. This might be because the H2O2 present within the cabinet or trapped in the HEPA filters was gently released in the room through the action of the cabinet exhaust system. Small levels of H2O2 (10 ppm or less) could be detected up to 10 hours after the start of the decontamination. As shown in Figure 4C, the temperature remained more or less constant during the process, whereas, as expected, the relative humidity of the room rose alongside the increase in aerosolized hydrogen peroxide levels to reach a peak (around 70% to 80% rh) 1 hour after the start of the decontamination before slowly declining as H2O2 particles degraded.
Figure 4.
Aerosolized hydrogen peroxide (aHP)–mediated decontamination of a biosafety cabinet (BSC). (A) Setting of the experiment. The BSC is positioned against the wall opposite of the entry door. The aHP generator is placed on the floor, facing the BSC, approximately 3 m away. Tyvek pouches containing Geobacillus stearothermophilus spores were placed within the containment of the BSC (samples 1 to 6) and just outside (samples 7 and 8). Sample 1 is placed inside a dish closed with a lid and sealed with a parafilm. Sample 2 is located next to sample 1. Samples 3 and 4 are positioned in the center of the BSC working surface and just in front of the back aspiration grid, respectively. Samples 5 and 6 are put into dishes (with no cover) that are attached with isolation tape to the top grid that protects the HEPA filter. Sample 7 is placed immediately in front of the opening of the BSC and cupule 8 is located on the floor, in front of the BSC. The crossed boxes indicate the position of the H2O2 probes. (B) Monitoring of H2O2 levels. At time zero (inverted gray triangle), an equivalent of 12 V of plasma-activated hydrogen peroxide was dispersed in the test room in 1 aerosolization episode followed by a contact time of 60 minutes (horizontal black arrow), after which the spores were collected and put into culture (blue vertical arrow). H2O2 levels (in ppm) were continuously recorded. (C) Recording of room temperature (°C) and relative humidity (% rh) during the complete decontamination process. (D) Growth results 6 days after aHP treatment. Samples 1 and c are nonexposed and nontreated, respectively. Samples 2 to 8 are treated with aHP. The yellow and the blue color of the medium indicate growth and absence of growth, respectively.
In addition, all the spores exposed to aHP were inactivated (Figure 4D), demonstrating that aHP aerosolized particles were not retained by the HEPA filter and could target any microbial contamination inside the enclosure of the safety cabinet.
Discussion
Benefits of Aerosolized Plasma-Activated Hydrogen Peroxide Surface Decontamination
In this study, we used an aHP approach to successfully inactivate spores of G stearothermophilus. The hydrogen peroxide aerosol is generated by a generator equipped with a venturi pump system dispersing 4 μm in size droplets of a plasma-activated H2O2 solution (Saniswiss). In these series of experiments, we could show that aerosolized hydrogen peroxide could inhibit spore and bacterial growth. The initial experiments were performed using a 1.5%, 3%, and 6% solution of plasma-activated H2O2. They all failed to produce a 6-log10 reduction in the number of viable spores. Only partial inactivation was achieved (4- to 5-log10; data not shown). Those observations were in concordance with studies by several groups12,20,23,26,30 in which a 5% to 6% hydrogen peroxide aerosol failed to get to the 6-log10 reduction limit. In our hands, a consistent and reproducible 6-log10 reduction was obtained with a 12% H2O2 solution.
It is interesting to note that the peroxide solution used in our study does not contain silver cations, an element known for its biocidal (antibacterial) properties.42,43 However, some studies also showed that silver particles might be toxic for higher organisms and human cells.44,45 This makes plasma-activated hydrogen peroxide a safer alternative in surface decontamination, especially if used for frequent treatment of hospital or laboratory rooms. Indeed, H2O2 degrades safely into water and oxygen, whereas the silver cations present in some peroxide preparations might deposit on walls and surfaces, leaving potentially problematic residues for patient health or cell culture activities.
Plasma activation is used with growing interest in the development of biocide products. Microbial inactivation could be obtained by using plasma-activated water.46 Plasma-activated coatings also showed antibacterial and antifungal activities.47 Moreover, plasma-activated media have been used for cancer treatment48 probably through activation of pathways inducing programmed cell death.49 Taken together, these observations highlight the biocidal potential of plasma-activated solutions. Studies have reported elevations of intracellular reactive oxygen or nitrogen species after plasma treatment50 that could interact in the liquid phase with the cell surface or move into the cell and interact with key molecules controlling cell growth, cell death, or DNA repair.51 Thus, plasma-activated hydrogen peroxide represents a promising way to decontaminate surfaces.
In some prospective experiments, we used the approach of Holmdahl et al,28 in which a total of 6 V of hydrogen peroxide aerosols were generated in 3 episodes (3 × 2 V) separated by a 60-minute contact period. The monitoring of H2O2 levels indicated, however, that the succession of 3 successive cycles did not end up in a significant accumulation of active products (data not shown). Therefore, for the whole study, we decided to deliver the whole volume (the equivalent of 6 V) in 1 cycle. This way, we managed to maintain a short plateau of hydrogen peroxide around 170 to 200 ppm for approximately 20 minutes (Figures 2, 3, and 4B). In our hands, maintaining a plateau of H2O2 above 150 ppm during several minutes proved to be more efficient than generating 3 successive brief peaks of a similar amount of decontaminant. During the course of our experiments, we also noticed that it was important to work with fresh solution of plasma-activated hydrogen peroxide. Indeed, once the bottle was opened, the product had to be used within 1 week; otherwise, the amount of active H2O2 measured dropped rapidly and concentrations less than 120 ppm had a poor biocidal effect on G stearothermophilus spores (data not shown).
We could also demonstrate that the aHP technology used in this study was efficient enough to decontaminate BSCs (Figure 4). BSCs require decontamination before maintenance and repairs, and often they are decontaminated when room fumigation takes place. Formaldehyde gas, hydrogen peroxide vapors, and chlorine dioxide gas are primary methods to do so, but with various constraints.52 Using formaldehyde or chlorine dioxide gas is work intensive (if not treated during the decontamination of the room, the cabinet face and exhaust system must be sealed with a leak-proof panel or with plastic sheeting and masking tape prior to decontamination) and hazardous due to the high toxicity of the chemicals.53 Indeed, formaldehyde is considered a probable carcinogen2 by the US Environmental Protection Agency (EPA) and is classified as a carcinogen to humans by the International Agency for Research on Cancer. In addition, formaldehyde vapors must be neutralized by ammonia before ventilating the cabinet with fresh air or, if the BSC is ducted, must be eliminated through the exhaust system, with a high risk of uncontrolled release through the air duct system and with the delicate question of releasing the product in the environment. However, since formaldehyde is a true gas at room temperature, it will expand and occupy the whole internal volume of the BSC and pass through the HEPA filter, which generally shows excellent compatibility for formaldehyde under typical decontamination cycles. To be efficient, formaldehyde gas requires a relative humidity of 60% to 85%. Although similarly efficient, the use of chlorine dioxide gas is facing similar challenges.54 VHP was also evaluated for decontamination of BSCs with mixed results.52,55,56 Fey and colleagues57 showed that spore inactivation could be achieved only if parameters such as dehumidification (relative humidity should be below 40% to prevent the vaporized hydrogen peroxide from condensing out but high enough to kill spores), conditioning (vapor injection), and decontamination time were perfectly set up. In constrast to formaldehyde or VPH, aHP does not require any adjustment of the relative humidity or of the temperature in the room or in the cabinet. Indeed, the room relative humidity varies during the aHP cycle, going up with the increase in H2O2 concentrations and returning to ambient values as H2O2 gently degrades. The generation of the aerosol does not depend on heat or on the production of vapors so that the temperature remains constant during the whole decontamination process. One must concede, however, that systems used to generate formaldehyde or VPH can be branched on the BSC and, providing proper sealing of the enclosure, allow treating the unit independently from the rest of the room. The aHP generator used in this study produces aerosols by means of a venturi pump system that generates a cone of concentrated aerosols. A minimal distance (approximately 1 m) must be kept to get an aerosol spray that is then randomly distributed within the room. There is room to put the aHP generator in the BSC chamber, but there is not enough distance between the walls to evenly distribute the aerosols, which condense on the surfaces inside the cabinet and no decontamination is achieved. Nevertheless, our experiments showed that it was possible to decontaminate BSCs during a room decontamination. The spores contained in the Tyvek pouches that were stuck to the grid underneath the HEPA filter on the chamber side were inactivated with plasma-activated hydrogen peroxide (Figure 4, samples 5 and 6), showing that H2O2 aerosols were going through the filter and decontaminating it. The outcome of this experiment is somehow unsettling since HEPA filters are meant to efficiently trap particles 4 μm in size, like the ones generated by the aHP generator. However, it was stated that capture mechanisms during filtration are influenced by physical properties such as the velocity of particles and their electric charge,58 and some authors stated that HEPA filters might retain charged particles with much lesser efficiency.59,60 Plasma activation of hydrogen peroxide generates large amounts of superoxide anions ( ), hydroxyl radicals (OH–), and peroxide ions ( ) that might thus affect the retention of these reactive species by the HEPA filter. It is also possible that the aerosol particles of aHP were captured by the HEPA filter, but the active chemistry from those particles was pushed down across the filter by the air moving down.
Off-Gassing
The whole series of aHP decontamination cycles that we performed in our 25-m3 test room showed that the levels of H2O2 dropped below 10 ppm, which is the lower detection limit of the sensor probe calibrated to measure high variation levels of hydrogen peroxide, within a time frame of 5 to 6 hours. However, using Dräger tubes, we could still detect 1 to 3 ppm of H2O2 in the room even though the reading of the sensor probe was 0 ppm (data not shown). The OSHA PEL value for hydrogen peroxide being 1 ppm, it would not have been safe to liberate the room for resuming normal activities. An additional period of 5 to 6 hours was generally necessary to get H2O2 levels below 0.5 ppm without active ventilation. For instance, engaging the ventilation system 60 minutes after the end of the aerosolization phase (that is at the time of biological samples collection in our assay) speeds up the degradation and elimination of the hydrogen peroxide in such a way that a complete room decontamination could be achieved in around 6 hours. It is also theoretically possible that some material like paper, cardboard, or clothing adsorbs H2O2 that could be slowly released and represents a long-term health issue for the workers. Since the whole process is run at room temperature, it is unlikely that the peroxide solution captured by adsorbing material will be released before the product is completely degraded. Until complete degradation, the peroxide deposited on surfaces would be more of a contact hazard, not a respiratory hazard, that could be easily solved by wearing appropriate safety gloves.
Peroxide Chemical Safety
One particular safety concern is the explosiveness of hydrogen peroxide. H2O2 itself is not flammable, but it can cause spontaneous combustion of flammable materials. It has indeed, according to the National Fire Protection Association (NFPA) 704 standard system, a reactivity code 3, meaning that the product is capable of detonation or explosive decomposition, but it requires a strong initiating source or must be heated under confinement before initiation. Thus, explosive vapor phases can only be formed of aqueous hydrogen peroxide solutions with concentrations higher than 70% (w/w) at temperatures above 110°C.61 The system used in this study generates hydrogen peroxide aerosols 4 μm in size from a room temperature 12% (w/w) solution at atmospheric pressure. We do not know if aerosols have intrinsic properties that make them more reactive, but by comparison with the reactivity of vapors, the risk of explosion under our experimental setting might be considered very low. In addition, during the decontamination process, H2O2 will gently settle on surfaces and either react with organisms or material or degrade. In a worst-case scenario, one could postulate that the complete volume of H2O2 aerosolized in our more stringent procedure (34.4 mL/m3; Figure 4) would spontaneously transform into water and oxygen. The dismutation reaction would produce 1.5 L of O2/m3, leading to an increase of 0.15% in oxygen level (Table 1). The total amount of oxygen would amount to 21.1%, which is negligible compared to the 23.45% upper safety limit set by the NFPA.62
Table 1.
Risk Due to an Oxygen Level Increase After Hydrogen Peroxide Decomposition Reaction.
| Calculation of the increase of oxygen (O2) level within the room if H2O2 is completely degraded by the dismutation reaction 2H2O2 → 2H2O + O2. This situation should be the worst-case scenario. |
| Experimental setting: |
| Use of 34.4 mL of 12%w aq. H2O2/m3 |
| Initial concentration of O2 in air = 20.95% |
| Density of 12%w aq. H2O2 = 1.03 g/mL |
| Molecular weight = 34 g/mole |
| Molar volume (at 20°C) = 24 L/mole |
| The increase of the oxygen level is calculated by: |
| 34.4 mL 12%w aq. H2O2 → 35.4 g 12%w aq. H2O2 → 4.25 g of H2O2 → 0.125 mole of H2O2 → 0.063 mole of O2 → generation of 1.5 L of O2/m3 → increase of 0.15% of O2 |
| The final concentration of O2 will therefore not exceed 21.1% within the room. |
| According to the National Fire Protection Association standards, the maximal oxygen concentration that can be safely used for oxygen enrichment at various atmospheric pressure is given by the following equation: |
| O2 (%) = 23.45 / (P 0.5) |
| where P is the total pressure in atmosphere (atm). |
| At sea level (P = 1 atm), the maximal oxygen concentration considered safe is therefore 23.45%. In our worst-case scenario, the increase in oxygen levels is therefore negligible in terms of inflammability safety concerns. |
Conclusion
aHP proved to be an efficient, easy to set up, and cost-effective method to decontaminate rooms and BSCs potentially contaminated with organisms. Indeed, the cost of the aHP generator is 10 to 20 times lower than HPV or VHP generators (catalog prices), and the cost of the 12% plasma-activated hydrogen peroxide solution is more or less equivalent to hydrogen peroxide solution sold for vaporizer devices. It is quick to set up, environmentally friendly (hydrogen peroxide degrades into oxygen and water), and well tolerated by standard laboratory equipment, including electronics. aHP will probably become a method of choice in the near future, when additional decontamination data with a larger selection of microorganisms and more stringent conditions (eg, organic load; type of surfaces) will show the robustness of the system. Nowadays, we regularly treat small- to medium-sized biosafety level 2 research laboratories before routine maintenance or change of affectation, sometimes running 2 decontamination cycles in a row when larger volumes need to be treated.
Acknowledgments
Our thanks go to Nicole Paduwat (EPFL) for her invaluable technical support and to Dr Urs Pauli (Institute of Immunology and Virology, Mittelhäusern, Switzerland) for carefully reading the manuscript.
Author Contribution
Camille Freyssenet and Stéphane Karlen contributed equally to this work.
Ethical Approval statement
The research did not involve human subjects or laboratory animals and was therefore not subjected to a formal approval by a relevant ethics committee or by an institutional review board.
Statement of Human and Animal Rights
No human subjects or animals were used.
Statement of Informed Consent
No written or oral informed consent statements were required.
Declaration of Conflicting Interests
The authors declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding
The authors disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This work was supported financially by the EPFL School of life sciences, service of biosafety.
References
- 1. Beswick AJ, Farrant J, Makison C, et al. Comparison of multiple systems for laboratory whole room fumigation. Appl Biosaf. 2011;16(3):139–157. [Google Scholar]
- 2. McLaughlin JK. Formaldehyde and cancer: a critical review. Int Arch Occup Environ Health. 1994;66(5):295–301. [DOI] [PubMed] [Google Scholar]
- 3. Abdel-Rahman MS, Couri D, Bull RJ. Toxicity of chlorine dioxide in drinking water. J Am Coll Toxicol. 1984;3(4):277–284. [PubMed] [Google Scholar]
- 4. Jin R, Hu S, Zhang Y, Bo T. Concentration-dependence of the explosion characteristics of chlorine dioxide gas. J Hazard Mater. 2009;166(2-3):842–847. [DOI] [PubMed] [Google Scholar]
- 5. Malik DJ, Shaw CM, Rielly CD, Shama G. The inactivation of Bacillus subtilis spores at low concentrations of hydrogen peroxide vapour. J Food Eng. 2013;114(3):391–396. [Google Scholar]
- 6. Falagas ME, Thomaidis PC, Kotsantis IK, Sgouros K, Samonis G, Karageorgopoulos DE. Airborne hydrogen peroxide for disinfection of the hospital environment and infection control: a systematic review. J Hosp Infect. 2011;78(3):171–177. [DOI] [PubMed] [Google Scholar]
- 7. Health Protection Scotland. Literature Review and Practice Recommendations: Existing and Emerging Technologies Used for Decontamination of the Healthcare Environment: Hydrogen Peroxide. Glasgow: National Services Scotland; 2016. [Google Scholar]
- 8. Pottage T, Richardson C, Parks S, Walker JT, Bennett AM. Evaluation of hydrogen peroxide gaseous disinfection systems to decontaminate viruses. J Hosp Infect. 2010;74(1):55–61. [DOI] [PubMed] [Google Scholar]
- 9. Weber DJ, Rutala WA. Self-disinfecting surfaces: review of current methodologies and future prospects. Am J Infect Control. 2013;41(5):S31–S35. [DOI] [PubMed] [Google Scholar]
- 10. Cobb TC. Methicillin-resistant Staphylococcus aureus decontamination: is ultraviolet radiation more effective than vapor-phase hydrogen peroxide? Rev Med Microbiol. 2017;28(2):69–74. [Google Scholar]
- 11. Hall L, Otter JA, Chewins J, Wengenack NL. Use of hydrogen peroxide vapor for deactivation of Mycobacterium tuberculosis in a biological safety cabinet and a room. J Clin Microbiol. 2007;45(3):810–815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Grare M, Dailloux M, Simon L, Dimajo P, Laurain C. Efficacy of dry mist of hydrogen peroxide (DMHP) against Mycobacterium tuberculosis and use of DMHP for routine decontamination of biosafety level 3 laboratories. J Clin Microbiol. 2008;46(9):2955–2958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Otter JA, French GL. Survival of nosocomial bacteria and spores on surfaces and inactivation by hydrogen peroxide vapor. J Clin Microbiol. 2009;47(1):205–207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Malik DJ, Shaw CM, Shama G, Clokie MR, Rielly CD. An investigation into the inactivation kinetics of hydrogen peroxide vapor against clostridium difficile endospores. Chem Eng Commun. 2016;203(12):1615–1624. [Google Scholar]
- 15. Krishnan J, Berry J, Fey G, Wagener S. Vaporized hydrogen peroxide–based biodecontamination of a high-containment laboratory under negative pressure. Appl Biosaf. 2006;11(2):74–80. [Google Scholar]
- 16. Wood JP, Calfee MW, Clayton M, et al. A simple decontamination approach using hydrogen peroxide vapour for Bacillus anthracis spore inactivation. J Appl Microbiol. 2016;121(6):1603–1615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Rij RE, Forney CF. Phytotoxocity of vapour phase hydrogen peroxide to Thompson seedless grapes and Botrytis cinerea spores. Crop Prot. 1995;14(2):131–135. [Google Scholar]
- 18. Fichet G, Comoy E, Duval C, et al. Novel methods for disinfection of prion-contaminated medical devices. Lancet. 2004;364(9433):521–526. [DOI] [PubMed] [Google Scholar]
- 19. Kahnert A, Seiler P, Stein M, Aze B, McDonnell G, Kaufmann SH. Decontamination with vaporized hydrogen peroxide is effective against Mycobacterium tuberculosis . Lett Appl Microbiol. 2005;40(6):448–452. [DOI] [PubMed] [Google Scholar]
- 20. Andersen BM, Syversen G, Thoresen H, et al. Failure of dry mist of hydrogen peroxide 5% to kill Mycobacterium tuberculosis . J Hosp Infect. 2010;76(1):80–83. [DOI] [PubMed] [Google Scholar]
- 21. Andersen BM. Does ‘airborne’ hydrogen peroxide kill Mycobacterium tuberculosis? J Hosp Infect. 2011;77(1):81–83. [DOI] [PubMed] [Google Scholar]
- 22. Otter JA, Yezli S. A call for clarity when discussing hydrogen peroxide vapour and aerosol systems. J Hosp Infect. 2011;77(1):83–84. [DOI] [PubMed] [Google Scholar]
- 23. Barbut F, Menuet D, Verachten M, Girou E. Comparison of the efficacy of a hydrogen peroxide dry-mist disinfection system and sodium hypochlorite solution for eradication of clostridium difficile spores. Infect Control Hosp Epidemiol. 2009;30(6):507–514. [DOI] [PubMed] [Google Scholar]
- 24. Steindl G, Fiedler A, Huhulescu S, Wewalka G, Allerberger F. Effect of airborne hydrogen peroxide on spores of Clostridium difficile . Wien Klin Wochenschr. 2015;127(11-12):421–426. [DOI] [PubMed] [Google Scholar]
- 25. Boyce JM, Havill NL, Otter JA, et al. Impact of hydrogen peroxide vapor room decontamination on Clostridium difficile environmental contamination and transmission in a healthcare setting. Infect Control Hosp Epidemiol. 2008;29(8):723–729. [DOI] [PubMed] [Google Scholar]
- 26. Shapey S, Machin K, Levi K, Boswell TC. Activity of a dry mist hydrogen peroxide system against environmental Clostridium difficile contamination in elderly care wards. J Hosp Infect. 2008;70(2):136–141. [DOI] [PubMed] [Google Scholar]
- 27. Fu TY, Gent P, Kumar V. Efficacy, efficiency and safety aspects of hydrogen peroxide vapour and aerosolized hydrogen peroxide room disinfection systems. J Hosp Infect. 2012;80(3):199–205. [DOI] [PubMed] [Google Scholar]
- 28. Holmdahl T, Lanbeck P, Wullt M, Walder MH. A head-to-head comparison of hydrogen peroxide vapor and aerosol room decontamination systems. Infect Control Hosp Epidemiol. 2011;32(9):831–836. [DOI] [PubMed] [Google Scholar]
- 29. Andersen BM, Rasch M, Hochlin K, Jensen FH, Wismar P, Fredriksen JE. Decontamination of rooms, medical equipment and ambulances using an aerosol of hydrogen peroxide disinfectant. J Hosp Infect. 2006;62(2):149–155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Orlando P, Cristina ML, Dallera M, Ottria G, Vitale F, Badolati G. Surface disinfection: evaluation of the efficacy of a nebulization system spraying hydrogen peroxide. J Prev Med Hyg. 2008;49(3):116–119. [PubMed] [Google Scholar]
- 31. Pottage T, Macken S, Giri K, Walker JT, Bennett AM. Low-temperature decontamination with hydrogen peroxide or chlorine dioxide for space applications. Appl Environ Microbiol. 2012;78(12):4169–4174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Phoenix Controls. HVAC Considerations for Gaseous Decontamination of Laboratory Spaces Using Hydrogen Peroxide Vapor. Acton, MA: Phoenix Controls; 2006. [Google Scholar]
- 33. McDonnell G, Russell AD. Antiseptics and disinfectants: activity, action, and resistance. Clin Microbiol Rev. 1999;12(1):147–179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Otter JA, Yezli S, Perl TM, Barbut F, French GL. The role of ‘no-touch’ automated room disinfection systems in infection prevention and control. J Hosp Infect. 2013;83(1):1–13. [DOI] [PubMed] [Google Scholar]
- 35. Kokubo M, Inoue T, Akers J. Resistance of common environmental spores of the genus Bacillus to vapor hydrogen peroxide. PDA J Pharm Sci Technol. 1998;52(5):228. [PubMed] [Google Scholar]
- 36. Maillard JY. Innate resistance to sporicides and potential failure to decontaminate. J Hosp Infect. 2011;77(3):204–209. [DOI] [PubMed] [Google Scholar]
- 37. Sk MH, Overfelt RA, Haney RL, Fergus JW. Hydrogen embrittlement of 4340 steel due to condensation during vaporized hydrogen peroxide treatment. Mater Sci Eng A. 2011;528(10-11):3639–3645. [Google Scholar]
- 38. Carling Philip C. What is the role of mobile no-touch disinfection technology in optimizing healthcare environmental hygiene? In: Bearman G, Munoz-Price S, Morgan D, Murthy R, eds. Infection Prevention: New Perspectives and Controversies. Springer, Cham: Springer International; 2018:67–82. [Google Scholar]
- 39. Pedahzur R, Shuval HI, Ulitzur S. Silver and hydrogen peroxide as potential drinking water disinfectants: their bactericidal effects and possible modes of action. Water Sci Technol. 1997;35(11-12):87–93. [Google Scholar]
- 40. Ali S, Muzslay M, Bruce M, Jeanes A, Moore G, Wilson APR. Efficacy of two hydrogen peroxide vapour aerial decontamination systems for enhanced disinfection of meticillin-resistant Staphylococcus aureus, Klebsiella pneumoniae and Clostridium difficile in single isolation rooms. J Hosp Infect. 2016;93(1):70–77. [DOI] [PubMed] [Google Scholar]
- 41. Bartels MD, Kristoffersen K, Slotsbjerg T, Rohde SM, Lundgren B, Westh H. Environmental methicillin-resistant Staphylococcus aureus (MRSA) disinfection using dry-mist-generated hydrogen peroxide. J Hosp Infect. 2008;70(1):35–41. [DOI] [PubMed] [Google Scholar]
- 42. Clement JL, Jarrett PS. Antibacterial silver. Met Based Drugs. 1994;1(5-6):467–482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Wakshlak RBK, Pedahzur R, Avnir D. Antibacterial activity of silver-killed bacteria: the “zombies” effect. Sci Rep. 2015;5:9555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Akter M, Sikder MT, Rahman MM, et al. A systematic review on silver nanoparticles-induced cytotoxicity: physicochemical properties and perspectives. J Adv Res. 2018;9:1–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Kvitek L, Panacek A, Prucek R, et al. Antibacterial activity and toxicity of silver—nanosilver versus ionic silver. J Phys Conf Ser. 2011;304(1):012029. [Google Scholar]
- 46. Kamgang-Youbi G, Herry JM, Meylheuc T, et al. Microbial inactivation using plasma-activated water obtained by gliding electric discharges. Lett Appl Microbiol. 2009;48(1):13–18. [DOI] [PubMed] [Google Scholar]
- 47. Akhavan B, Michl TD, Giles C, et al. Plasma activated coatings with dual action against fungi and bacteria. Appl Mater Today. 2018;12:72–84. [Google Scholar]
- 48. Tanaka H, Mizuno M, Ishikawa K, et al. Plasma with high electron density and plasma-activated medium for cancer treatment. Plasma Liq Interact. 2015;3(2):72–76. [Google Scholar]
- 49. Tanaka H, Mizuno M, Ishikawa K, et al. Plasma-activated medium selectively kills glioblastoma brain tumor cells by down-regulating a survival signaling molecule. AKT Kinase. 2011;1(3-4):265–277. [Google Scholar]
- 50. Graves DB. The emerging role of reactive oxygen and nitrogen species in redox biology and some implications for plasma applications to medicine and biology. J Phys Appl Phys. 2012;45(26):263001. [Google Scholar]
- 51. Kalghatgi S, Kelly CM, Cerchar E, et al. Effects of non-thermal plasma on mammalian cells. PLoS One. 2011;6(1):e16270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Czarneski MA, Lorcheim K. A discussion of biological safety cabinet decontamination methods: formaldehyde, chlorine dioxide, and vapor phase hydrogen peroxide. Appl Biosaf. 2011;16(1):26–33. [Google Scholar]
- 53. Munro K, Lanser J, Flower R. A comparative study of methods to validate formaldehyde decontamination of biological safety cabinets. Appl Environ Microbiol. 1999;65(2):873–876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Luftman HS, Regits MA, Lorcheim P, Lorcheim K, Paznek D. Validation study for the use of chlorine dioxide gas as a decontaminant for biological safety cabinets. Appl Biosaf. 2008;13(4):199–212. [Google Scholar]
- 55. Jia HQ, Li YJ, Bei S, et al. Evaluation of vaporized hydrogen peroxide fumigation as a method for the bio-decontamination of the high efficiency particulate air filter unit. Biomed Environ Sci. 2013;26(2):110–117. [DOI] [PubMed] [Google Scholar]
- 56. Hillman D. Vapor phase hydrogen peroxide gas decontamination of a BSC. Perform Rev. 2004;10(3):10–11. [Google Scholar]
- 57. Fey G, Klassen S, Theriault S, Krishnan J. Decontamination of a worst-case scenario class II biosafety cabinet using vaporous hydrogen peroxide. Appl Biosaf. 2010;15(3):142–150. [Google Scholar]
- 58. Hirling J, Gaal J. Comparison of some particulate air filter testing methods used in normal and unfavourable conditions (IAEA-SR-72/23). 1982. http://www.iaea.org/inis/collection/NCLCollectionStore/_Public/14/772/14772951.pdf. Accessed October 20, 2018.
- 59. Mainelis G, Willeke K, Baron P, et al. Electrical charges on airborne microorganisms. J Aerosol Sci. 2001;32(9):1087–1110. [Google Scholar]
- 60. Wenke C, Pospiech J, Reutter T, Truyen U, Speck S. Efficiency of different air filter types for pig facilities at laboratory scale. PLoS One. 2017;12(10):e0186558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Commission Regulation (EC). Regulation (EU) No 528/2012 Concerning the Making Available on the Market and Use of Biocidal Products: Evaluation of Active Substances. Assessment Report Official Journal of the European Union, Eur-lex, 2012. [Google Scholar]
- 62. West JB. Safe upper limits for oxygen enrichment of room air at high altitude. High Alt Med Biol. 2001;2(1):47–51. [DOI] [PubMed] [Google Scholar]