Prone positioning (PP) has been used in Acute Respiratory Distress Syndrome (ARDS), including patients with COVID-19 [1]. While it improves oxygenation, the impact of PP on cerebral hemodynamics remains controversial [2,3]. Of considerable relevance, patients with COVID-19 have shown a high incidence of delirium and neurological complications, that could suggest involvement of cerebral autoregulation (CA) [4]. Above all, it is not clear how CA is affected by PP in patients with COVID-19. In a pilot prospective study, we tested the feasibility of continuously monitoring key cerebral hemodynamic parameters in COVID-19 patients undergoing PP.
After ethical approval, subjected to availability of transcranial Doppler (TCD) operator, we included consecutive patients with ARDS due to COVID-19, undergoing PP, who provided written informed consent. Cerebral blood flow velocity (CBFV) in the middle cerebral artery and intra-arterial blood pressure (BP) were measured over 5 min supine and PP. CA was assessed by autoregulation index (ARI), with impaired CA defined as ARI < 4 [4,5]. Effects of PP, compared to the supine position, were assessed using paired t-tests. Fourteen patients (10 males, 55.1 ± 14.9 years) had admission SOFA score 3 [2.0–4.5]. Patients were placed in PP 9.5 ± 4.7 days after COVID-19 symptoms. PP increased PaO2/FiO2 ratio from 89.6 ± 28.0 to 220.6 ± 68.5 (p < 0.001). Blood lactate, base excess and arterial CO2 were not altered (Table 1 ). Due to the lack of differences, parameters were averaged between the right and left hemispheres. ARI was 2.2 [0.19–3.9] in supine position, with 11 patients showing impaired CA, without difference in PP. CBFV increased at PP as compared to supine. None of the other parameters showed differences due to body position (Table 1).
Table 1.
Cerebral, pulmonary, and systemic parameters of patients during supine and prone position.
| Supine | Prone | p-value | |
|---|---|---|---|
| pH | 7.34 ± 0.11 | 7.32 ± 0.10 | 0.630 |
| PaO2, mmHg | 89.6 ± 28.0 | 220.6 ± 68.5 | <0.001 |
| PaCO2, mmHg | 51.5 ± 9.8 | 52.9 ± 8.1 | 0.905 |
| PaO2/FiO2 | 128.0 ± 21.4 | 220.6 ± 68.5 | 0.002 |
| Lactate, mg/dL | 9.8 ± 4.2 | 9.9 ± 4.2 | 0.547 |
| Bicarbonate, mmol/L | 28.5 ± 6.4 | 28.3 ± 6.3 | 0.816 |
| Haemoglobin, mg/dL | 11.9 ± 2.4 | 12.2 ± 2.4 | 0.286 |
| SatO2, % | 96 [92–98] | 100 [99.1–100] | 0.007 |
| BP mean, mmHg | 81.7 ± 12.7 | 88.2 ± 15.5 | 0.140 |
| HR, bpm | 75.8 ± 16.9 | 80.7 ± 15.0 | 0.052 |
| Respiratory rate, bpm | 28 ± 40 | 28 ± 4 | 0.337 |
| VTe, ml | 366 [341–411] | 359 [318–421] | 0.110 |
| PEEP, mmHg | 12 [12−13] | 12 [12–13] | 1.0 |
| CBFV, cm/s | 61.3 ± 18.3 | 72.3 ± 19.7 | 0.039 |
| ARI | 2.2 [0.2–3.9] | 1.6 [0.4–3.8] | 0.433 |
Values are mean ± SD or median [interquartile range]. PaO2, partial pressure of oxygen; PaCO2, partial pressure of carbon dioxide; VTe: exhaled tidal volume; PEEP: positive end-expiratory pressure; BP, blood pressure; CBFV: cerebral blood flow velocity; HR: heart rate; ARI: autoregulation index.
The feasibility of studying cerebral hemodynamics with TCD in COVID patients during PP was demonstrated by the acquisition of key cerebrovascular parameters. TCD-based assessment leads to reliable estimates that could be used in further investigations of these patients, mainly in the context of prospective studies of the effects of PP, mechanical ventilation, and COVID complications, often associated with delirium, deterioration of cognitive function and ischemic/hemorrhagic stroke.
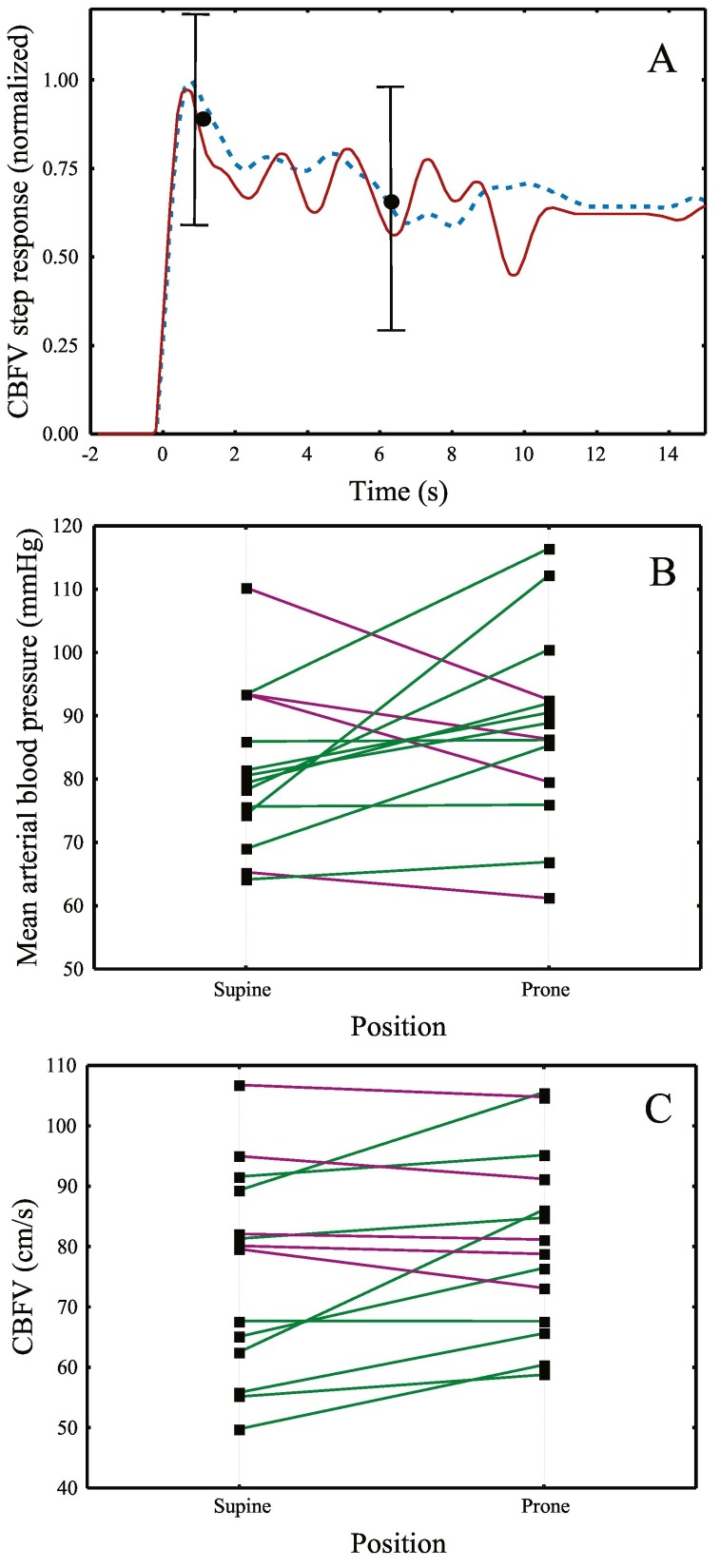
PP is an effective intervention to improve oxygenation and reduce mortality in ARDS patients [1], however previous studies showed that it can result in a significant increase in intracranial pressure given the reduced head elevation and potential inhibition of cerebral venous drainage due to compression of neck veins [2]. CA plays an important role in safeguarding adequate cerebral perfusion and there is a close link with delirium in critical patients [4]. Our findings suggest that impaired CA might be a common finding in COVID patients, independent of body position. The observation that CA was not improved with the recovery of oxygenation, warrants further investigation of the extent to which it could contribute to the occurrence of brain dysfunction in these patients. Moreover, the CBFV increase in PP, is likely to be caused by systemic alterations in patients with impaired CA [5], as suggested by the slight trend in HR and BP (Fig. 1 and Table 1).
Fig. 1.
A. Population averages (n = 14) of the temporal change in CBFV following a hypothetical step change in blood pressure in the supine (red line) and prone (blue dashed line) positions. Error bars represent the largest ±1 SE at the point of occurrence. In healthy subjects, a fast return of CBFV to its original value is normally observed [5], but in these patients, the slow return of the CBFV curves to their baseline values suggest that CA was depressed, as expressed by autoregulation index (ARI) [4,5] values of 2.2 [0.2–3.9] in the supine position and 1.6 [0.4–3.8] in the prone position. Blood pressure (B) and CBFV (C) values at supine and prone positions. Positive changes are represented by the green lines and negative trends are represented by purple lines.
COVID patients present hypoxia and, in some cases, as in our cohort, hypercapnia is part of mechanical ventilation strategies. Arterial CO2 has a strong influence on CBF regulation [5]. The hypercapnia shown in this group could contribute to CA impairment and we could speculate that myogenic BP signalling or vascular smooth muscle receptors might also be affected [5].
ptARDS can lead to acute brain injury and vice-versa, which suggests the existence of cross-talk between lung and brain [6], but the mechanism of lung-mediated brain injury is not well understood. Further studies are also needed of the influences of mechanical ventilation and sedation on CA in these patients. Protecting the lung should remain the main goal in COVID-19 patients, however, monitoring cerebrovascular function, especially in patients at risk for neurological complications may become clinically relevant with further studies.
Brain monitoring by TCD is possible during PP and impairment of CA should be a concern. Better understanding of the pathophysiology of CBF regulation is of paramount importance for developing brain-protective strategies.
Author contributions
J.R.C., R.H·P. AND J.G.R. conceived and designed research; J.R.C performed experiments; L.S collected data J.R.C. and R.B.P. analyzed data; J.R.C. and R.B.P. interpreted results of experiments; J.R.C. prepared figures; J.R.C. and R.B.P. drafted manuscript; J.R.C., R.B.P., J.G.R., R.H.P. edited and revised manuscript; J.R.C., R.B.P. R.H.P, J.G.R AND L.S approved final version of manuscript.
Conflict of interest
The authors declare that they have no conflict of interest.
Acknowledgements
The participation of all COVID-19 patients is gratefully acknowledged.
References
- 1.Marini J.J., Gattinoni L. Management of COVID-19 respiratory distress. JAMA. 2020;323(22):2329–2330. doi: 10.1001/jama.2020.6825. [DOI] [PubMed] [Google Scholar]
- 2.Roth C., Ferbert A., Deinsberger W., Kleffmann J., Kästner S., Godau J., et al. Does prone positioning increase intracranial pressure? A retrospective analysis of patients with acute brain injury and acute respiratory failure. Neurocrit Care. 2014;21(2):186–191. doi: 10.1007/s12028-014-0004-x. [DOI] [PubMed] [Google Scholar]
- 3.Thelandersson A., Cider A., Nellgård B. Prone position in mechanically ventilated patients with reduced intracranial compliance. Acta Anaesthesiol Scand. 2006;50(8):937–941. doi: 10.1111/j.1399-6576.2006.01037.x. [DOI] [PubMed] [Google Scholar]
- 4.Caldas J.R., Panerai R.B., Bor-Seng-Shu E., Ferreira G.S.R., Camara L., Passos R.H., et al. Dynamic cerebral autoregulation: a marker of post-operative delirium? Clin Neurophysiol Off J Int Fed Clin Neurophysiol. 2019;130(1):101–108. doi: 10.1016/j.clinph.2018.11.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Claassen J.A.H.R., Thijssen D.H.J., Panerai R.B., Faraci F.M. Regulation of cerebral blood flow in humans: physiology and clinical implications of autoregulation. Physiol Rev. 2021;101(4):1487–1559. doi: 10.1152/physrev.00022.2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Blanch L., Quintel M. Lung-brain cross talk in the critically ill. Intensive Care Med. 2017;43(4):557–559. doi: 10.1007/s00134-016-4583-1. [DOI] [PubMed] [Google Scholar]