Abstract
Frameless stereotactic brain biopsy (FSB) with navigation system has been widely used. We reported preliminary experience of FSB with intraoperative computed tomography (iCT) and examined the usefulness of this novel adjuvant technique and real target registration error (rTRE) of FSB. The FSB with 5-aminolevulinic acid (5-ALA) and iCT was performed on 10 patients. The gadolinium-enhanced lesions on magnetic resonance image were defined as the biopsy target. In the procedure, iCTs were scanned twice, for autoregistration of the navigation system and for confirmation of the position of the actual inserted biopsy needle. The red fluorescence of the samples was observed under excitation with violet-blue light through a low-cut filter of neurosurgical microscope. The distance between the planned target and the tip of the biopsy needle in the image of iCT was calculated in a workstation for the assessment of rTRE. The median volume of the target was 12.13 mL (0.06-39.15 mL). We performed the surgical procedure in a prone position in four patients. None to faint 5-ALA-induced fluorescence was observed in six samples. There existed no sampling errors. The mean target distance between the planned and real targets of the mean rTRE of FSB was 2.7 ± 0.56 mm. The real TRE of FSB was first reported and was larger than the reported rTRE exactly calculated from the fiducial registration error. iCT guarantees accurate tumor sampling with autoregistration regardless of the surgical position and prevents inaccurate biopsy to occur even with ALA fluorescence assistance.
Keywords: 5-aminolevulinic acid, frameless stereotactic biopsy, intraoperative computed tomography, navigation system, target registration error
Introduction
The frameless stereotactic brain biopsy (FSB) with navigation system has been widely used instead of frame-based stereotactic biopsy in recent years.1) In FSB, errors due to inaccurate registration, incorrect targeting, and incorrect coordinates may decrease the accuracy of the procedure. Once the apparatus is set, there is no way to reconfirm the accuracy of the target.2) Navigus frameless brain biopsy kit (Medtronic, Minneapolis, MN, USA) is a skull-mounted trajectory guide for navigation-guided brain biopsy without a trajectory adjustable arm and permits using an intraoperative computed tomography (iCT) or magnetic resonance image (iMRI) during the surgical procedures. In this paper, we reported preliminary experiences of FSB with iCT and examined the real target registration error (TRE) of FSB and the usefulness of this adjunctive technique.
Materials and Methods
Patient population and data collection
No patient consent was required, and this retrospective study was approved by the Institutional Review Board of our institution (No. 2146-2). The study included 10 patients who underwent a navigation-guided FSB with iCT in our hospital between January 2018 and August 2020. We retrospectively collected the patients' clinical and pathological features including preoperative symptoms, biopsy target's localization and volume, patients' surgical position, the iCT scanning time that interrupted the surgical procedure, permanent pathological diagnosis, postoperative hemorrhage evaluated with postoperative computed tomography (CT), and additional postoperative neurological deficits. Additionally, objective fluorescence intensity with 5-aminolevulinic acid (5-ALA) of the biopsy specimen was assessed as described in the following.
Preoperative preparation
The preoperative imaging including T1-weighted magnetic resonance image (MRI), T2-weighted MRI, T1-weighted MRI with gadolinium administration, plain CT, and CT angiography was obtained. These image data were transported to a workstation for the navigation system (Stealth Station S7; Medtronic, Minneapolis, MN, USA). The contrast-enhancing lesions on MRI were defined as the biopsy target. In the workstation, until 1 day before the biopsy, the trajectory from the entry point to the biopsy target was planned with avoidance of eloquent cortexes, cortical sulci, and major vessels including feeders or drainer of the tumor. Approximately 4 h before sampling of the biopsy specimen, all patients were administered with transoral 20 mg/kg body weight 5-ALA.
Autoregistration for the navigation system with iCT scanning
The patients were positioned with their heads fixed with a carbon-based three-pin head holder under general anesthesia. For minimalization of brain shift with cerebrospinal fluid leakage, the patients' heads were positioned where the planned entry points are the highest in them as possible. With a carbon-based reference frame (Cranial StealthAiR; Medtronic, Minneapolis, MN, USA) attached to the head holder and iCT (SOMATOM Definition; Siemens, Munich, Germany), the patients were scanned. Immediately after scanning, the data of the scanned iCT were transported to the navigation system, and the iCT images were aligning the position with the integrated images semiautomatically. With these procedures, the registration for the navigation was automatically completed.
Stereotactic frameless biopsy with iCT
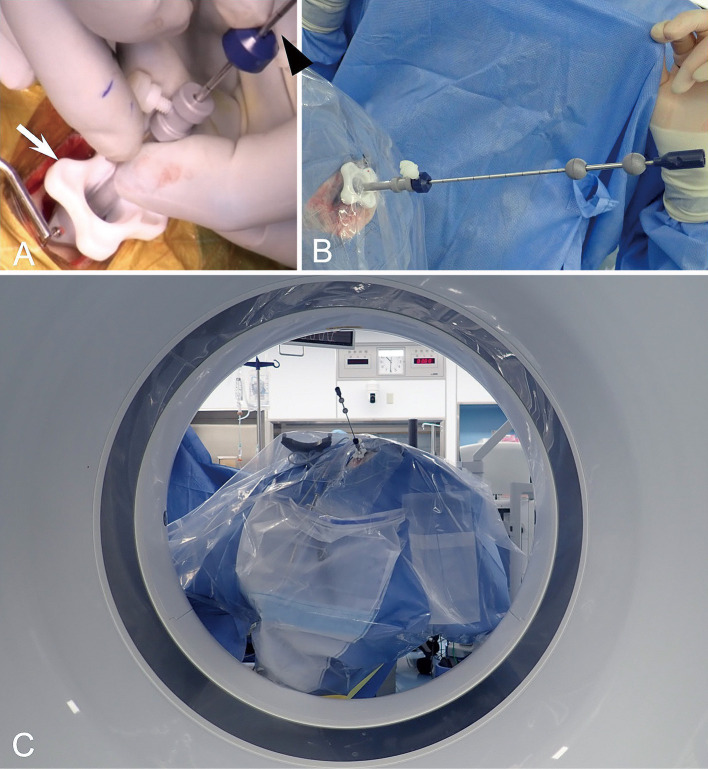
The entry point on the scalp along the planned trajectory was confirmed with a handheld probe for the navigation (Scope Probe; Medtronic, Minneapolis, MN, USA), and a small linear skin incision and a burr hole were made. After the dural opening, the Navigus trajectory guide (Medtronic, Minneapolis, MN, USA) was placed over the burr hole and fixed to the skull with screws (Fig. 1A). Navigus probe was inserted into the trajectory guide, and the probe was directed to the planned target under the navigation system guidance and fixed trajectory guide. The precalibrated biopsy needles are provided, which should be inserted through the instrument guide that was inserted into the fixed trajectory guide (Fig. 1B). Upon reaching the target, the biopsy needle is fixed to the trajectory guide tightly, and iCT was performed (Fig. 1C). While the patient's head was inserted to the gantry of the iCT scanner, several staff, including the operator, the assistants, and the radiology technicians, confirmed that the operation table, the patient's head, and the biopsy probe were certainly included within the gantry. The image data of iCT were transferred to the navigation system, and the iCT images were aligning the position with the integrated images. Then, the biopsy samples were aspirated through the biopsy needle after confirmation that the biopsy needle reached near the planned biopsy target.
Fig. 1.
Navigus trajectory guide (Medtronic, Minneapolis, MN, USA) (white arrow) was placed over the burr hole and fixed to the skull with screws (A). The precalibrated biopsy needle (black arrowhead) was inserted under real-time guidance with the navigation system (A). Upon reaching the target, the biopsy needle is fixed in the instrument adapter (white arrow) (B), and intraoperative computed tomography (CT) was performed. Intraoperative photograph was taken through the gantry of CT (C).
Assessment of 5-ALA fluorescence of biopsy specimen
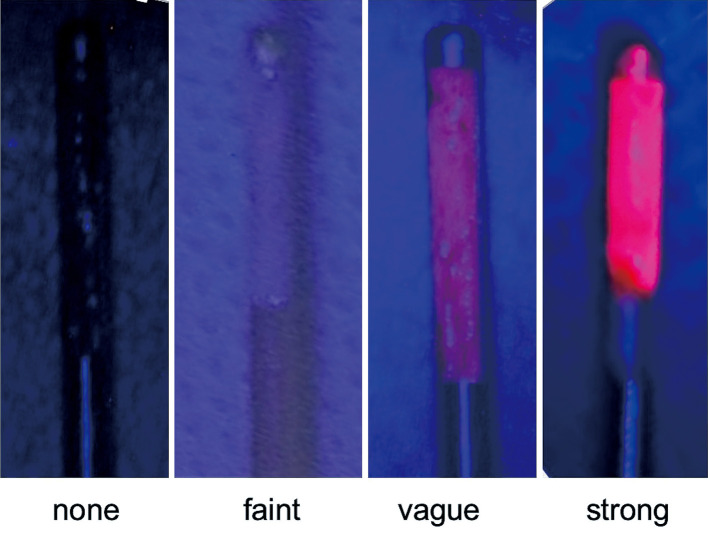
Immediately after the biopsy sampling, red fluorescence of the biopsy samples was observed under surgical microscope with blue light excitation (OPMI Pentero; Carl Zeiss, Jena, Germany). The operator and the assistant evaluated the fluorescence intensity of the biopsy specimen objectively in four levels: “strong,” “vague,” “faint,” and “none” (Fig. 2).
Fig. 2.
The representative objective four-level protoporphyrin IX fluorescence intensity of the biopsy specimens in the present study.
Assessment of real TRE of navigation-guided stereotactic biopsy with iCT
iCT images were aligning the position with a gadolinium-enhanced T1-weighted MR images to define the biopsy target immediately after iCT scanning intraoperatively. The distance from the tip of the biopsy needle in the image of iCT scanned after insertion of the needle to the planned target point was measured with the workstation of the navigation system for the assessment of real TRE. Data are presented as mean ± standard deviation.
Statistical analysis
All statistical analyses were performed using the Statistical Package for JMP Pro ver. 15 (SAS Institute Inc., Cary, NC, USA). The discrepancy of the tip of the biopsy needle and the planned target in surgical positions (supine position or prone position) and in localization of the target (deep-seated lesions (corpus callosum and basal ganglia) or another lesions) was compared by utilizing Student's t-test. We obtained a statistically significant result with p < 0.05.
Results
Table 1 shows the demographic and clinical characteristics of each of the 10 patients. Ten patients (six males and four females with a mean age of 77.0 ± 6.22 years) underwent the stereotactic frameless biopsy with iCT. The localization of the biopsy targets was the deep white matter in six patients (frontal lobe in three patients, parietal lobe in two patients, and occipital lobe in one patient), cerebral-basal ganglia in two patients (caudate head and thalamus), and corpus callosum in two patients. The mean volume with a standard deviation of the gadolinium-enhanced targets on T1-weighted MRI was 12.1 ± 11.8 mL. In three of them, the volume was less than 2 mL. Six patients were positioned in a supine position during the surgical procedure, and four patients were in a prone position. The meantime with the standard deviation of interruption of the surgical procedure with iCT scanning was 6.3 ± 1.7 min. Fortunately, because the tip of the biopsy probes was positioned almost near the planned target in the iCT images, there were no cases that the biopsy probe was repositioned.
Table 1.
The characteristics of the patients, targets, surgical position, target registration error, and postoperative course of navigation-guided frameless stereotactic brain biopsies with intraoperative CT
| Case no. | Age, years | Sex | Localization of target | Surgical position | Target volume (mL) | Histological diagnosis | Fluorescence intensity | Real TRE (mm) | Postoperative hemorrhage | Postoperative additional neurological deficit |
|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 75 | M | Rt. frontal | Supine | 1.58 | DLBCL | Vague | 1.8 | − | − |
| 2 | 82 | M | Corpus callosum | Prone | 15.06 | DLBCL | None | 2.7 | − | − |
| 3 | 81 | F | Corpus callosum | Supine | 6.35 | DLBCL | None | 3.0 | − | − |
| 4 | 80 | M | Lt. frontal | Supine | 1.81 | DLBCL | Faint | 3.2 | − | − |
| 5 | 87 | M | Rt. frontal | Supine | 21.71 | GBM | Faint | 2.5 | − | − |
| 6 | 71 | M | Rt. thalamus | Supine | 9.80 | GBM | Strong | 2.0 | + | − |
| 7 | 79 | F | Lt. parietal | Prone | 15.64 | DLBCL | None | 2.1 | − | − |
| 8 | 74 | F | Lt. parietal | Prone | 10.21 | DLBCL | Vague | 2.7 | + | − |
| 9 | 65 | F | Lt. caudate head | Supine | 0.06 | DLBCL | Vague | 2.9 | + | − |
| 10 | 76 | M | Lt. occipital | Prone | 39.15 | DLBCL | Faint | 3.6 | + | − |
DLBCL, diffuse large B-cell lymphoma; GBM, glioblastoma; TRE, target registration error.
Accuracy, diagnostic yield, and surgical complication of frameless stereotactic biopsy with iCT
The mean deviation of the planned target and the tip of the biopsy needle in images of iCT measured with the workstation (real TRE) was 2.7 ± 0.56 mm, which was not statistically significantly different according to the surgical position (supine vs. prone position) (p = 0.59) (Table 1). Additionally, there was no significant difference between deep-seated lesions (corpus callosum and cerebral-basal ganglia) and white matter lesions (p = 0.96). In 10 patients, histological diagnosis was confirmed (the overall diagnostic yield was 100%). Final histological diagnosis revealed diffuse large B-cell lymphoma (DLBCL) in eight patients and glioblastoma multiforme (GBM) in two patients. Immediately after the operation, the CT scan revealed small hemorrhage around the biopsy site in four patients; however, fortunately, a follow-up imaging study (CT or MRI a day after the operation) confirmed that the size of hematomas was not increased. As a result, after the surgery, all patients experienced no additional neurological deficit (Table 1). There were no intraoperative surgical procedure-related complications in this case series.
5-ALA-induced fluorescence of the surgical specimens
Objective 5-ALA-induced fluorescence of the biopsy specimen was “strong” in two samples, “vague” in two samples, “faint” in three samples, and “none” in three samples (Table 1). The relationship between the fluorescence intensity and the final histological diagnosis was as follows: in GBM, “faint” and “strong” fluorescence were observed in one sample each, and in DLBCL, “strong” fluorescence was observed in one sample, “vague” fluorescence was observed in two samples, “faint” fluorescence was observed in two samples, and “none” fluorescence was observed in three samples, respectively.
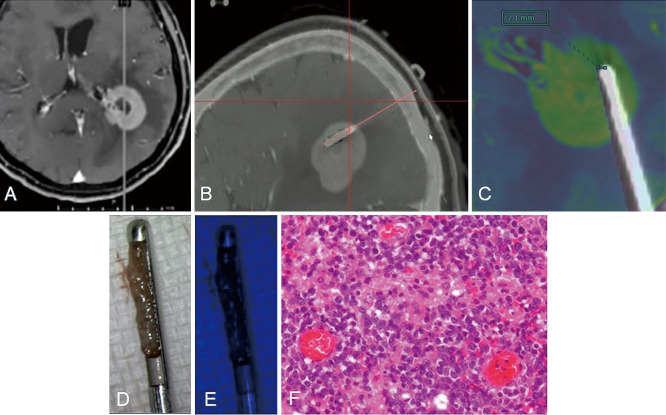
Illustrative case 1 (case 7)
The preoperative T1-weighted MRI with intravenous gadolinium administration revealed a homogeneous enhanced lesion (Fig. 3). The lesion was hyperintense on the diffusion-weighted image. Intraoperatively, iCT was performed, and biopsy specimen was sampled after confirmation that the tip of the biopsy needle reached the planned biopsy target. Although the red fluorescence was not observed when the biopsy specimen was excited with blue light, the biopsy sample was histologically diagnosed as DLBCL.
Fig. 3.
Illustrative case 1 (case 7): preoperative T1-weighted magnetic resonance image (MRI) with intravenous gadolinium administration (A) revealed a homogeneous enhanced lesion. The planned trajectory (orange line) and target (end of the orange line) corresponded to the inserted biopsy needle. The biopsy specimen under white light (B). A fusion image of preoperative T1-weighted MRI with intravenous gadolinium administration (colored image) and intraoperative CT and deviation of the planned target (black bar) and the tip of the biopsy needle in the intraoperative CT was 2.1 mm (C). Red fluorescence was not observed in the biopsy specimen under blue light excitation (D). Intraoperative sagittal merged image of the preoperative MRI with gadolinium administration and intraoperative computed tomography exactly after insertion of the biopsy needle (E). The biopsy sample was histologically diagnosed as diffuse large B-cell lymphoma. Hematoxylin–eosin stain, original magnification is ×400 (F).
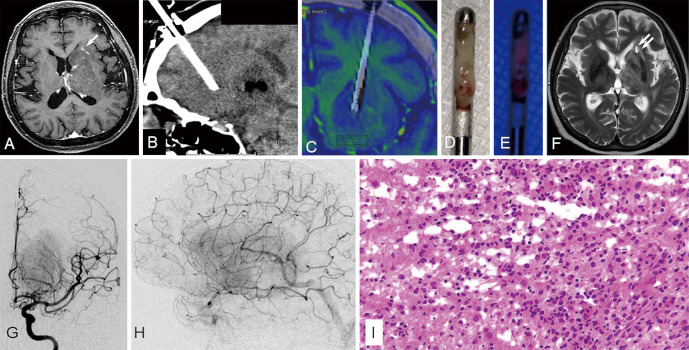
Illustrative case 2 (case 9)
T2-weighted MRI showed hyperintense lesion spreading in the left cerebral-basal ganglia and the deep white matter (Fig. 4). In the lesion, there were multiple dot-enhanced lesions with intravenous gadolinium administration. Cerebral angiography showed tumor stain with high-flow arteriovenous shunting. The extremely small, enhanced lesion of the caudate head that did not appear to be tumor-related vessels was defined as the biopsy target, and the biopsy trajectory was planned with avoidance of the cortical sulci and these vessels. The iCT was performed during the needle biopsy with confirmation that the tip of the biopsy needle reached the planned biopsy target, and the biopsy specimen was sampled. The biopsy specimen was excited with blue light, and vague red fluorescence was observed. The biopsy sample was histologically diagnosed as DLBCL. The patient received three courses of high dose methotrexate (3.5 g/m2, 2-week interval) followed by whole brain irradiation. Three months after the biopsy, T2-weighted MRI revealed a reduction in the tumor size and the hyperintensity part corresponding to the biopsy site.
Fig. 4.
Illustrative case 2 (case 9): preoperative T2-weighted magnetic resonance image (MRI) revealed hyperintense lesion spreading in the left cerebral-basal ganglia and the deep white matter. In the lesion, there were multiple dot-enhanced lesions with intravenous gadolinium administration (white arrow) (A). Preoperative angiography revealed tumor stain with high-flow arteriovenous shunting (G, H). Intraoperatively, we confirmed that the tip of the biopsy needle reached the planned biopsy target with an intraoperative computed tomography (CT) image (B). A fusion image of preoperative T1-weighted MRI with intravenous gadolinium administration (colored image) and intraoperative CT and deviation of the planned target (black bar) and the tip of the biopsy needle in the intraoperative CT was 2.9 mm (C). The biopsy specimen under white light (D). Vague red fluorescence was observed under blue light excitation in the biopsy specimen (E). The biopsy sample was histologically diagnosed as diffuse large B-cell lymphoma. Hematoxylin–eosin stain, original magnification is ×200 (I). Three months after the biopsy, T2-weighted MRI revealed a reduction in the tumor size and a small hyperintensity lesion (double white arrow) corresponding to the biopsy site (F).
Discussion
A review of 16 papers regarding FSB with a navigation system by Khatab et al. revealed that the diagnostic yield was 93.8% ranging from 87% to 100%.3) However, the FSB is basically a blind procedure; that is, no real-time radiographic feedback exists, which confirms that the biopsy needle is in fact in the target tissue. Therefore, the deviation of the reference frame (e.g., with surgical draping) or registration error would directly lead to not only inaccurate biopsy but also serious complications in addition to injuring the eloquent neural fiber or important vessels by the insertion of the biopsy needle through false trajectory. In the usual manual registration, the reference images are obtained before induction of general anesthesia and head fixation (usually a day or a few days before the operation), especially in prone or lateral positioning in surgery, which easily results in registration error. In the present study, the navigation system's autoregistration with iCT was performed exactly before the operative procedure with scanning the carbon-based three-pin head holder and the reference frame fixed to it. This registration method might provide more correct registration than the manual registration especially in the patients positioned in a lateral or prone position. Indeed, the present study included 4 (40%) patients who performed FSB in a prone position, and immediately after insertion of the biopsy needle in the prone position group, the discrepancies of the planned target and tip of the biopsy needle in iCT image were not statistically different compared with those in the supine position group.
Intraoperative histological diagnosis with frozen samples is the gold standard for confirmation of actual sampling in the biopsy operation. Dammers et al. compared a historical series of biopsied patients without an intraoperative frozen section with a recent series with frozen section, which showed a significant increase of diagnostic yield.4,5) Intraoperative histological diagnosis is time-consuming, and, sometimes, it takes a longer time than the actual surgical procedures. Therefore, this prevents the surgeons from submitting biopsy samples to the pathologists several times for intraoperative histological diagnosis in biopsy operations. To overcome these issues, the efficacy of FSBs with 5-ALA using violet-blue light excitation was reported.6,7) Widhalm et al. proved that strong fluorescence of the biopsy samples would provide an intraoperative histological diagnosis. However, the fluorescence of the tissue sample examined using the microscope with violet-blue light provided immediate information on the nature of the lesion. In malignant glioma, over 90% of the tissue samples exhibited strong fluorescence;8) on the other hand, in malignant lymphoma that might be the best indication of FSB, Kiesel et al. reported 41 cases of navigation-guided FSB with 5-ALA and that the fluorescence was not observed in 21% of these cases.9) Especially in an extremely small biopsy target, if the vague fluorescence was observed, despite the fact that the tissue around the planned target was sampled, the sampling and the surgical procedure were finished, and this might lead to misdiagnosis. As a result, although all of the patients in the present study were diagnosed histologically, none or faint fluoresce was observed in 60% of the cases. In these cases, to avoid inaccurate biopsy, the intraoperative histological diagnosis should be more important. The iCT scanning immediately after insertion of the biopsy needle will compensate for these shortcomings of 5-ALA assistance in FSB.
iMRI was also reported as an adjuvant modality for the stereotactic biopsy or electrode placement of deep brain stimulation (DBS).1,10,11) Giordano et al. also reported the same approach with iMRI and 5-ALA.6) The superiority of iMRI compared with iCT is overpoweringly good image resolution and avoiding radiation exposure. However, the efficacy of their procedure is questionable because iMRI is also time-consuming as well as the intraoperative histological diagnosis. We can confirm that the trajectory and the tip of the biopsy needle correspond to the planned trajectory and the targeted lesion by iCT images merging with MRI images scanned preoperatively in the workstation of the navigation system.
TRE of the surgical navigation systems has an influence on the accuracy of biopsy directly in small biopsy targets, especially in FSB. However, because the real TRE was difficult to measure directly in actual clinical cases for various factors, especially in the case of surgeries with craniotomy,12) only surface registration errors (i.e., deviation of the surface makers) are assessed. A recent meta-analysis revealed that TRE in DBS, which was calculated with a mean deviation between the inserted DBS lead tip and planned target, was 1.59-3.54 mm.13) Though it may be applied as a TRE for the FSB, as discussed in the paper, TRE in DBS was affected by repositioning the lead to appropriate target after intraoperative electrophysiologic testing, including microelectrode recording. Since surgeons elected to place greater trust in the physiologic target than in an anatomical-based target, this is not the “real” TRE of the navigation-guided stereotactic procedure. In our study, taking intraoperative imaging immediately after biopsy probe insertion allowed us to provide “real” TRE with the elimination of these various factors as much as possible. Our presented data suggested that special attention should be paid if the biopsy target is in a diameter of <3 mm. Furthermore, this real TRE would be helpful for other procedures with navigation system.
The major shortcoming of our presented adjunctive technique is the twice radiation exposure in one surgery. In the present study, iCT with normal radiation exposure was used. Carl et al. succeeded and reported reliable navigation autoregistration with iCT with low radiation exposure.14,15) Since it is predicted that the biopsy needle is enough for visual recognition in low-dose-irradiated iCT, not only in scanning for autoregistration but also in scanning for confirming the actual insertion of the biopsy needle, we should apply iCT with low radiation exposure in the future.
The main limitation of the present report is its retrospective design and single-center nature. Furthermore, the number of patients is relatively low. Further prospective study is needed to clarify the improvement of biopsy accuracy.
Conclusion
The use of FSB with iCT guarantees accurate brain biopsy for accurate registration with autoregistration regardless of the surgical position. Additionally, intraoperative scanning with iCT prevents inaccurate biopsy occurrence even with ALA fluorescence assistance.
Yoshihide Katayama and Naokado Ikeda contributed equally to this work.
Conflicts of Interest Disclosure
All authors have no conflict of interest.
Acknowledgments
A part of this work was supported by the Japan Society for the Promotion of Science Grant-in-Aid for Scientific Research (C) (18K09011) to Naokado Ikeda. The authors would like to thank Enago (www.enago.jp) for the English language review.
References
- 1). Bradac O, Steklacova A, Nebrenska K, Vrana J, de Lacy P, Benes V: Accuracy of VarioGuide Frameless Stereotactic System Against Frame-Based Stereotaxy: Prospective, Randomized, Single-Center Study. World Neurosurg 104: 831-840, 2017 [DOI] [PubMed] [Google Scholar]
- 2). Satoh M, Nakajima T, Yamaguchi T, Watanabe E, Kawai K: Application of Augmented Reality to Stereotactic Biopsy. Neurol Med Chir (Tokyo) 59: 444-447, 2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3). Khatab S, Spliet W, Woerdeman PA: Frameless image-guided stereotactic brain biopsies: emphasis on diagnostic yield. Acta Neurochir (Wien) 156: 1441-1450, 2014 [DOI] [PubMed] [Google Scholar]
- 4). Shooman D, Belli A, Grundy PL: Image-guided frameless stereotactic biopsy without intraoperative neuropathological examination. J Neurosurg 113: 170-178, 2010 [DOI] [PubMed] [Google Scholar]
- 5). Dammers R, Schouten JW, Haitsma IK, Vincent AJ, Kros JM, Dirven CM: Towards improving the safety and diagnostic yield of stereotactic biopsy in a single centre. Acta Neurochir (Wien) 152: 1915-1921, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6). Giordano M, Gallieni M, Zaed I, Samii A: Use of Frameless Stereotactic Navigation System Combined with Intraoperative Magnetic Resonance Imaging and 5-Aminolevulinic Acid. World Neurosurg 131: 32-37, 2019 [DOI] [PubMed] [Google Scholar]
- 7). Moriuchi S, Yamada K, Dehara M, et al. : Use of 5-aminolevulinic acid for the confirmation of deep-seated brain tumors during stereotactic biopsy. Report of 2 cases. J Neurosurg 115: 278-280, 2011 [DOI] [PubMed] [Google Scholar]
- 8). Widhalm G, Minchev G, Woehrer A, et al. : Strong 5-aminolevulinic acid-induced fluorescence is a novel intraoperative marker for representative tissue samples in stereotactic brain tumor biopsies. Neurosurg Rev 35: 381-391; discussion 391, 2012 [DOI] [PubMed] [Google Scholar]
- 9). Kiesel B, Millesi M, Woehrer A, et al. : 5-ALA-induced fluorescence as a marker for diagnostic tissue in stereotactic biopsies of intracranial lymphomas: experience in 41 patients. Neurosurg Focus 44: E7, 2018 [DOI] [PubMed] [Google Scholar]
- 10). Mohyeldin A, Lonser RR, Elder JB: Real-time magnetic resonance imaging-guided frameless stereotactic brain biopsy: technical note. J Neurosurg 124: 1039-1046, 2016 [DOI] [PubMed] [Google Scholar]
- 11). Czyz M, Tabakow P, Weiser A, Lechowicz-Glogowska BE, Zub LW, Jarmundowicz W: The safety and effectiveness of low field intraoperative MRI guidance in frameless stereotactic biopsies of brain tumours-design and interim analysis of a prospective randomized trial. Neurosurg Rev 37: 127-137, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12). West JB, Fitzpatrick JM, Toms SA, Maurer CR Jr., Maciunas RJ: Fiducial point placement and the accuracy of point-based, rigid body registration. Neurosurgery 48: 810-816; discussion 816-817, 2001 [DOI] [PubMed] [Google Scholar]
- 13). Philipp LR, Matias CM, Thalheimer S, Mehta SH, Sharan A, Wu C: Robot-Assisted Stereotaxy Reduces Target Error: A Meta-Analysis and Meta-Regression of 6056 Trajectories. Neurosurgery 88: 222-233, 2021 [DOI] [PubMed] [Google Scholar]
- 14). Carl B, Bopp M, Sass B, et al. : Reliable navigation registration in cranial and spine surgery based on intraoperative computed tomography. Neurosurg Focus 47: E11, 2019 [DOI] [PubMed] [Google Scholar]
- 15). Carl B, Bopp M, Sass B, Nimsky C: Intraoperative computed tomography as reliable navigation registration device in 200 cranial procedures. Acta Neurochir (Wien) 160: 1681-1689, 2018 [DOI] [PubMed] [Google Scholar]