Abstract
Increasing number of different novel positron emission tomography (PET) radiopharmaceuticals poses challenges for their manufacturing procedures at different PET research facilities. Recent commercial available radiochemistry units with disposable cassettes are becoming common stations to produce radiopharmaceuticals with high specifications to understand the critical PET imaging outputs of the study. Therefore, several radiochemists across the PET research centers develop and optimize their own radiochemistry protocols to develop a novel or routine radiopharmaceutical at their lab. In this report, we describe the general procedure and steps followed to develop a (clinical-grade) radiopharmaceutical on a commercially available radiochemistry unit, TRASIS AIO. As an example, we use our routine protocol followed for production of [11C]acetate, a fatty acid metabolic PET imaging ligand for several cancer imaging studies.
Keywords: PET, radiopharmaceutical, hot cell, acetate, Quality control
1. Introduction
Positron Emission Tomography (PET) is a non-invasive, sensitive imaging modality used for imaging biological processes in vivo including blood flow, metabolic pathways, receptor expressions, and multiple cancer mechanisms [1]. It is a powerful tool particularly on oncology due to its high molar concentration resolution (from 10−11 to 10−12 mol/L), along with relevant functional information and quantitative capabilities [2,3]. PET imaging requires the injection of a radiopharmaceutical, labeled with a short-lived radioisotope (generated by cyclotron) that emits a positron, which annihilates with an electron in the biological tissue to release gamma rays captured by a PET camera [4]. Advances in nuclear medicine imaging have led to an increased demand for PET radiopharmaceuticals for early and accurate diagnosis of cancer and other diseases [4]. Here we report the common and basic steps involved in production of radiopharmaceuticals in a typical PET research lab, and illustrate it with an example of [11C]acetate production. The radiopharmaceutical, [11C]acetate is widely used for imaging fatty acid metabolism in several types of cancers including brain, prostate, hepatocellular carcinoma, renal adenocarcinoma, urinary bladder, and head and neck cancers [5–9]. Owing its increasing clinical significance in oncology and cardiology imaging,[8] several research groups have reported [11C]acetate radiolabeling procedures using both in-house and commercially available radiochemistry modules, including TRASIS AIO, GE and Synthera systems [10–13]. TRASIS AIO is an automated commercially available radiochemistry module that produces clinical grade PET radiopharmaceuticals using cassette-based protocols [13,14]. We use TRASIS AIO module for [11C]acetate production [13] at Wake Forest PET imaging facility.
2. Materials
PET radiopharmaceutical production need two key components (a) equipment and (b) reagents and supplies [15].
2.1. Equipments
Cyclotron, hot cell, radiochemistry module with HPLC and radiation detectors, chemistry hood (optional).
2.2. Reagents and supplies
Precursor, anhydrous solvents, SepPak/resins, HPLC semi-preparative and QC columns, mobile phases, needles, syringes, final production vial and 0.22 μm filter units. For example with [11C]acetate production, methylmagnesium chloride (3.0 M, THF), anhydrous THF, acetic acid (1.0 M), and citric acid buffers were purchased from Sigma Aldrich, MO, USA. Ion-exchange column resins were directly purchased from Labnet Inc. USA. All sterile filters were purchased from Thermo Scientific Inc, USA.
3. Methods
To avoid any possible contamination and ensure safety, these radiopharmaceuticals are typically produced using an automated radiochemistry module located in specialized hood, called ‘hot cell’[15]. Recently several radiochemistry modules are available to simplify the task of complying with clinical-grade requirements and allowing radiochemists to produce multiple productions by simple cleaning processes [16,15]. Radiochemistry modules are very efficient and heavily used production units that perform all the key steps of radiochemistry including receiving PET radioactive isotopes, radiolabeling reaction, purification, separation and final elution. Typical radiopharmaceutical production can be separated into three phases: a. Pre-labeling setup, b. Radiochemistry, and c. QC testings (Figure 1).
Figure 1:
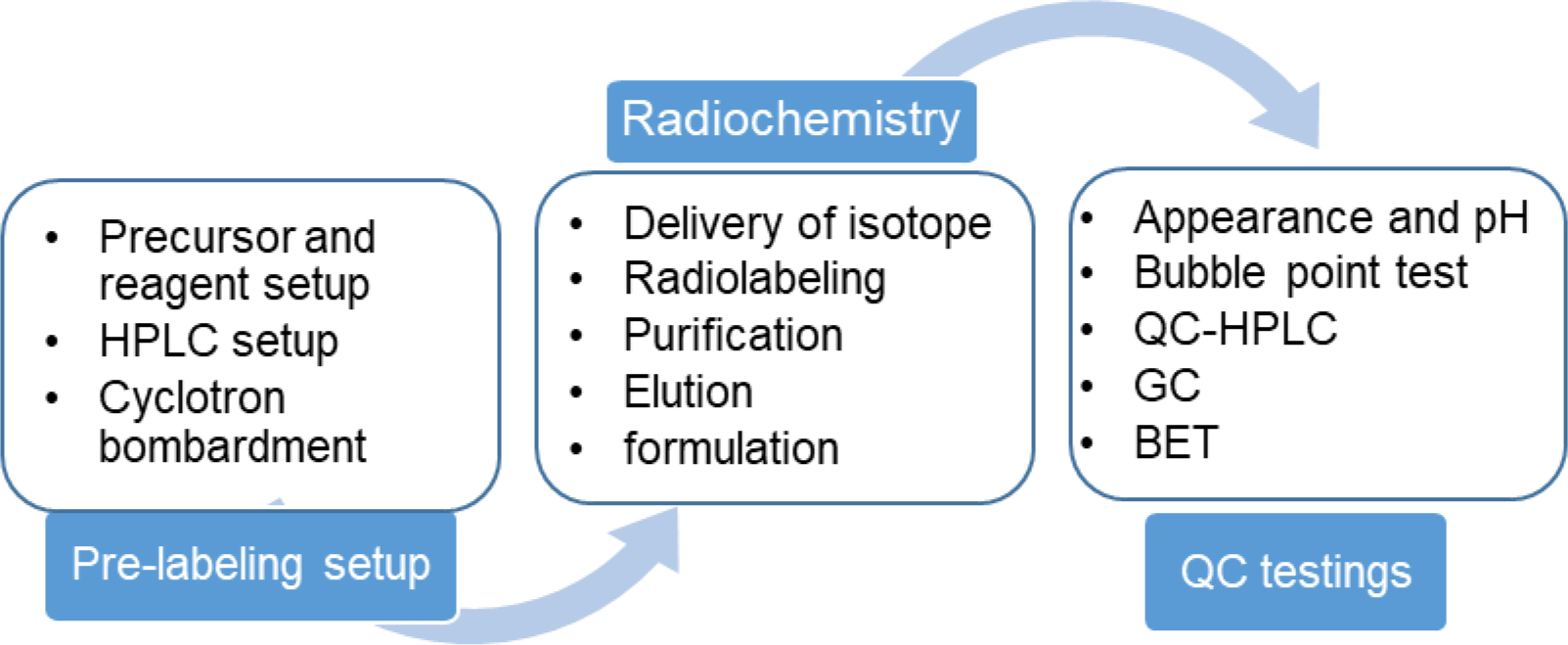
Flow chart of common steps involved in a typical PET radiopharmaceutical production
3.1. Pre-labeling setup
Most of the precursors for radiopharmaceutical productions are commercially available and can be purchased directly from the vendor. These precursors need to be refrigerated and thawed at least for 10–15 min at room temperature before starting production. Few radiopharmaceuticals need freshly prepared precursors, including [11C]acetate and [11C]acetoacetate [13,14].
Turn the cyclotron master computer ON to [11C]-target (Note 1)
Place all the required materials including starting materials, activated sepPaks, solvents, and final sterile vial with filtration setups. For [11C]acetate production, make the following items available: methylmagnesium chloride (3M, 0.12 mL) in anhydrous tetrahydrofuran (0.6 mL), acetic acid (1 M, 10 mL), aqueous citrate buffer pH 4.5 (5 mL), activated ion exchange column resin sepPak (chromofix PS-H+, PS-AG+, PS-OH−), and sterile pyrogen-free 0.22 μm sterile filter.
Activate the SepPaks ~15 min before the production. C18 SepPaks are commonly activated with 5 mL of ethanol (or acetonitrile) and 10 mL of water. For [11C]acetate production, activate Chromofix PS-H+ and PS-AG+ ion-exchange resins with 10 mL deionized water, and PS-OH- with 1 M NaOH (10 mL) followed by 10 mL deionized water elutions.
Turn ON the power switch to the module/hot cell and the necessary gas valves. For [11C]acetate on TRASIS AIO module, turn ON the power switch, and valves for compressed air and ultra-pure nitrogen gases (Note 2).
Place the right HPLC semi-preparative column with the corresponding freshly-made filtered mobile phase solutions. Also select the right UV wavelength for the HPLC purification (Note 3). No HPLC purification is needed for [11C]acetate production.
Open the TRASIS AIO software system and select the right radiopharmaceutical production program. Follow the steps for production and acknowledge each step, after completion of performance of the respective tasks.
All productions in TRASIS AIO involves leak checks in gases, vacuum leak checks and then cassette compatibility checks (Note 4). Load the cassette after the right prompt. For [11C]acetate, once the system checks for all possible leaks, load the reagents at the right place in the cassette following the schematic representation in the program (Figure 2).
Now the system checks for leaks with the reagents (Note 4). Make sure all the components of the cassette are leak-tight and ready to be proceeded for next steps of cyclotron bombardment.
Make sure all the gases, vacuum, water temperatures, and valves on the cyclotron are turned ON and then login to the cyclotron computer (Note 1). For our GE PETtrace 800 series cyclotron we leave all the gases, vacuum and water valves always ON.
Login into the system and select the right PET isotope. For [11C]acetate, select C11 target (Note 1).
Input the right current (μA) and bombardment time (min). For a typical clinical production of [11C]acetate we use 59 μA of bombardment for 25 min.
Make sure that the beam is turned ON and the right delivery path/hot cell is selected for the release of the corresponding PET isotope. For example, [11C]acetate production needs [11C]CO2 to be released in hot cell containing TRASIS AIO module.
Make arrangements of precursor setup, ~5–8 min of the radioisotope delivery from cyclotron. [11C]acetate needs the Grignard reagent, methylmagnesium bromide (3M, 0.12 mL) in anhydrous THF (0.6 mL) at lower temperatures (0 to −10 °C) in a dry reaction vial of the TRASIS AIO module.
Figure 2:
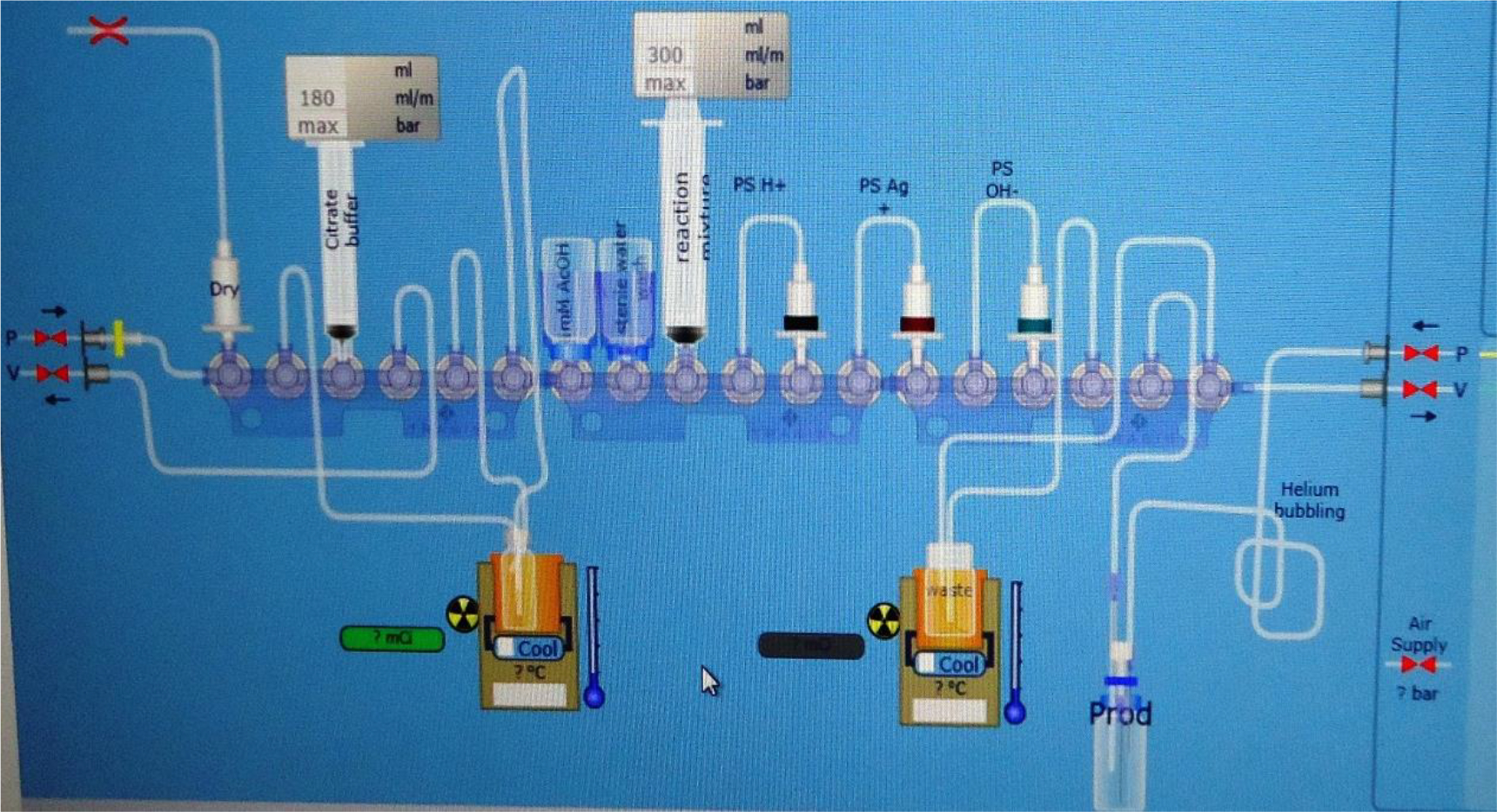
TRASIS AIO reaction program screenshot for [11C]acetate production
3.2. Radiochemistry
Select ‘delivery’ option from the cyclotron computer after the completion time of target bombardment, for example at 25 min for [11C]acetate.
Visually inspect the delivery of radioactive material into the radiochemistry module. TRAIS AIO system shows an increase in reaction vial radioactivity during the delivery process (Note 2). For [11C]acetate production, [11C]CO2 from cyclotron will be directly bubbled into the closed reaction vial containing the precursor, methylmagnesium bromide/THF (Figure 3).
Acknowledge the prompt on TRASIS AIO of ‘receiving all the radioactivity’ and record the radioactivity numbers after achieving the maximum load and then ‘start the radiolabeling reaction’.
Acknowledge and terminate the production on cyclotron system accordingly. It usually takes ~2.5 min to completely deliver all the radioactive [11C]CO2 into the TRASIS AIO module (Note 4).
Allow the radiolabeling to proceed and record the radioactivity along with time (Note 5). For [11C]acetate radiolabeling, [11C]CO2 carboxylation of methylmagnesium bromide is needed ~2 min at room temperature.
Quench the reaction mixture after the radiolabeling step with some aqueous solutions. [11C]acetate is quenched with 1M aqueous acetic acid solution (10 mL).
Load the crude reaction mixture onto the semi-preparative HPLC chromatography for purification (Note 3, 6). Collect the desired radioactive peak into a flask/vial/syringe with 25 ± 10 mL of deionized water. Pass the collected pure aqueous radioactive solution through the activated sepPak/resins into the waste. As [11C]acetate production does not involve any HPLC purification, it is purified by ion-exchange Chromofix resins.
The pure radioactive product will be trapped into the activated SepPak (Note 7). For [11C]acetate, the crude acidic reaction mixture will be passed through activated chromofix PS-H+, PS-AG+ and PS-OH− ion-exchange resin sepPaks. [11C]acetate will be trapped in the last PS-OH− resin.
Elute the final radiopharmaceutical from the SepPak/resin using ethanol or acetonitrile or aqueous buffer solutions. For [11C]acetate, the final product will be eluted from PS-OH− ion exchange resin using aqueous citrate buffer pH~ 4.5 solution (6 mL) into the sterile final product vial via a pyrogen-free 0.22 μm sterile filter unit.
Degas if necessary to remove any excess undesired gases, including CO2, CO from the final product vial (Note 7). For [11C]acetate, the final product vial needs to be degassed for an additional 4 min using nitrogen gas to remove any excess CO2.
Figure 3:
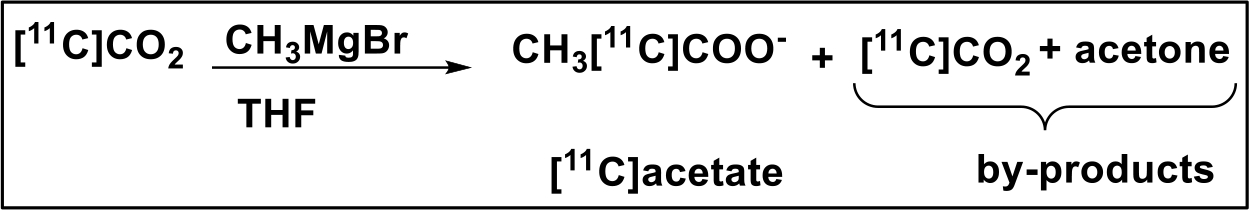
Radiolabeling route for [11C]acetate production
3.3. Quality Control testings
Release of final radiopharmaceutical dose takes place after a series of QC testings and the common tests are listed in Table 1.
Table 1.
List of common QC testings performed for a typical PET radiopharmaceutical production
| QC testings | Typical acceptable parameters for [11C]acetate release |
|---|---|
| Appearance | Clear, colorless and particle-free |
| pH | 4.0–5.5 |
| Filter bubble point test | >30 psi |
| Radiochemical purity (HPLC) | >90% |
| Chemical mass (HPLC) | < 100 ppm |
| Chemical purity (HPLC) | >90% |
| Specific activity (HPLC) | >300 mCi/μmol at injection |
| Bacterial Endotoxin Test | Pass |
| Radionuclide half-life | 20±1 min |
| GC solvent analysis | One peak, <100 ppm citrate salts |
| Sterility | pass |
Remove the formulated radiopharmaceutical from the hot cell by unplugging the filter units and other tubing materials (Note 8).
Measure and record the final weight and radioactivity of the final dose
Withdraw two ~0.5 mL of radioactive samples following the sterile processes into pre-labeled sterile syringes inside the sterile UV chamber. One sample will be used for immediate QC testing and the other for sterility testing
Record the appearance by visual inspection
Measure the pH of the solution using a pH indicator paper
Withdraw ~10 μL of sample from sterility for Bacterial Endotoxin Test (BET) test
Perform the bubble point test for the 0.22 μm filter unit used for eluting the final radiopharmaceutical into the final product vial (Note 9).
Assess the half-life of the radioactive isotope by measuring its activity at two different time points and fitting the radioactive isotope decay curve. For example, [11C]acetate exhibits half-life of 20 min.
First develop an analytical HPLC QC method for the radiopharmaceutical produced, using the non-radioactive standard i.e., establish the right mobile phase, HPLC column, retention time, UV wavelength, and calibration curve (Note 8). Validate the method by performing the analytical runs at different concentrations. For [11C]acetate, Aminex HPX-87 H analytical column (300 X 70 mm) at 44°C (column heater) with aqueous sulfuric acid (5mM) as the mobile phase at 210 nm with a flow rate of 1.0 mL/min was used.
Run the standard injections of non-radioactive standards, reaction solvents, and mobile phases on the day of radiopharmaceutical production.
Inject ~20 μL of the radioactive solution from the 0.5 mL sample into the QC HPLC system for assessing the radiochemical and chemical purity.
Calculate the UV mass, specific activity and radiochemical purity of the final dose.
Inject another fresh aliquot of ~20 μL of radioactive solution premixed with non-radioactive standard (~20 μL) to authenticate the purity of the final dose. [11C]acetate retention time was ~6.2–7.1 min at the above mentioned QC conditions in both single and co-injections (with non-radioactive acetate) validating its final purity.
Assess residual solvent(s) of the final radiopharmaceutical formulation by performing gas chromatography. For [11C]acetate, one peak with a retention time at 3.3–3.6 min corresponds to the citrate buffer solution.
Release the final radioactive drug for subject injection-PET scanning, following the test results from the release criteria for radiopharmaceutical QC testings.
Consult the head or the lead contact personnel of the PET facility immediately for any deviation in the results from release criteria of QC testings, before releasing the final dose.
Shut down the cyclotron production on the cyclotron computer.
Print the module-generated report that includes the semi-preparative HPLC chromatogram and other radioactivity steps after the completion of production. For [11C]acetate production, TRASIS AIO generates a complete production report with all the detailed parameters starting from leak tests, receiving [11C]CO2 radioactivity, radiolabeling, purification, and elutions at specific timings.
Remove the used cassette and place it in a safe lead shielded storage unit to allow for complete decay of the PET isotope used before disposal.
Radiochemistry unit should be ready to use for the next production
4. Notes
The following steps are suggested for any possible ‘troubleshooting tasks’ at different steps of radiopharmaceutical production
If there is an issue with cyclotron bombardment, first restart the cyclotron computer and make sure the connections on vacuum, gases, and water are good. If the issue doesn’t get resolved contact the cyclotron engineer immediately
If the radiochemistry unit fails the leak tests, make sure the vacuum bottle is empty and the tubing lines are tightly connected
Make sufficient amount of semiprep HPLC mobile phase for any unexpected HPLC-based issues and possible repetitions
If the system fails on the cassette leak tests, make sure the cassette components are connected right and tight to the radiochemistry module
If the reaction mixture moves slowly during the radiolabeling, check for nitrogen gas tank occupancy. If the pressure is too low (< 5.5 bar), module doesn’t work efficiently.
If the HPLC pressure is too high or too low, check the HPLC connections and make sure there are no leaks and no solid particulates in the loop.
- If the radiochemical yields are low, there could be issues with the following
- Cyclotron: always dry the cyclotron delivery lines efficiently with helium and notify the cyclotron engineer/manufacturer for unresolved cyclotron-based issues.
- Reagents: make sure all the reagents including precursor, solvents, aqueous acids/base, and buffer solutions are fresh and not expired or contaminated.
- Moisture: Make sure all the organic solvents used are anhydrous, as any moisture in the solvent and reaction vial can lower the radiolabeling yields and purity drastically.
- QC trouble shooting steps
- If the final purity of the radiopharmaceutical is unreasonably high or low, make sure the injection syringes and septa of HPLC/GC are clean and dry.
- If the QC-HPLC system shows peaks of carbonic acid/CO2 (for [11C]acetate production), perform degassing using nitrogen gas for another additional 3–4 min.
- Make sure to run the non-radioactive standards at least 2–3 times to establish the right retention time of the radiopharmaceutical
- Prepare sufficient amount of HPLC mobile phase to avoid any issues with high pressures and unexpected repeating procedures
If the bubble point pressure of the filter unit fails, re-filter the lot using a new sterile filter unit and redo the bubble point test.
References
- 1.Jerusalem G, Hustinx R, Beguin Y, Fillet G (2003) PET scan imaging in oncology. European journal of cancer 39 (11):1525–1534 [DOI] [PubMed] [Google Scholar]
- 2.Davidson CQ, Phenix CP, Tai TC, Khaper N, Lees SJ (2018) Searching for novel PET radiotracers: imaging cardiac perfusion, metabolism and inflammation. Am J Nucl Med Mol Imaging 8 (3):200–227 [PMC free article] [PubMed] [Google Scholar]
- 3.Sai KKS, Jones LA, Mach RH (2013) Development of 18F-labeled PET probes for imaging cell proliferation. Current topics in medicinal chemistry 13 (8):892–908 [DOI] [PubMed] [Google Scholar]
- 4.Vāvere AL, Scott PJH (2017) Clinical Applications of Small-molecule PET Radiotracers: Current Progress and Future Outlook. Seminars in Nuclear Medicine 47 (5):429–453. doi: 10.1053/j.semnuclmed.2017.05.001 [DOI] [PubMed] [Google Scholar]
- 5.Seltzer MA, Jahan SA, Sparks R, Stout DB, Satyamurthy N, Dahlbom M, Phelps ME, Barrio JR (2004) Radiation Dose Estimates in Humans for 11C-Acetate Whole-Body PET. Journal of Nuclear Medicine 45 (7):1233–1236 [PubMed] [Google Scholar]
- 6.Liu R-S, Chang C-P, Chu L-S, Chu Y-K, Hsieh H-J, Chang C-W, Yang B-H, Yen S-H, Huang M-C, Liao S-Q, Yeh S-H (2006) PET imaging of brain astrocytoma with 1–11C-acetate. European Journal of Nuclear Medicine and Molecular Imaging 33 (4):420–427. doi: 10.1007/s00259-005-0023-0 [DOI] [PubMed] [Google Scholar]
- 7.Park J-W, Kim JH, Kim SK, Kang KW, Park KW, Choi J-I, Lee WJ, Kim C-M, Nam BH (2008) A Prospective Evaluation of 18F-FDG and 11C-Acetate PET/CT for Detection of Primary and Metastatic Hepatocellular Carcinoma. Journal of Nuclear Medicine 49 (12):1912–1921 [DOI] [PubMed] [Google Scholar]
- 8.Czernin J, Benz MR, Allen-Auerbach MS (2009) PET Imaging of Prostate Cancer Using C-Acetate. PET Clin 4 (2):163–172. doi: 10.1016/j.cpet.2009.05.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Spick C, Herrmann K, Czernin J (2016) Evaluation of Prostate Cancer with 11C-Acetate PET/CT. Journal of Nuclear Medicine 57 (Supplement 3):30S–37S [DOI] [PubMed] [Google Scholar]
- 10.Le Helleix S, Dollé F, Kuhnast B (2013) Easy upgrade of the TRACERLab FX C Pro for [11C]carboxylation reactions: Application to the routine production of [1–11C]acetate. Applied Radiation and Isotopes 82:7–11. doi: 10.1016/j.apradiso.2013.06.020 [DOI] [PubMed] [Google Scholar]
- 11.Moerlein S, Gaehle G, Welch M (2002) Robotic preparation of Sodium Acetate C 11 Injection for use in clinical PET. Nuclear medicine and biology 29:613–621. doi: 10.1016/S0969-8051(02)00307-4 [DOI] [PubMed] [Google Scholar]
- 12.Pike VW, Horlock PL, Brown C, Clark JC (1984) The remotely-controlled preparation of a 11C-labelled radiopharmaceutical-[1–11C]acetate. The International Journal Of Applied Radiation And Isotopes 35 (7):623–627. doi: 10.1016/0020-708X(84)90107-8 [DOI] [Google Scholar]
- 13.Solingapuram Sai KK, Cartwright J, Mintz A (2017) Automated synthesis of 1-[11C]acetate on a commercially available TRASIS AIO synthesizer. Journal of Nuclear Medicine 58 (supplement 1):873 [Google Scholar]
- 14.Solingapuram Sai KK, Donald Gage H, Almaguel F, Neth B, Hughes TM, Tremblay S, Castellano C-A, Cunnane SC, Jorgensen MJ, Craft S, Mintz A (2017) Automated synthesis of 1-[11C]acetoacetate on a TRASIS AIO module. Applied Radiation and Isotopes 129:57–61. doi: 10.1016/j.apradiso.2017.07.066 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Au - Schopf E, Au - Waldmann CM, Au - Collins J, Au - Drake C, Au - Slavik R, Au - van Dam RM (2018) Automation of a Positron-emission Tomography (PET) Radiotracer Synthesis Protocol for Clinical Production. JoVE (140):e58428. doi:doi: 10.3791/58428 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Li S, Schmitz A, Lee H, Mach RH (2017) Automation of the Radiosynthesis of Six Different (18)F-labeled radiotracers on the AllinOne. EJNMMI Radiopharm Chem 1 (1):15–15. doi: 10.1186/s41181-016-0018-0 [DOI] [PMC free article] [PubMed] [Google Scholar]


