PURPOSE:
The purpose of this study was to evaluate the implementation of pressure injury (PI) prevention strategies in adult acute care settings in the United States using the data from the 2018/2019 International Pressure Ulcer Prevalence (IPUP) Survey.
DESIGN:
Observational, cohort study with cross-sectional data collection and retrospective data analysis.
SUBJECTS AND SETTING:
The sample comprised 296,014 patients hospitalized in 1801 acute care facilities in the United States that participated in the 2018 and/or 2019 IPUP Survey. Slightly less than half (49.4%, n = 146,231) were male, 50% (n = 148,997) were female, 0.6% (n = 17,760) were unknown. Their mean age was 64.29 (SD 17.2) years.
METHODS:
Data from the 2018/2019 IPUP database were analyzed to evaluate the implementation of prevention strategies including repositioning, support surface use, head-of-bed (HOB) elevation, heel elevation, moisture management, minimizing linen layers, and nutritional support. Practices were analyzed for differences between patients without pressure injuries, and patients with Stage 1 and 2 hospital-acquired pressure injury (HAPI), and those with severe HAPIs (Stage 3, Stage 4, unstageable, and deep tissue pressure injury). Acute care unit types included critical or intensive care units, medical-surgical inpatient care units, and step-down units.
RESULTS:
Compliance rates to PI prevention strategies varied among patients at risk for HAPIs (Braden Scale for Pressure Sore Risk score ≤18). Daily skin assessment was performed for 86% of patients with no HAPIs and 96.8% of patients with severe HAPIs. Pressure redistribution was used in 74.6% of all patients and in over 90% of patients with severe HAPIs; however, compliance to routine repositioning was reported at lower levels between 67% and 84%, respectively. Heel elevation was reported for over 60% of the patients with severe HAPIs while 31.9% did not receive heel elevation, though only 6% were reported as not needing elevation. The majority of patients had HOB greater than the 30° at the time of the data collection; compliance with minimizing linen layers (≤3) was reported in 76% or more. Moisture management strategies were reportedly used in more than 71% of all patients and 89% for patients with severe HAPIs. Nutrition support was used for 55% to 82% of the patients and only documented as contraindicated in fewer than 2% of all groups.
CONCLUSION:
Study findings revealed substantial compliance rates to PI prevention strategies. Nevertheless, there is potential for improvement in the implementation of some of the most basic prevention strategies including repositioning, heel elevation, nutritional support, and moisture management.
Keywords: Acute care, Critical care, Hospital acquired, iPUP, Pressure injuries, Pressure ulcer, Prevalence, Prevention strategies
INTRODUCTION
Pressure injuries develop when tissue loading and/or duration of loading exceed individual tissue tolerance.1 The risk factors for the development of pressure injuries include mobility/activity limitations, skin status, perfusion, circulation, oxygenation, moisture/microclimate, age, and nutrition.1 Patients in critical care settings have additional risk such as vasopressor administration, prolonged admission in a critical care unit, diabetes mellitus, cardiovascular disease, hypotension, prolonged mechanical ventilation, hemodialysis, and sedation, confirming the complex nature of pressure injury (PI) development.2–4 Prevention strategies have been developed to mitigate modifiable risk factors, though other risk factors such as age or use of life saving measures such as mechanical ventilation cannot be altered.
Overall, hospital-acquired pressure injuries (HAPIs) decreased from 2006 to 2013 from 6.6% to 3.6%.5 However, the HAPI rate has not decreased any further since 2013 and analysis of 2013-2019 rates showed an upward trend within a range of 2.5% to 3.2% for all inpatient care units and an upward trend of 5.6% to 6.4% among critical care units.5 Any analysis of HAPIs invites the question of which prevention strategies were implemented for those patients. We examined prevention strategies used in adult patients in acute care settings in the United States using data from the International Pressure Ulcer Prevalence (IPUP) database.
The following research question guided this study: Is there a difference in the implementation of prevention strategies (skin assessment, repositioning, support surface implementation, head-of-bed [HOB] elevation, heel elevation, linen layers, moisture management, and nutritional support) between patients with no pressure injuries, Stage 1 and 2 HAPIs, or severe HAPIs (Stage 3, Stage 4, unstageable, and deep tissue pressure injury [DTPI])?
MATERIALS AND METHODS
We analyzed data from the 2018/2019 International Pressure Ulcer Prevalence (IPUP) Survey, an observational, cross-sectional cohort database. The IPUP has been facilitated by HillRom, Inc (Batesville, Indiana) since 1989 and participation is open to all healthcare facilities. This study was approved by the Institutional Review Board of Rutgers University through exempt status (#2019001057).
Data Collection
Hospital-based clinical teams were trained, prior to the survey, on the data collection procedure and proper completion of the data abstraction record. The goal of the survey was to assess all patients admitted to the hospital over a specific preselected 24-hour period within a 2- to 3-day window. We analyzed the following variables: demographic and clinical characteristics (age, gender, type of care unit, length of hospital stay prior to the IPUP Survey date, and Braden Scale for Pressure Sore Risk score on the day of the survey), along with selected PI characteristics (overall and hospital-acquired prevalence rates, stage, and anatomic location).
The following prevention practices were analyzed: mobility status, support surface type in use, number of linen layers present, heel elevation status, and HOB angle. Additional prevention practices, which were based on the National Database of Nursing Quality Indicators (NDNQI), were also evaluated from the IPUP database; this included skin assessment, repositioning, pressure redistribution (support surface), moisture management, and nutrition support (Table 1). The prevention practices outlined in the NDNQI data collection were evaluated only for patients with a Braden score of 18 or less within this same 24-hour assessment timeframe. Compliance to prevention practices was determined by the survey teams at the time of the data collection based on documented and observed implementation of each of these prevention practices.
TABLE 1. Anatomical Location and Stage of HAPIs.
| Location | Stage 1 (n = 2212)n (%) | Stage 2 (n = 3369)n (%) | Stage 3 (n = 491)n (%) | Stage 4 (n = 172)n (%) | Unstageable (n = 1373)n (%) | DTPI (n = 2878)n (%) | Indeterminable (n = 45)n (%) | Mucosal Membrane (n = 150) n (%) | All Stages (n = 10,690)n (%) |
|---|---|---|---|---|---|---|---|---|---|
| Sacrum/coccyx | 735 (33.2) | 1399 (41.5) | 235 (47.9) | 107 (62.2) | 440 (32.0) | 723 (25.1) | 12 (26.7) | 2 (1.3) | 3653 (34.2) |
| Heel | 461 (20.8) | 219 (6.5) | 23 (4.7) | 5 (2.9) | 202 (14.7) | 851 (29.6) | 8 (17.8) | 0 (0.0) | 1769 (16.5) |
| Buttocks | 315 (14.2) | 845 (25.1) | 88 (17.9) | 17 (9.9) | 129 (9.4) | 377 (13.1) | 4 (8.9) | 5 (3.3) | 1780 (16.7) |
| Trochanter | 22 (1.0) | 25 (0.7) | 7 (1.4) | 7 (4.1) | 26 (1.9) | 27 (0.9) | 1 (2.2) | 0 (0.0) | 115 (1.1) |
| Scapula | 10 (0.5) | 17 (0.5) | 2 (0.4) | 1 (0.6) | 6 (0.4) | 10 (0.3) | 0 (0.0) | 0 (0.0) | 46 (0.4) |
| Occiput | 2 (0.1) | 14 (0.4) | 9 (1.8) | 0 (0.0) | 33 (2.4) | 19 (0.7) | 0 (0.0) | 0 (0.0) | 77 (0.7) |
Abbreviations: DTPI, deep tissue pressure injury; HAPI, hospital-acquired pressure injury.
Specific trade names of support surfaces in use by the patient were recorded as part of the IPUP database collection. For purposes of this study, surfaces were categorized as foam, gel, gel and foam, self-adjusting technology (SAT), air, low-air loss (LAL), or air fluidized based on features listed in the manufacturer's website. Specific surfaces that had fewer than 100 survey patients placed on them were not included in the analysis; this decision led to exclusion of 4105 patients (<2% of sample).
Data Analysis
Descriptive statistics, which included frequency distributions, mean, and standard deviation, for study variables were analyzed using R Version 4.0.2 (R Foundation for Statistical Computing, Vienna, Austria). Differences in prevention practices among patients with no PIs, Stage 1 and Stage 2 PIs, or severe PIs (Stages 3, Stage 4, DTPI, and unstageable) were analyzed using the χ2 test.
RESULTS
The study sample comprised 296,014 patients hospitalized in 1801 acute care facilities in the United States that participated in 2018 (n = 914 facilities) and 2019 (n = 887 facilities) IPUP data collection. The cohort included 26,562 patients with PIs and 7631 with HAPIs. Pressure injury prevalence was 8.97% and HAPI prevalence 2.58%.5 The total number of PIs in the cohort was 45,672 and the number of HAPI was 10,690. Slightly less than half of patients (n = 146,231, 49.4%) were male and 50% were female (n = 148,007), and the sex of 0.6% (n = 1776) was not identified. The average age of the patients was 64.29 (SD 17.2) years; patients free from a PI had an average age of 63.74 (SD 17.2) years, whereas those with PIs had an average age of 69.91 (SD 15.45) years. The mean hospital length of stay (LOS) was 10.5 (SD 83.7) days and the median LOS was 3 days. Acute care units were grouped into 3 categories of care and included general medical-surgical, critical care, and step-down patients.
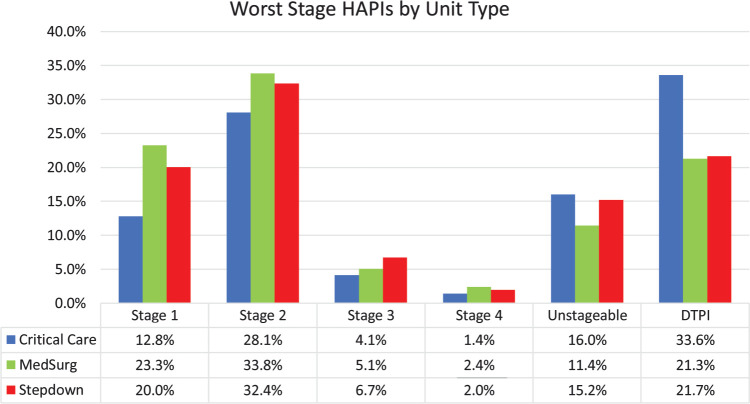
The worst stage HAPIs in the study varied by unit type. For purposes of this study, pressure injury was ranked from highest to lowest severity as Stage 4, unstageable, DTPI, Stage 3, Stage 2, and Stage 1. Critical care units had the greatest percentage of DTPIs (33.6%, n = 823), followed by Stage 2 (28.1%, n = 688). Medical-surgical units had the greatest percentage of Stage 2 (n = 1234, 33.8%), along with 21.3% (n = 777) of DTPIs and 23.3% (n = 849) of Stage 1 PIs. Step-down settings also had a similar breakdown with Stage 2 as the greatest percentage (32.4%, n = 260) and Stage 1 (20%, n = 161) and DTPI (21.7%, n = 174) (Figure 1). With regard to anatomic location, most HAPIs occurred on the sacrum, followed by the buttocks and heel. Deep tissue pressure injuries occurred most commonly on the heels (Table 1).
Figure 1.
HAPIs by setting and stage. HAPI indicates hospital-acquired pressure injury.
Immobility
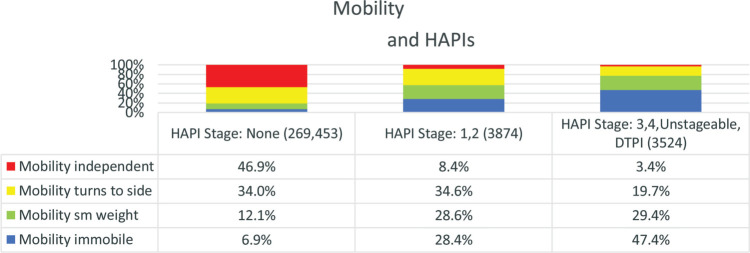
Immobility is the greatest risk factor for the development of PIs.6 For this project, mobility was evaluated in 4 categories based on the IPUP descriptors. Patients classified as “completely immobile” were able to make only small movements of arms or feet, but unable to reposition entire leg or move to modestly reposition pressure on body. Those classified as “capable of small weight shifts” could not turn to their side, but were able to reposition a leg or make other movements that reposition pressure on body. Patients classified as “turns to side” were able to turn to their side on their own, but required help to stand or get out of bed. Finally, patients classified as “independent” got in and out of bed and repositioned themselves while in bed without assistance. The analysis of mobility in all acute care settings resulted in findings consistent with immobility being the greatest risk factor for the development of PI. The greatest number of patients with immobility also had the most severe pressure injuries (Figure 2).
Figure 2.
Patients in the highest mobility category had the fewest HAPIs and the least severe HAPIs. For each mobility group there were significant differences between all 3 PI stage groups (P = .000). HAPI indicates hospital-acquired pressure injury; PI, pressure injury.
Preventive Interventions: Repositioning and Support Surfaces for at-Risk Patients
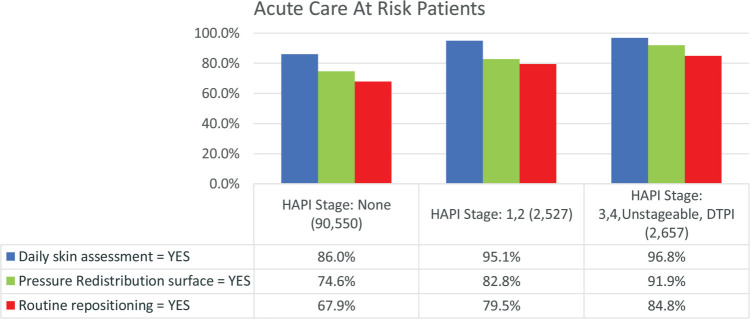
As noted earlier, use of preventive interventions was analyzed for patients with a Braden Scale score of 18 or less. Strategies to prevent pressure injuries that target decreased mobility included repositioning and use of support surfaces; data concerning the use of these preventive interventions were acquired from the NDNQI portion of the IPUP data collection. Routine repositioning was reported in 67.9% (n = 61,469) of patients with no HAPIs, and 79.5% (n = 2010) of all at-risk (Braden ≤18) patients with Stage 1 or 2 HAPIs (P = .000). Repositioning was also reported in 84.8% (n = 2254) for at-risk patients with severe PIs (P = .000, Figure 3). Pressure redistribution surfaces were implemented for 74.6% (n = 67,588) for at-risk patients with no HAPIs, 82.8% (n = 2093) for at-risk patients with Stage 1 or 2 HAPIs, and 91.9% (n = 2443) for at-risk patients with severe HAPIs (P = .000). Daily skin assessment was performed for 86% (n = 77,841) of at-risk patients with a Braden Scale score of 18 or less and with no HAPI present and in 96.8% (n = 2573) of those with a severe HAPI, and 95.1% (n = 2403) for those with Stage 1 or 2 HAPIs. Further analysis revealed that inpatients without HAPI 0.6% (n = 534) were not assessed as were 1.1 % (n = 30) of those with the severe HAPI (Table 2). Routine repositioning was documented as no in 9.1% to 11% of the groups (Table 3).
Figure 3.
Prevention practices implemented within the last 24 hours for patients with Braden Scores 18 or less. For each question there were significant differences between all 3 PI stage groups (P = .000). PI indicates pressure injury.
TABLE 2. Daily Skin Assessment Responses.
| HAPI Stage: None (n = 90,550) n (%) | HAPI Stage: 1 and 2 (n = 2,527) n (%) | HAPI Stage: 3, 4, Unstageable, and DTPI (n = 2,657) n (%) | |
|---|---|---|---|
| Yes | 77,841 (86.0) | 2,403 (95.1) | 2,573 (96.8) |
| No | 534 (0.6) | 26 (1.0%) | 30 (1.1) |
| Documented contraindication | 26 (0.03) | 3 (0.1%) | 2 (0.1) |
Abbreviations: DTPI, deep tissue pressure injury; HAPI, hospital-acquired pressure injury.
TABLE 3. Routine Repositioning Responses.
| HAPI Stage: None (n = 90,550) n (%) | HAPI Stage: 1 and 2 (n = 2,527) n (%) | HAPI Stage: 3, 4, Unstageable, and DTPI (n = 2,657) n (%) | |
|---|---|---|---|
| Yes | 61,469 (67.9) | 2,010 (79.5) | 2,254 (84.8) |
| No | 8,287 (9.2) | 279 (11.0) | 241 (9.1) |
| Documented contraindication | 276 (0.3) | 8 (0.3) | 10 (0.4) |
| Unnecessary for patient | 7,062 (7.8) | 87 (3.4) | 48 (1.8) |
| Patient refused | 620 (0.7) | 25 (1.0) | 32 (1.2) |
Abbreviations: DTPI, deep tissue pressure injury; HAPI, hospital-acquired pressure injury.
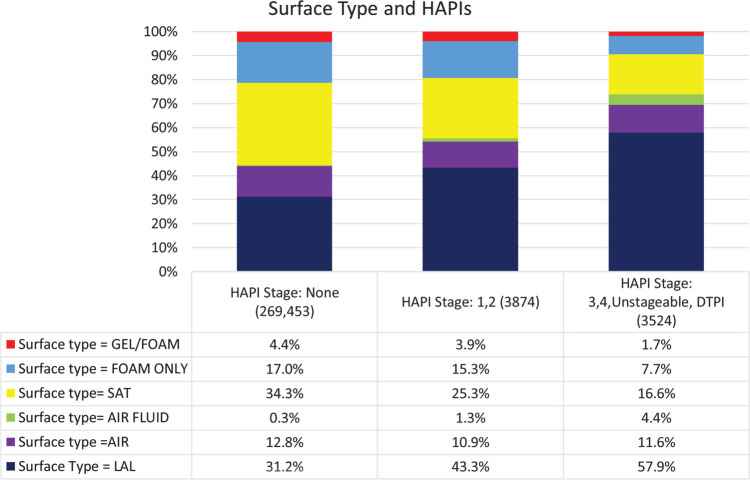
Support surfaces were grouped into several categories: foam only, gel, gel and foam combined, static air, SAT, LAL, and air fluidized. The greatest percentage of patients with Stage 1 and 2 HAPIs (43.3%, n = 1327) and severe HAPIs (57.9%, n = 1601) were place on LAL surfaces (Figure 4). There were significant differences across all 3 PI stage groups (P = .000). For patients, with no pressure injuries, SAT was the most frequently identified support surface, which is described as a pressure redistribution surface that can sense patient movements and automatically adjust pressure redistribution properties.
Figure 4.
Support surfaces implemented for patients. For each surface type there were significant differences between all 3 PI stage groups (P = 0.000). AF indicates air fluidized; Air, air-filled bladders; LAL, low-air-loss surface; PI, pressure injury; SAT, self-adjusting technology.
Preventive Interventions: Minimizing Linen Layers and Moisture Management
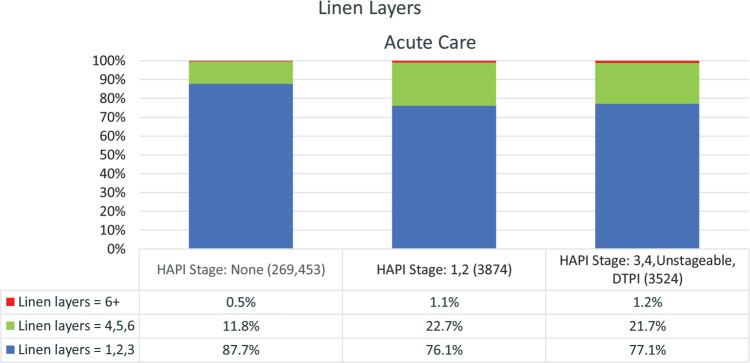
Linens are routinely used under patients, but they can impact the features on support surfaces such as the ability of the mattress to provide microclimate management by reducing air flow on a low-air-loss system, as well as the ability of the mattress to provide pressure redistribution. Current guidelines recommend limiting linen layers.1 In this sample, a very small percentage (≤1.2%) were using more than 6 layers of linens and the majority (≥76%) were using 3 or less layers (Figure 5).
Figure 5.
Linen layers in use at time of survey for all acute settings. For each set of linen layers, there were significant differences between all 3 PI stage groups (P = .000). PI indicates pressure injury.
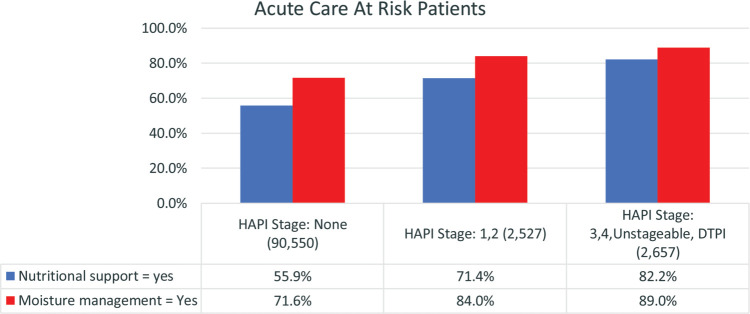
Moisture management strategies were implemented in 72% (n = 69,337) of all patients, including 84% (n = 2122) of patients with Stage 1 and 2 HAPIs and 89% (n = 2365) of those with the most severe HAPIs (Figure 6). Moisture management was reported as unnecessary for 7.8% (n = 7062) of patients with no HAPIs, 3.7% (n = 94) of patients with Stage 1 and 2 HAPIs, and only 2.6% (n = 70) of those with the most severe HAPIs (Table 4).
Figure 6.
Moisture management and nutritional support. For each question there were significant differences between all 3 PI stage groups (P = .000). PI indicates pressure injury.
TABLE 4. Moisture Management.
| HAPI Stage: None (n = 90,550) n (%) | HAPI Stage: 1 and 2 (n = 2,527) n (%) | HAPI Stage: 3, 4, Unstageable, and DTPI (n = 2,657) n (%) | |
|---|---|---|---|
| Yes | 64,850 (71.6) | 2,122 (84.0) | 2,365 (89.0) |
| No | 5,543 (6.1) | 188 (7.4) | 146 (5.5) |
| Documented contraindication | 59 (0.1) | 1 (0.04) | 0 (0.0) |
| Unnecessary for patient | 7,062 (7.8) | 94 (3.7) | 70 (2.6) |
| Patient refused | 150 (0.2) | 6 (0.2) | 4 (0.2) |
Abbreviations: DTPI, deep tissue pressure injury; HAPI, hospital-acquired pressure injury.
Preventive Interventions: Nutrition Support, Heel, and Head-of-Bed Elevation
Assessment for nutritional deficits is strongly recommended in current guidelines.1 A nutritional support consultation or plan was present for 82.7% (n = 2185) of patients with severe HAPIs and 71.4% (n = 1805) for patients with Stage 1 or 2 HAPIs (Figure 6). Nutritional support was not provided for 10.7% (n = 285) of those with the most severe HAPIs (Table 5).
TABLE 5. Nutritional Support.
| HAPI Stage: None (n = 90,550) n (%) | HAPI Stage: 1 and 2 (n = 2,527) n (%) | HAPI Stage: 3, 4, Unstageable, and DTPI (n = 2,657) n (%) | |
|---|---|---|---|
| Yes | 50,578 (55.9) | 1,805 (71.4) | 2,185 (82.7) |
| No | 16,910 (18.7) | 436 (17.3) | 285 (10.7) |
| Documented contraindication | 1,552 (1.7) | 35 (1.4) | 34 (1.3) |
| Unnecessary for patient | 8,133 (9.0) | 110 (4.4) | 70 (2.6) |
| Patient refused | 201 (0.2) | 8 (0.3) | 11 (0.4) |
Abbreviations: DTPI, deep tissue pressure injury; HAPI, hospital-acquired pressure injury.
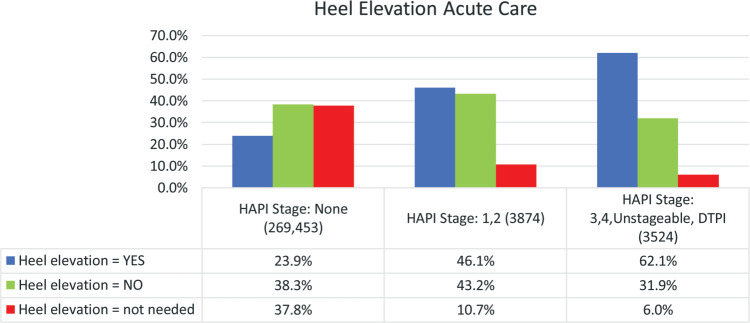
Heel elevation was implemented for 23.9% (n = 41,715) of patients with no HAPIs and deemed unnecessary for 37.8% (n = 65,854). It was implemented for 46.1% (n = 1375) of patients with Stage 1 or 2 HAPIs and deemed unnecessary for 10.7% (n = 320). In patients with severe HAPIs, heel elevation was implemented for 62.1% (n = 1604) and not needed in 6.0% (n = 156, Figure 7).
Figure 7.
Utilization of heel elevation in acute care patients. For each heel elevation response, there were significant differences between all 3 PI stage groups (P = .000).
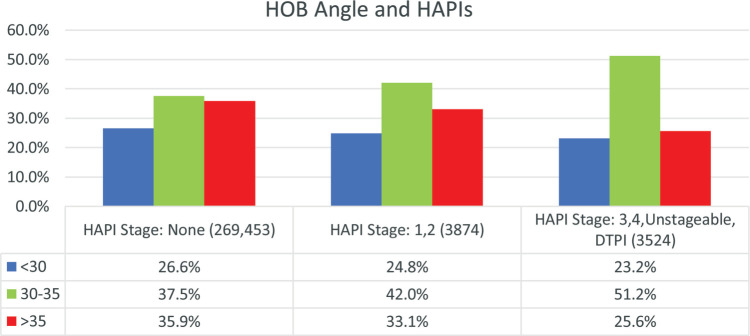
Current International PI Prevention and Treatment Guidelines recommend head elevation of 30° or less for patients at risk for PIs unless medically contraindicated.1 In this study the greatest percentage of patients were at 30° to 35° HOB elevation for all groups, with greater than 35° the second greatest (Figure 8).
Figure 8.
Head-of-bed (HOB) angles for all patients with HAPIs in acute care settings. For each HOB angle subgroup, there were significant differences between all 3 PI stage groups (P = .02 for less than 30 and .00 for others). HAPI indicates hospital-acquired pressure injury; PI, pressure injury.
DISCUSSION
This study evaluated the implementation of best practice prevention strategies in a large sample of patients cared for in 1801 acute care settings in 2018-2019. Compliance rates for pressure injury prevention strategies were very good for skin assessment and pressure redistribution. Findings indicated room for improvement in preventive interventions repositioning, heel elevation, moisture management, and nutritional support.
Prevention strategies have been developed over the years to reduce HAPI occurrences by mitigating modifiable risk factors. To better understand the number of HAPIs in acute care settings since 2013, a brief history of PI preventive interventions is helpful. Since 2009, the National Pressure Injury Advisory Panel (NPIAP), European Pressure Ulcer Advisory Panel (EPUAP), and Pan Pacific Pressure Injury Alliance (PPPIA) have develop and updated guidelines for the prevention and treatment of pressure injuries based on research evidence and expert opinion.1 The last 20 years of PI research has seen a broadening of research in various care settings beyond the initial focus on long-term care facilities.
Immobility is a necessary condition for pressure injury development according to the 2019 International Guidelines.1 A prospective study of over 500 hospitalized patients found that immobility was a strong risk factor for the development of a PI.6 Previous analysis of data from the 2011 to 2016 IPUP surveys also revealed that patients unable to self-ambulate were more likely to have severe PIs.7 Gray and Giuliano8 also reported that immobility was a statistically significant factor related to prevalence of full-thickness pressure injury in the sacral area. Our study found that individuals with the least mobility had the most severe pressure injuries; this finding is similar to results reported by Lahmann and Kottner.9 Our analysis indicated that most Stage 1 and 2 HAPIs occurred in patients classified as “turns to side” (requiring help to stand or get out of bed). Also, of those patients in the mobility category of “capable of only small weight shifts,” nearly 30% acquired Stage 1 or 2 PI. The high percentage of Stage 1 and 2 PIs in both of these mobility categories suggest that these injuries may be due to shear forces at the upper layers of the tissue created by patient movements, which correspond with the findings of prior research.9
Daily skin assessments were performed for 86% of the patients with a Braden Scale score of 18 or less and with no HAPI present, versus 95.1% to 96.8% of those with Stage 1 or 2 or severe HAPIs. Only a small percentage (∼1.1 %) were not assessed. These numbers are encouraging because they are approaching the goal of 100% daily skin assessment identified in the NPIAP/EPUAP/PPPIA guidelines.1
Although support surfaces for pressure redistribution were used in greater than 74.6% of all patients and in over 90% of patients with severe HAPIs, compliance to routine repositioning was lower at 67% to 84%. Routine repositioning was not completed for 9% to 11% of the groups although it was noted as unnecessary for only 1.8% of those with the most severe HAPIs. Routine repositioning has been reported to reduce the odds for HAPI by 14%.10 Best practice recommendations prescribe regular repositioning of all patients regardless of support surface use.1 Skin assessment and repositioning are basic PI prevention strategies, and study findings indicate for improvement in use of this preventive intervention. The reported high use of support surfaces and the moderate compliance to repositioning raises the potential concern that use of support surfaces might be usurping repositioning in some facilities.
There is no universal recommendation for the selection of a support surface for patients who are at risk for or who have PIs. To guide clinicians when choosing an appropriate support surface, the Wound, Ostomy and Continence Nurses Society developed a support surface algorithm to be used in clinical practice.11 In this study, LAL support surfaces were the most commonly selected for all stages of HAPIs and the second most commonly used in patients without HAPIs. We assert that patients should be assessed and a surface selected based on how the surface meets overall care goals including treatment of prevention of PI. Support surface selection is also recommended as an early intervention for patients who move from a low stage (Stage 1) to higher stage (more severe) PI and routine reevaluation is indicated when treating existing PIs.1 Guidelines also recommend that support surfaces use be limited to their therapeutic life span.
We observed use of LAL surfaces combined with use of 4 to 6 linen layers in greater than 20% of the patients with HAPIs. This finding supports the need for further education regarding the negative impact of linen layers on the pressure redistributing and moisture management properties of LAL surfaces. Research has shown that increased linen layers significantly increase the mean peak sacral pressure when compared to a single layer on both the low-air-loss surface and the foam surface.12 Additionally, this increase in peak sacral pressure was greater on LAL versus foam support surfaces.12
Moisture management was used in more than 71% of all patients and in 89% of those with severe HAPIs. Moisture management was not completed for 7.4% of patients with reported Stage 1 or 2 HAPIs. The presence of moisture changes both the tolerance of tissue to loading and the loads present.1 The impact of moisture on the development of severe pressure injuries at the sacrum was reported in work by Gray and Guiliano.8,13 We plan to examine the effects of incontinence on preventive interventions in a future article.
Nutrition support was used in 55% to 82% of patients; it was deemed contraindicated in less than 2% of all groups. It was noted as unnecessary for 9% of the no HAPI group, but only 2.6% of the severe HAPI group. Adequate nutrition plays a role in the treatment and prevention of pressure injuries.1
Heels were among the most common location for HAPIs and accounted for the greatest percentage of DTPIs in the study; this finding is similar to outcomes of prior research.14 Heel elevation was reported for over 60% of the patients with the most severe HAPIs, but 31.9% of this group did not receive heel elevation, even though only 6.0% were reported as not requiring elevation. The use of heel elevation was similar in patients with Stage 1 and 2 HAPIs (46.1% vs 43.2%, respectively). It is not known why 31.9% of individuals with severe HAPIs did not receive heel elevation. In addition, we were unable to determine the proportion of patients with severe heel HAPIs in this subgroup.
A majority of patients had a higher HOB angle than the recommended 30° for pressure injury prevention.1 Several factors may have influenced this finding; for example, acute and critically ill patients have competing care priorities that may influence HOB elevation. Ventilator-associated pneumonia (VAP) is a major complication in critically ill adults and is associated with increased likelihood of death or extended stay.15 Burk and Grap16 outlined the conflicting recommendations for HOB relative to VAP and PI prevention. Grap and coworkers17 found that HOB elevation affects tissue interface pressure, but that relationship was also impacted by knee angle, body mass index, and patient mobility. Schallom and colleagues18 also reported no development of PIs in patients with greater than 30° HOB.
In 2010, the NPIAP held a consensus conference where 100% of respondents agreed that not all pressure injuries are avoidable.19 In addition, 91% reached consensus that certain situations or conditions limit the use of PI preventive interventions. In 2014, the NPIAP hosted a consensus conference to explore the issue of PI unavoidability within an organ system framework.20 Respondents unanimously reached consensus that in hemodynamically unstable or critically ill/critically injured individuals, when management of life-threatening conditions must take precedence over skin preservation interventions, development of an unavoidable PI can occur. When sustained head-of-bed of greater than 30° elevation is medically necessary, an unavoidable PI also can occur (>85% consensus).
We searched the literature and found no studies comparing the level of compliance to prevention strategies identified in our study. Given the plateau in HAPI reduction observed over the last 5 years, additional research is needed to determine adherence to best practices and strategies to promote adherence in this area of preventive care.5 Such research may also provide further insight into the persistent HAPI rates in acute care hospitals in the United States.
Limitations
This observational, cross-sectional cohort study has several limitations. Facilities self-report their patient data and errors in data reporting and response bias can occur. Fortunately, most facilities use their wound care team, which is likely to increase the accuracy of data collected. The prevention strategies reported in the current study document whether the strategy was in place at the time of the patient's assessment during the survey. The survey has a 24-hour timeframe for data collection and it is not known if HAPI development may have been the result of inconsistent prevention practices prior to that data collection period.
It has been well established that the implementation of evidence-based PI prevention bundles does result in decreases in PI rates.21 From a healthcare economics perspective, PI prevention has been found to be less costly than the treatment of PIs internationally, however less in known about the economics of PI prevention in the United States.22,23 Overall annual costs associated with hospital acquired PIs have been reported at almost US $27 billion, not to mention the human suffering endured when a PI develops.24 Future studies focusing on a cost analysis of PI prevention in US hospitals would provide valuable information to both healthcare providers and administrators regarding the efficacy of these interventions when compared to hospital-acquired PI rates.
CONCLUSION
In 2019, the Agency for Healthcare Research and Quality reported that HAPI rates are rising, while all other hospital-acquired conditions are decreasing.25 It is possible that despite the implementation of best practice prevention strategies some PI are unavoidable. Nevertheless, there is room for improvement in the implementation of some of the most basic PI prevention strategies including repositioning, heel elevation, layers of bed linens, nutritional support, and moisture management. Future research should seek to build on the current study by focusing on prospective and longitudinal studies evaluating risk factors and prevention practices in acute care settings to better understand the occurrence of HAPIs in this population as well as provide insight into the potential occurrence of unavoidable PIs.
Footnotes
The authors have no conflicts of interest to declare.
Contributor Information
Laura E. Edsberg, Email: Ledsberg@daemen.edu.
Jill Cox, Email: jillcox@sn.rutgers.edu.
Kimberly Koloms, Email: Kimberly.koloms@hillrom.com.
Catherine A. VanGilder-Freese, Email: Cvangilder7@gmail.com.
REFERENCES
- 1.National Pressure Ulcer Advisory Panel EPUAP and PPPIA. Prevention and Treatment of Pressure Ulcers: Clinical Practice Guideline. EPUAP/NPIAP/PPPIA; 2019. [Google Scholar]
- 2.Cox J. Pressure injury risk factors in adult critical care patients: a review of the literature. Ostomy Wound Manag. 2017;63(11):30–43. [PubMed] [Google Scholar]
- 3.Alderden J, Rondinelli J, Pepper G, Cummins M, Whitney JA. Risk factors for pressure injuries among critical care patients: a systematic review. Int J Nurs Stud. 2017;71:97–114. doi:10.1016/j.ijnurstu.2017.03.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Lima Serrano M, González Méndez MI, Carrasco Cebollero FM, Lima Rodríguez JS. Risk factors for pressure ulcer development in intensive care units: a systematic review. Med Intensiva (English Ed). 2017;41(6):339–346. doi:10.1016/j.medine.2017.04.006. [DOI] [PubMed] [Google Scholar]
- 5.Vangilder CA, Cox J, Edsberg LE, Koloms K. Pressure injury prevalence in acute care hospitals with unit-specific analysis: results from the International Pressure Ulcer Prevalence (IPUP) Survey database. J Wound Ostomy Continence Nurs. 2021;48(12):492–503. doi:10.1097/WON.0000000000000817. [DOI] [PubMed] [Google Scholar]
- 6.Lindgren M, Unosson M, Fredrikson M, Ek AC. Immobility—a major risk factor for development of pressure ulcers among adult hospitalized patients: a prospective study. Scand J Caring Sci. 2004;18(1):57–64. doi:10.1046/j.0283-9318.2003.00250.x. [DOI] [PubMed] [Google Scholar]
- 7.Kayser SA, VanGilder CA, Lachenbruch C. Predictors of superficial and severe hospital-acquired pressure injuries: a cross-sectional study using the International Pressure Ulcer Prevalence™ Survey. Int J Nurs Stud. 2019;89:46–52. doi:10.1016/j.ijnurstu.2018.09.003. [DOI] [PubMed] [Google Scholar]
- 8.Gray M, Giuliano KK. Incontinence-associated dermatitis, characteristics and relationship to pressure injury. J Wound Ostomy Continence Nurs. 2018;45(1):63–67. doi:10.1097/WON.0000000000000390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lahmann NA, Kottner J. Relation between pressure, friction and pressure ulcer categories: a secondary data analysis of hospital patients using CHAID methods. Int J Nurs Stud. 2011;48(12):1487–1494. doi:10.1016/j.ijnurstu.2011.07.004. [DOI] [PubMed] [Google Scholar]
- 10.Berquist-beringer S. Pressure ulcers and prevention among acute care hospitals in the United States. Jt commision J Qual Patient Saf. 2013;39(13):404–414. [DOI] [PubMed] [Google Scholar]
- 11.Wound, Ostomy, Continence Nurses Society™ (WOCN). An Evidence- and Consensus- Based Support Surface Algorithm. https://algorithm.wocn.org/#home.
- 12.Williamson R, Lachenbruch C, Vangilder C. The effect of multiple layers of linens on surface interface pressure: results of a laboratory study. Ostomy Wound Manag. 2013;59(6):38–47. [PubMed] [Google Scholar]
- 13.Lachenbruch C, Ribble D, Emmons K, VanGilder C. Pressure ulcer risk in the incontinent patient analysis of incontinence and hospital-acquired pressure ulcers from the International Pressure Ulcer Prevalence™ Survey. J Wound Ostomy Continence Nurs. 2016;43(3):235–241. doi:10.1097/WON.0000000000000225. [DOI] [PubMed] [Google Scholar]
- 14.Vanderwee K, Clark M, Dealey C, Gunningberg L, Defloor T. Pressure ulcer prevalence in Europe: a pilot study. J Eval Clin Pract. 2007;13(2):227–235. doi:10.1111/j.1365-2753.2006.00684.x. [DOI] [PubMed] [Google Scholar]
- 15.Safdar N, Dezfulian C, Collard HR, Saint S. Clinical and economic consequences of ventilator-associated pneumonia: a systematic review. Crit Care Med. 2005;33(10):2184–2193. doi:10.1097/01.CCM.0000181731.53912.D9. [DOI] [PubMed] [Google Scholar]
- 16.Burk RS, Grap MJ. Backrest position in prevention of pressure ulcers and ventilator-associated pneumonia: conflicting recommendations. Hear Lung. 2012;41(6):536–545. doi:10.1016/j.hrtlng.2012.05.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Grap MJ, Munro CL, Wetzel PA, et al. Backrest elevation and tissue interface pressure by anatomical location during mechanical ventilation. Am J Crit Care. 2016;25(3):e56–e63. doi:10.4037/ajcc2016317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Schallom M, Dykeman B, Metheny N, Kirby J, Pierce J. Head-of-bed elevation and early outcomes of gastric reflux, aspiration and pressure ulcers: a feasibility study. Am J Crit Care. 2015;24(1):57–66. doi:10.4037/ajcc2015781. [DOI] [PubMed] [Google Scholar]
- 19.Black JM, Edsberg LE, Baharestani MM, et al. Pressure ulcers: avoidable or unavoidable? Results of the National Pressure Ulcer Advisory Panel Consensus Conference. Ostomy Wound Manage. 2011;57(2):24–37. http://www.ncbi.nlm.nih.gov/pubmed/21350270. [PubMed] [Google Scholar]
- 20.Edsberg LE, Langemo D, Baharestani MM, Posthauer ME, Goldberg M. Unavoidable pressure injury. J Wound Ostomy Continence Nurs. 2014;41(4):313–334. doi:10.1097/WON.0000000000000050. [DOI] [PubMed] [Google Scholar]
- 21.Kelleher AD, Moorer A, Makic MF. Peer-to-peer nursing rounds and hospital-acquired pressure ulcer prevalence in a surgical intensive care unit: a quality improvement project. J Wound Ostomy Continence Nurs. 2012;39(2):152–157. doi:10.1097/WON.0b013e3182435409. [DOI] [PubMed] [Google Scholar]
- 22.McEvoy N, Avsar P, Patton D, Curley G, Kearney CJ, Moore Z. The economic impact of pressure ulcers among patients in intensive care units. A systematic review. J Tissue Viability. 2021;30(2):168–177. doi:10.1016/j.jtv.2020.12.004. [DOI] [PubMed] [Google Scholar]
- 23.Demarré L, Van Lancker A, Van Hecke A, et al. The cost of prevention and treatment of pressure ulcers: a systematic review. Int J Nurs Stud. 2015;52(11):1754–1774. doi:10.1016/j.ijnurstu.2015.06.006. [DOI] [PubMed] [Google Scholar]
- 24.Padula WV, Delarmente BA. The national cost of hospital-acquired pressure injuries in the United States. Int Wound J. 2019;16(3):634–640. doi:10.1111/iwj.13071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Agency for Healthcare Research and Quality. AHRQ National Scorecard on Hospital-Acquired Conditions. Final Results for 2014 Through 2017. Rockville, MD: Agency for Healthcare Research and Quality; 2020. [Google Scholar]