Abstract
Diffuse midline glioma, H3 K27M-mutant (H3 K27M-mt DMG), is a rare and highly aggressive tumor that is more common in children than in adults. Few studies have compared the differences between pediatric and adult patients with this rare tumor. We here report our retrospective study of 94 adult and 70 pediatric cases of diffuse midline glioma. Surgical tumor samples were analyzed by routine histopathology and immunohistochemistry for H3 K27M, IDH1 R132H, ATRX, p53, OLIG2, glial fibrillary acidic protein, and Ki-67; Sanger sequencing for hot mutation spots in genes including H3F3A, HIST1H3B, IDH1, IDH2, TERT, and BRAF; and methylation-specific polymerase chain reaction for O 6 -methylguanine DNA methyltransferase promoter methylation. The most frequent anatomic locations in adult and pediatric patients were the thalamus and brainstem, respectively. Molecular profiling revealed higher frequencies of ATRX loss and H3.3 mutation in adult than in pediatric H3 K27M-mt DMGs. TERT promoter mutations and O 6 -methylguanine DNA methyltransferase promoter methylation were not detected in pediatric patients but were present in a few adult patients. During the follow-up period, 93/122 patients (70.1%) died from the disease, with a median survival time of 10.5 months (range: 1 to 104 mo). Kaplan-Meier analyses demonstrated that the prognosis was better for adult patients than the pediatric cohort (P=0.0003). Multivariate analyses indicated that patient age, primary tumor size, status of ATRX expression, and Ki-67 index were independent prognosticators. The present study showed that there were differences between adult and pediatric H3 K27M-mt DMGs in terms of the anatomic location of tumor, molecular changes, and prognosis.
Key Words: diffuse midline gliomas, H3 K27M-mutant, adult, pediatric, prognosis
Diffuse midline glioma, H3 K27M-mutant (H3 K27M-mt DMG), is an entity introduced in the 2016 World Health Organization (WHO) Classification of Tumors of the Central Nervous System (CNS),1 which corresponds to WHO grade 4 with poor prognosis. These tumors harbor somatic mutations in the H3F3A or HIST1H3B/C genes, resulting in lysine to methionine substitution at amino acid residue 27 (K27M) in the histone H3 variants H3.3 or H3.1.1 The revised criteria outlined in the Consortium to Inform Molecular and Practical Approaches to CNS Tumor Taxonomy-Not Official WHO update 2 (cIMPACT-NOW2) require that H3 K27M-mt DMGs must have a diffuse growth pattern and are restricted to midline structures, such as the thalamus, brainstem, cerebellum, and spinal cord.2 In a summary of the 2021 WHO Classification of Tumors of the CNS, the diffuse midline glioma is renamed to “diffuse midline glioma, H3 K27-altered,” to incorporate recent findings that other molecular changes (such as EZHIP overexpression) also result in H3 K27 alterations. This entity mainly presents as a pediatric diffuse high-grade glioma, primarily seen in children and less frequently in adults.3
H3 K27M-mt DMGs are histopathologically heterogenous and may show bland to highly malignant morphologies.4 Large cohort studies of H3 K27M-mt DMGs were limited and were mainly focused on pediatric patients.5,6 Published data on adult H3 K27M-mt DMGs and comparisons of characteristics in adult and pediatric patients in terms of clinical, pathologic, and molecular features are scarce.
The present study is a large cohort study of 164 patients with H3 K27M-mt DMG, including 94 adult and 70 pediatric patients, in one of the largest comprehensive research medical centers in China. We characterized the clinical, histopathologic, and molecular features and prognosis of the H3 K27M-mt DMGs, with an emphasis on comparison between adult and pediatric patients. These data provide further information on this tumor entity and highlight the differences of adult H3 K27M-mt DMGs from that in children, particularly in prognosis.
MATERIALS AND METHODS
Patient Cohort
We retrospectively reviewed data on 164 cases of molecularly confirmed H3 K27M-mutant diffuse midline gliomas diagnosed and treated at West China Hospital, Sichuan University, Chengdu, China, from 2012 to 2020. The inclusion criteria were as follows: (1) radiologic and pathologic diagnosis of diffuse glioma, (2) tumor with midline location, and (3) H3 K27M mutation confirmed by sequence analysis. The clinical, radiologic, pathologic, and molecular characteristics of the patients were reviewed. Raw data can be found in Table S1 (Supplemental Digital Content 1, http://links.lww.com/PAS/B333). A total of 156 patients (95.1%) underwent surgical resection, and the remaining 8 patients (4.9%) underwent stereotactic biopsy. The histopathologic findings were reviewed by 2 neuropathologists according to the 2016 WHO Classification of CNS tumors and cIMPACT-NOW2. This study was approved by the institutional review board. Informed consent for translational research was obtained from each patient or the patient’s legal guardian.
Immunohistochemistry
The following primary antibodies were used: isocitrate dehydrogenase (IDH1) R132H (mouse monoclonal, clone H09; Dianova, Germany; 1:100), p53 (mouse monoclonal, clone DO-7; DakoCytomation, Glostrup, Denmark; 1:200), Ki-67 (mouse monoclonal, clone MIB-1; DakoCytomation; 1:100), glial fibrillary acidic protein (GFAP) (rabbit monoclonal, clone EP13; ZSGB-BIO, Beijing, China; 1:200), OLIG2 (rabbit monoclonal, clone EP112; ZSGB-BIO; 1:200), alpha-thalassemia/mental retardation syndrome X-linked protein (ATRX) (rabbit polyclonal, ZA-0016; ZSGB-BIO; ready-to-use) and H3 K27M (rabbit monoclonal, clone RM192; ZSGB-BIO; ready-to-use). All immunohistochemical staining was performed using the Roche BenchMark ULTRA automated staining system (Roche, Basel, Switzerland) according to the manufacturer’s protocols.
H3 K27M nuclear staining were interpreted as diffusely strong positivity (strong nuclear staining in almost all tumor cells), mixed strong and weak positivity (mixed strong or weak nuclear staining in almost all tumor cells), and “mosaic positive pattern” (only a minority of tumor cells being positive). For GFAP and OLIG2, >50% of strong positive tumor cells were interpreted as diffusely positive, and <50% were considered as partly positive. IDH1 R132H positivity was cytoplasmic and nuclear. For p53, strong nuclear positive staining in >30% of tumor cell nuclei was considered as p53 positive. For ATRX, loss of nuclear staining was assessed.
Molecular Studies
DNA was extracted from formalin-fixed, paraffin-embedded (FFPE) tumor tissues using a DNA FFPE Tissue Kit (Qiagen, Hilden, Germany). The polymerase chain reaction (PCR) primers for IDH1, IDH2, H3F3A, HIST1H3B, BRAF, and promoter regions of telomerase reverse transcriptase (TERT) were designed as previously described.7 Standard buffer conditions, 50 ng of DNA, and Taq DNA polymerase (TaKaRa, China) were used. The reaction mixture was subjected to an initial denaturation at 95°C for 10 minutes, followed by 35 cycles of amplification (denaturation at 95°C for 30 s, annealing at 56°C for 15 s, and extension at 72°C for 20 s). PCR products were sequenced in both the sense and antisense directions by Sangon Biotech (Chengdu, China).
Methylation-specific quantitative PCR was used to investigate the status of the O6-methylguanine DNA methyltransferase (MGMT) promoter methylation. Genomic DNA was extracted from FFPE sections. DNA Bisulfite Conversion Kit (SpaceGen, Xiamen, China) and MGMT methylation Kit (SpaceGen, Xiamen, China) were used according to the manufacturer’s instructions. Real-time quantitative PCR was performed at 95°C for 5 minutes, followed by 45 cycles at 95°C for 20 seconds, 56°C for 30 seconds, and 72°C for 20 seconds. The MGMT methylation status was considered positive when C t value was <28.
Statistical Analysis
Between-group differences were analyzed by using the χ2 test and Fisher exact test. Kaplan-Meier analyses were performed for survival data using the log-rank test. Multivariate survival analysis was performed with the Cox proportional hazard model. Statistical analysis was performed using SPSS 26.0 and GraphPad Prism 7. P-value <0.05 was considered statistically significant.
RESULTS
Clinical Characteristics
One hundred sixty-four patients with molecularly confirmed H3 K27M-mt DMGs were collected, including 94 (57.3%) adult (age above 18 y) and 70 (42.7%) pediatric (age 18 y or younger) patients.
The overall median age of the patients was 24.0 years (range: 3 to 71 y) and was 33.0 years (range: 19 to 71 y) in the adult group, and 9.0 years (range: 3 to 18 y) in the pediatric group. There were 88 males and 76 females (male-to-female ratio, 1.2:1). The mean maximum diameter of the tumor was 3.9 cm (1.4 to 8.0 cm). Tumors were anatomically located in the brainstem (n=75), the thalamus (n=46), the ventricles (n=16), the spinal cord (n=12), basal ganglia (n=7), pineal gland (n=4), cerebellum (n=2), and sellar region (n=2) (Figs. 1A, D). Notably, the most frequent anatomic location was the brainstem in children (44/70, 62.9%), whereas in adults, it was the thalamus (33/94, 35.1%). The distribution ratio of the location was significantly different (P<0.05) between the 2 groups (Figs. 1B, C, E, F; Table 1).
FIGURE 1.
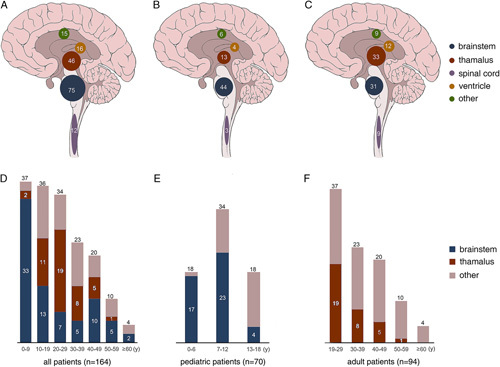
Anatomic and age distribution of H3 K27M-mt DMGs in all the current cohort (A, D), and in the pediatric (B, E), and adult (C, F) groups, respectively.
TABLE 1.
Comparison of Clinical and Molecular Characteristics Between Pediatric and Adult H3 K27M-mt DMGs
| n (%) | |||
|---|---|---|---|
| Pediatric | Adult | P * | |
| No. cases | 70 (42.7) | 94 (57.3) | |
| Sex | 0.621 | ||
| Male | 36 (51.4) | 52 (55.3) | |
| Female | 34 (48.6) | 42 (44.7) | |
| Anatomic location | |||
| Brainstem | 44 (62.9) | 31 (33.0) | <0.001 |
| Thalamus | 13 (18.6) | 33 (35.1) | 0.020 |
| Spinal cord | 3 (4.3) | 9 (9.6) | 0.239 |
| Ventricle | 4 (5.7) | 12 (12.8) | 0.184 |
| Others | 6 (8.6) | 9 (9.6) | 0.826 |
| Tumor diameter (cm) | |||
| ≤3 | 8 (21.1) | 17 (31.5) | 0.268 |
| >3 | 30 (78.9) | 37 (68.5) | |
| Dissemination | |||
| Yes | 4 (6.3) | 13 (17.1) | 0.121 |
| No | 59 (93.7) | 63 (82.9) | |
| Conventional histologic grade | |||
| Grade 2 | 13 (18.6) | 20 (21.3) | 0.669 |
| Grade 3 | 23 (32.9) | 25 (26.6) | 0.383 |
| Grade 4 | 34 (48.6) | 49 (52.1) | 0.652 |
| Molecular characteristics | |||
| ATRX expression | |||
| Lost | 10 (16.1) | 25 (34.2) | 0.017 |
| Intact | 52 (83.9) | 48 (65.8) | |
| p53 | |||
| Positive | 41 (74.5) | 53 (68.8) | 0.475 |
| Negative | 14 (25.5) | 24 (31.2) | |
| Ki-67 (%) | |||
| ≤5 | 6 (8.8) | 12 (13.3) | 0.377 |
| >5 | 62 (91.2) | 78 (86.7) | |
| H3 mutation | |||
| H3.3 K27M | 61 (87.1) | 91 (96.8) | 0.031 |
| H3.1 K27M | 9 (12.9) | 3 (3.2) | |
| TERT promoter | |||
| Mutated | 0 (0.0) | 4 (7.5) | 0.304 |
| WT | 24 (100.0) | 49 (92.5) | |
| MGMT promoter | |||
| Methylated | 0 (0.0) | 6 (12.8) | 0.175 |
| Unmethylated | 18 (100.0) | 41 (87.2) | |
| OS (mo) | 5.0 | 16.0 | <0.001 |
Bold P values <0.05 were considered statistically significant.
P-values represent comparison between pediatric and adult patients.
Magnetic resonance imaging showed leptomeningeal or subependymal dissemination on preoperative images in 8 patients, and dissemination developed with tumor recurrence on follow-up after surgery in another 9 patients. Among these patients with leptomeningeal/subependymal spread, 4 were children (6.3% of the pediatric group), and 13 were adults (17.1% of the adult group) (P=0.121) (Fig. 3, Table 1).
FIGURE 3.
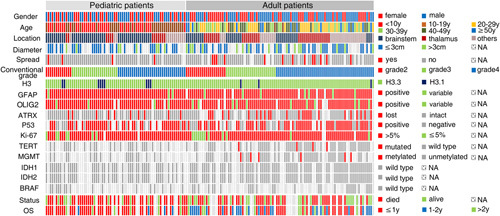
Schematic summary of clinical and molecular characteristics of 164 H3 K27M-mt DMGs.
The most common symptoms were headache and dizziness (94/164, 57.3%), dysesthesia or dyskinesia (66/164, 40.2%), vomiting (34/164, 20.7%), and visual abnormalities (27/164, 16.5%). Other rare symptoms included tinnitus and a sudden disturbance of consciousness. The majority of patients (156/164, 95.1%) underwent surgical resection, and the remaining 8 patients (4.9%) had a stereotactic biopsy.
Pathologic Characteristics of H3 K27M-mt DMGs in Adults and Children
Although H3 K27M-mt DMGs are all assigned grade 4 in the 2016 WHO Classification, we also evaluated these tumors by conventional histologic grades 2, 3, and 4 based on conventional morphologic criteria (as described in the 2007 WHO blue book8). There were 83 cases (50.6%) corresponding to conventional histologic grade 4, 48 cases (29.3%) to grade 3, and 33 cases (20.1%) to grade 2, with 79.9% of cases showing high-grade morphology (Figs. 2A–D). In the adult H3 K27M-mt DMGs, conventional histologic grades 4, 3, and 2 consisted of 49 (52.1%), 25 (26.6%), and 20 (21.3%) cases, respectively. In the pediatric group, 34 (48.6%), 23 (32.9%), and 13 (18.6%) cases showed morphology consistent with conventional histologic grades 4, 3, and 2, respectively. No significant differences were observed in morphology spectrum or distribution of “conventional histologic grades” of H3 K27M-mt DMGs between the adult and pediatric patients.
FIGURE 2.
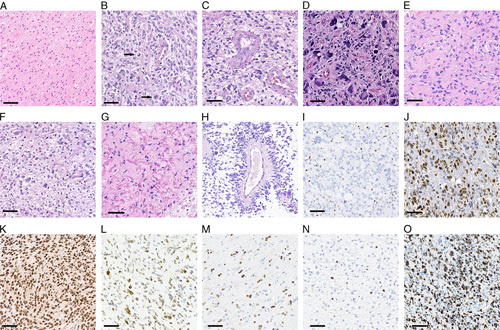
Histomorphology and immunohistochemistry in H3 K27M-mt DMGs. A, Diffuse infiltrating glioma, conventional histologic grade 2. B, Diffuse infiltrating glioma, conventional histologic grade 3 (arrow showing atypical mitosis). C and D, Conventional histologic grade 4 glioma, with microvascular proliferation and tumor giant cells. E, Epithelioid astrocytes with abundant eosinophilic cytoplasm. F, Oligodendroglial-like morphology. G, Rosenthal fibers. H, Pseudorosettes. I, Loss of ATRX expression in tumor cells (internal control being positive). J, Strong nuclear positivity of p53. K, Diffusely strong nuclear positivity of H3 K27M. L, Mixed strong and weak positivity of H3 K27M. M, H3 K27M staining in a case consisted with “mosaic pattern.” N, Low Ki-67 index (<5%). O, High Ki-67 index (>70%). Scale bar=50 μm.
The majority of the cases displayed astrocytic morphology, whereas 9 (5.5%) cases also contained oligodendroglial-like features. Microvascular proliferation (77/164, 47.0%), tumor cell necrosis (53/164, 32.3%), and multinucleated tumor cells (38/164, 23.2%) were also commonly observed. Pseudorosettes (13/164, 7.9%), calcification (9/164, 5.5%), neuropil-like islands (4/164, 2.4%), Rosenthal fibers (2/164, 1.2%), and primitive neuronal components (1/164, 0.6%) were present less frequently. Representative histologic morphologies were shown in Figures 2E–H. No significant differences in the histopathologic spectrum were observed between adult and pediatric patients.
H3 K27M immunohistochemistry was performed in 80/164 cases. Among them, 79/80 cases showed diffuse positive staining of H3 K27M, in which 60/80 cases showed diffusely strong positivity (Fig. 2K) and 19/80 cases showed mixed strong and weak positivity (Fig. 2L). Only 1 case showed a staining pattern consistent with the “mosaic pattern” as described by Lopez et al9 (Fig. 2M).
GFAP staining (n=157) showed diffuse positivity in 147 (93.6%), and the other 10 (6.4%) tumors showed positivity in various proportions. For OLIG2 immunohistochemistry (n=151), 136 (90.1%) tumors showed diffuse positivity, and the other 15 (9.9%) were variously positive. ATRX immunohistochemical staining (n=137) found a loss of ATRX expression in 35 cases (35/135, 25.9%) (Fig. 2I). The proportion of adult H3 K27M-mutant DMG cases with loss of ATRX was substantially greater in adult cases (25/73, 34.2%) compared with that in pediatric cases (10/62, 16.1%) (P=0.017). p53 was immunopositive in 94 (94/132, 71.2%) of the tested cases, with a positive rate of 66.2% in adults and 80.0% in children (Fig. 2J). The Ki-67 proliferation index ranged from 2% to 70% (Figs. 2N, O), and most tested cases (140/158, 88.6%) showed Ki-67 proliferation index >5%. IDH1 R132H was immunonegative in all tested cases (n=130).
Molecular Characteristics of H3 K27M-mt DMGs in Adults and Children
H3.3 or H3.1 K27M mutations were identified in 152/164 (92.7%) and 12/164 (7.3%) cases, respectively. The rate of H3.3 K27M mutation in adult cases was 96.8% (91/94), which was significantly higher than that in children (61/70, 87.1%) (P=0.031). In contrast, adult patients had a lower rate of H3.1 K27M mutation than children (3.2% vs. 12.9%, P=0.031). The median age of patients with H3.3 K27M or H3.1 K27M was 24.0 years (range: 4 to 71 y) and 6.0 years (range: 3 to 54 y), respectively. Anatomic locations of H3.3 K27M-mutant tumors include the brainstem (n=64), the thalamus (n=45), the ventricles (n=16), the spinal cord (n=12), and others (n=15). In contrast to H3.3 K27M-mt DMGs, the anatomic locations of H3.1 K27M-mt DMGs were restricted to the brainstem (n=11) and the thalamus (n=1) in the current cohort.
TERT promoter mutations were found in 4/75 (5.3%) tested cases, including 3 C250T and 1 C228T mutations. Methylation of the promoter region of the MGMT gene was detected in 6/70 (8.6%) tested cases. Importantly, all cases with either TERT promoter mutation or methylation of the MGMT promoter were adults. No mutation was found in IDH1 R132 (0/106), IDH2 R172 (0/106), and BRAF V600 (0/43) in cases tested (Fig. 3).
Survival Data and Prognostic Factors
Among the 164 patients, follow-up data were available for 122 patients (follow-up time: 1 to 104 mo). Among the followed up patients, 93/122 patients (76.2%) died from the disease during the follow-up period, with a median survival time of 10.5 months. The 1-year overall survival (OS) of the entire cohort was 41.9%, which was 54.5% in adult and 25.3% in pediatric patients. The 2-year OS of all patients was 26.7%, with 36.9% in adult and 14.2% in pediatric patients.
Significantly, the median survival time for adult patients was 16.0 months, whereas it was 5.0 months for the pediatric patients, indicating a better prognosis for the adult group (P=0.0003) (Fig. 4A). The prognosis of patients with tumor diameter >3 cm was significantly worse than that of patients with tumor diameter ≤3 cm (P=0.0468) (Fig. 4B). When the data were stratified by anatomic location, patients with tumors in the brainstem showed a worse prognosis than those with tumors in other locations (P=0.0089) (Fig. 4C).
FIGURE 4.
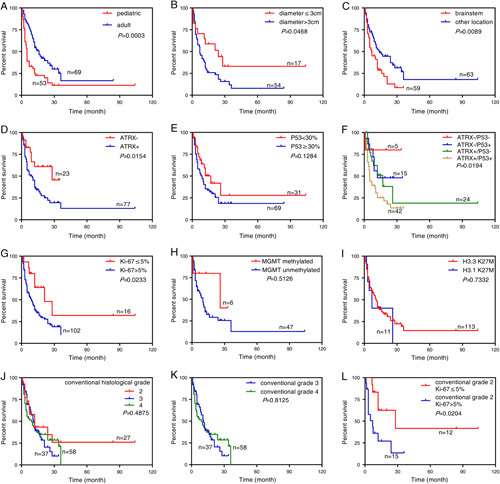
Kaplan-Meier survival curves and log-rank tests for H3 K27M and associated factors. Patients with age less than or equal to 18 (A), tumor diameter >3 cm (B), or tumor located in the brainstem (C) were associated with poorer OS. Loss of ATRX expression was associated with longer OS (D). Expression of p53 was not significantly associated with prognosis (E), but patients with intact ATRX and positive p53 staining conferred significant worse OS (F). Patients with Ki-67 index ≤5% had longer OS (G). There were no statistically significant differences among cases with MGMT methylated and unmethylated (H) or between H3.3 and H3.1 mutation (I). No differences were observed for conventional tumor grade 2/3/4 alone (J, K), however, tumors showing conventional grade 2 histology and low Ki-67 index (≤5%) did fare relatively better (L).
Compared with cases whose ATRX was intact, those with loss of ATRX expression had a significantly better prognosis (P=0.0154) (Fig. 4D). p53 expression status did not independently affect the prognosis of H3 K27M-mt DMGs. However, the combined expression status of ATRX and p53 conferred prognostic significance, with the ATRX (+)/p53 (+) group showing the worst prognosis than all other ATRX/p53 expression patterns (P=0.0194) (Figs. 4E, F). Besides, those with a Ki-67 index >5% had a worse prognosis than patients with a lower Ki-67 index (P=0.0233) (Fig. 4G). Differences in survival was not observed between H3.3 K27M and H3.1 K27M mutations (Fig. 4I), MGMT methylated and unmethylated (Fig. 4H), males and females, with and without dissemination, or other factors analyzed. No differences were observed for conventional histologic tumor grade 2/3/4 alone (Figs. 4J, K), however, tumors showing conventional grade 2 histology and low Ki-67 index (≤5%) did fare relatively better (Fig. 4L).
Multivariate survival analysis with the Cox proportional hazard model showed that patient age, anatomic location of tumor, loss of ATRX expression, and Ki-67 labeling index were significant prognosticators. Among them, age above 18 years (P=0.007), loss of ATRX expression (P=0.032), and Ki-67 index ≤5% (P=0.039) were independent favorable prognosticators for longer survival across the entire cohort of H3 K27M-mt DMGs, whereas tumor location could not be used as an independent prognostic factor (Table 2).
TABLE 2.
Univariate and Multivariate Cox Analysis of Clinicopathologic Features in H3 K27M-mt DMGs
| 95% Confidence Interval | 95% Confidence Interval | |||||||
|---|---|---|---|---|---|---|---|---|
| Univariate Hazard Ratio | Lower | Upper | P | Multivariate Hazard Ratio | Lower | Upper | P | |
| Age (>18 vs. ≤18 y) | 0.979 | 0.965 | 0.993 | 0.003 | 0.483 | 0.286 | 0.817 | 0.007 |
| Sex (male vs. female) | 1.008 | 0.654 | 1.553 | 0.971 | — | — | — | — |
| Anatomic location (brainstem vs. others) | 0.567 | 0.364 | 0.883 | 0.012 | 0.799 | 0.466 | 1.371 | 0.415 |
| Tumor diameter (>3 vs. ≤3 cm) | 1.954 | 0.977 | 3.909 | 0.058 | — | — | — | — |
| Dissemination (yes vs. no) | 0.755 | 0.347 | 1.639 | 0.477 | — | — | — | — |
| Conventional histologic grade (2/3/4) | 1.954 | 0.977 | 3.909 | 0.303 | — | — | — | — |
| H3.1 vs. H3.3 | 1.140 | 0.524 | 2.482 | 0.741 | — | — | — | — |
| ATRX expression (lost vs. intact) | 2.265 | 1.156 | 4.436 | 0.017 | 2.235 | 1.074 | 4.653 | 0.032 |
| p53 positivity (positive vs. negative) | 1.256 | 0.703 | 2.244 | 0.441 | — | — | — | — |
| Ki-67 (>5% vs. ≤5%) | 2.242 | 1.029 | 4.889 | 0.042 | 2.488 | 1.049 | 5.903 | 0.039 |
| MGMT (methylated vs. unmethylated) | 0.471 | 0.112 | 1.983 | 0.305 | ||||
Bold P values <0.05 were considered statistically significant.
DISCUSSION
Since “diffuse midline glioma, H3 K27M-mutant” was introduced as a distinct entity in the 2016 edition of the WHO classification of tumors of CNS, ∼400 cases have been reported in the literature, although large cohort studies are still limited.6,10–27 The clinicopathologic features of pediatric H3 K27M-mt DMGs have been relatively well characterized over the past few years. However, the clinicopathologic and molecular characteristics of H3 K27M-mt DMGs in adults have only been described in a couple of studies, and few compared characteristics between adult and pediatric cohorts.28 We characterized the clinical, histopathologic, and molecular features with prognostic analysis of H3 K27M-mt DMG with an emphasis on comparison between adult and pediatric patients.
Earlier studies indicated that H3 K27M-mt DMGs predominantly occurred in children and young adults.29 In recent cohort studies with no age limit on patient selection, the ratios of pediatric to adult patients range from 1:0.87 to 1:2.42.28,30–32 The present cohort of 164 consecutive patients diagnosed in West China Hospital over 8 years showed that 57.3% (94/164) of H3 K27M-mt DMGs occurred in adults; 34.8% and 8.5% of the patients were above 30 and 50 years of age, respectively. The oldest patient with H3 K27M-mt DMG reported in the literature was 81 years old.33 It appears that the incidence of H3 K27M-mt DMG in adults may be similar to (if not higher than) that in children. Hence, assessing H3 status in midline gliomas in adult patients should also be considered regardless of conventional histologic grades.
A large cohort study of H3 K27M-mt DMG, including 23 patients with H3.1 K27M and 56 patients with H3.3 K27M, showed that patients with H3.1 K27M had a median age of 5.1 years, which was approximately 2 years younger than that of patients with H3.3 K27M (median age: 7.4 y), and were associated with a better prognosis.5 In our data, patients with H3.1 K27M represented a small fraction and were much younger than patients with H3.3 K27M (6.0 vs. 24.0 y). We have not observed significant differences in prognosis between these 2 groups. These data imply that patients with H3.3 K27M mutations are more frequently seen, often of older age, and have more varied anatomic locations. Future prognostic analysis of more patients may better define the prognostic implications among patients with different mutations.
H3 K27M-mt DMG may show a wide range of histopathologic presentations, mostly of high grade.4 Our data also showed that most H3 K27M-mt DMGs were of high-grade histology, and the distribution of “histologic grade” was similar between adults and pediatric patients. Tumor cells in H3 K27M-mt DMGs may show various morphologies, including giant multinucleated cells, epithelial/rhabdoid, ependymoma-like, and pleomorphic xanthoastrocytoma-like cells.4,6 Perivascular lymphocytic infiltrates and neuropil-like islands can also be seen. Since H3 K27M-mt DMGs have a wide morphologic spectrum, morphologic changes could not reliably point to the correct final diagnosis or patient outcome.
A recent study indicated that H3 K27M could be specifically identified with high specificity and sensitivity using an H3 K27M antibody and immunohistochemistry, and most cases showed strong nuclear positivity in >80% of tumor cells.34 In rare cases, a mosaic pattern of H3 K27M staining can be seen9,19,35 in which only a minority of tumor cells were positive, whereas most tumor cells were negative. Two cases with H3 K27M mosaic expression were reported to carry subclonal H3F3A K27M mutations.9 Our study showed that most cases demonstrated diffusely strong (60/80) or mixed strong and weak positivity (19/80) in almost all tumor cells, and there was only 1 case which showed staining for H3 K27M similar to the “mosaic pattern” described by Lopez et al.9 The patient (case 42) was a 50-year-old male with a survival of 36 months. Sanger sequencing showed that the tumor harbored H3 K27M mutation in the H3F3A gene which confirmed the diagnosis of diffuse midline glioma, H3 K27M-mutant, WHO grade 4. We used whole-exome sequencing to assess the variant allelic frequency, which showed mutant H3 K27M variant allelic frequency to be 25% (Fig. S1, Supplemental Digital Content 2, http://links.lww.com/PAS/B334, Table S2, Supplemental Digital Content 3, http://links.lww.com/PAS/B335), possibly indicating a subclonal allelic frequency.
Loss of ATRX expression was more common in adult than pediatric patients in our series. It has been reported that more than half of IDH wild-type (WT)/H3-WT gliomas have TERT promoter mutations and MGMT promoter methylation,1 whereas the lower frequency of TERT promoter mutations was observed in H3 K27M-mt DMGs, ranging from 0% to 10%.10,36 In our cases, TERT promoter mutation was observed in 8.2% of the adult group, and no TERT mutation was detected in the pediatric group. MGMT promoter methylation in patients with DMG has been reported to range from 0% to 23.6%, significantly lower than that in IDH-WT/H3-WT gliomas. The present study indicated coexistent TERT promoter mutation and/or MGMT promoter methylation was mainly seen in adult patients.
H3 K27M-mt DMGs are known to have poor OS compared with H3 K27 WT gliomas, independent of conventional histologic grade.16,17,37 However, whether there were potential prognostic factors for H3 K27M-mt DMGs have not been elucidated. In one study which included 15 pediatric and 13 adult H3 K27M-mt DMGs, adult patients had the same poor prognosis as pediatric patients.32 Our data indicated the prognosis of pediatric patients was significantly worse than that of adult patients. This may be related to the differences in anatomic location and ATRX status between the adult and pediatric patients. It has been reported that patients with H3 K27M-mt DMGs in the brainstem had a poorer prognosis than those with tumors in other anatomic locations.19 Loss of ATRX expression and p53 overexpression as negative prognostic factors for OS have also been reported.30 Future studies on more patients should shed more light on this issue.
Higher grade gliomas tend to have higher Ki-67 labeling indexes. For H3 K27M-mt DMGs, although conventional histologic grade (grades 2, 3, 4) alone was not prognostically useful, the Ki-67 index (Ki-67 ≤5% vs. Ki-67 >5%) showed prognostic value. Indeed, similar results were found in other gliomas, such as IDH-mutant astrocytic gliomas38 and pediatric high-grade gliomas,39 for which prognoses were related to Ki-67 index but not conventional histologic grade. One study showed a strong association between higher Ki-67 and a greater tumor volume for glioblastoma.40 Another study found higher Ki-67 index was associated with a high variant allele frequency in H3 K27M-mt DMGs, and that a high variant allele frequency was associated with poorer survival.41 These data indicated that Ki-67 labeling index may have use in stratification of high-grade gliomas and H3 K27M-mt DMGs. In addition, our data also indicated that patients with tumors showing both conventional histologic grade 2 morphology and lower Ki-67 index (≤5%) might have relatively better survival.
Leptomeningeal and subventricular spread was reported typically later in the course of DMGs.42,43 One study reported that 2 H3 K27M-mt DMG patients showed leptomeningeal dissemination at the time of onset.32 Our data also showed there were 8 patients with tumor spread at initial admission and 9 patients who developed dissemination later. These results indicated higher risks of dissemination for DMGs, which could occur in the early stages of the disease, although no significant influence on prognosis was observed, most probably due to the already dire outcome of DMGs.
To our knowledge, the present study is by far the largest series that evaluated the clinicopathologic characteristics and prognosis of adult and pediatric H3 K27M-mt diffuse midline gliomas. In addition to confirmation of earlier findings that H3 K27M-mt DMGs had a wide morphologic spectrum, we showed that DMGs had low frequency of mutation of the TERT promoter and MGMT promoter methylation, in contrast to IDH-WT or H3-WT gliomas. We also showed that patient age, anatomic location, tumor diameter, loss of ATRX expression, p53 overexpression, and Ki-67 index were significant for the prognosis of H3 K27M-mt DMGs. Most interestingly, our data showed that adult H3 K27M-mt DMGs were more common in the thalamus, had higher frequency of ATRX loss and H3.3 K27M mutation, as well as better prognosis. These characteristics were different from DMGs in children. Future research with even larger cohorts, including both pediatric and adult cases, should provide more insights into this highly aggressive group of gliomas.
Supplementary Material
Supplemental Digital Content is available for this article. Direct URL citations appear in the printed text and are provided in the HTML and PDF versions of this article on the journal's website, www.ajsp.com.
Footnotes
L.Z. and J.G. are co-first authors.
N.C. and Q.Z. are co-corresponding authors.
L.Z. and J.G.: analyzed the data and performed the experiments. T.Y., Y.Z., M.Z., L.N., and X.C.: provided essential material and pathologic data. Q.Y.: analyzed the imaging features. Y.L. and Q.M.: provided clinical data. J.G., and N.C.: reviewed the pathologic material and diagnosis. L.Z.: prepared the draft of the manuscript. Q.Z. and N.C.: designed and supervised the study, wrote and revised the manuscript.
Conflicts of Interest and Source of Funding: Supported by grants from the National Natural Science Foundation of China (NSFC 81872107, 81872108) and Sichuan STA Key Programs (2021YFS0114). The authors have disclosed that they have no significant relationships with, or financial interest in, any commercial companies pertaining to this article.
Contributor Information
Linmao Zheng, Email: 18842628348@163.com.
Jing Gong, Email: gongjingpath@163.com.
Tianping Yu, Email: 290567761@qq.com.
Yan Zou, Email: 474774926@qq.com.
Mengni Zhang, Email: 469426231@qq.com.
Ling Nie, Email: lingling830209@163.com.
Xueqin Chen, Email: 147552104@qq.com.
Qiang Yue, Email: qiangmoon@126.com.
Yanhui Liu, Email: 827005432@qq.com.
Qing Mao, Email: qingmao2000@163.com.
Qiao Zhou, Email: zhou_qiao@hotmail.com.
Ni Chen, Email: chenni1@163.com.
REFERENCES
- 1. Louis DN, Ohgaki H, Wiestler OD, et al. WHO Classification of Tumours of the Central Nervous System, revised 4th ed. Lyon, France: IARC Press; 2016. [Google Scholar]
- 2. Louis DN, Giannini C, Capper D, et al. cIMPACT-NOW update 2: diagnostic clarifications for diffuse midline glioma, H3 K27M-mutant and diffuse astrocytoma/anaplastic astrocytoma, IDH-mutant. Acta Neuropathol. 2018;135:639–642. [DOI] [PubMed] [Google Scholar]
- 3. Louis DN, Perry A, Wesseling P, et al. The 2021 WHO classification of tumors of the central nervous system: a summary. Neuro Oncol. 2021;23:1231–1251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Solomon DA, Wood MD, Tihan T, et al. Diffuse midline gliomas with histone H3-K27M mutation: a series of 47 cases assessing the spectrum of morphologic variation and associated genetic alterations. Brain Pathol. 2016;26:569–580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Castel D, Philippe C, Calmon R, et al. Histone H3F3A and HIST1H3B K27M mutations define two subgroups of diffuse intrinsic pontine gliomas with different prognosis and phenotypes. Acta Neuropathol. 2015;130:815–827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Bozkurt SU, Dagcinar A, Tanrikulu B. Significance of H3K27M mutation with specific histomorphological features and associated molecular alterations in pediatric high-grade glial tumors. Childs Nerv Syst. 2018;34:107–116. [DOI] [PubMed] [Google Scholar]
- 7. Zheng L, Zhang M, Hou J, et al. High-grade gliomas with isocitrate dehydrogenase wild-type and 1p/19q codeleted: atypical molecular phenotype and current challenges in molecular diagnosis. Neuropathology. 2020;40:599–605. [DOI] [PubMed] [Google Scholar]
- 8. Louis DN, Ohgaki H, Wiestler OD, et al. The 2007 WHO classification of tumours of the central nervous system. Acta Neuropathol. 2007;114:97–109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Lopez GY, Oberheim Bush NA, Phillips JJ, et al. Diffuse midline gliomas with subclonal H3F3A K27M mutation and mosaic H3.3 K27M mutant protein expression. Acta Neuropathol. 2017;134:961–963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Meyronet D, Esteban-Mader M, Bonnet C, et al. Characteristics of H3 K27M-mutant gliomas in adults. Neuro Oncol. 2017;19:1127–1134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Nakata S, Nobusawa S, Yamazaki T, et al. Histone H3 K27M mutations in adult cerebellar high-grade gliomas. Brain Tumor Pathol. 2017;34:113–119. [DOI] [PubMed] [Google Scholar]
- 12. Daoud EV, Rajaram V, Cai C, et al. Adult brainstem gliomas with H3K27M mutation: radiology, pathology, and prognosis. J Neuropathol Exp Neurol. 2018;77:302–311. [DOI] [PubMed] [Google Scholar]
- 13. Funata N, Nobusawa S, Nakata S, et al. A case report of adult cerebellar high-grade glioma with H3.1 K27M mutation: a rare example of an H3 K27M mutant cerebellar tumor. Brain Tumor Pathol. 2018;35:29–35. [DOI] [PubMed] [Google Scholar]
- 14. Gao Y, Feng YY, Yu JH, et al. Diffuse midline gliomas with histone H3-K27M mutation: a rare case with PNET-like appearance and neuropil-like islands. Neuropathology. 2018;38:165–170. [DOI] [PubMed] [Google Scholar]
- 15. Gilbert AR, Zaky W, Gokden M, et al. Extending the neuroanatomic territory of diffuse midline glioma, K27M mutant: pineal region origin. Pediatr Neurosurg. 2018;53:59–63. [DOI] [PubMed] [Google Scholar]
- 16. Huang T, Garcia R, Qi J, et al. Detection of histone H3 K27M mutation and post-translational modifications in pediatric diffuse midline glioma via tissue immunohistochemistry informs diagnosis and clinical outcomes. Oncotarget. 2018;9:37112–37124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Karremann M, Gielen GH, Hoffmann M, et al. Diffuse high-grade gliomas with H3 K27M mutations carry a dismal prognosis independent of tumor location. Neuro Oncol. 2018;20:123–131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Picart T, Barritault M, Berthillier J, et al. Characteristics of cerebellar glioblastomas in adults. J Neurooncol. 2018;136:555–563. [DOI] [PubMed] [Google Scholar]
- 19. Wang L, Li Z, Zhang M, et al. H3 K27M-mutant diffuse midline gliomas in different anatomical locations. Hum Pathol. 2018;78:89–96. [DOI] [PubMed] [Google Scholar]
- 20. Aboian MS, Tong E, Solomon DA, et al. Diffusion characteristics of pediatric diffuse midline gliomas with histone H3-K27M mutation using apparent diffusion coefficient histogram analysis. Am J Neuroradiol. 2019;40:1804–1810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Alvi MA, Ida CM, Paolini MA, et al. Spinal cord high-grade infiltrating gliomas in adults: clinico-pathological and molecular evaluation. Mod Pathol. 2019;32:1236–1243. [DOI] [PubMed] [Google Scholar]
- 22. Jung JS, Choi YS, Ahn SS, et al. Differentiation between spinal cord diffuse midline glioma with histone H3 K27M mutation and wild type: comparative magnetic resonance imaging. Neuroradiology. 2019;61:313–322. [DOI] [PubMed] [Google Scholar]
- 23. Liu Y, Zhang Y, Hua W, et al. Clinical and molecular characteristics of thalamic gliomas: retrospective report of 26 cases. World Neurosurg. 2019;126:e1169–e1182. [DOI] [PubMed] [Google Scholar]
- 24. Qiu T, Chanchotisatien A, Qin Z, et al. Imaging characteristics of adult H3 K27M-mutant gliomas. J Neurosurg. 2019;15:1–9. [DOI] [PubMed] [Google Scholar]
- 25. Schreck KC, Ranjan S, Skorupan N, et al. Incidence and clinicopathologic features of H3 K27M mutations in adults with radiographically-determined midline gliomas. J Neurooncol. 2019;143:87–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Yi S, Choi S, Shin DA, et al. Impact of H3.3 K27M mutation on prognosis and survival of grade IV spinal sord glioma on the basis of new 2016 world health organization classification of the central nervous system. Neurosurgery. 2019;84:1072–1081. [DOI] [PubMed] [Google Scholar]
- 27. Chai RC, Zhang YW, Liu YQ, et al. The molecular characteristics of spinal cord gliomas with or without H3 K27M mutation. Acta Neuropathol Commun. 2020;8:40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Ebrahimi A, Skardelly M, Schuhmann MU, et al. High frequency of H3 K27M mutations in adult midline gliomas. J Cancer Res Clin Oncol. 2019;145:839–850. [DOI] [PubMed] [Google Scholar]
- 29. Wu G, Broniscer A, McEachron TA, et al. Somatic histone H3 alterations in pediatric diffuse intrinsic pontine gliomas and non-brainstem glioblastomas. Nat Genet. 2012;44:251–253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Wang Y, Feng LL, Ji PG, et al. Clinical features and molecular markers on diffuse midline gliomas with H3K27M mutations: a 43 cases retrospective cohort study. Front Oncol. 2020;10:602553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Su X, Chen N, Sun H, et al. Automated machine learning based on radiomics features predicts H3 K27M mutation in midline gliomas of the brain. Neuro Oncol. 2020;22:393–401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Kleinschmidt-DeMasters BK, Mulcahy Levy JM. H3 K27M-mutant gliomas in adults vs. children share similar histological features and adverse prognosis. Clin Neuropathol. 2018;37:53–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Low JT, Wang SH, K BP. Diffuse midline glioma with H3 K27M-mutation in an 83-year-old woman. CNS Oncol. 2021;10:CNS71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Bechet D, Gielen GG, Korshunov A, et al. Specific detection of methionine 27 mutation in histone 3 variants (H3K27M) in fixed tissue from high-grade astrocytomas. Acta Neuropathol. 2014;128:733–741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Zhao H, Fang X, Xue B. Four methods to analyze H3K27M mutation in diffuse midline gliomas. Pathol Res Pract. 2020;216:153065. [DOI] [PubMed] [Google Scholar]
- 36. Reuss DE, Kratz A, Sahm F, et al. Adult IDH wild type astrocytomas biologically and clinically resolve into other tumor entities. Acta Neuropathol. 2015;130:407–417. [DOI] [PubMed] [Google Scholar]
- 37. Khuong-Quang DA, Buczkowicz P, Rakopoulos P, et al. K27M mutation in histone H3.3 defines clinically and biologically distinct subgroups of pediatric diffuse intrinsic pontine gliomas. Acta Neuropathol. 2012;124:439–447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Shirahata M, Ono T, Stichel D, et al. Novel, improved grading system(s) for IDH-mutant astrocytic gliomas. Acta Neuropathol. 2018;136:153–166. [DOI] [PubMed] [Google Scholar]
- 39. Varlet P, Le Teuff G, Le Deley MC, et al. WHO grade has no prognostic value in the pediatric high-grade glioma included in the HERBY trial. Neuro Oncol. 2020;22:116–127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Armocida D, Frati A, Salvati M, et al. Is Ki-67 index overexpression in IDH wild type glioblastoma a predictor of shorter progression free survival? A clinical and molecular analytic investigation. Clin Neurol Neurosurg. 2020;198:106126. [DOI] [PubMed] [Google Scholar]
- 41. Maeda S, Ohka F, Okuno Y, et al. H3F3A mutant allele specific imbalance in an aggressive subtype of diffuse midline glioma, H3 K27M-mutant. Acta Neuropathol Commun. 2020;8:8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Caretti V, Bugiani M, Freret M, et al. Subventricular spread of diffuse intrinsic pontine glioma. Acta Neuropathol. 2014;128:605–607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Tinkle CL, Orr BA, Lucas JT, Jr, et al. Rapid and fulminant leptomeningeal spread following radiotherapy in diffuse intrinsic pontine glioma. Pediatr Blood Cancer. 2017;64:e26416. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental Digital Content is available for this article. Direct URL citations appear in the printed text and are provided in the HTML and PDF versions of this article on the journal's website, www.ajsp.com.


