BACKGROUND:
Cholinergic stimulation of prefrontal cortex (PFC) can reverse anesthesia. Conversely, inactivation of PFC can delay emergence from anesthesia. PFC receives cholinergic projections from basal forebrain, which contains wake-promoting neurons. However, the role of basal forebrain cholinergic neurons in arousal from the anesthetized state requires refinement, and it is currently unknown whether the arousal-promoting effect of basal forebrain is mediated through PFC. To address these gaps in knowledge, we implemented a novel approach to the use of chemogenetic stimulation and tested the role of basal forebrain cholinergic neurons in behavioral arousal during sevoflurane anesthesia. Next, we investigated the effect of tetrodotoxin-mediated inactivation of PFC on behavioral arousal produced by electrical stimulation of basal forebrain during sevoflurane anesthesia.
METHODS:
Adult male and female transgenic rats (Long-Evans-Tg [ChAT-Cre]5.1 Deis; n = 22) were surgically prepared for expression of excitatory hM3D(Gq) receptors or mCherry in basal forebrain cholinergic neurons, and activation of these neurons by local delivery of compound 21, an agonist for hM3D(Gq) receptors. The transgenic rats were fitted with microdialysis probes for agonist delivery into basal forebrain and simultaneous prefrontal acetylcholine measurement. Adult male and female Sprague Dawley rats were surgically prepared for bilateral electrical stimulation of basal forebrain and tetrodotoxin infusion (156 μM and 500 nL) into PFC (n = 9) or bilateral electrical stimulation of piriform cortex (n = 9) as an anatomical control. All rats were implanted with electrodes to monitor the electroencephalogram. Heart and respiration rates were monitored using noninvasive sensors. A 6-point scale was used to score behavioral arousal (0 = no arousal and 5 = return of righting reflex).
RESULTS:
Compound 21 delivery into basal forebrain of rats with hM3D(Gq) receptors during sevoflurane anesthesia produced increases in arousal score (P < .001; confidence interval [CI], 1.80–4.35), heart rate (P < .001; CI, 36.19–85.32), respiration rate (P < .001; CI, 22.81–58.78), theta/delta ratio (P = .008; CI, 0.028–0.16), and prefrontal acetylcholine (P < .001; CI, 1.73–7.46). Electrical stimulation of basal forebrain also produced increases in arousal score (P < .001; CI, 1.85–4.08), heart rate (P = .018; CI, 9.38–98.04), respiration rate (P < .001; CI, 24.15–53.82), and theta/delta ratio (P = .020; CI, 0.019–0.22), which were attenuated by tetrodotoxin-mediated inactivation of PFC.
CONCLUSIONS:
This study validates the role of basal forebrain cholinergic neurons in behavioral arousal and demonstrates that the arousal-promoting effects of basal forebrain are mediated in part through PFC.
KEY POINTS.
Question: Is the arousal-promoting effect of basal forebrain mediated through prefrontal cortex?
Findings: Inactivation of prefrontal cortex attenuates basal forebrain-mediated arousal.
Meaning: This provides further evidence that the prefrontal cortex is part of the arousal circuitry.
See Article, p 1114
Cholinergic stimulation of prefrontal cortex (PFC)—via local carbachol delivery—in sevoflurane-anesthetized rats induces a wake-like state despite continuous anesthetic administration.1 Similarly, cholinergic stimulation of PFC during slow-wave sleep decreases the latency to wakefulness and increases the time spent in wakefulness.2 Conversely, tetrodotoxin (TTX)-mediated inactivation of PFC, but not parietal cortex, delays emergence from sevoflurane anesthesia.3 Notably, reversal of general anesthesia after carbachol delivery into PFC was associated with an increase in local acetylcholine levels,1 which is consistent with a wide array of studies demonstrating high cortical acetylcholine levels in association with cortical activation and wakefulness.4–10 In contrast, cortical acetylcholine levels are known to be low during slow-wave sleep and ether- or propofol-induced unconsciousness,4–10 and decreases in endogenous forebrain acetylcholine levels increase anesthetic sensitivity.11
The primary source of acetylcholine in PFC is cholinergic neurons in basal forebrain,12,13 which have been shown to be active during the wake state and which are known to produce behavioral arousal.14–17 Although manipulation of both PFC and basal forebrain has been shown to produce behavioral arousal,1,2,14,16,17 the direct cholinergic projections from basal forebrain to PFC12,13 and the increase in prefrontal acetylcholine in association with wakefulness1,4–10 suggest that the PFC might gate the arousal-promoting effect of basal forebrain. However, there is no direct evidence for an interplay between PFC and basal forebrain in behavioral arousal. Furthermore, although recent studies showed a role for basal forebrain cholinergic neurons in passive emergence from anesthesia,18,19 a direct role of cholinergic basal forebrain neurons in reversing the state of anesthesia has not been demonstrated.
Therefore, in this study, we first investigated whether chemogenetic activation of cholinergic neurons in basal forebrain was sufficient to reverse the state of general anesthesia and produce behavioral arousal in the continued presence of sevoflurane anesthesia. Next, we conducted electrical stimulation of basal forebrain in sevoflurane-anesthetized rats with or without concurrent inactivation of PFC to determine whether the arousal-promoting effects of basal forebrain are mediated through PFC. We demonstrate that chemogenetic activation of basal forebrain cholinergic neurons in sevoflurane-anesthetized rats induces a wake-like state, and inactivation of PFC attenuates behavioral arousal induced by electrical stimulation of basal forebrain.
METHODS
Rats
The experiments were approved by the Institutional Animal Care and Use Committee at the University of Michigan and were in compliance with the Guide for the Care and Use of Laboratory Animals (Ed 8, National Academies Press) and the Animal Research: Reporting of In Vivo Experiments (ARRIVE) guidelines. The rats were maintained on a 12-hour light/12-hour dark cycle (lights on at 8:00 am) with ad libitum food and water in a temperature-controlled animal care and housing facility at the University of Michigan. To take advantage of the chemogenetic cell-specific targeting, we used adult male and female transgenic rats (Long-Evans-Tg[ChAT-Cre]5.1 Deis, referred herein as the ChAT-Cre rats, ~6 months of age; n = 22)20 that express Cre recombinase in cholinergic neurons (Rat Resource & Research Center, University of Missouri, Columbia, Missouri). We infused 500 nL of AAV5:pAAV-hSyn-DIO-hM3D(Gq)-mCherry bilaterally into the basal forebrain of these rats (n = 18) to express excitatory hM3D(Gq) receptors in the local cholinergic neurons, which were subsequently activated with compound 21 (C21), a selective agonist for hM3D(Gq) receptors.21–24 Of these 18 rats, 13 were used in chemogenetic experiments wherein C21 was dialyzed directly into basal forebrain while 5 rats received C21 as intravenous bolus. A subgroup of ChAT-Cre rats (n = 4) and a wild-type male Long-Evans rat received AAV5:pAAV-hSyn-DIO-mCherry via bilateral injection (500 nL) into basal forebrain to express mCherry, the fluorescent protein/tag, in the local cholinergic neurons. The electrical stimulation experiments were conducted in adult male and female nontransgenic Sprague Dawley rats (n = 18; 300–350 g; Charles River Laboratories).
Experimental Design
Chemogenetic Activation of Basal Forebrain Cholinergic Neurons
The experimental design is illustrated in Figure 1. The rats (n = 10 males and 8 females) were connected to the electrophysiological recording system at least 30 minutes before the start of experimental sessions (9:30 am–10:30 am), and microdialysis probes being perfused (2 μL/min) with artificial cerebrospinal fluid were lowered into the basal forebrain (bilateral) and PFC (unilateral). Baseline electroencephalographic data were collected for 50 minutes while keeping the behavioral state constant by introducing novel objects and gentle tapping on the recording chamber. Thereafter, sevoflurane administration was started and titrated (1.7%–2.3%) to produce loss of righting reflex—a behavioral surrogate for unconsciousness in rodents—along with complete immobility and high-amplitude slow waves in electroencephalogram (EEG). Sevoflurane concentration was continuously monitored using anesthesia monitors (Datex Medical Instrumentation) connected to the anesthesia inlet and outlet ports of the recording chamber. At the onset of loss of righting reflex, a rectal probe connected to a far-infrared heating pad (Kent Scientific) through a feedback temperature controller (Physitemp, Model RET-3) was positioned to monitor and maintain the body temperature at 37.0°C ± 1°C. In addition, a pulse oximetry sensor (MouseOx, Starr Life Science) was positioned on the foot or around the neck to record the changes in respiration rate, heart rate, and oxygen saturation levels during anesthesia.
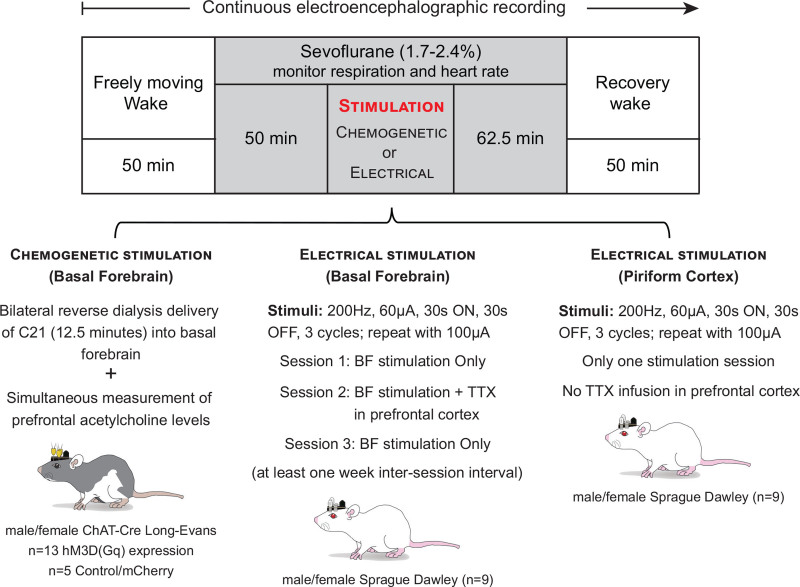
Figure 1.
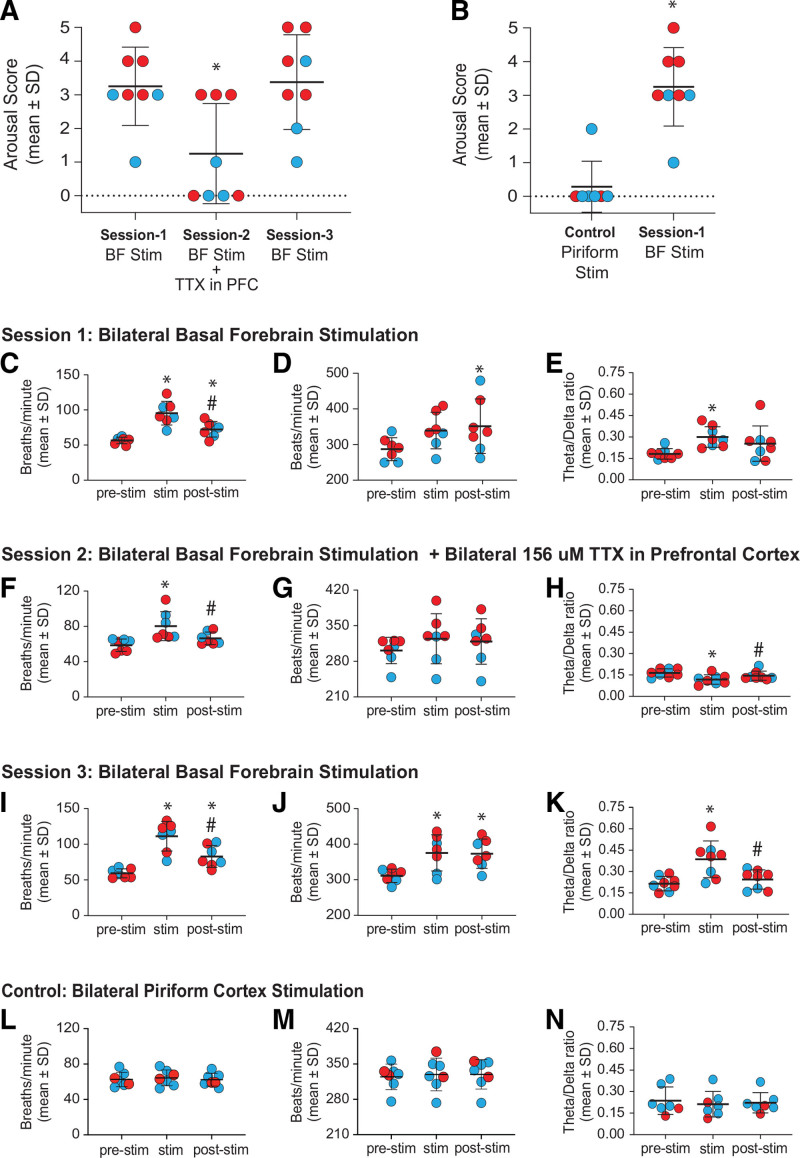
Schematic to show the experimental design and timeline. The chemogenetic experiments were conducted in adult male and female Long-Evans-Tg(ChAT-Cre)5.1 Deis rats (referred herein as ChAT-Cre rats). The EEG data were recorded continuously, while the microdialysis samples from prefrontal cortex were collected every 12.5 min. Baseline wake data were collected for 50 min, after which the recording chamber was sealed and sevoflurane adminstration (1.7%–2.4%) was started. Respiration and heart rate were then monitored using a rodent pulse oximeter until the end of sevoflurane exposure. After 50 min of sevoflurane anesthesia, compound 21 (C21), the agonist for hM3D(Gq) receptors, was reverse dialyzed for 12.5 min into the basal forebrain of ChAT-Cre rats. Female rats received 0.5 mM C21, while the male rats received 1 mM C21. At the completion of C21 delivery, the rats were maintained at the same sevoflurane concentration until the postsevoflurane recovery epoch. Postsevoflurane recovery data were collected for 50 min. Adult male and female Sprague Dawley rats were used for bilateral electrical stimulation of basal forebrain; electrical stimulation of piriform cortex was conducted to confirm the specificity of response to electrical stimulation of basal forebrain. The experimental design was similar to that followed for chemogenetic experiments except that instead of C21 administration, the rats received electrical stimuli in basal forebrain with or without bilateral infusion of 500 nL of 156 μM TTX into prefrontal cortex. BF indicates basal forebrain; ChAT, choline acetyl transferase; TTX, tetrodotoxin.
After 50 minutes of sevoflurane administration, C21 was dialyzed (12.5 minutes) into the basal forebrain while the rats continued to inhale the same concentration of sevoflurane, which continued for 62.5 minutes. The EEG data were collected for another 50 minutes in postsevoflurane period. Our pilot experiments showed that dialysis delivery of C21 at equimolar concentration (1.0 mM) during sevoflurane anesthesia produced profound respiratory depression in female, but not male rats (data not included). Therefore, we used 1.0 mM C21 in male rats and 0.5 mM C21 in female rats. Electroencephalographic recording and microdialysis were conducted simultaneously and continuously across the experimental session, but the microdialysis samples were collected in 12.5-minute bins, as described in our recent publications.1,10
Electrical Stimulation of Basal Forebrain With or Without Concurrent Infusion of TTX Into PFC
As illustrated in Figure 1, 3 experimental sessions were conducted in each rat (n = 4 males and 5 females), separated by at least 1 week. For session 1, the rats were connected to the EEG recording and electrical stimulation system at least 30 minutes before the start of the experimental session (9:30 am–10:30 am). The baseline electroencephalographic data collection, sevoflurane administration (1.7%–2.4%), and physiologic monitoring were conducted as described earlier for the chemogenetic experiments. After 50 minutes of sevoflurane administration, a constant current electrical stimulation protocol was initiated to deliver stimuli bilaterally to the basal forebrain: 200-Hz square wave, 60 μA, 30 seconds on, and 30 seconds off (rest period) for 3 cycles, after which the protocol was repeated for another 3 cycles, but the current amplitude was increased to 100 μA. The electrical stimulation parameters were informed by previous studies showing the effect of subcortical electrical stimulation on behavioral arousal25,26 and pilot studies conducted in our laboratory (data not included). After cessation of sevoflurane administration, the electroencephalographic data were collected for another 50 minutes.
For session 2, 40 minutes before the start of baseline electroencephalographic recording, the rats received 500 nL of 156 μM TTX bilaterally into the PFC. Thereafter, the experimental protocol as described for session 1 was repeated. The concentration of TTX was based on previous studies.27,28 For session 3, the experimental protocol as described for session 1 was repeated to confirm that the electrical stimulation in session 1 did not cause damage to basal forebrain that may have resulted in the lack of arousal phenotype in session 2 experiments. One rat was excluded from analysis because of unusual resistance to sevoflurane anesthesia.
Electrical Stimulation of Piriform Cortex
A separate group of rats with bipolar stimulation electrodes implanted in piriform cortex (n = 7 males and 2 females), a site adjacent to basal forebrain, was used as anatomical control. These rats underwent only 1 experimental session, which was the same as session 1 described above for the basal forebrain group. Two rats were excluded from analysis because the stimulation electrodes were found to be outside the target area.
Surgical and Experimental Procedures
The detailed surgical methods are provided in Supplemental Digital Content 1, Materials and Methods, http://links.lww.com/AA/D917. In brief, the ChAT-Cre rats were implanted with 15 stainless-steel screw electrodes to record EEG from across the cortical hemisphere. The electrodes were arranged in 2 columns along the rostral-caudal axis (interelectrode distance of 2 mm). The lateral distance from sagittal suture was 2 and 4 mm for the first and second electrode columns, respectively. Thereafter, the rats received bilateral injection (500 nL) of either the excitatory or the control viral construct into substantia innominata region of the basal forebrain (Bregma: 0.48 mm posterior, 2.0 mm mediolateral, and 8.2 mm ventral).29 After the infusion of viral constructs, a pair of microdialysis guide tubes was implanted 1 mm above the substantia innominata for dialysis delivery of C21, and a single microdialysis guide tube was implanted 1 mm above the PFC (Bregma: 3.0 mm anterior, 0.5 mm mediolateral, and 3.0 mm ventral)29 for simultaneous quantification of local acetylcholine levels.
Table.
Quantification of Behavioral Arousal
| Arousal score | Criteria |
|---|---|
| 0 | Complete absence of behavioral arousal |
| 1 | Orofacial movements or eye blinks |
| 2 | Isolated body movements (ie, movement of only 1 set of limbs, either front or hind) or head or tail movements |
| 3 | Coordinated body movements (ie, simultaneous movement of 1 or more sets of limbs along with head and/or tail movements) |
| 4 | Attempt at regaining righting reflex |
| 5 | Return of righting reflex |
For the electrical stimulation studies, the rats were implanted with 15 stainless-steel screw electrodes arranged in 2 columns, 1 on either side (2 mm) of the sagittal suture along the rostral-caudal axis (interelectrode distance 2 mm). Thereafter, the rats were bilaterally implanted with bipolar wire electrodes either into substantia innominata or piriform cortex (from Bregma: 0.48 mm posterior, 5.0 mm mediolateral, and 9.0 mm ventral).29 The rats with stimulation electrode in substantia innominata were also implanted with a pair of microinjection guide cannula 1 mm above the PFC for bilateral infusion of TTX. A stainless-steel screw implanted over the nasal sinus served as reference electrode for all EEG recordings.
Quantification of Electroencephalographic Activation and Behavioral Arousal
The electroencephalographic activation was quantified using power spectral changes in theta and delta bandwidths (theta/delta ratio), as described in Supplemental Digital Content 1, Materials and Methods, http://links.lww.com/AA/D917. Behavioral arousal was quantified using a 6-level scoring scale (Table) that was modified from previous studies assessing responses to brain stimulation in rodents under anesthesia.30,31 The arousal scores were quantified by investigators not directly involved in data collection and blinded to the experimental conditions.
Statistical Analyses
Distribution of response variables was investigated graphically. All variables appeared to be normally distributed except acetylcholine, which was positively skewed and was, therefore, analyzed on a logarithmic scale.
To compare between-rat arousal scores, we used a linear regression controlling for sex, ie, treatment (experimental versus control rats)-by-sex interaction. We used linear mixed models for the statistical comparison of within-rat (repeated) observations of prefrontal acetylcholine levels, theta/delta ratio, and heart and respiration rates, before, during, and after C21 administration or electrical stimulation. The linear mixed models included an epoch-(wake, sevoflurane, C21/electrical stimulation, and recovery wake)-by-sex interaction term to investigate differential sex effects. The interaction term was not found to be statistically significant either in linear regression or linear mixed models. In cases with comparison of >2 epochs, Tukey’s correction was applied to post hoc pairwise comparisons (eg, respiration rate between prestimulation versus stimulation, prestimulation versus poststimulation, and stimulation versus poststimulation).
The respiration and heart rates were statistically compared 300 seconds before and 300 seconds after the visible change in EEG following C21 delivery, and 300 seconds before, during (330 seconds), and 300 seconds following electrical stimulation of basal forebrain or piriform cortex. The effect of C21 delivery on prefrontal acetylcholine levels was assessed by statistical comparison of the following 12.5-minute epochs: (1) wakefulness—last epoch in the wake condition, (2) sevoflurane anesthesia—last epoch during sevoflurane exposure and before C21 delivery, (3) C21 administration—epoch after C21 administration and the appearance of first signs of arousal, and (4) recovery wake state—the second 12.5-minute epoch after sevoflurane cessation by which all the animals were ambulatory. For graphical purposes, the acetylcholine data were normalized and plotted as percent change with respect to baseline wake values. A P value of <.05 was considered statistically significant. The data in the results section are reported as mean ± standard deviation (SD) along with 95% confidence interval (CI) of ratios of the means. The descriptive and inferential statistics for all experiments are provided in a tabular format in Supplemental Digital Content 1, Tables S1–S10, http://links.lww.com/AA/D917. Statistical analyses were conducted with R (Version 3.3.1) and in consultation with the Consulting for Statistics, Computing and Analytics Research unit at the University of Michigan. We designed the chemogenetic study to have 80% power (α = 0.05/6, Bonferroni correction for 6 pairwise tests) and to detect an effect size (difference in means divided by SD of difference) of 1.2 or larger, predicted based on a recently published similar study from our laboratory.1 The sample size for electrical stimulation study was based on similar, previously published rodent studies.25,26,32
RESULTS
Chemogenetic Stimulation of Basal Forebrain Cholinergic Neurons During Sevoflurane Anesthesia Caused Electroencephalographic and Physiologic Activation, Increased Prefrontal Acetylcholine, and Produced Behavioral Arousal
Histological analysis confirmed the expression of hM3D(Gq) receptors in basal forebrain cholinergic neurons and localization of microdialysis sites in basal forebrain and PFC (Figure 2). Bilateral delivery of C21 into basal forebrain—expressing hM3D(Gq) receptors in cholinergic neurons—of sevoflurane-anesthetized rats produced electroencephalographic activation and increased the theta/delta ratio (P = .008; CI, 0.028–0.16; Figure 3A). There was no statistical change in theta/delta ratio following C21 delivery into the basal forebrain of rats expressing only mCherry in cholinergic neurons (control group; P = .17; CI, −0.020 to 0.10). The electroencephalographic activation was followed by behavioral arousal (Supplemental Digital Content 2, Video 1, http://links.lww.com/AA/D918). The arousal score following C21 administration was significantly higher (mean ± SD, 3.1 ± 1.3; P < .001; CI, 1.80–4.35) than that observed after C21 administration in the control rats lacking hM3D(Gq) receptors (mean ± SD, 0.0 ± 0.0; Figure 3B). Of 13 rats, 2 regained righting reflex, 4 attempted righting, 1 showed coordinated body movements, 5 showed isolated body movements, and 1 rat showed orofacial movements and eye blinks. The mean time to electroencephalographic activation following the start of C21 delivery was longer for female rats (mean ± SD, 34.8 ± 17.9 minutes) than for male rats (mean ± SD, 18.4 ± 16.9 minutes). Behavioral arousal after C21 administration in rats with hM3D(Gq) receptors was accompanied by significant increase in respiration rate (mean ± SD, from 56 ± 9.0 to 97 ± 33 breaths/min; P < .001; CI, 22.81–58.78) and heart rate (mean ± SD, from 325 ± 25 to 386 ± 50 beats/min; P < .001; CI, 36.19–85.32; Figure 3C, E). There was no significant change in heart and respiration rates after C21 delivery in the control rats (Figure 3D, F).
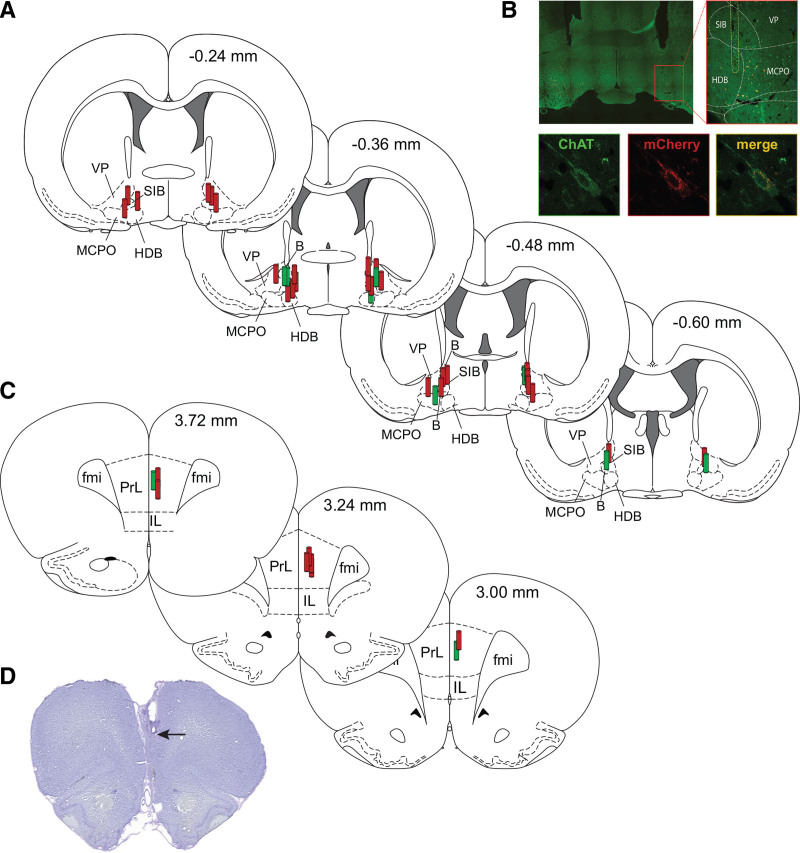
Figure 2.
Histological verification of the sites of dialysis delivery of compound 21 (C21) into basal forebrain, and microdialysis sites in prefrontal cortex for quantification of acetylcholine levels. A, Stereotaxic maps from rat brain atlas show the location of microdialysis probes (red and green cylinders) in basal forebrain for C21 delivery. B, Top-left panel shows a representative coronal brain section (30 μm) through basal forebrain immunostained for ChAT and mCherry, the fluorescent tag for hM3D(Gq) receptors. The inset encompasses the basal forebrain area and is enlarged in the panel on the right to show the outline of dialysis probe (white vertical broken lines). The bottom panels show a close-up of an individual neuron stained for ChAT (green), mCherry (red), and overlay of both (yellow) to show colocalization. C, Stereotaxic maps from rat brain atlas show the location of microdialysis probes in prefrontal cortex. D, Cresyl violet-stained representative coronal brain section (30 μm) through prefrontal cortex; arrow indicates the tip of the microdialysis probe. In A and C, red cylinders represent the microdialysis probe locations in rats with hM3D(Gq) receptors expressed in cholinergic neurons (experimental group), while the green cylinders show probe locations in rats that expressed only mCherry (control group). The numbers on the top right of stereotaxic maps show the anteroposterior distance from Bregma: positive numbers are anterior to Bregma, while the negative numbers are posterior to Bregma. B indicates basal nucleus (Meynert); ChAT, choline acetyl transferase; fmi, forceps minor of the corpus callosum; HDB, nucleus of the horizontal limb of the diagonal band; IL, infralimbic area; MCPO, magnocellular preoptic nucleus; PrL, prelimbic cortex; SIB, substantia innominata; VP, ventral pallidum.
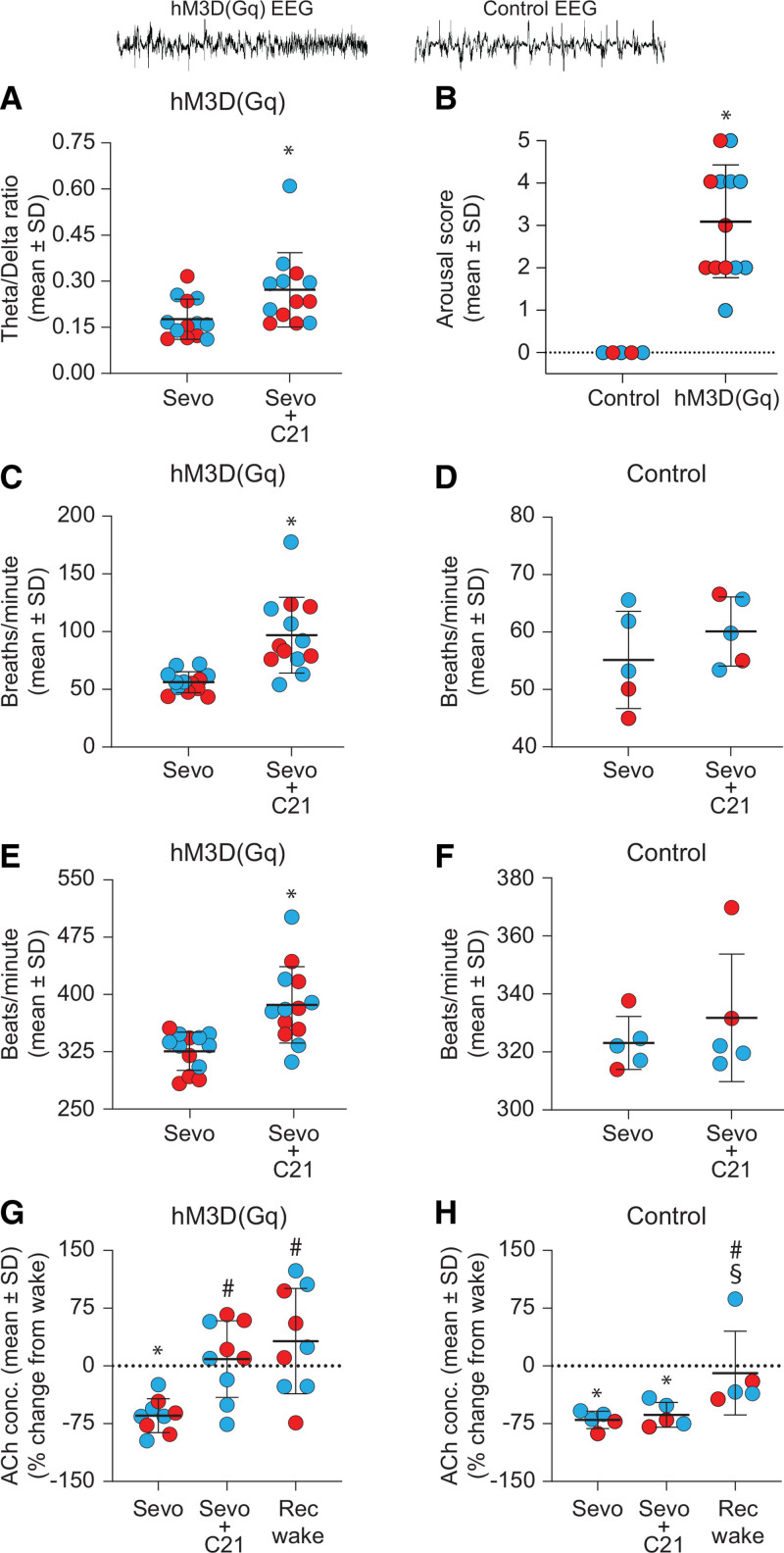
Figure 3.
Chemogenetic stimulation of basal forebrain cholinergic neurons during sevoflurane anesthesia caused electroencephalographic and physiologic activations, increased prefrontal acetylcholine, and produced behavioral arousal. The EEG traces on top are representative frontal EEG segments to show activation in ChAT-Cre rats expressing hM3D(Gq) receptors (left) and the lack of activation in ChAT-Cre rats (control) without hM3D(Gq) receptors (right). Each red (female) and blue (male) dot represents data from 1 rat. Male rats received 1.0 mM C21, while female rats received 0.5 mM C21. Dialysis delivery of compound 21 (C21) into basal forebrain of sevoflurane-anesthetized ChAT-Cre rats expressing hM3D(Gq) receptors significantly increased theta/delta ratio (A) and behavioral arousal score (B). C and E, Respiration rate and heart rate 300 s before (sevoflurane – Sevo) and 300 s after (Sevo + C21) C21-induced EEG changes in ChAT-Cre rats expressing hM3D(Gq) receptors. Dialysis delivery of C21 into basal forebrain significantly increased respiration (C) and heart (E) rates. D and F, C21 delivery into basal forebrain of ChAT-Cre rats expressing only mCherry (control group) failed to elicit significant changes in respiration or heart rate. G and H, Changes in ACh levels in prefrontal cortex (% change from baseline wake state) during Sevo, Sevo + C21, and recovery wake (Rec wake) epochs. The C21 delivery into basal forebrain during sevoflurane anesthesia caused a significant increase in ACh levels to baseline wake levels in ChAT-Cre rats expressing hM3D(Gq) receptors (G) but no such increase was observed in ACh levels in the control group (H). A linear mixed model controlling for sex was used for within-rat statistical comparisons, while a linear regression controlling for sex was used for between-rat (B) statistical comparisons. Post hoc comparisons were Tukey corrected. For B, C, and E: *compared to Control/Sevo. For G and H: *compared to baseline wake state, #compared to Sevo, and §compared to Sevo + C21. The P values are shown at <.05, but the actual P values are reported in the main text and in Supplemental Digital Content 1, Tables S1–S3, http://links.lww.com/AA/D917. A video showing representative behavior after chemogenetic stimulation of basal forebrain cholinergic neurons in ChAT-Cre rats is provided in Supplemental Digital Content 2, Video 1, http://links.lww.com/AA/D918. ACh indicates acetylcholine; EEG, electroencephalogram.
Sevoflurane anesthesia reduced prefrontal acetylcholine levels to below waking levels (P < .001; CI, 0.13–0.54; Figure 3G), which is consistent with previous reports from our and other laboratories.1,7 EEG activation and behavioral arousal induced by C21 delivery into basal forebrain were accompanied by significant increase in prefrontal acetylcholine levels (P < .001; CI, 1.73–7.46, compared to sevoflurane), which was not significantly different than that observed during baseline wake state (P = 1; CI, 0.45–1.97; Figure 3G). Acetylcholine level during postsevoflurane recovery wake state remained significantly higher than that observed during sevoflurane anesthesia (P < .001; CI, 2.05–8.85; Figure 3G) but was not significantly different compared to that observed during baseline wake state (P = .97; CI, 0.54–2.32) or C21-induced behavioral arousal (P = .92; CI, 0.57–2.46; Figure 3G).
In the control group, C21 delivery into basal forebrain did not produce any change in EEG in 4 of 5 rats; 1 female rat showed transient activation of EEG. Sevoflurane anesthesia in control rats decreased prefrontal acetylcholine levels compared to that observed during wake state (P = .001; CI, 0.13–0.58; Figure 3H). The delivery of C21 into basal forebrain during sevoflurane anesthesia did not have any significant effect on prefrontal acetylcholine levels (P = .80; CI, 0.58–2.59, compared to sevoflurane), which remained significantly below that observed during baseline wake state (P = .005; CI, 0.16–0.71; Figure 3H). After cessation of sevoflurane administration, prefrontal acetylcholine levels returned to waking levels (P = .85; CI, 0.38–1.72) and were significantly higher than that observed during sevoflurane anesthesia (P = .005; CI, 1.40–6.23) or C21 delivery (P = .019; CI, 1.15–5.10; Figure 3H).
A separate group of ChAT-Cre rats (n = 5 males) received intravenous bolus of C21 (5 mg/kg) under similar conditions as described above for the rats that received C21 via dialysis into basal forebrain. These rats showed electroencephalographic activation and behavioral arousal (Supplemental Digital Content 1, Figure S1, http://links.lww.com/AA/D917). Surprisingly, intravenous C21 (5 mg/kg) in wild-type Long-Evans rats that lacked hM3D(Gq) receptors (n = 2 males) also showed similar electroencephalographic activation and behavioral arousal, thus demonstrating nonspecific off-target effects of systemic C21 as has been reported recently24 (Supplemental Digital Content 1, Figure S1, http://links.lww.com/AA/D917).
Bilateral Electrical Stimulation of Basal Forebrain During Sevoflurane Anesthesia Caused Electroencephalographic and Physiologic Activation and Produced Behavioral Arousal
Histological analysis confirmed the electrical stimulation sites to be within basal forebrain (Figure 4A, B). Stimulation of basal forebrain (session 1) during sevoflurane anesthesia produced clear behavioral arousal and statistically significant increase in arousal score: 1 rat fully regained the righting reflex, 2 attempted righting, 4 showed coordinated body movements, and 1 showed orofacial movements and eye blinks (Figure 5A; Supplemental Digital Content 3, Video 2, http://links.lww.com/AA/D919). In contrast, bilateral stimulation of piriform cortex, a site adjacent to basal forebrain, did not produce behavioral arousal in any but 1 female rat, in which isolated hindlimb movement was observed. The arousal score after piriform cortex stimulation remained significantly lower than that observed after basal forebrain stimulation in session 1 (mean ± SD, 0.3 ± 0.8 for piriform cortex versus 3.3 ± 1.2 for basal forebrain; P < .001; CI, 1.85–4.08; Figure 5B). Behavioral arousal after bilateral electrical stimulation of basal forebrain was accompanied by increase in respiration rate (mean ± SD, from 57 ± 4.7 to 95 ± 17 breaths/min; P < .001; CI, 24.15–53.82) but did not produce a statistically significant increase in heart rate (Figure 5C, D); there was a statistically significant increase in theta/delta ratio (P = .020; CI, 0.019–0.22; Figure 5E). The respiration and heart rates were significantly elevated during the poststimulation period (mean ± SD, 72 ± 11 breaths/min; P = .038; CI, 0.88–30.55; and 377 ± 64 beats/min; P = .018; CI, 9.38–98.04; Figure 5C, D).
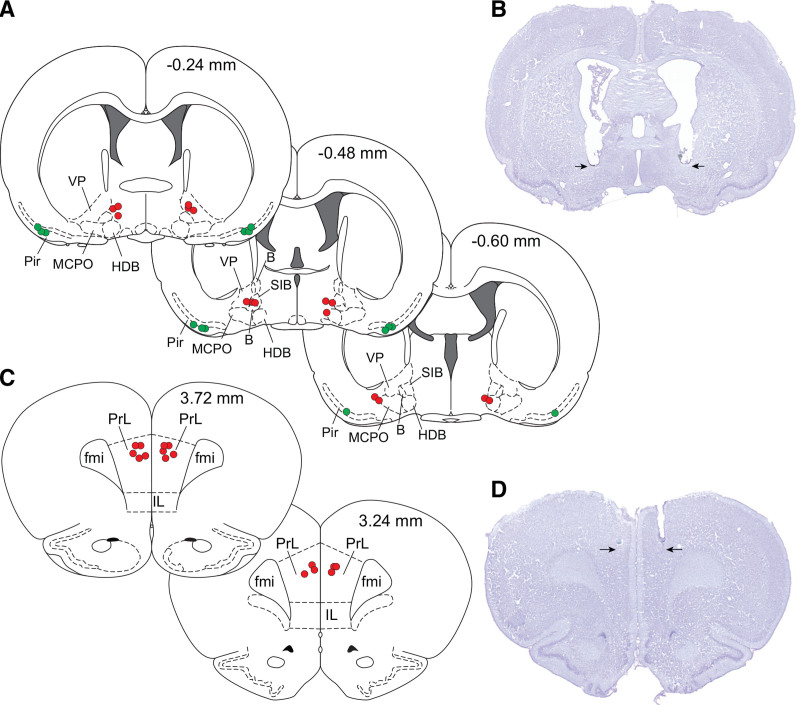
Figure 4.
Histological verification of the sites of electrical stimulation in basal forebrain and piriform cortex, and tetrodotoxin microinjection sites in prefrontal cortex. A, Stereotaxic maps from rat brain atlas show the sites of electrical stimulation in basal forebrain (red dots) and piriform cortex (green dots). B, Cresyl violet-stained representative coronal brain section (30 μm) through basal forebrain shows the site of bilateral electrical stimulation (arrows). C, Stereotaxic maps from rat brain atlas show the sites of tetrodotoxin microinjection into prefrontal cortex (red dots). D, Cresyl violet-stained representative coronal brain section (30 μm) through prefrontal cortex shows the site of bilateral tetrodotoxin microinjection (arrows). The numbers on the top right of stereotaxic maps show the anteroposterior distance from Bregma: positive numbers are anterior to Bregma, while the negative numbers are posterior to Bregma. B, basal nucleus (Meynert); fmi, forceps minor of the corpus callosum; HDB, nucleus of the horizontal limb of the diagonal band; IL, infralimbic area; MCPO, magnocellular preoptic nucleus; PrL, prelimbic cortex; SIB, substantia innominata; VP, ventral pallidum.
Figure 5.
Inactivation of prefrontal cortex attenuates electroencephalographic, physiologic, and behavioral arousal induced by bilateral electrical stimulation of basal forebrain during sevoflurane anesthesia. Each red dot (female) and blue dot (male) show the individual rat data. A, Comparison of behavioral arousal score after bilateral electrical stimulation of basal forebrain with or without concurrent inactivation of prefrontal cortex. Compared to the bilateral electrical stimulation of basal forebrain without prefrontal inactivation (session 1), bilateral electrical stimulation of basal forebrain with concurrent prefrontal inactivation (session 2) caused a significant decrease in the arousal score. Repeat of session 1 (ie, session 3) showed the arousal score to be significantly higher than that observed in session 2 and reached the levels observed during session 1. B, Behavioral arousal score after bilateral electrical stimulation of basal forebrain (session 1) was significantly higher than the arousal score after bilateral electrical stimulation of piriform cortex (anatomical control). Bilateral electrical stimulation of piriform cortex did not produce behavioral arousal as in session 1 rats. Session 1—bilateral electrical stimulation of basal forebrain in sevoflurane-anesthetized rats increased respiration rate (C), heart rate (D), and theta/delta ratio (E). The prestimulation data were quantified 300 s before stimulation (prestim), and the poststimulation data were quantified 300 s after the stimulation (poststim). Session 2—bilateral basal forebrain stimulation in the presence of TTX in prefrontal cortex produced a significant increase in respiration rate (F), but no significant change was observed in heart rate (G); theta/delta ratio showed a significant decrease during electrical stimulation (H). Session 3—bilateral electrical stimulation of basal forebrain in the same rats used in sessions 1 and 2 produced increase in respiration rate (I), heart rate (J), and theta/delta ratio (K). A video showing representative behavior after stimulation of (1) basal forebrain and (2) basal forebrain along with concurrent inactivation of prefrontal cortex is provided in Supplemental Digital Content 3, Video 2, http://links.lww.com/AA/D919. Bilateral electrical stimulation of piriform cortex during sevoflurane anesthesia did not produce any statistical change in respiration rate (L), heart rate (M), or theta/delta ratio (N). A linear mixed model controlling for sex was used for within-rat statistical comparisons, while a linear regression controlling for sex was used for between-rat (B) statistical comparisons. Post hoc comparisons were Tukey corrected. The P values are shown at <.05, but the actual P values are reported in the main text and in Supplemental Digital Content 1, Tables S4–S8, http://links.lww.com/AA/D917. For panel A: *compared to session 1. For panel B: *compared to control. For panels C–N: *compared to prestimulation, #compared to stimulation. BF indicates basal forebrain; PFC, prefrontal cortex; Piriform, piriform cortex; post-stim, 300 seconds following stimulation period; pre-stim, 300 seconds before stimulation; SD, standard deviation; stim, stimulation period; TTX, tetrodotoxin.
Inactivation of PFC Attenuated the Electroencephalographic, Physiologic, and Behavioral Arousal Induced by Bilateral Electrical Stimulation of Basal Forebrain During Sevoflurane Anesthesia
The sites of TTX microinjection into PFC and electrical stimulation sites in basal forebrain were histologically confirmed (Figure 4). Basal forebrain stimulation during sevoflurane anesthesia, in the presence of TTX in PFC, attenuated electroencephalographic, physiologic, and behavioral arousal as was observed in session 1 (without TTX; Figure 5A, F–H; Supplemental Digital Content 3, Video 2, http://links.lww.com/AA/D919). The arousal score was significantly reduced (mean ± SD, 1.3 ± 1.5; P < .001; CI, −2.98 to −1.02) compared to that observed after electrical stimulation of basal forebrain without concurrent prefrontal inactivation in session 1 (Figure 5A). There was a significant increase in respiration rate following electrical stimulation (mean ± SD, from 59 ± 7.1 to 80 ± 16 breaths/min; P < .001; CI, 11.50–31.82; Figure 5F) but no significant effect on heart rate was observed (mean ± SD, from 301 ± 26 to 324 ± 49 beats/min; P = .063; CI, −1.19 to 47.35; Figure 5G). Moreover, TTX administration decreased theta/delta ratio compared to during sevoflurane anesthesia alone in the same experiment (P = .002; CI, −0.073 to −0.018; Figure 5H). After about a week, in session 3, we repeated session 1 experiments in these rats to ensure that the lack of arousal phenotype in session 2 (ie, stimulation with TTX in PFC) was not because of any potential damage to basal forebrain during the session 1. Repeating the electrical stimulation protocol without TTX in PFC (session 3) produced behavioral arousal: 2 rats regained the righting reflex, 2 attempted righting, 2 showed coordinated body movements, 1 showed isolated body movements, and 1 showed orofacial movements and eye blinks. The arousal score (mean ± SD, 3.4 ± 1.4) was not significantly different from that observed in session 1 (P = .94; CI, −0.86 to 1.11; Figure 5A). Along with behavioral arousal, there was an increase in respiration rate (mean ± SD, from 59 ± 6.3 to 111 ± 21 breaths/min; P < .001; CI, 31.77–72.00), heart rate (mean ± SD, from 311 ± 19 to 375 ± 51 beats/min; P < .001; CI, 30.98–97.85), and theta/delta ratio (P < .001; CI, 0.087–0.25; Figure 5I–K).
Bilateral stimulation of piriform cortex failed to produce any statistical change in heart rate (P = .57; CI, −7.49 to 16.92), respiration rate (P = .53; CI, −2.48 to 5.98), or theta/delta ratio (P = .26; CI, −0.064 to 0.015; Figure 5L, M).
DISCUSSION
We demonstrate that stimulation of basal forebrain, including the specific chemogenetic excitation of cholinergic neurons, is sufficient to induce behavioral arousal in sevoflurane-anesthetized rats. Furthermore, concurrent inactivation of PFC attenuated the arousal-promoting effect of basal forebrain stimulation. The results related to stimulation of cholinergic neurons are consistent with 2 murine studies published over the last year. Luo et al18 showed that genetic lesions of basal forebrain cholinergic neurons delayed the emergence from isoflurane and propofol anesthesia, while chemogenetic stimulation of basal forebrain cholinergic neurons had an opposite effect. In a similar and more recent study, Wang et al19 demonstrated that optogenetic stimulation of cholinergic and glutamatergic neurons in basal forebrain accelerated the emergence from propofol anesthesia. Although the results from these 2 studies and our findings from the current study converge, it is important to note that, unlike facilitating passive emergence from anesthesia as was done in past studies, we were able to actively reverse the state of general anesthesia (in the continued presence of sevoflurane) and provide the most robust evidence for a direct role of basal forebrain cholinergic neurons in promoting behavioral arousal and emergence from anesthesia. Our data comparing intravenous and localized dialysis delivery of C21 suggest that administration of chemogenetic agonists directly into a brain site represents a powerful new approach to chemogenetic study design. Furthermore, we used C21, which demonstrates a lower EC-50 at hM3D(Gq) receptors (ie, is more potent), less activity at endogenous hM3 receptors, and higher brain concentrations on administration compared to clozapine-N-oxide.21–23 These considerations may underlie the marked arousal phenotype observed in our study. The systemic off-target effects reported previously24 and as observed after intravenous C21 in this study further emphasize the need for appropriate controls and suggest that none of the chemogenetic ligands (eg, clozapine-N-oxide, clozapine, and C21) offer complete target selectivity.
Studies from multiple laboratories have revealed several subcortical sites that could be manipulated to alter behavioral arousal. For example, electrical stimulation of ventral tegmental area as well as the selective activation of ventral tegmental dopaminergic neurons have been shown to facilitate passive emergence and/or active reversal of the state of general anesthesia.26,33,34 Similarly, activation of neurons in parabrachial nucleus has been shown to both facilitate passive recovery from general anesthesia35 as well as induce active emergence in the continued presence of general anesthetic.25 Despite these remarkable insights into subcortical sites, relatively few studies have attempted to map the neural circuits involving the cortex that may play a role in behavioral arousal. Our results show that the chemogenetic stimulation of basal forebrain cholinergic neurons produced behavioral arousal and simultaneously increased acetylcholine levels in PFC. There are abundant data to show a close association between cortical acetylcholine and behavioral arousal,4–10 and given that basal forebrain is the primary source of prefrontal acetylcholine,12,13 we tested the hypothesis that the PFC gates the arousal-promoting effect of basal forebrain stimulation. We show that bilateral electrical stimulation of basal forebrain during sevoflurane anesthesia was sufficient to induce behavioral arousal, and concurrent inactivation of PFC attenuated these arousal-promoting effects.
Although dialysis delivery of C21 directly into basal forebrain allowed us to circumvent any systemic effects, combining this approach with simultaneous TTX-mediated inactivation of PFC would have required multiple insertions of microdialysis probes into basal forebrain (3 times, once for each experimental session over a span of 3 weeks). The scar tissue formed due to the probe insertion can prevent efficient diffusion of C21 molecules from the probe membrane into the surrounding tissue, which can affect the response to C21, as was observed in our pilot studies (data not shown). In our study, it was critical to demonstrate that the lack of arousal phenotype after TTX-mediated inactivation of PFC was not caused by damage to neural circuitry. Therefore, we decided to use electrical stimulation, which allows repeated experimental manipulations without the need for multiple insertions of microdialysis probes and multiple drug administrations. A major drawback of electrical stimulation is that it is not specific to neuronal phenotype and can excite the passing fibers. Therefore, it is not possible to rule out that the effect of electrical stimulation in our study could have been mediated through additional circuits. However, it is highly unlikely that electrical stimulation excited the passing fibers while completely sparing the cholinergic neurons. Furthermore, given the similarity between the effects of basal forebrain electrical stimulation and chemogenetic stimulation of cholinergic neurons, it is likely that the behavioral arousal after basal forebrain electrical stimulation is at least partially mediated via local cholinergic neurons. Further studies combining cholinesterase delivery to PFC during basal forebrain stimulation may provide more definitive data on the role of cholinergic pathway from basal forebrain to PFC in behavioral arousal.
Of note, despite the clear behavioral arousal induced by chemogenetic and electrical stimulation of basal forebrain, only 2 of 13 rats in the chemogenetic cohort and 2 of 8 rats in the electrical stimulation cohort regained righting reflex. This demonstrates the stringent experimental conditions presented by continued exposure to the anesthetic and also precluded us from statistically comparing the return of righting reflex between experimental and control groups, which is a limitation in our study.
Although data from this study and previous reports18,19 provide compelling evidence that the basal forebrain is an arousal-promoting area, we cannot discount the possibility that—rather than being a source of arousal, per se—the basal forebrain may be a point of convergence for the arousal-promoting influence of other subcortical nuclei and/or that the basal forebrain may be activating other brain regions to promote behavioral arousal. For example, selective activation of orexinergic terminals in basal forebrain was shown to accelerate the emergence from isoflurane anesthesia,36 and orexin administration in basal forebrain has been shown to increase cortical acetylcholine and facilitate recovery from anesthesia.37 Similarly, disinhibition of medullary glutamatergic neurons in anterior nucleus gigantocellularis induced emergence from isoflurane anesthesia and increased the activity of several subcortical nuclei, including basal forebrain.30
In summary, we report a novel, reverse-dialysis approach to chemogenetic activation that circumvents off-target effects and that demonstrated a direct causal role for basal forebrain cholinergic neurons in behavioral arousal during general anesthesia. We also provide evidence that supports the role of the PFC as a cortical mediator of basal forebrain-regulated arousal.
ACKNOWLEDGMENTS
The authors thank Chris Andrews, PhD (Consultant, Consulting for Statistics, Computing & Analytics Research, University of Michigan) for help with statistical analysis.
DISCLOSURES
Name: Jon G. Dean, PhD.
Contribution: This author helped design the study, conduct the experiments, analyze and interpret the data, and write the manuscript.
Name: Christopher W. Fields, BS.
Contribution: This author helped conduct the experiments, analyze and interpret the data, and write the manuscript.
Name: Michael A. Brito, PhD.
Contribution: This author helped design and conduct the experiments and write the manuscript.
Name: Brian H. Silverstein, PhD.
Contribution: This author helped analyze and interpret the data, and write the manuscript.
Name: Chloe Rybicki-Kler, BS.
Contribution: This author helped conduct the experiments, analyze the data, and write the manuscript.
Name: Anna M. Fryzel, MS.
Contribution: This author helped conduct the experiments, analyze the data, and write the manuscript.
Name: Trent Groenhout, BA.
Contribution: This author helped conduct the experiments and write the manuscript.
Name: Tiecheng Liu, MD.
Contribution: This author helped conduct the experiments and write the manuscript.
Name: George A. Mashour, MD, PhD.
Contribution: This author helped design the study, analyze and interpret the data, and write the manuscript.
Name: Dinesh Pal, PhD.
Contribution: This author helped design the study, analyze and interpret the data, and write the manuscript.
This manuscript was handled by: Oluwaseun Johnson-Akeju, MD, MMSc.
Supplementary Material
FOOTNOTES
GLOSSARY
- ARRIVE
- Animal Research: Reporting of In Vivo Experiments
- B
- basal nucleus (Meynert)
- BF
- basal forebrain
- C21
- compound 21
- ChAT
- choline acetyl transferase
- ChAT-Cre
- choline acetyl transferase-Cre recombinase
- CI
- confidence interval
- EEG
- electroencephalogram
- fmi
- forceps minor of the corpus callosum
- HDB
- nucleus of the horizontal limb of the diagonal band
- IL
- infralimbic area
- MCPO
- magnocellular preoptic nucleus
- PFC
- prefrontal cortex
- Piriform
- piriform cortex
- post-stim
- 300 seconds after stimulation period
- pre-stim
- 300 seconds before stimulation
- PrL
- prelimbic cortex
- SD
- standard deviation
- SIB
- substantia innominata
- stim
- stimulation period
- TTX
- tetrodotoxin
- VP
- ventral pallidum
Funding: This study was supported by a National Institutes of Health (Bethesda, Maryland) grant (R01 GM111293) to G.A.M. and D.P., and by the Department of Anesthesiology, University of Michigan Medical School, Ann Arbor, Michigan.
The authors declare no conflicts of interest.
Supplemental digital content is available for this article. Direct URL citations appear in the printed text and are provided in the HTML and PDF versions of this article on the journal’s website (www.anesthesia-analgesia.org).
Reprints will not be available from the authors.
REFERENCES
- 1.Pal D, Dean JG, Liu T, et al. Differential role of prefrontal and parietal cortices in controlling level of consciousness. Curr Biol. 2018;28:2145–2152.e5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Parkar A, Fedrigon DC, Alam F, Vanini G, Mashour GA, Pal D. Carbachol and nicotine in prefrontal cortex have differential effects on sleep-wake states. Front Neurosci. 2020;14:567849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Huels ER, Groenhout T, Fields CW, Liu T, Mashour GA, Pal D. Inactivation of prefrontal cortex delays emergence from sevoflurane anesthesia. Front Syst Neurosci. 2021;15:690717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Celesia GG, Jasper HH. Acetylcholine released from cerebral cortex in relation to state of activation. Neurology. 1966;16:1053–1063. [DOI] [PubMed] [Google Scholar]
- 5.Marrosu F, Portas C, Mascia MS, et al. Microdialysis measurement of cortical and hippocampal acetylcholine release during sleep-wake cycle in freely moving cats. Brain Res. 1995;671:329–332. [DOI] [PubMed] [Google Scholar]
- 6.Kikuchi T, Wang Y, Sato K, Okumura F. In vivo effects of propofol on acetylcholine release from the frontal cortex, hippocampus and striatum studied by intracerebral microdialysis in freely moving rats. Br J Anaesth. 1998;80:644–648. [DOI] [PubMed] [Google Scholar]
- 7.Shichino T, Murakawa M, Adachi T, Arai T, Miyazaki Y, Mori K. Effects of inhalation anaesthetics on the release of acetylcholine in the rat cerebral cortex in vivo. Br J Anaesth. 1998;80:365–370. [DOI] [PubMed] [Google Scholar]
- 8.Lapierre JL, Kosenko PO, Lyamin OI, Kodama T, Mukhametov LM, Siegel JM. Cortical acetylcholine release is lateralized during asymmetrical slow-wave sleep in northern fur seals. J Neurosci. 2007;27:11999–12006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lydic R, Baghdoyan HA. Sleep, anesthesiology, and the neurobiology of arousal state control. Anesthesiology. 2005;103:1268–1295. [DOI] [PubMed] [Google Scholar]
- 10.Pal D, Silverstein BH, Lee H, Mashour GA. Neural correlates of wakefulness, sleep, and general anesthesia: an experimental study in rat. Anesthesiology. 2016;125:929–942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Leung LS, Chu L, Prado MAM, Prado VF. Forebrain acetylcholine modulates isoflurane and ketamine anesthesia in adult mice. Anesthesiology. 2021;134:588–606. [DOI] [PubMed] [Google Scholar]
- 12.Briand LA, Gritton H, Howe WM, Young DA, Sarter M. Modulators in concert for cognition: modulator interactions in the prefrontal cortex. Prog Neurobiol. 2007;83:69–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Henny P, Jones BE. Projections from basal forebrain to prefrontal cortex comprise cholinergic, GABAergic and glutamatergic inputs to pyramidal cells or interneurons. Eur J Neurosci. 2008;27:654–670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Zant JC, Kim T, Prokai L, et al. Cholinergic neurons in the basal forebrain promote wakefulness by actions on neighboring non-cholinergic neurons: an opto-dialysis study. J Neurosci. 2016;36:2057–2067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lee MG, Hassani OK, Alonso A, Jones BE. Cholinergic basal forebrain neurons burst with theta during waking and paradoxical sleep. J Neurosci. 2005;25:4365–4369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Han Y, Shi YF, Xi W, et al. Selective activation of cholinergic basal forebrain neurons induces immediate sleep-wake transitions. Curr Biol. 2014;24:693–698. [DOI] [PubMed] [Google Scholar]
- 17.Xu M, Chung S, Zhang S, et al. Basal forebrain circuit for sleep-wake control. Nat Neurosci. 2015;18:1641–1647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Luo TY, Cai S, Qin ZX, et al. Basal forebrain cholinergic activity modulates isoflurane and propofol anesthesia. Front Neurosci. 2020;14:559077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Wang L, Zhang W, Wu Y, et al. Cholinergic-induced specific oscillations in the medial prefrontal cortex to reverse propofol anesthesia. Front Neurosci. 2021;15:664410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Witten IB, Steinberg EE, Lee SY, et al. Recombinase-driver rat lines: tools, techniques, and optogenetic application to dopamine-mediated reinforcement. Neuron. 2011;72:721–733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Chen X, Choo H, Huang XP, et al. The first structure-activity relationship studies for designer receptors exclusively activated by designer drugs. ACS Chem Neurosci. 2015;6:476–484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Thompson KJ, Khajehali E, Bradley SJ, et al. DREADD agonist 21 is an effective agonist for muscarinic-based DREADDs in vitro and in vivo. ACS Pharmacol Transl Sci. 2018;1:61–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Jendryka M, Palchaudhuri M, Ursu D, et al. Pharmacokinetic and pharmacodynamic actions of clozapine-N-oxide, clozapine, and compound 21 in DREADD-based chemogenetics in mice. Sci Rep. 2019;9:4522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Goutaudier R, Coizet V, Carcenac C, Carnicella S. Compound 21, a two-edged sword with both DREADD-selective and off-target outcomes in rats. PLoS One. 2020;15:e0238156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Muindi F, Kenny JD, Taylor NE, et al. Electrical stimulation of the parabrachial nucleus induces reanimation from isoflurane general anesthesia. Behav Brain Res. 2016;306:20–25. [DOI] [PubMed] [Google Scholar]
- 26.Solt K, Van Dort CJ, Chemali JJ, Taylor NE, Kenny JD, Brown EN. Electrical stimulation of the ventral tegmental area induces reanimation from general anesthesia. Anesthesiology. 2014;121:311–319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Sanford LD, Yang L, Liu X, Tang X. Effects of tetrodotoxin (TTX) inactivation of the central nucleus of the amygdala (CNA) on dark period sleep and activity. Brain Res. 2006;1084:80–88. [DOI] [PubMed] [Google Scholar]
- 28.Tang X, Yang L, Liu X, Sanford LD. Influence of tetrodotoxin inactivation of the central nucleus of the amygdala on sleep and arousal. Sleep. 2005;28:923–930. [DOI] [PubMed] [Google Scholar]
- 29.Paxinos G, Watson C. The Rat Brain in Stereotaxic Coordinates, 6th ed. Academic Press; 2007. [Google Scholar]
- 30.Gao S, Proekt A, Renier N, Calderon DP, Pfaff DW. Activating an anterior nucleus gigantocellularis subpopulation triggers emergence from pharmacologically induced coma in rodents. Nat Commun. 2019;10:2897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Alkire MT, McReynolds JR, Hahn EL, Trivedi AN. Thalamic microinjection of nicotine reverses sevoflurane-induced loss of righting reflex in the rat. Anesthesiology. 2007;107:264–272. [DOI] [PubMed] [Google Scholar]
- 32.Pal D, Mallick BN. GABA in pedunculopontine tegmentum increases rapid eye movement sleep in freely moving rats: possible role of GABA-ergic inputs from substantia nigra pars reticulata. Neuroscience. 2009;164:404–414. [DOI] [PubMed] [Google Scholar]
- 33.Gui H, Liu C, He H, Zhang J, Chen H, Zhang Y. Dopaminergic projections from the ventral tegmental area to the nucleus accumbens modulate sevoflurane anesthesia in mice. Front Cell Neurosci. 2021;15:671473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Taylor NE, Chemali JJ, Brown EN, Solt K. Activation of D1 dopamine receptors induces emergence from isoflurane general anesthesia. Anesthesiology. 2013;118:30–39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Luo T, Yu S, Cai S, et al. Parabrachial neurons promote behavior and electroencephalographic arousal from general anesthesia. Front Mol Neurosci. 2018;11:420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Wang D, Guo Y, Li H, et al. Selective optogenetic activation of orexinergic terminals in the basal forebrain and locus coeruleus promotes emergence from isoflurane anaesthesia in rats. Br J Anaesth. 2021;126:279–292. [DOI] [PubMed] [Google Scholar]
- 37.Zhang LN, Yang C, Ouyang PR, et al. Orexin-A facilitates emergence of the rat from isoflurane anesthesia via mediation of the basal forebrain. Neuropeptides. 2016;58:7–14. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.