Abstract
Enterocin I (ENTI) is a novel bacteriocin produced by Enterococcus faecium 6T1a, a strain originally isolated from a Spanish-style green olive fermentation. The bacteriocin is active against many olive spoilage and food-borne gram-positive pathogenic bacteria, including clostridia, propionibacteria, and Listeria monocytogenes. ENTI was purified to homogeneity by ammonium sulfate precipitation, binding to an SP-Sepharose fast-flow column, and phenyl-Sepharose CL-4B and C2/C18 reverse-phase chromatography. The purification procedure resulted in a final yield of 954% and a 170,000-fold increase in specific activity. The primary structure of ENTI was determined by amino acid and nucleotide sequencing. ENTI consists of 44 amino acids and does not show significant sequence similarity with any other previously described bacteriocin. Sequencing of the entI structural gene, which is located on the 23-kb plasmid pEF1 of E. faecium 6T1a, revealed the absence of a leader peptide at the N-terminal region of the gene product. A second open reading frame, ORF2, located downstream of entI, encodes a putative protein that is 72.7% identical to ENTI. entI and ORF2 appear to be cotranscribed, yielding an mRNA of ca. 0.35 kb. A gene encoding immunity to ENTI was not identified. However, curing experiments demonstrated that both enterocin production and immunity are conferred by pEF1.
Bacteriocins are bacterial proteins or peptides that inhibit strains and species that are usually, but not always, closely related to the producing bacteria (47). In recent years, several bacteriocins from gram-positive bacteria, in particular the lactic acid bacteria (LAB), have been identified and characterized (24, 30, 36). Some of these bacteriocins display fairly broad inhibitory spectra and have potential as food preservatives (13, 22, 24). Apart from the lantibiotic nisin, the most promising are those belonging to the pediocin family of bacteriocins (36). These bacteriocins are active against a broad spectrum of food spoilage and food-borne gram-positive pathogenic bacteria, including Listeria monocytogenes. Pediocin-like bacteriocins have been identified for various genera of LAB, including Carnobacterium (carnobacteriocins BM1 and B2 [42]), Lactobacillus (sakacins A [21] and P [48]), Leuconostoc (leucocin A [17], and mesentericin Y105 [19]), and Pediococcus (pediocin PA-1 [20]) and, more recently, in Enterococcus faecium (enterocins A [4], B [8], and P [10]). The bacteriocins produced by Enterococcus species (the enterocins) show considerable diversity. Based on their amino acid sequence similarity and their inhibitory spectra, most of these bacteriocins have been included in the pediocin-like group (36). According to the Klaenhammer classification, this group belongs to the class II bacteriocins, the small heat-stable nonlantibiotic bacteriocins (30). Although there are also lantibiotic enterocins that belong to the class I bacteriocins (7) and cyclic enterocins (45), they all have in common their pediocin-like broad spectrum of activity. Consequently, the enterocins have become attractive in recent years as natural additives for food preservation and safety (13).
All bacteriocins known to date are synthetized as prepeptides with an N-terminal leader sequence that directs their export outside the cell and that is removed before the active bacteriocin can be detected (36). Bacteriocins can be secreted by an ATP-binding cassette transport system (18) or by the general secretory pathway (10, 41, 49, 52). Bacteriocins that use the first system have a leader peptide that contains a conserved double-glycine motif that serves as a signal for processing and secretion (50). The leader peptide for the general secretory pathway is usually positively charged and has a hydrophobic core and a cleavage region (16, 23, 51). This peptide is processed by a signal peptidase during translocation across the cytoplasmic membrane (41, 51).
The organization of the genes for bacteriocin production and immunity is generally highly conserved, with the bacteriocin structural gene followed by a cotranscribed open reading frame (ORF) that encodes a putative immunity protein (30, 36). However, there are exceptions: the structural genes for carnobacteriocin A (52) and enterocin B (8) are followed by a putative rho-independent terminator with no recognizable ORF downstream.
In this paper, we describe the identification, purification, and genetic characterization of a new plasmid-carried enterocin, enterocin I (ENTI). ENTI has the same inhibitory spectrum as the pediocin-like enterocins but does not show significant sequence similarity with these bacteriocins. Other unusual features of ENTI are also described.
MATERIALS AND METHODS
Bacterial strains and media.
The ENTI producer E. faecium 6T1a was isolated from a Spanish-style green olive fermentation. It was maintained as a frozen stock at −20°C in distilled water plus 20% (vol/vol) glycerol and propagated twice in MRS broth (Oxoid, Basingstoke, Hampshire, England) at 30°C before use.
The bacterial strains used as indicator organisms for the evaluation of ENTI activity are listed in Table 1. All LAB used, as well as the Enterococcus faecalis strains, were propagated in MRS broth at 30°C. Listeria and Bacillus strains were grown in brain heart infusion (Oxoid) at 30 and 37°C, respectively. Clostridia were cultivated anaerobically in RCM medium (Difco Laboratories, Detroit, Mich.) at 37°C. Propionibacterium strains were propagated anaerobically in YGL medium (6) at 37°C. Gram-negative bacteria were propagated in tryptone soya broth (Oxoid) at 37°C. Escherichia coli DH5α (3) was used for all genetic manipulations.
TABLE 1.
Inhibitory spectrum of ENTI from E. faecium 6T1a for gram-positive bacteria
| Indicator species | Strain | Sourcea | Diam of inhibition (mm) |
|---|---|---|---|
| Bacillus cereus | 9139 | ATCC | 8 |
| B. subtilis | BD630 | TNO | 11.5 |
| B. subtilis | 1A510 | BGSC | 10 |
| Clostridium sporogenes | C 22/10 | TNO | 12 |
| Enterococcus faecalis | 1353 | NCDO | 12 |
| E. faecalis | BM 4100 WT | TNO | 13 |
| E. faecalis | 610 | NCDO | 16 |
| E. faecalis | 19433 | ATCC | 16 |
| Lactobacillus fermentum | 9338 | ATCC | 11 |
| L. fermentum | 14933 | ATCC | 16 |
| L. plantarum | 128/2 | Our strain collection | 19 |
| L. plantarum | 1/43/1 | Our strain collection | 17 |
| L. plantarum | LPS-5 | Our strain collection | 19 |
| L. plantarum | LPS-10 | Our strain collection | 14 |
| Lactococcus lactis | MG 1363 | TNO | 10 |
| L. Lactis | MG 1614 | TNO | 14 |
| L. Lactis | CNRZ150 | INRA | 20 |
| L. Lactis | CNRZ117 | INRA | 9 |
| L. Lactis | CNRZ148 | INRA | 15 |
| Leuconostoc mesenteroides | 808 | NCDO | 0 |
| L. mesenteroides | CNRZ8015 | INRA | 0 |
| Listeria innocua | BL86/26 | TNO | 9 |
| L. monocytogenes | 7973 | NCTC | 13 |
| L. monocytogenes | L15sv 1/2 | FVM | 0 |
| L. monocytogenes | 5105 | NCTC | 14.3 |
| L. monocytogenes | LI1sv 4 | FVM | 13 |
| L. monocytogenes | Scott A | FVM | 14.3 |
| Pediococcus pentosaceus | FBB63 | TNO | 13 |
| P. Pentosaceus | PC1 | TNO | 9 |
| Propionibacterium acidi-propionici | 563 | NCDO | 0 |
| Propionibacterium spp. | P6 | Our strain collection | 9 |
Abbreviations: ATCC, American Type Culture Collection (Rockville, Md.); BGSC, Bacillus Genetic Stock Center (Ohio State University, Columbus); FVM, Facultad de Veterinaria, Universidad Complutense (Madrid, Spain); INRA, Station de Recherches Laitières (Jouy-en-Josas, France); NCDO, National Collection of Dairy Organisms (Reading, United Kingdom); NCTC, National Collection of Type Cultures, Central Public Health Laboratory (London, United Kingdom); TNO, Nutrition and Food Research (Zeist, The Netherlands).
Bacterial characterization.
E. faecium 6T1a was examined by phase-contrast microscopy for cell morphology determination and by Gram staining. The strain was identified as E. faecium 6T1a by use of the Gram-Positive Identification Card (BioMérieux Vitek, Inc., Hazelwood, Mo.) in conjunction with the Vitek System (BioMérieux) for automated identification and by sodium dodecyl sulfate (SDS)-polyacrylamide gel electrophoresis (PAGE) protein pattern analysis.
The Gram-Positive Identification Card included tests for growth on and resistance to bacitracin, optochin (ethylhydrocupreine hydrochloride), and novobiocin (ethylhydrocupreine hydrochloride), tolerance of bile (10 and 40%) and NaCl (6%); reduction of tetrazolium red; esculin hydrolysis; arginine hydrolase, catalase, urease, beta-hemolytic, and coagulase activities; and fermentation of dextrose, lactose, mannitol, raffinose, salicin, sorbitol, sucrose, trehalose, arabinose, hemicellulose, pyruvic acid, pullulan, inulin, melibiose, melezitose, cellobiose, ribose, and xylose.
Preparation of cell extracts and SDS-PAGE were carried out by the Research Group of the Laboratory for Microbiology (Ghent University) (39). The normalized and digitized protein patterns were numerically analyzed and clustered with the reference profiles in the LAB database (29, 40).
Bacteriocin assays.
The bacteriocin producer 6T1a was grown in MRS broth at 30°C. The supernatant from late-log-phase cultures was adjusted to pH 7.0 with 5 M NaOH and filter sterilized through a 0.22-μm-pore-size Millex-GV filter (Millipore SA, Molsheim, France). The antimicrobial activity of the supernatant was determined by the well diffusion method (47). Fifty microliters of the supernatant was placed in wells (6 mm in diameter) cut in MRS, brain heart infusion, RCM, or YGL agar plates (25 ml) seeded (ca. 105 CFU/ml) with the indicator microorganisms listed in Table 1. The plates were kept for 2 to 4 h at 4°C to allow diffusion of the supernatants and then were incubated at 30 or 37°C for 18 h; the diameters of the zones of growth inhibition were then measured.
During purification, ENTI activity was quantified with a microtiter plate assay system (14). E. faecium 20, a non-enterocin-producing, enterocin-sensitive E. faecium 6T1a derivative obtained as described below, was used as the indicator strain. Each well of the microtiter plate contained 25 μl of twofold-concentrated MRS broth, 25 μl of ENTI fractions at serial two- or threefold dilutions, and 10 μl of the indicator strain (A600, 0.01 [ca. 106 CFU/ml]). As a turbidity control, E. faecium 20 was incubated as described above but with sterile distilled water in place of the ENTI fractions. The microtiter plate cultures were incubated for 7 h at 37°C, after which growth inhibition of the indicator strain was measured spectrophotometrically at 600 nm with a microplate reader (model 450; Bio-Rad Laboratories, Hercules, Calif.). One ENTI unit (ENTIU) was arbitrarily defined as the amount of ENTI that inhibited the growth of the indicator strain by 50% (50% of the turbidity of the control culture without bacteriocin). This amount was expressed as the reciprocal of the highest dilution exhibiting 50% inhibition of the indicator strain per milliliter (ENTIU per milliliter). The results obtained with the two- and threefold dilution series for every sample were averaged. This method was also used to study the stability of the bacteriocin in the presence of heat and enzymes.
Sensitivity of ENTI to heat and enzymes.
Cell-free, filter-sterilized, log-phase E. faecium 6T1a MRS culture supernatants were neutralized with 5 M NaOH and treated with solid ammonium sulfate (80% saturation at 0°C). The mixture was stirred for 2 h at 4°C and centrifuged at 20,000 × g for 30 min at 4°C. The precipitate was resuspended in citrate-phosphate buffer (50 mM, pH 5.0) and then desalted through PD10 gel filtration columns (Pharmacia Biotech, Uppsala, Sweden) equilibrated with the same buffer.
To test for heat sensitivity, samples containing 20,000 ENTIU/ml were heated to 100°C for 5 min or autoclaved (121°C, 1 atm) for 1, 5, 10, and 20 min, and the remaining activity was determined with the microtiter plate assay with Lactobacillus plantarum 128/2 as the indicator strain (25).
To test for enzyme sensitivity, samples containing 3,200 ENTIU/ml were treated with trypsin, pronase E, α-chymotrypsin, thermolysin, subtilopeptidase A, proteinase K, lysozyme, RNase A, α-amylase, papain, lipase, or ficin at a final concentration of 0.1 mg/ml. Buffers used were those recommended by the supplier (Sigma Chemical Co., St. Louis, Mo.). Samples were incubated at 37°C for 1 h, and the residual activity was determined with the microtiter plate assay. To exclude potential inhibition by hydrogen peroxide, a sample was treated with catalase (Sigma) at a final concentration of 100 U/ml (37). It was maintained at 25°C for 35 min, and its ENTI activity was then determined.
Bacteriocin purification.
All the purification steps were carried out at room temperature, and all of the chromatographic equipment and media were purchased from Pharmacia Biotech. The bacteriocin was purified from a 1-liter MRS broth culture of E. faecium 6T1a by the same method as that described for the bacteriocin plantaricin S (26); fractions showing activity after the C2/C18 reverse-phase column step were pooled and subjected to a second run. ENTI activity was eluted with 3 ml of 30% 2-propanol containing 0.1% trifluoroacetic acid, and the samples were stored at −80°C.
SDS-PAGE.
C2/C18 reverse-phase column-purified ENTI was analyzed by SDS-PAGE (46) with an 18.5% acrylamide resolving gel. A molecular weight marker (range, 2,512 to 16,946) kit (Pharmacia Biotech) was used for size standards. After electrophoresis, the gel was divided in two; one part was silver stained (34), and the other was used for the detection of antimicrobial activity (6) with L. plantarum 128/2 as the indicator strain.
N-terminal amino acid sequencing.
Amino acid sequencing was performed by automated Edman degradation with a Beckman LF3000 sequencer/phenylthiohydantoin amino acid analyzer (System Gold) by F. Canals, Institut de Biologia Fonamental “Vicent Villar Palasí,” University of Barcelona, Barcelona, Spain.
Plasmid profiles and curing of plasmids from E. faecium 6T1a.
The protocol of Anderson and McKay (2) for isolating large-plasmid DNA from lactic streptococci was followed. L. plantarum LPCO10 was used as a source of plasmid markers (44).
To test if ENTI production and immunity were plasmid determined, novobiocin (0.125 to 0.5 μg/ml) and ethidium bromide (10 to 50 μg/ml) were used to treat MRS broth cultures of E. faecium 6T1a as described previously (44). Cultures were plated on MRS agar plates to yield individual colonies. After 18 h at 30°C, MRS soft agar (0.75% agar) containing the indicator strain L. plantarum 128/2 (final concentration, ca. 105 CFU/ml) was poured onto the plates, which were incubated for an additional 24 h. Colonies without clear zones of inhibition were purified on MRS agar and repeatedly transferred into MRS broth, and their ability to inhibit the growth of L. plantarum 128/2 was determined. As controls, MRS broth cultures of E. faecium 6T1a that had not been treated with novobiocin or ethidium bromide were processed at the same time. The immunity of the nonproducing variants to ENTI was examined by spotting active E. faecium 6T1a MRS culture supernatants on lawns of these derivatives. Plasmid DNA was isolated from both producing and nonproducing variants of E. faecium and analyzed.
Other DNA manipulations.
Total DNA was prepared from E. faecium as described previously (9), and plasmid purification was done by CsCl gradient centrifugation. Isolation of E. coli plasmid DNA and subsequent nucleic acid manipulations were done as described by Maniatis et al. (32).
Cloning and sequencing of ENTI.
From the amino acid sequence of ENTI, the degenerate primer ent1 (5′-GGNGAYCCNATHGTNAARAAR-3′) was designed and synthesized (Pharmacia Biotech). This oligonucleotide was 3′ end labelled with fluorescein-11-dUTP by use of a 3′-end-labelling ECL kit (Amersham Life Science, Little Chalfont, Buckinghamshire, United Kingdom) in accordance with the manufacturer’s instructions and was used as a probe in Southern blot analysis of CsCl-purified, HindIII-digested plasmid pEF1. Hybridization and detection conditions were in accordance with the manufacturer’s instructions. Nucleotide sequencing was performed by the MediGene Sequencing Service (Martinsried, Germany) with standard primers and primers designed from the deduced sequence (see Fig. 4). Analysis of ORFs and amino acid alignments were performed with programs in the Sequence Analysis Software Package (version 9.0) licensed from the Genetics Computer Group, University of Wisconsin, Madison (12).
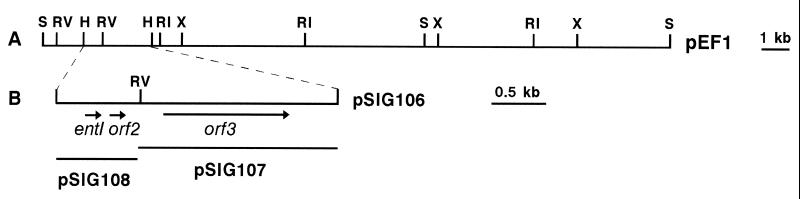
FIG. 4.
Restriction map of pEF1 (A) and pSIG subclones (B) containing the entI locus. Restriction sites: H, HindIII; RI, EcoRI; RV, EcoRV; S, SalI; X, XbaI.
RNA isolation and Northern blotting.
Total RNA was isolated from E. faecium 6T1a cultures grown at 30°C in MRS broth at different phases of growth by the method of Anba et al. (1). Northern blot analysis was done as described by Ausubel et al. (3) with 40 μg of RNA from each sample. The RNA was blotted onto nylon membranes (Pharmacia Biotech), which were stained with methylene blue (0.02% [wt/vol] in sodium acetate [0.5 M, pH 5.2]) to check the amount of RNA transferred. RNA molecular weight marker I (Boehringer GmbH, Mannheim, Germany) was used to provide size standards. The 0.7-kb EcoRV-HindIII fragment contained in plasmid pSIG108 (see Fig. 4B) was used as a probe after being labelled with [α-32P]CTP by use of a Ready-to-Go labelling kit (Pharmacia Biotech). Hybridization was carried out at 55°C for 16 h.
Nucleotide sequence accession number.
The nucleotide sequence presented in this article has EMBL accession no. Y16413.
RESULTS
Identification of bacteriocin-producing strain 6T1a.
The isolated bacteriocinogenic strain was a gram-positive, catalase-negative coccus which fermented glucose but did not produce gas. The strain was also able to ferment l-arabinose, cellobiose, mannitol, melibiose, ribose, sucrose, and trehalose but did not ferment inulin, lactose, melezitose, pullulan, d-raffinose, sorbitol, or xylose. Furthermore, it was able to grow in the presence of 6% NaCl; was resistant to bacitracin, bile, esculin, and optochin; produced ammonia from the hydrolysis of arginine; and could not reduce triphenyltetrazolium chloride. The strain did not show urease activity, and it was not betahemolytic. All of these characteristics, together with the SDS-PAGE protein pattern analysis (29, 39, 40), identified strain 6T1a as an E. faecium isolate.
Antimicrobial spectrum of ENTI.
The antimicrobial activity of ENTI, determined by the agar diffusion assay, is summarized in Table 1. The E. faecium 6T1a inhibitory activity was directed against the natural flora present in olive fermentations, including Lactobacillus spp., Lactococcus lactis, and Pediococcus pentosaceus strains. Among gram-positive olive spoilage organisms, the compound of interest showed activity against Propionibacterium spp., Clostridium spp., and Bacillus strains. E. faecalis, a frequent contaminant in olive fermentations, was also sensitive to the bacteriocin. The bacteriocin also inhibited Listeria innocula and four of five L. monocytogenes strains tested. Gram-negative bacteria (E. coli, Klebsiella spp., and Pseudomonas spp.) were not inhibited by ENTI (data not shown).
Sensitivity of ENTI to heat and enzymes.
ENTI was completely stable in the presence of heating at 100°C for 5 min but was partially inactivated by autoclaving. Thus, after 1 min of treatment, the activity was reduced from 20,000 to 5,000 ENTIU/ml, and prolonged autoclaving (5, 10, and 20 min) resulted in 2,500 ENTIU of residual activity per ml.
The inhibitory activity of ENTI was completely abolished after treatment with α-chymotrypsin, pronase E, proteinase K, subtilopeptidase A, thermolysin, and trypsin, thus suggesting a proteinaceous nature for the inhibitory compound. Other enzymes, such as α-amylase, catalase, ficin, lysozyme, and RNase A, did not affect the activity of ENTI.
Purification of ENTI.
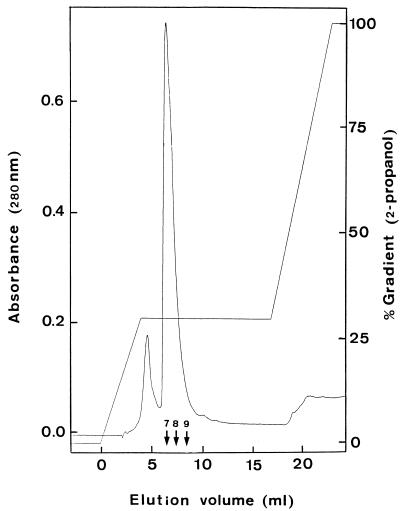
Maximum inhibitory activity in the growth medium was observed during the early stationary phase of growth (data not shown). The purification scheme for ENTI is shown in Table 2. After the second reverse-phase chromatographic step, a final yield of 954% the initial activity and a 170,000-fold increase in the specific activity of ENTI was obtained. The overall purification procedure resulted in a single peak upon C2/C18 reverse-phase liquid chromatography (Fig. 1). SDS-PAGE analysis showed an electrophoretically pure protein with an apparent molecular size of ca. 5 kDa and with inhibitory activity against L. plantarum 128/2 (Fig. 2).
TABLE 2.
Purification of ENTI from E. faecium 6T1a
| Fraction | Vol (ml) | Total A280a | Total activity (107 BU)b | Sp actc | Increase in sp act | Yield (%) |
|---|---|---|---|---|---|---|
| Culture supernatant | 1,000 | 33,273 | 0.22 | 67.3 | 1 | 100 |
| Ammonium sulfate precipitation (fraction I) | 67 | 2,640 | 1.10 | 4.2 × 103 | 62.4 | 500 |
| Binding to SP-Sepharose fast flow (fraction II) | 50 | 11.80 | 26.20 | 2.2 × 107 | 3.3 × 105 | 1.2 × 104 |
| Binding to phenyl-Sepharose CL-4B (fraction III) | 37 | 7.60 | 0.15 | 2.0 × 105 | 3.0 × 103 | 68.2 |
| FPLCd (C2/C18 reverse-phase chromatography) | ||||||
| First run | 12 | 2.60 | 0.20 | 7.7 × 105 | 1.1 × 104 | 91.0 |
| Second run | 3 | 1.84 | 2.10 | 1.1 × 107 | 1.7 × 105 | 954 |
Total A280 is the A280 multiplied by the volume in milliliters.
BU, bacteriocin units.
Specific activity is BU divided by the A280.
FPLC, fast-performance liquid chromatography.
FIG. 1.
C2/C18 reverse-phase chromatography of ENTI (fraction V). Numbers above the arrows indicate the fractions (1 ml each) exhibiting ENTI activity. Maximum bacteriocin activity was detected in fraction 8.
FIG. 2.
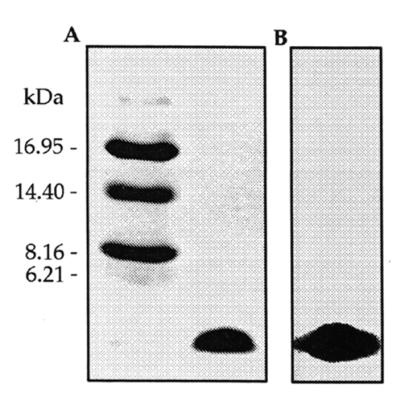
SDS-PAGE of ENTI and detection of antimicrobial activity. (A) Silver-stained gel. Left lane, size standards (sizes indicated on the left); right lane, purified ENTI sample. (B) Gel fixed in 20% isopropanol–10% acetic acid and washed in deionized water as described by Bhunia et al. (6). The gel was then placed on an MRS agar plate and overlaid with MRS soft agar containing L. plantarum 128/2.
N-terminal sequencing of purified ENTI allowed determination of the first 40 amino acid residues (see Fig. 5), which revealed a highly hydrophobic protein, as expected.
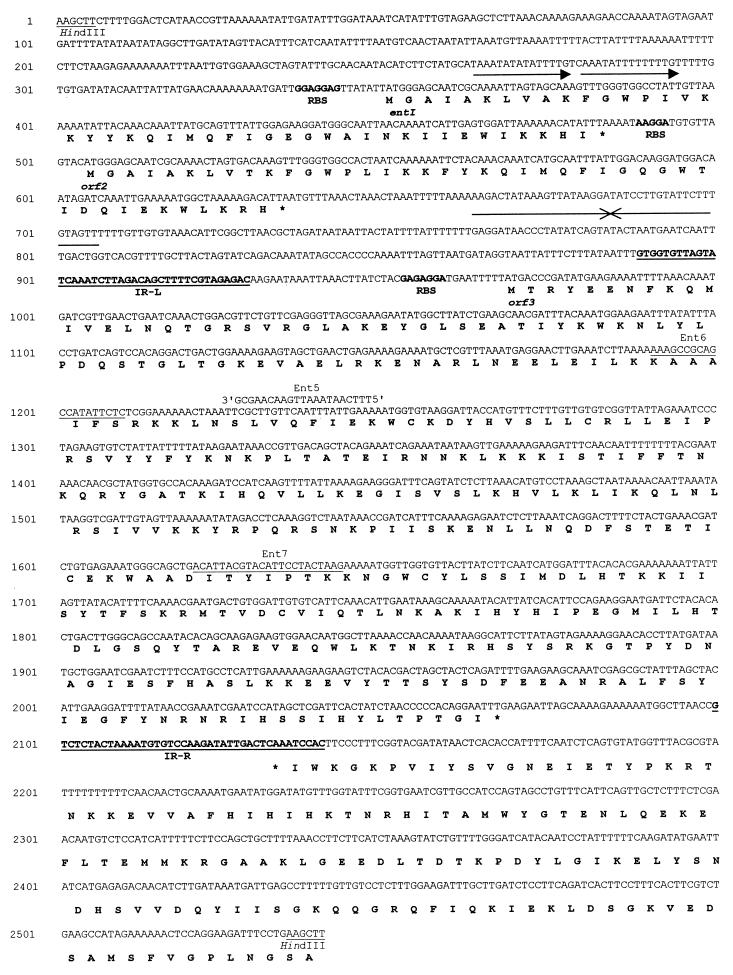
FIG. 5.
Nucleotide sequence of the entI locus and deduced protein sequences. Ribosome binding sites (RBS) are indicated in boldface letters. Direct repeats upstream of entI are indicated by arrows. Sequences that have dyad symmetry and might serve as transcription terminators are indicated by facing arrows downstream of orf2. Inverted repeats upstream and downstream of ORF3 that could serve as the right (IR-R) and left (IR-L) arms for an IS-like element are underlined and shown in boldface letters. Oligonucleotides used for sequencing are shown. Oligonucleotides Ent6 and Ent7 used for sequencing are underlined. Other oligonucleotides (e.g., Ent5) are shown.
Plasmid-curing experiments.
After treatment with novobiocin or ethidium bromide, 2,637 or 683 colonies, respectively, of strain 6T1a were tested for ENTI production on MRS agar. Totals of 21.5 and 2.5% of the colonies from the novobiocin- and ethidium bromide-treated cultures, respectively, failed to induce clear zones of inhibition of lawns of L. plantarum 128/2. A total of 1,583 isolated colonies of 6T1a that had not been treated with a plasmid-curing agent were also tested for ENTI production; 15 of these (0.95%) lost inhibitory activity. When the suspected non-ENTI producers were purified on MRS agar and repeatedly subcultured in MRS broth, none of them regained the ability to produce ENTI. All of these ENTI-deficient derivatives were also sensitive to ENTI.
Plasmid profile analysis of the non-ENTI-producing derivatives isolated after novobiocin or ethidium bromide treatment, as well as those appearing spontaneously, showed that the 23-kb plasmid pEF1 harbored by parental strain 6T1a had been lost in all cases (Fig. 3).
FIG. 3.
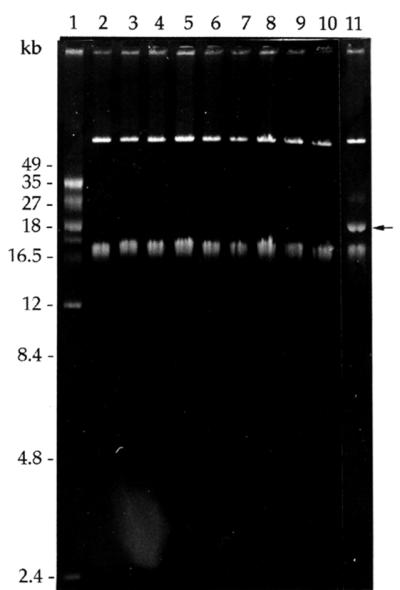
Agarose gel electrophoresis of plasmid DNAs from novobiocin- and ethidium bromide-treated variants of E. faecium 6T1a. Lane 1, plasmid profile from L. plantarum LPCO10 used as a size marker (sizes indicated on the left); lanes 2 to 10, non-ENTI-producing, ENTI-sensitive variants from E. faecium 6T1a lacking the 23-kb plasmid pEF1; lane 11, E. faecium 6T1a (the pEF1 plasmid is indicated by an arrow).
Genetic analysis and DNA sequencing of entI.
Southern analysis of restriction fragments of pEF1 with the degenerate oligonucleotide ent1 confirmed that the entI structural gene was located on pEF1 (data not shown). A 2.5-kb HindIII restriction fragment from pEF1 that hybridized to ent1 was identified, purified from an agarose gel, and ligated with HindIII-cleaved pUC18 to give the recombinant plasmid pSIG106 (Fig. 4). By use of an internal EcoRV restriction site in the 2.5-kb HindIII fragment, 1.8-kb HindIII-EcoRV and 0.7-kb EcoRV-HindIII fragments were cloned separately into pBluescript II SK(+), giving the recombinant plasmids pSIG107 and pSIG108, respectively (Fig. 4).
Analysis of the sequences of the inserts revealed several ORFs preceded by putative ribosome binding sites (Fig. 5). Two direct repeats of 15 nucleotides separated by 1 nucleotide, with the consensus sequence 5′-AAATATxTxTTTTGT-3′, are present 55 nucleotides upstream of the first ORF, named entI, which codes for a protein identical to ENTI. The protein encoded by entI has a molecular weight of 5,190 and a theoretical pI of 10.82. The N-terminal sequence of the protein deduced from the nucleotide sequence revealed the absence of any kind of leader peptide. A second ORF, ORF2, which encodes a putative protein that is very similar to ENTI (72.7% identity), is located 19 bp downstream of entI. ORF2 encodes a 5,178-Da protein with a theoretical pI of 11.0. An inverted repeat which may function as a rho-independent transcription terminator was found 27 nucleotides downstream of ORF2.
A 1,254-bp insertion sequence (IS)-like element lies downstream of ORF2 (Fig. 5). Two 41-bp imperfect inverted repeats flank a DNA sequence containing an ORF (ORF3). ORF3 encodes a protein of 366 amino acids that shows 36% identity to the transposase of the lactococcal insertion element IS904 (43). Identities of 30 to 36% were found with other known transposases (reference 31 and data not shown). The number of copies of the IS-like element in the genome of E. faecium 6T1a was then determined. Total DNA from strain 6T1a was digested with several restriction enzymes, and the resulting fragments were analyzed by Southern hybridization with oligonucleotide ent7 as a probe (Fig. 5). The results revealed that only one copy of the putative IS-like element was present in 6T1a and that it was located on pEF1 (data not shown). Finally, an incomplete ORF (ORF5) was identified downstream of the IS-like element; this ORF is transcribed in the direction opposite that of the putative transposase. The putative protein encoded by ORF5 is homologous to proteins involved in the control of plasmid partitioning (protein STBA) (15).
Transcription analysis of the sequenced ORFs.
Northern analysis performed on total RNA isolated from E. faecium 6T1a at different times of culturing in MRS broth at 30°C showed a unique mRNA of ca. 0.35 kb (Fig. 6). This transcript is long enough to encode both entI and ORF2. The level of this mRNA reached a maximum at the end of logarithmic growth (32,000 ENTIU/ml) and then declined during the stationary phase, although the level of ENTI activity remained constant (16,000 ENTIU/ml).
FIG. 6.
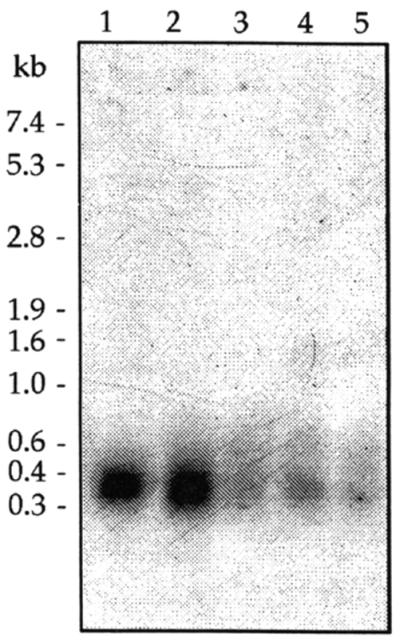
Northern blot analysis of the entI operon. The purified DNA fragment that was cloned in pSIG108 and that contained both entI and orf2 was used as a probe. RNA molecular weight markers are indicated on the left. Forty micrograms of RNA was loaded in each lane. Lane 1, log phase of growth; lane 2, transition from log to stationary phase; lane 3, early stationary phase; lanes 4 and 5, late stationary phase (25 and 40 h of incubation, respectively).
DISCUSSION
In this paper, we have described the purification and genetic characterization of a new plasmid-carried bacteriocin produced by E. faecium 6T1a, which was isolated from a Spanish-style green olive fermentation. To our knowledge, this is the first time that a bacteriocinogenic member of the genus Enterococcus has been isolated from such a fermentation. Almost nothing is known about the role of this genus in fermentation, but the spectrum of inhibitory activity of ENTI suggests a potentially useful means for controlling the growth of spoilage microorganisms that are often found in olive brines.
ENTI exhibits a broad inhibitory spectrum that includes most of the gram-positive bacteria that constitute the natural flora present in olive fermentations. Like other enterococcal bacteriocins and other members of the pediocin-like family of bacteriocins (4, 7, 8, 10, 30, 36, 45), ENTI strongly inhibited several L. monocytogenes strains. In addition, ENTI also inhibited several L. monocytogenes strains tested, including the nisin-producing strains CNRZ148 and CNRZ150, whereas other enterococcal bacteriocins are not active against lactococci.
Purification of ENTI was accomplished with the protocol described for plantaricin S (26), consistent with the conserved biochemical characteristics of many bacteriocins; e.g., they are generally small, cationic, and highly hydrophobic. As has been noted for other bacteriocins (4, 5, 10, 26, 27, 35, 38), a marked increase in specific activity occurred after some of the purification steps. This finding presumably reflects the presence in culture supernatants of inhibitory compounds that are removed during ENTI purification and/or the dissociation of high-molecular-weight ENTI aggregates into their smaller, more active forms.
Amino acid sequence comparisons indicated that ENTI is very different from other enterococcal and pediocin-like bacteriocins (4, 7, 8, 10, 17, 19, 20, 21, 42, 45, 48, 52). Thus, ENTI does not contain the highly conserved YGNGVxC motif found in the N-terminal part of most of these bacteriocins (enterocin B [8] and carnobacteriocin A [52] are also exceptions). ENTI also lacks cysteine residues, which are always present in the pediocin-like bacteriocins, including the enterocins. Cys content has been related to the antibacterial efficiency of bacteriocins (24); those with two or more cysteines capable of forming disulfide bridges have a wide inhibitory spectrum, while those with no cysteines have a narrow inhibitory spectrum. Within the enterocins, enterocin 4, bacteriocin AS-48 (28, 33, 45), and ENTI are the only bacteriocins that do not contain cysteine but show a broad inhibitory spectrum. Together with the absence of significant amino acid sequence similarity to other bacteriocins, this finding suggests that the mechanism of action of ENTI may be different from that proposed for the pediocin-like family.
Cloning and sequencing of the structural gene entI revealed some unusual features about the enterocin and its genetic organization. Whereas all bacteriocins described thus far have a leader peptide, the N terminus of ENTI deduced from the nucleotide sequence was identical to that obtained from amino acid sequencing, indicating the absence of a leader sequence. Such leader peptides are believed to be signals for export and processing, through the use of either an ATB-binding cassette-type or a sec-dependent transport system (30, 36). Thus, the mechanism by which ENTI is transported outside the cell is unknown and is likely to be novel.
Production of and immunity to ENTI are plasmid associated, as the loss of plasmid pEF1 led to phenotypes of non-ENTI production and ENTI sensitivity. Unusual also was that no recognizable immunity gene was found downstream of entI; instead, ORF2, which encodes an ENTI homolog, was found. Perhaps the protein encoded by ORF2 functions as an immunity protein. Based on its homology to ENTI, this protein may bind to the putative receptors for ENTI which would be present on the surface of 6T1a cells, thus preventing ENTI binding and providing immunity to ENTI. Further experiments are in progress to identify the protein(s) that confers immunity to ENTI.
The nucleotide sequence downstream of the entI locus appeared to contain an IS-like element. Many IS elements have been described for LAB, with most strains carrying multiple copies of at least two (11). It is interesting to note that nisin production is associated with a 68-kb transposon that is flanked by two IS904 elements (43). In E. faecium 6T1a, the entI locus does not seem to be associated with a transposon, since the IS-like sequence was present only once in pEF1. Whether the IS-like element is active or not is unknown. The absence of direct repeats flanking the IS-like element suggested that these flanking regions may have undergone secondary mutations following insertion.
The work presented here increases our knowledge about the bacteriocins made by LAB and suggests that ENTI represents a novel class of bacteriocins. This conclusion is based on the absence of any signal peptide, which could indicate that its secretion mechanism is different from any other previously described secretion systems for the known bacteriocins. This fact may prove advantageous for the heterologous expression of ENTI in other bacteria. Another attractive feature of ENTI is its plasmid-carried nature. Thus, pEF1 could be used as a cloning vector in E. faecium to produce not only ENTI but also other broad-spectrum bacteriocins to overcome bacterial resistance to many bacteriocins. With these facts and ideas this in mind, studies to define the minimal replicon of pEF1 in E. faecium 6T1a are in progress. Finally, the IS-like element found in pEF1 could be a potential tool for mutagenesis in E. faecium 6T1a.
ACKNOWLEDGMENTS
We are grateful to Antonio Márquez Cabeza of the Biochemistry Department of the Chemistry Faculty, University of Seville, for allowing us to use the radioactive laboratory at that facility. We are also grateful to M. J. Bibb of the John Innes Institute, Norwich, United Kingdom, for critical reading of the manuscript.
This work was supported by CICYT project ALI97-0658-CO3-01. B.F. is the recipient of a contract from the Spanish Ministry of Education and Culture.
ADDENDUM
While the manuscript was under review, a paper dealing with the same topic by Cintas et al. was published (10a); our results are basically in agreement, except for ORF2, which was described by them as encoding enterocin L50B.
REFERENCES
- 1.Anba J, Bidnenko E, Hillier A, Ehrlich D, Chopin M C. Characterization of the lactococcal abiD1 gene coding for phage abortive infection. J Bacteriol. 1995;177:3818–3823. doi: 10.1128/jb.177.13.3818-3823.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Anderson D G, McKay L L. Simple and rapid method for isolating large-plasmid DNA from lactic streptococci. Appl Environ Microbiol. 1983;46:548–552. doi: 10.1128/aem.46.3.549-552.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ausubel F M, Brent R, Kingston R E, Moore D D, Seidman J G, Smith J A, Struhl K. Current protocols in molecular biology. New York, N.Y: Greene Publishing and Wiley-Interscience; 1992. [Google Scholar]
- 4.Aymerich T, Holo H, Håvarstein L S, Hugas M, Garriga M, Nes I F. Biochemical and genetic characterization of enterocin A from Enterococcus faecium, a new antilisterial bacteriocin in the pediocin family of bacteriocins. Appl Environ Microbiol. 1996;62:1676–1682. doi: 10.1128/aem.62.5.1676-1682.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Barefoot S F, Klaenhammer T R. Detection and activity of lactacin B, a bactericin produced by Lactobacillus acidophilus. Antimicrob Agents Chemother. 1984;26:328–334. doi: 10.1128/aac.26.3.328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bhunia A K, Johnson M C, Ray B. Direct detection of an antimicrobial peptide of Pediococcus acidilactici in sodium dodecyl sulphate-polyacrylamide gel electrophoresis. J Ind Microbiol. 1987;2:319–322. [Google Scholar]
- 7.Booth M C, Bogie C P, Sahl H-G, Siezen R J, Hatter K L, Gilmore M S. Structural analysis and proteolytic activation of Enterococcus faecalis cytolysin, a novel lantibiotic. Mol Microbiol. 1996;21:1175–1184. doi: 10.1046/j.1365-2958.1996.831449.x. [DOI] [PubMed] [Google Scholar]
- 8.Casaus P, Nilsen T, Cintas L M, Nes I F, Hernández P E, Holo H. Enterocin B, a new bacteriocin from Enterococcus faecium T136 which can act synergistically with enterocin A. Microbiology. 1997;143:2287–2294. doi: 10.1099/00221287-143-7-2287. [DOI] [PubMed] [Google Scholar]
- 9.Cathcart D P. Purification, characterization and molecular analysis of plantaricin S, a two peptide bacteriocin from olive fermenting Lactobacillus plantarum strains. Ph.D. thesis. Cranfield, Bedford, United Kingdom: Cranfield University; 1995. [Google Scholar]
- 10.Cintas L M, Casaus P, Håvarstein L V, Hernández P E, Nes I F. Biochemical and genetic characterization of enterocin P, a novel sec-dependent bacteriocin from Enterococcus faecium P13 with a broad antimicrobial spectrum. Appl Environ Microbiol. 1997;63:4321–4330. doi: 10.1128/aem.63.11.4321-4330.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10a.Cintas L M, Casaus P, Holo H, Hernandez P E, Nes I F, Havarstein L S. Enterocins L50A and L50B, two novel bacteriocins from Enterococcus faecium L50, are related to staphylococcal hemolysins. J Bacteriol. 1998;180:1988–1994. doi: 10.1128/jb.180.8.1988-1994.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Davidson B E, Kordias N, Dobos M, Hillier A J. Genomic organization of lactic acid bacteria. Antoine Leeuwenhoek. 1996;70:161–183. doi: 10.1007/BF00395932. [DOI] [PubMed] [Google Scholar]
- 12.Devereux J, Haeberli P, Smithies O. A comprehensive set of sequence analysis programs for the VAX. Nucleic Acids Res. 1984;12:387–395. doi: 10.1093/nar/12.1part1.387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.de Vuyst L, Vandamme E J. Bacteriocins of lactic acid bacteria. London, England: Blackie Academie & Professional; 1994. [Google Scholar]
- 14.Geis A, Singh J, Teuber M. Potential of lactic streptococci to produce bacteriocin. Appl Environ Microbiol. 1993;45:205–211. doi: 10.1128/aem.45.1.205-211.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Gerdes K, Molin S. Partitioning of plasmid R1. Structural and functional analysis of the parA locus. J Mol Biol. 1986;190:269–279. doi: 10.1016/0022-2836(86)90001-x. [DOI] [PubMed] [Google Scholar]
- 16.Gierasch L M. Signal sequences. Biochemistry. 1989;28:923–930. doi: 10.1021/bi00429a001. [DOI] [PubMed] [Google Scholar]
- 17.Hastings J W, Sailer M, Johnson K, Roy K L, Vederas J C, Stiles M E. Characterization of leucocin A-UAL 187 and cloning of the bacteriocin gene from Leuconostoc gelidum. J Bacteriol. 1991;173:7491–7500. doi: 10.1128/jb.173.23.7491-7500.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Håverstein L S, Diep D B, Nes I F. A family of bacteriocin ABC transporters carry out proteolytic processing of their substrates concomitant with export. Mol Microbiol. 1995;16:229–240. doi: 10.1111/j.1365-2958.1995.tb02295.x. [DOI] [PubMed] [Google Scholar]
- 19.Hechard Y, Derijard D B, Letellier F, Cenatiempo Y. Characterization and purification of mesentericin Y105, an anti-Listeria bacteriocin from Leuconostoc mesenteroides. J Gen Microbiol. 1992;138:2725–2731. doi: 10.1099/00221287-138-12-2725. [DOI] [PubMed] [Google Scholar]
- 20.Henderson J T, Chopko A L, van Wasserman P D. Characterization and primary structure of pediocin PA-1 produced by Pediococcus acidilactici PAC1.0. Arch Biochem Biophys. 1992;295:5–12. doi: 10.1016/0003-9861(92)90480-k. [DOI] [PubMed] [Google Scholar]
- 21.Holck A, Axelsson L, Birkeland S E, Aukrust T, Blom H. Purification and amino acid sequence of sakacin A, a bacteriocin from Lactobacillus sake Lb 706. J Gen Microbiol. 1992;138:2715–2720. doi: 10.1099/00221287-138-12-2715. [DOI] [PubMed] [Google Scholar]
- 22.Hoover G D, Steenson L R. Bacteriocins of lactic acid bacteria. San Diego, Calif: Academic Press, Inc.; 1993. [Google Scholar]
- 23.Izard J W, Kendall D A. Signal peptides: exquisitely designed transport promoters. Mol Microbiol. 1994;13:765–773. doi: 10.1111/j.1365-2958.1994.tb00469.x. [DOI] [PubMed] [Google Scholar]
- 24.Jack R W, Tagg J R, Ray B. Bacteriocins of gram-positive bacteria. Microbiol Rev. 1995;59:171–200. doi: 10.1128/mr.59.2.171-200.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Jiménez-Díaz R, Rios-Sánchez R M, Desmazeau M, Ruiz-Barba J L, Piard J C. Plantaricins S and T, two new bacteriocins produced by Lactobacillus plantarum LPCO10 isolated from a green olive fermentation. Appl Environ Microbiol. 1993;59:1416–1424. doi: 10.1128/aem.59.5.1416-1424.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Jiménez-Díaz R, Ruiz-Barba J L, Cathcart D P, Holo H, Nes I F, Sletten K H, Warner P J. Purification and partial amino acid sequence of plantaricin S, a bacteriocin produced by Lactobacillus plantarum LPCO10, the activity of which depends on the complementary action of two peptides. Appl Environ Microbiol. 1995;61:4459–4463. doi: 10.1128/aem.61.12.4459-4463.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Joeger M, Klaenhammer T R. Characterization and purification of helveticin J and evidence for a chromosomally determined bacteriocin produced by Lactobacillus helveticus 48l. J Bacteriol. 1986;167:439–446. doi: 10.1128/jb.167.2.439-446.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Joosten H M L, Núñez M, Devreese B, van Beeumen J, Marugg J D. Purification and characterization of enterocin 4, a bacteriocin produced by Enterococcus faecalis INIA 4. Appl Environ Microbiol. 1996;62:4220–4223. doi: 10.1128/aem.62.11.4220-4223.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kersters K, de Ley J. Identification and grouping of bacteria by numerical analysis of their electrophoretic protein patterns. J Gen Microbiol. 1975;87:333–342. doi: 10.1099/00221287-87-2-333. [DOI] [PubMed] [Google Scholar]
- 30.Klaenhammer T R. Genetics of bacteriocins produced by lactic acid bacteria. FEMS Microbiol Rev. 1993;12:39–86. doi: 10.1111/j.1574-6976.1993.tb00012.x. [DOI] [PubMed] [Google Scholar]
- 31.Lipman D J, Pearson W R. Rapid and sensitive protein similarity searches. Science. 1985;227:1435–1441. doi: 10.1126/science.2983426. [DOI] [PubMed] [Google Scholar]
- 32.Maniatis T, Fritsch E F, Sambrook J. Molecular cloning: a laboratory manual. Cold Spring Harbor, N.Y: Cold Spring Harbor Laboratory; 1982. [Google Scholar]
- 33.Martínez-Bueno M, Maqueda M, Gálvez A, Samyn B, Van Beeumen J, Coyette J, Valdivia E. Determination of the gene sequence and the molecular structure of the enterococcal peptide antibiotic AS-48. J Bacteriol. 1994;176:6334–6339. doi: 10.1128/jb.176.20.6334-6339.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Merril C R, Goldman D, Sedman S A, Ebert M H. Ultrasensitive stain for proteins in polyacrylamide gels shows regional variation in cerebrospinal fluid proteins. Science. 1981;211:1437–1438. doi: 10.1126/science.6162199. [DOI] [PubMed] [Google Scholar]
- 35.Mortvedt C I, Nissen-Meyer J, Sletten K, Nes I F. Purification and amino acid sequence of lactocin S, a bacteriocin produced by Lactobacillus sake L45. Appl Environ Microbiol. 1991;57:1829–1834. doi: 10.1128/aem.57.6.1829-1834.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Nes I F, Diep D B, Håvarstein L S, Brurberg M B, Eijsink V, Holo H. Biosynthesis of bacteriocins in lactic acid bacteria. Antonie Leeuwenhoek. 1996;70:113–128. doi: 10.1007/BF00395929. [DOI] [PubMed] [Google Scholar]
- 37.Piard J-C, Delorme F, Giraffa G, Commisaire J, Desmazeaud M. Evidence for a bacteriocin produced by Lactococcus lactis CNRZ 481. Neth Milk Diary J. 1990;44:143–158. [Google Scholar]
- 38.Piard J-C, Muriana P M, Desmazeaud M J, Klaenhammer T R. Purification and partial characterization of lactacin 481, a lanthionine-containing bacteriocin produced by Lactococcus lactis subsp. lactis CNRZ 481. Appl Environ Microbiol. 1992;58:279–284. doi: 10.1128/aem.58.1.279-284.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Pot B, Hertel C, Ludwig W, Descheemaeker P, Kersters K, Schleifer K H. Identification and classification of Lactobacillus acidophilus, L. gasseri and L. johnsonii strains by SDS-PAGE and rRNA-targeted oligonucleotide probe hybridization. J Gen Microbiol. 1993;139:513–517. doi: 10.1099/00221287-139-3-513. [DOI] [PubMed] [Google Scholar]
- 40.Pot B, Vandamme P, Kersters K. Analysis of electrophoretic whole-organism protein fingerprints. In: Goodfellow M, O’Donnel A G, editors. Chemical methods in prokaryotic systematics. Chichester, England: John Wiley & Sons, Inc.; 1994. pp. 493–521. [Google Scholar]
- 41.Pugsley A P. The complete general secretory pathway in gram-negative bacteria. Microbiol Rev. 1993;57:50–108. doi: 10.1128/mr.57.1.50-108.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Quadri L E N, Sailer M, Roy K L, Vederas J C, Stiles M E. Chemical and genetic characterization of bacteriocins produced by Carnobacterium piscicola LV17B. J Biol Chem. 1994;269:12204–12211. [PubMed] [Google Scholar]
- 43.Rauch P J G, Beerthuyzen M M, De Vos W M. Nucleotide sequence of IS904 Lactococcus lactis subsp. lactis strain NIZO R5. Nucleic Acids Res. 1990;18:4253–4254. doi: 10.1093/nar/18.14.4253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Ruiz-Barba J L, Piard J C, Jiménez-Díaz R. Plasmid profile and curing of plasmids in Lactobacillus plantarum strains isolated from green olive fermentations. J Appl Bacteriol. 1991;71:417–421. doi: 10.1111/j.1365-2672.1991.tb03810.x. [DOI] [PubMed] [Google Scholar]
- 45.Samyn B, Martínez-Bueno M, Devreese B, Maqueda M, Gálvez A, Valdivia E, Coyette J, van Beeumen J. The cyclic structure of the enterococcal peptide antibiotic AS-48. FEBS Lett. 1994;352:87–90. doi: 10.1016/0014-5793(94)00925-2. [DOI] [PubMed] [Google Scholar]
- 46.Schägger H, Von Jagow G. Tricine-sodium dodecyl sulphate-polyacrylamide gel electrophoresis for the separation of proteins in the range from 1 to 100 kDa. Anal Biochem. 1987;166:368–379. doi: 10.1016/0003-2697(87)90587-2. [DOI] [PubMed] [Google Scholar]
- 47.Tagg J R, Dajani A S, Wannamaker L W. Bacteriocins of gram-positive bacteria. Bacteriol Rev. 1976;40:722–756. doi: 10.1128/br.40.3.722-756.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Tichaczek P S, Vogel R F, Hammes W P. Cloning and sequencing of sakP encoding sakacin P, the bacteriocin produced by Lactobacillus sake LTH 673. Microbiology. 1994;140:361–367. doi: 10.1099/13500872-140-2-361. [DOI] [PubMed] [Google Scholar]
- 49.Tomita H, Fujimoto S, Tanimoto K, Ike Y. Cloning and genetic organization of the bacteriocin 31 determinant encoded on the Enterococcus faecalis pheromone-responsive conjugative plasmid pYI17. J Bacteriol. 1996;178:3585–3593. doi: 10.1128/jb.178.12.3585-3593.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.van Belkum M J, Worobo R W, Stiles M E. Double-glycine-type leader peptides direct secretion of bacteriocins by ABC transporters: colicin V secretion in Lactococcus lactis. Mol Microbiol. 1997;23:1293–1301. doi: 10.1046/j.1365-2958.1997.3111677.x. [DOI] [PubMed] [Google Scholar]
- 51.von Heijne G. Net N-C charge imbalance may be important for signal sequence function in bacteria. J Mol Biol. 1986;192:287–290. doi: 10.1016/0022-2836(86)90365-7. [DOI] [PubMed] [Google Scholar]
- 52.Worobo R W, Henkel T, Sailer M, Roy K L, Vederas J C, Stiles M E. Characteristics and genetic determinant of a hydrophobic peptide bacteriocin, carnobacteriocin A, produced by Carnobacterium piscicola LV17A. Microbiology. 1994;140:517–526. doi: 10.1099/00221287-140-3-517. [DOI] [PubMed] [Google Scholar]