Abstract
Introduction:
Some jurisdictions have implemented nicotine vaping product (NVP) flavor restrictions because of concerns about rising adolescent use. However, little is known how these restrictions may impact adult vapers. This study describes the level of support and predictive behavioral responses to a hypothetical NVP ban on non-tobacco flavors among regular adult vapers who use the flavors that would be banned.
Methods:
Data came from 851 regular vapers (all current or ex-smokers) participating in the 2020 ITC Four Country Smoking and Vaping Survey in Canada, England, and the United States (US). A random sample of respondents in each country received and completed the questions about flavor bans: (1) do you support or oppose a ban on all non-tobacco flavors; and (2) what would you do if all flavors were banned, with the exception of tobacco in the US, and tobacco and menthol in Canada and England. Those who used tobacco-flavored or unflavored NVPs were excluded from all analyses, and additionally, vapers of menthol flavor in Canada and England were excluded from Aim 2.
Results:
Overall, 53.6% of vapers were strongly opposed to flavor bans, 28.2% were opposed, 9.3% were in support, 3.6% were in strong support, and 5.2% did not know. Predicted behavioral responses were: 28.8% would continue vaping an available flavor, 28.3% would find a way to get their banned flavor(s), 17.1% would stop vaping and smoke instead, 12.9% said that they would stop vaping and not smoke, and 12.9% do not know what they would do. Responses to a potential flavor ban largely varied by smoking and vaping status, and by the level of support of a flavor restriction policy.
Conclusions:
At this time, it is not clear what net population-level consequences would occur if non-tobacco flavored NVPs were prohibited. While a majority of vapers in this study opposed this policy, and many vapers would not be willing to switch to available flavors, there was considerable variability in predicted behavioral responses.
Keywords: e-cigarettes, nicotine vaping products, tobacco, smoking cessation
1. Introduction
Over the last decade, the nicotine vaping product (NVP) market has increased exponentially [1–3] largely due to the availability of a variety of brands, device-types, and flavors, all of which are appealing to potential and current consumers [4]. In 2014, it was estimated that there were over 400 brands and nearly 8000 unique flavored products available online in the United States (US) [5], and since then the variety has increased dramatically due to customization options and the ongoing development of new products [1,2]. In the US, NVPs are primarily used by younger people [2], with the greatest proportion of vapers being between 18-29 years. NVPs have also become popular with youth [2,6–8], leading to concerns within public health groups that the rise of NVP use will result in a new generation becoming dependent on nicotine [8–11]. Consistent with data from the US, NVPs are popular among Canadian youth [12]. However, in the United Kingdom (UK), where public health organizations have encouraged adult smokers to switch to NVPs as an alternative to continued cigarette smoking, the majority of NVP users are middle-aged current and ex-smokers [2,13–14].
Adult vapers have self-reported using NVPs for a variety of reasons, such as they are helpful in replacing cigarettes and/or quitting smoking, greater social acceptance, they are less harmful, and they are enjoyable and affordable [15–17]. Research has also consistently demonstrated that flavors play a large role in NVP initiation as well as continued use among adult current smokers and ex-smokers [17–21], as well as facilitating switching from cigarettes to NVPs [20,21]. Adult vapers prefer the availability of a range of flavors, and commonly use non-tobacco flavored NVPs, such as fruit and menthol/mint flavors [17–20,22–25]. However, while flavors appear to be one of the most appealing NVP features among adults, attractive flavors are also one of the reasons for use by youth [8,17,26,27]. Rising NVP use among youth in Canada and the US has raised significant concern among public health officials and youth front groups [10,11,28,29], and has further prompted some jurisdictions [25,30–33] to implement, or consider implementing, NVP flavor restrictions.
While bans on the sale of non-tobacco flavored NVPs may in theory help prevent some youth from taking up vaping, the evidence to support this claim is lacking at this time. Moreover, there may be unintended consequences associated with restricting flavors for adults. Such consequences could reduce harm reduction potential for those who wish to use a NVP as a cessation aid, or completely switch from cigarettes to NVPs. For instance, two flavors that have been primarily exempted from flavor bans—tobacco and menthol— have been found to be less satisfying than other flavors (e.g., fruit and candy), especially among exclusive vapers who have quit smoking [18,22]. And while some studies have found little evidence at this time of whether NVP flavors increase smoking cessation [17, 27], other studies have shown that non-tobacco flavored NVPs were associated with increased quit smoking attempts [34], and with transitioning away from smoking [35,36].
The investigation into vapers’ intentions to continue vaping or engage in other behaviors in the case of flavor restrictions (e.g., stop vaping and smoke instead, make their own e-juice, or purchase flavored NVPs from illicit sources), could inform policy makers of the possible consequences of these regulatory interventions. For any policy measure, it is important to evaluate not only the intended effectiveness of the policy, but also any possible unintended consequences. Where policies have not yet been implemented, hypothetical scenarios can provide valuable information [37–42]. Notably, NVP flavor restriction policies are relatively new, and data conclusively assessing actual impacts are not readily available, thus we have relied on vapers’ hypothetical (and predictive) behavioural responses in this study.
This study was conducted among regular adult vapers (all of whom were current smokers or ex-smokers) in Canada, England, and the US who use non-tobacco flavored products. The two study aims were to: (1) assess vapers’ level of support or opposition to banning all NVP flavors except for tobacco; and (2) measure vapers’ predicted behavioral responses if a ban was implemented on all flavors except for tobacco in the US, and all flavors except for tobacco and menthol in Canada and England.
2. METHODS
2.1. Design, setting, and participants
The International Tobacco Control Project Four Country Smoking and Vaping (ITC 4CV) Survey is a cohort study of parallel online surveys conducted in Canada, US, England, and Australia. Respondents (adults ≥ 18 years) were recruited by commercial panel firms in each country as established cigarette smokers (smoke ≥ monthly, and smoked at least 100 cigarettes in their lifetime), recent ex-smokers (quit ≤ 2 years), or vapers (vape ≥ weekly). Informed consent was obtained from all respondents. This study received ethics approval at the University of Waterloo (Canada), King’s College London (UK), Cancer Council Victoria (Australia), University of Queensland (Australia), and the Medical University of South Carolina (US). Full details of the ITC 4CV study can be found at: https://itcproject.org/methods/technical-reports/.
For the current study, data are from the Wave 3 ITC 4CV Survey (February-June 2020) [43]. Respondents were eligible for inclusion, if at time of the survey they: (1) were from Canada, England, or the US (Australian respondents were excluded as the sale of NVPs is prohibited [44], and vapers were not asked the relevant survey questions); (2) were current smokers or ex-smokers who vaped at least weekly; (3) received the relevant outcome questions (asked among a random sample of half the respondents in each country); and (4) used only non-tobacco NVP flavors (those who used tobacco or unflavored were excluded). Additionally, for Aim 2, vapers also reporting using menthol flavor were excluded in Canada and England (because they received a different set of questions than US vapers). Overall, 851 vapers were eligible for inclusion, resulting in 845 vapers who had complete data for Aim 1, and 703 regular vapers who were eligible and had complete data for Aim 2. A study flow diagram is presented in Figure 1.
Figure 1.
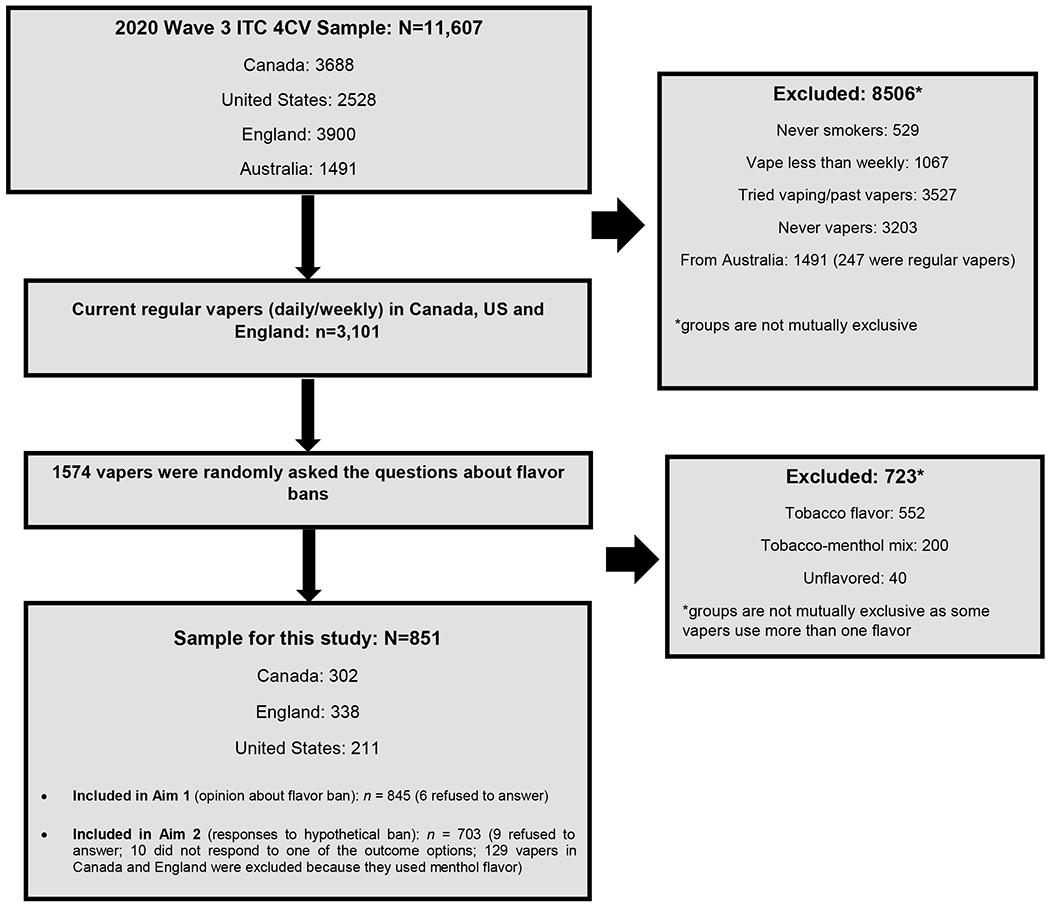
4CV3 study flow diagram
2.2. Measures
All 4CV3 survey measures can be found at: https://itcproject.org/surveys/. The following variables were included in the current study:
2.2.1. Sociodemographic data
Age, sex, and country of residence data collected by the commercial panels and verified by respondents at the time of survey completion.
2.2.2. Smoking status
Smoking status was verified by each respondent. Those who indicated that they were current smokers (at least monthly), or ex-smokers [either as a recent quitter (quit smoking ≤ 2years ago) or long-term quitter (quit >2 years ago)] were included. Vapers who reported smoking less-than-monthly were included in the ex-smoker analytical sample (n=27).
2.2.3. NVP use
Those who indicated that they were currently using NVPs at the time of the survey were asked about their frequency of use. If vapers used a NVP at least on a weekly basis, they were defined as being a regular vaper and included in this study. Respondents who also smoked cigarettes were sub-classified as ‘concurrent users’ and those who formerly smoked cigarettes (or smoked < monthly) were classified as ‘exclusive vapers’.
2.2.4. NVP flavors
Respondents were asked to select from a list of 11 categories, any flavors they had used in the last 30 days. Those who selected any flavor except for tobacco, tobacco-menthol mix, or unflavored were eligible for initial inclusion.
2.2.5. Outcomes
The following outcomes were tested in this study: (1) support or oppose a ban on all non-tobacco flavors; and (2) predicted behavioral responses. The survey questions and details can be found in Box 1.
Box 1.
1. Support or oppose a ban on all non-tobacco flavors:
Would you support or oppose a law that bans menthol, fruit, candy, and other flavors in vaping products and/or e-liquid, so that ONLY TOBACCO flavor would be available. Responses included: ‘strongly support’, ‘support, ‘oppose’, ‘strongly oppose’, ‘don’t know’, or refused to answer. Those who refused to answer were excluded.
2. Predicted behavioral responses:
If fruit, candy, and other flavors were banned in vaping products and e-liquid, and ONLY [TOBACCO flavor (US)/ TOBACCO and MENTHOL flavors (Canada, England)] would be available, what would you do? Respondents could select one of the following response options: ‘find a way to get the flavors that are banned’; ‘vape available flavor(s)’; ‘quit vaping and smoke cigarettes instead’; ‘quit vaping and not smoke cigarettes’; ‘do something else (open-ended: specify)’, ‘don’t know’; or ‘refused’. The country difference for this question was because a blanket flavor ban (the only exception being tobacco flavor) was being discussed nationally at the time of survey development in the US (but not in Canada or the UK [45]), and several public health organizations have been strongly advocating for the FDA to propose a ban on menthol and mint flavors for all tobacco products, including NVPs [46,47].
For the second aim, the following respondents were excluded: (1) those who refused to answer (n=9); (2) those who used menthol flavor in Canada and England (n=123), as well as those who could not distinguish between menthol and mint flavor (n=6); and (3) those who reported ‘do something else’ due to the small sample size (n=10).
2.3. Analyses
Unweighted descriptive statistics were used to describe the sample of vapers from each of the three countries. All other estimates were obtained using adjusted regression analyses on weighted data. All confidence intervals were computed at the 95% confidence level. Analyses were conducted using SAS Version 9.4 (SAS Institute Inc. 2013, Cary, North Carolina, USA). The analyses for the current study were not pre-registered, thus the results herein should be considered exploratory.
For Aim 1, an adjusted multinomial regression model was used to examine whether vapers would support or oppose a non-tobacco flavor ban (first overall, and then by country). This was first done with the original response options (strongly oppose, oppose, support, strongly support, or don’t know), and then by a dichotomized outcome: ‘strongly oppose/oppose’ vs. ‘otherwise’ (strongly support/support/don’t know). The models controlled for country, age, sex, vaping/smoking status [referred to herein now as ‘vaping status’: concurrent users (also smoke) vs. exclusive vapers (ex-smokers)], and vaping frequency (daily vs. weekly).
For Aim 2, an adjusted multinomial regression model was used, with the multiple outcomes being the five possible behavioral responses. The response ‘continue vaping with available flavor(s)’ was the reference group. The model controlled for age, sex, vaping status, vaping frequency, and country. Country comparisons were not made because the question differed between Canada/England and the US. In addition to the main model, further analyses were conducted that examined differences in predictive behavioral responses by: (1) vaping status; (2) vaping frequency; and (3) level of support for NVP flavor bans (support, oppose or don’t know). The same covariates described in Aim 1 were used in these analyses.
3. Results
3.1. Respondents’ Characteristics
Respondents’ sample characteristics (overall and by country) are presented in Table 1.
Table 1.
Respondent characteristics (unweighted %)
| Canada n=302 | England n=338 | US n=211 | Overall N=851 | ||
|---|---|---|---|---|---|
| Respondent type | |||||
| Recontact (n=324) | 34.4 | 34.9 | 48.3 | 38.1 | |
| Replenishment (n=521) | 65.6 | 65.1 | 51.7 | 61.9 | |
|
| |||||
| Age group | |||||
| 18-24 (n=379) | 50.3 | 39.1 | 45.0 | 44.5 | |
| 25-39 (n=214) | 24.8 | 28.4 | 20.4 | 25.2 | |
| 40-54 (n=150) | 18.5 | 18.6 | 14.7 | 17.6 | |
| 55+ (n=108) | 6.3 | 13.9 | 19.9 | 12.7 | |
|
| |||||
| Mean age | 31.0 | 34.8 | 35.6 | 33.7 | |
|
| |||||
| Sex | |||||
| Male (n=435) | 47.7 | 54.7 | 50.2 | 51.1 | |
| Female (n=416) | 52.3 | 45.3 | 49.8 | 48.9 | |
|
| |||||
| Smoking status | |||||
| Current smoker (n=626) | 78.5 | 76.9 | 61.1 | 73.6 | |
| Vapes daily | 43.9 | 60.0 | 59.7 | 53.8 | |
| Ex-smoker (n=225) | 21.5 | 23.1 | 38.9 | 26.4 | |
| Vapes daily | 72.3 | 74.4 | 82.9 | 76.9 | |
|
| |||||
| Vaping frequency | |||||
| Daily (n=510) | 50.0 | 63.3 | 68.7 | 59.9 | |
| Weekly (n=341) | 50.0 | 36.7 | 31.3 | 40.1 | |
|
| |||||
| Non-tobacco/unflavored flavor(s) used in the last 30 days* | |||||
| Fruit (n=540) | 70.2 | 60.4 | 58.8 | 63.5 | |
| Menthol/Mint (n=328) | 35.1 | 39.6 | 41.7 | 38.5 | |
| Menthol only (n=135) | 9.3 | 18.6 | 20.9 | 15.9 | |
| Mint only (n=140) | 19.9 | 15.1 | 13.7 | 16.5 | |
| Menthol and mint mix (n=44) | 4.6 | 5.3 | 5.7 | 5.2 | |
| Don’t know if menthol or mint (n=9) | 1.3 | 0.6 | 1.4 | 1.1 | |
| Candy (n=296) | 33.8 | 32.5 | 39.8 | 34.8 | |
| Other flavors (n162) | 18.9 | 20.7 | 16.6 | 19.0 | |
| Complete data for outcomes | n | n | n | N | |
| Outcome 1 | |||||
| Included | 301 | 337 | 207 | 845 | |
| Refused | 1 | 1 | 4 | 6 | |
|
|
|||||
| Outcome 2 | |||||
| Included | 250 | 248 | 205 | 703 | |
| Refused | 1 | 5 | 3 | 9 | |
| Used menthol in Canada or England (excluded) ┼ | 46 | 83 | N/A | – | |
| Reported response: ‘do something else’ (excluded) | 5 | 1 | 4 | 10 | |
Self-reported use of flavors are not mutually exclusive. Candy: sweets, desserts, chocolate; Other flavors: coffee and other non-alcoholic beverages, alcoholic beverages, cloves, spices, vanilla, food.
4 vapers in Canada and 2 in England did not know if their flavor was menthol or mint.
3.2. Support for a Non-Tobacco NVP Flavor Ban
Overall, 845 regular vapers completed the question about their level of support in banning all non-tobacco flavored NVPs. Estimates for original response options can be found in Figure 2. Figure 3 reports these findings by vaping status and vaping frequency.
Figure 2.
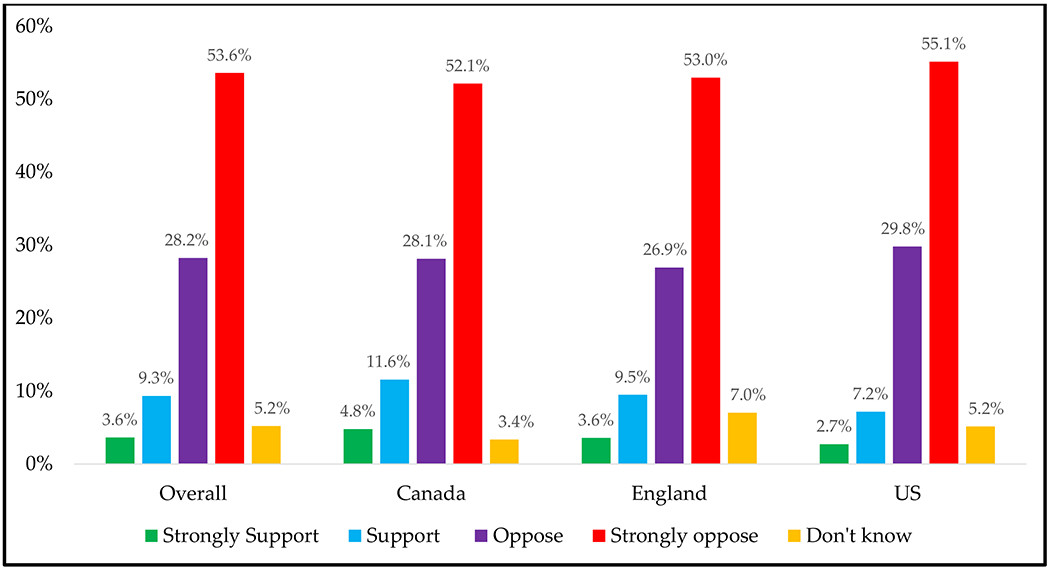
Regular vapers’ opposition or support for banning non-tobacco NVP flavors, overall and by country
N=845; Data are weighted and adjusted. Wald: 2.09, p<0.001. Covariates: country: p=0.79, age: 0.59, sex: p=0.83, vaping status: p=0.07, vaping frequency: p=0.007). Responses (n): Strongly support: n=51; support: n=120; oppose: n=224; strongly oppose: n=392, don’t know: n=58.
Figure 3.
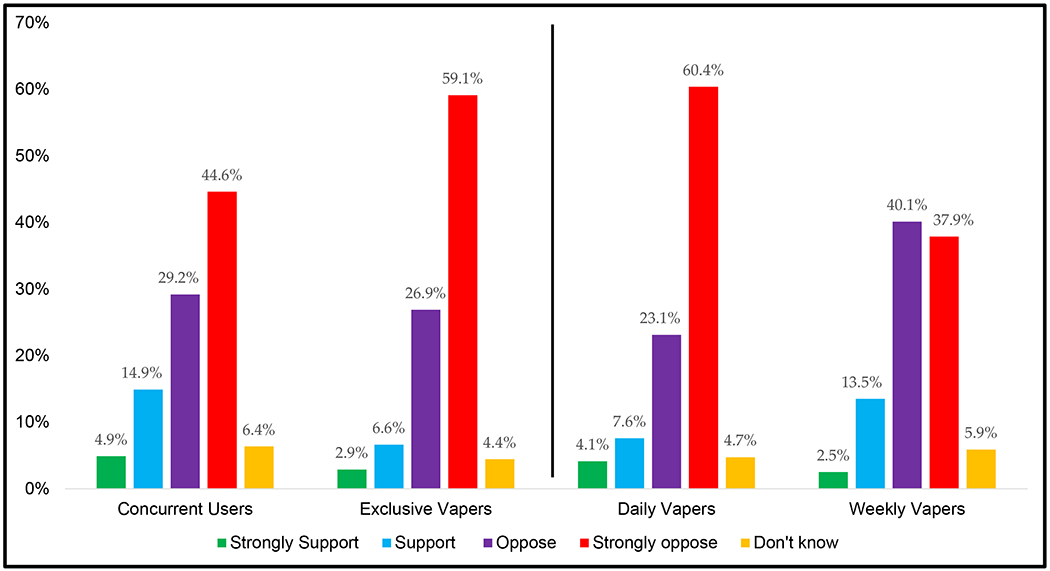
Regular vapers’ opposition or support for banning non-tobacco NVP flavors by vaping status and vaping frequency
Data are weighted and adjusted.
In summary, 53.6% of vapers were strongly opposed to the flavor ban, 28.2% were opposed, 9.3% were in support, 3.6% were in strong support, and 5.2% did not know. Daily vapers were more likely than weekly vapers to strongly oppose a flavor ban (60.4% vs. 37.9%, p=0.01). A greater proportion of exclusive vapers strongly opposed to a flavor ban (59.1%) relative to concurrent users (44.6%), but this only approached statistical significance (p=0.07). There were no differences by country (p=0.78), age (p=0.59) or sex (p=0.83).
When the response options were combined (oppose vs. otherwise), 81.4% opposed to the policy, with exclusive vapers being more likely than concurrent users to oppose the flavor ban (85.6% vs. 73.3%, p=0.041).
3.3. Projected Behavioral Responses to a Hypothetical Flavor Ban
Table 2 shows the responses by regular vapers (n=703) to hypothetical NVP flavor restrictions, overall and by country. Figure 4 shows the responses by vaping status and vaping frequency, and Figure 5 shows the responses by whether vapers support or oppose NVP flavor bans. Table 3 further subdivided vaping status by frequency of use.
Table 2.
Potential reactions to NVP flavor restrictions by non-tobacco flavor regular vapers.
| n | Overall (n=703) | Canada (n=250) | England (n=248) | US (n=205) | |
|---|---|---|---|---|---|
|
| |||||
| Weighted % (95% confidence interval) | |||||
| Vape available flavor(s) | 202 | 28.8% (22.6-34.9) | 45.4% (32.8-58.1) | 30.5% (17.2-43.8) | 20.9% (12.9-28.9) |
| Find a way to get restricted flavor(s) | 169 | 28.3% (22.3-34.3) | 22.3% (13.1-31.5) | 28.4% (15.8-41.0) | 33.1% (23.4-42.8) |
| Quit vaping and smoke instead | 147 | 17.1% (12.8-21.4) | 12.3% (5.8-18.8) | 14.8% (8.5-21.1) | 21.0% (10.5-31.5) |
| Not smoke or vape | 98 | 12.9% (8.8-17.0) | 10.4% (3.5-17.2) | 9.6% (1.6-17.6) | 9.2% (4.1-14.2) |
| Don’t know | 87 | 12.9% (8.3-17.5) | 9.6% (1.4-17.8) | 16.8% (8.2-25.4) | 15.8% (7.2-24.5) |
Data are weighted and adjusted. Vape available flavors: tobacco or menthol in Canada and England, and tobacco in the US. Overall model: Reference group: vape available flavor(s); Wald: 2.65, p<0.0001. Covariates: country: p=0.17; age: p=0.41; sex: p=0.01; smoking/vaping status: p=0.001; vaping frequency: p=0.03.
Figure 4.
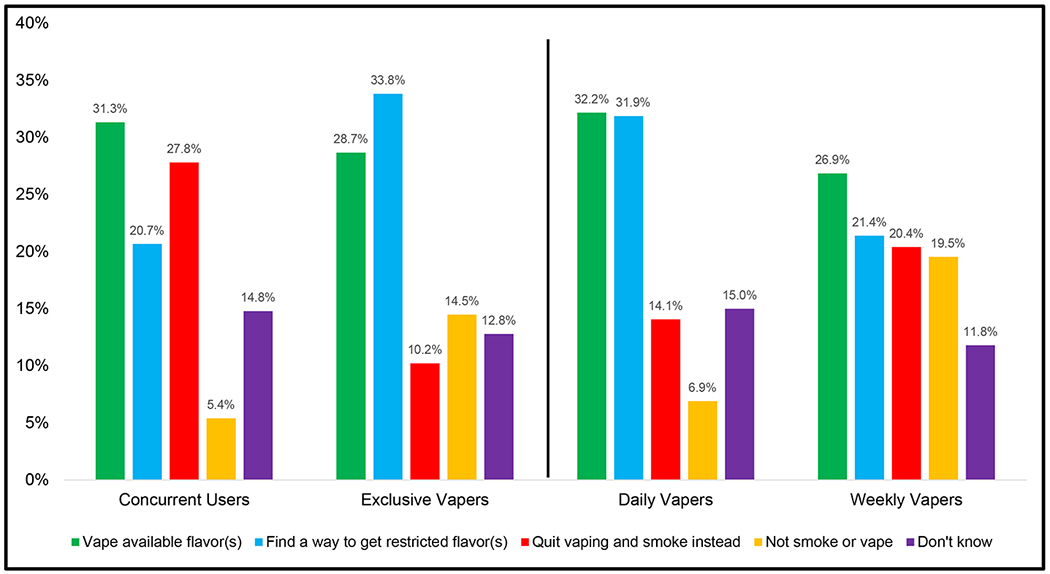
Predictive behavioral responses to NVP flavor restrictions stratified by vaping status and vaping frequency
Data are weighted and adjusted.
Figure 5.
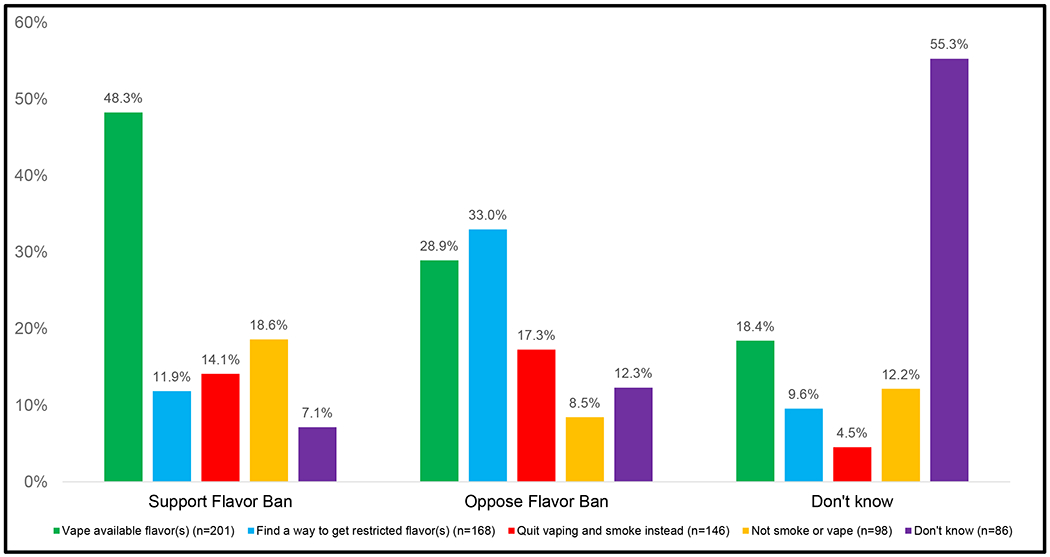
Predictive behavioral responses to NVP flavor restrictions stratified by whether vapers support or oppose NVP flavor bans
Data are weighted an adjusted. Wald: 3.35, p<0.0001. Support vs. oppose NVP flavor ban (support, oppose or don’t know): p<0.0001; country: p=0.35; age: p=0.23; sex: p=0.01; smoking/vaping status: p=0.001; vaping frequency: p=0.06.
Table 3.
Potential reactions to NVP flavor restrictions by non-tobacco flavor regular vapers stratified by vaping status and vaping frequency.
| Outcome response = yes (vs. otherwise) | Concurrent Users (n=516) | Exclusive Vapers (n=187) | ||
|---|---|---|---|---|
|
| ||||
| Daily vapers (n=287) | Weekly vapers (n=229) | Daily vapers (n=142) | Weekly vapers (n=45) | |
| Vape available flavors, n=202 | 30.1% | 26.9% | 26.9% | 22.3% |
| p-value | 0.59 | 0.69 | ||
|
| ||||
| Find a way to get the banned flavors, n=169 |
23.6% | 15.6% | 35.2% | 19.2% |
| p-value | 0.11 | 0.17 | ||
|
| ||||
| Quit vaping and smoke instead, n=147 |
26.0% | 29.5% | 5.4% | 12.0% |
| p-value | 0.56 | 0.30 | ||
|
| ||||
| Not smoke or vape, n=98 |
3.8% | 8.5% | 8.6% | 26.8% |
| p-value | 0.05 | 0.03 | ||
|
| ||||
| Don’t know, n=87 |
12.4% | 14.0% | 12.8% | 5.6% |
| p-value | 0.73 | 0.28 | ||
Data are weighted and adjusted.
In summary, there were varied responses to the hypothetical flavor ban, where 28.8% would plan to continue vaping with an available flavor, 28.3% would find a way to get banned flavors, 17.1% would stop vaping and smoke cigarettes instead, 12.9% said that they would stop vaping and not smoke, and 12.9% do not know what they would do. There were no differences by age (p=0.41). Females were more likely than males to report that they would find a way to get their preferred flavor (p=0.04) and stop vaping and not smoke (p=0.03), compared to vaping available flavors. Concurrent users were more likely to say that they would stop vaping and smoke instead (p=0.04) and less likely to stop vaping and not smoke (no nicotine use) (p=0.02). Daily vapers were less likely than weekly vapers to say that they would to stop vaping and not smoke (no nicotine use) (p=0.01). No other comparisons were statistically significant.
When predictive behavioral responses to NVP flavor restrictions were examined by whether vapers support or oppose a NVP flavor ban, about half of those who support flavor bans said that they would vape an available flavor, whereas only 28.9% of those who oppose flavor bans reported this. A much higher proportion of those who oppose flavor bans said that they would find a way to get their banned flavor(s) (33.0%) relative to those who are in support of the bans (11.9%) or do not know if they would support such a ban or not (9.6%). Those who oppose a flavor ban had the highest proportion of vapers say that they would stop vaping and smoke instead (17.3%) relative to those who support flavor bans (14.1%) and those who do not know (4.5%). Slightly more than half of those who do not know whether they would support a flavor ban reported that they do not know what they would do if their flavor were banned.
4. Discussion
The current study evaluated the level of support or opposition for NVP flavor restrictions among vapers who use non-tobacco flavors, as well as vaper’s predictive reactions if the flavors they use were to be prohibited. We found that in all three countries, the vapers who would be most affected by flavor restrictions, overwhelmingly opposed the adoption of this policy. We also found varied projected behavioral responses to the hypothetical flavor restrictions.
Flavor restrictions have been met with strong resistance by various groups, including vaping advocates, manufacturers, vape shop owners, and current NVP users [48–53]. Such groups argue that a ban on preferred flavors would reduce the number of smokers who initiate vaping, make switching less likely among those who are using them to try and quit smoking, trigger relapse back to smoking among those who have already switched, and therefore reduce the number of lives that could be saved by providing an appealing and less harmful alternative to smoking. And while, health agencies such as Health Canada and the US FDA explicitly state that there is a trade-off between protecting youth from nicotine use and protecting adults from the harms of continued smoking [45,53,54], the overall net effect of such policies require rigorous evaluation (e.g., using quasi-experimental cohort studies that compare changes in smoking and vaping behaviors between jurisdictions that have implemented flavor bans relative to others that have not). It is common practice in tobacco control research to evaluate support for tobacco regulatory policies among those who would be most impacted by the regulations (e.g., smokers) [55–57]. By doing so, we can make inferences about whether there is an important relationship between support for these policies that would clearly make it harder to continue tobacco use (e.g., not allowing smoking in all public places, by making cigarettes less accessible, by making cigarettes less addictive etc.). This study applied this concept to the current paper, where we evaluated support for NVP flavor bans among vapers who would be the most impacted by such a policy.
Our study found that about 80% of vapers opposed banning all non-tobacco flavored NVPs. This was especially true among ex-smokers who have already completely switched to vaping. The opposition to such flavor bans appears to be similar to a 2016 ITC study in Canada which found that 74% of weekly vapers and 70% of daily vapers (all concurrent users) were opposed to bans on fruit and candy-flavored NVPs [60]. This is identical to our 2020 estimate of 73% of concurrent users who oppose the policy, demonstrating that opinions about flavor bans have not changed over the last four years. In contrast, about 13% of vapers in our study supported flavor restrictions. We are uncertain why this may be the case; however, our findings show that half of those who support the policy would be willing to vape available flavors; thus this latter subset of vapers may be less affected by flavor variety. Other reasons may be that they may want to quit vaping and this would provide them an opportunity to do so (e.g., not being able to access their preferred flavors may encourage vapers to stop vaping and not use nicotine at all), and/or they believe that flavor variety is indeed problematic for youth, and flavor bans would be effective in reducing youth NVP use.
Researchers have used different methods to evaluate the impact of tobacco control policies that have not yet been implemented, or have recently been implemented but have not yet been extensively evaluated (e.g., surveys to assess smokers’ predictive behaviours, simulation modelling and experimental studies) [37–42,58–60]. And while predicted behaviors may not actually predict future behavior, the use of surveys and other designs based on hypothetical scenarios helps to fill a gap in the evidence given the limited evaluations of existing policies. At this time, NVP flavor restriction policies are relatively new in Canada and the US, and data conclusively assessing actual impacts on youth and adults are limited.
Our current study directly asked vapers what they would do if flavor restrictions were placed on NVPs, which has the potential to show the direct impact on actual behaviours of individual vapers. We found that just over a quarter of the sample said that they would vape available flavors, but a similar proportion said that they would find a way to obtain their preferred flavor. The latter was more common among exclusive vapers (ex-smokers), daily vapers, and those who opposed the flavor ban. The latter response is an important measure of possible illicit purchasing behavior, which is a significant concern among policy-makers when considering bans on tobacco or vaping products [61,62]. For example, experimental marketplace studies have examined the effects of a hypothetical vaping ban and a flavored vaping ban on the probability of vapers purchasing illicit vaping products, and demonstrated that NVP users were more likely to purchase from the illegal experimental market when product availability in the legal market was more restricted [63,64].
In addition to some experimental studies, two population studies to our knowledge have evaluated if vapers would be able to access their preferred flavor during NVP flavor bans in the US. First, Yang et al. assessed the impact of San Francisco’s ban on all flavored tobacco products except tobacco-flavored NVPs and found most NVP users reported being able to obtain flavored products in a variety of ways, especially by purchasing vaping flavors on the internet [65]. A study by Katchmar et al. examined the impact of the Massachusetts law that imposed a 75% excise tax on NVPs and banned the sale of all flavored tobacco products (for both combustible tobacco and NVPs) in June 2020 [66]. The findings showed that there was an increase in the number of vapers who purchased their NVPs in another state (from 53% before the ban to 69% post-ban). Therefore, there appears to be ways for vapers to circumvent flavor bans, and our study suggests that a large subset of vapers would be willing and motivated to turn to online purchasing, accessing flavors in other jurisdictions where flavors are not banned, and possibly use illicit market products to continue accessing their preferred flavor(s).
The most negative potential consequence of a NVP flavor ban would be that it may drive some current vapers to resume or increase their use of cigarettes. At this time, there are only two studies to our knowledge that have assessed the impact of flavor bans on tobacco use among youth [67] and one on young adults [65] in one jurisdiction in the US (San Francisco). Freidman et al. found an increase in cigarette smoking among youth after the NVP ban, but did not test if vaping decreased among this subset of the population [67]. Yang et al. reported that after the NVP flavor ban, cigarette smoking increased among young adults aged 18–24, and the prevalence of flavored NVPs decreased among 18-24 and 25-34 year old’s [65]. Data on the impact of flavor bans among adults are limited, and the available literature (mostly unpublished) has focused solely on changes in consumer purchasing data (e.g., cigarette sales) in the US [66,68–70], and not on changes in use of NVPs or cigarettes. Specifically, actual behaviors in the wake of NVP flavor restrictions, as well as vapers’ predictions of their own responses to flavor bans is lacking. The findings from our study—across a broad sample of adult vapers from several countries— found that about one in five vapers (17%) would plan to quit vaping and smoke cigarettes instead. A much larger proportion of concurrent users (28%) compared to exclusive vapers (10%) reported that they would stop vaping and continue to smoke, thus, creating uncertainty about how many current smokers would have eventually transitioned away from smoking if they had continued to vape. And although we did not test country differences, our findings suggest that if the US were to prohibit all non-tobacco flavours of NVPs, this might have the potential for negative consequences, in that 21% of vapers said that they would stop vaping and smoke instead. In contrast, in England and Canada, where the hypothetical ban would still allow menthol flavor, respectively, 15% of vapers in England and 12% of vapers in Canada said that they would stop vaping and smoke instead. This lower percentage of vapers who might switch to smoking in England and Canada suggests that permitting greater flavor diversity (e.g., menthol) may reduce this potential negative outcome.
From a public health perspective, the most ideal scenario under a flavor ban would be that vapers would give up both vaping and smoking (transition towards not using any nicotine at all). We found that 13% of the sample reported that they would stop vaping and not smoke if their preferred flavor was not available, with a higher proportion of ex-smokers saying so (15% vs. 5% of concurrent users). Under this hypothetical scenario, the most positive response—no nicotine use— appears to be an unlikely outcome among the majority of regular vapers (who are likely highly dependent on nicotine). However, it is possible that some of these vapers would switch to other tobacco/nicotine products instead (e.g., cigars, NRT, heated tobacco or oral tobacco/nicotine products), a reaction that we did not include in our scenario.
This study has some limitations. First, the survey questions were hypothetical in nature, and as noted above, may not translate to actual behaviors. Second, some respondents in the US and Canada were already exposed to NVP flavor bans, and thus may have formed a different opinion based on lived, rather than hypothetical, experiences. We were unable to analyze these data by region, therefore more quasi-experimental studies on real-life population outcomes are critical. Third, this study is only generalizable to adult current and former smokers. In order to obtain a net impact on the population, data on both youth and adults, smokers and non-smokers, needs to be considered. Fourth, this is a cross-sectional study, which is subject to inherent limitations. Longitudinal analyses are warranted to explore possible consequences to NVP flavor restrictions, particularly the impact on smoking. Fifth, differences in flavor inclusion for the hypothetical flavor ban question, where menthol was permitted in Canada and England, and not the US, poses a significant limitation for country comparisons. Sixth, due to sample size constraints, we included what we considered to be key covariates into the models. Other potentially important sociodemographic covariates (e.g., income, education, and ethnicity) were tested, and we did not see evidence of the presented conclusions changing. Further studies are warranted to explore if behaviors would differ by sociodemographic differences (e.g. if low income vapers would behave differently than their higher income counterparts) in the case of NVP flavor bans. Finally, these results should be taken in context that the field work occurred during the onset of the COVID-19 pandemic. We are unable to empirically test if this had a material impact on our findings, but we do not see a reason why it would specifically impact responses to a hypothetical vaping flavor restriction policy.
6. Conclusions
At this time, it is not yet clear exactly how NVP flavor bans will play out as they are implemented across national and sub-national jurisdictions. However, by extrapolating from this study and the other limited studies available, it appears that the outcomes will be mixed and complex. While our study suggests most vapers are opposed to a ban, and some vapers indicated that such a ban would lead to unintended consequences (e.g., taking up cigarette smoking again, or turning to illicit channels), many vapers said that they would be willing to vape available flavors. Thus, it is important for policy makers to weigh the potential for public health consequences, as well as possible benefits.
Highlights.
Most vapers who use non-tobacco flavors oppose flavor bans
There was considerable variability in responses by vapers to a hypothetical flavor ban
Predictive behaviors largely varied by smoking and vaping status
Many vapers would not be willing to use available flavors if their preferred flavor was banned
Footnotes
Declarations of interest: The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper. KMC has served as paid expert witness in litigation filed against cigarette manufacturers. GTF and DH have served as expert witnesses on behalf of governments in litigation involving the tobacco industry. AM is a UK National Institute for Health Research (NIHR) Senior Investigator. The views expressed in this article are those of the authors and not necessarily those of the NIHR, or the UK Department of Health and Social Care.
References
- 1.Foundation for a Smoke-Free World. (2018). Global trends in nicotine. Retrieved from https://www.smokefreeworld.org/sites/default/files/fsfw-report-trends-in-nicotine-1005201811.pdf
- 2.Baker P (2021). Vaping facts and statistics 2020 / 2021. Retrieved from https://www.allencarr.com/easyway-stop-vaping/facts-statistics/#world
- 3.Statista. (2021). E-cigarettes. Retrieved from https://www.statista.com/outlook/50040000/100/e-cigarettes/worldwide#market-marketDriver
- 4.Grand View Research. (2020). E-cigarette And vape market size, share & trends analysis report by product (disposable, rechargeable), by component (vape mod, e-liquid), by distribution channel, and segment forecasts, 2020 – 2027. Retrieved from https://www.grandviewresearch.com/industry-analysis/e-cigarette-vaping-market
- 5.Zhu SH, Sun JY, Bonnevie E, Cummins SE, Gamst A, Yin L, & Lee M (2014). Four hundred and sixty brands of e-cigarettes and counting: implications for product regulation. Tobacco Control, 23(Suppl 3), iii3–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Wang TW, Gentzke AS, Creamer MR, Cullen KA, Holder-Hayes E, Sawdey MD, Anic GM, Portnoy DB, Hu S, Homa DM, et al. (2019). Tobacco product use and associated factors among middle and high school students - United States, 2019. MMWR Surveillance Summaries, 68(12), 1–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Wang TW, Neff LJ, Park-Lee E, Ren C, Cullen KA, King BA (2020). E-cigarette use among middle and high school students — United States, 2020. Morbidity and Mortality Weekly Report, 69(37), 1310–1312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.National Academies of Sciences Engineering and Medicine. (2018). Public health consequences of e-cigarettes. The National Academies Press (US). Retrieved from 10.17226/24952. [DOI] [PubMed] [Google Scholar]
- 9.Campaign for Tobacco-Free Kids. (2020). Electronic cigarettes and youth. Retrieved from https://www.tobaccofreekids.org/assets/factsheets/0382.pdf
- 10.Centers for Disease Control and Prevention (CDC). (2020). Youth and tobacco use. Retrieved from https://www.cdc.gov/tobacco/data_statistics/fact_sheets/youth_data/tobacco_use/index.htm
- 11.United States Food and Drug Administration (FDA). (2018). FDA takes new steps to address epidemic of youth e-cigarette use, including a historic action against more than 1,300 retailers and 5 major manufacturers for their roles perpetuating youth access. Retrieved from https://www.fda.gov/news-events/press-announcements/fda-takes-new-steps-address-epidemic-youth-e-cigarette-use-including-historic-action-against-more
- 12.Cole AG, Aleyan S, Battista K, & Leatherdale S (2021). Trends in youth e-cigarette and cigarette use between 2013 and 2019: insights from repeat cross-sectional data from the COMPASS study. Canadian Journal of Public Health, 112(1), 60–69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Action on Smoking and Health (ASH UK). (2021). Fact sheets. Retrieved from https://ash.org.uk/fact-sheets/
- 14.McNeill A, Brose LS, Calder R, Simonavicius E & Robson D (2021). Vaping in England: An evidence update including vaping for smoking cessation: A report commissioned by PHE. Retrieved from https://www.gov.uk/government/publications/vaping-in-england-evidence-update-february-2021/vaping-in-england-2021-evidence-update-summary
- 15.Nicksic NE, Snell LM, Barnes AJ (2019). Reasons to use e-cigarettes among adults and youth in the Population Assessment of Tobacco and Health (PATH) study. Addictive Behaviors, 93, 93–99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Yong HH, Borland R, Cummings KM, Gravely S, Thrasher JF, McNeill A, et al. (2019). Reasons for regular vaping and for its discontinuation among smokers and recent ex-smokers: findings from the 2016 ITC Four Country Smoking and Vaping Survey. Addiction, 114(Suppl 1), 35–48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Zare S, Nemati M, & Zheng YA (2018). A systematic review of consumer preference for e-cigarette attributes: Flavor, nicotine strength, and type. PLoS One, 13(3), e0194145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Landry RL, Groom AL, Vu TT, Stokes AC, Berry KM, Kesh A, et al. (2019). The role of flavors in vaping initiation and satisfaction among U.S. adults. Addictive Behaviors, 99, 106077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Gendall P, & Hoek J (2021). Role of flavors in vaping uptake and cessation among New Zealand smokers and non-smokers: a cross-sectional study. Tobacco Control, 30(1), 108–110. [DOI] [PubMed] [Google Scholar]
- 20.Russell C, McKeganey N, Dickson T, & Nides M (2018). Changing patterns of first e-cigarette flavor used and current flavors used by 20,836 adult frequent e-cigarette users in the USA. Harm Reduction Journal, 15(1), 33,018–0238-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Notley C, Ward E, Dawkins L, & Holland R (2018). The unique contribution of e-cigarettes for tobacco harm reduction in supporting smoking relapse prevention. Harm Reduction Journal,15(1), 31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Gravely S, Cummings KM, Hammond D, Lindblom E, Smith DM, Martin N, et al. (2020). The association of e-cigarette flavors with satisfaction, enjoyment, and trying to quit or stay abstinent from smoking among regular adult vapers from Canada and the United States: Findings from the 2018 ITC Four Country Smoking and Vaping Survey. Nicotine Tobacco Research, 22(10), 1831–1841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Goldenson NI, Leventhal AM, Simpson KA, & Barrington-Trimis JL (2019). A review of the use and appeal of flavored electronic cigarettes. Current Addiction Reports, 6(2), 98–113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Reid JL, Hammond D, Tariq U, Burkhalter R, Rynard VL, & Douglas O (2019). Tobacco use in Canada: Patterns and trends, 2019 edition. Retrieved from https://uwaterloo.ca/tobacco-use-canada/tobacco-use-canada-patterns-and-trends
- 25.Government of Canada. (2019). What we heard: Reducing youth access and appeal of vaping products: Consultation summary. Retrieved from https://www.canada.ca/en/health-canada/services/publications/healthy-living/consultation-summary-reducing-youth-access-appeal-vaping-products.html
- 26.Campaign for Tobacco-Free Kids. (2017). The flavor trap: How tobacco companies are luring kids with candy-flavored e-cigarettes and cigars. Retrieved from https://www.tobaccofreekids.org/microsites/flavortrap/#summary
- 27.Meernik C, Baker HM, Kowitt SD, Ranney LM, & Goldstein AO (2019). Impact of non-menthol flavors in e-cigarettes on perceptions and use: An updated systematic review. BMJ Open, 9(10), e031598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Drazen JM, Morrissey S, & Campion EW (2019). The dangerous flavors of e-cigarettes. New England Journal of Medicine, 380(7), 679–680. [DOI] [PubMed] [Google Scholar]
- 29.Heart and Stroke Foundation of Canada. (2020). Positions statement: Protecting youth from the vaping crisis. Retrieved from https://www.heartandstroke.ca/-/media/pdf-files/canada/2020-position-statement/youth-vaping-position-statement-web.ashx?rev=1b133488f2f44b1fa001b7cb7bea69ec&hash=2CE7ED86FD82907E0D8950EF7A2574F3&hash=2CE7ED86FD82907E0D8950EF7A2574F3
- 30.O’Connell M, & Kephart L (2020). Local and state policy action taken in the United States to address the emergence of e-cigarettes and vaping: A scoping review of literature. Health Promotion Practice, 1524839920963691. [DOI] [PubMed] [Google Scholar]
- 31.Campaign for Tobacco-Free Kids. (2020). States and localities that have restricted the sale of flavored tobacco product. Retrieved from https://www.tobaccofreekids.org/assets/factsheets/0398.pdf
- 32.United States Food and Drug Administration (FDA). (2020). FDA news release. FDA finalizes enforcement policy on unauthorized flavored cartridge-based e-cigarettes that appeal to children, including fruit and mint. Retrieved from https://www.fda.gov/news-events/press-announcements/fda-finalizes-enforcement-policy-unauthorized-flavored-cartridge-based-e-cigarettes-appeal-children
- 33.Physicians for a Smoke-free Canada. (2020). At-a-glance: Provincial restrictions on vaping products. Retrieved from http://www.smoke-free.ca/SUAP/2020/Provincial%20regulations%20on%20vaping%20promotions.pdf
- 34.Kasza KA, Edwards KC, Gravely S, Coleman B, Kimmel H, Everard C, et al. (2021). Adults’ e-cigarette flavor use and cigarette quit attempts: Population assessment of tobacco and health study findings. American Journal of Preventive Medicine, 60(2), 300–302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Friedman AS, & Xu S (2020). Associations of flavored e-cigarette uptake with subsequent smoking initiation and cessation. JAMA Network Open, 1;3(6), e203826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Li P, Borland R, Cummings KM, Fong GT, Gravely S, Smith DM, et al. (2021). How does the use of flavored nicotine vaping products relate to progression towards quitting smoking? Findings from the 2016 and 2018 ITC 4CV surveys. Nicotine & Tobacco Research, ntab, 033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.O’Connor RJ, Bansal-Travers M, Carter LP, & Cummings KM (2021). What would menthol smokers do if menthol in cigarettes were banned? Behavioral intentions and simulated demand. Addiction,107(7):1330–1338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.MacKillop J, Murphy JG, Ray LA, Eisenberg DT, Lisman SA, Lum JK, et al. (2008). Further validation of a cigarette purchase task for assessing the relative reinforcing efficacy of nicotine in college smokers. Experimental and Clinical Psychopharmacology, 16(1):57–65. [DOI] [PubMed] [Google Scholar]
- 39.Ross H, Blecher E, Yan L, & Cummings KM (2011). Predictors of what smokers say they will do in response to future price increases. Findings from the International Tobacco Control (ITC) Four Country Survey. Nicotine & Tobacco Research, 13(6):419–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Czoli CD, Goniewicz M, Islam T, Kotnowski K, & Hammond D (2016). Consumer preferences for electronic cigarettes: results from a discrete choice experiment. Tobacco Control, 25(e1), e30–6. [DOI] [PubMed] [Google Scholar]
- 41.Rousu MC, & Thrasher J (2012). Demand reduction from plain and pictorial cigarette warning labels: Evidence from experimental auctions. Applied Economic Perspectives and Policy, 35(1), 171–184. [Google Scholar]
- 42.Heckman BW, Cummings KM, Nahas GJ, Willemsen MC, O’Connor RJ, Borland R, et al. (2019). Behavioral economic purchase tasks to estimate demand for novel nicotine/tobacco products and prospectively predict future use: Evidence from the Netherlands. Nicotine & Tobacco Research, 21(6), 784–791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.ITC Project. (2020). ITC Four Country Smoking and Vaping Survey, Wave 3 (4CV3, 2020), preliminary technical report. Retrieved from https://itcproject.org/methods/technical-reports/itc-four-country-smoking-and-vaping-survey-4cv-wave-3-2020-preliminary-technical-report-oct-2020/
- 44.Greenhalgh EM, Grace C, & Scollo MM (2018). InDepth 18B: Electronic cigarettes (e-cigarettes). In Scollo MM and Winstanley MH [editors]. Tobacco in Australia: Facts and issues. Melbourne: Cancer Council Victoria. Retrieved from http://www.tobaccoinaustralia.org.au/chapter-18-harm-reduction/indepth-18b-e-cigarettes [Google Scholar]
- 45.United States Food and Drug Administration (FDA), HHS. (2018). Regulation of flavors in tobacco products. A proposed rule by the food and drug administration on 03/21/2018. Retrieved from https://www.federalregister.gov/documents/2018/03/21/2018-05655/regulation-of-flavors-in-tobacco-products
- 46.Action on Smoking and Health (ASH) (2021). Coalition letter urging the FDA to proceed with its rule characterizing menthol as an added flavor in tobacco products. Retrieved from Coalition letter urging the FDA to proceed with its rule characterizing menthol as an added flavor in tobacco products. (ada.org).
- 47.Campaign for Tobacco-Free Kids. (2019). FDA restrictions on flavored e-cigarettes fall short of what Is needed to reverse youth epidemic. Retrieved from https://www.tobaccofreekids.org/press-releases/2019_03_13_fda
- 48.Ramer H (2020). Vape shop owners oppose New Hampshire flavor ban proposal. Retrieved from https://apnews.com/article/c3a187e7a31aeb240e6fe9bb4bf39b3e [Google Scholar]
- 49.Beaglehole R, Bates C, Youdan B, & Bonita R (2019). Nicotine without smoke: Fighting the tobacco epidemic with harm reduction. Lancet, 394, 718–20. [DOI] [PubMed] [Google Scholar]
- 50.Kirkpatrick MG, Dormanesh A, Rivera V, Majmundar A, Soto DW, Chen-Sankey JC, et al. (2021). #FlavorsSaveLives: An analysis of Twitter posts opposing flavored e-cigarette restrictions. Nicotine & Tobacco Research, ntaa, 276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Lamorena A (2021). In opposition to SB 24: An act relating to restricting flavored tobacco products and e-liquids. Retrieved from https://www.rstreet.org/2021/02/25/in-opposition-to-sb-24-an-act-relating-to-banning-flavored-tobacco-products-and-e-liquids/
- 52.Bentley G (2018). A question of taste: The public health case for e-cigarette flavors. Retrieved from https://reason.org/policy-brief/the-public-health-case-for-e-cigarette-flavors/
- 53.Callard C (2019). Canada: government releases consumer research on vaping and tobacco. Blog. Tobacco Control. Retrieved from https://blogs.bmj.com/tc/2019/09/28/canada-government-releases-consumer-research-on-vaping-and-tobacco/
- 54.Maloney J (2017). FDA chief: menthol, tobacco flavors could do both ‘harm and good’. Retrieved from https://www.wsj.com/articles/fda-chief-menthol-tobacco-flavors-could-do-both-harm-and-good-1508432700
- 55.Smith TT, Nahhas GJ, Borland R, et al. (2021). Which tobacco control policies do smokers support? Findings from the International Tobacco Control Four Country Smoking and Vaping Survey. Prev Med, 149,106600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Fong GT, Yuan J, Craig LV, et al. (2021). Achieving the Goals of Healthy China 2030 Depends on Increasing Smoking Cessation in China: Comparative Findings from the ITC Project in China, Japan, and the Republic of Korea. China CDC Weekly, [Published online May 28, doi: 10.46234/ccdcw2021.120]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Chung-Hall J, Fong GT, Driezen P, Craig L (2018). Smokers’ support for tobacco endgame measures in Canada: findings from the 2016 International Tobacco Control Smoking and Vaping Survey. CMAJ Open, 6(3), E412–E422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Levy DT, Pearson JL, Villanti AC, et al. (2011). Modeling the future effects of a menthol ban on smoking prevalence and smoking-attributable deaths in the United States. Amercian Journal of Public Health,101,1236–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Institute of Medicine. 2015. Public Health Implications of Raising the Minimum Age of Legal Access to Tobacco Products. Washington, DC: The National Academies Press. 10.17226/18997. [DOI] [PubMed] [Google Scholar]
- 60.Kronfli C Guiding policy principals for tobacco and vaping products. Ontario Chamber of Commerce. 2019. https://occ.ca/wp-content/uploads/Guiding-Policy-Principles-for-Tobacco-and-Vaping-Products.pdf [Google Scholar]
- 61.Freitas-Lemos R, Stein JS, Tegge AN, et al. (2021). The Illegal Experimental Tobacco Marketplace I: Effects of VapingProduct Bans. Nicotine Tobacco Research, 6, ntab088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Government of Canada. Canada Gazette, Part I, Volume 155, Number 25: Order Amending Schedules 2 and 3 to the Tobacco and Vaping Products Act (Flavours). Regulatory Impact Statement. 2021. [Google Scholar]
- 63.Griffiths CJ (2018). Illicit trade in tobacco products after implementation of an FDA product standard. Center for Tobacco Products U.S. Food and Drug Administration. [Google Scholar]
- 64.Bickel W, Freitas-Lemos R, Stein JS, Tegge AN (2021). The Illegal Experimental Tobacco Marketplace III: Effects of Vaping Product Bans. Findings from the 2020 International Tobacco Control Project. Abstract submitted to the 2022 Society for Research on Nicotine and Tobacco. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Freitas-Lemos R, Stein JS, Tegge AN, et al. (2021). The Illegal Experimental Tobacco Marketplace I: Effects of VapingProduct Bans. Nicotine Tobacco Research, 6, ntab088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Yang Y, Lindblom EN, Salloum RG, & Ward KD (2020). The impact of a comprehensive tobacco product flavor restrictions in San Francisco among young adults. Addictive Behaviors Reports, 11, 100273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Katchmar A, Gunawan A, & Siegel M (2021). Effect of Massachusetts House Bill No. 4196 on electronic cigarette use: A mixed-methods study. Harm Reduction Journal, 18(1), 50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Friedman AS (2021). A Difference-in-differences analysis of youth smoking and a ban on sales of flavored tobacco products in San Francisco, California. JAMA Pediatrics, doi: 10.1001/jamapediatrics.2021.0922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Jiang L, Xu Y, & Prakash S (2020). The impact of restricting ENDS products on combustible cigarette sales: Initial evidence from U.S. state-level policies. Retrieved from NoSmoke_ENDSBan_Jiang_2020_v4 (juullabsscience.com)
- 70.Liber A (2021). The EVALI outbreak and tobacco sales in the US. Presented to tobacco control researchers on March 16, 2021 (data not published). [Google Scholar]
- 71.Cahn Z, Liber LC, Donovan EM, Diaz MC, Vallone DM, Schillo BA (2021). The EVALI outbreak and tobacco cigarette sales in the United States, 2014—2020 (data not published). [DOI] [PubMed] [Google Scholar]


