Abstract
Purpose:
In this first-in-human phase 1 study (NCT02132754), we explored MK-4166 (humanized IgG1 agonist monoclonal antibody targeting GITR) with and without pembrolizumab in advanced solid tumors.
Experimental Design:
MK-4166 was tested alone (0.0015–900 mg IV Q3W for four doses) or with pembrolizumab (200 mg IV Q3W for ≤35 doses) in patients with metastatic solid tumors (dose-escalation/confirmation) and advanced melanoma (expansion). Primary objectives were to evaluate the safety and tolerability and establish the maximum-tolerated dose of MK-4166. Exploratory endpoints: objective response rate (ORR) and T-cell–inflamed gene expression profile (GEP) analysis using RNA from baseline tumor samples.
Results:
One hundred and thirteen patients were enrolled (monotherapy, n=48; combination therapy, n=65 [20 in the expansion]). Forty-six patients (40.7%) had grade ≥3 adverse events, 9 (8.0%) of which were treatment-related. No treatment-related deaths were observed. One dose-limiting toxicity event with monotherapy (bladder perforation in patient with neobladder) was considered related to study drug. Maximum-tolerated dose was not reached. MK-4166 pharmacodynamics showed decreased GITR availability on circulating T cells with increasing doses. One objective response (ORR, 2.2%) was achieved with combination therapy in the dose-escalation/confirmation (n=45). In the expansion, 8/13 patients with ICI-naive melanoma achieved a response (ORR, 62%; 95% CI, 32%−86%; five complete responses and three partial responses). None of the ICI-pretreated patients (n=7) responded. High response rates were observed in ICI-naive patients irrespective of GEP status.
Conclusions:
MK-4166 900 mg IV Q3W as monotherapy and with pembrolizumab was tolerable. Responses were observed with combination therapy, mostly in patients with ICI-naive melanoma.
Keywords: solid tumors, GITR, MK-4166, immunotherapy, pembrolizumab
INTRODUCTION
Immune checkpoint inhibitors (ICI) targeting cytotoxic T lymphocyte antigen 4 (CTLA-4) or programmed death 1/programmed death ligand 1 have improved outcomes for patients across a wide range of cancers (1–5); however, a substantial proportion of patients do not respond to these treatments. This has led clinical research to focus on new combinations that might enhance the efficacy of ICI (6).
The glucocorticoid-induced tumor necrosis factor receptor (GITR) is a member of the tumor necrosis factor receptor superfamily (7–10). GITRs are constitutively expressed at high levels on regulatory T cells (Tregs) and at low levels on resting CD4+ T cells, CD8+ T cells, natural killer cells, and natural killer T cells (8, 9). After T-cell activation, GITR expression is upregulated on CD4+ and CD8+ T cells and natural killer cells, including tumor-infiltrating lymphocytes (10). The natural ligand for GITR, GITR ligand, is expressed at low levels by antigen-presenting cells and is upregulated upon activation (11). Activating the GITR pathway promotes antitumor effects of T cells by enhancing CD4+ and CD8+ T-cell proliferation and effector functions, and may protect T cells from activation-induced cell death (12). GITR ligation enhances T-cell survival by upregulating interleukin-2, interleukin-2 receptor alpha, and IFN-γ (13); activation downregulates the immunosuppressive activity of Treg cells (14). A recent pan-tumor study demonstrated GITR expression variability on immune cells (Tregs and/or tumor-infiltrating lymphocytes) across tumor types and suggested that non–small cell lung cancer, renal cell carcinoma, and melanoma should be prioritized in the development of anti-GITR therapies (15).
MK-4166 is a humanized immunoglobulin G1 agonist monoclonal antibody that targets GITR (10). MK-4166 binds to a region on human GITR that is comparable to the region where a functionally active surrogate monoclonal antibody, DTA-1, binds to mouse GITR. DTA-1 augments antitumor T-cell responses, inhibits or depletes Tregs, and induces tumor rejection in syngeneic mouse tumor models (16–18). Given the different mechanisms of action of MK-4166, which stimulates effector immune cells and inhibits or depletes Tregs, and of pembrolizumab, which counteracts programmed death 1–mediated T-cell exhaustion in tumors, the two may have synergistic antitumor effects when used in combination (18, 19). In a murine model of ovarian cancer, the combination of DTA-1 and anti–programmed death 1 conferred greater antitumor efficacy than either antibody alone and shifted the tumor milieu from that of an immunosuppressive to an immunostimulatory state (20). In B16F10-melanoma mice, resistance to anti-GITR monotherapy was overcome by T-cell reinvigoration with programmed death 1 blockade (19). These results provide a strong rationale for combining GITR– and programmed death 1–targeted therapy.
Herein, we investigated the safety and tolerability and explored the preliminary antitumor activity of MK-4166 when administered as monotherapy or in combination with pembrolizumab in patients with advanced solid tumors.
MATERIAL AND METHODS
Study Design
This phase I study (NCT02132754) was a nonrandomized, multicenter, two-arm, open-label trial of MK-4166 monotherapy (Arm 1) and MK-4166 in combination with pembrolizumab (Arm 2) in patients with metastatic solid tumors (Supplemental Figure S1). Patients were enrolled at four centers across three countries. The study was conducted in accordance with the protocol and protocol amendments, the Good Clinical Practice guidelines, and the Declaration of Helsinki. The protocol and subsequent amendments were approved by the appropriate institutional review board or ethics committee at each participating institution. All patients provided written informed consent to participate.
Patients
Eligible patients were aged ≥18 years with a histologically/cytologically confirmed metastatic solid tumor, measurable disease according to Response Evaluation Criteria in Solid Tumors version 1.1 (RECIST v1.1) (21), Eastern Cooperative Oncology Group performance status (ECOG PS) of 0 or 1, and adequate organ function. Patients in the expansion cohort were required to have advanced melanoma (treatment-naive or pretreated), excluding uveal melanoma. Patients who received chemotherapy, radiation, or biological cancer therapy ≤4 weeks before the first dose of study drug or who had not yet recovered to grade ≤1 (based on the National Cancer Institute Common Terminology Criteria for Adverse Events version 4 [NCI CTCAE v4]) (22) from adverse events resulting from cancer therapies received ≥4 weeks prior to the first dose of study drug were excluded. Other key exclusion criteria were current treatment with immunosuppressive therapy or chronic systemic steroid therapy, known active central nervous system metastases and/or carcinomatous meningitis, active autoimmune disease, active infection requiring therapy, and current pneumonitis or history of (noninfectious) pneumonitis that required steroids.
Treatments
The study used an accelerated dose escalation followed by a 3+3 design for the monotherapy arm and a 3+3 design followed by dose confirmation for the combination arm. Until the planned maximum dose level was reached in the monotherapy arm, patients were allocated to the monotherapy and combination therapy arms to ensure that dose escalation in the combination arm was at least two dose levels lower than that in the monotherapy arm.
MK-4166 monotherapy was administered intravenously at 18 dose levels, ranging from 0.0015 to 900 mg every 3 weeks for up to four cycles. When delivered in combination with pembrolizumab (at a flat dose of 200 mg intravenously every 3 weeks for up to 35 doses [~2 years]), MK-4166 was administered at doses ranging from 1.1 to 900 mg intravenously every 3 weeks for up to four cycles. In the monotherapy arm, accelerated dose escalation in single-patient cohorts proceeded, based on safety events, from a starting dose of 0.0015 mg to a maximum dose of 10.0 mg. Dose escalation then continued at 30.0 mg with a 3+3 design to identify a preliminary maximum-tolerated dose for MK-4166. In the combination arm, a 3+3 design was used for dose escalation, with a MK-4166 starting dose of 1.1 mg and a pembrolizumab fixed dose of 200 mg. In the expansion cohort, patients with advanced melanoma received combination therapy at the maximum-tolerated dose.
During dose escalation, an initial cohort of three patients were enrolled at each dose level. If no patients experienced dose-limiting toxicity, escalation to the next dose occurred; if one patient experienced dose-limiting toxicity, another three patients were enrolled at that dose level; and if one dose-limiting toxicity was observed among six patients, dose escalation continued. If two of three or two of six patients at a dose level experienced dose-limiting toxicity, dose escalation was stopped and the study proceeded to dose confirmation at the previous dose level. Each dose escalation was based on the safety and tolerability observed at each dose level.
Assessments
Adverse events were graded according to NCI CTCAE v4 (22). Adverse events were recorded throughout the study period and for 30 days after the last dose of study therapy (90 days for serious adverse events or before initiation of a new anticancer therapy, whichever occurred first). Patients with a grade >1 adverse event were followed further until resolution of the adverse event to grade 0 or 1 or until initiation of a new anticancer therapy. Tumor imaging (computed tomography or magnetic resonance imaging) was performed at baseline (≤28 days prior to enrollment and assessed using RECIST v1.1), and every 9 weeks from the first dose of treatment until confirmed disease progression, start of new anticancer therapy, withdrawal of consent, death, or end of study, whichever occurred first.
Blood was sampled for pharmacokinetic and pharmacodynamic (receptor availability) assessments at screening, in Cycles 1–4 on day 1 predose, at the end of MK-4166 infusion (+10 minutes), 2 hours after the start of the MK-4166 infusion (±10 minutes), and on days 2, 3, 5, 8, and 15 for patients treated at the 9 lowest dose levels in Arm 1. Starting at the tenth dose level in Arm 1 and all dose levels in Arm 2, samples were drawn at screening, in Cycles 1–4 on day 1 predose, at the end of the MK-4166 infusion (+10 minutes), 2 hours after the start of MK-4166 infusion (±10 minutes), and on days 2, 3, 8, and 15. ln addition, in Arm 2, samples were drawn predose in Cycles 5–6. MK-4166 quantitation in human serum was determined using Singulex Erenna and MesoScale Discovery (MSD) platform-based immunoassays to measure total MK-4166 concentrations. Due to GITR internalization upon binding by MK-4166, direct measurement of GITR receptor occupancy was not feasible. A novel pharmacodynamic assay was developed and used to detect GITR on CD4+CD25+ and CD4+CD95+ T-cell subpopulations using flow cytometry; postdose levels were compared with levels at baseline. Blood was also sampled for determination of development of antidrug antibodies against MK-4166 and pembrolizumab at regular intervals throughout treatment and follow-up.
Outcomes
The primary objectives were to evaluate the safety and tolerability of MK-4166 as monotherapy and in combination with pembrolizumab, including dose-limiting toxicity and maximum-tolerated dose, in patients with advanced solid tumors. Safety was assessed by quantifying the toxicities and grades thereof (per NCI CTCAE v4) experienced by patients who received either study therapy, including serious adverse events. Pharmacodynamic end points included evaluation of GITR target engagement on peripheral blood lymphocytes before and after administration of MK-4166 using a receptor availability assay via flow cytometry; antidrug antibody response at the beginning of each cycle of therapy; and serum cytokine levels. An exploratory objective was antitumor activity (per RECIST v1.1) of MK-4166 monotherapy or in combination with pembrolizumab. Tumor biopsy samples were also obtained from patients before treatment for preplanned, exploratory, correlative biomarker analyses, such as gene expression profiling. An additional biopsy was requested, but not required, in Cycle 1 between day 8 and day 15. An 18-gene T-cell–inflamed gene expression profile developed on the NanoString platform to evaluate the combined expression pattern of IFN-γ responsive genes linked to antigen presentation, chemokine expression, cytotoxicity, and adaptive immune resistance in the tumor microenvironment was measured for association with clinical response (23).
Statistical Analyses
Safety was analyzed in all patients who received ≥1 dose of study treatment (All-Patients-as-Treated [APaT]). Dose-limiting toxicity was evaluated in patients in the APaT population who were observed for safety for the first cycle (21 days) after the first dose of assigned treatment or who experienced a dose-limiting toxicity <21 days after the first dose. Safety and tolerability were assessed by clinical review of adverse events, serious adverse events, laboratory tests, vital signs, electrocardiogram measurements, and physical examinations. Dose-limiting toxicities and adverse events were summarized as counts and frequencies for each MK-4166 dose level (alone and in combination with pembrolizumab) with at least three patients treated and listed for other dose levels.
For pharmacokinetic analyses, serum concentrations of MK-4166 in the monotherapy arm and of MK-4166 and pembrolizumab in the combination arm were summarized and analyzed using descriptive statistics.
The exploratory efficacy analysis population (full analysis set]) comprised all patients with measurable disease at baseline (scan assessed by investigator) who received MK-4166 monotherapy (Arm 1; full analysis set 1) or MK-4166 with pembrolizumab (Arm 2; full analysis set 2). Duration of response was based on data from all confirmed responders. The database cutoff date was July 31, 2019.
RESULTS
Patients
Of the 113 patients with advanced solid tumors enrolled in this study, 48 received MK-4166 monotherapy and 65 received MK-4166 and pembrolizumab combination therapy (Supplementary Table S1). From the combination arm, 20 patients with melanoma (13 ICI–naive and seven ICI–treated) were included in the expansion cohort. Thirty-five of 48 patients (72.9%) in the monotherapy arm and 63 of 65 (96.9%) in the combination arm discontinued study treatment; four patients in the combination arm were transferred to the follow-up study with the option to complete therapy with pembrolizumab according to the protocol. Most patients discontinued because of disease progression (monotherapy arm, 60.4%; combination arm, 63.1%). Baseline characteristics were generally well balanced between the two treatment arms, with the exception of ECOG PS and primary diagnosis (Table 1). The most common primary diagnoses were gastrointestinal cancer (31.3%), pancreatic cancer (14.6%), and lung cancer (10.4%) in the monotherapy arm, and melanoma (30.8%), gastrointestinal cancer (23.1%), and pancreatic cancer (12.3%) in the combination arm.
Table 1.
Baseline Characteristics for Total Population
| Characteristic | Monotherapy Arma n = 48 |
Combination Therapy Armb n = 65 |
|---|---|---|
| Age, median (range), years | 64.0 (34–84) | 62.0 (19–86) |
| ≥65 years, n (%) | 24 (50.0) | 27 (41.5) |
| Male, n (%) | 23 (47.9) | 34 (52.3) |
| Race, n (%)c | ||
| White | 46 (95.8) | 60 (92.3) |
| Asian | 0 | 2 (3.1) |
| Black or African American | 1 (2.1) | 0 |
| ECOG PS, n (%) | ||
| 0 | 18 (37.5) | 41 (63.1) |
| 1 | 30 (62.5) | 24 (36.9) |
| Primary diagnosis, n (%) | ||
| Gastrointestinal cancer | 15 (31.3) | 15 (23.1) |
| Pancreatic cancer | 7 (14.6) | 8 (12.3) |
| Lung cancer | 5 (10.4) | 3 (4.6) |
| Other§ | 5 (10.4) | 3 (4.6) |
| Gynecologic cancer | 4 (8.3) | 7 (10.8) |
| Breast cancer | 3 (6.3) | 1 (1.5) |
| Genitourinary cancer | 3 (6.3) | 0 |
| Neuroendocrine cancer | 2 (4.2) | 1 (1.5) |
| Primary unknown | 2 (4.2) | 1 (1.5) |
| Head and neck cancer | 1 (2.1) | 3 (4.6) |
| Sarcoma | 1 (2.1) | 3 (4.6) |
| Melanoma | 0 | 20 (30.8) |
| Prior lines of therapy, n (%)d | ||
| 0 | 9 (19.2) | 12 (21.8) |
| 1 | 11 (23.4) | 17 (30.9) |
| 2 | 9 (19.2) | 6 (10.9) |
| ≥3 | 18 (37.5) | 20 (30.8) |
| Prior therapy with ICI, n (%)d | 6 (12.5) | 12 (18.5) |
Abbreviations: ECOG PS, Eastern Cooperative Oncology Group performance status; ICI, immune checkpoint inhibitor; RECIST v1.1, Response Evaluation Criteria in Solid Tumors version 1.1.
Includes all patients from the dose acceleration, dose escalation, and dose confirmation phases.
Includes all patients from the dose escalation/confirmation cohort and the expansion cohort.
Race was missing for four patients (one in the monotherapy arm and three in the combination therapy arm).
Tumor types occurring in two or fewer patients: renal cancer (n = 2 in monotherapy arm]), prostate cancer (n = 1 in each arm), liver cancer (n = 1 in combination therapy arm), mesothelioma (n = 1 in monotherapy arm), thyroid cancer (n = 1 in combination therapy arm), and skin cancer other than melanoma (n = 1 in monotherapy arm).
Information on prior lines of therapy and prior ICI therapy missing for eleven patients.
Safety and Tolerability
Adverse events occurred in 44 of 48 patients (91.7%) in the monotherapy arm and 63 of 65 (96.9%) in the combination arm (Table 2). The most frequent adverse events were fatigue (31.3%), abdominal pain (20.8%), and infusion-related reaction (18.8%) in the monotherapy arm, and fatigue (56.9%), infusion-related reaction (33.8%), and nausea (24.6%) in the combination arm. Grade 3–4 adverse events occurred in 46 patients (40.7%): 15 patients (31.3%) in the monotherapy arm and 31 patients (47.7%) in the combination arm.
Table 2.
All-Grade and Grade 3 or 4 Adverse Events in Patients Receiving MK-4166 Monotherapy and in Combination With Pembrolizumab
| Adverse events in ≥10% of Patients in Either Arm, n (%) | Monotherapy Arma n = 48 |
Combination Therapy Armb n = 65 |
||
|---|---|---|---|---|
| All | Grade 3 or 4 | All | Grade 3 or 4 | |
| Any | 44 (91.7) | 15 (31.3) | 63 (96.9) | 31 (47.7) |
| Fatigue | 15 (31.3) | 1 (2.1) | 37 (56.9) | 0 |
| Infusion-related reaction | 9 (18.8) | 1 (2.1) | 22 (33.8) | 0 |
| Nausea | 8 (16.7) | 0 | 16 (24.6) | 0 |
| Abdominal pain | 10 (20.8) | 2 (4.2) | 12 (18.5) | 2 (3.1) |
| Pruritus | 7 (14.6) | 0 | 15 (23.1) | 1 (1.5) |
| Decreased appetite | 8 (16.7) | 0 | 12 (18.5) | 0 |
| Vomiting | 6 (12.5) | 0 | 13 (20.0) | 0 |
| Anemia | 6 (12.5) | 1 (2.1) | 12 (18.5) | 4 (6.2) |
| Dyspnea | 7 (14.6) | 1 (2.1) | 11 (16.9) | 0 |
| Back pain | 3 (6.3) | 0 | 13 (20.0) | 0 |
| Constipation | 7 (14.6) | 0 | 8 (12.3) | 0 |
| Hypothyroidism | 0 | 0 | 10 (15.4) | 0 |
Includes all patients from the dose acceleration, dose escalation, and dose confirmation phases.
Includes all patients from the dose escalation/confirmation cohort and the expansion cohort.
Treatment-related adverse events occurred in 30 of 48 patients (62.5%) in the monotherapy arm and 47 of 65 (72.3%) in the combination arm (Table 3). The most common treatment-related adverse events in the monotherapy arm were infusion-related reaction (18.8%), pruritus (12.5%), and fatigue (8.3%), and in the combination arm were infusion-related reaction (33.8%), fatigue (33.8%), and pruritus (16.9%). Grade 3–4 treatment-related adverse events occurred in nine patients (8.0%): two (4.2%) in the monotherapy arm and seven (10.8%) in the combination arm. Grade 3 bladder perforation and infusion-related reaction were reported in the monotherapy arm (n = 1 each); grade 3 decreased lymphocyte count (n = 2), increased lipase (n = 1), nephritis (n = 1), pneumonitis (n = 1), and pruritus (n = 1) and grade 4 type 1 diabetes mellitus (n = 1) were reported in the combination arm. Serious treatment-related adverse events occurred in four patients (3.5%), including one patient in the monotherapy arm (bladder perforation) and three patients in the combination arm (type 1 diabetes mellitus, nephritis, and pneumonitis in one patient each); two of three patients in the combination arm required treatment with systemic steroids. One patient (2.1%) in the monotherapy arm and five patients (7.7%) in the combination arm discontinued study treatment owing to treatment-related adverse events, including infusion-related reaction (n = 3), pneumonitis (n = 1), increased lipase (n = 1), and colitis (n = 1). No treatment-related deaths occurred.
Table 3.
All-Grade and Grade 3 or 4 Treatment-Related Adverse Events in Patients Receiving MK-4166 Monotherapy and in Combination With Pembrolizumab
| Treatment-Related Adverse Events in ≥5% of Patients in Either Arm, n (%) | Monotherapy Arma n = 48 |
Combination Therapy Armb n = 65 |
||
|---|---|---|---|---|
| All | Grade 3 or 4 | All | Grade 3 or 4 | |
| Any | 30 (62.5) | 2 (4.2) | 47 (72.3) | 7 (10.8) |
| Infusion-related reaction | 9 (18.8) | 1 (2.1) | 22 (33.8) | 0 |
| Pruritus | 6 (12.5) | 0 | 11 (16.9) | 1 (1.5) |
| Fatigue | 4 (8.3) | 0 | 22 (33.8) | 0 |
| Arthralgia | 3 (6.3) | 0 | 6 (9.2) | 0 |
| Nausea | 3 (6.3) | 0 | 6 (9.2) | 0 |
| Rashc | 2 (4.2) | 0 | 8 (12.3) | 0 |
| Hypothyroidism | 0 | 0 | 8 (12.3) | 0 |
| Pneumonitis | 0 | 0 | 4 (6.2) | 1 (1.5) |
Includes all patients from the dose acceleration, dose escalation, and dose confirmation phases.
Includes all patients from the dose escalation/confirmation cohort and the expansion cohort.
Includes rash and maculopapular rash.
The only dose-limiting toxicity possibly related to MK-4166 was the grade 3 bladder perforation that occurred in one patient with neobladder in the monotherapy arm who received MK-4166 at the 30-mg dose; this resolved without intervention. Maximum-tolerated dose was not reached, inclusive of the planned maximum administered dose of 900 mg.
Pharmacokinetics/Pharmacodynamics
MK-4166 pharmacokinetics/pharmacodynamics exhibited target-mediated drug disposition concomitant with decreased GITR availability on T cells in blood with increasing doses (Fig. 1A; Supplementary Table S2). Saturation occurred at a MK-4166 dose ≥10 mg. The incidence of antidrug antibodies was 33%; no correlation was observed between the development of antidrug antibodies and incidence of infusion-related reactions. At a serum MK-4166 concentration of 0.217 μg/mL, 90% GITR engagement was achieved (Fig. 1B). The MK-4166 dose of 10 mg achieved >90% GITR engagement at the trough.
Figure 1.
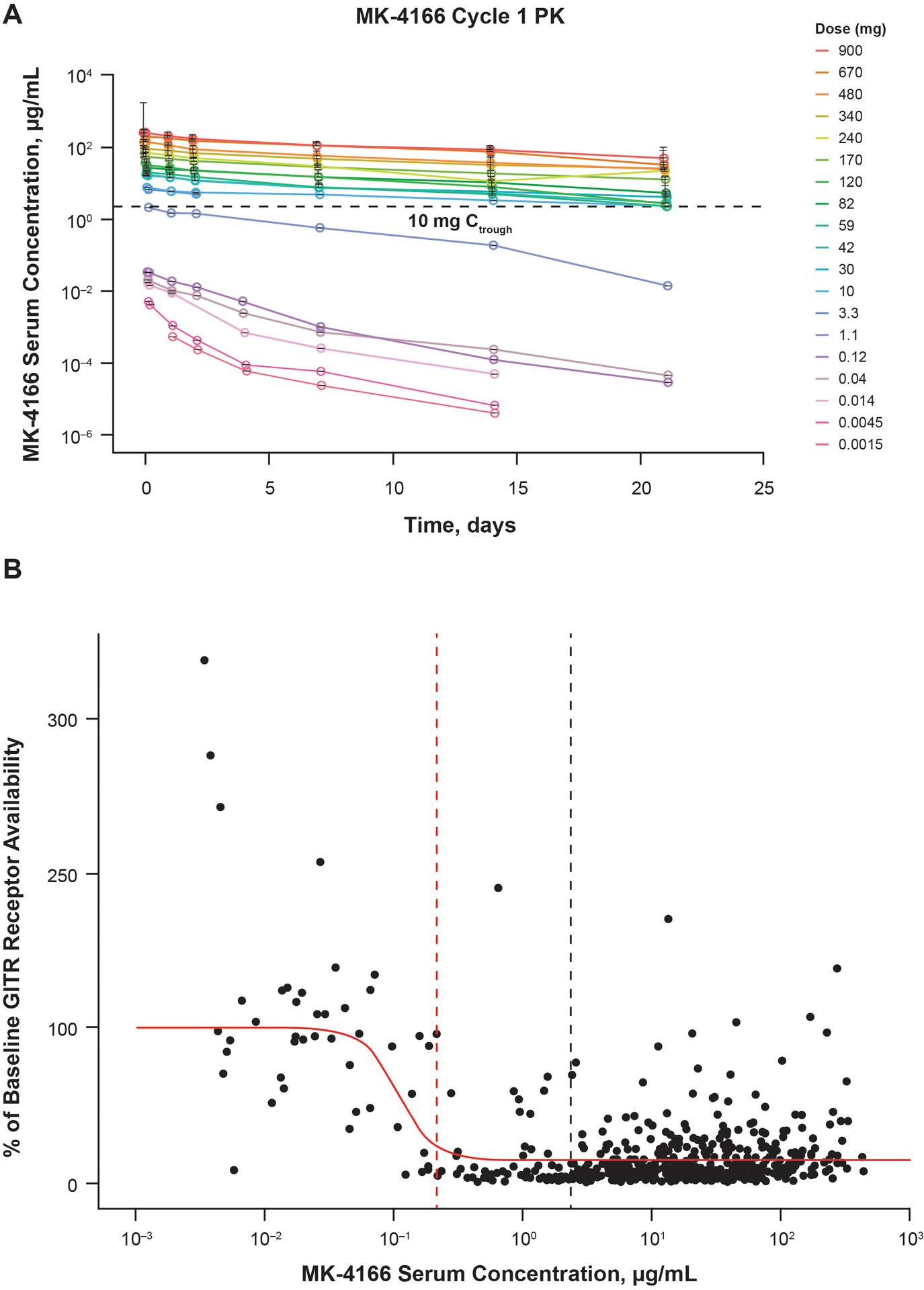
(A) Target-mediated drug disposition and (B) GITR receptor availability in patients with solid tumors treated with MK-4166 alone (0.0015 to 10 mg) and with pembrolizumab (MK-4166 30 to 900 mg). The vertical dashed red line indicates the serum concentration of MK-4166 required for 90% target engagement. The vertical dashed black line indicates the cycle 1 trough serum MK-4166 concentration at a 10-mg dose. Abbreviation: GITR, glucocorticoid-induced tumor necrosis factor receptor.
Efficacy in the Dose Escalation/Confirmation Cohort
Antitumor activity was observed with combination therapy but not with monotherapy. One patient with ICI–naive ovarian carcinoma achieved a confirmed partial response (objective response rate, 2.2%). Three unconfirmed partial responses were reported in patients with anaplastic thyroid carcinoma, head and neck cancer, and anal squamous cell carcinoma; all of these patients were ICI naive. Stable disease was observed in 22.9% of patients in the monotherapy arm and 24.6% in the combination arm. Disease control rate (complete response or partial response or stable disease for ≥6 months) was 0% in the monotherapy arm and 13.8% in the combination arm. Progressive disease was observed in 64.6% of patients in the monotherapy arm and in 52.3% in the combination arm.
Patients and Response in the Expansion Cohort, Including Response by Baseline Gene Expression Profile
Baseline characteristics of the expansion cohort, which comprised 20 patients with advanced melanoma, are reported in Supplementary Table S3. Thirteen patients (65.0%) were naive to ICI therapy and seven (35.0%) had received prior ICI treatment. Disease progression was the primary reason for discontinuing study treatment in the expansion cohort (ICI–naive patients, 30.8%; ICI–treated patients, 85.7%) (Supplementary Table S1).
In the expansion cohort, the confirmed objective response rate was 61.5% in ICI–naive patients, including five complete responses (38.5%) and three partial responses (23.1%); no ICI–treated patients achieved a response (Table 4). Of the three patients with confirmed partial response, decrease in tumor size ranged from −50% to −83% (Fig. 2A; Supplementary Fig. S2). Median time to response in ICI-naive patients who achieved a response was 1.9 months (range, 1.9–4.1). Median duration of response in these patients was 14.5 months (range, 4.3–16.8+ months) (Fig. 2B). All responses lasted at least 3 months, and seven of eight responders (87.5%) had a response duration of ≥6 months.
Table 4.
Summary of Confirmed Responses in the Expansion Cohort (melanoma only)
| ICI naive n = 13 |
ICI treated n = 7 |
|||
|---|---|---|---|---|
| n (%) | 95% CI | n (%) | 95% CI | |
| Objective response rate | 8 (61.5) | 31.6–86.1 | 0 | – |
| Complete response | 5 (38.5) | 13.9–68.4 | 0 | – |
| Partial response | 3 (23.1) | 5.0–53.8 | 0 | – |
| Disease control ratea | 8 (61.5) | 31.6–86.1 | 0 | – |
| Stable disease | 2 (15.4) | 1.9–45.4 | 0 | – |
| Progressive disease | 3 (23.1) | 5.0–53.8 | 7 (100) | 59.0–100 |
Abbreviation: ICI, immune checkpoint inhibitor.
Complete response + partial response + (stable disease ≥6 months).
Figure 2.

(A) Change in baseline tumor size over time in the expansion cohort in patients with advanced melanoma and (B) response and duration of response in ICI-naive patients.
Abbreviations: CR, complete response; ICI, immune checkpoint inhibitor; PR, partial response; SD, stable disease.
Evaluable baseline tumor samples from a proportion of the expansion cohort (n = 17) were assessed using the 18-gene T-cell–inflamed gene expression profile. Six of 17 patients (35.3%) had tumors with low gene expression profile expression status (<–0.318); 4 of 12 (33.3%) of ICI–naive patients had gene expression profile–low tumors. High response rates were observed in ICI–naive patients with both low–gene expression profile (three of four patients; 75%) and non-low–gene expression profile tumors (six of eight patients; 75%) (Supplementary Table S4; Supplementary Fig. S3).
DISCUSSION
This study explored the safety and preliminary efficacy of the GITR agonist monoclonal antibody MK-4166 as monotherapy and in combination with the anti– programmed death 1 monoclonal antibody pembrolizumab in patients with advanced solid tumors. At a dose up to 900 mg, MK-4166 as monotherapy and in combination with pembrolizumab had a tolerable safety profile; however, objective responses were only demonstrated with the combination. Rates of stable disease were similar between the two treatment arms (monotherapy arm, 22.9%; combination arm, 24.6%). In ICI–naive patients with advanced melanoma, we observed responses (objective response rate, 61.5% with five complete responses and three partial responses) and durable antitumor activity (median duration of response, 14.5 months) irrespective of T-cell–inflamed gene expression profile expression status in this small number of patients. This compares favorably with studies of pembrolizumab monotherapy in treatment-naive patients with advanced melanoma, in which objective response rates ranged from 32% to 42% (24–26) and where higher responses were seen in patients with high expression of T-cell–inflamed signature. Cross-trial comparisons should be made with caution, however, as the ICI–naive patients in this study had good baseline prognostic characteristics (low tumor burden and normal lactate dehydrogenase in ≥77% of patients). Although a complete response or partial response was not achieved with MK-4166 monotherapy and the objective response rate was low with MK-4166 plus pembrolizumab in the dose escalation/confirmation cohort, it should be noted that the predominant tumor types in enrolled patients (gastrointestinal [31%] and pancreatic [15%] cancers) are known to respond poorly to currently available immunotherapies (27, 28).
MK-1248, which has the same complementary-determining regions as MK-4166 but with significantly reduced Fc effector function (10), demonstrated a manageable safety profile and some antitumor activity when used in combination with fixed-dose pembrolizumab (200 mg intravenously every 3 weeks) for the treatment of patients with advanced tumors in a phase I trial (NCT02553499) (29). One complete response and two partial responses were observed among 17 patients in the combination arm. These results and the present study may be helpful for designing clinical studies to investigate the antitumor activity of anti–GITR agonist antibodies in combination with ICI in select patients with advanced solid tumors (18, 30).
Anti-GITR antibodies other than MK-4166 and MK-1248 have been evaluated in early-phase clinical trials as monotherapy or combination therapy with ICI (19, 30–32). While these were tolerable, with safety profiles comparable to MK-4166, no objective responses were observed with monotherapy (31–33) and overall response ranged from 0% to 11.1% when used in combination with ICI (31).
In the current study, 33% of patients treated with MK-4166 alone or in combination with pembrolizumab developed antidrug antibodies, a higher incidence than the 2.6%–6.7% reported with other anti-GITR monoclonal antibodies (31, 32). Neutralizing antidrug antibodies were observed in eleven patients (22.9%) in the monotherapy arm and in nine (13.8%) in the combination arm. The evidence to date indicates that antidrug antibody formation does not appear to have a significant impact on the safety or efficacy of MK-4166.
Currently, there are no data supporting the efficacy of monotherapy with anti-GITR antibodies across various tumor types. The results of the current study demonstrated antitumor activity with the combination of MK-4166 and pembrolizumab in patients with ICI–naive melanoma, but this needs to be interpreted with caution owing to the small sample size and the good baseline prognostic characteristics of the patients.
In conclusion, combination therapy with MK-4166 and pembrolizumab has a tolerable safety profile in patients with solid tumors. More data are needed to confirm whether the combination of MK-4166 and pembrolizumab may potentially improve efficacy compared with pembrolizumab alone in ICI–naive patients with advanced melanoma.
Supplementary Material
Translational Relevance.
In this first-in-human phase 1 study, we explored the safety and preliminary efficacy of the GITR agonist monoclonal antibody MK-4166 as monotherapy and in combination with the anti– programmed death 1 monoclonal antibody pembrolizumab in patients with advanced solid tumors. At a dose up to 900 mg, MK-4166 monotherapy and in combination with pembrolizumab had a tolerable safety profile; maximum-tolerated dose was not reached. Responses were observed with combination therapy, primarily in patients with ICI-naive melanoma. More data are needed to determine if the combination of MK-4166 and pembrolizumab improves efficacy compared with pembrolizumab alone in immune checkpoint inhibitor–naive patients with advanced melanoma.
ACKNOWLEDGMENTS
The authors thank the patients and their families and caregivers, all primary investigators, and their site personnel. Funding for this research was provided by Merck Sharp & Dohme Corp., a subsidiary of Merck & Co., Inc., Kenilworth, NJ, USA. Medical writing and/or editorial assistance was provided by Holly C. Cappelli, PhD, CMPP, and Dana Francis, PhD, of the ApotheCom pembrolizumab team (Yardley, PA, USA). This assistance was funded by Merck Sharp & Dohme Corp, a subsidiary of Merck & Co., Inc., Kenilworth, NJ, USA. Karen Autio was supported in part by grants from the NIH/NCI (Cancer Center Support Grant P30 CA008748). The authors also thank Linda Telliho for substantial contribution to operational performance during the study, Terri McClananhan, Wendy Blumenschein, Kate Bohrer, Jennifer Yearley, and Qing Zhao for contributions to GEP biomarker analyses, and Tommy Ruosi Li and Kapil Mayawala for contributions to PK/PD analyses.
Financial Support:
This study was sponsored by Merck Sharp & Dohme Corp., a subsidiary of Merck & Co., Inc., Kenilworth, NJ, USA
Disclosure of Potential Conflicts of Interest
Kyriakos P. Papadopoulos reports funding to START for conduct of clinical trials from AbbVie, Amgen, ArQule, ARMO Biosciences, ADC Therapeutics, Anheart, 3D Medicines, Basilea, Bayer, Calithera Biosciences, Daiichi Sankyo, EMD Serono, F-star, Incyte, Jounce Therapeutics, Linnaeus, MabSpace Biosciences, Merck, Mirati Therapeutics, MedImmune, Mersana, Peleton Therapeutics, Regeneron, Syros Pharmaceuticals, and Tempest Therapeutics; and advisory board fees from ArQule, Basilea, and Bayer.
Karen Autio reports grant support from Merck, Pfizer, Amgen, Astra Zeneca, Tizona, and CytomX for study conduct to institution,
Talia Golan reports grant/research support from AstraZeneca and MSD Merck; consultancy fees from AbbVie, AstraZeneca, Bayer, MSD Merck, and Teva; speakers bureau fees from AbbVie, Bioline, and Roche; and travel fees from AstraZeneca and MSD Merck.
Konstantin Dobrenkov is a stockholder of Merck & Co., Inc., Kenilworth, NJ, USA, and an employee of Merck Sharp & Dohme Corp., a subsidiary of Merck & Co., Inc., Kenilworth, NJ, USA. Elliot Chartash is a stockholder of Merck & Co., Inc., Kenilworth, NJ, USA, and an employee of Merck Sharp & Dohme Corp., a subsidiary of Merck & Co., Inc., Kenilworth, NJ, USA.Qiusheng Chen is an employee of Merck Sharp & Dohme Corp., a subsidiary of Merck & Co., Inc., Kenilworth, NJ, USA.
Richard Wnek is an employee of Merck Sharp & Dohme Corp., a subsidiary of Merck & Co., Inc., Kenilworth, NJ, USA.
Georgina V. Long received consultant fees from Aduro, Amgen, Bristol-Myers Squibb, MassARRAY, Merck, MDS, Novartis, OncoSec Medical, Pierre Fabre, Roche, QBiotics, SkylineDx, and Sandoz.
REFERENCES
- 1.Topalian SL, Drake CG, Pardoll DM. Immune checkpoint blockade: a common denominator approach to cancer therapy. Cancer Cell 2015;27:450–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bavencio (avelumab) injection, for intravenous use [package insert]. Rockland, MA: EMD Serono Inc; 2019. [Google Scholar]
- 3.KEYTRUDA® (pembrolizumab) injection, for intravenous use, 06/2020. Merck Sharp & Dohme, Corp., Whitehouse Station, NJ, USA: 2020. [Google Scholar]
- 4.Yervoy (ipilimumab) injection, for intravenous use [package insert]. Princeton, NJ: Bristol-Myers Squibb Company; 2020. [Google Scholar]
- 5.Opdivo (nivolumab) injection, for intravenous use [package insert]. Princeton, NJ: Bristol-Myers Squibb Company; 2020. [Google Scholar]
- 6.Farkona S, Diamandis EP, Blasutig IM. Cancer immunotherapy: the beginning of the end of cancer? BMC Med 2016;14:73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Nocentini G, Giunchi L, Ronchetti S, Kruasz LT, Bartoli A, Moraca R, et al. A new member of the tumor necrosis factor/nerve growth factor receptor family inhibits T cell receptor-induced apoptosis. Proc Natl Acad Sci U S A 1997;94:6216–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Liu B, Li Z, Mahesh SP, Pantanelli S, Hwang FS, Siu WO, et al. Glucocorticoid-induced tumor necrosis factor receptor negatively regulates activation of human primary natural killer (NK) cells by blocking proliferative signals and increasing NK cell apoptosis. J Biol Chem 2008;283:8202–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Schoenhals JE, Cushman TR, Barsoumian HB, Li A, Cadena AP, Niknam S, et al. Anti-glucocorticoid-induced tumor necrosis factor-related protein (GITR) therapy overcomes radiation-induced Treg immunosuppression and drives abscopal effects. Front Immunol 2018;9:2170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Sukumar S, Wilson DC, Yu Y, Wong J, Naravula S, Ermakov G, et al. Characterization of MK-4166, a clinical agonistic antibody that targets human GITR and inhibits the generation and suppressive effects of t regulatory cells. Cancer Res 2017;77:4378–88. [DOI] [PubMed] [Google Scholar]
- 11.Schaer DA, Murphy JT, Wolchok JD. Modulation of GITR for cancer immunotherapy. Curr Opin Immunol 2012;24:217–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Narumi K, Miyakawa R, Shibasaki C, Henmi M, Mizoguchi Y, Ueda R, et al. Local administration of GITR agonistic antibody induces a stronger antitumor immunity than systemic delivery. Sci Rep 2019;9:5562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Clouthier DL, Watts TH. Cell-specific and context-dependent effects of GITR in cancer, autoimmunity, and infection. Cytokine Growth Factor Rev 2014;25:91–106. [DOI] [PubMed] [Google Scholar]
- 14.Shimizu J, Yamazaki S, Takahashi T, Ishida Y, Sakaguchi S. Stimulation of CD25(+)CD4(+) regulatory T cells through GITR breaks immunological self-tolerance. Nat Immunol 2002;3:135–42. [DOI] [PubMed] [Google Scholar]
- 15.Vence L, Bucktrout SL, Fernandez Curbelo I, Blando J, Smith BM, Mahne AE, et al. Characterization and comparison of GITR expression in solid tumors. Clin Cancer Res 2019;25:6501–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ko K, Yamazaki S, Nakamura K, Nishioka T, Hirota K, Yamaguchi T, et al. Treatment of advanced tumors with agonistic anti-GITR mAb and its effects on tumor-infiltrating Foxp3+CD25+CD4+ regulatory T cells. J Exper Med 2005;202:885–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Cohen AD, Diab A, Perales MA, Wokchok JD, Rizzuto G, Merghoub T, et al. Agonist anti-GITR antibody enhances vaccine-induced CD8(+) T-cell responses and tumor immunity. Cancer Res 2006;66:4904–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Knee DA, Hewes B, Brogdon JL. Rationale for anti-GITR cancer immunotherapy. Eur J Can 2016;67:1–10. [DOI] [PubMed] [Google Scholar]
- 19.Zappasodi R, Sirard C, Li Y, Budhu S, Abu-Akeel M, Liu C, et al. Rational design of anti-GITR-based combination immunotherapy. Nat Med 2019;25:759–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lu L, Xu X, Zhang B, Hongzan J, Wang X. Combined PD-1 blockade and GITR triggering induce a potent antitumor immunity in murine cancer models and synergizes with chemotherapeutic drugs. J Transl Med 2014;12:36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Eisenhauer EA, Therasse P, Bogaerts J, Schwartz LH, Sargent D, Ford R, et al. New response evaluation criteria in solid tumours: revised RECIST guideline (version 1.1). Eur J Cancer 2009;45:228–47. [DOI] [PubMed] [Google Scholar]
- 22.National Cancer Institute: Common Terminology Criteria for Adverse Events (CTCAE) Version 4. https://evs.nci.nih.gov/ftp1/CTCAE/CTCAE_4.03/Archive/CTCAE_4.0_2009-05-29_QuickReference_8.5×11.pdf. Published May 28, 2009. Accessed June 24, 2020
- 23.Ayers M, Lunceford J, Nebozhyn M, Murphy E, Loboda A, Kaufman DR, et al. IFN-gamma-related mRNA profile predicts clinical response to PD-1 blockade. J Clin Invest 2017;127:2930–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hamid O, Robert C, Daud A, Hodi FS, Hwu WJ, Kefford R, et al. Five-year survival outcomes for patients with advanced melanoma treated with pembrolizumab in KEYNOTE-001. Ann Oncol 2019;30:582–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Robert C, Ribas A, Schachter J, Arance A, Grob J-J, Mortier L, et al. Pembrolizumab versus ipilimumab in advanced melanoma (KEYNOTE-006): post-hoc 5-year results from an open-label, multicentre, randomised, controlled, phase 3 study. Lancet Oncol 2019;20:1239–51. [DOI] [PubMed] [Google Scholar]
- 26.Long G, Dummer R, Hamid O, Gajewski TF, Caglevic C, Dalle S, et al. Epacadostat plus pembrolizumab versus placebo plus pembrolizumab in patients with unresctable or metatstic melanome (ECHO-301/KEYNOTE-252): a phase 3, randomised, double-blind study. Lancet Oncol 2019;20:1083–97. [DOI] [PubMed] [Google Scholar]
- 27.Procaccio L, Schirripa M, Fassan M, Vecchione L, Bergamo F, Prete AA, et al. Immunotherapy in gastrointestinal cancers. Biomed Res Int 2017;4346576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Upadhrasta S, Zheng L. Strategies in developing immunotherapy for pancreatic cancer: recognizing and correcting multiple immune “defects” in the tumor microenvironment. J Clin Med 2019;8:1472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Geva R, Voskoboynik M, Beebe AM, Gwo J, Dobrenkov K, Chartash EK, et al. First-in-human phase 1 study of MK-1248, an anti-human glucocorticoid-induced tumor necrosis factor receptor (GITR) monoclonal antibody, as monotherapy or in combination with pembrolizumab in patients with advanced solid tumors. J Clin Oncol 2018;36:3029. [DOI] [PubMed] [Google Scholar]
- 30.Spranger S, Gajewski TF. Mechanisms of tumor cell–intrinsic immune evasion. Ann Rev Cancer Biol 2018;2:213–28. [Google Scholar]
- 31.Heinhuis KM, Carlino M, Joerger M, Di Nicola M, Meniawy T, Rottey S, et al. Safety, tolerability, and potential clinical activity of a glucocorticoid-induced TNF receptor-related protein agonist alone or in combination with nivolumab for patients with advanced solid tumors: a phase 1/2a dose-escalation and cohort-expansion clinical trial. JAMA Oncol 2020;6:100–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Tran B, Carvajal RD, Marabelle A, Pravin Patel S, LoRusso PM, Rasmussen E, et al. Dose escalation results from a first-in-human, phase 1 study of glucocorticoid-induced TNF receptor-related protein agonist AMG 228 in patients with advanced solid tumors. J Immunother Cancer 2018;6:93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Denlinger CS, Infante JR, Aljumaily R, Neing A, Chintakuntlawar A, Rizvi NA, et al. A phase I study of MEDI1873, a novel GITR agonist, in advanced solid tumors. Ann Oncol 2018;29:viii400–1. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


