Abstract
Purpose:
While infections are common following left ventricular assist device (LVAD) implant, the relationship between timing and category of first infection and mortality is less well understood.
Methods:
The Society of Thoracic Surgeons Intermacs patients receiving a primary LVAD from 4/2012 to 5/2017 were included, with follow-up through 10/2017. The primary exposure was defined in 3 ways: any infection, timing of first infection (early: ≤90 days; intermediate: 91–180 days; late: >180 days), and type of first infection (VAD-specific, VAD-related, non-VAD). The association between first infection and all-cause mortality was estimated using Cox proportional hazards regression, adjusting for comorbidities & post-implant adverse events.
Results:
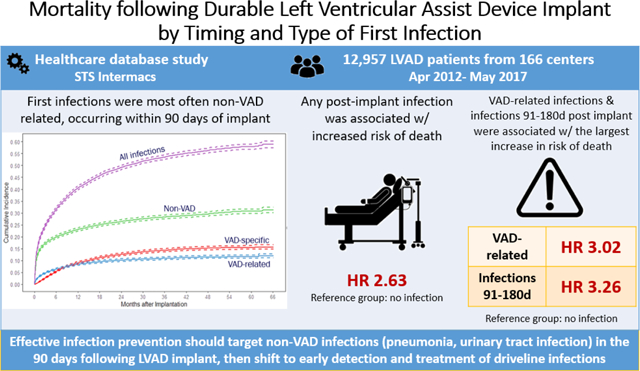
The study cohort included 12,957 patients (228,742 person-months) implanted (destination therapy: 47.4%, bridge-to-transplant: 41.2%) at 166 centers. First infections were most often non-VAD (54.2%), followed by VAD-specific (25.4%) & VAD-related (20.4%). Rates of first infection (per 100 person-months) were highest in the early interval (10.7) versus intermediate (3.7), or late (2.7), p<0.001. Relative to patients without infection, patients with any infection had a significantly higher adjusted hazard of death [HR 2.63 (2.46, 2.86)]. First infection in the intermediate interval was associated with the largest increase in adjusted hazard of death [HR 3.26 (2.82–3.78)], followed by late [HR 3.13 (2.77, 3.53)] and early intervals [HR 2.37 (2.16, 2.60)]. VAD-related infections were associated with the largest increase in hazard of death [HR 3.02 (2.69, 3.40)], followed by VAD-specific [HR 2.92 (2.57, 3.32)], and non-VAD [HR 2.42 (2.20, 2.65)].
Conclusions:
Relative to those without infection, patients with any post-implant infection had an increased risk of death. VAD-related infections and infections occurring in the intermediate interval were associated with the largest increase in risk of death.
Keywords: mechanical circulatory support, ventricular assist device, infection, mortality
Graphical Abstract
Central Picture Legend:First infections were most often non-VAD related, occurring within 90 days of implant
INTRODUCTION
Left ventricular assist devices (LVAD) provide life-saving and quality of life benefits for patients with advanced heart failure.1 Large-scale interhospital variability exists in infections and associated spending, both of which hinder broad dissemination of this therapy.2 Newer LVAD pump technology has significantly reduced the risk of post-implant pump thrombosis and stroke, yet the risk of infections remains a leading cause of morbidity and mortality over the duration of a patient’s LVAD support.1 At 2 years, major infections occurred among 58% of HeartMate 3 and 56% HeartMate II patients (p=0.57) in the recent MOMENTUM 3 (Multi-center Study of MagLev Technology in Patients Undergoing MCS Therapy With HeartMate 3™) trial3 and 52% of all patients in the most recent The Society of Thoracic Surgeons Interagency Registry for Mechanically Assisted Circulatory Support (“STS-Intermacs”) experience.4
Despite higher mortality rates at hospitals with high versus low infection rates,5 further investigation is warranted to account for other contributing factors. First, prior reports have not fully adjusted for the effect of a patient’s comorbid conditions and other post-implant adverse events.3,6 Second, the role of timing and VAD-relatedness (VAD-specific, VAD-related, non-VAD) of a first infection on subsequent mortality has not been fully evaluated.3,6 Addressing these knowledge gaps would both inform targets for infection prevention and treatment strategies, as well as advance efforts to more broadly disseminate this life-saving therapy to diverse target populations.
Using STS-Intermacs data to identify patients who received a primary, durable LVAD from April 1, 2012, to May 4, 2017, the objectives of this study were to investigate: 1) the timing of first infection stratified by infection type, and 2) the risk of death associated with infection stratified by the timing and type of infection. It was hypothesized that post-implant infections would be associated with an increased risk of death and that the risk of death would vary as a function of the time of occurrence of the first infection and infection type (i.e., VAD-related or non-VAD related).
Methods
Data source
The structure and registry process for data collection and elements within STS-Intermacs have previously been reported.7 The study sample included adult patients ≥19 years of age implanted with a durable, primary continuous flow LVAD, with or without a right ventricular assist device (RVAD). Patients were excluded if they were implanted with a pulsatile device, received isolated RVAD placement, total artificial heart, or if the LVAD was not placed as a primary implant (Supplemental figure 1). Records were obtained between April 1, 2012 through May 4, 2017, with follow up through October 31, 2017, where available.
Using STS-Intermacs definitions, patient-level data were obtained, including demographics (e.g. age, sex, race/ethnicity), characteristics pre-implant (height, weight, Intermacs profile), VAD implant indication, comorbidities (e.g. hypertension, diabetes, history of stroke), and laboratory studies. Follow-up data of post-implant adverse events included the occurrence of infections, right heart failure, device exchange, re-operation for any reason, renal dysfunction (including new acute renal injury and dialysis), stroke, as well as cause and date of death.
Informed consent for registrant participation in STS-Intermacs was required until the implementation of protocol v4.0 (February 27, 2014). The research use of STS-Intermacs data was approved by the University of Michigan Medical School Institutional Review Board (HUM00155687, approved 2/4/2019).
Exposure and Clinical Outcomes
The exposure of interest was the first post-implant infection (using STS-Intermacs’ definition for Major Infection Adverse Event). Infection types were categorized as: VAD-specific (exit cannula, pump pocket, pump interior, or driveline), VAD-related (positive blood culture, mediastinum, or line sepsis), and non-VAD (pulmonary, urine, gastrointestinal, peripheral wound, other, or unknown). A priority rule was a priori implemented to classify multiple concomitant infections based on assumed infection severity: VAD-specific > VAD-related > non-VAD.
The timing of first infection following implant was divided into early (≤90 days), intermediate (91–180 days), and late (>180 days) intervals.
The primary outcome was all-cause mortality following the first infection. Patients were censored at transplant, explant (no new device) or device decommissioning (i.e., termination of pump support without removal), lost to follow-up, or at end of follow-up at study closure.
Statistical Analysis
Baseline patient characteristics were summarized as the mean (standard deviation) for continuous variables and number (%) for categorical variables. Group comparison for continuous and categorical variables was conducted using the nonparametric Kruskal-Wallis test and the Chi-square test, respectively. The crude incidence rates of first infection per 100 patient-months of support by timing and category of the first infection were calculated.
Crude cause-specific cumulative incidence functions of the first infection during the entire follow-up period were calculated, with death, transplant, and explant (no new device or decommissioning) treated as competing risks of the first infection. Stratified Cox proportional hazards models were used to study the effect of first infection (vs. no infection) on mortality, by timing and category of the first infection. The Cox models were stratified by hospitals and adjusted for baseline patient risk factors and post-implant time-dependent covariates including stroke, new right heart failure (in the 30d following implant), renal dysfunction or dialysis, device exchange, and re-operation (for any reason). The likelihood ratio test was used to examine whether the effect of first infection on mortality differed by timing and type of the first infection.
Analyses were conducted using R 3.2.5 and SAS 9.4 software. All statistical tests are two-sided with the significance level set at 0.05.
RESULTS
Patient demographics
The cohort included 12,957 patients at 166 centers who were enrolled in STS-Intermacs between April 1, 2012 through May 4, 2017, Supplemental Figure 1. Patients were predominantly male (78.4%) and white (66.1%) with a mean age of 57.3 (SD 12.8), Table 1. Intermacs Profile was 2 or 3 for the majority of patients (34.7% and 34.48%, respectively). The most common device strategy was destination therapy (47.4%) followed by bridge-to-transplant (41.2%). Axial flow devices were implanted in 73.8% of patients. There were statistical, although not clinically meaningful, differences between the first infection type and some baseline characteristics. The baseline characteristics for patients who did and did not have an infection in the follow-up period are reported in Supplemental Table 1.
Table 1.
Patient Characteristics for the Overall Cohort (N=12,957) and by Infection Type
| Overall | First Infection Type (n=6,378) | p-value | |||
|---|---|---|---|---|---|
| VAD-specific | VAD-related | Non-VAD | |||
| Number of Patients | 12,957 | 1,617 | 1,303 | 3,458 | |
| Demographics | |||||
| Age (years), mean (SD) | 57.3 (12.8) | 53.9 (13.7) | 57.6 (12.9) | 58.7 (12.4) | <.0001 |
| Female Sex, n (%) | 2765 (21.4) | 352 (21.8) | 245 (18.8) | 864 (25.0) | <.0001 |
| Race, n (%) | 0.0009 | ||||
| White | 8558 (66.1) | 998 (61.7) | 868 (66.6) | 2291 (66.3) | |
| African-American | 3181 (24.6) | 482 (29.8) | 326 (25.0) | 843 (24.4) | |
| Other | 1218 (9.4) | 137 (8.5) | 109 (8.4) | 324 (9.4) | |
| Hispanic Ethnicity, n (%) | 836 (6.6) | 102 (6.4) | 82 (6.4) | 229 (6.7) | 0.8510 |
| Indication | |||||
| Device Strategy, n (%) | <.0001 | ||||
| Group 1 (Bridge to Transplant) | 5338 (41.2) | 663 (41.0) | 508 (39.0) | 1242 (35.9) | |
| Group 2 (Bridge to Candidacy) | 1482 (11.4) | 219 (13.5) | 138 (10.6) | 389 (11.3) | |
| Group 3 (Destination therapy) | 6137 (47.4) | 735 (45.5) | 657 (50.4) | 1827 (52.8) | |
| Device Brand, n (%) | 0.0009 | ||||
| HeartMate II LVAS (Axial) | 9562 (73.8) | 1253 (77.5) | 933 (71.6) | 2617 (76.7) | |
| HeartWare HVAD (Centrifugal) | 3395 (26.2) | 364 (22.5) | 370 (28.4) | 841 (24.3) | |
| Patient Characteristics | |||||
| Body mass index (Kg/M2), mean (SD) | 28.6 (7.2) | 29.1 (7.4) | 29.5 (8.4) | 28.8 (7.0) | 0.1164 |
| INTERMACS Profile, n (%) | <.0001 | ||||
| Profile 1 | 1805 (13.9) | 169 (10.5) | 186 (14.3) | 533 (15.3) | |
| Profile 2 | 4522 (34.9) | 524 (32.4) | 480 (36.8) | 1273 (36.8) | |
| Profile 3 | 4563 (35.2) | 653 (40.4) | 453 (34.8) | 1109 (32.1) | |
| Profile 4–7 | 2061 (15.9) | 271 (16.8) | 184 (14.1) | 543 (15.7) | |
| Primary Diagnosis, n (%) | <.0001 | ||||
| Etiology 1 | 61 (0.5) | 7 (0.4) | 6 (0.5) | 20 (0.6) | |
| Etiology 2 | 5806 (45.2) | 657 (40.3) | 581 (44.9) | 1670 (48.6) | |
| Etiology 3 | 6734 (52.4) | 918 (57.2) | 681 (52.7) | 1679 (49.9) | |
| Etiology 4 | 258 (2.0) | 32 (2.0) | 25 (1.9) | 67 (2.0) | |
| Left ventricular ejection fraction, n (%) | 0.0014 | ||||
| >=50% | 15 (0.1) | 1 (0.1) | 3 (0.2) | 3 (0.1) | |
| 40–49% | 60 (0.5) | 6 (0.4) | 7 (0.5) | 16 (0.5) | |
| 30–39% | 456 (3.5) | 45 (2.8) | 43 (3.3) | 138 (4.0) | |
| 20–29% | 3173 (24.5) | 352 (21.8) | 364 (27.9) | 899 (26.0) | |
| <20% | 8569 (66.1) | 1118 (69.1) | 831 (63.8) | 2213 (64.0) | |
| Not Recorded/Documented or Unknown | 684 (5.3) | 95 (5.9) | 55 (4.2) | 189 (5.5) | |
| Co-morbidities | |||||
| Severe diabetes, n (%) | 1252 (9.7) | 148 (9.2) | 138 (10.6) | 416 (12.1) | 0.0078 |
| Frailty, n (%) | 840 (6.6) | 60 (3.8) | 96 (7.4) | 263 (7.7) | <.0001 |
| Pulmonary disease, n (%) | 1229 (9.6) | 150 (9.4) | 136 (10.5) | 405 (11.9) | 0.0251 |
| Peripheral vascular disease, n (%) | 604 (4.7) | 71 (4.4) | 66 (5.1) | 211 (6.2) | 0.0307 |
| Laboratory Studies | |||||
| Serum creatinine (mg/dL), mean (SD) | 1.4 (0.7) | 1.3 (0.6) | 1.5 (0.7) | 1.4 (0.7) | <.0001 |
| Albumin (g/dL), mean (SD) | 3.4 (0.6) | 3.5 (0.6) | 3.4 (0.6) | 3.4 (0.6) | 0.0306 |
| Total bilirubin (mg/dL), mean (SD) | 1.3 (1.8) | 1.2 (1.1) | 1.4 (1.8) | 1.4 (2.1) | 0.3743 |
| Implant Year, n (%) | 0.0003 | ||||
| 2012 | 1593 (12.3) | 266 (16.5) | 164 (12.6) | 501 (14.5) | |
| 2013 | 2516 (19.4) | 356 (22.0) | 276 (21.2) | 770 (22.3) | |
| 2014 | 2615 (20.2) | 375 (23.2) | 280 (21.5) | 752 (21.8) | |
| 2015 | 2888 (22.3) | 372 (23.0) | 325 (24.9) | 731 (21.1) | |
| 2016 | 2568 (19.8) | 211 (13.1) | 206 (15.8) | 573 (16.6) | |
| 2017 | 777 (6.0) | 37 (2.3) | 52 (4.0) | 131 (3.8) | |
Primary Diagnosis: Etiology 1 includes congenital heart disease. Etiology 2 includes coronary artery disease and ischemic dilated cardiomyopathy. Etiology 3 includes non-ischemic dilated cardiomyopathy ( e.g., adriamycin, alcoholic, familial, idiopathic, myocarditis, post-partum, viral and valvular). Etiology 4 includes cancer and restrictive myopathies (e.g., amyloidosis, sarcoidosis, idiopathic, hypertrophic cardiomyopathy, and radiation).
P-value refers to the comparison between the three first infection categories and calculated from Kruskal-Wallis test (continuous variables) and Pearson's Chi-squared test (categorical variables)
A priority rule was established a priori to classify multiple concomitant infections based on assumed infection severity: VAD-specific > VAD-related > non-VAD.
Among the 6378 patients who developed an infection, 2,287 (35.86%) died. Neurological dysfunction was the most common cause of death for patients with VAD-specific and VAD-related infections, while multisystem organ failure was the most common cause of death for Non-VAD infections (Supplemental Table 2).
First Infection by Type and Timing of Onset
A total of 6378 patients (49.2%) developed an infection during the follow-up period. Non-VAD infections were the most common first infection (54.2%), followed by VAD-specific (25.3%), and VAD-related (20.4%) infections (Table 1).
Overall, the rate (per 100 person-months) of the first infection was 4.54 (Table 2). Rates of first infections (per 100 person-months) were highest in the early interval (10.7), followed by the intermediate (3.7) and late intervals (2.7), p<0.001.
Table 2.
Rates of First infection by Type, Location, and Timing
| First VAD Infection Type | Location | Overall | Early ( ≤ 90 days) | Intermediate (91–180 days) | Late ( > 180 days) | p-value | ||||
|---|---|---|---|---|---|---|---|---|---|---|
| Events (N) | Rate | Events (N) | Rate | Events (N) | Rate | Events (N) | Rate | |||
| All First Infection | 6378 | 4.54 | 3194 | 10.72 | 849 | 3.67 | 2335 | 2.66 | <.0001 | |
| VAD specific | 1617 | 1.15 | 347 | 1.16 | 330 | 1.43 | 940 | 1.07 | <.0001 | |
| Pump/Related: Driveline or Percutaneous Lead | 1454 | 1.03 | 289 | 0.97 | 302 | 1.31 | 863 | 0.98 | <.0001 | |
| Pump/Related: Pump Pocket | 181 | 0.13 | 61 | 0.2 | 32 | 0.14 | 88 | 0.1 | 0.0002 | |
| Pump/Related: Exit Cannula | 30 | 0.02 | 11 | 0.04 | 5 | 0.02 | 14 | 0.02 | 0.1300 | |
| Pump/Related: Pump Interior | 23 | 0.02 | 5 | 0.02 | 3 | 0.01 | 15 | 0.02 | 0.9000 | |
| VAD related | 1517 | 1.08 | 675 | 2.27 | 242 | 1.05 | 600 | 0.68 | <.0001 | |
| Blood Cultures, Positive | 1284 | 0.91 | 507 | 1.7 | 219 | 0.95 | 558 | 0.64 | <.0001 | |
| Mediastinum | 166 | 0.12 | 134 | 0.45 | 15 | 0.06 | 17 | 0.02 | <.0001 | |
| Line Sepsis | 145 | 0.1 | 83 | 0.28 | 18 | 0.08 | 44 | 0.05 | <.0001 | |
| Non-VAD | 3921 | 2.79 | 2452 | 8.23 | 398 | 1.72 | 1071 | 1.22 | <.0001 | |
| Pulmonary | 1441 | 1.02 | 1036 | 3.48 | 95 | 0.41 | 310 | 0.35 | <.0001 | |
| Urine | 1062 | 0.76 | 676 | 2.27 | 102 | 0.44 | 284 | 0.32 | <.0001 | |
| Gastrointestinal | 433 | 0.31 | 257 | 0.86 | 55 | 0.24 | 121 | 0.14 | <.0001 | |
| Peripheral Wound | 145 | 0.1 | 65 | 0.22 | 22 | 0.1 | 58 | 0.07 | <.0001 | |
| Other | 847 | 0.6 | 411 | 1.38 | 132 | 0.57 | 304 | 0.35 | <.0001 | |
| Unknown | 222 | 0.16 | 150 | 0.5 | 20 | 0.09 | 52 | 0.06 | <.0001 | |
Infection rates are represented as per 100 person-months.
P-value refers to the comparison between early, intermediate and late infection rates and calculated from the likelihood ratio test.
In the early interval, the rate of first non-VAD infections (8.23/100 person-months) was significantly higher than both VAD-related (2.27/100 person-months) and VAD-specific infections (1.16/100 person-months), p<0.0001. Pneumonia (rate:1.02/100 person-months) and urinary tract infections (rate:0.76/100 person-months) were the most common non-VAD infections.
In the intermediate interval, rates of first non-VAD infection remained highest among all infection categories (1.72/100 person-months), followed by VAD specific (1.43/100 person-months) and VAD-related (1.05/100 person-months) infections.
In the late interval, rates of first non-VAD infection were highest (1.22/100 person-months), followed by VAD specific (1.07/100 person-months) and VAD-related (0.68/100 person-months) infections.
The early interval (≤90d) had the highest first infection rates in all infection categories (VAD-specific, VAD-related, non-VAD). Compared to the early interval, rates of non-VAD and VAD-related first infections decreased in the intermediate interval (91–180d), with non-VAD infections seeing a much larger decline (8.23 to 1.72/100 person-months) compared to VAD-related (2.27 to 1.05/100 person-months). Conversely, rates of VAD-specific infection saw an increase from the early (1.16/100 person-months) to intermediate (1.43/100 person-months) interval, mostly driven by an increase in driveline infections (p<.0001, Table 2). In the late interval (>180d), infection rates decreased in all three categories.
The cumulative incidence of first infection by category over the post-implant follow-up period is displayed in Figure 1. Non-VAD infections had the highest cumulative incidence, followed by VAD-specific and VAD-related infections.
Figure 1.
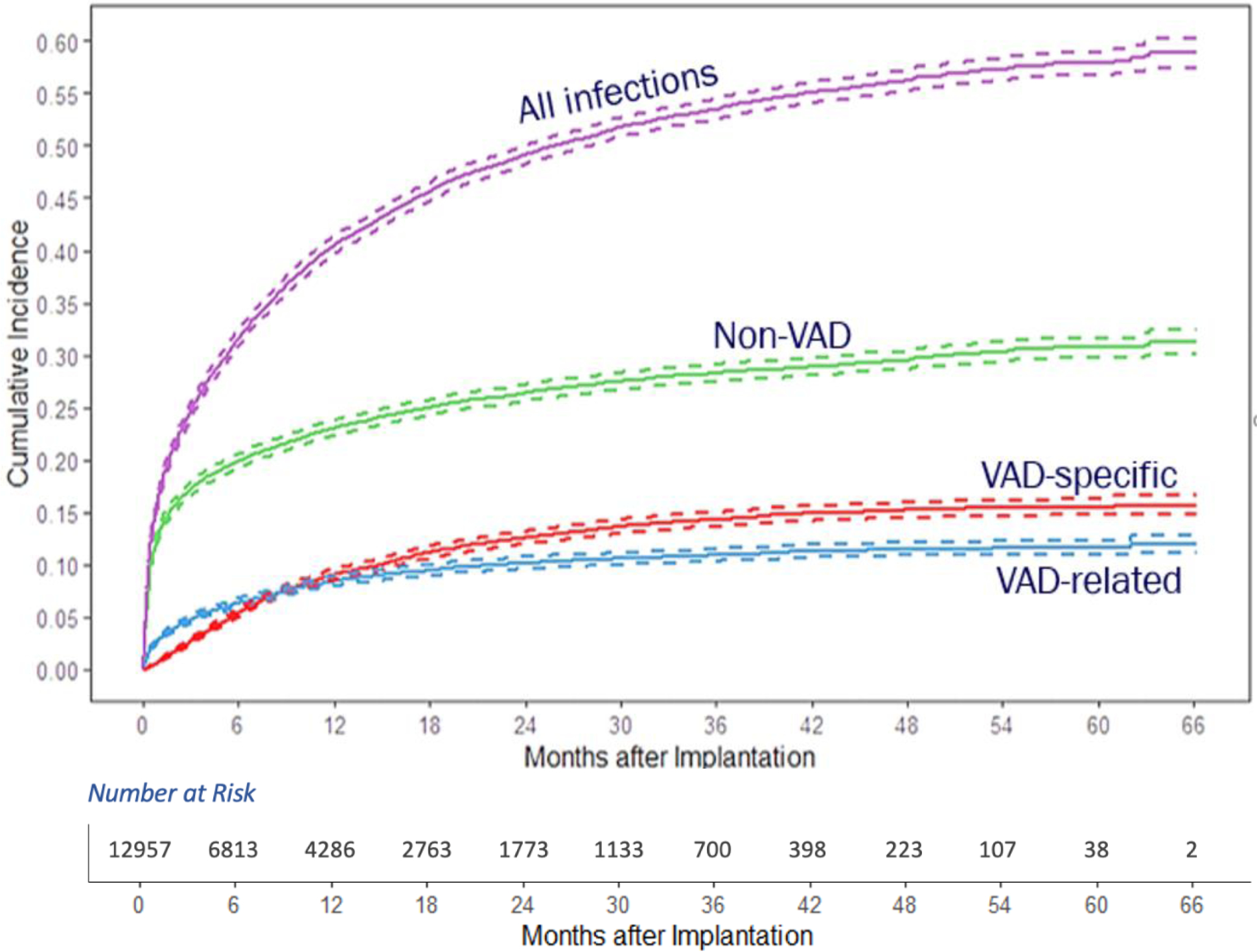
Cumulative Incidence of First Infection Following VAD Implant by Infection Type
Impact of First Infection on Mortality
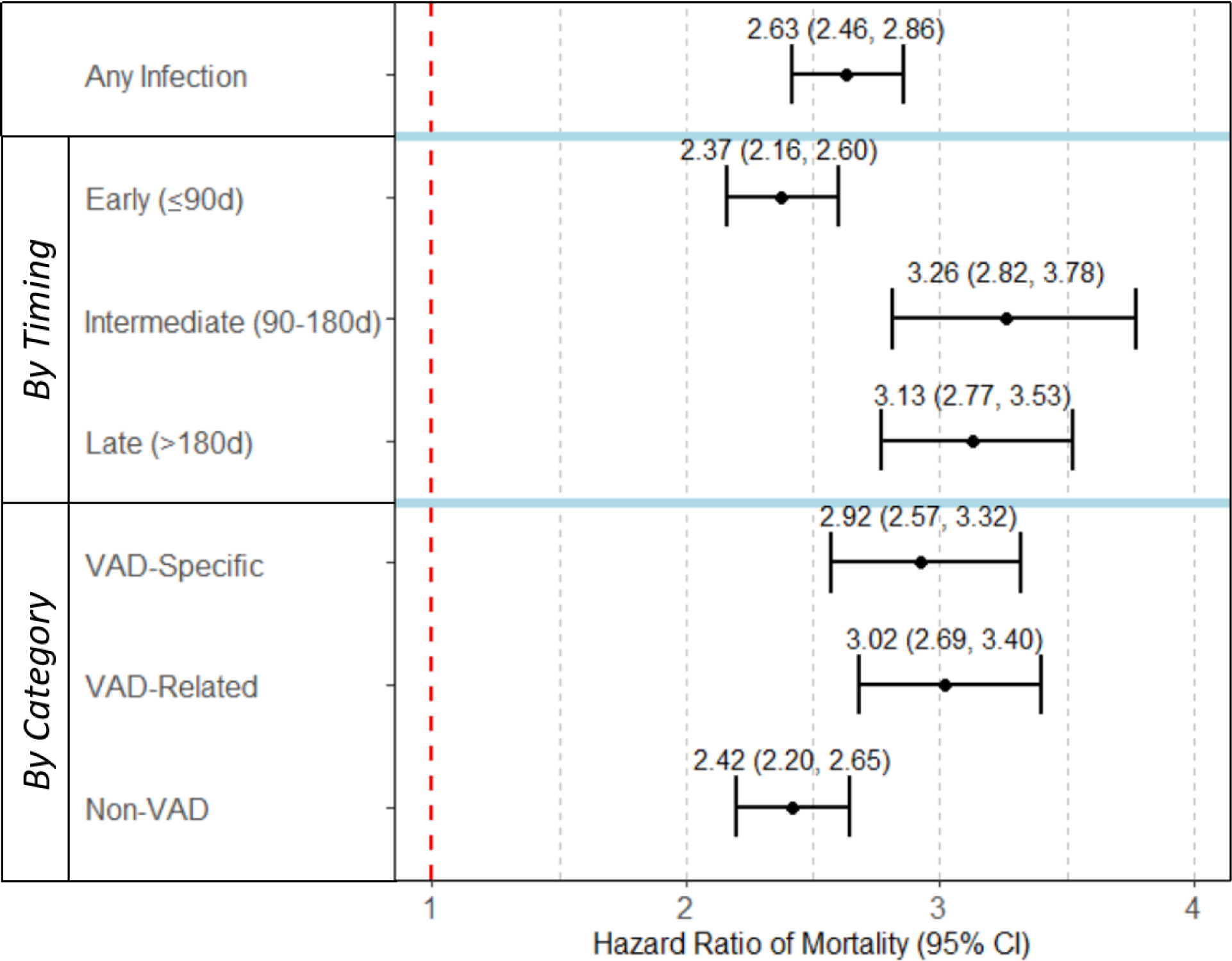
Compared to those without infection, patients who had any infection during the follow-up time had a significantly higher adjusted hazard of death (HR 2.63, 95% CI 2.46 – 2.86), Table 3. The adjusted hazard ratios of any infection, by timing and infection category, are displayed in Figure 2.
Table 3.
Impact of First Infection on Mortality by 3 Primary Exposures: Any Infection, by First Infection Timing, and by First Infection Category
| HR (95% CI) | p-value | ||
|---|---|---|---|
| Model 1 | Any infection | 2.63 (2.46, 2.86) | <.0001 |
| (Any Infection) | |||
| Model 2 | Early interval first infection | 2.37 (2.16, 2.60) | <.0001 |
| (By Timing) | Intermediate interval first infection | 3.26 (2.82, 3.78) | <.0001 |
| Late interval first infection | 3.13 (2.77, 3.53) | <.0001 | |
| LRT: p <.0001 | |||
| Model 3 | VAD-specific first infection | 2.92 (2.57, 3.32) | <.0001 |
| (By Category) | VAD-related first infection | 3.02 (2.69, 3.40) | <.0001 |
| Non-VAD first infection | 2.42 (2.20, 2.65) | <.0001 | |
| LRT: p <.0001 | |||
The reference group is the group that had no infection in the entire follow up time period
P-value is calculated from the Wald-test.
LRT: likelihood ratio test, p value refers to the significance compare whether the effect of first infection on mortality is different by timing/by category.
Figure 2.
The Effect of First Infection following LVAD-implant on Subsequent Mortality
Compared to those without infection, patients whose first infection occurred in the intermediate interval had the highest increase in adjusted hazard of death (HR 3.26, 95% CI 2.82 – 3.78), followed by the late interval (HR 3.13, 95% CI 2.77 – 3.53, Table 3). The adjusted hazard of mortality differed by the timing of first infection, p<0.001. Of the three time intervals, an infection in the early interval carried the lowest adjusted hazard of death (HR 2.37, 95% 2.16 – 2.60).
Compared to patients without infection, those who had a VAD-related first infection had the highest increase in adjusted hazard of death (HR 3.02, 95% CI 2.69 – 3.40), followed by VAD-specific (HR 2.92 95% CI 2.57 – 3.32) infections, Table 3. The adjusted hazard of mortality differed by category of the first infection, p<0.001). Of the three infection categories, non-VAD first infections carried the lowest adjusted hazard of death (HR 2.42, 95% CI 2.20 – 2.65).
DISCUSSION
Three key findings emerged from this large, population-based evaluation of mortality following the first infection after durable VAD implant. First, relative to patients without infection, patients with any infection had a significantly higher risk of death (HR 2.63). Second, first infections in the intermediate interval (90–180d post-implant) were associated with the largest increase in risk of death (HR 3.26), followed by late (>180d post-implant, HR 3.13) and early intervals (≤90d post-implant, HR 2.37). Third, VAD-related infections were associated with the largest increase in risk of death (HR 3.02), followed by VAD-specific (HR 2.92), and non-VAD infections (HR 2.42).
To our knowledge, this is the largest observational study evaluating the relationship between post-implant infections and long-term mortality. Previous work by Hannan and colleagues, using the International Society of Heart and Lung Transplantation Mechanically Assisted Circulatory Support (IMACS) registry, evaluated the epidemiology of infections among 10,171 patients undergoing VAD implant between January 2013 and December 2015.8 Among these patients, infection was associated with increased mortality; the 2-year unadjusted all-cause mortality of VAD patients with infection was 41.0% compared to 25.2% for those without infection.8 The present analysis advances this prior work by adjusting for baseline demographic and disease characteristics as well as post-implant adverse effects that might have accounted for some of the deaths among patients with infections. After doing so, patients with any infection following VAD implant had an associated adjusted risk of death more than twice as high (HR 2.6) as those without an infection.
Further, this study is among the first to evaluate the relationship between later-developing post-implant infections and mortality. Holman and colleagues reported the relationship between first infections and mortality among 593 patients undergoing VADs (LVAD with or without RVAD) among 88 centers participating in STS-Intermacs between 2006–2008.6 Patients developing an infection within one month of implant had significantly lower survival than those developing an infection between one to three months after implant (p=0.04). In the present analysis, early interval infections were associated with a 2.37-fold associated increased risk of death. Compared to the other pre-defined time intervals, early infections had the lowest associated risk of death. A higher risk of death associated with late occurring infections, likely because the intermediate and late intervals had a larger proportion of VAD-related and VAD-specific infections, both of which have been previously identified with elevated risks of death.9
Last, this study further advances the relationship between the type of first infection and mortality. Hannan and colleagues also observed that patients with infections varied by the location of infection, with the highest mortality occurring among patients developing VAD pump/cannula infections (60.1% at 18 months) and VAD pocket infections (54.8% at 2 years); both VAD-specific infections. In another study from the IMACS registry, Aslam and colleagues evaluated the impact of bloodstream infections (BSIs) on 10,171 patients undergoing mechanical circulatory support device implant between January 2013 and December 2015.10 Patients with BSI within the first 3 months of implant had increased risk of death compared to those without BSI (HR 2.56). In the present analysis, the largest increase in risk of death was observed among VAD-related infections, 95% of which are bloodstream infections (HR 3.02), followed closely by VAD-specific (HR 2.92), then by non-VAD infections (HR 2.42).5
Further, neurological dysfunction was the most common cause of death among those developing VAD-specific and VAD-related infections, while multisystem organ failure was the most common cause of death among patients developing non-VAD infections. These findings suggest that a very different pathway between VAD-specific and VAD-related infections and death relative to non-VAD infections. It has been postulated that some of the increase in mortality following BSIs may be attributed to strokes and device thrombosis subsequent to the infection.11–13 In the work by Hannan, the five most common primary causes of death among all VAD patients were multisystem organ failure, cardiovascular causes, neurologic causes, withdrawal of support, and major infection. These were also the same five most common causes of death among our cohort of patients.
These findings have important implications for patients and providers. As an example, these findings can be used to enhance a patient’s understanding of the changing risk of death associated with early, intermediate, and late-occurring first infections. We believe that our findings can be used to inform different strategies to prevent infections across the spectrum of the patient’s post-implant course. For example, during the early interval when rates of non-VAD infections are highest, infection prevention efforts may be appropriately directed towards healthcare-associated infection, such as implementing bundles to prevent hospital-acquired urinary and pulmonary infections (e.g., expedited extubation, expeditious removal of post-implant urinary catheters). In the intermediate post-implant period, when rates of all infections decreased except for that of driveline infections, close surveillance of the driveline site through routine VAD clinic visits, adoption of innovative wound care techniques, and aggressive treatment upon first symptoms may be beneficial. Improvements in device technology with adoption of totally implantable systems, eliminating the need for the percutaneous lead, may therefore have significant impact on later mortality related to device infection.14
Future directions
Further work is needed to make these data more actionable to individual institutions seeking to improve infection practices. A mixed-methods exploration of infection practices at higher performing hospitals could help identify and elucidate best practices for infection prevention and treatment, which may be generalized and implemented at other institutions.15 We believe a similar approach may be helpful to define best practices for prevention of post-VAD infections across the spectrum of post-VAD care.
Benchmarking rates of health-care associated infections (e.g. pneumonia, surgical site infection, urinary tract infection) following coronary artery bypass grafting (CABG) surgery is standard practice. Prior work in the CABG and thoracic surgery literature has shown that collaborative quality improvement initiatives have led to large-scale reductions in healthcare-associated infections.16,17 Our prior analysis of variability of infections between VAD centers showed that higher tertile hospitals had more than four times higher bacterial infections, as well as higher device and non-device related infections compared to the low tercile hospitals. The creation of similar benchmarks in VAD patients for bloodstream infection and driveline infections using a standardized infection ratio, as well as provision of institution-specific rates of infection to various hospitals would create more transparency regarding the variability of HAI rates between centers, and highlight local opportunities for targeted interventions and quality improvement.
Limitations
Several limitations are worthy of discussion. First, while the dataset used in this study reflects practice between 2012–2017 with limited inclusion of newer HeartMate3 devices, the rate of post-implant infection in this cohort (49.2%) is comparable to that of the HeartMate 3 arm of MOMENTUM 3 (58.3%) clinical study.1,3 Second, while a large, multicenter North American experience, findings from this study may not reflect experiences among non-FDA approved devices. Nonetheless, rates of infection and device exchange are largely consistent with the existing literature. It is also important to note that the STS-Intermacs registry does not collect data on microbiology, susceptibility, and antibiotic therapy for patients with infections. In our cohort, the overall bacterial infections were by far the most common, similar to previous studies.18–20 Last, as with any observational study, it is important to recognize the potential influence of unmeasured confounding. While we took into account pre-implant characteristics and a number of post-implant adverse events that may affect the relationship between first infection and mortality, we recognize that other confounders may remain.
CONCLUSION
In this large, multinational cohort of VAD patients, first infections were most often non-VAD related and rates were highest in the early interval after implant. We found that compared to those without infection, patients with any infection post-implant had an increased risk of death in the follow-up time. VAD-related infections and infections occurring in the intermediate interval were associated with the largest increase in risk of death. These findings suggest that the focus of infection prevention in the early interval should be directed towards non-VAD related infections, but shift to VAD-specific and VAD-related infections as the patients come to the intermediate/later intervals following VAD implant.
Supplementary Material
Central message:
Effective infection prevention should target non-VAD infections in the 90 days following LVAD implant, then shift to early detection and treatment of driveline infections.
Perspective Statement:
Patients with any infections post durable implantation had a higher all-cause mortality compared to those without infections after adjusting for other post-implant factors (HR 2.63). VAD-related first infections (HR 3.02) and first infections occurring in the intermediate interval (HR 3.26) were associated with the largest increase in risk of death.
Acknowledgments
The authors thank the feedback received from members of the Michigan Society of Thoracic and Cardiovascular Surgeons Quality Collaborative.
Sources of Funding
This project was supported by grant number R01HS026003 from the Agency for Healthcare Research and Quality (AHRQ), U.S. Department of Health and Human Services (HHS). The content is solely the responsibility of the authors and does not necessarily represent the official views of AHRQ or HHS. Support for the Michigan Society of Thoracic and Cardiovascular Surgeons Quality Collaborative (MSTCVS-QC) is provided by Blue Cross and Blue Shield of Michigan and Blue Care Network as part of the BCBSM Value Partnerships program. Although Blue Cross Blue Shield of Michigan and MSTCSV-QC work collaboratively, the opinions, beliefs, and viewpoints expressed by the author do not necessarily reflect the opinions, beliefs, and viewpoints of BCBSM or any of its employees.
Data for this study were provided, in part, by STS-Intermacs, previously funded, in part, by the National Heart, Lung, and Blood Institute (NHLBI), NIH, under Contract No. HHSN268201100025C. This study was undertaken prior to the acquisition of Intermacs by the Society of Thoracic Surgeons. Opinions expressed in this manuscript do not represent those of STS-Intermacs, NHLBI, AHRQ, or the US Department of Health and Human Services.
Disclosures
Donald S. Likosky received extramural support from the Agency for Healthcare Research and Quality (AHRQ, R01HS026003). Keith Aaronson serves on a Medtronic Independent Physician Quality Panel. Francis D. Pagani is a member of the scientific advisory board of FineHeart, Inc., member of the Data Safety Monitoring Board for Carmat, Inc., member of the Data Safety Monitoring Board for the National Heart, Blood, and Lung Institute PumpKIN clinical trial, and Chair of The Society of Thoracic Surgeons, Intermacs Task Force.
Glossary of abbreviations:
- CI
confidence interval
- FDA
US Food and Drug Administration
- HR
hazard ratio
- Intermacs
Interagency Registry for Mechanically Assisted Circulatory Support
- STS
The Society of Thoracic Surgeons
- VAD
ventricular assist device
Footnotes
IRB #HUM00155687; Date of approval 02/04/2019.
REFERENCES
- 1.Mehra MR, Uriel N, Naka Y, et al. A Fully Magnetically Levitated Left Ventricular Assist Device - Final Report. N Engl J Med. 2019;380(17):1618–1627. [DOI] [PubMed] [Google Scholar]
- 2.Pagani FD, Aaronson KD, Kormos R, et al. The NHLBI REVIVE-IT study: Understanding its discontinuation in the context of current left ventricular assist device therapy. J Heart Lung Transplant. 2016;35(11):1277–1283. [DOI] [PubMed] [Google Scholar]
- 3.Patel CB, Blue L, Cagliostro B, et al. Left ventricular assist systems and infection-related outcomes: A comprehensive analysis of the MOMENTUM 3 trial. J Heart Lung Transplant. Published online March 20, 2020. doi: 10.1016/j.healun.2020.03.002 [DOI] [PubMed] [Google Scholar]
- 4.Molina EJ, Shah P, Kiernan MS, et al. The Society of Thoracic Surgeons Intermacs 2020 Annual Report. Ann Thorac Surg. Published online January 16, 2021. doi: 10.1016/j.athoracsur.2020.12.038 [DOI] [PubMed] [Google Scholar]
- 5.Likosky DS, Yang G, Zhang M, et al. Interhospital Variability in Healthcare-Associated Infections and Payments After Durable Ventricular Assist Device Implant among Medicare Beneficiaries. J Thorac Cardiovasc Surg {2021, Under Review). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Holman WL, Kirklin JK, Naftel DC, et al. Infection after implantation of pulsatile mechanical circulatory support devices. J Thorac Cardiovasc Surg. 2010;139(6):1632–1636.e2. [DOI] [PubMed] [Google Scholar]
- 7.Kirklin JK, Naftel DC, Stevenson LW, et al. INTERMACS database for durable devices for circulatory support: first annual report. J Heart Lung Transplant. 2008;27(10):1065–1072. [DOI] [PubMed] [Google Scholar]
- 8.Hannan MM, Xie R, Cowger J, et al. Epidemiology of infection in mechanical circulatory support: A global analysis from the ISHLT Mechanically Assisted Circulatory Support Registry. J Heart Lung Transplant. 2019;38(4):364–373. [DOI] [PubMed] [Google Scholar]
- 9.Zierer A, Melby SJ, Voeller RK, et al. Late-onset driveline infections: the Achilles’ heel of prolonged left ventricular assist device support. Ann Thorac Surg. 2007;84(2):515–520. [DOI] [PubMed] [Google Scholar]
- 10.Aslam S, Xie R, Cowger J, et al. Bloodstream infections in mechanical circulatory support device recipients in the International Society of Heart and Lung Transplantation Mechanically Assisted Circulation Support Registry: Epidemiology, risk factors, and mortality. J Heart Lung Transplant. 2018;37(8):1013–1020. [DOI] [PubMed] [Google Scholar]
- 11.Trachtenberg BH, Cordero-Reyes AM, Aldeiri M, et al. Persistent blood stream infection in patients supported with a continuous-flow left ventricular assist device is associated with an increased risk of cerebrovascular accidents. J Card Fail. 2015;21(2):119–125. [DOI] [PubMed] [Google Scholar]
- 12.Aggarwal A, Gupta A, Kumar S, et al. Are blood stream infections associated with an increased risk of hemorrhagic stroke in patients with a left ventricular assist device? ASAIO J. 2012;58(5):509–513. [DOI] [PubMed] [Google Scholar]
- 13.Frontera JA, Starling R, Cho S-M, et al. Risk factors, mortality, and timing of ischemic and hemorrhagic stroke with left ventricular assist devices. J Heart Lung Transplant. 2017;36(6):673–683. [DOI] [PubMed] [Google Scholar]
- 14.Pya Y, Maly J, Bekbossynova M, et al. First human use of a wireless coplanar energy transfer coupled with a continuous-flow left ventricular assist device. J Heart Lung Transplant. 2019;38(4):339–343. [DOI] [PubMed] [Google Scholar]
- 15.Chandanabhumma PP, Fetters MD, Pagani FD, et al. Understanding and Addressing Variation in Health Care-Associated Infections After Durable Ventricular Assist Device Therapy: Protocol for a Mixed Methods Study. JMIR Res Protoc. 2020;9(1):e14701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Likosky DS, Harrington SD, Cabrera L, et al. Collaborative Quality Improvement Reduces Postoperative Pneumonia After Isolated Coronary Artery Bypass Grafting Surgery. Circ Cardiovasc Qual Outcomes. 2018;11(11):e004756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hemmila MR, Cain-Nielsen AH, Jakubus JL, Mikhail JN, Dimick JB. Association of Hospital Participation in a Regional Trauma Quality Improvement Collaborative With Patient Outcomes. JAMA Surg. 2018;153(8):747–756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Gordon SM, Schmitt SK, Jacobs M, et al. Nosocomial bloodstream infections in patients with implantable left ventricular assist devices. Ann Thorac Surg. 2001;72(3):725–730. [DOI] [PubMed] [Google Scholar]
- 19.Nienaber JJC, Kusne S, Riaz T, et al. Clinical manifestations and management of left ventricular assist device-associated infections. Clin Infect Dis. 2013;57(10):1438–1448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Gordon RJ, Weinberg AD, Pagani FD, et al. Prospective, multicenter study of ventricular assist device infections. Circulation. 2013;127(6):691–702. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


