Abstract
Ovarian carcinoma is one of the most common causes for cancer death in women; lack of early diagnosis and acquired resistance to platinum-based chemotherapy account for its poor prognosis and high mortality rate. As with other cancer types, ovarian cancer is characterized by dysregulated signaling pathways and protein synthesis, which together contribute to rapid cellular growth and invasiveness. The mechanistic/mammalian target of rapamycin (mTOR) pathway represents the core of different signaling pathways regulating a number of essential steps in the cell, among which protein synthesis and the eukaryotic initiation factor 4E (eIF4E), the mRNA cap binding protein, is one of its downstream effectors. eIF4E is a limiting factor in translation initiation and its overexpression is a hallmark in many cancers. Because its action is regulated by a number of factors that compete for the same binding site, eIF4E is an ideal target for developing novel antineoplastic drugs. Several inhibitors targeting the mTOR signaling pathway have been designed thus far, however most of these molecules show poor stability and high toxicity in vivo. This minireview explores the possibility of targeting mTOR and eIF4E proteins, thus impacting on translation initiation in ovarian cancer, describing the most promising experimental strategies and specific inhibitors that have been shown to have an effect on other kinds of cancers.
Keywords: Ovarian cancer, targeted therapy, mTOR pathway, eIF4E, inhibitors
INTRODUCTION
Ovarian cancer ranks 5th among the most common causes of cancer death in women worldwide[1]. The American Cancer Society estimates that in 2021 about 21,410 new cases of ovarian cancer will be diagnosed and about 13,770 women will die from ovarian cancer in the United States[2]. With its overall 5-year survival rate of 30% for the advanced stage disease[3,4], ovarian cancer has the worst prognosis and the highest mortality rate among gynecologic cancers[5,6]. This is due to lack of early diagnosis tools and to the fact that the earliest symptoms are easy to overlook, as they can be confused with other common illnesses. Symptoms usually become more severe by the time the tumor has spread to the surface of the peritoneal cavity, making it much harder to remove by surgical intervention. Indeed, the standard approach in ovarian cancer therapy is surgery, followed by platinum-based chemotherapy, in which cisplatin or carboplatin is combined with taxanes[7-9].
Despite the initial efficacy of treatment, more than 65% of patients relapse and develop acquired resistance to platinum-based chemotherapy[10,11]. Platinum-based drugs, such as cisplatin, interact with DNA and form intra- or inter-strand DNA cross-links, thereby activating cell death but also DNA repair pathways[12-14]. Despite the large number of studies[15,16] and the evidence of the multiple mechanisms of sensitivity and resistance to platinum agents[17], there is an urgent need to target additional mechanisms underlying ovarian cancer platinum-resistance.
Drug resistance is the outcome of the deregulation of several molecular mechanisms, such as drug inactivation, apoptotic stimulation, expression of pro-survival or anti-survival proteins, and alteration of the expression of growth factor receptors[18-21]. For the treatment of cisplatin-resistant ovarian cancer, a few options are available with the need to improve treatment efficacy and reduce toxicity[10,22,23].
To overcome these limitations, new analogs of conventional drugs and new therapeutic options are being developed, among which several are currently being tested in clinical trials and others have recently been approved[24,25]; however, overcoming drug resistance constantly requires new molecular targets that may provide, in addition to conventional treatment, a more selective therapeutic approach to fight ovarian cancer. To overcome drug resistance and formulate customized individual therapies to improve early diagnosis, research needs to focus on the molecular background of ovarian cancer[11]. Translation and signaling pathways represent ideal molecular targets for the development of novel therapeutic strategies. Protein synthesis regulates every aspect of cell phenotype, growth, and metabolism and is tightly controlled by several signaling pathways in response to different external stimuli[26]. In human cancer, the dysregulation of these processes has an impact in overall protein synthesis, leading to cancer development and growth[27].
This minireview focuses on the recent research reporting the use of inhibitors of the mechanistic/mammalian target of rapamycin (mTOR) and the eukaryotic translation initiation factor 4E (eIF4E) as adjuvants in cancer treatment; despite the limited number of studies performed on ovarian cancer cell models, the encouraging results obtained in vitro and in pre-clinical studies with some inhibitors might establish the use in ovarian cancer therapy.
THE mTOR PATHWAY: A POTENTIAL THERAPEUTIC TARGET
The AKT/mTOR pathway is crucial for the regulation of transcription, translation, cell growth, motility, survival, proliferation, autophagy, and angiogenesis[28,29]. Not surprisingly, this pathway is largely deregulated in cancer, where several genes coding for proteins of this axis are frequently mutated, leading to pathway hyperactivation in disease. The majority of the cancer-associated mutations occur in the mechanistic/mammalian target of rapamycin (mTOR) gene[30]. Because it is found to also be upregulated in ovarian carcinoma[31,32], and its deregulation has been associated with platinum-drug resistance[33,34], mTOR represents an attractive biological target for ovarian cancer therapy.
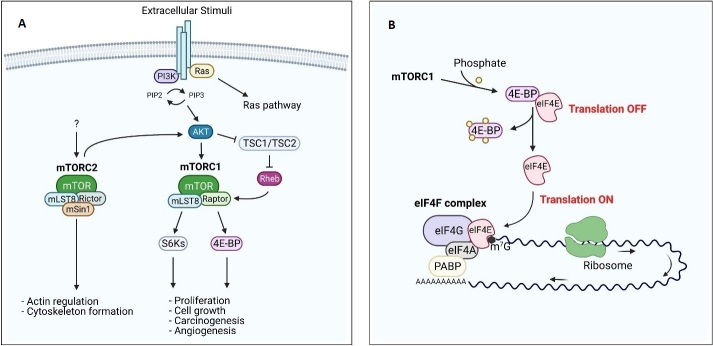
mTOR is a 289-kDa serine/threonine kinase of the phosphatidylinositol 3-kinase related kinase (PIKK) family. In mammals, mTOR is the core component of two functionally different multi-proteins complexes: mTOR Complex 1 (mTORC1) and mTOR Complex 2 (mTORC2). mTORC1, or rapamycin-sensitive complex, includes the proteins mTOR, mLST8 (or GβL), and Raptor (regulatory-associated protein of mTOR) and plays a key role in the regulation of mRNA translation and cell growth[31,35]. mTORC2 (rapamycin-insensitive complex), consisting of mTOR, mLST8, Rictor (rapamycin-independent companion of mTOR), and mSin1 (or mitogen-activated protein-kinase-associated protein 1), is mainly involved in actin cytoskeleton dynamics [Figure 1A][31,35]. mTOR is a downstream mediator in the phosphatidylinositol-3-kinase (PI3K)/AKT pathway; in fact, as a consequence of extracellular stimuli, PIP2 is phosphorylated to produce PIP3 by PI3K. This leads to the activation of the protein kinase B (AKT) via phosphorylation by phosphatidylinositol-dependent kinase 1 and 2 (PDK1 and PDK2)[36]. Activated AKT phosphorylates mTOR on Ser2448, or indirectly by phosphorylation of tuberin protein or tuberous sclerosis complex 2 (TSC2). TSC2 is complexed to TSC1, and, when active, its function is to convert Rheb (Ras homolog enriched in brain)-GTP to Rheb-GDP, inactivating mTORC1. Once phosphorylated, TSC2 loses its affinity for TSC1, leading to activation of mTORC1[29,36]. Activated mTORC1 phosphorylates the translation regulating factor ribosomal S6 kinase-1 (S6K-1) and the eukaryotic translation initiation factor 4E (eIF4E) binding family of proteins (4E-BPs) [Figure 1A]. The phosphorylation of S6K-1 leads to the translation of ribosomal proteins, elongation factors, and other proteins involved in the cell cycle, while phosphorylation of 4E-BPs causes the release of eIF4E [Figure 1B][37,38]. eIF4E is a crucial translation initiation factor, also involved in the onset of a number of cancer types (see discussion below)[39,40].
Figure 1.
PI3K/mTOR pathway and regulation of eIF4F complex. (A) PI3K and its downstream effectors AKT and mTOR are activated by many extracellular stimuli. The serine/threonine protein kinase mTOR forms, by association with several binding partners (mLST8, Raptor/Rictor, and mSIN1), 2 distinct complexes mTORC1 and mTORC2. mTORC2 activation leads to actin regulation and cytoskeleton organization, while mTORC1 allows the cap-dependent translation through the 4E-BPs phosphorylation, resulting in the dissociation of these proteins from eIF4E. PI3K pathway dysregulation results in an altered proliferation, carcinogenesis, and angiogenesis. (B) The cap-binding protein eIF4E is released by 4E-BPs as a result of its phosphorylation by mTORC1, allowing eIF4F complex formation and thus permitting translation of eIF4E-sensitive mRNAs.
Contrary to mTORC1, the mechanism by which mTORC2 is activated remains elusive. PI3K stimulates mTORC2 by responding to growth factors. Activated mTORC2 is found to be associated to ribosomes[41], and it directly activates AKT, through phosphorylation of Ser473. This, in turn, activates mTOR, protein kinase C-α, and serum- and glucocorticoid-induced protein kinase 1, acting on actin regulation, as well as on metabolism and cell survival[29,36].
It is clear that the mTOR signaling pathway has an important role in malignancy, as it is frequently hyperactivated in a wide range of tumors, including ovarian cancer[11,42]. As a consequence, it represents an attractive candidate for drug design. To date, several inhibitors developed against different proteins of AKT/mTOR pathway are already available at different stages in clinical research, as shown in Table 1[39,43]. The best characterized mTOR inhibitor is rapamycin (or sirolimus), a macrolide produced by the bacterium Streptomyces hygroscopicus. Rapamycin binds selectively to mTORC1 interacting with a specific binding domain present in mTORC1, but not in mTORC2. Rapamycin inhibits the serine/threonine kinase activity of mTORC1 with an allosteric mechanism[43]. Despite the activity shown by rapamycin in many tumor types[29,44], its clinical use is unsuccessful because of its poor water solubility[29]. To overcome this limitation, several analogs of rapamycin, called rapalogs, have been formulated, some of which show encouraging pharmacological features on several cancers. Among rapalogs, there are CCI-779 (temsirolimus), RAD001 (everolimus), and AP23573 (deforolimus)[43,45-49]. Some rapalogs are FDA-approved, while others are still in clinical trials[40,43]. However, the therapeutic efficacy of rapalogs is hindered by the appearance of negative feedback loops that trigger the activation of AKT in the mTOR pathway[29,39]. Second-generation inhibitors are thus being developed, and many of them are already under clinical trial. These compounds have shown promising preliminary results, as dual-specificity inhibitors (targeting both mTOR and PI3K) and inhibitors targeting directly mTOR, inhibiting both mTORC1 and mTORC2[37,39,43] [Table 1]. Among the downstream effectors of mTOR, the p70S6 kinase is also implicated in fundamental cellular processes, such as cell growth and proliferation[38]. p70S6k, together with 4E-BPs and eIF4E, is frequently activated in a wide range of cancer types and could be a driver or malignancy, also for ovarian cancer[50-52]. Thus, targeting p70S6K using specific inhibitors already developed represents another alternative strategy against rapamycin-resistant tumors[53].
Table 1.
Main inhibitors of mTOR and eIF4E
| Target | Category | Compound | Clinical trials in ovarian cancer | Ref. |
| mTORC1 | Antibiotic | Rapamycin | [54] | |
| Rapalogs | Temsirolimus | Completed | [45,46] | |
| Everolimus | Phase I/II | [47,48] | ||
| Deferolimus | [49] | |||
| mTORC1 and mTORC2 | Second generations inhibitors | Torin1 | [55] | |
| INK128 | [56] | |||
| AZD8055 | [57] | |||
| AZD2014 | Phase I/II | [58] | ||
| PI3K and mTOR | Dual inhibitors | PI-103 | [59] | |
| NVP-BEZ235 | Completed | [60] | ||
| SF1126 | [61] | |||
| GNE-477 | [62] | |||
| XL765 | [63] | |||
| eIF4E expression | Antisense oligonucleotides | ISIS 183750 | [64] | |
| LY2275796 | [65] | |||
| miRNA | miR-768-3p | [66] | ||
| eIF4E phosphorylation | MEK inhibitor | U0126 | [67] | |
| Small molecule | CGP57380 | [68] | ||
| Natural product | cercosporamide | [69] | ||
| eIF4E-partners interactions | Nucleoside analog | Ribavirin | [70-78] | |
| Small molecules | 4Ei-1 | [79,80] | ||
| 4EGI-1 | [81,82] | |||
| 4E1RCat | [83] | |||
| Oligopeptides | GnRH-4EBP fusion peptide | [84] | ||
| 4E-BP mimetics peptides | [85-87] |
TARGETING mTOR PROTEIN IN OVARIAN CANCER
On the basis of the promising activity of these compounds on other cancer types, mTOR inhibitors have been tested on ovarian cancer[35,36,88]. Rapamycin treatment of several ovarian cancer cell lines resulted in a decrease in the phosphorylation levels of mTOR and 4E-BPs, together with an increase of p-AKT[42]. These effects lead to an accumulation of eIF4E-4E-BPs complexes, which blocks protein translation and inhibits ovarian cancer cells proliferation[42]. Despite the above-mentioned promising results, rapamycin’s clinical use is currently restricted due to adverse bioavailability[36]. The rapalogs Temsirolimus and Everolimus have been tested in clinical trials, showing anti-proliferative and antiangiogenic actions on ovarian cancer, especially when administrated in combination with carboplatin and paclitaxel[35,36]. However, as found in other cancers, the critical limitation of these inhibitors is the occurrence of resistance during the treatment, with a molecular mechanism that remains elusive. Loss of the negative feedback loops associated to PI3K/AKT/mTOR might trigger drug resistance mechanisms, one of which concerns the activation of mTORC2 that in turn activates AKT in response to mTORC1 inhibition[35]. In addition, differences in AKT activity observed in different ovarian cancer cell lines contribute to the cell-specific sensitivity to pharmacological treatment by rapamycin and, more in general, by mTOR inhibitors[16]. In fact, as mentioned above, AKT signaling is the central hub of cellular proliferation and growth as well as activates alternative pathways[29]. Besides molecules able to target both mTORC1 and mTORC2 complexes, AZD2014 and AZD8055 have been tested in different ovarian cancer models, showing antitumor activities in combination with others drugs[57,58].
Testing the effects of natural products in ovarian cancer therapy is of clinical interest. Resveratrol (3,4’,5-trihydroxy-trans-stilbene) is a polyphenol found in several plants and wines and thus naturally present in the human diet[89]. It has been shown to have anti-neoplastic effects on several types of carcinomas[90], likely via inhibition of hypoxia-inducible factor 1α (HIF-1α) and vascular endothelial growth factor[91], both of which are expressed at higher levels in many human cancers, including ovarian cancer[11,92]. The mechanisms of inhibition by resveratrol are multiple; among them, its interference in the AKT/mTOR pathway, leading to a phosphorylation of S6K1 and 4E-BPs. Resveratrol also increases apoptosis and autophagy and decreases proliferation and invasiveness of ovarian cancer cells[93,94].
Further strategies in the development of anti-cancer compounds targeting ovarian malignancies include the suppression of proteins that affect the mTOR cascade, using siRNA[95], or the inactivation of AKT, PI3K, or simultaneous block of mTOR and PI3K, designing dual mTOR/PI3K inhibitors. Simultaneously targeting 2 kinases belonging to the same signaling pathway should lead to a more efficient inhibition of the PI3K/AKT/mTOR cascade and lower the probability of developing drug resistance. In addition, these compounds are developed to prevent AKT hyperactivation due to rapamycin or rapalog treatment[88]. The most clinically relevant dual drugs are PI-103, SF1126, GNE-477, XL765, and NVP-BEZ235, the latter being the only one that has been tested in a clinical trial[35,36,60,88]. In vitro and in vivo studies in ovarian cancer models have shown that these inhibitors decrease tumor cell growth and proliferation, also in combination with paclitaxel[96-98]. Interestingly, all these findings show that platinum resistance of ovarian cancer cells can be reversed by inhibiting mTOR (mTORC1/mTORC2), as specific mRNAs encoding survival, cell cycle, and other functions are inhibited[57,99].
However, despite the promising results obtained targeting the mTOR pathway, there is a constant need to hunt for new molecular targets and therapeutic approaches. Therefore, the downstream mediator of mTOR pathway, the cap-dependent translation factor eIF4E, represents a valid candidate, being also a confluence point of multiple pathways.
eIF4E INHIBITION: THE FUTURE OF OVARIAN CANCER THERAPY?
Translation of proteins promoting cancer progression and invasiveness, such as kinases, transcription factors, and vascular growth factors, is mediated by the mRNA 5’-cap-binding complex, a heterotrimeric protein complex consisting of eIF4E, eIF4G, and eIF4A, which recruits the translation apparatus to the mRNA [Figure 1B][37]. eIF4G acts as a scaffold protein, binding both the mRNA helicase eIF4A and eIF4E, while the function of eIF4E is to bind the 5’ 7-methylguansine (m7G) mRNA cap, thereby selecting the mRNAs to be translated. Under normal conditions, eIF4E is expressed at low levels and is therefore the limiting factor in the formation of the cap-binding complex. Overexpression of eIF4E is, however, a signature of several aggressive cancer types, including triple-negative breast cancer and ovarian cancer[100-106]. eIF4E undergoes phosphorylation at Ser209 by the mitogen-activated protein kinase-interacting kinases 1 and 2 (MNK1/2), which target the eIF4F complex[107,108]. Phosphorylation of eIF4E seems to have no functional role under physiological conditions[43,109] but is an independent prognostic factor in several types of cancer[110]. The activity of eIF4E is modulated by a heterogeneous group of 4E-BPs, phosphoproteins that compete with eIF4G for the same binding site on eIF4E. When phosphorylated by the mTORC1 pathway, the affinity of 4E-BPs for eIF4E decreases, facilitating translational initiation [Figure 1B][37]. Because of its tight control, a 2.5-fold increased expression of eIF4E is enough to induce transformation, metastatic progression, and suppression of apoptosis[102,111]. Conversely, more and more studies have shown that decreasing eIF4E levels in the cell reduces cell growth and invasiveness in several models of cancers[112-116]. It is therefore of utmost importance to develop drugs that hamper the activity of eIF4E by either reducing its expression and phosphorylation levels or competing with its biological partners, namely the m7G cap, eIF4G, and the 4E-BPs [Table 1].
Because the activity of eIF4E is strictly dependent on its interaction with the mRNA cap, m7G analogs represent attractive drug candidates. By mimicking the mRNA cap structure, m7G analogs compete for eIF4E binding, thereby decreasing the rate of translation initiation. Ribavirin, an anti-viral drug commonly used to treat hepatitis C, is a guanosine ribonucleoside analogue, initially characterized as cap-mimetic[76], but this is controversial[77,78]. It was tested against acute myeloid leukemia[71,72], ribavirin showed promising results in several cancer models, including breast and ovarian cancer[74,75,117]. Notably, even though eIF4E levels are not homogeneous among ovarian cancer cell lines and tissues, ribavirin treatment reduced the growth and survival of ovarian cancer cell lines and increased the efficacy of cisplatin treatment both in vitro and in vivo[74], an observation that raises the possibility of combining it with other drugs for ovarian cancer therapy. Following the encouraging results obtained with ribavirin, several molecules extracted from m7G analog libraries have been selected for further studies. Among them, 7-benzyl guanosine monophosphate (Bn7GMP)[118] and 4Ei-1[119] are currently being studied in vitro and in vivo and might represent ideal adjuvants in ovarian cancer therapy[79,80,118,119].
The balance between active and inactive eIF4E is controlled by the relative abundance of 4E-BPs and eIF4G in the cell. Thus, to reduce the levels of eIF4E, drug design should focus on finding potential ligands able to outcompete cellular eIF4G and/or mimic the 4E-BPs. 4EGI-1, a small molecule discovered through high-throughput screening of compound libraries[81], is an allosteric eIF4E inhibitor which binds eIF4E in a different region with respect to eIF4G[82], inhibiting eIF4G-eIF4E complex formation and facilitating the binding of 4E-BP1[120]. Furthermore, several short peptides have been designed, based on the sequence of eIF4E binding partners, to specifically destabilize the eIF4E-eIF4G complex in the cell, representing a valuable approach to target eIF4E-mediated translation in cancer models[86,87,121,122]. The increasing evidence that eIF4E targeting slows down cancer progression is having a prompt response in ovarian cancer research. Recently, a novel strategy to specifically target eIF4E activity in ovarian cancer models has been reported, where 4E-BP1 peptides were fused to an agonist of the gonadotropin-releasing hormone (GnRH)[84], thereby mediating the uptake and showing a marked decrease in tumor cells growth.
Taken together, these studies suggest that eIF4E represents a promising candidate for the research on ovarian tumors, whereas downregulating eIF4E expression might be a useful and feasible approach to improve the therapeutic responsiveness of ovarian cancer. Future research effort must be employed in designing and testing potential drugs against eIF4E, to evaluate the possibility of a cancer-specific drug.
CONCLUSION
Patients diagnosed with ovarian cancer have poor prognosis, owing to the advanced status of malignancy at the time of diagnosis and the development of resistance to the standard therapy treatment. Thereby, new therapeutic strategies are urgently needed. The mTOR signaling pathway is frequently overactivated in ovarian cancer and converge to the increased levels of cap-dependent translation, being eIF4E the focal point of this pathway. Thus, specific inhibitors targeting mTOR and eIF4E represent promising and valid adjuvants for clinical management of ovarian cancer.
DECLARATIONS
Authors’ contributions
The concept of the review was conceived by: Di Marino D, Romagnoli A
Wrote the manuscript: Romagnoli A, Maracci C
Supervision: Di Marino D, La Teana A
Prepared the figure: D’Agostino M
Availability of data and materials
Not applicable.
Financial support and sponsorship
Cristina Maracci was supported by Fondazione Umberto Veronesi.
Conflicts of interest
All authors declared that there are no conflicts of interest.
Ethical approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Copyright
© The Author(s) 2021.
References
- 1.Siegel RL, Miller KD, Jemal A. Cancer statistics, 2016. CA Cancer J Clin. 2016;66:7–30. doi: 10.3322/caac.21332. [DOI] [PubMed] [Google Scholar]
- 2. American Cancer Society, 2021 Available from: https://www.cancer.org/cancer/ovarian-cancer/about/key-statistics.html. [Last accessed on 6 May 2021]
- 3.Yurkovetsky Z, Skates S, Lomakin A, et al. Development of a multimarker assay for early detection of ovarian cancer. J Clin Oncol. 2010;28:2159–66. doi: 10.1200/JCO.2008.19.2484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Torre LA, Trabert B, DeSantis CE, et al. Ovarian cancer statistics, 2018. CA Cancer J Clin. 2018;68:284–96. doi: 10.3322/caac.21456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lloyd KL, Cree IA, Savage RS. Prediction of resistance to chemotherapy in ovarian cancer: a systematic review. BMC Cancer. 2015;15:117. doi: 10.1186/s12885-015-1101-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Momenimovahed Z, Tiznobaik A, Taheri S, Salehiniya H. Ovarian cancer in the world: epidemiology and risk factors. Int J Womens Health. 2019;11:287–99. doi: 10.2147/IJWH.S197604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Helm CW, States JC. Enhancing the efficacy of cisplatin in ovarian cancer treatment - could arsenic have a role. J Ovarian Res. 2009;2:2. doi: 10.1186/1757-2215-2-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Agarwal R, Kaye SB. Ovarian cancer: strategies for overcoming resistance to chemotherapy. Nat Rev Cancer. 2003;3:502–16. doi: 10.1038/nrc1123. [DOI] [PubMed] [Google Scholar]
- 9.Metzger-Filho O, Moulin C, D'Hondt V. First-line systemic treatment of ovarian cancer: a critical review of available evidence and expectations for future directions. Curr Opin Oncol. 2010;22:513–20. doi: 10.1097/CCO.0b013e32833ae99c. [DOI] [PubMed] [Google Scholar]
- 10.Armstrong DK. Relapsed ovarian cancer: challenges and management strategies for a chronic disease. Oncologist. 2002;7 Suppl 5:20–8. doi: 10.1634/theoncologist.7-suppl_5-20. [DOI] [PubMed] [Google Scholar]
- 11.Bast RC Jr, Hennessy B, Mills GB. The biology of ovarian cancer : new opportunities for translation. Nat Rev Cancer. 2009;9:415–28. doi: 10.1038/nrc2644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Eckstein N. Platinum resistance in breast and ovarian cancer cell lines. J Exp Clin Cancer Res. 2011;30:91. doi: 10.1186/1756-9966-30-91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Damia G, Broggini M. Platinum Resistance in Ovarian Cancer: Role of DNA Repair. Cancers (Basel) 2019;11:119. doi: 10.3390/cancers11010119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.El-Shaimaa A. Arafa, Zhu Q, et al. Tangeretin Sensitizes Cisplatin-resistant Human Ovarian Cancer Cells through Down-regulation of PI3K/Akt Signaling Pathway. Cancer Res. 2009;23:8910–7.. doi: 10.1158/0008-5472.CAN-09-1543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Chen SH, Chang JY. New Insights into Mechanisms of Cisplatin Resistance: From Tumor Cell to Microenvironment. Int J Mol Sci. 2019;20:4136. doi: 10.3390/ijms20174136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Xing D, Orsulic S. Modeling Resistance to Pathway-Targeted Therapy in Ovarian Cancer. Cell Cycle. 2005;4:1004–6. doi: 10.4161/cc.4.8.1869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Rottenberg S, Disler C, Perego P. The rediscovery of platinum-based cancer therapy. Nat Rev Cancer. 2021;21:37–50. doi: 10.1038/s41568-020-00308-y. [DOI] [PubMed] [Google Scholar]
- 18.Beale PJ, Rogers P, Boxall F, Sharp SY, Kelland LR. BCL-2 family protein expression and platinum drug resistance in ovarian carcinoma. Br J Cancer. 2000;82:436–40. doi: 10.1054/bjoc.1999.0939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Bieg D, Sypniewski D, Nowak E, Bednarek I. Morin decreases galectin-3 expression and sensitizes ovarian cancer cells to cisplatin. Arch Gynecol Obstet. 2018;298:1181–94. doi: 10.1007/s00404-018-4912-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Mansouri A, Zhang Q, Ridgway LD, Tian L, Claret FX. Cisplatin resistance in an ovarian carcinoma is associated with a defect in programmed cell death control through XIAP regulation. Oncol Res. 2003;13:399–404. doi: 10.3727/096504003108748410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Li J, Feng Q, Kim JM, et al. Human ovarian cancer and cisplatin resistance: possible role of inhibitor of apoptosis proteins. Endocrinology. 2001;142:370–80. doi: 10.1210/endo.142.1.7897. [DOI] [PubMed] [Google Scholar]
- 22.Gordon AN, Fleagle JT, Guthrie D, Parkin DE, Gore ME, Lacave AJ. Recurrent epithelial ovarian carcinoma: a randomized phase III study of pegylated liposomal doxorubicin versus topotecan. J Clin Oncol. 2001;19:3312–22. doi: 10.1200/JCO.2001.19.14.3312. [DOI] [PubMed] [Google Scholar]
- 23.Mutch DG, Orlando M, Goss T, et al. Randomized phase III trial of gemcitabine compared with pegylated liposomal doxorubicin in patients with platinum-resistant ovarian cancer. J Clin Oncol. 2007;25:2811–8. doi: 10.1200/JCO.2006.09.6735. [DOI] [PubMed] [Google Scholar]
- 24.Gogineni V, Morand S, Staats H, et al. Current Ovarian Cancer Maintenance Strategies and Promising New Developments. J Cancer. 2021;12:38–53. doi: 10.7150/jca.49406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Cortez AJ, Tudrej P, Kujawa KA, Lisowska KM. Advances in ovarian cancer therapy. Cancer Chemother Pharmacol. 2018;81:17–38. doi: 10.1007/s00280-017-3501-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Tahmasebi S, Sonenberg N, Hershey JWB, Mathews MB. Protein Synthesis and Translational Control: A Historical Perspective. Cold Spring Harb Perspect Biol. 2019;11:a035584. doi: 10.1101/cshperspect.a035584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Grzmil M, Hemmings BA. Translation regulation as a therapeutic target in cancer. Cancer Res. 2012;72:3891–900. doi: 10.1158/0008-5472.CAN-12-0026. [DOI] [PubMed] [Google Scholar]
- 28.Liu GY, Sabatini DM. mTOR at the nexus of nutrition, growth, ageing and disease. Nat Rev Mol Cell Biol. 2020;21:183–203. doi: 10.1038/s41580-019-0199-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Laplante M, Sabatini DM. mTOR signaling in growth control and disease. Cell. 2012;149:274–93. doi: 10.1016/j.cell.2012.03.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Grabiner BC, Nardi V, Birsoy K, et al. A diverse array of cancer-associated mTOR mutations are hyperactivating and can predict rapamycin sensitivity. Cancer Discov. 2014;4:554–63. doi: 10.1158/2159-8290.CD-13-0929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Sabatini DM. mTOR and cancer: insights into a complex relationship. Nat Rev Cancer. 2006;6:729–34. doi: 10.1038/nrc1974. [DOI] [PubMed] [Google Scholar]
- 32.Fruman DA, Rommel C. PI3K and Cancer: Lessons, Challenges and Opportunities. Nat Rev Drug Discov. 2014;13:140–56. doi: 10.1038/nrd4204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Cossa G, Lanzi C, Cassinelli G, et al. Differential outcome of MEK1/2 inhibitor-platinum combinations in platinum-sensitive and -resistant ovarian carcinoma cells. Cancer Lett. 2014;347:212–24. doi: 10.1016/j.canlet.2014.02.016. [DOI] [PubMed] [Google Scholar]
- 34.Deng J, Bai X, Feng X, et al. Inhibition of PI3K/Akt/mTOR signaling pathway alleviates ovarian cancer chemoresistance through reversing epithelial-mesenchymal transition and decreasing cancer stem cell marker expression. BMC Cancer. 2019;19:618. doi: 10.1186/s12885-019-5824-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Mabuchi S, Kuroda H, Takahashi R, Sasano T. The PI3K/AKT/mTOR pathway as a therapeutic target in ovarian cancer. Gynecol Oncol. 2015;137:173–9. doi: 10.1016/j.ygyno.2015.02.003. [DOI] [PubMed] [Google Scholar]
- 36.Mabuchi S, Hisamatsu T, Kimura T. Targeting mTOR signaling pathway in ovarian cancer. Curr Med Chem. 2011;18:2960–8. doi: 10.2174/092986711796150450. [DOI] [PubMed] [Google Scholar]
- 37.Siddiqui N, Sonenberg N. Signalling to eIF4E in cancer. Biochem Soc Trans. 2015;43:763–72. doi: 10.1042/BST20150126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Hay N, Sonenberg N. Upstream and downstream of mTOR. Genes Dev. 2004;18:1926–45. doi: 10.1101/gad.1212704. [DOI] [PubMed] [Google Scholar]
- 39.Bhat M, Robichaud N, Hulea L, Sonenberg N, Pelletier J, Topisirovic I. Targeting the translation machinery in cancer. Nat Rev Drug Discov. 2015;14:261–78. doi: 10.1038/nrd4505. [DOI] [PubMed] [Google Scholar]
- 40.Wendel HG, Silva RL, Malina A, et al. Dissecting eIF4E action in tumorigenesis. Genes Dev. 2007;21:3232–7. doi: 10.1101/gad.1604407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Zinzalla V, Stracka D, Oppliger W, Hall MN. Activation of mTORC2 by association with the ribosome. Cell. 2011;144:757–68. doi: 10.1016/j.cell.2011.02.014. [DOI] [PubMed] [Google Scholar]
- 42.Noske A, Lindenberg JL, Darb-esfahani S. Activation of mTOR in a subgroup of ovarian carcinomas : Correlation with p-eIF-4E and prognosis. Oncol Rep. 2008;20:1409–17. [PubMed] [Google Scholar]
- 43.Lu C, Makala L, Wu D, Cai Y. Targeting translation: eIF4E as an emerging anticancer drug target. Expert Rev Mol Med. 2016;18:e2. doi: 10.1017/erm.2015.20. [DOI] [PubMed] [Google Scholar]
- 44.Li J, Kim SG, Blenis J. Rapamycin: one drug, many effects. Cell Metab. 2014;19:373–9. doi: 10.1016/j.cmet.2014.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Hudes G, Carducci M, Tomczak P, et al. Global ARCC Trial. Temsirolimus, interferon alfa, or both for advanced renal-cell carcinoma. N Engl J Med. 2007;356:2271–81. doi: 10.1056/NEJMoa066838. [DOI] [PubMed] [Google Scholar]
- 46.Rini BI. Temsirolimus, an inhibitor of mammalian target of rapamycin. Clin Cancer Res. 2008;14:1286–90. doi: 10.1158/1078-0432.CCR-07-4719. [DOI] [PubMed] [Google Scholar]
- 47.Houghton PJ. Everolimus. Clin Cancer Res. 2010;16:1368–72. doi: 10.1158/1078-0432.CCR-09-1314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Mabuchi S, Altomare DA, Connolly DC, et al. RAD001 (Everolimus) delays tumor onset and progression in a transgenic mouse model of ovarian cancer. Cancer Res. 2007;67:2408–13. doi: 10.1158/0008-5472.CAN-06-4490. [DOI] [PubMed] [Google Scholar]
- 49.Mita M, Sankhala K, Abdel-Karim I, Mita A, Giles F. Deforolimus (AP23573) a novel mTOR inhibitor in clinical development. Expert Opin Investig Drugs. 2008;17:1947–54. doi: 10.1517/13543780802556485. [DOI] [PubMed] [Google Scholar]
- 50.Pópulo H, Lopes JM, Soares P. The mTOR signalling pathway in human cancer. Int J Mol Sci. 2012;13:1886–918. doi: 10.3390/ijms13021886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Pon YL, Zhou HY, Cheung AN, Ngan HY, Wong AS. p70 S6 kinase promotes epithelial to mesenchymal transition through snail induction in ovarian cancer cells. Cancer Res. 2008;68:6524–32. doi: 10.1158/0008-5472.CAN-07-6302. [DOI] [PubMed] [Google Scholar]
- 52.Kurgan N, Tsakiridis E, Kouvelioti R, Moore J, Klentrou P, Tsiani E. Inhibition of Human Lung Cancer Cell Proliferation and Survival by Post-Exercise Serum Is Associated with the Inhibition of Akt, mTOR, p70 S6K, and Erk1/2. Cancers (Basel) 2017;9:46. doi: 10.3390/cancers9050046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Ip CK, Wong AS. Exploiting p70 S6 kinase as a target for ovarian cancer. Expert Opin Ther Targets. 2012;16:619–30. doi: 10.1517/14728222.2012.684680. [DOI] [PubMed] [Google Scholar]
- 54.Lamming DW. Inhibition of the Mechanistic Target of Rapamycin (mTOR)-Rapamycin and Beyond. Cold Spring Harb Perspect Med. 2016;6:a025924. doi: 10.1101/cshperspect.a025924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Thoreen CC, Sabatini DM. Rapamycin inhibits mTORC1, but not completely. Autophagy. 2009;5:725–6. doi: 10.4161/auto.5.5.8504. [DOI] [PubMed] [Google Scholar]
- 56.Hsieh AC, Liu Y, Edlind MP, et al. The translational landscape of mTOR signalling steers cancer initiation and metastasis. Nature. 2013;485:55–61. doi: 10.1038/nature10912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Hsieh AC, Liu Y, Edlind MP, et al. The translational landscape of mTOR signalling steers cancer initiation and metastasis. Nature. 2012;485:55–61. doi: 10.1038/nature10912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Wong Te Fong AC, Thavasu P, Gagrica S, et al. Evaluation of the combination of the dual m-TORC1/2 inhibitor vistusertib (AZD2014) and paclitaxel in ovarian cancer models. Oncotarget. 2017;8:113874–84. doi: 10.18632/oncotarget.23022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Zou ZQ, Zhang XH, Wang F, et al. A novel dual PI3Kalpha/mTOR inhibitor PI-103 with high antitumor activity in non-small cell lung cancer cells. Int J Mol Med. 2009;24:97–101. doi: 10.3892/ijmm_00000212. [DOI] [PubMed] [Google Scholar]
- 60.Maira SM, Stauffer F, Brueggen J, et al. Identification and characterization of NVP-BEZ235, a new orally available dual phosphatidylinositol 3-kinase/mammalian target of rapamycin inhibitor with potent in vivo antitumor activity. Mol Cancer Ther. 2008;7:1851–63. doi: 10.1158/1535-7163.MCT-08-0017. [DOI] [PubMed] [Google Scholar]
- 61.Garlich JR, De P, Dey N, et al. A vascular targeted pan phosphoinositide 3-kinase inhibitor prodrug, SF1126, with antitumor and antiangiogenic activity. Cancer Res. 2008;68:206–15. doi: 10.1158/0008-5472.CAN-07-0669. [DOI] [PubMed] [Google Scholar]
- 62.Heffron TP, Berry M, Castanedo G, et al. Identification of GNE-477, a potent and efficacious dual PI3K/mTOR inhibitor. Bioorg Med Chem Lett. 2010;20:2408–11. doi: 10.1016/j.bmcl.2010.03.046. [DOI] [PubMed] [Google Scholar]
- 63.Yu K, Toral-Barza L, Shi C, et al. Biochemical, cellular, and in vivo activity of novel ATP-competitive and selective inhibitors of the mammalian target of rapamycin. Cancer Res. 2009;69:6232–40. doi: 10.1158/0008-5472.CAN-09-0299. [DOI] [PubMed] [Google Scholar]
- 64.Duffy AG, Makarova-Rusher OV, Ulahannan SV, et al. Modulation of tumor eIF4E by antisense inhibition: A phase I/II translational clinical trial of ISIS 183750-an antisense oligonucleotide against eIF4E-in combination with irinotecan in solid tumors and irinotecan-refractory colorectal cancer. Int J Cancer. 2016;139:1648–57. doi: 10.1002/ijc.30199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Hong DS, Kurzrock R, Oh Y, et al. A phase 1 dose escalation, pharmacokinetic, and pharmacodynamic evaluation of eIF-4E antisense oligonucleotide LY2275796 in patients with advanced cancer. Clin Cancer Res. 2011;17:6582–91. doi: 10.1158/1078-0432.CCR-11-0430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Jiang CC, Croft A, Tseng HY, et al. Repression of microRNA-768-3p by MEK/ERK signalling contributes to enhanced mRNA translation in human melanoma. Oncogene. 2014;33:2577–88. doi: 10.1038/onc.2013.237. [DOI] [PubMed] [Google Scholar]
- 67.Liu S, Zha J, Lei M. Inhibiting ERK/Mnk/eIF4E broadly sensitizes ovarian cancer response to chemotherapy. Clin Transl Oncol. 2018;20:374–81. doi: 10.1007/s12094-017-1724-0. [DOI] [PubMed] [Google Scholar]
- 68.Diab S, Kumarasiri M, Yu M, et al. MAP kinase-interacting kinases--emerging targets against cancer. Chem Biol. 2014;21:441–52. doi: 10.1016/j.chembiol.2014.01.011. [DOI] [PubMed] [Google Scholar]
- 69.Altman JK, Szilard A, Konicek BW, et al. Inhibition of Mnk kinase activity by cercosporamide and suppressive effects on acute myeloid leukemia precursors. Blood. 2013;121:3675–81. doi: 10.1182/blood-2013-01-477216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Hofmann WP, Herrmann E, Sarrazin C, Zeuzem S. Ribavirin mode of action in chronic hepatitis C: from clinical use back to molecular mechanisms. Liver Int. 2008;28:1332–43. doi: 10.1111/j.1478-3231.2008.01896.x. [DOI] [PubMed] [Google Scholar]
- 71.Assouline S, Culjkovic B, Cocolakis E, et al. Molecular targeting of the oncogene eIF4E in acute myeloid leukemia (AML): a proof-of-principle clinical trial with ribavirin. Blood. 2009;114:257–60. doi: 10.1182/blood-2009-02-205153. [DOI] [PubMed] [Google Scholar]
- 72.Borden KL, Culjkovic-Kraljacic B. Ribavirin as an anti-cancer therapy: acute myeloid leukemia and beyond? Leuk Lymphoma. 2010;51:1805–15. doi: 10.3109/10428194.2010.496506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Casaos J, Gorelick NL, Huq S, et al. The Use of Ribavirin as an Anticancer Therapeutic: Will It Go Viral? Mol Cancer Ther. 2019;18:1185–94. doi: 10.1158/1535-7163.MCT-18-0666. [DOI] [PubMed] [Google Scholar]
- 74.Jin J, Xiang W, Wu S, Wang M, Xiao M, Deng A. Targeting eIF4E signaling with ribavirin as a sensitizing strategy for ovarian cancer. Biochem Biophys Res Commun. 2019;510:580–6. doi: 10.1016/j.bbrc.2019.01.117. [DOI] [PubMed] [Google Scholar]
- 75.Dai D, Chen H, Tang J, Tang Y. Inhibition of mTOR/eIF4E by anti-viral drug ribavirin effectively enhances the effects of paclitaxel in oral tongue squamous cell carcinoma. Biochem Biophys Res Commun. 2017;482:1259–64. doi: 10.1016/j.bbrc.2016.12.025. [DOI] [PubMed] [Google Scholar]
- 76.Kentsis A, Topisirovic I, Culjkovic B, Shao L, Borden KL. Ribavirin suppresses eIF4E-mediated oncogenic transformation by physical mimicry of the 7-methyl guanosine mRNA cap. Proc Natl Acad Sci U S A. 2004;101:18105–10. doi: 10.1073/pnas.0406927102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Yan Y, Svitkin Y, Lee JM, Bisaillon M, Pelletier J. Ribavirin is not a functional mimic of the 7-methyl guanosine mRNA cap. RNA. 2005;11:1238–44. doi: 10.1261/rna.2930805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Westman B, Beeren L, Grudzien E, et al. The antiviral drug ribavirin does not mimic the 7-methylguanosine moiety of the mRNA cap structure in vitro. RNA. 2005;11:1505–13. doi: 10.1261/rna.2132505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Chen EZ, Jacobson BA, Patel MR, et al. Small-molecule inhibition of oncogenic eukaryotic protein translation in mesothelioma cells. Invest New Drugs. 2014;32:598–603. doi: 10.1007/s10637-014-0076-7. [DOI] [PubMed] [Google Scholar]
- 80.Li S, Jia Y, Jacobson B, et al. Treatment of breast and lung cancer cells with a N-7 benzyl guanosine monophosphate tryptamine phosphoramidate pronucleotide (4Ei-1) results in chemosensitization to gemcitabine and induced eIF4E proteasomal degradation. Mol Pharm. 2013;10:523–31. doi: 10.1021/mp300699d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Moerke NJ, Aktas H, Chen H, et al. Small-molecule inhibition of the interaction between the translation initiation factors eIF4E and eIF4G. Cell. 2007;128:257–67. doi: 10.1016/j.cell.2006.11.046. [DOI] [PubMed] [Google Scholar]
- 82.Papadopoulos E, Jenni S, Kabha E, et al. Structure of the eukaryotic translation initiation factor eIF4E in complex with 4EGI-1 reveals an allosteric mechanism for dissociating eIF4G. Proc Natl Acad Sci U S A. 2014;111:E3187–95. doi: 10.1073/pnas.1410250111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Cencic R, Hall DR, Robert F, et al. Reversing chemoresistance by small molecule inhibition of the translation initiation complex eIF4F. Proc Natl Acad Sci U S A. 2011;108:6689. doi: 10.1073/pnas.1011477108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Ko SY, Guo H, Barengo N, Naora H. Inhibition of ovarian cancer growth by a tumor-targeting peptide that binds eukaryotic translation initiation factor 4E. Clin Cancer Res. 2009;15:4336–47. doi: 10.1158/1078-0432.CCR-08-2924. [DOI] [PubMed] [Google Scholar]
- 85.Lama D, Quah ST, Verma CS, et al. Rational optimization of conformational effects induced by hydrocarbon staples in peptides and their binding interfaces. Sci Rep. 2013;3:3451. doi: 10.1038/srep03451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Lama D, Quah ST, Brown CJ, Lane DP, Verma CS. 159 Stapled-peptides targeting the protein-binding interface of eukaryotic Translation Initiation Factor 4E (eIF4E) protein. J Biomol Struct Dyn. 2015;33:102–3. doi: 10.1080/07391102.2015.1032796. [DOI] [Google Scholar]
- 87.Lama D, Liberatore AM, Frosi Y, et al. Structural insights reveal a recognition feature for tailoring hydrocarbon stapled-peptides against the eukaryotic translation initiation factor 4E protein. Chem Sci. 2019;10:2489–500. doi: 10.1039/c8sc03759k. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Mazzoletti M, Broggini M. PI3K/AKT/mTOR inhibitors in ovarian cancer. Curr Med Chem. 2010;17:4433–47. doi: 10.2174/092986710794182999. [DOI] [PubMed] [Google Scholar]
- 89.Langcake P, Pryce RJ. The production of resveratrol and the viniferins by grapevines in response to ultraviolet irradiation. Phytochemistry. 1977;16:1193–6. doi: 10.1016/S0031-9422(00)94358-9. [DOI] [Google Scholar]
- 90.Jang M, Cai L, Udeani GO, et al. Cancer chemopreventive activity of resveratrol, a natural product derived from grapes. Science. 1997;275:218–20. doi: 10.1126/science.275.5297.218. [DOI] [PubMed] [Google Scholar]
- 91.Cao Z, Fang J, Xia C, Shi X, Jiang BH. trans-3,4,5'-Trihydroxystibene inhibits hypoxia-inducible factor 1alpha and vascular endothelial growth factor expression in human ovarian cancer cells. Clin Cancer Res. 2004;10:5253–63. doi: 10.1158/1078-0432.CCR-03-0588. [DOI] [PubMed] [Google Scholar]
- 92.Zhong H, De Marzo AM, Laughner E, et al. Overexpression of Hypoxia-inducible Factor 1α in Common Human Cancers and Their Metastase. Cancer Res. 2000;59:5830–5. [PubMed] [Google Scholar]
- 93.Zhong LX, Zhang Y, Wu ML, et al. Resveratrol and STAT inhibitor enhance autophagy in ovarian cancer cells. Cell Death Discov. 2016;2:15071. doi: 10.1038/cddiscovery.2015.71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Stakleff KS, Sloan T, Blanco D, Marcanthony S, Booth TD, Bishayee A. Resveratrol exerts differential effects in vitro and in vivo against ovarian cancer cells. Asian Pac J Cancer Prev. 2012;13:1333–40. doi: 10.7314/apjcp.2012.13.4.1333. [DOI] [PubMed] [Google Scholar]
- 95.Altman MK, Alshamrani AA, Jia W, et al. Suppression of the GTPase-activating protein RGS10 increases Rheb-GTP and mTOR signaling in ovarian cancer cells. Cancer Lett. 2015;369:175–83.. doi: 10.1016/j.canlet.2015.08.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Lheureux S, Lecerf C, Briand M, et al. (18)F-FDG Is a Surrogate Marker of Therapy Response and Tumor Recovery after Drug Withdrawal during Treatment with a Dual PI3K/mTOR Inhibitor in a Preclinical Model of Cisplatin-Resistant Ovarian Cancer. Transl Oncol. 2013;6:586–95. doi: 10.1593/tlo.13100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Fabi F, Adam P, Parent S, Tardif L, Cadrin M, Asselin E. Pharmacologic inhibition of Akt in combination with chemotherapeutic agents effectively induces apoptosis in ovarian and endometrial cancer cell lines. Mol Oncol. 2020 doi: 10.1002/1878-0261.12888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Hu L, Hofmann J, Lu Y, Mills GB, Jaffe RB. Inhibition of phosphatidylinositol 3’-kinase increases efficacy of paclitaxel in in vitro and in vivo ovarian cancer models. Cancer Res 2002;62:1087-92. [PubMed] [Google Scholar]
- 99.David-West G, Ernlund A, Gadi A, Schneider RJ. mTORC1/2 inhibition re-sensitizes platinum-resistant ovarian cancer by disrupting selective translation of DNA damage and survival mRNAs. Oncotarget. 2018;9:33064–76. doi: 10.18632/oncotarget.25869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Jacobson BA, Alter MD, Kratzke MG, et al. Repression of cap-dependent translation attenuates the transformed phenotype in non-small cell lung cancer both in vitro and in vivo. Cancer Res. 2006;66:4256–62. doi: 10.1158/0008-5472.CAN-05-2879. [DOI] [PubMed] [Google Scholar]
- 101.Pelletier J, Graff J, Ruggero D, Sonenberg N. Targeting the eIF4F translation initiation complex: a critical nexus for cancer development. Cancer Res. 2015;75:250–63. doi: 10.1158/0008-5472.CAN-14-2789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Graff JR, Zimmer SG. Translational control and metastatic progression: Enhanced activity of the mRNA cap-binding protein eIF-4E selectively enhances translation of metastasis-related mRNAs. Clin Exp Metastasis. 2003;20:265–73. doi: 10.1023/a:1022943419011. [DOI] [PubMed] [Google Scholar]
- 103.Pettersson F, Del Rincon SV, Emond A, et al. Genetic and pharmacologic inhibition of eIF4E reduces breast cancer cell migration, invasion, and metastasis. Cancer Res. 2015;75:1102–12. doi: 10.1158/0008-5472.CAN-14-1996. [DOI] [PubMed] [Google Scholar]
- 104.Asimomytis A, Karanikou M, Rodolakis A, et al. mTOR downstream effectors, 4EBP1 and eIF4E, are overexpressed and associated with HPV status in precancerous lesions and carcinomas of the uterine cervix. Oncol Lett. 2016;12:3234–40. doi: 10.3892/ol.2016.5056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Yang S, Hewitt S, Steinberg S, Liewehr D, Swain S. Expression levels of eIF4E, VEGF, and cyclin D1, and correlation of eIF4E with VEGF and cyclin D1 in multi-tumor tissue microarray. Oncol Rep. 2007;17:281–7. [PubMed] [Google Scholar]
- 106.Zheng J, Li X, Zhang C, Zhang Y. eIF4E Overexpression Is Associated with Poor Prognoses of Ovarian Cancer. Anal Cell Pathol (Amst) 2020;2020:8984526. doi: 10.1155/2020/8984526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Ueda T, Watanabe-Fukunaga R, Fukuyama H, Nagata S, Fukunaga R. Mnk2 and Mnk1 are essential for constitutive and inducible phosphorylation of eukaryotic initiation factor 4E but not for cell growth or development. Mol Cell Biol. 2004;24:6539–49. doi: 10.1128/MCB.24.15.6539-6549.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Waskiewicz AJ, Johnson JC, Penn B, Mahalingam M, Kimball SR, Cooper JA. Phosphorylation of the cap-binding protein eukaryotic translation initiation factor 4E by protein kinase Mnk1 in vivo. Mol Cell Biol. 1999;19:1871–80. doi: 10.1128/mcb.19.3.1871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.McKendrick L, Morley SJ, Pain VM, Jagus R, Joshi B. Phosphorylation of eukaryotic initiation factor 4E (eIF4E) at Ser209 is not required for protein synthesis in vitro and in vivo. Eur J Biochem. 2001;268:5375–85. doi: 10.1046/j.0014-2956.2001.02478.x. [DOI] [PubMed] [Google Scholar]
- 110.Carter JH, Deddens JA, Spaulding NR IV, et al. Phosphorylation of eIF4E serine 209 is associated with tumour progression and reduced survival in malignant melanoma. Br J Cancer. 2016;114:444–53. doi: 10.1038/bjc.2015.450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Schneider RJ, Sonenberg N. Translational Control in Cancer Development and Progression. Cold Spring Harb Monogr Arch. 2007;48:401. doi: 10.1101/087969767.48.401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Wan J, Shi F, Xu Z, Zhao M. Knockdown of eIF4E suppresses cell proliferation, invasion and enhances cisplatin cytotoxicity in human ovarian cancer cells. Int J Oncol. 2015;47:2217–25. doi: 10.3892/ijo.2015.3201. [DOI] [PubMed] [Google Scholar]
- 113.Zhan Y, Dahabieh MS, Rajakumar A, et al. The role of eIF4E in response and acquired resistance to vemurafenib in melanoma. J Invest Dermatol. 2015;135:1368–76. doi: 10.1038/jid.2015.11. [DOI] [PubMed] [Google Scholar]
- 114.Zhou FF, Yan M, Guo GF, et al. Knockdown of eIF4E suppresses cell growth and migration, enhances chemosensitivity and correlates with increase in Bax/Bcl-2 ratio in triple-negative breast cancer cells. Med Oncol. 2011;28:1302–7. doi: 10.1007/s12032-010-9630-0. [DOI] [PubMed] [Google Scholar]
- 115.Choi CH, Lee JS, Kim SR, et al. Direct inhibition of eIF4E reduced cell growth in endometrial adenocarcinoma. J Cancer Res Clin Oncol. 2011;137:463–9. doi: 10.1007/s00432-010-0902-z. [DOI] [PubMed] [Google Scholar]
- 116.Cao J, Sun X, Zhang X, Chen D. Inhibition of eIF4E cooperates with chemotherapy and immunotherapy in renal cell carcinoma. Clin Transl Oncol. 2018;20:761–7. doi: 10.1007/s12094-017-1786-z. [DOI] [PubMed] [Google Scholar]
- 117.Pettersson F, Yau C, Dobocan MC, et al. Ribavirin treatment effects on breast cancers overexpressing eIF4E, a biomarker with prognostic specificity for luminal B-type breast cancer. Clin Cancer Res. 2011;17:2874–84. doi: 10.1158/1078-0432.CCR-10-2334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Jia Y, Chiu TL, Amin EA, Polunovsky V, Bitterman PB, Wagner CR. Design, synthesis and evaluation of analogs of initiation factor 4E (eIF4E) cap-binding antagonist Bn7-GMP. Eur J Med Chem. 2010;45:1304–13. doi: 10.1016/j.ejmech.2009.11.054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Ghosh B, Benyumov AO, Ghosh P, et al. Nontoxic chemical interdiction of the epithelial-to-mesenchymal transition by targeting cap-dependent translation. ACS Chem Biol. 2009;4:367–77. doi: 10.1021/cb9000475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Sekiyama N, Arthanari H, Papadopoulos E, Rodriguez-Mias RA, Wagner G, Léger-Abraham M. Molecular mechanism of the dual activity of 4EGI-1: Dissociating eIF4G from eIF4E but stabilizing the binding of unphosphorylated 4E-BP1. Proc Natl Acad Sci U S A. 2015;112:E4036–45. doi: 10.1073/pnas.1512118112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Marino D, Chillemi G, De Rubeis S, Tramontano A, Achsel T, Bagni C. MD and Docking Studies Reveal That the Functional Switch of CYFIP1 is Mediated by a Butterfly-like Motion. J Chem Theory Comput. 2015;11:3401–10. doi: 10.1021/ct500431h. [DOI] [PubMed] [Google Scholar]
- 122.Marino D, D'Annessa I, Tancredi H, Bagni C, Gallicchio E. A unique binding mode of the eukaryotic translation initiation factor 4E for guiding the design of novel peptide inhibitors. Protein Sci. 2015;24:1370–82. doi: 10.1002/pro.2708. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Not applicable.