Abstract
The COVID-19 pandemic has presented many challenges that have spurred biotechnological research to address specific problems. Diagnostics is one area where biotechnology has been critical. Diagnostic tests play a vital role in managing a viral threat by facilitating the detection of infected and/or recovered individuals. From the perspective of what information is provided, these tests fall into two major categories, molecular and serological. Molecular diagnostic techniques assay whether a virus is present in a biological sample, thus making it possible to identify individuals who are currently infected. Additionally, when the immune system is exposed to a virus, it responds by producing antibodies specific to the virus. Serological tests make it possible to identify individuals who have mounted an immune response to a virus of interest and therefore facilitate the identification of individuals who have previously encountered the virus. These two categories of tests provide different perspectives valuable to understanding the spread of SARS-CoV-2. Within these categories, different biotechnological approaches offer specific advantages and disadvantages. Here we review the categories of tests developed for the detection of the SARS-CoV-2 virus or antibodies against SARS-CoV-2 and discuss the role of diagnostics in the COVID-19 pandemic.
3. Introduction
Since the emergence of Severe acute respiratory syndrome-like coronavirus 2 (SARS-CoV-2) in late 2019, significant international efforts have focused on managing the spread of the virus. Identifying individuals who have contracted coronavirus disease 2019 (COVID-19) and may be contagious is crucial to reducing the spread of the virus. Given the high transmissibility of SARS-CoV-2 and the potential for asymptomatic or presymptomatic individuals to be contagious [1], the development of rapid, reliable, and affordable methods to detect SARS-CoV-2 infection is and was vitally important for understanding and controlling spread. For instance, test-trace-isolate procedures were an early cornerstone of many nations’ efforts to control the outbreak [2,3,4]. Such efforts depend on diagnostic testing.
The genetic sequence of the SARS-CoV-2 virus was first released by Chinese officials on January 10, 2020 [5], and the first test to detect the virus was released about 13 days later [6]. The genomic information was critical to the development of diagnostic approaches. There are two main classes of diagnostic tests: molecular tests, which can diagnose an active infection by identifying the presence of SARS-CoV-2, and serological tests, which can assess whether an individual was infected in the past via the presence or absence of antibodies against SARS-CoV-2. Over the course of the COVID-19 pandemic, a variety of tests have emerged within these two categories.
Molecular tests detect either viral RNA or protein in a patient sample. They are essential to identifying infected individuals, which can be important for determining courses of action related to treatment, quarantine, and contact tracing. Tests for viral RNA are done by reverse transcription (RT) of viral RNA to DNA followed by DNA amplification, usually with polymerase chain reaction (PCR) [7]. Tests for viral proteins typically use an antibody pair for detection as implemented in techniques such as lateral flow tests (LFTs) and enzyme-linked immunosorbent assays (ELISAs) [8,9]. Molecular tests require the viral genome sequence in order to develop DNA primers for viral RNA detection or to express a viral protein for use as an antigen in antibody production.
Serological tests, on the other hand, detect the presence of antibodies in blood plasma samples or other biological samples, providing insight into whether an individual has acquired immunity against SARS-CoV-2. Assays that can detect the presence of antibodies in blood plasma samples include ELISA, lateral flow immunoassay, and chemiluminescence immunoassay (CLIA) [10]. To distinguish past infection from vaccination, serological tests detect antibodies that bind the nucleocapsid protein of the SARS-CoV-2 virus [11]. They are useful for collecting population-level information for epidemiological analysis, as they can be used to estimate the extent of the infection in a given area. Thus, serological tests may be useful to address population-level questions, such as the percent of cases that manifest as severe versus mild and for guiding public health and economic decisions regarding resource allocation and counter-disease measures.
Molecular and serological tests therefore offer distinct, complementary perspectives on COVID-19 infections. Some of the same technologies are useful to both strategies, and different technologies have been employed to varying extents throughout the world since the start of the COVID-19 pandemic. Two of the primary metrics used to evaluate these tests are sensitivity and specificity. Sensitivity refers to a test’s ability to correctly identify a true positive; for example, a test with 50% sensitivity would identify SARS-CoV-2 in only one of every two positive samples. On the other hand, specificity refers to how well a test is able to identify a negative sample as negative. This metric can be relevant both in terms of understanding the risk of false positives and in discussing whether a test is susceptible to identifying other coronaviruses. Here, we review the different types of tests within each category that have been developed and provide perspective on their applications.
4. Molecular Tests to Identify SARS-CoV-2
Molecular tests are used to identify distinct genomic subsequences of a viral molecule in a sample or the presence of viral protein, and they thus can be used to diagnose an active viral infection. An important first step is identifying which biospecimens are likely to contain the virus in infected individuals and then acquiring these samples from the patient(s) to be tested.
Common sampling sources for molecular tests include nasopharyngeal cavity samples, such as throat washes, throat swabs, and saliva [12], and stool samples [13]. Once a sample is acquired from a patient, molecular tests detect SARS-CoV-2 based on the presence of either viral nucleic acids or viral proteins.
4.1. PCR-Based Tests
When testing for RNA from viruses like SARS-CoV-2, the first step involves pre-processing in order to create complementary DNA (cDNA) from the RNA sample using RT. The second step involves the amplification of a region of interest in the cDNA using successive cycles of heating and cooling. Depending on the application, this amplification is achieved using variations of PCR. Reverse transcription polymerase chain reaction (RT-PCR) tests determine whether a target is present by amplifying a region of interest of cDNA [14]. Some tests use the results of the PCR itself (e.g., a band on a gel) to determine whether the pathogen is present. However, this approach has not been employed widely in diagnostic testing, and instead most PCR-based tests are quantitative.
4.1.1. Quantitative Real-Time PCR
In contrast to RT-PCR, quantitative, real-time PCR uses fluorescent dyes that bind to the amplified DNA, thereby allowing a real time assessment of the amplification procedure [14] (in this manuscript we refer to quantitative real-time PCR as qPCR, following the Minimum Information for Publication of Quantitative Real-Time PCR Experiments guidelines [15], and when combined with reverse transcriptase steps, as is required for the evaluation of RNA, it is known as RT-qPCR.) The time resolution provided by qPCR and RT-qPCR is useful because the amount of fluorescence emitted by the sample is proportional to the amount of DNA amplified, and therefore the amount of virus present can be indirectly measured using the cycle threshold (Ct) determined by qPCR.
The first test developed and validated for the detection of SARS-CoV-2 used RT-qPCR to detect several regions of the viral genome: the ORF1b of the RNA-dependent RNA polymerase (RdRP), the envelope protein gene (E), and the nucleocapsid protein gene (N) [6]. The publication reporting this test was released on January 23, 2020, less than two weeks after the sequence of the virus was first reported [6]. In collaboration with several other labs in Europe and in China, the researchers confirmed the specificity of this test with respect to other coronaviruses against specimens from 297 patients infected with a broad range of respiratory agents. Specifically, this test uses two probes against RdRP, one of which is specific to SARS-CoV-2 [6]. Importantly, this assay was not found to return false positive results.
In January 2020, Chinese researchers developed a test that used RT-qPCR to identify two gene regions of the viral genome, ORF1b and N [16]. This assay was tested on samples from two COVID-19 patients and a panel of positive and negative controls consisting of RNA extracted from several cultured viruses. The assay uses the N gene to screen patients, while the ORF1b gene region is used to confirm the infection [16]. The test was designed to detect sequences conserved across sarbecoviruses, or viruses within the same subgenus as SARS-CoV-2. Considering that Severe acute respiratory syndrome-related coronavirus 1 (SARS-CoV-1) and SARS-CoV-2 are the only sarbecoviruses currently known to infect humans, a positive test can be assumed to indicate that the patient is infected with SARS-CoV-2, although this test is not able to discriminate the genetics of viruses within the sarbecovirus clade. The fact that the targets are so conserved offers the advantage of reduced concern about sensitivity in light of the evolution of SARS-CoV-2.
qPCR tests have played an important role in diagnostics during the COVID-19 pandemic. For SARS-CoV-2, studies have typically considered a patient to be infectious if the Ct is below 33 or sometimes 35 [1,17,18]. A lower Ct corresponds to fewer qPCR cycles needed to reach a detectable level, indicating that higher amounts of virus were present in the initial reaction. Interpretations of the Ct values obtained from these tests have raised some interesting questions related to viral load and contagiousness. Lower Ct values correspond to a higher probability of a positive viral culture, but no threshold could discriminate all positive from all negative cultures [19]. Additionally, because of the variability introduced by sample collection and clinical components of testing, Ct is not a proxy for viral load [20]. Positive PCR results have also been reported for extended periods of time from symptom onset and/or the first positive PCR test [21], meaning that in some cases, a positive PCR may not indicate that someone is contagious [1].
In addition to the nuance required to interpret PCR results, there are also factors that influence their accuracy. The specificity of these tests is very high [22], meaning that a positive RT-PCR result is very likely to indicate SARS-CoV-2 infection. The weight given to these tests as an indicator of SARS-CoV-2 infection regardless of other clinical considerations is not typical [23]. In fact, while the analytical specificity of the assay is extremely high, the challenges of implementing testing can introduce variability that results in a lower clinical specificity [23]. Several factors may influence the sensitivity and specificity, with sample collection being a critically important factor in the reliability of RT-PCR results. The most reliable results were found to come from nasopharyngeal swabs and from pooled nasal and throat swabs, with lower accuracies produced by saliva or by throat or nasal swabs alone [22,24]. Differences in experimental parameters such as the use of primers more specific to SARS-CoV-2 has been found to improve sensitivity in these specimens [25]. Additionally, the impact of viral evolution on RT-PCR sensitivity is a concern [26,27]. Using a panel that includes multiple targets can mitigate these effects [28]. Additionally, a test designed to incorporate genomic differences with SARS-CoV-1 was found to offer improved sensitivity and specificity [25]. Thus, while various factors can influence the exact parameters of testing accuracy, RT-PCR is known to have very high specificity and lower, but still high, sensitivity.
4.1.2. Digital PCR
Digital PCR (dPCR) is a new generation of PCR technologies offering an alternative to traditional qPCR [29]. In dPCR, a sample is partitioned into thousands of compartments, such as nanodroplets (droplet dPCR or ddPCR) or nanowells, and a PCR reaction takes place in each compartment. This design allows for a digital read-out where each partition is either positive or negative for the nucleic acid sequence being tested for, allowing for absolute target quantification through Poisson statistics. While dPCR equipment is not yet as common as that for qPCR, dPCR for DNA targets generally achieves higher sensitivity than other PCR technologies while maintaining high specificity, though sensitivity is slightly lower for RNA targets [30].
High sensitivity is particularly relevant for SARS-CoV-2 detection, since low viral load in clinical samples can lead to false negatives. In one study, Suo et al. [31] performed a double-blind evaluation of ddPCR for SARS-CoV-2 detection. They evaluated on 63 samples collected from suspected positive outpatients and 14 from supposed convalescent patients. Of the 63 outpatients, only 21 (33%) were identified as positive for SARS-CoV-2 with qPCR. However, ddPCR identified 49 (78%) as positive, 10 (16%) as negative, and 4 (6%) as suspected/borderline for SARS-CoV-2 infection. While both qPCR and ddPCR were found to have very high specificity (100%), this analysis reported that the sensitivity was 40% with qPCR compared to 94% with ddPCR. Analysis of serial dilutions of a linear DNA standard suggested that ddPCR was approximately 500 times more sensitive than qPCR [31]. Thus, this study suggests that ddPCR provides an extremely sensitive molecular test that is able to detect SARS-CoV-2 even at very low viral loads.
A second study [32] confirmed that RT-ddPCR is able to detect SARS-CoV-2 at a lower threshold for viral load relative to RT-PCR. This study analyzed 196 samples, including 103 samples from suspected patients, 77 from contacts and close contacts, and 16 from suspected convalescents, using both RT-qPCR and RT-ddPCR. First, the authors evaluated samples from the 103 suspected cases. Using RT-qPCR, 29 (28%) were identified as positive, 25 (24%) as negative, and 49 (48%) as borderline, i.e., the Ct value was higher than the positive threshold of 35 but lower than the negative threshold of 40. When the 61 negative and borderline samples were reanalyzed with ddPCR, 19 (31%) of the negative and 42 (69%) of the borderline samples were identified as positive. All of the suspected cases were later confirmed to be COVID-19 through a combination of symptom development and RT-qPCR resampling, indicating that ddPCR improved the overall detection rate compared to RT-qPCR from 28.2% to 87.4%.
They repeated this analysis in patient samples from contacts and close contacts. Patients who tested negative with both methods (n = 48) were observed to remain healthy over a period of 14 days. Among the remaining 29 samples from contacts, RT-qPCR identified 12 as positive, 1 as negative, and 16 as borderline. All of the samples that tested positive using RT-qPCR also tested positive using ddPCR. In contrast, the negative result and all but one of the borderline results were identified as positive by RT-ddPCR, and these patients were later determined to be SARS-CoV-2 positive based on clinical evaluation and repeated molecular sampling. Similarly, in the final group, 16 convalescent patients, RT-qPCR identified 12 as positive, three as suspect, and one as negative, but RT-dPCR identified all as positive. The evidence from this study therefore supports a lower limit of detection with ddPCR. Overall, these studies suggest that ddPCR is a promising tool for overcoming the problem of false negatives in SARS-CoV-2 RNA testing, but this method is unlikely to affect the current pandemic due to its lack of availability.
4.1.3. Sequencing
In some cases, the DNA amplified with PCR is sequenced. Sequencing requires an additional sample pre-processing step called library preparation. Library preparation is the process of preparing the sample for sequencing, typically by fragmenting the sequences and adding adapters [33]. In some cases, library preparation can involve other modifications of the sample, such as adding barcodes to identify a particular sample within the sequence data. Barcoding can therefore be used to pool samples from multiple sources. There are different reagents used for library preparation that are specific to identifying one or more target sections with PCR [34]. Sequential pattern matching is then used to identify unique subsequences of the virus, and if sufficient subsequences are found, the test is considered positive. Therefore, tests that use sequencing require a number of additional molecular and analytical steps relative to tests that use PCR alone.
Sequencing has been an important strategy for discovery of SARS-CoV-2 variants (e.g., see [35]). Sequencing elucidates any genetic variants located between the PCR primers. For this reason, it is critical to genomic surveillance efforts. Genomic surveillance is an important complement to epidemiological surveillance efforts [36], as described below. Through genomic surveillance, it has become possible to monitor the emergence of variants of interest and variants of concern (VOC) that may pose additional threats due to increased contagiousness, virulence, or immune escape [36,37]. Sequencing also allows for analysis of the dominant strains in an area at a given time. Worldwide, the extent of genomic surveillance varies widely, with higher-income countries typically able to sequence a higher percentage of cases [38]. Sequencing efforts are important for identifying variants containing mutations that might affect the reliability of molecular diagnostic tests, as well as mitigation measures such as therapeutics and prophylactics [26,27]. Therefore, sequencing is an important component of diagnostics: while it is not necessary for diagnosing an individual case, it is critical to monitoring trends in the variants affecting a population and to staying aware of emerging variants that may pose additional challenges.
4.1.4. Pooled and Automated PCR Testing
Due to limited supplies and the need for more tests, several labs have found ways to pool or otherwise strategically design tests to increase throughput. The first such result came from Yelin et al. [39], who reported that they could pool up to 32 samples in a single qPCR run. This was followed by larger-scale pooling with slightly different methods [40]. Although these approaches are also PCR based, they allow for more rapid scaling and higher efficiency for testing than the initial PCR-based methods developed. Conceptually, pooling could also be employed in analysis with RT-qPCR [41], and this strategy has been evaluated in settings such as schools [42] and hospitals [43].
0.4.2. RT-LAMP
RT-PCR remains the gold standard for detection of SARS-CoV-2 RNA from infected patients, but the traditional method requires special equipment and reagents, including a thermocycler. Loop-mediated isothermal amplification (LAMP) is an alternative to PCR that does not require specialized equipment [44]. In this method, nucleic acids are amplified in a 25 μL reaction that is incubated and chilled on ice [44]. It uses primers designed to facilitate auto-cycling strand displacement DNA synthesis [44]. LAMP can be combined with reverse transcription (RT-LAMP) to enable the detection of RNA.
One study showed that RT-LAMP is effective for detection of SARS-CoV-2 with excellent specificity and sensitivity and that this method can be applied to unprocessed saliva samples [45]. This method was benchmarked against RT-PCR using 177 human nasopharyngeal RNA samples, of which 126 were COVID positive. The authors break down the sensitivity of their test according to the Ct value from RT-PCR of the same samples; RT-LAMP performs at 100% sensitivity for samples with a Ct from RT-PCR of 32 or less. The performance is worse when considering all RT-PCR positive samples (including those with Ct values between 32–40). However, there is some evidence suggesting that samples obtained from individuals that achieve Ct values >30 measured using RT-PCR tend to be less infective that those that record a C t value <30 [46,47,48], so RT-LAMP is still a useful diagnostic tool. Various combinations of reagents are available, but one example is the WarmStart Colorimetric LAMP 2X Master Mix with a set of six primers developed previously by Zhang et al. [49]. To determine assay sensitivity, serial tenfold dilutions of in vitro transcribed N-gene RNA standard were tested using quantities from 105 copies down to 10 copies. The assay readout is the color of the dye changing from pink to yellow due to binding to the DNA product over 30 minutes. The RT-LAMP assay was then applied to clinical nasopharyngeal samples. For viral loads above 100 copies of genomic RNA, the RT-LAMP assay had a sensitivity of 100% and a specificity of 96.1% from purified RNA. The sensitivity of the direct assay of saliva by RT-LAMP was 85%. Sensitivity and specificity metrics were obtained by comparison with results from RT-PCR. RT-LAMP pilot studies for detection of SARS-CoV-2 were reviewed in a meta-analysis [50]. In the meta-analysis of all 2,112 samples, the cumulative sensitivity of RT-LAMP was calculated at 95.5%, and the cumulative specificity was 99.5%.
This test aims to bring the sensitivity of nucleic acid detection to the point of care or home testing setting. It could be applied for screening, diagnostics, or as a definitive test for people who are positive based on LFTs (see below). The estimated cost per test is about 2 euros when RNA extraction is included. The main strength of this test over RT-PCR is that it can be done isothermally, but the main drawback is that it is about 10-fold less sensitive than RT-PCR. The low cost, excellent sensitivity/specificity, and quick readout of RT-LAMP makes this an attractive alternative to RT-PCR. Alternative strategies like RT-LAMP are needed to bring widespread testing away from the lab and into under-resourced areas.
4.3. CRISPR-based Detection
Technology based on CRISPR (clustered regularly interspaced short palindromic repeats) [51] has also been instrumental in scaling up testing protocols. Two CRISPR-associated nucleases, Cas12 and Cas13, have been used for nucleic acid detection. Multiple assays exploiting these nucleases have emerged as potential diagnostic tools for the rapid detection of SARS-CoV-2 genetic material and therefore SARS-CoV-2 infection. The SHERLOCK method (Specific High-sensitivity Enzymatic Reporter unLOCKing) from Sherlock Biosciences relies on Cas13a to discriminate between inputs that differ by a single nucleotide at very low concentrations [52]. The target RNA is amplified by real-time recombinase polymerase amplification (RT-RPA) and T7 transcription, and the amplified product activates Cas13a. The nuclease then cleaves a reporter RNA, which liberates a fluorescent dye from a quencher. Several groups have used the SHERLOCK method to detect SARS-CoV-2 viral RNA. An early study reported that the method could detect 7.5 copies of viral RNA in all 10 replicates, 2.5 copies in 6 out of 10, and 1.25 copies in 2 out of 10 runs [53]. It also reported 100% specificity and sensitivity on 114 RNA samples from clinical respiratory samples (61 suspected cases, among which 52 were confirmed and nine were ruled out by metagenomic next-generation sequencing, 17 SARS-CoV-2-negative but human coronavirus (HCoV)-positive cases, and 36 samples from healthy subjects) and a reaction turnaround time of 40 minutes. A separate study screened four designs of SHERLOCK and extensively tested the best-performing assay. They determined the limit of detection to be 10 copies/μl using both fluorescent and lateral flow detection [54].
LFT strips are simple to use and read, but there are limitations in terms of availability and cost per test. Another group therefore proposed the CREST (Cas13-based, Rugged, Equitable, Scalable Testing) protocol, which uses a P51 cardboard fluorescence visualizer, powered by a 9-volt battery, for the detection of Cas13 activity instead of immunochromatography [55]. CREST can be run, from RNA sample to result, with no need for AC power or a dedicated facility, with minimal handling in approximately 2 hours. Testing was performed on 14 nasopharyngeal swabs. CREST picked up the same positives as the CDC-recommended TaqMan assay with the exception of one borderline sample that displayed low-quality RNA. This approach may therefore represent a rapid, accurate, and affordable procedure for detecting SARS-CoV-2.
The DETECTR (DNA Endonuclease-Targeted CRISPR Trans Reporter) method from Mammoth Biosciences involves purification of RNA extracted from patient specimens, amplification of extracted RNAs by loop-mediated amplification, and application of their Cas12-based technology. In this assay, guide RNAs (gRNAs) were designed to recognize portions of sequences corresponding to the SARS-CoV-2 genome, specifically the N2 and E regions [56]. In the presence of SARS-CoV-2 genetic material, sequence recognition by the gRNAs results in double-stranded DNA cleavage by Cas12, as well as cleavage of a single-stranded DNA molecular beacon. The cleavage of this molecular beacon acts as a colorimetric reporter that is subsequently read out in a lateral flow assay and indicates the presence of SARS-CoV-2 genetic material and therefore SARS-CoV-2 infection. The 40-minute assay is considered positive if there is detection of both the E and N genes or presumptive positive if there is detection of either of them. The assay had 95% positive predictive agreement and 100% negative predictive agreement with the US Centers for Disease Control and Prevention SARS-CoV-2 RT-qPCR assay. The estimated limit of detection was 10 copies per μl reaction, versus 1 copy per μl reaction for the CDC assay.
These results have been confirmed by other DETECTR approaches. Using RT-RPA for amplification, another group detected 10 copies of synthetic SARS-CoV-2 RNA per μl of input within 60 minutes of RNA sample preparation in a proof-of-principle evaluation [57]. Through a similar approach, another group reported detection at 1 copy per μl [58]. The DETECTR protocol was improved by combining RT-RPA and CRISPR-based detection in a one-pot reaction that incubates at a single temperature and by using dual CRISPR RNAs, which increases sensitivity. This new assay, known as All-In-One Dual CRISPR-Cas12a, detected 4.6 copies of SARS-CoV-2 RNA per μl of input in 40 minutes [59]. Another single-tube, constant-temperature approach using Cas12b instead of Cas12a achieved a detection limit of 5 copies/μl in 40–60 minutes [60].
It was also reported that electric field gradients can be used to control and accelerate CRISPR assays by co-focusing Cas12-gRNA, reporters, and target [61]. The authors generated an appropriate electric field gradient using a selective ionic focusing technique known as isotachophoresis (ITP) implemented on a microfluidic chip. They also used ITP for automated purification of target RNA from raw nasopharyngeal swab samples. Combining this ITP purification with loop-mediated isothermal amplification, their ITP-enhanced assay achieved detection of SARS-CoV-2 RNA (from raw sample to result) in 30 minutes.
All these methods require upstream nucleic acid amplification prior to CRISPR-based detection. They rely on type V (Cas12-based) and type IV (Cas13-based) CRISPR systems. In contrast, type III CRISPR systems have the unique property of initiating a signaling cascade, which could boost the sensitivity of direct RNA detection. In type III CRISPR systems, guide CRISPR RNAs (crRNAs) are bound by several Cas proteins [62] and can target both DNA and RNA molecules [63,64]. A study tested this hypothesis using the type III-A crRNA-guided surveillance complex from Thermus thermophilus [65]. The authors showed that activation of the Cas10 polymerase generates three products (cyclic nucleotides, protons, and pyrophosphates) that can all be used to detect SARS-CoV-2 RNA. Detection of viral RNA in patient samples still required an initial nucleic acid amplification step, but improvements may in the future remove that requirement.
This goal of amplification-free detection was later achieved for a Cas13a-based system [66]. This approach combined multiple CRISPR RNAs to increase Cas13a activation, which is detected by a fluorescent reporter. Importantly, because the viral RNA is detected directly, the test yields a quantitative measurement rather than a binary result. The study also shows that fluorescence can be measured in a custom-made dark box with a mobile phone camera and a low-cost laser illumination and collection optics. This approach is a truly portable assay for point-of-care diagnostics. The authors achieved detection of 100 copies/μl of pre-isolated RNA in 30 minutes, and correctly identified all SARS-CoV-2-positive patient RNA samples tested in 5 minutes (n = 20).
There is an increasing body of evidence that CRISPR-based assays offer a practical solution for rapid, low-barrier testing in areas that are at greater risk of infection, such as airports and local community hospitals. In the largest study to date, DETECTR was compared to RT-qPCR on 378 patient samples [67]. The authors reported 95% reproducibility. Both techniques were equally sensitive in detecting SARS-CoV-2. Lateral flow strips showed 100% correlation to the high-throughput DETECTR assay. Importantly, DETECTR was 100% specific for SARS-CoV-2 and did not detect other human coronaviruses. A method based on a Cas9 ortholog from Francisella novicida known as FnCas9 achieved 100% sensitivity and 97% specificity in clinical samples, and the diagnostic kit is reported to have completed regulatory validation in India [68].
4.4. Immunoassays for the Detection of Antigens
Immunoassays can detect molecular indicators of SARS-CoV-2 infection, such as the proteins that act as antigens from the SARS-CoV-2 virus. They offer the advantage of generally being faster and requiring less specialized equipment than other molecular tests, especially those involving PCR. As a result, immunoassays hold particular interest for implementation at home and in situations where resources for PCR testing are limited. The trade-off is that these tests typically have a lower sensitivity, and sometimes a lower specificity, than other molecular tests. However, these tests tend to return a positive result five to 12 days after symptom onset, which may therefore correlate more closely with the timeframe during which viral replication occurs [69]. Immunoassays for the detection of the SARS-CoV-2 antigen can include LFTs and ELISA, as discussed here, as well as CLIA and chromatographic immunoassays [70], as described in the serological testing section below.
4.4.1. Lateral Flow Tests
LFTs provide distinct value relative to PCR tests. They can return results within 30 minutes and can be performed without specialized equipment and at low cost. They also do not require training to operate and are cheap to produce. Thus, they can be distributed widely to affected populations making them an important public health measure to curb pandemic spread. LFTs rely on the detection of viral protein with an antibody. Often this is done with an antibody sandwich format, where one antibody conjugated to a dye binds at one site on the antigen, and an immobilized antibody on the strip binds at another site [8]. This design allows the dye to accumulate to form a characteristic positive test line on the strip [8]. Outside of COVID-19 diagnostics, the applications of LFTs are broad; they are routinely used for home pregnancy tests, disease detection, and even drugs of abuse detection in urine [71].
A recent review surveyed the performance of LFTs for detection of current SARS-CoV-2 infection [72]. This review covered 24 studies that included more than 26,000 total LFTs. They reported significant heterogeneity in test sensitivities, with estimates ranging from 37.7% to 99.2%. The estimated specificities of these tests were more homogeneous, spanning 92.4% to 100.0%.
Despite having lower sensitivity than PCR tests, LFTs occupy an important niche in the management of SARS-CoV-2. Current infection detection by LFTs enables the scale and speed of testing that is beneficial to managing viral spread. LFTs were available freely to citizens in the United Kingdom until April 1, 2022 [73] and to citizens of the United States in early 2022 [74]. These tests are particularly useful for ruling out SARS-CoV-2 infection in cases where the likelihood of infection is low (e.g., asymptomatic individuals) and positives (including false positives) can be validated with testing by alternate means [75].
4.4.2. Enzyme-Linked Immunosorbent Assay
ELISA is a very sensitive immunoassay that can be considered a gold standard for the detection of biological targets, including antibodies and antigenic proteins [9]. It can be used to generate either quantitative or qualitative results that can be returned within a few hours [76]. ELISA builds on the idea that antibodies and antigens bind together to form complexes [9] and utilizes an enzyme covalently linked to an antibody against the antigen to produce assay signal, usually a color change [77]. The main advantage of ELISA is that it enables signal amplification through the enzyme’s activity, which increases sensitivity. With sandwich ELISA, antibodies are immobilized on a surface such as a plate, and viral protein antigens in the sample bind and are retained [78]. A second antibody is added that binds to another site on the antigen is then added, and that second antibody is covalently linked to an enzyme. A substrate for that enzyme is then added to produce signal, usually light or a color change The exact strategy for tagging with a reporter enzyme varies among different types of ELISA [9,78]. For COVID-19 diagnostics, ELISAs have been designed to detect the antigenic Spike protein [79].
One of these assays uses two monoclonal antibodies specific to the nucleocapsid of SARS-CoV-2 to evaluate the relationship between the effect of (estimated) viral load on the ability of the assay to detect the SARS-CoV-2 antigen [80]. This study analyzed 339 naso-oropharyngeal samples that were also analyzed with RT-qPCR as a gold standard. RT-qPCR identified 147 samples as positive and 192 as negative. The authors estimated the overall sensitivity and specificity to be 61.9% and 99.0%, respectively. Sensitivity increased with higher Ct. This study also assessed the performance of the ELISA test under different conditions in order to evaluate how robust it would be to the challenges of testing in real-world settings globally. Higher sensitivity was achieved for samples that were stored under ideal conditions (immediate placement in −80° C). Therefore, while immediate access to laboratory equipment is an advantage, it is not strictly necessary for ELISA to detect the antigen.
4.5. Limitations of Molecular Tests
Tests that identify SARS-CoV-2 using molecular technologies will identify only individuals with current infections and are not appropriate for identifying individuals who have recovered from a previous infection. Among molecular tests, different technologies have different sensitivities and specificities. In general, specificity is high, and even then, the public health repercussions of a false positive can generally be mitigated with follow-up testing. On the other hand, a test’s sensitivity, which indicates the risk of a false-negative response, can pose significant challenge to large-scale testing. False negatives are a significant concern for several reasons. Importantly, clinical reports indicate that it is imperative to exercise caution when interpreting the results of molecular tests for SARS-CoV-2 because negative results do not necessarily mean a patient is virus-free [81]. To reduce occurrence of false negatives, correct execution of the analysis is crucial [82]. Additionally, PCR-based tests can remain positive for a much longer time than the virus is likely to be actively replicating [69], raising concerns about their informativeness after the acute phase of the disease. Hence, the CDC has advised individuals who suspect they have been re-infected with SARS-CoV-2 to avoid using diagnostic tests within 90 days of receiving a previous positive test [83].
Additionally, the emerging nature of the COVID-19 pandemic has introduced some challenges related to uncertainty surrounding interactions between SARS-CoV-2 and its human hosts. For example, viral shedding kinetics are still not well understood but are expected to introduce a significant effect of timing of sample collection on test results [82]. Similarly, the type of specimen could also influence outcomes, as success in viral detection varies among clinical sample types [22,24,82]. With CRISPR-based testing strategies, the gRNA can recognize off-target interspersed sequences in the viral genome [84], potentially resulting in false positives and a loss of specificity.
There are also significant practical and logistical concerns related to the widespread deployment of molecular tests. Much of the technology used for molecular tests is expensive, and while it might be available in major hospitals and/or diagnostic centers, it is often not available to smaller facilities [85]. At times during the pandemic, the availability of supplies for testing, including swabs and testing media, has also been limited [86]. Similarly, processing times can be long, and tests might take up to 4 days to return results [85], especially during times of high demand, such as spikes in case numbers [87]. Countries have employed various and differing molecular testing strategies as a tool to reduce viral transmission, even among high-income countries [88]. The rapid development of molecular tests has provided a valuable, albeit imperfect, tool to identify active SARS-CoV-2 infections.
5. Serological Tests to Identify Recovered Individuals
Although several molecular diagnostic tests to detect viral genetic material have high specificity and sensitivity, they provide information only about active infection, and therefore offer just a snapshot-in-time perspective on the spread of a disease. Most importantly, they would not work on a patient who has fully recovered from the virus at the time of sample collection. In such contexts, serological tests are informative.
Serological tests use many of the same technologies as the immunoassays used to detect the presence of an antigen but are instead used to evaluate the presence of antibodies against SARS-CoV-2 in a serum sample. These tests are particularly useful for insight into population-level dynamics and can also offer a glimpse into the development of antibodies by individual patients during the course of a disease. Immunoassays can detect antibodies produced by the adaptive immune system in response to viral threat. Understanding the acquisition and retention of antibodies is important both to the diagnosis of prior (inactive) infections and to the development of vaccines. The two immunoglobulin classes that are most pertinent to these goals are immunoglobulin M (IgM), which are the first antibodies produced in response to an infection, and immunoglobulin G (IgG), which are the most abundant antibodies [89,90]. Serological tests detect these antibodies, offering a mechanism through which prior infection can be identified. However, the complexity of the human immune response means that there are many facets to such analyses.
In general, SARS-CoV-2 infection will induce the immune system to produce antibodies fairly quickly. Prior research is available about the development of antibodies to SARS-CoV-1 during the course of the associated disease, severe acute respiratory syndrome (SARS). IgM and IgG antibodies were detected in the second week following SARS-CoV-1 infection. IgM titers peaked by the first month post-infection, and then declined to undetectable levels after day 180. IgG titers peaked by day 60 and persisted in all donors through the two-year duration of study [91]. Such tests can also illuminate the progression of viral disease, as IgM are the first antibodies produced by the body and indicate that the infection is active. Once the body has responded to the infection, IgG are produced and gradually replace IgM, indicating that the body has developed immunogenic memory [92]. Therefore, it was hoped that the development of assays to detect the presence of IgM and IgG antibodies against SARS-CoV-2 would allow the identification of cases from early in the infection course (via IgM) and for months or years afterwards (via IgG). Several technologies have been used to develop serological tests for COVID-19, including ELISA, lateral flow immunoassay, chemiluminescence immunoassay, and neutralizing antibody assays [93].
5.1. ELISA
The application of ELISA to serological testing is complementary to its use in molecular diagnostics (see above). Instead of using an enzyme-labeled antibody as a probe that binds to the target antigen, the probe is an antigen and the target is an antibody. The enzyme used for detection and signal amplification is on a secondary antibody raised generally against human IgG or IgM. In March 2020, the Krammer lab proposed an ELISA test that detects IgG and IgM that react against the receptor-binding domain (RBD) of the spike proteins (S) of the virus [94]. A subsequent ELISA test developed to detect SARS-CoV-2 IgG based on the RBD reported a specificity of over 99% and a sensitivity of up to 88.24%, which was observed in samples collected 21 to 27 days after the onset of infection (approximated with symptom onset or positive PCR test) [95]. Earlier in the disease course, sensitivity was lower: 53.33% between days 0 and 13 and 80.47% between days 14 and 20. This study reported that their laboratory ELISA outperformed two commercial kits that also used an ELISA design [95]. Therefore, while analysis with ELISA requires laboratory support and equipment, these results do suggest that ELISA achieves relatively high sensitivity, especially in the weeks following infection. Efforts have been made to develop low-cost strategies for conducting these tests that will make them more accessible worldwide [96].
5.2. Chemiluminescence Immunoassay
Another early approach investigated for detection of antibodies against SARS-CoV-2 was CLIA. Like ELISA, CLIA is a type of enzyme immunoassay (EIA) [97]. While the technique varies somewhat, in one approach, a bead is coated with the antigen and then washed with the sample [98]. If the antibody is present in the sample, it will bind to the bead. Then the bead is exposed to a label, a luminescent molecule that will bind to the antigen/antibody complex and can therefore be used as an indicator [98]. One CLIA approach to identify COVID-19 used a synthetic peptide derived from the amino acid sequence of the SARS-CoV-2 S protein [99]. It was highly specific to SARS-CoV-2 and detected IgM in 57.2% and IgG in 71.4% of serum samples from 276 COVID-19 cases confirmed with RT-qPCR. IgG could be detected within two days of the onset of fever, but IgM could not be detected any earlier [99], which has been supported by other analyses as well [100]. This pattern was consistent with observations in Middle East respiratory syndrome, which is also caused by an HCoV. In comparisons of different commercial immunoassays, accuracy of CLIA tests were often roughly comparable to other EIAs [101], although one CLIA did not perform as well as several other EIAs [100,102]. The sensitivity and specificities reported vary among CLIA tests and for the detection of IgM versus IgG, but sensitivities and specificities as high as 100% have been reported among various high-throughput tests [102,103,104]. CLIA has previously been used to develop tests that can be used at point of care (e.g., [97]) which may allow for this technique to become more widely accessible in the future.
5.3. Lateral Flow Immunoassay
The first serological test approved for emergency use in the United States was developed by Cellex [105]. The Cellex qSARS-CoV-2 IgG/IgM Rapid Test is a chromatographic immunoassay, also known as a lateral flow immunoassay, designed to qualitatively detect IgM and IgG antibodies against SARS-CoV-2 in the plasma of patients suspected to have developed a SARS-CoV-2 infection [105]. The Cellex test cassette contains a pad of SARS-CoV-2 antigens and a nitrocellulose strip with lines for each of IgG and IgM, as well as a control (goat IgG) [105]. In a specimen that contains antibodies against the SARS-CoV-2 antigen, the antibodies will bind to the strip and be captured by the IgM and/or IgG line(s), resulting in a change of color [105]. With this particular assay, results can be read within 15 to 20 minutes [105]. Lateral flow immunoassays are often available at point of care but can have very low sensitivity [102].
5.4. Neutralizing Antibody Assays
Neutralizing antibody assays play a functional role in understanding immunity that distinguishes them from other serological tests. The tests described above are all binding antibody tests. On the other hand, rather than simply binding an antibody to facilitate detection, neutralizing antibody assays determine whether an antibody response is present that would prevent infection [106,107]. Therefore, these tests serve the purpose of evaluating the extent to which a sample donor has acquired immunity that will reduce susceptibility to SARS-CoV-2. As a result, neutralizing antibody assays have been used widely to characterize the duration of immunity following infection, to assess vaccine candidates, and to establish correlates of protection against infection and disease [108,109,110]. These tests are typically performed in a laboratory [106], and in SARS-CoV-2, the results of neutralizing antibody assays are often correlated with the results of binding antibody tests [106].
The gold standard for assessing the presence of neutralizing antibodies is the plaque reduction neutralization test (PRNT), but this approach does not scale well [107]. An early high-throughput neutralizing antibody assay designed against SARS-CoV-2 used a fluorescently labeled reporter virus that was incubated with different dilutions of patient serum [107]. The cells used for incubation would turn green if antibodies were not present. Essentially, this assay evaluates whether the virus is able to infect the cell in the presence of the serum. The specificity of this assay was 100%, and the correlation between the results of this assay and of PRNT was 0.85 with the results suggesting that the sensitivity of the high-throughput approach was higher than that of PRNT [107]. While this approach was performed on a plate and using cells, other methods have been developed using methods such as bead arrays [111].
5.5. Duration of Immune Indicators
While the adaptive immune system produces antibodies in response to SARS-CoV-2 viral challenge, these indicators of seroconversion are unlikely to remain in circulation permanently. Previously, a two-year longitudinal study following convalesced SARS patients with a mean age of 29 found that IgG antibodies were detectable in all 56 patients surveyed for at least 16 months and remained detectable in all but 4 patients (11.8%) through the full two-year study period [112]. These results suggest that immunity to SARS-CoV-1 is sustained for at least a year. Circulating antibody titers to other coronaviruses have been reported to decline significantly after one year [113]. Evidence to date suggests that sustained immunity to the SARS-CoV-2 virus remains for a shorter period of time but at least 6 to 8 months after infection [114,115,116,117]. However, this does not mean that all serological evidence of infection dissipates, but rather that the immune response becomes insufficient to neutralize the virus.
In order to study the persistence of SARS-CoV-2 antibodies, one study assessed sustained immunity using 254 blood samples from 188 COVID-19 positive patients [115]. The samples were collected at various time points between 6 and 240 days post-symptom onset; some patients were assessed longitudinally. Of the samples, 43 were collected at least 6 months after symptom onset. After one month, 98% of patients were seropositive for IgG to S. Moreover, S IgG titers were stable and heterogeneous among patients over a period of 6 to 8 months post-symptom onset, with 90% of subjects seropositive at 6 months. Similarly, at 6 to 8 months 88% of patients were seropositive for RBD IgG, and 90% were seropositive for SARS-CoV-2 neutralizing antibodies. Another study examined 119 samples from 88 donors who had recovered from mild to severe cases of COVID-19 [117]. A relatively stable level of IgG and plasma neutralizing antibodies was identified up to 6 months post diagnosis. Significantly lower but considerable levels of anti-SARS-CoV-2 IgG antibodies were still present in 80% of samples obtained 6 to 8 months post-symptom onset.
Titers of IgM and IgG antibodies against the RBD were found to decrease from 1.3 to 6.2 months post infection in a study of 87 individuals [118]. However, the decline of IgA activity (15%) was less pronounced than that of IgM (53%) or IgG (32%). It was noted that higher levels of anti-RBD IgG and anti-N total antibodies were detected in individuals that reported persistent post-acute symptoms at both study visits. Moreover, plasma neutralizing activity decreased five-fold between 1.3 and 6.2 months in an assay of HIV-1 virus pseudotyped with SARS-CoV-2 S protein, and this neutralizing activity was directly correlated with IgG anti-RBD titers [118]. These findings are in accordance with other studies that show that the majority of seroconverters have detectable, albeit decreasing, levels of neutralizing antibodies at least 3 to 6 months post infection [119,120,121].
Determining the potency of anti-RBD antibodies early in the course of an infection may be important moving forward, as their neutralizing potency may be prognostic for disease severity and survival [122]. The duration of immunity might also vary with age [123] or ABO blood type [124]. Autopsies of lymph nodes and spleens from severe acute COVID-19 patients showed a loss of T follicular helper cells and germinal centers that may explain some of the impaired development of antibody responses [125]. Therefore, serological testing may be time-limited in its ability to detect prior infection.
Other immune indicators of prior infection have also been evaluated to see how they persist over time. SARS-CoV-2 memory CD8+ T cells were slightly decreased (50%) 6 months post-symptom onset. In this same subset of COVID-19 patients, 93% of subjects had detectable levels of SARS-CoV-2 memory CD4+ T cells, of which 42% had more than 1% SARS-CoV-2-specific CD4+ T cells. At 6 months, 92% of patients were positive for SARS-CoV-2 memory CD4+ T cells. Indeed, the abundance of S-specific memory CD4+ T cells over time was similar to that of SARS-CoV-2-specific CD4+ T cells overall [115]. T cell immunity to SARS-CoV-2 at 6 to 8 months following symptom onset has also been confirmed by other studies [117,126,127]. In another study, T cell reactivity to SARS-CoV-2 epitopes was also detected in some individuals never been exposed to SARS-CoV-2. This finding suggests the potential for cross-reactive T cell recognition between SARS-CoV-2 and pre-existing circulating HCoV that are responsible for the “common cold” [128], but further research is required. Therefore, whether T cells will provide a more stable measure through which to assess prior infection remains unknown. Notably, commercial entities have tried to develop tests specifically for T cells, some of which have been authorized by the United States Food and Drug Administration [129,130] to identify people with adaptive T cell immune responses to SARS-CoV-2, either from a previous or ongoing infection.
5.6. Applications of Serological Tests
In addition to the limitations posed by the fact that antibodies are not permanent indicators of prior infection, serological immunoassays carry a number of limitations that influence their utility in different situations. Importantly, false positives can occur due to cross-reactivity with other antibodies according to the clinical condition of the patient [105]. Due to the long incubation times and delayed immune responses of infected patients, serological immunoassays are insufficiently sensitive for a diagnosis in the early stages of an infection. Therefore, such tests must be used in combination with RNA detection tests if intended for diagnostic purposes [131]. False positives are particularly harmful if they are erroneously interpreted to mean that a population is more likely to have acquired immunity to a disease [132]. Similarly, while serological tests may be of interest to individuals who wish to confirm they were infected with SARS-CoV-2 in the past, their potential for false positives means that they are not currently recommended for this use. However, in the wake of vaccines becoming widely available, accurate serological tests that could be administered at point of care were investigated in the hope that they could help to prioritize vaccine recipients [133]. Another concern with serological testing is the potential for viral evolution to reduce the sensitivity of assays, especially for neutralizing antibody assays. Chen et al. performed a systematic re-analysis of published data examining the neutralizing effect of serum from vaccinated or recovered individuals on four VOC [134]. They found reduced neutralizing titers against these variants relative to the lineages used for reference. These findings suggest that such techniques will need to be modified over time as SARS-CoV-2 evolves.
These limitations make serological tests far less useful for diagnostics and for test-and-trace strategies; however, serological testing is valuable for public health monitoring at the population level. Serosurveys provide a high-level perspective of the prevalence of a disease and can provide insight into the susceptibility of a population as well as variation in severity, e.g., between geographic regions [132]. From a public health perspective, they can also provide insight into the effectiveness of mitigation efforts and to gain insight into risk factors influencing susceptibility [135]. EIA methods are high-throughput [136,137], and, as with molecular tests, additional efforts have been made to scale up the throughput of serological tests [138]. Therefore, serological tests can be useful to developing strategies for the management of viral spread.
Early in the course of the pandemic, it was also hoped that serological tests would provide information relevant to advancing economic recovery. Some infectious agents can be controlled through “herd immunity”, which is when a critical mass within the population acquires immunity through vaccination and/or infection, preventing an infectious agent from spreading widely. It was hoped that people who had recovered and developed antibodies might be able to return to work [139,140]. This strategy would have relied on recovered individuals acquiring long-term immunity, which has not been borne out [141]. Additionally, it was hoped that identifying seroconverters and specifically those who had mounted a strong immune response would reveal strong candidates for convalescent plasma donation [94]; however, convalescent plasma has not been found to offer therapeutic benefit (reviewed in [142]). While these hopes have not been realized, serological tests have been useful for gaining a better understanding of the pandemic [135].
6. Possible Alternatives to Current Diagnostic Practices
One possible alternative or complement to molecular and serological testing would be diagnosing COVID-19 cases based on symptomatology. COVID-19 can present with symptoms similar to other types of pneumonia, and symptoms can vary widely among COVID-19 patients; therefore, clinical presentation is often insufficient as a sole diagnostic criterion. In addition, identifying and isolating mild or asymptomatic cases is critical to efforts to manage outbreaks. Even among mildly symptomatic patients, a predictive model based on clinical symptoms had a sensitivity of only 56% and a specificity of 91% [143]. More problematic is that clinical symptom-based tests are only able to identify already symptomatic cases, not presymptomatic or asymptomatic cases. They may still be important for clinical practice and for reducing tests needed for patients deemed unlikely to have COVID-19.
In some cases, clinical signs may also provide information that can inform diagnosis. Using computed tomography of the chest in addition to RT-qPCR testing was found to provide a higher sensitivity than either measure alone [144]. X-ray diagnostics have been reported to have high sensitivity but low specificity in some studies [145]. Other studies have shown that specificity varies between radiologists [146], though the sensitivity reported here was lower than that published in the previous paper. While preliminary machine-learning results suggested that chest X-rays might provide high sensitivity and specificity and potentially facilitate the detection of asymptomatic and presymptomatic infections (e.g., [147]), further investigation suggested that such approaches are prone to bias and are unlikely to be clinically useful [148]. Given the above, the widespread use of X-ray tests on otherwise healthy adults is likely inadvisable.
Finally, in addition to genomic and serological surveillance, other types of monitoring have proven useful in managing the pandemic [149]. One that has received significant attention is wastewater surveillance. This approach can use several of the technologies described for molecular testing, such as qPCR and dPCR, as well as in vitro culturing [150] and can provide insight into trends in the prevalence of SARS-CoV-2 regionally.
7. Strategies and Considerations for Testing
Deciding whom to test, when to test, and which test to use have proven challenging as the COVID-19 pandemic has unfolded. Early in the COVID-19 pandemic, testing was typically limited to individuals considered high risk for developing serious illness [151]. This approach often limited testing to people with severe symptoms and people showing mild symptoms that had been in contact with a person who had tested positive. Individuals who were asymptomatic (i.e., potential spreaders) and individuals who were able to recover at home were thus often unaware of their status. Therefore, this method of testing administration misses a high proportion of infections and does not allow for test-and-trace methods to be used. For instance, a study from Imperial College estimates that in Italy, the true number of infections was around 5.9 million in a total population of ~60 million, compared to the 70,000 detected as of March 28, 2020 [152]. Another analysis, which examined New York state, indicated that as of May 2020, approximately 300,000 cases had been reported in a total population of approximately 20 million [153]. This corresponded to ~1.5% of the population, but ~12% of individuals sampled statewide were estimated as positive through antibody tests (along with indications of spatial heterogeneity at higher resolution) [153]. Technological advancements that facilitate widespread, rapid testing would therefore be valuable for accurately assessing the rate of infection and aid in controlling the virus’ spread. Additionally, the trade off of accessibility, sensitivity, and time to results has raised some complex questions around which tests are best suited to certain situations. Immunoassays, including serological tests, have much higher limits of detection than PCR tests do [154].
Changes in public attitudes and the lifting of COVID-19 restrictions due to the multifactorial desire to stimulate economic activities has required a shift of testing paradigms in 2022, despite warnings from public health officials against a hard exit from public health restrictions [155,156]. An important strategy for testing moving forward is to determine when someone becomes infectious or is no longer infectious following a positive test for COVID-19. Generally, patient specimens tend to not contain culturable virus past day 5 of symptom onset [157,158]. However, due to their sensitivity to post-infectious viral RNA in specimens, PCR-based methods may mislead individuals to believe that they are still infectious several days after symptom onset [131]. Furthermore, detection of viral RNA can occur days and weeks after an active infection due to the sensitivity of PCR-based methods [18,159,160].
In contrast, LFTs were thought to have poor sensitivity and their value for identifying infections and managing the pandemic was questioned [161,162]. However, LFTs do reliably detect SARS-CoV-2 proteins when there is a high viral load, which appears to correlate with a person’s infectiousness [69,163]. Therefore, LFTs are an important diagnostic tool to determine infectiousness with fast turnaround times, ease of use, and accessibility by the general public [131,164]. One study has suggested that the test sensitivity of LFTs appears to be less important than accessibility to LFTs, frequent testing, and fast reporting times for reducing the impact of viral spread [165]. While PCR-based methods are important for COVID-19 surveillance, their use is labor intensive and time consuming, and laboratories are often slow to report results, rendering such methods limited in their surveillance capacity [131].
These limitations are demonstrated by the estimated 10-fold under-reporting of cases in the United States in 2020 due to shortages in testing and slow rollout of testing and slow reporting of results [166]. However, one strategy that may balance the strengths and weaknesses of both types of tests is to corroborate a positive LFT result using a PCR-based method. Indeed, in December 2021 sufficient surveillance and reduction of COVID-19 spread using this joint LFT-PCR strategy was demonstrated in Liverpool, U.K., where there was an estimated 21% reduction of cases [164,167].
8. What Lies Ahead
Diagnostic tools have played an important role during the COVID-19 pandemic. Different tests offer different advantages (Figure 1). Specifically, the results of SARS-CoV-2 diagnostic tests (typically qPCR or LFT-based tests) have been used to estimate the number of infections in the general population, thus informing public health strategies around the globe [26]. During the surges caused by the different SARS-CoV-2 variants between 2020 and 2021, government-sponsored efforts to conduct mass testing and to provide free diagnostic tests to the population were a common occurrence in many parts of the world [168,169,170]. However, recent reports indicate that such public health policies are starting to change during 2022. For example, it is known that the UK plans to dismantle its COVID-19 testing program and scale back its daily reporting requirements [171,172]. A similar approach can be seen in the US as well, where multiple state-run testing facilities are closing, despite some groups advocating to keep them open [173,174]. These ongoing changes in testing policy are likely to have a direct effect on how the pandemic is managed moving forward. SARS-CoV-2 diagnostic tests can be used effectively to slow the spread of the disease only when 1) they are used to share testing results in a timely manner so that they can reasonably be used to approximate the number of infections in the population and 2) those tests are easily accessible by the general public.
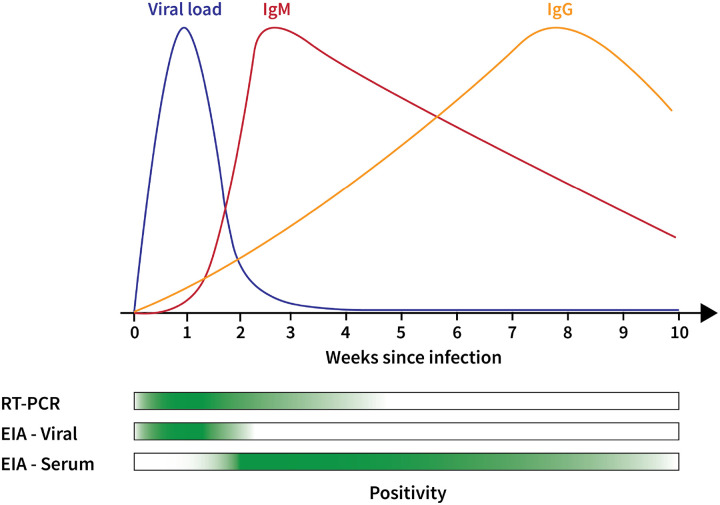
Figure 1: Summary of Diagnostic Technologies used in COVID-19 Testing.
The immune response to SARS-CoV-2 means that different diagnostic approaches offer different views of COVID-19. Early in the infection course, viral load is high. This means that PCR-based testing and EIA testing for antigens are likely to return positives (as indicated by the green bars at the bottom). As viral load decreases, EIA antigen tests become negative, but PCR-based tests can still detect even very low viral loads. From a serological perspective, IgM peaks in the first few weeks following infection and then decreases, while IgG peaks much later in the infection course. Therefore, serological tests are likely to return positives in first few months following the acute infection course. Additional detail is available above and in several analyses and reviews [1,131,160,175].
Children are one segment of the population where the importance of the two aforementioned conditions can be exemplified. This group is particularly vulnerable as there are ongoing challenges with testing in schools, increased COVID-19 mortality rates, and COVID-19-associated orphanhood. In this regard, although there is evidence of the efficacy of routine diagnostic testing to reduce the probability of having infectious students [176,177], as of March of 2022 there is an increasing number of schools that have stopped or plan to stop contact tracing efforts [178,179], in line with an announcement made by the CDC where it no longer recommended contact tracing as a strategy to contain the virus [180]. An estimated 197 children have died in the US from COVID-19 during the first three months of 2022 [181], compared to 735 deaths in the preceding 20 months of the pandemic [182], and millions of children have been orphaned as a consequence of parent or caregiver death due to COVID-19 [183]. It is likely that reducing or eliminating testing capacity in schools will directly exacerbate these negative outcomes for the remainder of 2022.
The SARS-CoV-2 diagnostic tools presented in this paper are far less useful if they are difficult to obtain, or if their limited use results in biased data that would lead to ill-informed public health strategies. Under conditions of limited supply, different strategies for testing are needed [184]. The pandemic is still an ongoing public health threat and it is worrying that active testing and tracing efforts are a low priority for public health authorities in many countries. If this trend continues, the lack of testing could result in increased morbidity and mortality and an overall failure to manage the pandemic.
2. Importance.
Testing is critical to pandemic management. Among molecular tests, messaging about testing strategies has varied widely between countries, with the United States in particular emphasizing the higher sensitivity of polymerase chain reaction tests above immunoassays. However, these tests offer different advantages, and a holistic view of the testing landscape is needed to identify the information provided by each test and its relevance to addressing different questions. Another important consideration is the ease of use and ability to scale for each test, which determines how widely they can be deployed. Here we describe the different diagnostic technologies available as well as the information they provide about SARS-CoV-2 and COVID-19.
Acknowledgements
We thank Yael Evelyn Marshall, who contributed writing (original draft) as well as reviewing and editing of pieces of the text but who did not formally approve the manuscript, as well as Ronnie Russell, who contributed text to and helped develop the structure of the manuscript early in the writing process. We also thank Matthias Fax, Joshua Blake, and consortium members Simina Boca, Lucy D’Agostino McGowan, and Greg Szeto, who helped with writing and editing text. We are also grateful to Nadia Danilova, James Eberwine and Ipsita Krishnan for feedback on early versions of the text.
COVID-19 Review Consortium:
Vikas Bansal, John P. Barton, Simina M. Boca, Joel D Boerckel, Christian Brueffer, James Brian Byrd, Stephen Capone, Shikta Das, Anna Ada Dattoli, John J. Dziak, Jeffrey M. Field, Soumita Ghosh, Anthony Gitter, Rishi Raj Goel, Casey S. Greene, Marouen Ben Guebila, Daniel S. Himmelstein, Fengling Hu, Nafisa M. Jadavji, Jeremy P. Kamil, Sergey Knyazev, Likhitha Kolla, Alexandra J. Lee, Ronan Lordan, Tiago Lubiana, Temitayo Lukan, Adam L. MacLean, David Mai, Serghei Mangul, David Manheim, Lucy D’Agostino McGowan, Jesse G. Meyer, Ariel I. Mundo, Amruta Naik, YoSon Park, Dimitri Perrin, Yanjun Qi, Diane N. Rafizadeh, Bharath Ramsundar, Halie M. Rando, Sandipan Ray, Michael P. Robson, Vincent Rubinetti, Elizabeth Sell, Lamonica Shinholster, Ashwin N. Skelly, Yuchen Sun, Yusha Sun, Gregory L Szeto, Ryan Velazquez, Jinhui Wang, Nils Wellhausen
Footnotes
| Author | Competing Interests | Last Reviewed |
|---|---|---|
| Halie M. Rando | None | 2021-01-20 |
| Christian Brueffer | Employee and shareholder of SAGA Diagnostics AB. | 2020-11-11 |
| Ronan Lordan | None | 2020-11-03 |
| Anna Ada Dattoli | None | 2020-03-26 |
| David Manheim | None | 2022-03-15 |
| Jesse G. Meyer | None | 2022-01-06 |
| Ariel I. Mundo | None | 2021-12-19 |
| Dimitri Perrin | None | 2020-11-11 |
| David Mai | None | 2021-01-08 |
| Nils Wellhausen | None | 2020-11-03 |
| COVID-19 Review Consortium | None | 2021-01-16 |
| Anthony Gitter | Filed a patent application with the Wisconsin Alumni Research Foundation related to classifying activated T cells | 2020-11-10 |
| Casey S. Greene | None | 2021-01-20 |
Contributor Information
Halie M. Rando, Department of Systems Pharmacology and Translational Therapeutics, University of Pennsylvania, Philadelphia, Pennsylvania, United States of America; Department of Biochemistry and Molecular Genetics, University of Colorado School of Medicine, Aurora, Colorado, United States of America; Center for Health AI, University of Colorado School of Medicine, Aurora, Colorado, United States of America · Funded by the Gordon and Betty Moore Foundation (GBMF 4552); the National Human Genome Research Institute (R01 HG010067).
Christian Brueffer, Department of Clinical Sciences, Lund University, Lund, Sweden.
Ronan Lordan, Institute for Translational Medicine and Therapeutics, Perelman School of Medicine, University of Pennsylvania, Philadelphia, PA 19104-5158, USA; Department of Medicine, Perelman School of Medicine, University of Pennsylvania, Philadelphia, PA 19104, USA; Department of Systems Pharmacology and Translational Therapeutics, Perelman School of Medicine, University of Pennsylvania; Philadelphia, PA 19104, USA.
Anna Ada Dattoli, Department of Pathology and Laboratory Medicine, The Children’s Hospital of Philadelphia, Philadelphia, PA, USA; Department of Systems Pharmacology & Translational Therapeutics, Perelman School of Medicine, University of Pennsylvania, Philadelphia, PA, USA.
David Manheim, 1DaySooner, Delaware, United States of America; Risk and Health Communication Research Center, School of Public Health, University of Haifa, Haifa, Israel; Technion, Israel Institute of Technology, Haifa, Israel · Funded by Center for Effective Altruism, Long Term Future Fund.
Jesse G. Meyer, Department of Biochemistry, Medical College of Wisconsin, Milwaukee, Wisconsin, United States of America · Funded by National Institute of General Medical Sciences (R35 GM142502).
Ariel I. Mundo, Department of Biomedical Engineering, University of Arkansas, Fayetteville, Arkansas, USA.
Dimitri Perrin, School of Computer Science, Queensland University of Technology, Brisbane, Australia; Centre for Data Science, Queensland University of Technology, Brisbane, Australia.
David Mai, Department of Bioengineering, University of Pennsylvania, Philadelphia, PA, USA; Center for Cellular Immunotherapies, Perelman School of Medicine, and Parker Institute for Cancer Immunotherapy at University of Pennsylvania, Philadelphia, PA, USA.
Nils Wellhausen, Department of Systems Pharmacology and Translational Therapeutics, University of Pennsylvania, Philadelphia, Pennsylvania, United States of America.
Anthony Gitter, Department of Biostatistics and Medical Informatics, University of Wisconsin-Madison, Madison, Wisconsin, United States of America; Morgridge Institute for Research, Madison, Wisconsin, United States of America · Funded by John W. and Jeanne M. Rowe Center for Research in Virology.
Casey S. Greene, Department of Systems Pharmacology and Translational Therapeutics, University of Pennsylvania, Philadelphia, Pennsylvania, United States of America; Childhood Cancer Data Lab, Alex’s Lemonade Stand Foundation, Philadelphia, Pennsylvania, United States of America; Department of Biochemistry and Molecular Genetics, University of Colorado School of Medicine, Aurora, Colorado, United States of America; Center for Health AI, University of Colorado School of Medicine, Aurora, Colorado, United States of America · Funded by the Gordon and Betty Moore Foundation (GBMF 4552); the National Human Genome Research Institute (R01 HG010067).
References
- 1.Pathogenesis, Symptomatology, and Transmission of SARS-CoV-2 through Analysis of Viral Genomics and Structure Rando Halie M, MacLean Adam L, Lee Alexandra J, Lordan Ronan, Ray Sandipan, Bansal Vikas, Skelly Ashwin N, Sell Elizabeth, Dziak John J, Shinholster Lamonica, … Greene Casey S mSystems (2021-October-26) https://pubmed.ncbi.nlm.nih.gov/34698547/ DOI: 10.1128/msystems.00095-21 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Aggressively find, test, trace and isolate to beat COVID-19 Matukas Larissa M, Dhalla Irfan A, Laupacis Andreas Canadian Medical Association Journal (2020-September-09) https://doi.org/gh2jvk DOI: 10.1503/cmaj.202120 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.COVID-19 in New Zealand and the impact of the national response: a descriptive epidemiological study Jefferies Sarah, French Nigel, Gilkison Charlotte, Graham Giles, Hope Virginia, Marshall Jonathan, McElnay Caroline, McNeill Andrea, Muellner Petra, Paine Shevaun, … Priest Patricia The Lancet Public Health (2020-November) https://doi.org/ftzx DOI: 10.1016/s2468-2667(20)30225-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Potential lessons from the Taiwan and New Zealand health responses to the COVID-19 pandemic Summers Jennifer, Cheng Hao-Yuan, Lin Hsien-Ho, Barnard Lucy Telfar, Kvalsvig Amanda, Wilson Nick, Baker Michael G The Lancet Regional Health - Western Pacific (2020-November) https://doi.org/ghrzb4 DOI: 10.1016/j.lanwpc.2020.100044 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Novel Coronavirus - China. https://www.who.int/emergencies/disease-outbreak-news/item/2020-DON233 .
- 6.Detection of 2019 novel coronavirus (2019-nCoV) by real-time RT-PCR Corman Victor M, Landt Olfert, Kaiser Marco, Molenkamp Richard, Meijer Adam, Chu Daniel KW, Bleicker Tobias, Brünink Sebastian, Schneider Julia, Schmidt Marie Luisa, … Drosten Christian Eurosurveillance (2020-January-23) https://doi.org/ggjs7g DOI: 10.2807/1560-7917.es.2020.25.3.2000045 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Polymerase Chain Reaction Garibyan Lilit, Avashia Nidhi Journal of Investigative Dermatology (2013-March) https://doi.org/ggtkkc DOI: 10.1038/jid.2013.1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Development and application of lateral flow test strip technology for detection of infectious agents and chemical contaminants: a review Ngom Babacar, Guo Yancheng, Wang Xiliang, Bi Dingren Analytical and Bioanalytical Chemistry (2010-April-27) https://doi.org/cn8cn9 DOI: 10.1007/s00216-010-3661-4 [DOI] [PubMed] [Google Scholar]
- 9.Enzyme Linked Immunosorbent Assay Alhajj Mandy, Farhana Aisha StatPearls (2022-February-02) https://pubmed.ncbi.nlm.nih.gov/32310382 [PubMed] [Google Scholar]
- 10.Evaluation and Comparison of Serological Methods for COVID-19 Diagnosis Gong Fanwu, Wei Hua-xing, Li Qiangsheng, Liu Liu, Li Bofeng Frontiers in Molecular Biosciences (2021-July-23) https://doi.org/gnxn82 DOI: 10.3389/fmolb.2021.682405 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Sensitivity in Detection of Antibodies to Nucleocapsid and Spike Proteins of Severe Acute Respiratory Syndrome Coronavirus 2 in Patients With Coronavirus Disease 2019 Burbelo Peter D, Riedo Francis X, Morishima Chihiro, Rawlings Stephen, Smith Davey, Das Sanchita, Strich Jeffrey R, Chertow Daniel S, Davey Richard T Jr, Cohen Jeffrey I The Journal of Infectious Diseases (2020-May-19) https://doi.org/ggxz2f DOI: 10.1093/infdis/jiaa273 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Temporal profiles of viral load in posterior oropharyngeal saliva samples and serum antibody responses during infection by SARS-CoV-2: an observational cohort study To Kelvin Kai-Wang, Tsang Owen Tak-Yin, Leung Wai-Shing, Tam Anthony Raymond, Wu Tak-Chiu, Lung David Christopher, Yip Cyril Chik-Yan, Cai Jian-Piao, Chan Jacky Man-Chun, Chik Thomas Shiu-Hong, … Yuen Kwok-Yung The Lancet Infectious Diseases (2020-May) https://doi.org/ggp4qx DOI: 10.1016/s1473-3099(20)30196-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Fecal specimen diagnosis 2019 novel coronavirus-infected pneumonia Zhang JingCheng, Wang SaiBin, Xue YaDong Journal of Medical Virology (2020-March-12) https://doi.org/ggpx6d DOI: 10.1002/jmv.25742 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.A beginner’s guide to RT-PCR, qPCR and RT-qPCR Adams Grace The Biochemist (2020-June-15) https://doi.org/gm8nfz DOI: 10.1042/bio20200034 [DOI] [Google Scholar]
- 15.The MIQE Guidelines: Minimum Information for Publication of Quantitative Real-Time PCR Experiments Bustin Stephen A, Benes Vladimir, Garson Jeremy A, Hellemans Jan, Huggett Jim, Kubista Mikael, Mueller Reinhold, Nolan Tania, Pfaffl Michael W, Shipley Gregory L, … Wittwer Carl T Clinical Chemistry (2009-April-01) https://doi.org/fkkjq5 DOI: 10.1373/clinchem.2008.112797 [DOI] [PubMed] [Google Scholar]
- 16.Molecular Diagnosis of a Novel Coronavirus (2019-nCoV) Causing an Outbreak of Pneumonia Chu Daniel KW, Pan Yang, Cheng Samuel MS, Hui Kenrie PY, Krishnan Pavithra, Liu Yingzhi, Ng Daisy YM, Wan Carrie KC, Yang Peng, Wang Quanyi, … Poon Leo LM Clinical Chemistry (2020-April) https://doi.org/ggnbpp DOI: 10.1093/clinchem/hvaa029 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Viral RNA load as determined by cell culture as a management tool for discharge of SARS-CoV-2 patients from infectious disease wards Scola Bernard La, Bideau Marion Le, Andreani Julien, Van Thuan Hoang, Grimaldier Clio, Colson Philippe, Gautret Philippe, Raoult Didier European Journal of Clinical Microbiology & Infectious Diseases (2020-April-27) https://doi.org/ghf8cj DOI: 10.1007/s10096-020-03913-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Duration and key determinants of infectious virus shedding in hospitalized patients with coronavirus disease-2019 (COVID-19) van Kampen Jeroen JA, van de Vijver David AMC, Fraaij Pieter LA, Haagmans Bart L, Lamers Mart M, Okba Nisreen, van den Akker Johannes PC, Endeman Henrik, Gommers Diederik AMPJ, Cornelissen Jan J, … van der Eijk Annemiek A Nature Communications (2021-January-11) https://doi.org/gjm8g2 DOI: 10.1038/s41467-020-20568-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Duration of infectiousness and correlation with RT-PCR cycle threshold values in cases of COVID-19, England, January to May 2020 Singanayagam Anika, Patel Monika, Charlett Andre, Bernal Jamie Lopez, Saliba Vanessa, Ellis Joanna, Ladhani Shamez, Zambon Maria, Gopal Robin Eurosurveillance (2020-August-13) https://doi.org/gg9jt7 DOI: 10.2807/1560-7917.es.2020.25.32.2001483 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ct values from SARS-CoV-2 diagnostic PCR assays should not be used as direct estimates of viral load Dahdouh Elias, Lázaro-Perona Fernando, Romero-Gómez María Pilar, Mingorance Jesús, García-Rodriguez Julio Journal of Infection (2021-March) https://doi.org/gjkr5s DOI: 10.1016/j.jinf.2020.10.017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Virological assessment of hospitalized patients with COVID-2019 Wölfel Roman, Corman Victor M, Guggemos Wolfgang, Seilmaier Michael, Zange Sabine, Müller Marcel A, Niemeyer Daniela, Jones Terry C, Vollmar Patrick, Rothe Camilla, … Wendtner Clemens Nature (2020-April-01) https://doi.org/ggqrv7 DOI: 10.1038/s41586-020-2196-x [DOI] [PubMed] [Google Scholar]
- 22.Estimating the false-negative test probability of SARS-CoV-2 by RT-PCR Wikramaratna Paul S, Paton Robert S, Ghafari Mahan, Lourenço José Eurosurveillance (2020-December-17) https://doi.org/gmb6n3 DOI: 10.2807/1560-7917.es.2020.25.50.2000568 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Diagnosing SARS-CoV-2 infection: the danger of over-reliance on positive test results Cohen Andrew N, Kessel Bruce, Milgroom Michael G Cold Spring Harbor Laboratory (2020-May-01) https://doi.org/gh3xk7 DOI: 10.1101/2020.04.26.20080911 [DOI] [Google Scholar]
- 24.Diagnostic performance of different sampling approaches for SARS-CoV-2 RT-PCR testing: a systematic review and meta-analysis Tsang Nicole Ngai Yung, So Hau Chi, Ng Ka Yan, Cowling Benjamin J, Leung Gabriel M, Ming Ip Dennis Kai The Lancet Infectious Diseases (2021-September) https://doi.org/gjrfns DOI: 10.1016/s1473-3099(21)00146-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Improved Molecular Diagnosis of COVID-19 by the Novel, Highly Sensitive and Specific COVID-19-RdRp/Hel Real-Time Reverse Transcription-PCR Assay Validated In Vitro and with Clinical Specimens Chan Jasper Fuk-Woo, Yip Cyril Chik-Yan, To Kelvin Kai-Wang, Tang Tommy Hing-Cheung, Wong Sally Cheuk-Ying, Leung Kit-Hang, Fung Agnes Yim-Fong, Ng Anthony Chin-Ki, Zou Zijiao, Tsoi Hoi-Wah, … Yuen Kwok-Yung Journal of Clinical Microbiology (2020-April-23) https://doi.org/ggpv64 DOI: 10.1128/jcm.00310-20 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Testing at scale during the COVID-19 pandemic Mercer Tim R, Salit Marc Nature Reviews Genetics (2021-May-04) https://doi.org/gjvw2n DOI: 10.1038/s41576-021-00360-w [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.SARS-CoV-2 samples may escape detection because of a single point mutation in the N gene Ziegler Katharina, Steininger Philipp, Ziegler Renate, Steinmann Jörg, Korn Klaus, Ensser Armin Eurosurveillance (2020-October-01) https://doi.org/ghnwss DOI: 10.2807/1560-7917.es.2020.25.39.2001650 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Multiple assays in a real-time RT-PCR SARS-CoV-2 panel can mitigate the risk of loss of sensitivity by new genomic variants during the COVID-19 outbreak Peñarrubia Luis, Ruiz Maria, Porco Roberto, Rao Sonia N, Juanola-Falgarona Martí, Manissero Davide, López-Fontanals Marta, Pareja Josep International Journal of Infectious Diseases (2020-August) https://doi.org/gmb6rv DOI: 10.1016/j.ijid.2020.06.027 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Droplet Digital PCR versus qPCR for gene expression analysis with low abundant targets: from variable nonsense to publication quality data Taylor Sean C, Laperriere Genevieve, Germain Hugo Scientific Reports (2017-May-25) https://doi.org/gbgsjw DOI: 10.1038/s41598-017-02217-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.dPCR: A Technology Review Quan Phenix-Lan, Sauzade Martin, Brouzes Eric Sensors (2018-April-20) https://doi.org/ggr39c DOI: 10.3390/s18041271 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.ddPCR: a more accurate tool for SARS-CoV-2 detection in low viral load specimens Suo Tao, Liu Xinjin, Feng Jiangpeng, Guo Ming, Hu Wenjia, Guo Dong, Ullah Hafiz, Yang Yang, Zhang Qiuhan, Wang Xin, … Chen Yu Emerging Microbes & Infections (2020-June-07) https://doi.org/ggx2t2 DOI: 10.1080/22221751.2020.1772678 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Highly accurate and sensitive diagnostic detection of SARS-CoV-2 by digital PCR Dong Lianhua, Zhou Junbo, Niu Chunyan, Wang Quanyi, Pan Yang, Sheng Sitong, Wang Xia, Zhang Yongzhuo, Yang Jiayi, Liu Manqing, … Fang Xiang Talanta (2021-March) https://doi.org/gh2jvj DOI: 10.1016/j.talanta.2020.121726 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Library preparation for next generation sequencing: A review of automation strategies Hess JF, Kohl TA, Kotrová M, Rönsch K, Paprotka T, Mohr V, Hutzenlaub T, Brüggemann M, Zengerle R, Niemann S, Paust N Biotechnology Advances (2020-July) https://doi.org/ggth2v DOI: 10.1016/j.biotechadv.2020.107537 [DOI] [PubMed] [Google Scholar]
- 34.Diagnosing COVID-19: The Disease and Tools for Detection Udugama Buddhisha, Kadhiresan Pranav, Kozlowski Hannah N, Malekjahani Ayden, Osborne Matthew, Li Vanessa YC, Chen Hongmin, Mubareka Samira, Gubbay Jonathan B, Chan Warren CW ACS Nano (2020-March-30) https://doi.org/ggq8ds DOI: 10.1021/acsnano.0c02624 [DOI] [PubMed] [Google Scholar]
- 35.Emergence of a novel SARS-CoV-2 strain in Southern California, USA Zhang Wenjuan, Davis Brian D, Chen Stephanie S, Martinez Jorge MSincuir, Plummer Jasmine T, Vail Eric Cold Spring Harbor Laboratory (2021-January-20) https://doi.org/ghvq48 DOI: 10.1101/2021.01.18.21249786 [DOI] [Google Scholar]
- 36.Genomic surveillance to combat COVID-19: challenges and opportunities Robishaw Janet D, Alter Scott M, Solano Joshua J, Shih Richard D, DeMets David L, Maki Dennis G, Hennekens Charles H The Lancet Microbe (2021-September) https://doi.org/hkwr DOI: 10.1016/s2666-5247(21)00121-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Cov-Lineages. https://cov-lineages.org/
- 38.Global disparities in SARS-CoV-2 genomic surveillance Brito Anderson F, Semenova Elizaveta, Dudas Gytis, Hassler Gabriel W, Kalinich Chaney C, Kraemer Moritz UG, Ho Joses, Tegally Houriiyah, Githinji George, Agoti Charles N, … Cold Spring Harbor Laboratory (2021-August-26) https://doi.org/gn2tht DOI: 10.1101/2021.08.21.21262393 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Evaluation of COVID-19 RT-qPCR Test in Multi sample Pools Yelin Idan, Aharony Noga, Tamar Einat Shaer, Argoetti Amir, Messer Esther, Berenbaum Dina, Shafran Einat, Kuzli Areen, Gandali Nagham, Shkedi Omer, … Kishony Roy Clinical Infectious Diseases (2020-May-02) https://doi.org/ggtx9r DOI: 10.1093/cid/ciaa531 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Analytical Validation of a COVID-19 qRT-PCR Detection Assay Using a 384-well Format and Three Extraction Methods Nelson Andrew C, Auch Benjamin, Schomaker Matthew, Gohl Daryl M, Grady Patrick, Johnson Darrell, Kincaid Robyn, Karnuth Kylene E, Daniel Jerry, Fiege Jessica K, … Yohe Sophia Cold Spring Harbor Laboratory (2020-April-05) https://doi.org/ggs45d DOI: 10.1101/2020.04.02.022186 [DOI] [Google Scholar]
- 41.Two-Stage Adaptive Pooling with RT-QPCR for Covid-19 Screening Heidarzadeh Anoosheh, Narayanan Krishna ICASSP 2021 - 2021 IEEE International Conference on Acoustics, Speech and Signal Processing (ICASSP) (2021-June-06) https://doi.org/gppbs9 DOI: 10.1109/icassp39728.2021.9413685 [DOI] [Google Scholar]
- 42.Pooled RT-qPCR testing for SARS-CoV-2 surveillance in schools - a cluster randomised trial Joachim Alexander, Dewald Felix, Suárez Isabelle, Zemlin Michael, Lang Isabelle, Stutz Regine, Marthaler Anna, Bosse Hans Martin, Lübke Nadine, Münch Juliane, … Kern Anna EClinicalMedicine (2021-September) https://doi.org/gpzfjz DOI: 10.1016/j.eclinm.2021.101082 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Pooling RT-qPCR testing for SARS-CoV-2 in 1000 individuals of healthy and infection-suspected patients Hirotsu Yosuke, Maejima Makoto, Shibusawa Masahiro, Nagakubo Yuki, Hosaka Kazuhiro, Amemiya Kenji, Sueki Hitomi, Hayakawa Miyoko, Mochizuki Hitoshi, Tsutsui Toshiharu, … Omata Masao Scientific Reports (2020-November-03) https://doi.org/gpzfj4 DOI: 10.1038/s41598-020-76043-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Loop-mediated isothermal amplification of DNA Notomi T Nucleic Acids Research (2000-June-15) https://doi.org/bx567n DOI: 10.1093/nar/28.12.e63 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.A molecular test based on RT-LAMP for rapid, sensitive and inexpensive colorimetric detection of SARS-CoV-2 in clinical samples Amaral Catarina, Antunes Wilson, Moe Elin, Duarte Américo G, Lima Luís MP, Santos Cristiana, Gomes Inês L, Afonso Gonçalo S, Vieira Ricardo, Teles Helena Sofia S, … Pimentel Catarina Scientific Reports (2021-August-12) https://doi.org/gnx4h3 DOI: 10.1038/s41598-021-95799-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Can the cycle threshold (Ct) value of RT-PCR test for SARS CoV2 predict infectivity among close contacts? Bayat Soha Al, Mundodan Jesha, Hasnain Samina, Sallam Mohamed, Khogali Hayat, Ali Dina, Alateeg Saif, Osama Mohamed, Elberdiny Aiman, Hamad Al-Romaihi, Al-Thani Mohammed Hamad J Journal of Infection and Public Health (2021-September) https://doi.org/gnx4jw DOI: 10.1016/j.jiph.2021.08.013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Evaluation of cycle threshold values at deisolation Mowrer Clayton T, Creager Hannah, Cawcutt Kelly, Birge Justin, Lyden Elizabeth, Van Schooneveld Trevor C, Rupp Mark E, Hewlett Angela Infection Control & Hospital Epidemiology (2021-April-06) https://doi.org/gnx4jx DOI: 10.1017/ice.2021.132 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.To Interpret the SARS-CoV-2 Test, Consider the Cycle Threshold Value Tom Michael R, Mina Michael J Clinical Infectious Diseases (2020-May-21) https://doi.org/ggxf2s DOI: 10.1093/cid/ciaa619 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Rapid Molecular Detection of SARS-CoV-2 (COVID-19) Virus RNA Using Colorimetric LAMP Zhang Yinhua, Odiwuor Nelson, Xiong Jin, Sun Luo, Nyaruaba Raphael Ohuru, Wei Hongping, Tanner Nathan A Cold Spring Harbor Laboratory (2020-February-29) https://doi.org/ggx3wn DOI: 10.1101/2020.02.26.20028373 [DOI] [Google Scholar]
- 50.Reverse Transcriptase Loop Mediated Isothermal Amplification (RT-LAMP) for COVID-19 diagnosis: a systematic review and meta-analysis. Subali Anita Dominique, Wiyono Lowilius Pathogens and global health (2021-June-04) https://www.ncbi.nlm.nih.gov/pubmed/34086539 DOI: 10.1080/20477724.2021.1933335 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.The CRISPR tool kit for genome editing and beyond Adli Mazhar Nature Communications (2018-May-15) https://doi.org/gdj266 DOI: 10.1038/s41467-018-04252-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Nucleic acid detection with CRISPR-Cas13a/C2c2 Gootenberg Jonathan S, Abudayyeh Omar O, Lee Jeong Wook Essletzbichler Patrick, Dy Aaron J, Joung Julia, Verdine Vanessa, Donghia Nina, Daringer Nichole M, Freije Catherine A, … Zhang Feng Science (2017-April-28) https://doi.org/f93x8p DOI: 10.1126/science.aam9321 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Development and evaluation of a rapid CRISPR-based diagnostic for COVID-19 Hou Tieying, Zeng Weiqi, Yang Minling, Chen Wenjing, Ren Lili, Ai Jingwen, Wu Ji, Liao Yalong, Gou Xuejing, Li Yongjun, … Xu Teng PLOS Pathogens (2020-August-27) https://doi.org/ghn7rp DOI: 10.1371/journal.ppat.1008705 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.CRISPR-based surveillance for COVID-19 using genomically-comprehensive machine learning design Metsky Hayden C, Freije Catherine A, Kosoko-Thoroddsen Tinna-Solveig F, Sabeti Pardis C, Myhrvold Cameron Cold Spring Harbor Laboratory (2020-March-02) https://doi.org/ggr3zf DOI: 10.1101/2020.02.26.967026 [DOI] [Google Scholar]
- 55.A Scalable, Easy-to-Deploy Protocol for Cas13-Based Detection of SARS-CoV-2 Genetic Material Rauch Jennifer N, Valois Eric, Solley Sabrina C, Braig Friederike, Lach Ryan S, Audouard Morgane, Ponce-Rojas Jose Carlos, Costello Michael S, Baxter Naomi J, Kosik Kenneth S, … Wilson Maxwell Z Journal of Clinical Microbiology (2021-March-19) https://doi.org/gh3nm3 DOI: 10.1128/jcm.02402-20 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.CRISPR-Cas12-based detection of SARS-CoV-2 Broughton James P, Deng Xianding, Yu Guixia, Fasching Clare L, Servellita Venice, Singh Jasmeet, Miao Xin, Streithorst Jessica A, Granados Andrea, Alicia Sotomayor-Gonzalez, … Chiu Charles Y Nature Biotechnology (2020-April-16) https://doi.org/ggv47f DOI: 10.1038/s41587-020-0513-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.An ultrasensitive, rapid, and portable coronavirus SARS-CoV-2 sequence detection method based on CRISPR-Cas12 Lucia Curti, Federico Pereyra-Bonnet, Alejandra Gimenez Carla Cold Spring Harbor Laboratory (2020-March-02) https://doi.org/gg7km6 DOI: 10.1101/2020.02.29.971127 [DOI] [Google Scholar]
- 58.Rapid detection of SARS-CoV-2 with CRISPR-Cas12a Xiong Dan, Dai Wenjun, Gong Jiaojiao, Li Guande, Liu Nansong, Wu Wei, Pan Jiaqiang, Chen Chen, Jiao Yingzhen, Deng Huina, … Tang Guanghui PLOS Biology (2020-December-15) https://doi.org/gh3nm6 DOI: 10.1371/journal.pbio.3000978 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Ultrasensitive and visual detection of SARS-CoV-2 using all-in-one dual CRISPR-Cas12a assay Ding Xiong, Yin Kun, Li Ziyue, Lalla Rajesh V, Ballesteros Enrique, Sfeir Maroun M, Liu Changchun Nature Communications (2020-September-18) https://doi.org/ghwjb2 DOI: 10.1038/s41467-020-18575-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.SARS-CoV-2 detection with CRISPR diagnostics Guo Lu, Sun Xuehan, Wang Xinge, Liang Chen, Jiang Haiping, Gao Qingqin, Dai Moyu, Qu Bin, Fang Sen, Mao Yihuan, … Li Wei Cell Discovery (2020-May-19) https://doi.org/ggx2wt DOI: 10.1038/s41421-020-0174-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Electric field-driven microfluidics for rapid CRISPR-based diagnostics and its application to detection of SARS-CoV-2 Ramachandran Ashwin, Huyke Diego A, Sharma Eesha, Sahoo Malaya K, Huang ChunHong, Banaei Niaz, Pinsky Benjamin A, Santiago Juan G Proceedings of the National Academy of Sciences (2020-November-04) https://doi.org/gh3nms DOI: 10.1073/pnas.2010254117 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Annotation and Classification of CRISPR-Cas Systems Makarova Kira S, Koonin Eugene V Methods in Molecular Biology (2015) https://doi.org/gppg52 DOI: 10.1007/978-1-4939-2687-9_4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Type III CRISPR-Cas systems: when DNA cleavage just isn’t enough Pyenson Nora C, Marraffini Luciano A Current Opinion in Microbiology (2017-June) https://doi.org/gcx7r3 DOI: 10.1016/j.mib.2017.08.003 [DOI] [PubMed] [Google Scholar]
- 64.Type III CRISPR-Cas Immunity: Major Differences Brushed Aside Tamulaitis Gintautas, Venclovas Česlovas, Siksnys Virginijus Trends in Microbiology (2017-January) https://doi.org/f9nj96 DOI: 10.1016/j.tim.2016.09.012 [DOI] [PubMed] [Google Scholar]
- 65.Intrinsic signal amplification by type III CRISPR-Cas systems provides a sequence-specific SARS-CoV-2 diagnostic Santiago-Frangos Andrew, Hall Laina N, Nemudraia Anna, Nemudryi Artem, Krishna Pushya, Wiegand Tanner, Wilkinson Royce A, Snyder Deann T, Hedges Jodi F, Cicha Calvin, … Wiedenheft Blake Cell Reports Medicine (2021-June) https://doi.org/gpmv6n DOI: 10.1016/j.xcrm.2021.100319 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Amplification-free detection of SARS-CoV-2 with CRISPR-Cas13a and mobile phone microscopy Fozouni Parinaz, Son Sungmin, Derby María Díaz de León, Knott Gavin J, Gray Carley N, D’Ambrosio Michael V, Zhao Chunyu, Switz Neil A, Kumar GRenuka, Stephens Stephanie I, … Ott Melanie Cell (2021-January) https://doi.org/ghnszx DOI: 10.1016/j.cell.2020.12.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Rapid, Sensitive, and Specific Severe Acute Respiratory Syndrome Coronavirus 2 Detection: A Multicenter Comparison Between Standard Quantitative Reverse-Transcriptase Polymerase Chain Reaction and CRISPR-Based DETECTR Brandsma Eelke, Verhagen Han JMP, van de Laar Thijs JW, Claas Eric CJ, Cornelissen Marion, van den Akker Emile The Journal of Infectious Diseases (2020-October-10) https://doi.org/gh3nmt DOI: 10.1093/infdis/jiaa641 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Rapid, accurate, nucleobase detection using FnCas9 Azhar Mohd, Phutela Rhythm, Kumar Manoj, Ansari Asgar Hussain, Rauthan Riya, Gulati Sneha, Sharma Namrata, Sinha Dipanjali, Sharma Saumya, Singh Sunaina, … Cold Spring Harbor Laboratory (2020-September-14) https://doi.org/gh3nmv DOI: 10.1101/2020.09.13.20193581 [DOI] [Google Scholar]
- 69.Rapid Diagnostic Testing for SARS-CoV-2 Drain Paul K New England Journal of Medicine (2022-January-20) https://doi.org/gn2sfk DOI: 10.1056/nejmcp2117115 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.The Performance of Two Rapid Antigen Tests During Population-Level Screening for SARS-CoV-2 Infection Alghounaim Mohammad, Bastaki Hamad, Essa Farah Bin, Motlagh Hoda, Salman Al-Sabah Frontiers in Medicine (2021-December-23) https://doi.org/gpp4xw DOI: 10.3389/fmed.2021.797109 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Lateral flow (immuno)assay: its strengths, weaknesses, opportunities and threats. A literature survey Posthuma-Trumpie Geertruida A, Korf Jakob, van Amerongen Aart Analytical and Bioanalytical Chemistry (2008-August-13) https://doi.org/bcsjdw DOI: 10.1007/s00216-008-2287-2 [DOI] [PubMed] [Google Scholar]
- 72.A systematic review of the sensitivity and specificity of lateral flow devices in the detection of SARS-CoV-2. Mistry Dylan A, Wang Jenny Y, Moeser Mika-Erik, Starkey Thomas, Lee Lennard YW BMC infectious diseases (2021-August-18) https://www.ncbi.nlm.nih.gov/pubmed/34407759 DOI: 10.1186/s12879-021-06528-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. GOV.UK . Government sets out next steps for living with COVID. https://www.gov.uk/government/news/government-sets-out-next-steps-for-living-with-covid .
- 74.Fact Sheet: The Biden Administration to Begin Distributing At-Home, Rapid COVID-19 Tests to Americans for Free The White House; (2022-January-14) https://www.whitehouse.gov/briefing-room/statements-releases/2022/01/14/fact-sheet-the-biden-administration-to-begin-distributing-at-home-rapid-covid-19-tests-to-americans-for-free/ [Google Scholar]
- 75.Scaling up COVID-19 rapid antigen tests: promises and challenges Peeling Rosanna W, Olliaro Piero L, Boeras Debrah I, Fongwen Noah The Lancet Infectious Diseases (2021-September) https://doi.org/hk34 DOI: 10.1016/s1473-3099(21)00048-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Detection technologies and recent developments in the diagnosis of COVID-19 infection Rai Praveen, Kumar Ballamoole Krishna, Deekshit Vijaya Kumar, Karunasagar Indrani, Karunasagar Iddya Applied Microbiology and Biotechnology (2021-January) https://doi.org/gnvtp9 DOI: 10.1007/s00253-020-11061-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Enzyme Immunoassay (EIA)/Enzyme-Linked Immunosorbent Assay (ELISA) Lequin Rudolf M Clinical Chemistry (2005-December-01) https://doi.org/dts5xp DOI: 10.1373/clinchem.2005.051532 [DOI] [PubMed] [Google Scholar]
- 78.Enzyme-Immunoassay: A Powerful Analytical Tool Schuurs AHWM, Van Weemen BK Journal of Immunoassay (1980-January) https://doi.org/btqbvh DOI: 10.1080/01971528008055786 [DOI] [PubMed] [Google Scholar]
- 79.Antibody testing for COVID-19: A report from the National COVID Scientific Advisory Panel Adams Emily R, Ainsworth Mark, Anand Rekha, Andersson Monique I, Auckland Kathryn, Baillie JKenneth, Barnes Eleanor, Beer Sally, Bell John I, Berry Tamsin, … Wellcome Open Research (2020-June-11) https://doi.org/gpp4wq DOI: 10.12688/wellcomeopenres.15927.1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Detection of SARS-CoV-2 by antigen ELISA test is highly swayed by viral load and sample storage condition Adnan Nihad, Khandker Shahad Saif, Haq Ahsanul, Chaity Mousumi Akter, Khalek Abdul, Nazim Anawarul Quader, Kaitsuka Taku, Tomizawa Kazuhito, Mie Masayasu, Kobatake Eiry, … Jamiruddin MohdRaeed Expert Review of Anti-infective Therapy (2021-September-11) https://doi.org/gprjx9 DOI: 10.1080/14787210.2021.1976144 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Negative Nasopharyngeal and Oropharyngeal Swabs Do Not Rule Out COVID-19 Winichakoon Poramed, Chaiwarith Romanee, Liwsrisakun Chalerm, Salee Parichat, Goonna Aree, Limsukon Atikun, Kaewpoowat Quanhathai Journal of Clinical Microbiology (2020-April-23) https://doi.org/ggpw9m DOI: 10.1128/jcm.00297-20 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Coronavirus and the race to distribute reliable diagnostics Sheridan Cormac Nature Biotechnology (2020-February-19) https://doi.org/ggm4nt DOI: 10.1038/d41587-020-00002-2 [DOI] [PubMed] [Google Scholar]
- 83.Quarantine & Isolation CDC Centers for Disease Control and Prevention (2022-March-30) https://www.cdc.gov/coronavirus/2019-ncov/your-health/quarantine-isolation.html [Google Scholar]
- 84.CRISPR-Cas System: An Approach With Potentials for COVID-19 Diagnosis and Therapeutics Kumar Prashant, Malik Yashpal Singh, Ganesh Balasubramanian, Rahangdale Somnath, Saurabh Sharad, Natesan Senthilkumar, Srivastava Ashish, Sharun Khan, Yatoo MohdIqbal, Tiwari Ruchi, … Dhama Kuldeep Frontiers in Cellular and Infection Microbiology (2020-November-02) https://doi.org/ghz57p DOI: 10.3389/fcimb.2020.576875 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.The standard coronavirus test, if available, works well—but can new diagnostics help in this pandemic? Service Robert Science (2020-March-22) https://doi.org/ggq9wm DOI: 10.1126/science.abb8400 [DOI] [Google Scholar]
- 86.Laboratory Diagnosis of COVID-19: Current Issues and Challenges Tang Yi-Wei, Schmitz Jonathan E, Persing David H, Stratton Charles W Journal of Clinical Microbiology (2020-May-26) https://doi.org/ggq7h8 DOI: 10.1128/jcm.00512-20 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.The Plane Is Boarding, Where Are Your Test Results? Sloss Lauren The New York Times (2021-December-31) https://www.nytimes.com/2021/12/31/travel/covid-test-chaos.html [Google Scholar]
- 88.A Stark Contrast Between the U.S. and Europe on Tests Kresge Naomi Bloomberg (2022-January-07) https://www.bloomberg.com/news/newsletters/2022-01-07/a-stark-contrast-between-the-u-s-and-europe-on-tests [Google Scholar]
- 89.IgG Subclasses and Allotypes: From Structure to Effector Functions Vidarsson Gestur, Dekkers Gillian, Rispens Theo Frontiers in Immunology (2014-October-20) https://doi.org/gc6vx6 DOI: 10.3389/fimmu.2014.00520 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.The distribution and functions of immunoglobulin isotypes Janeway Charles A Jr, Travers Paul, Walport Mark, Shlomchik Mark J (editors) Immunobiology: The Immune System in Health and Disease (2001) https://www.ncbi.nlm.nih.gov/books/NBK27162 ISBN: 978-0815336426 [Google Scholar]
- 91.Longitudinal profile of antibodies against SARS-coronavirus in SARS patients and their clinical significance Hongying MO, ZENG Guangqiao, REN Xiaolan, LI Hui, KE Changwen, TAN Yaxia, CAI Chaoda, LAI Kefang, CHEN Rongchang, Moira CHAN-YEUNG, Nanshan ZHONG Respirology (2006-January) https://doi.org/dn23vj DOI: 10.1111/j.1440-1843.2006.00783.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Detection of antibodies against SARS CoV 2 in patients with COVID 19 Du Zhe, Zhu Fengxue, Guo Fuzheng, Yang Bo, Wang Tianbing Journal of Medical Virology (2020-April-10) https://doi.org/ggq7m2 DOI: 10.1002/jmv.25820 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Review of Current Advances in Serologic Testing for COVID-19 Espejo Andrea P, Akgun Yamac, Al Mana Abdulaziz F, Tjendra Youley, Millan Nicolas C, Carmen Gomez-Fernandez, Cray Carolyn American Journal of Clinical Pathology (2020-June-25) https://doi.org/gkzfrn DOI: 10.1093/ajcp/aqaa112 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.A serological assay to detect SARS-CoV-2 seroconversion in humans Amanat Fatima, Stadlbauer Daniel, Strohmeier Shirin, Nguyen Thi HO, Chromikova Veronika, McMahon Meagan, Jiang Kaijun, Arunkumar Guha Asthagiri, Jurczyszak Denise, Polanco Jose, … Krammer Florian Nature Medicine (2020-May-12) https://doi.org/ggx28b DOI: 10.1038/s41591-020-0913-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Development of a Fast SARS-CoV-2 IgG ELISA, Based on Receptor-Binding Domain, and Its Comparative Evaluation Using Temporally Segregated Samples From RT-PCR Positive Individuals Mehdi Farha, Chattopadhyay Souvick, Thiruvengadam Ramachandran, Yadav Sarla, Kumar Manjit, Sinha Sangita Kumari, Goswami Sandeep, Kshetrapal Pallavi, Wadhwa Nitya, Natchu Uma Chandramouli, … Batra Gaurav Frontiers in Microbiology (2021-January-20) https://doi.org/gpp5md DOI: 10.3389/fmicb.2020.618097 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Development and evaluation of a low cost IgG ELISA test based in RBD protein for COVID-19 Villafañe Luciana, Vaulet Lucía Gallo, Viere Florencia M, Klepp Laura I, Forrellad Marina A, Bigi María M, Romano María I, Magistrelli Giovanni, Fermepin Marcelo Rodríguez, Bigi Fabiana Journal of Immunological Methods (2022-January) https://doi.org/gpp4wh DOI: 10.1016/j.jim.2021.113182 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.A sensitive and rapid chemiluminescence immunoassay for point-of-care testing (POCT) of copeptin in serum based on high-affinity monoclonal antibodies via cytokine-assisted immunization Wang Yu, Dzakah Emmanuel Enoch, Kang Ye, Cai Yanxue, Wu Peidian, Tang Bo, Li Run, He Xiaowei International Journal of Nanomedicine (2019-June) https://doi.org/gpp5mc DOI: 10.2147/ijn.s200556 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Chemiluminescent immunoassay technology: what does it change in autoantibody detection? Cinquanta Luigi, Fontana Desré Ethel, Bizzaro Nicola Autoimmunity Highlights (2017-June-24) https://doi.org/gh6hcm DOI: 10.1007/s13317-017-0097-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.A Peptide-Based Magnetic Chemiluminescence Enzyme Immunoassay for Serological Diagnosis of Coronavirus Disease 2019 Cai Xue-fei, Chen Juan, Hu Jie-li, Long Quanxin, Deng Hai-jun, Liu Ping, Fan Kai, Liao Pu, Liu Bei-zhong, Wu Gui-cheng, … Wang De-qiang The Journal of Infectious Diseases (2020-July-15) https://doi.org/ggv2fx DOI: 10.1093/infdis/jiaa243 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Comparison of SARS-CoV-2 serological tests with different antigen targets Coste Alix T, Jaton Katia, Matthaios Papadimitriou-Olivgeris, Greub Gilbert, Croxatto Antony Journal of Clinical Virology (2021-January) https://doi.org/gk8s5q DOI: 10.1016/j.jcv.2020.104690 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Assessment of SARS-CoV-2 serological tests for the diagnosis of COVID-19 through the evaluation of three immunoassays: Two automated immunoassays (Euroimmun and Abbott) and one rapid lateral flow immunoassay (NG Biotech) Nicol Thomas, Lefeuvre Caroline, Serri Orianne, Pivert Adeline, Joubaud Françoise, Dubée Vincent, Kouatchet Achille, Ducancelle Alexandra, Françoise Lunel-Fabiani, Hélène Le Guillou-Guillemette Journal of Clinical Virology (2020-August) https://doi.org/gg2ks6 DOI: 10.1016/j.jcv.2020.104511 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Evaluation of Six Commercial Mid- to High-Volume Antibody and Six Point-of-Care Lateral Flow Assays for Detection of SARS-CoV-2 Antibodies Charlton Carmen L, Kanji Jamil N, Johal Kam, Bailey Ashley, Plitt Sabrina S, MacDonald Clayton, Kunst Andrea, Buss Emily, Burnes Laura E, Fonseca Kevin, … Tipples Graham Journal of Clinical Microbiology (2020-September-22) https://doi.org/gpp5mb DOI: 10.1128/jcm.01361-20 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Diagnostic accuracy of an automated chemiluminescent immunoassay for anti-SARS-CoV-2 IgM and IgG antibodies: an Italian experience Infantino Maria, Grossi Valentina, Lari Barbara, Bambi Riccardo, Perri Alessandro, Manneschi Matteo, Terenzi Giovanni, Liotti Irene, Ciotta Giovanni, Taddei Cristina, … Manfredi Mariangela Journal of Medical Virology (2020-May-10) https://doi.org/ggv4c6 DOI: 10.1002/jmv.25932 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.SARS-CoV-2 serology: Validation of high-throughput chemiluminescent immunoassay (CLIA) platforms and a field study in British Columbia Sekirov Inna, Barakauskas Vilte E, Simons Janet, Cook Darrel, Bates Brandon, Burns Laura, Masud Shazia, Charles Marthe, McLennan Meghan, Mak Annie, … Morshed Muhammad Journal of Clinical Virology (2021-September) https://doi.org/gpp5kb DOI: 10.1016/j.jcv.2021.104914 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Cellex qSARS-CoV-2 IgG/IgM Rapid Test Cellex; (2020-April-07) https://www.fda.gov/media/136625/download [Google Scholar]
- 106.Evaluation of Humoral Immune Response after SARS-CoV-2 Vaccination Using Two Binding Antibody Assays and a Neutralizing Antibody Assay Nam Minjeong, Seo Jong Do, Moon Hee-Won, Kim Hanah, Hur Mina, Yun Yeo-Min Microbiology Spectrum (2021-December-22) https://doi.org/gnkz5s DOI: 10.1128/spectrum.01202-21 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.A high-throughput neutralizing antibody assay for COVID-19 diagnosis and vaccine evaluation Muruato Antonio E, Fontes-Garfias Camila R, Ren Ping, Garcia-Blanco Mariano A, Menachery Vineet D, Xie Xuping, Shi Pei-Yong Nature Communications (2020-August-13) https://doi.org/gjkvr9 DOI: 10.1038/s41467-020-17892-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Neutralizing antibody levels are highly predictive of immune protection from symptomatic SARS-CoV-2 infection Khoury David S, Cromer Deborah, Reynaldi Arnold, Schlub Timothy E, Wheatley Adam K, Juno Jennifer A, Subbarao Kanta, Kent Stephen J, Triccas James A, Davenport Miles P Nature Medicine (2021-May-17) https://doi.org/gj3h47 DOI: 10.1038/s41591-021-01377-8 [DOI] [PubMed] [Google Scholar]
- 109.Neutralizing Antibodies Correlate with Protection from SARS-CoV-2 in Humans during a Fishery Vessel Outbreak with a High Attack Rate Addetia Amin, Crawford Katharine HD, Dingens Adam, Zhu Haiying, Roychoudhury Pavitra, Huang Meei-Li, Jerome Keith R, Bloom Jesse D, Greninger Alexander L Journal of Clinical Microbiology (2020-October-21) https://doi.org/gk7n4d DOI: 10.1128/jcm.02107-20 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Neutralising antibody titres as predictors of protection against SARS-CoV-2 variants and the impact of boosting: a meta-analysis Cromer Deborah, Steain Megan, Reynaldi Arnold, Schlub Timothy E, Wheatley Adam K, Juno Jennifer A, Kent Stephen J, Triccas James A, Khoury David S, Davenport Miles P The Lancet Microbe (2022-January) https://doi.org/gnx76z DOI: 10.1016/s2666-5247(21)00267-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.A cell-free high throughput assay for assessment of SARS-CoV-2 neutralizing antibodies Mravinacova Sara, Jönsson Malin, Christ Wanda, Klingström Jonas, Yousef Jamil, Hellström Cecilia, Hedhammar My, Havervall Sebastian, Thålin Charlotte, Pin Elisa, … Hober Sophia New Biotechnology (2022-January) https://doi.org/gm9pkb DOI: 10.1016/j.nbt.2021.10.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Two-Year Prospective Study of the Humoral Immune Response of Patients with Severe Acute Respiratory Syndrome Liu Wei, Fontanet Arnaud, Zhang Pan He, Zhan Lin, Xin Zhong-Tao, Baril Laurence, Tang Fang, Lv Hui, Cao Wu-Chun The Journal of Infectious Diseases (2006-March-15) https://doi.org/cmzn2k DOI: 10.1086/500469 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.The time course of the immune response to experimental coronavirus infection of man Callow KA, Parry HF, Sergeant M, Tyrrell DAJ Epidemiology and Infection (2009-May-15) https://doi.org/c9pnmg DOI: 10.1017/s0950268800048019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Antibody Responses 8 Months after Asymptomatic or Mild SARS-CoV-2 Infection Choe Pyoeng Gyun, Kim Kye-Hyung, Kang Chang Kyung, Suh Hyeon Jeong, Kang EunKyo, Lee Sun Young, Kim Nam Joong, Yi Jongyoun, Park Wan Beom, Oh Myoung-don Emerging Infectious Diseases (2021-March) https://doi.org/ghs9kq DOI: 10.3201/eid2703.204543 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Immunological memory to SARS-CoV-2 assessed for up to 8 months after infection Dan Jennifer M, Mateus Jose, Kato Yu, Hastie Kathryn M, Yu Esther Dawen, Faliti Caterina E, Grifoni Alba, Ramirez Sydney I, Haupt Sonya, Frazier April, … Crotty Shane Science (2021-January-06) https://doi.org/ghrv9b DOI: 10.1126/science.abf4063 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Rapid generation of durable B cell memory to SARS-CoV-2 spike and nucleocapsid proteins in COVID-19 and convalescence. Hartley Gemma E, Edwards Emily SJ, Aui Pei M, Varese Nirupama, Stojanovic Stephanie, McMahon James, Peleg Anton Y, Boo Irene, Drummer Heidi E, Hogarth PMark, … van Zelm Menno C Science immunology (2020-December-22) https://www.ncbi.nlm.nih.gov/pubmed/33443036 DOI: 10.1126/sciimmunol.abf8891 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Persistence of SARS-CoV-2-specific B and T cell responses in convalescent COVID-19 patients 6–8 months after the infection Sherina Natalia, Piralla Antonio, Du Likun, Wan Hui, Makiko Kumagai-Braesch, Andréll Juni, Sten Braesch-Andersen, Cassaniti Irene, Percivalle Elena, Sarasini Antonella, … Pan-Hammarström Qiang Med (2021-March) https://doi.org/gh3xkz DOI: 10.1016/j.medj.2021.02.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Evolution of antibody immunity to SARS-CoV-2 Gaebler Christian, Wang Zijun, Lorenzi Julio CC, Muecksch Frauke, Finkin Shlomo, Tokuyama Minami, Cho Alice, Jankovic Mila, Dennis Schaefer-Babajew, Oliveira Thiago Y, … Nussenzweig Michel C Nature (2021-January-18) https://doi.org/fq6k DOI: 10.1038/s41586-021-03207-w [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Robust neutralizing antibodies to SARS-CoV-2 infection persist for months Wajnberg Ania, Amanat Fatima, Firpo Adolfo, Altman Deena R, Bailey Mark J, Mansour Mayce, McMahon Meagan, Meade Philip, Mendu Damodara Rao, Muellers Kimberly, … Carlos Cordon-Cardo Science (2020-December-04) https://doi.org/fgfs DOI: 10.1126/science.abd7728 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Longitudinal observation and decline of neutralizing antibody responses in the three months following SARS-CoV-2 infection in humans Seow Jeffrey, Graham Carl, Merrick Blair, Acors Sam, Pickering Suzanne, Steel Kathryn JA, Hemmings Oliver, O’Byrne Aoife, Kouphou Neophytos, Galao Rui Pedro, … Doores Katie J Nature Microbiology (2020-October-26) https://doi.org/fh7j DOI: 10.1038/s41564-020-00813-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Evolution of immune responses to SARS-CoV-2 in mild-moderate COVID-19 Wheatley Adam K, Juno Jennifer A, Wang Jing J, Selva Kevin J, Reynaldi Arnold, Tan Hyon-Xhi, Lee Wen Shi, Wragg Kathleen M, Kelly Hannah G, Esterbauer Robyn, … Kent Stephen J Nature Communications (2021-February-19) https://doi.org/gh9vd5 DOI: 10.1038/s41467-021-21444-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.COVID-19-neutralizing antibodies predict disease severity and survival Garcia-Beltran Wilfredo F, Lam Evan C, Astudillo Michael G, Yang Diane, Miller Tyler E, Feldman Jared, Hauser Blake M, Caradonna Timothy M, Clayton Kiera L, Nitido Adam D, … Balazs Alejandro B Cell (2021-January) https://doi.org/gh9vdx DOI: 10.1016/j.cell.2020.12.015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Children develop robust and sustained cross-reactive spike-specific immune responses to SARS-CoV-2 infection Dowell Alexander C, Butler Megan S, Jinks Elizabeth, Tut Gokhan, Lancaster Tara, Sylla Panagiota, Begum Jusnara, Bruton Rachel, Pearce Hayden, Verma Kriti, … Ladhani Shamez Nature Immunology (2021-December-22) https://doi.org/gnz6x6 DOI: 10.1038/s41590-021-01089-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.ABO blood group is involved in the quality of the specific immune response anti-SARS-CoV-2 Sergio Gil-Manso, Blanco Iria Miguens, Motyka Bruce, Halpin Anne, López-Esteban Rocío, Pérez-Fernández Verónica Astrid, Carbonell Diego, López-Fernández Luis Andrés, West Lori, Rafael Correa-Rocha, Pion Marjorie Virulence (2021-December-30) https://doi.org/gpphhv DOI: 10.1080/21505594.2021.2019959 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Loss of Bcl-6-Expressing T Follicular Helper Cells and Germinal Centers in COVID-19 Kaneko Naoki, Kuo Hsiao-Hsuan, Boucau Julie, Farmer Jocelyn R, Hugues Allard-Chamard, Mahajan Vinay S, Alicja Piechocka-Trocha, Lefteri Kristina, Osborn Matthew, Bals Julia, … Pillai Shiv Cell (2020-October) https://doi.org/gg9rdv DOI: 10.1016/j.cell.2020.08.025 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Robust SARS-CoV-2-specific T-cell immunity is maintained at 6 months following primary infection Zuo J, Dowell A, Pearce H, Verma K, Long HM, Begum J, Aiano F, Amin-Chowdhury Z, Hallis B, Stapley L, … Moss P Cold Spring Harbor Laboratory (2020-November-02) https://doi.org/ghhrps DOI: 10.1101/2020.11.01.362319 [DOI] [Google Scholar]
- 127.Persistent Cellular Immunity to SARS-CoV-2 Infection Breton Gaëlle, Mendoza Pilar, Hagglof Thomas, Oliveira Thiago Y, Dennis Schaefer-Babajew, Gaebler Christian, Turroja Martina, Hurley Arlene, Caskey Marina, Nussenzweig Michel C Cold Spring Harbor Laboratory (2020-December-09) https://doi.org/ghs9kk DOI: 10.1101/2020.12.08.416636 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Targets of T Cell Responses to SARS-CoV-2 Coronavirus in Humans with COVID-19 Disease and Unexposed Individuals Grifoni Alba, Weiskopf Daniela, Ramirez Sydney I, Mateus Jose, Dan Jennifer M, Moderbacher Carolyn Rydyznski, Rawlings Stephen A, Sutherland Aaron, Premkumar Lakshmanane, Jadi Ramesh S, … Sette Alessandro Cell (2020-June) https://doi.org/ggzxz2 DOI: 10.1016/j.cell.2020.05.015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.COVID-19 testing turns to T cells Sheridan Cormac Nature Biotechnology (2021-May) https://doi.org/gprdmb DOI: 10.1038/s41587-021-00920-9 [DOI] [PubMed] [Google Scholar]
- 130.Coronavirus (COVID-19) Update: FDA Authorizes Adaptive Biotechnologies T-Detect COVID Test Office of the Commissioner FDA (2021-March-09) https://www.fda.gov/news-events/press-announcements/coronavirus-covid-19-update-fda-authorizes-adaptive-biotechnologies-t-detect-covid-test [Google Scholar]
- 131.Rethinking Covid-19 Test Sensitivity — A Strategy for Containment Mina Michael J, Parker Roy, Larremore Daniel B New England Journal of Medicine (2020-November-26) https://doi.org/ghdg6n DOI: 10.1056/nejmp2025631 [DOI] [PubMed] [Google Scholar]
- 132.The time to do serosurveys for COVID-19 is now Peeling Rosanna W, Olliaro Piero L The Lancet Respiratory Medicine (2020-September) https://doi.org/gg559s DOI: 10.1016/s2213-600(20)30313-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Accurate point-of-care serology tests for COVID-19 Schuler Charles F, Gherasim Carmen, O’Shea Kelly, Manthei David M, Chen Jesse, Giacherio Don, Troost Jonathan P, Baldwin James L, Baker James R PLOS ONE (2021-March-16) https://doi.org/gpq4mz DOI: 10.1371/journal.pone.0248729 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Neutralizing Antibodies Against Severe Acute Respiratory Syndrome Coronavirus 2 (SARS-CoV-2) Variants Induced by Natural Infection or Vaccination: A Systematic Review and Pooled Analysis Chen Xinhua, Chen Zhiyuan, Azman Andrew S, Sun Ruijia, Lu Wanying, Zheng Nan, Zhou Jiaxin, Wu Qianhui, Deng Xiaowei, Zhao Zeyao, … Yu Hongjie Clinical Infectious Diseases (2021-July-24) https://doi.org/gmdht2 DOI: 10.1093/cid/ciab646 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.COVID-19 serosurveys for public health decision making Murhekar Manoj V, Clapham Hannah The Lancet Global Health (2021-May) https://doi.org/gh7598 DOI: 10.1016/s2214-109x(21)00057-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.COVID-19 Serological Tests: How Well Do They Actually Perform? Ghaffari Abdi, Meurant Robyn, Ardakani Ali Diagnostics (2020-July-04) https://doi.org/gg4h62 DOI: 10.3390/diagnostics10070453 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Serological tests for COVID-19 Bond Katherine, Williams Eloise, Howden Benjamin P, Williamson Deborah A Medical Journal of Australia (2020-September-06) https://doi.org/gpqt86 DOI: 10.5694/mja2.50766 [DOI] [PubMed] [Google Scholar]
- 138.A high-throughput multiplexed microfluidic device for COVID-19 serology assays Rodriguez-Moncayo Roberto, Cedillo-Alcantar Diana F, Guevara-Pantoja Pablo E, Chavez-Pineda Oriana G, Hernandez-Ortiz Jose A, Amador-Hernandez Josue U, Gustavo Rojas-Velasco, Sanchez-Muñoz Fausto, Daniel Manzur-Sandoval, Patino-Lopez Luis D, … Garcia-Cordero Jose L Lab on a Chip (2021) https://doi.org/gpqt7j DOI: 10.1039/d0lc01068e [DOI] [PubMed] [Google Scholar]
- 139.A systematic review of antibody mediated immunity to coronaviruses: antibody kinetics, correlates of protection, and association of antibody responses with severity of disease Huang Angkana T, Bernardo Garcia-Carreras, Hitchings Matt DT, Yang Bingyi, Katzelnick Leah, Rattigan Susan M, Borgert Brooke, Moreno Carlos, Solomon Benjamin D, Isabel Rodriguez-Barraquer, … Cummings Derek AT Cold Spring Harbor Laboratory (2020-April-17) https://doi.org/ggsfmz DOI: 10.1101/2020.04.14.20065771 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Antibodies, Immunity, and COVID-19 Spellberg Brad, Nielsen Travis B, Casadevall Arturo JAMA Internal Medicine (2021-April-01) https://doi.org/gpphhs DOI: 10.1001/jamainternmed.2020.7986 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Protection and waning of natural and hybrid COVID-19 immunity Goldberg Yair, Mandel Micha, Bar-On Yinon M, Bodenheimer Omri, Freedman Laurence, Ash Nachman, Sharon Alroy-Preis, Huppert Amit, Milo Ron Cold Spring Harbor Laboratory (2021-December-05) https://doi.org/g9rq DOI: 10.1101/2021.12.04.21267114 [DOI] [Google Scholar]
- 142.Identification and Development of Therapeutics for COVID-19 Rando Halie M, Wellhausen Nils, Ghosh Soumita, Lee Alexandra J, Dattoli Anna Ada, Hu Fengling, Byrd James Brian, Rafizadeh Diane N, Lordan Ronan, Qi Yanjun, … Greene Casey S mSystems (2021-December-21) https://pubmed.ncbi.nlm.nih.gov/34726496/ DOI: 10.1128/msystems.00233-21 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Strong associations and moderate predictive value of early symptoms for SARS-CoV-2 test positivity among healthcare workers, the Netherlands, March 2020 Tostmann Alma, Bradley John, Bousema Teun, Yiek Wing-Kee, Holwerda Minke, Chantal Bleeker-Rovers, Oever Jaap ten, Meijer Corianne, Janette Rahamat-Langendoen, Hopman Joost, … Wertheim Heiman Eurosurveillance (2020-April-23) https://doi.org/ggthwx DOI: 10.2807/1560-7917.es.2020.25.16.2000508 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Application and optimization of RT-PCR in diagnosis of SARS-CoV-2 infection Ren Xiaoshuai, Liu Yan, Chen Hongtao, Liu Wei, Guo Zhaowang, Zhang Yaqin, Chen Chaoqun, Zhou Jianhui, Xiao Qiang, Jiang Guanmin, Shan Hong Cold Spring Harbor Laboratory (2020-February-27) https://doi.org/gpq4k9 DOI: 10.1101/2020.02.25.20027755 [DOI] [Google Scholar]
- 145.Correlation of Chest CT and RT-PCR Testing for Coronavirus Disease 2019 (COVID-19) in China: A Report of 1014 Cases Ai Tao, Yang Zhenlu, Hou Hongyan, Zhan Chenao, Chen Chong, Lv Wenzhi, Tao Qian, Sun Ziyong, Xia Liming Radiology (2020-August) https://doi.org/ggmw6p DOI: 10.1148/radiol.2020200642 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Performance of Radiologists in Differentiating COVID-19 from Non-COVID-19 Viral Pneumonia at Chest CT Bai Harrison X, Hsieh Ben, Xiong Zeng, Halsey Kasey, Choi Ji Whae, Tran Thi My Linh, Pan Ian, Shi Lin-Bo, Wang Dong-Cui, Mei Ji, … Liao Wei-Hua Radiology (2020-August) https://doi.org/ggnqw4 DOI: 10.1148/radiol.2020200823 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Covid-19: automatic detection from X-ray images utilizing transfer learning with convolutional neural networks Apostolopoulos Ioannis D, Mpesiana Tzani A Physical and Engineering Sciences in Medicine (2020-April-03) https://doi.org/ggs448 DOI: 10.1007/s13246-020-00865-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Common pitfalls and recommendations for using machine learning to detect and prognosticate for COVID-19 using chest radiographs and CT scans Roberts Michael, Driggs Derek, Thorpe Matthew, Gilbey Julian, Yeung Michael, Ursprung Stephan, Aviles-Rivero Angelica I, Etmann Christian, McCague Cathal, … Schönlieb Carola-Bibiane Nature Machine Intelligence (2021-March) https://doi.org/gjkjvw DOI: 10.1038/s42256-021-00307-0 [DOI] [Google Scholar]
- 149.Epidemiologic surveillance for controlling Covid-19 pandemic: types, challenges and implications Ibrahim Nahla Khamis Journal of Infection and Public Health (2020-November) https://doi.org/gk7ghp DOI: 10.1016/j.jiph.2020.07.019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Wastewater and public health: the potential of wastewater surveillance for monitoring COVID-19 Farkas Kata, Hillary Luke S, Malham Shelagh K, McDonald James E, Jones David L Current Opinion in Environmental Science & Health (2020-October) https://doi.org/gg4tb6 DOI: 10.1016/j.coesh.2020.06.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Healthcare Workers CDC Centers for Disease Control and Prevention (2020-February-11) https://www.cdc.gov/coronavirus/2019-ncov/hcp/testing-overview.html [Google Scholar]
- 152.Report 13: Estimating the number of infections and the impact of non-pharmaceutical interventions on COVID-19 in 11 European countries Flaxman S, Mishra S, Gandy A, Unwin H, Coupland H, Mellan T, Zhu H, Berah T, Eaton J, Guzman P Perez, … Bhatt S Imperial College London (2020-March-30) https://doi.org/ggrbmf DOI: 10.25561/77731 [DOI] [Google Scholar]
- 153.NY Forward: a guide to reopening New York & building back better (2020-May-15) https://www.governor.ny.gov/sites/governor.ny.gov/files/atoms/files/NYForwardReopeningGuide.pdf
- 154.Antigen tests for COVID-19 Kyosei Yuta, Yamura Sou, Namba Mayuri, Yoshimura Teruki, Watabe Satoshi, Ito Etsuro Biophysics and Physicobiology (2021) https://doi.org/gpp4wr DOI: 10.2142/biophysico.bppb-v18.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Covid-19: Show us evidence for lifting restrictions, doctors tell Johnson Waters Adele BMJ (2022-February-15) https://doi.org/gprm69 DOI: 10.1136/bmj.o383 [DOI] [PubMed] [Google Scholar]
- 156.Exit strategies from lockdowns due to COVID-19: a scoping review Misra Madhavi, Joshi Harsha, Sarwal Rakesh, Rao Krishna D BMC Public Health (2022-March-12) https://doi.org/gprm7c DOI: 10.1186/s12889-022-12845-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Viral Cultures for Coronavirus Disease 2019 Infectivity Assessment: A Systematic Review Jefferson Tom, Spencer Elisabeth A, Brassey Jon, Heneghan Carl Clinical Infectious Diseases (2020-December-03) https://doi.org/ghpwks DOI: 10.1093/cid/ciaa1764 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.SARS-CoV-2, SARS-CoV, and MERS-CoV viral load dynamics, duration of viral shedding, and infectiousness: a systematic review and meta-analysis Cevik Muge, Tate Matthew, Lloyd Ollie, Maraolo Alberto Enrico, Schafers Jenna, Ho Antonia The Lancet Microbe (2021-January) https://doi.org/ghk47x DOI: 10.1016/s2666-5247(20)30172-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Long-term SARS-CoV-2 RNA shedding and its temporal association to IgG seropositivity Agarwal Vineet, Venkatakrishnan AJ, Puranik Arjun, Kirkup Christian, Agustin Lopez-Marquez, Challener Douglas W, Theel Elitza S, O’Horo John C, Binnicker Matthew J, Kremers Walter K, … Soundararajan Venky Cell Death Discovery (2020-December) https://doi.org/gprm6q DOI: 10.1038/s41420-020-00375-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Trajectory of Viral RNA Load Among Persons With Incident SARS-CoV-2 G614 Infection (Wuhan Strain) in Association With COVID-19 Symptom Onset and Severity Stankiewicz Karita Helen C, Dong Tracy Q, Johnston Christine, Neuzil Kathleen M, Paasche-Orlow Michael K, Kissinger Patricia J, Bershteyn Anna, Thorpe Lorna E, Deming Meagan, Kottkamp Angelica, … Brown Elizabeth R JAMA Network Open (2022-January-10) https://doi.org/gprm58 DOI: 10.1001/jamanetworkopen.2021.42796 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Covid-19: Tests on students are highly inaccurate, early findings show Armstrong Stephen BMJ (2020-December-23) https://doi.org/gprm67 DOI: 10.1136/bmj.m4941 [DOI] [PubMed] [Google Scholar]
- 162.Covid-19: Controversial rapid test policy divides doctors and scientists Kmietowicz Zosia BMJ (2021-January-12) https://doi.org/gprm68 DOI: 10.1136/bmj.n81 [DOI] [PubMed] [Google Scholar]
- 163.Assessment of the Analytical Sensitivity of 10 Lateral Flow Devices against the SARS-CoV-2 Omicron Variant Deerain Joshua, Druce Julian, Tran Thomas, Batty Mitchell, Yoga Yano, Fennell Michael, Dwyer Dominic E, Kok Jen, Williamson Deborah A Journal of Clinical Microbiology (2022-February-16) https://doi.org/gnvpb8 DOI: 10.1128/jcm.02479-21 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Clarifying the evidence on SARS-CoV-2 antigen rapid tests in public health responses to COVID-19 Mina Michael J, Peto Tim E, García-Fiñana Marta, Semple Malcolm G, Buchan Iain E The Lancet (2021-April) https://doi.org/gnmbjd DOI: 10.1016/s0140-6736(21)00425-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Test sensitivity is secondary to frequency and turnaround time for COVID-19 screening Larremore Daniel B, Wilder Bryan, Lester Evan, Shehata Soraya, Burke James M, Hay James A, Tambe Milind, Mina Michael J, Parker Roy Science Advances (2021-January) https://doi.org/ghs8s7 DOI: 10.1126/sciadv.abd5393 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.CDC chief says coronavirus cases may be 10 times higher than reported. Washington Post; https://www.washingtonpost.com/health/2020/06/25/coronavirus-cases-10-times-larger/ [Google Scholar]
- 167. GOV.UK . Liverpool coronavirus (COVID-19) community testing pilot: full evaluation report summary. https://www.gov.uk/government/publications/liverpool-coronavirus-covid-19-community-testing-pilot-full-evaluation-report-summary/liverpool-coronavirus-covid-19-community-testing-pilot-full-evaluation-report-summary .
- 168.Covid-19: Government rolls out twice weekly rapid testing to all in England Iacobucci Gareth BMJ (2021-April-06) https://doi.org/gprcnx DOI: 10.1136/bmj.n902 [DOI] [PubMed] [Google Scholar]
- 169.A comparative study of COVID-19 responses in South Korea and Japan: political nexus triad and policy responses Moon M Jae, Suzuki Kohei, Park Tae In, Sakuwa Kentaro International Review of Administrative Sciences (2021-March-18) https://doi.org/gprcn2 DOI: 10.1177/0020852321997552 [DOI] [Google Scholar]
- 170.All things equal? Heterogeneity in policy effectiveness against COVID-19 spread in chile Bennett Magdalena World Development (2021-January) https://doi.org/gjggkh DOI: 10.1016/j.worlddev.2020.105208 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.UK ending Covid testing ‘very worrying’ as WHO chief warns pandemic ‘isn’t over’ The Independent; (2022-March-11) https://www.independent.co.uk/news/health/who-covid-testing-anil-soni-b2032884.html [Google Scholar]
- 172.UK scales back routine covid-19 surveillance Clarke Jonathan, Beaney Thomas, Majeed Azeem BMJ (2022-March-04) https://doi.org/gprcnz DOI: 10.1136/bmj.o562 [DOI] [PubMed] [Google Scholar]
- 173. https://www.9news.com/article/news/health/coronavirus/local-groups-continue-push-for-covid-testing-and-vaccinations/73-bbcd8384-d96a-425e-aaeb-16ac9f36e581 .
- 174.Utah will stop daily COVID case counts, close test sites in wind-down, Gov. Cox announces. The Salt Lake Tribune; https://www.sltrib.com/news/2022/02/18/utah-will-stop-daily/ [Google Scholar]
- 175.Safety, tolerability and viral kinetics during SARS-CoV-2 human challenge in young adults Killingley Ben, Mann Alex J, Kalinova Mariya, Boyers Alison, Goonawardane Niluka, Zhou Jie, Lindsell Kate, Hare Samanjit S, Brown Jonathan, Frise Rebecca, … Chiu Christopher Nature Medicine (2022-March-31) https://doi.org/gptbvp DOI: 10.1038/s41591-022-01780-9 [DOI] [PubMed] [Google Scholar]
- 176.Considerations for the Safe Operation of Schools During the Coronavirus Pandemic Lordan Ronan, Prior Samantha, Hennessy Elizabeth, Naik Amruta, Ghosh Soumita, Paschos Georgios K, Skarke Carsten, Barekat Kayla, Hollingsworth Taylor, Juska Sydney, … Grosser Tilo Frontiers in Public Health (2021-December-16) https://doi.org/gprq6z DOI: 10.3389/fpubh.2021.751451 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.SARS-CoV-2 infection and transmission in school settings during the second COVID-19 wave: a cross-sectional study, Berlin, Germany, November 2020 Theuring Stefanie, Thielecke Marlene, van Loon Welmoed, Hommes Franziska, Hülso Claudia, von der Haar Annkathrin, Körner Jennifer, Schmidt Michael, Böhringer Falko, Mall Marcus A, … Eurosurveillance (2021-August-26) https://doi.org/gprq6x DOI: 10.2807/1560-7917.es.2021.26.34.2100184 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.End to quarantines, contact tracing among changes in new Oregon guidance for schools, starting March 12 Miller Elizabeth OPB (2022-March-02) https://www.opb.org/article/2022/03/02/oregon-schools-guidance-mask-mandate-end/ [Google Scholar]
- 179.Palm Beach County public schools to stop COVID-19 contact tracing WPTV; (2022-March-11) https://www.wptv.com/news/education/palm-beach-county-public-schools-to-stop-covid-19-contact-tracing [Google Scholar]
- 180.Covid News: C.D.C. Drops Contact Tracing Recommendation Hassan Adeel The New York Times (2022-March-02) https://www.nytimes.com/live/2022/03/02/world/covid-19-tests-cases-vaccine [Google Scholar]
- 181.Children and COVID-19: State-Level Data Report. American Academy of Pediatrics; http://www.aap.org/en/pages/2019-novel-coronavirus-covid-19-infections/children-and-covid-19-state-level-data-report/ [Google Scholar]
- 182.A fifth of all US child Covid deaths occurred during Omicron surge Schreiber Melody The Guardian (2022-March-11) https://www.theguardian.com/world/2022/mar/11/us-child-covid-deaths-omicron-surge [Google Scholar]
- 183.Global, regional, and national minimum estimates of children affected by COVID-19-associated orphanhood and caregiver death, by age and family circumstance up to Oct 31, 2021: an updated modelling study Unwin HJuliette T, Hillis Susan, Cluver Lucie, Flaxman Seth, Goldman Philip S, Butchart Alexander, Bachman Gretchen, Rawlings Laura, Donnelly Christl A, Ratmann Oliver, … Sherr Lorraine The Lancet Child & Adolescent Health (2022-April) https://doi.org/gpr264 DOI: 10.1016/s2352-4642(22)00005-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184.Five Ways that COVID-19 Diagnostics Can Save Lives: Prioritizing Uses of Tests to Maximize Cost-Effectiveness Reed Tristan, Waites William, Manheim David, de Walque Damien, Vallini Chiara, Gatti Roberta, Hallett Timothy B World Bank (2021-February-23) https://openknowledge.worldbank.org/handle/10986/35150 [Google Scholar]