Abstract
COVID-19 pandemic-related shifts in healthcare utilization, in combination with trends in non-COVID-19 disease transmission and NPI use, had clear impacts on infectious and chronic disease hospitalization rates. Using a national healthcare billing database (C19RDB), we estimated the monthly incidence rate ratio of hospitalizations between March 2020 and June 2021 according to 19 ICD-10 diagnostic chapters and 189 subchapters. The majority of hospitalization causes showed an immediate decline in incidence during March 2020. Hospitalizations for diagnoses such as reproductive neoplasms, hypertension, and diabetes returned to pre-pandemic norms in incidence during late 2020 and early 2021, while others, like those for infectious respiratory disease, never returned to pre-pandemic norms. These results are crucial for contextualizing future research, particularly time series analyses, utilizing surveillance and hospitalization data for non-COVID-19 disease. Our assessment of subchapter level primary hospitalization codes offers new insight into trends among less frequent causes of hospitalization during the COVID-19 pandemic.
Keywords: COVID-19 Research Database, COVID-19, ecological, hospitalization, non-COVID, ICD-10, time series
Introduction
The emergence of COVID-19 in the United States had immediate impacts on healthcare utilization and rates of disease due to non-SARS-CoV-2 related illness. Between March and April 2020, when a majority of US states had enacted stay-at-home orders, non-COVID-19 related illness dropped approximately 20–30% [1,2]. These shifts in hospitalization rates could be attributed to a combination of factors influencing health and healthcare. During the early months of the pandemic, an estimated 41% of US adults avoided medical care due to COVID-19 related concerns [3]. At the same time, there were massive and long-lasting alterations to daily activities and contact patterns. Non-essential workers were required to work-from-home, businesses closed, students transitioned to remote learning, and outpatient healthcare settings favored telehealth visits over in-person visits when possible [4,5]. These sudden shifts, in addition to pathogen-pathogen interference, and non-pharmaceutical interventions (NPI) like face masks, had the potential to influence the transmission of other pathogens and to both directly and indirectly affect the rates of hospitalization for a multitude of non-COVID-19 conditions [6,7].
The incidence of many infectious diseases decreased during the pandemic period (starting in March 2020) compared to previous years [8,9]. Influenza and respiratory syncytial virus (RSV) sharply declined during the winter of 2020/2021 and this was largely attributed to a decline in transmission of these viruses [10–12]. Conversely, certain conditions, like those related to mental health, were predicted to increase in incidence during pandemic shutdowns. Initial studies of self-reported depression and anxiety recorded during April 2020 supported this hypothesis, yet there has been mixed evidence on how this has translated to mental health-related hospitalizations [13–15]. Analyses of trends in non-COVID-19 disease during the pandemic period have largely focused on specific subsets of health conditions and have not taken a comprehensive view of disease-specific trends in relation to overall trends in hospitalization through multiple waves of SARS-CoV-2. A systematic analysis of diagnoses prior to and during the pandemic period is necessary to provide the comprehensive estimates of disease burden and to aid in hospital preparation and resource allocation during subsequent waves of COVID-19 [16,17].
In this study, we leverage a large national healthcare billing database to estimate relative changes in the incidence of hospitalizations by diagnostic category as the COVID-19 pandemic emerged and progressed through multiple waves of SARS-COV-2 variants. Our examination identifies broad patterns, which inform hypotheses regarding the influence of NPIs, diagnostic testing, and healthcare seeking on the reported incidence of disease. The results of this research provide a unique high-level understanding of the role of disruptions to healthcare and social interactions on multiple illnesses and can help to interpret reported trends in surveillance and hospitalization data.
Methods
COVID-19 Research Database
Data were provided by the COVID-19 Research Database (C19RDB), which is a pro-bono public-private consortium that collects and aggregates de-identified claims data, such as healthcare claims data. For this study, we used a health insurance claims (i.e. billing) database that included identifiers for healthcare setting (inpatient, outpatient), primary diagnostic code, secondary diagnostic codes, billing site location (state), date of service, Current Procedural Terminology codes, age and gender, among other billing details. Reporting to this database was continually updated until September 2021, leading to variation in total claims per month as additional payers were incorporated over time. This database specifically captures billing data at the level of individual hospitalizations, along with associated demographic, diagnostic, and procedure code information. Information on the total population within each insurance payer (i.e. payees) was not available, limiting our ability to accurately assess a population denominator from which hospitalizations arose. This research has been approved by the Georgetown University Institutional Review Board.
Data Cleaning
We limited the data to inpatient records due to large and unexplained fluctuations in the volume of outpatient data entering the database in early 2019. Only hospitalizations with a corresponding procedure code were included for analysis. Based on anonymized patient identification numbers, entries were deduplicated to retain only the first hospitalization record within a 30-day rolling period for each patient. The month and year of diagnosis used for analysis was the “service from date” when available, otherwise “service to date” or “admission date” or “billing statement from” date was used (in order of first availability). “Service from date” was present in the majority (>90%) of records and the primary source for date information. Hospitalizations for COVID-19 were excluded from all analyses if a code for COVID-19 (ICD-10 “U07.1 – COVID-19” or “J12.82 – pneumonia due to SARS-associated coronavirus” or “M35.81 – COVID-19 related multisystem inflammatory syndrome” or “B97.2* - coronavirus as the cause of disease elsewhere classified”) was present, in order to properly assess trends in non-COVID-19 illnesses [18]. The final study period was January 1, 2017 to June 31, 2021 (Supplementary Figure S1).
The outcome of interest was the primary (i.e. first) diagnostic field recorded for each visit as categorized by the International Classification of Diseases-Clinical Modification (ICD-10-CM) codes. We included only the primary diagnostic code for each hospitalization in order to limit the influence of diagnoses not directly related to admission [19]. Primary diagnostic codes for all patients were grouped by ICD-10 chapter (2016 edition of ICD-10-CM), subchapter, month, and year. These chapters are organized according to illness type and/or body system affected, totaling 22 chapters, of which diagnostic codes falling into chapters 1 through 19 were assessed (excluding chapters for external morbidity, special purposed codes, and health services contact codes) (Supplementary Table S1). Subchapter groupings were created according to categories defined by the World Health Organization (WHO) and were primarily organized by type of infectious agent identified, type of illness, or part of body affected in addition to codes for “unspecified” or “unknown” illness [20]. The 19 chapters could be subdivided into 236 subchapters, but subchapters with fewer than 5000 diagnoses total between 2017 and 2019 were excluded from all analyses (47 exclusions) (Supplementary Table S2).
Analysis
The aim of this analysis was to provide a monthly estimate of the incidence rate ratio (IRR), defined as the observed number of monthly hospitalizations divided by the expected monthly hospitalizations, for each diagnostic chapter and subchapter of interest. Estimates were generated using Poisson regression models fit to the monthly count of diagnostic codes within each ICD-10 chapter (or subchapter) from the pre-pandemic period (January 1, 2017 – December 31, 2019) to estimate the number of hospitalizations per month had the pandemic not occurred. Time series for each chapter and subchapter were analyzed independently. All models adjusted for time trends (linear trend) and yearly seasonality (using sine and cosine harmonic terms). While we did not have a true population denominator to use as an offset term in the mode, due to a lack of information on payer population or hospital catchment areas, we estimated the size of the active patient population as the number of unique individuals with a recorded hospitalization per year for any cause. For the hospitalizations occurring between 2017 and 2019, the offset was the total number of unique individuals present in the same calendar year. Because there was a generally increasing trend in the number of individuals included in this database, the offset for 2020 and 2021 was estimated by fitting linear and seasonal trends to the monthly counts of unique individuals in the study prior to March 2020 and extrapolating these trends to the period from March 2020-June 2021. Prediction intervals for the final IRR estimates were calculated for each time point through a two-stage simulation using Monte Carlo resampling that accounted for parameter uncertainty and observation uncertainty [21,22].
Due to the numerous subchapters included in this analysis (N = 180), we implemented a hierarchical clustering algorithm (applying Ward’s minimum variance method) to systematically group diagnoses that shared temporal trends in IRRs during the period between Jan 2020 and Jun 2021. The optimal number of clusters (k) was chosen via the “elbow method”. This method attempts to maximize the variance (within-cluster sum of squares) explained by the clustering and minimize the number of clusters thereby excluding an increasing number of clusters that only provide a marginal gain in explained variance. Data queries and analyses were run in SQL and R version 4.0.2 using the packages data.table, DBI, dplyr, ggplot2, glue, gridExtra, lubridate, and readr.
Results
The total number of unique hospitalized individuals ranged from 2.8 million to 3.1 million per year between 2017 and 2020. Insurance payers covered patients from all fifty states and Puerto Rico. Between January 2017 and December 2019, the monthly number of unique hospitalizations (excluding repeat admissions occurring within a 30-day period and COVID-19 admissions) ranged from ~300,000 to ~400,000. In 2020, this number dropped from 455,000 unique hospitalizations in January to 263,000 in March and then declined again to 151,000 encounters in April 2020, before rebounding moderately to 213,000 in June.
Trends in broad disease classes
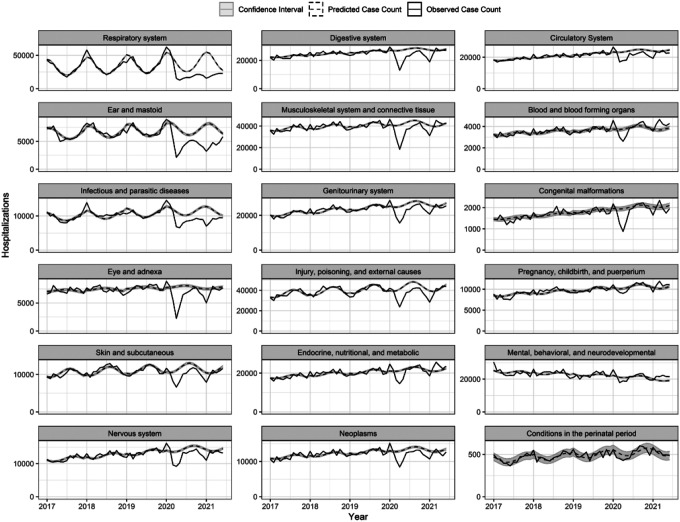
All ICD10 chapters experienced relative increases in incidence of hospitalizations during the pre-pandemic months of January and February 2020, with IRRs ranging from 0.96 to 1.21 in Jan 2020 (Figure 1; Supplemental Table S3), likely reflecting database reporting changes at the start of the new calendar year. Beginning in March 2020, the incidence of hospitalization among all diagnostic chapters exhibited a moderate decrease. For most of chapters, the greatest declines in incidence occurred during April 2020 (Supplementary Figure S2). This was especially true for diseases of the eye and diseases of the ear while the smallest changes in hospitalizations were found among conditions classified within mental health, perinatal period, and pregnancy, childbirth, and puerperium chapters. The incidence of hospitalizations for respiratory disease was lower than expected for all months following April 2020, reaching the greatest relative decline in January 2021, when seasonal hospitalizations would typically be increasing (IRR 0.29; 95% CI: 0.28, 0.29).
Figure 1.
Time series of observed hospitalizations per diagnostic chapter by month and year (solid line) with model predicted case counts (dashed line) and confidence intervals (grey).
Clustering of trends within diagnostic subchapters
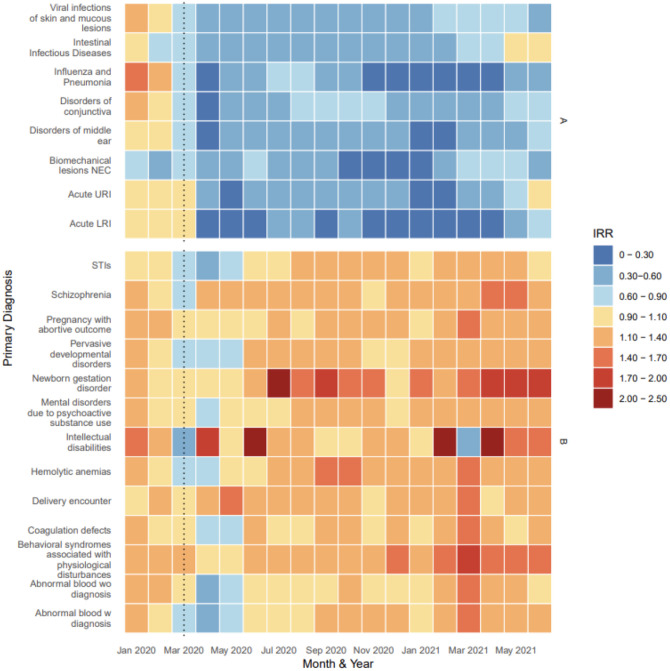
A cluster analysis revealed 4 distinct groupings of ICD-10 subchapters (Supplemental Figures S3–S4). Subchapters grouped in cluster A shared a sharp relative decrease in hospitalizations in April 2020 that continued to have below-expected rates throughout the remainder of the 2020. The average IRR of this cluster was 0.46 (range: 0.11, 1.09) between March 2020 and June 2021. These subchapters included diagnoses for Pneumonia & Influenza (P&I) (J09 -J18), Middle Ear (H65-H75), Intestinal Infectious diseases (A00-A09), Conjunctivitis (H10-H11), Viral skin and mucous infections (B00-B09), Biomechanical lesions (M99), Acute lower respiratory infection (LRI), and Acute upper respiratory infection (URI) (Figure 2A). The incidence of P&I in January and February 2021 was 89% (95%CI: 88.7%, 90.4%) lower than what would be expected given predicted trends in incidence from previous years and seasonal trends (Supplemental Table S4). There were just 9,427 unique diagnoses for P&I between Oct 2020 and Mar 2021 compared to 61,085 during the same months of 2019. The timing and intensity of the decline in incidence varied by subchapter within this cluster, with most subchapters experiencing the greatest decline in April 2020 or January 2021. Codes for biomechanical lesions (not elsewhere classified) were unique in that their lowest IRR was recorded in Nov 2020 of 0.20 (0.17, 0.23). While diagnoses for biomechanical lesions decreased during the summer of 2020 compared to what was expected, the magnitude of the declines for biomechanical lesions showed much greater uncertainty compared to the estimates for infectious diseases, reflecting the small case counts for primary diagnoses of this subchapter within the dataset. The only diagnostic subchapters from this cluster A to approach pre-pandemic incidence levels (i.e. RR approaching 1.0) were intestinal infectious diseases, acute URI, and acute LRI in May and June of 2021.
Figure 2A.
Estimated IRR per subchapter and month grouped according to hierarchical clustering, Jan 2020 to Jun 2021
In contrast to cluster A, cluster B had little to no decrease in hospitalizations during March, April or May of 2020. The average IRR in this cluster remained below expected levels only for the months of March, April, and May 2020, prior to returning to and exceeding pre-pandemic levels for the remainder of the study period. Diagnoses within this cluster included: Sexually Transmitted Infections (STIs) (A50-A64), intellectual disabilities (F70-F79), psychological disturbances (F50-F59), and coagulation defects (D65-D69) in addition to ICD-10 codes related to pregnancy such as delivery encounter (O80-O82), pregnancy with an abortive outcome (O00-O08), and newborn gestation disorder (P05-P08). The average IRR of this cluster between March 2020 and June 2021 was 1.20 (range: 0.46, 2.20).
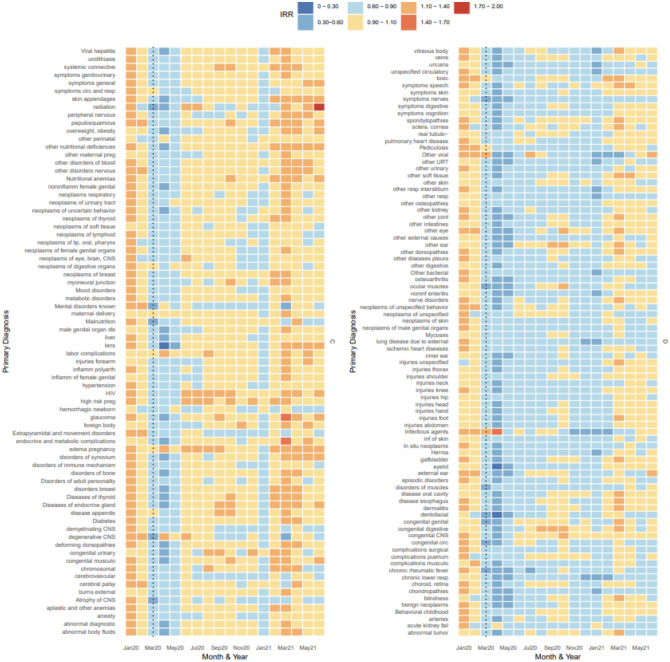
Cluster C included diagnoses that decreased in incidence during March, April, and May of 2020, and well as January of 2021, but hovered around pre-pandemic estimates of incidence for the remainder of the study period (Figure 2B). This grouping included diagnoses for hypertension (I10-I16), malnutrition (E40-E46), diseases of appendix (K35-K38), diabetes (E08-E13), and neoplasms of multiple systems (e.g. respiratory, CNS, reproductive organs, soft tissue, breast, digestive organs, etc.). The average IRR of this cluster between March 2020 and June 2021 was 0.95 (range: 0.12, 1.85). Subchapters of cluster C demonstrated an average IRR < 1.0 during 2020 but incidence began to increase gradually during 2021. Diseases of the lens, which experienced the largest drop in incidence during April 2020 (IRR: 0.12; 95% PI: 0.12, 0.13) was included in this cluster as it quickly returned to its pre-pandemic incidence range by June 2020.
Figure 2B.
Estimated IRR per subchapter and month grouped according to hierarchical clustering (clusters C and D), Jan 2020 to Jun 2021
Cluster D exhibited a relative decrease in hospitalizations during March, April, and May 2020 and continued to exhibit lower-than-expected incidence during the remainder of 2020 and early 2021 with occasional months near baseline, particularly during Aug, Sept, Oct (Figure 2B). This included diagnoses such as pulmonary heart disease (I26-I28), ischemic heart diseases (I20-I25), injuries of multiple areas of the body (thorax, shoulder, neck, knee, hip, head, hand, foot)(S00-S99), chronic lower respiratory disease (J40-J47), chronic rheumatic fever (I05-I09), and neoplasms of the skin (C43-C44). On average, the diseases in these clusters had an IRR of 0.83 (range: 0.29, 1.45) between March and June 2021.
Discussion
The COVID-19 pandemic has profoundly disrupted healthcare and disease dynamics in the United States. The wealth of data provided by this healthcare insurance billing database allowed for a novel, large scale examination of these disruptions for different disease categories. The vast majority of diagnostic categories experienced a decline in relative incidence of hospitalizations during April and May of 2020, while the timing at which incidence returned to pre-pandemic levels varied by diagnostic chapter, and some chapters have yet to reach pre-pandemic levels of incidence. The unique and robust data leveraged in this analysis allowed for exploration of trends in hospitalizations that we hypothesize might be due to a range of pandemic changes in healthcare utilization, lifestyle changes, and sanitation practices.
Our analysis was able to confirm findings from prior, smaller studies of illness using a national database, and was thus able to provide more substantial estimates of relative incidence. Hospitalizations with a primary diagnostic code for infectious respiratory disease (e.g. influenza, acute lower respiratory infections) and otitis media (the main contributor to the “middle ear diagnosis” subchapter) clustered in our analysis given their shared dramatic decrease in IRR during April and May 2020 and their uncharacteristically low relative incidence during fall/winter 2020/2021. These results substantiate prior studies of influenza, RSV and other upper and lower respiratory infections during the winter 2020/2021 season [10,23]. NPIs are believed to have reduced influenza transmission during the 2020/2021 influenza season while also contributing to the decline in mumps and other respiratory infections in some countries [12,23–25]. While the decline in influenza could be partially attributed to a decrease in the number of specimens sent for respiratory panel testing, as evidenced by an 61% decrease in US specimens sent for testing in 2020, studies have shown that the positivity rate among specimens sent had also declined, approximately 98%, during winter 2020/2021 [11]. Our finding that middle ear infections (otitis media, OM) remained low following pandemic lockdowns is supported by multiple studies in Milan where authors identified reductions in OM among OM-prone children of ~80% between Feb and May 2020 compared to the same time period in 2019 [26,27].
In contrast to the subchapters with large declines in incidence, the cluster of subchapters with increased rates (cluster B), which included diagnoses for behavioral syndromes associated with physiological disturbances (F50-F59), hemolytic anemias (D55-D59), and intellectual disabilities (F70-F79). Interestingly, these illnesses did not appear to have a clear shared mechanism to explain the pandemic-period trends. Potential drivers of the sporadic increases in disease incidences during the pandemic period include: 1) changes in the underlying population covered by the insurance payers included in our analysis or changes in which hospitals (or types of hospitals that) are within the insurance service areas, 2) diagnoses that are a direct result of a prior SARS-CoV-2 infection, and 3) mechanisms that cannot be elucidated using this dataset such as diagnostic screening, test ordering, and the prioritization of hospitalization vs outpatient care settings. Prior research has revealed that patients with intellectual disabilities are more likely to contract COVID-19 and more likely to require hospitalization compared to those without an established intellectual disability [28]. Our analysis excluded any diagnoses for COVID-19, but it is possible that the elevated incidence of hospitalizations where the primary diagnosis is for intellectual disability could be linked to the downstream effects of a prior COVID-19 infection or other pandemic-related changes in care for members of this population.
There has been limited research assessing the impact of the pandemic on behavioral syndromes associated with physiological disturbances (e.g. eating and sleep disorders); however, researchers at a children’s hospital did describe an increase in restrictive eating disorders during the first year of the pandemic [29]. Contrary to our results, psychiatric hospitalizations in Brazil decreased between March and August 2020 by 33%, demonstrating the complex relationship between physiological disturbances, schizophrenia, and behavioral syndromes incidence and hospitalization incidence [30]. Interestingly, schizophrenia is noted as a risk factor for COVID-19 mortality, but our study was limited by its inability to explore mortality information [31]. An increase in hemolytic anemias during the pandemic period was also identified; however, further research is needed to validate our initial findings given that no apparent mechanism for this trend could be elucidated from this data [32,33]. The development of autoimmune hemolytic anemias in conjunction with SARS-COV-2 infection have been reported; however, our analysis excluded any such hospitalizations so the mechanism responsible for this increase in our data is unknown [34,35].
The ICD-10 chapter for Pregnancy, Childbirth, and Puerperium (O00-O9A) only experienced an estimated decrease in incidence of 11% (95% PI: 9%,13%) in April of 2020. ICD-10 codes for labor and delivery would not be expected to drop in the early months of the pandemic as the number of expected births could not be influenced by pandemic-related changes in family planning. However, birth outcomes could be influenced by the level of prenatal care and pregnancy-related stress as the pandemic continued into late 2020 and early 2021. Throughout the study period, incidence of newborn gestation disorder was aberrant, vacillating between an estimate IRR of 0.68 and 2.20, and did not appear to clearly alter in relation to COVID-19 pandemic changes in health care. The increase in incidence during 2020 and 2021 for these diagnostic codes could be a product of an otherwise increasing trend in data reporting to the database or a true reflection of adverse newborn outcomes related to the COVID-19 pandemic.
Cluster D included diagnoses that lacked specificity, such as “symptoms for….”, “other disorders of….”, and “complications of…” diagnoses, and may not be commonly coded within the primary diagnosis field. Hospitalizations for injuries clustered in this category as well, with most experiencing a decline in incidence until Jan 2021. These findings are supported by a few studies detailing trauma center admissions, with large declines among injuries due to road traffic conditions which constitute a large portion of trauma admissions [36,37]. Conversely, increases in certain types of severe injuries (i.e. self-inflicted injuries) have been reported in some emergency settings during the early period of the pandemic, including “penetrating injuries” [38,39]. As our analysis relies on inpatient data, we are likely only seeing a small portion (and severe enough to require an inpatient hospitalization) of the total injuries that were presenting during this time.
In contrast to diagnoses that had a slow rebound in incidence of hospitalizations during 2020, like those in cluster D, hospitalizations for neoplasms (cluster C) had a short-lived decline between March and June 2020, followed by an extended period of time hovering around pre-pandemic levels. The largest decrease among all neoplasm diagnoses was among benign neoplasms in April 2020 (−77%, PI: −76%, −78%). Neoplasms the skin, thyroid, and male genital track also had a decrease in hospitalizations of 30% or more in April 2020. The most likely explanation for the fluctuations in neoplasm diagnoses are disruptions to cancer screenings and subsequent diagnoses resulting from healthcare access limitations during the early pandemic period followed by an influx of patients in need of delayed cancer screenings and referrals [40–43]. Cluster C, as a whole, could be characterized as declining in relative incidence in correspondence with waves of COVID-19 occurring in the US, primarily March and April of 2020 and January 2021. This supports the hypothesis that these hospitalizations are primarily those that could be delayed during peak COVID-19 transmission cycles.
Strengths and Limitations
Due to the passive nature of data reporting to the healthcare encounter billing clearinghouse and the differential reporting by the insurance payer, different payers may contribute to relative increases or decreases in disease rates in a manner that might not be reflective of true trends present across the US during the study period. Alternative explanations for the relative increases in disease diagnoses include payer catchment area redefinitions (and sudden inclusions of specialty facilities), reimbursement policies, and primary vs secondary diagnostic field coding practices. Our analysis was limited to primary diagnostic codes, thus the full range of diagnoses made during the study period were not captured and it is possible that IRR trends may vary if the full breadth of secondary codes were included for analysis. This study was also limited to inpatient data; therefore, we are unable to discern if relative increases or decreases in hospitalization may be linked to a shift in treatment location (i.e. cases being treated in an outpatient setting that would otherwise be hospitalized or vice versa). This phenomenon has been noted among cases of appendicitis such that more cases were treated with non-operative management prior to delayed operative treatment during the early pandemic period [44,45].
For most diagnostic chapters and subchapters, there was an increase in hospitalizations in Jan and Feb 2020 which indicated a fluctuation in the billing data reported to the database used for this analysis. This complicates the interpretation of elevated incidence rates among certain diagnostic subchapters during the pandemic period as it may be a reflection of the overall influx of data to this clearinghouse that began at the start of the 2020 calendar year. Additionally, some diagnostic chapters had a low frequency of events, particularly during the pandemic period, such that we could not obtain precise estimates of their monthly IRR.
Our analysis is strengthened by the large study population size (28 million hospitalizations) and the ability to investigate rare disorders that may otherwise not be captured in an analysis of disease trends. Our ability to access multiple years of data prior to the pandemic also strengthens our confidence in estimates of relative incidence during the pandemic period. Examination of billing data allowed this study to overcome limitations in disease reporting due to COVID-19 related disruptions, as has been demonstrated for the Nationally Notifiable Disease Surveillance System (NNDSS) data [8].
Conclusion
There were substantial changes in hospitalization patterns across many different disease categories during the early part of the COVID-19 pandemic, some of which persisted for at least a year. The broad trends identified here point to a number of hypotheses about the mechanisms driving these trends. Declines in infectious respiratory disease may likely be a result of NPI use, social distancing, and changes to daily life that altered contact patterns. Meanwhile, this research has highlighted potential areas for further investigation by experts in the field, such as trends in hospitalizations for intellectual disabilities, newborn gestation disorders, and mental health disorders. Understanding these patterns will be crucial to minimize disruptions to healthcare during future major health events.
Supplementary Material
Acknowledgements
The data, technology, and services used in the generation of these research findings were generously supplied pro bono by the COVID-19 Research Database partners, who are acknowledged at https://covid19researchdatabase.org/. We would also like to thank Dominic Copper-Wooten for his contributions regarding SQL querying.
Funding Statement
This work was supported by the Merck Investigator Studies Program (Study # 60274 and the National Institute of Allergy and Infectious Diseases of the National Institutes of Health under award numbers 1F31AI161971-01A1. These funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
DMW has received consulting fees from Pfizer, Merck, GSK, Affinivax, and Matrivax for work unrelated to this manuscript and is Principal Investigator on research grants from Pfizer and Merck to Yale University, unrelated to this manuscript.
Data Availability
The data that support the findings of this study are available from COVID-19 Research Database consortium, but restrictions apply to the availability of these data, which were used under license for the current study, and so are not publicly available. Data are however available from the authors upon reasonable request and with permission of COVID-19 Research Database consortium.
References
- 1.Heist T, Schwartz K, Oct 19 SBP, 2020. How Were Hospital Admissions Impacted by COVID-19? Trends in Overall and Non-COVID-19 Hospital Admissions Through August 8, 2020 [Internet]. KFF. 2020. [cited 2021 Nov 17]. Available from: https://www.kff.org/health-costs/issue-brief/how-were-hospital-admissions-impacted-by-covid-19-trends-in-overall-and-non-covid-19-hospital-admissions-through-august-8-2020/ [Google Scholar]
- 2.Nguyen JL, Benigno M, Malhotra D, Khan F, Angulo FJ, Hammond J, Swerdlow DL, Reimbaeva M, Emir B, McLaughlin JM. Pandemic-related declines in hospitalization for non-COVID-19-related illness in the United States from January through July 2020. PLOS ONE [Internet]. 2022. Jan 6 [cited 2022 Feb 1];17(1):e0262347. Available from: https://journals.plos.org/plosone/article?id=10.1371/journal.pone.0262347 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Czeisler MÉ. Delay or Avoidance of Medical Care Because of COVID-19–Related Concerns — United States, June 2020. MMWR Morb Mortal Wkly Rep [Internet]. 2020. [cited 2021 Sep 28];69. Available from: https://www.cdc.gov/mmwr/volumes/69/wr/mm6936a4.htm [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Park KH, Kim AR, Yang MA, Lim SJ, Park JH. Impact of the COVID-19 pandemic on the lifestyle, mental health, and quality of life of adults in South Korea. PLOS ONE [Internet]. 2012. Feb 26 [cited 2022 Feb 3];16(2):e0247970. Available from: https://journals.plos.org/plosone/article?id=10.1371/journal.pone.0247970 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Zhang J. People’s responses to the COVID-19 pandemic during its early stages and factors affecting those responses. Humanit Soc Sci Commun [Internet]. 2021. Feb 3 [cited 2022 Feb 3];8(1):1–13. Available from: https://www.nature.com/articles/s41599-021-00720-1 [Google Scholar]
- 6.Baker RE, Park SW, Yang W, Vecchi GA, Metcalf CJE, Grenfell BT. The impact of COVID-19 nonpharmaceutical interventions on the future dynamics of endemic infections. Proc Natl Acad Sci [Internet]. 2020. Dec 1 [cited 2021 Aug 29];117(48):30547–53. Available from: https://www.pnas.org/content/117/48/30547 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Nickbakhsh S, Mair C, Matthews L, Reeve R, Johnson PCD, Thorburn F, von Wissmann B, Reynolds A, McMenamin J, Gunson RN, Murcia PR. Virus–virus interactions impact the population dynamics of influenza and the common cold. Proc Natl Acad Sci [Internet]. 2019. Dec 26 [cited 2022 Apr 20];116(52):27142–50. Available from: https://www.pnas.org/doi/10.1073/pnas.1911083116 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Crane MA, Popovic A, Panaparambil R, Stolbach AI, Romley JA, Ghanem KG. Reporting of Infectious Diseases in the United States During the Coronavirus Disease 2019 (COVID-19) Pandemic. Clin Infect Dis [Internet]. [cited 2021 Nov 12]; Available from: https://academic.oup.com/cid/advance-article/doi/10.1093/cid/ciab529/6294158 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Chen B, Wang M, Huang X, Xie M, Pan L, Liu H, Liu Z, Zhou P. Changes in Incidence of Notifiable Infectious Diseases in China Under the Prevention and Control Measures of COVID-19. Front Public Health [Internet]. 2021. [cited 2022 Feb 1];9. Available from: https://www.frontiersin.org/article/10.3389/fpubh.2021.728768 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Solomon DA, Sherman AC, Kanjilal S. Influenza in the COVID-19 Era. JAMA [Internet]. 2020. Oct 6 [cited 2021 Aug 18];324(13):1342–3. Available from: 10.1001/jama.2020.14661 [DOI] [PubMed] [Google Scholar]
- 11.Olsen SJ, Azziz-Baumgartner E, Budd AP, Brammer L, Sullivan S, Pineda RF, Cohen C, Fry AM. Decreased influenza activity during the COVID-19 pandemic—United States, Australia, Chile, and South Africa, 2020. Am J Transplant [Internet]. 2020. [cited 2021 Aug 29];20(12):3681–5. Available from: https://onlinelibrary.wiley.com/doi/abs/10.1111/ajt.16381 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Soo RJJ, Chiew CJ, Ma S, Pung R, Lee V. Decreased Influenza Incidence under COVID-19 Control Measures, Singapore. Emerg Infect Dis [Internet]. 2020. Aug [cited 2021 Aug 18];26(8):1933–5. Available from: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC7392467/ [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Chandola T, Kumari M, Booker CL, Benzeval M. The mental health impact of COVID-19 and lockdown-related stressors among adults in the UK. Psychol Med [Internet]. 2020. Dec 7 [cited 2021 Nov 17];1–10. Available from: https://www.cambridge.org/core/journals/psychological-medicine/article/mental-health-impact-of-covid19-and-lockdownrelated-stressors-among-adults-in-the-uk/C1814A3228BCDBA5A9F697C231622628 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Leeb RT. Mental Health–Related Emergency Department Visits Among Children Aged 18 Years During the COVID-19 Pandemic — United States, January 1–October 17, 2020. MMWR Morb Mortal Wkly Rep [Internet]. 2020. [cited 2022 Feb 3];69. Available from: https://www.cdc.gov/mmwr/volumes/69/wr/mm6945a3.htm [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Panchal N, Kamal R, Feb 10 RGP, 2021. The Implications of COVID-19 for Mental Health and Substance Use [Internet]. KFF. 2021. [cited 2022 Feb 9]. Available from: https://www.kff.org/coronavirus-covid-19/issue-brief/the-implications-of-covid-19-for-mental-health-and-substance-use/ [Google Scholar]
- 16.Kohli P, Virani SS. Surfing the Waves of the COVID-19 Pandemic as a Cardiovascular Clinician. Circulation [Internet]. 2020. Jul 14 [cited 2021 Sep 28];142(2):98–100. Available from: https://www.ahajournals.org/doi/full/10.1161/CIRCULATIONAHA.120.047901 [DOI] [PubMed] [Google Scholar]
- 17.Hassan EM, Mahmoud HN. Impact of multiple waves of COVID-19 on healthcare networks in the United States. PLOS ONE [Internet]. 2021. Mar 3 [cited 2021 Sep 28];16(3):e0247463. Available from: https://journals.plos.org/plosone/article?id=10.1371/journal.pone.0247463 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.New COVID-19-related ICD-10-CM codes take effect | AAP News | American Academy of Pediatrics [Internet]. [cited 2022 Feb 9]. Available from: https://publications.aap.org/aapnews/news/12215/New-COVID-19-related-ICD-10-CM-codes-take-effect
- 19.ICD-10-CM official guidelines for coding and reporting FY 2016 [Internet]. [cited 2021 Nov 18]. Available from: https://stacks.cdc.gov/view/cdc/34313
- 20.ICD-10 Version:2019 [Internet]. [cited 2021 Nov 18]. Available from: https://icd.who.int/browse10/2019/en
- 21.Weinberger DM, Chen J, Cohen T, Crawford FW, Mostashari F, Olson D, Pitzer VE, Reich NG, Russi M, Simonsen L, Watkins A, Viboud C. Estimation of Excess Deaths Associated With the COVID-19 Pandemic in the United States, March to May 2020. JAMA Intern Med [Internet]. 2020. Oct 1 [cited 2021 Sep 8];180(10):1336–44. Available from: 10.1001/jamainternmed.2020.3391 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lauer SA, Sakrejda K, Ray EL, Keegan LT, Bi Q, Suangtho P, Hinjoy S, Iamsirithaworn S, Suthachana S, Laosiritaworn Y, Cummings DAT, Lessler J, Reich NG. Prospective forecasts of annual dengue hemorrhagic fever incidence in Thailand, 2010–2014. Proc Natl Acad Sci [Internet]. 2018. Mar 6 [cited 2022 Feb 9];115(10):E2175–82. Available from: https://www.pnas.org/content/115/10/E2175 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Geng MJ, Zhang HY, Yu LJ, Lv CL, Wang T, Che TL, Xu Q, Jiang BG, Chen JJ, Hay SI, Li ZJ, Gao GF, Wang LP, Yang Y, Fang LQ, Liu W. Changes in notifiable infectious disease incidence in China during the COVID-19 pandemic. Nat Commun [Internet]. 2021. Nov 26 [cited 2022 Feb 1];12(1):6923. Available from: https://www.nature.com/articles/s41467-021-27292-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Huang QS, Wood T, Jelley L, Jennings T, Jefferies S, Daniells K, Nesdale A, Dowell T, Turner N, Campbell-Stokes P, Balm M, Dobinson HC, Grant CC, James S, Aminisani N, Ralston J, Gunn W, Bocacao J, Danielewicz J, Moncrieff T, McNeill A, Lopez L, Waite B, Kiedrzynski T, Schrader H, Gray R, Cook K, Currin D, Engelbrecht C, Tapurau W, Emmerton L, Martin M, Baker MG, Taylor S, Trenholme A, Wong C, Lawrence S, McArthur C, Stanley A, Roberts S, Rahnama F, Bennett J, Mansell C, Dilcher M, Werno A, Grant J, van der Linden A, Youngblood B, Thomas PG, Webby RJ. Impact of the COVID-19 nonpharmaceutical interventions on influenza and other respiratory viral infections in New Zealand. Nat Commun [Internet]. 2021. Feb 12 [cited 2021 Aug 3];12(1):1001. Available from: https://www.nature.com/articles/s41467-021-21157-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Huh K, Jung J, Hong J, Kim M, Ahn JG, Kim JH, Kang JM. Impact of Nonpharmaceutical Interventions on the Incidence of Respiratory Infections During the Coronavirus Disease 2019 (COVID-19) Outbreak in Korea: A Nationwide Surveillance Study. Clin Infect Dis [Internet]. 2021. Apr 1 [cited 2022 Apr 12];72(7):e184–91. Available from: 10.1093/cid/ciaa1682 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Torretta S, Capaccio P, Coro I, Bosis S, Pace ME, Bosi P, Pignataro L, Marchisio P. Incidental lowering of otitis-media complaints in otitis-prone children during COVID-19 pandemic: not all evil comes to hurt. Eur J Pediatr [Internet]. 2021. Feb 1 [cited 2021 Aug 18];180(2):649–52. Available from: 10.1007/s00431-020-03747-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Aldè M, Di Berardino F, Marchisio P, Cantarella G, Ambrosetti U, Consonni D, Zanetti D. Effects of COVID-19 Lockdown on Otitis Media With Effusion in Children: Future Therapeutic Implications. Otolaryngol Neck Surg [Internet]. 2021. Jan 26 [cited 2021 Aug 18];0194599820987458. Available from: 10.1177/0194599820987458 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Gleason Jonathan Ross Wendy, Fossi Alexander Blonsky Heather, Tobias Jane Stephens Mary. The Devastating Impact of Covid-19 on Individuals with Intellectual Disabilities in the United States. NEJM Catal Innov Care Deliv [Internet]. 2021. Mar 5 [cited 2021 Nov 8]; Available from: https://catalyst.nejm.org/doi/full/10.1056/CAT.21.0051 [Google Scholar]
- 29.Otto AK, Jary JM, Sturza J, Miller CA, Prohaska N, Bravender T, Van Huysse J. Medical Admissions Among Adolescents With Eating Disorders During the COVID-19 Pandemic. Pediatrics [Internet]. 2021. Oct 1 [cited 2022 Feb 9];148(4):e2021052201. Available from: 10.1542/peds.2021-052201 [DOI] [PubMed] [Google Scholar]
- 30.Ornell F, Borelli WV, Benzano D, Schuch JB, Moura HF, Sordi AO, Kessler FHP, Scherer JN, Diemen L von. The next pandemic: impact of COVID-19 in mental healthcare assistance in a nationwide epidemiological study. Lancet Reg Health – Am [Internet]. 2021. Dec 1 [cited 2022 Feb 3];4. Available from: https://www.thelancet.com/journals/lanam/article/PIIS2667-193X(21)00057-0/fulltext [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Nemani K, Li C, Olfson M, Blessing EM, Razavian N, Chen J, Petkova E, Goff DC. Association of Psychiatric Disorders With Mortality Among Patients With COVID-19. JAMA Psychiatry [Internet]. 2021. Apr 1 [cited 2022 Feb 9];78(4):380–6. Available from: 10.1001/jamapsychiatry.2020.4442 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Salehinejad MA, Azarkolah A, Ghanavati E, Nitsche MA. Circadian disturbances, sleep difficulties and the COVID-19 pandemic. Sleep Med [Internet]. 2021. Jul 14 [cited 2021 Nov 12]; Available from: https://www.sciencedirect.com/science/article/pii/S1389945721003932 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Fernández‐Aranda F, Casas M, Claes L, Bryan DC, Favaro A, Granero R, Gudiol C, Jiménez-Murcia S, Karwautz A, Le Grange D, Menchón JM, Tchanturia K, Treasure J. COVID-19 and implications for eating disorders. Eur Eat Disord Rev [Internet]. 2020. May [cited 2021 Nov 18];28(3):239–45. Available from: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC7267370/ [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Capes A, Bailly S, Hantson P, Gerard L, Laterre PF. COVID-19 infection associated with autoimmune hemolytic anemia. Ann Hematol [Internet]. 2020. Jul 1 [cited 2021 Nov 12];99(7):1679–80. Available from: 10.1007/s00277-020-04137-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Hindilerden F, Yonal-Hindilerden I, Akar E, Yesilbag Z, Kart-Yasar K. Severe Autoimmune Hemolytic Anemia in COVID-19 İnfection, Safely Treated with Steroids. Mediterr J Hematol Infect Dis [Internet]. 2020. Jul 1 [cited 2021 Nov 12];12(1):e2020053. Available from: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC7340241/ [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Rozenfeld M, Peleg K, Givon A, Bala M, Shaked G, Bahouth H, Bodas M. COVID-19 Changed the Injury Patterns of Hospitalized Patients. Prehospital Disaster Med [Internet]. [cited 2022 Feb 10];1–9. Available from: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC7985901/ [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Giudici R, Lancioni A, Gay H, Bassi G, Chiara O, Mare C, Latronico N, Pesenti A, Faccincani R, Cabrini L, Fumagalli R, Chieregato A, Briani L, Sammartano F, Sechi G, Zoli A, Pagliosa A, Foti G, Borotto E, Palo A, Valoti O, Botteri M, Carlucci M, Reitano E, Bini R. Impact of the COVID-19 outbreak on severe trauma trends and healthcare system reassessment in Lombardia, Italy: an analysis from the regional trauma registry. World J Emerg Surg [Internet]. 2021. Jul 19 [cited 2022 Feb 10];16(1):39. Available from: 10.1186/s13017-021-00383-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Berg GM, Wyse RJ, Morse JL, Chipko J, Garland JM, Slivinski A, Lieser M, Biswas S, Carrick MM, Rhodes H, Acuna D, Watts DD, Wilson NY, Shen Y, Deas S, Fakhry SM. Decreased adult trauma admission volumes and changing injury patterns during the COVID-19 pandemic at 85 trauma centers in a multistate healthcare system. Trauma Surg Acute Care Open [Internet]. 2021. Feb 1 [cited 2022 Feb 10];6(1):e000642. Available from: https://tsaco.bmj.com/content/6/1/e000642 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Ghafil C, Matsushima K, Ding L, Henry R, Inaba K. Trends in Trauma Admissions During the COVID-19 Pandemic in Los Angeles County, California. JAMA Netw Open [Internet]. 2021. Feb 22 [cited 2022 Feb 10];4(2):e211320. Available from: 10.1001/jamanetworkopen.2021.1320 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Kaufman HW, Chen Z, Niles J, Fesko Y. Changes in the Number of US Patients With Newly Identified Cancer Before and During the Coronavirus Disease 2019 (COVID-19) Pandemic. JAMA Netw Open [Internet]. 2020. Aug 4 [cited 2021 Nov 18];3(8):e2017267. Available from: 10.1001/jamanetworkopen.2020.17267 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Corley DA, Sedki M, Ritzwoller DP, Greenlee RT, Neslund-Dudas C, Rendle KA, Honda SA, Schottinger JE, Udaltsova N, Vachani A, Kobrin S, Li CI, Haas JS. Cancer Screening During the Coronavirus Disease-2019 Pandemic: A Perspective From the National Cancer Institute’s PROSPR Consortium. Gastroenterology [Internet]. 2021. Mar 1 [cited 2021 Nov 18];160(4):999–1002. Available from: https://www.gastrojournal.org/article/S00165085(20)35317-8/fulltext [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Vincentiis LD, Carr RA, Mariani MP, Ferrara G. Cancer diagnostic rates during the 2020 ‘lockdown’, due to COVID-19 pandemic, compared with the 2018–2019: an audit study from cellular pathology. J Clin Pathol [Internet]. 2021. Mar 1 [cited 2021 Aug 24];74(3):187–9. Available from: https://jcp.bmj.com/content/74/3/187 [DOI] [PubMed] [Google Scholar]
- 43.Ferrara G, De Vincentiis L, Ambrosini-Spaltro A, Barbareschi M, Bertolini V, Contato E, Crivelli F, Feyles E, Mariani MP, Morelli L, Orvieto E, Pacella E, Venturino E, Saragoni L. Cancer Diagnostic Delay in Northern and Central Italy During the 2020 Lockdown Due to the Coronavirus Disease 2019 Pandemic: Assessment of the Magnitude of the Problem and Proposals for Corrective Actions. Am J Clin Pathol [Internet]. 2021. Jan 1 [cited 2021 Aug 24];155(1):64–8. Available from: 10.1093/ajcp/aqaa177 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Javanmard-Emamghissi H, Boyd-Carson H, Hollyman M, Doleman B, Adiamah A, Lund JN, Clifford R, Dickerson L, Richards S, Pearce L, Cornish J, Hare S, Lockwood S, Moug SJ, Tierney GM, Javanmard-Emamghissi H, Boyd-Carson H, Hollyman M, Doleman B, Adiamah A, Lund JN, Clifford R, Dickerson L, Richards S, Pearce L, Cornish J, Hare S, Lockwood S, Moug SJ, Tierney GM, Kulkarni N, Pereira I, Barlow S, Vanniasegaram S, Blencowe NS, Zucker BE, Tyer A, Hollyman M, Kosti A, Badenoch T, Wheatstone S, Jaffer M, Gerretsen H, Menon R, Sajid MS, Kennedy L, Malik A, Nada A, Ray K, Khan M, Varcada M, Froghi F, Khalil A, Kyprianou D, Tewari N, Sarma DR, Baig M, Sood S, Ng EYW, Ng V, Shortland T, Marangoni G, Khan S, Ahmad J, Brown S, Pannu A, Gemmill E, Boyd-Carson H, Herrod P, Shari SS, Mohammed MJS, Narbad V, Hanbali N, Kushairi A, Matthew MA, Downey C, Alamassi A, Wheatley T, Emslie K, Alcocer B, Lau S, Morgan R, Gala T, Ibrahim S, Stephanos M, Mithany R, Abdelkarim M, Venkatesan G, Aqsalan A, Taylor J, Fok M, Kattakayam A, Rajput K, Bevan K, Kim HK, Salih L, Sabaratnam R, Creanga M, Shafi A, Law J, Elniel M, Walmsley M, Ayyar S, Cornish J, Reeves N, Mowbray N, Mayo I, Chohda E, Mccaughran W, Beck E, Garikipati S, Lovett BE., Alkistawi F, Hadjitoffi C, Uddin A, Patel PK, Handa S, Parker J, Littlehales D, Belgaumkar AP., Oyewole B, Narayan P, Elahi Z, Gaukroger A, Dunne DFJ, Nita GE, Baron RD, Sochorova D, Szatmary P, Gahunia SA, Thomas AJ, Mann KS, Mcfall M, Farkas N, Siddig H, Camilleri-Brennan J, Rutherford D, Wilson M, Massie E, Mcgivern K, Mcguckin J, Mckee C, Marinos-Kouris S, Gammeri E, Patel N, Cillo G, Baldwin AJ, Magro T, Krishna K, Olivier J, Anyaugo N, Philip K, Pearce L, Al-Amin A, Thomas M, Anderson I, Clark R, Tierney G, Javanmard-Emamghissi H, Hope C, Gowda A, Photiou D, Malcolm F, Daliya P, Chia Z, Husain N, Thomas P, Urbonas T, Centea D, Moug S, Brown C, Mcguigan MC, Bisset C, Ingham A, Galbraith N, Clifford R, Dickerson L, Lockwood S, Johnston J, Shrestha AK., Pangen A, Balakumar C, Iqbal S, Prakash S, Rait J, Hanu A, Guy R, Majeed T, Young R, Shamim S, Mesri M, Patel R, Lewis S, Eigbadon AT, Thakrar D, Karamitsou E, Oyeyipo Y, Nadeem U, Ndlovu S, Fnshawe A, Henderson N, Payne C, Porter D, Brooks A, Lee RXN, Jackson J, Morton AJ, Oyende OE, Worku D, Koh A, Kanani T, Blackwell J, Shaw M, Lewis-Lloyd C, Blackburn L, Adiamah A, Shaikh S, Ghazanfar M, Elhusseini M, Abdelhamid A, Eley J, Nassar A, Farinella E, Mahmood Z, Policastro T, McGregor RJ., Damaskos D, Drogouti M, Tuharska Z, Bennett JM, Antakia R, O’neill R, Hardwick R, Fearnhead N, Xanthis A, Georgiades F, Hudson V, Ashcroft J, Singh A, Osborne L, Ryska O, Weber B, Searight F, McCoss C, Bignell M, Patel R, Bond-Smith G, Lewis C, Williams G, Whewell H, Smith L, Ooi R, Powell-Chandler A, Tang AM., Richards S. K, Thompson DB, Van Dellen J, Alberto V, Shirazi S, Arang H, Rahman N, Monaghan E, Dodds K, Babalola O, Airhunmwunde P, Alam I, Wang K, Artemis F, Wijetunga I, Kidd T, Nambiar K, Ng CE, Collier T, Ibrahim B, Khan K, Sriskandarajah K, Pelly T, Vance-Daniel J, Rao PD, Bateman K, Gavrila A, Aslam MI, Amin V, Wilkins R, Zafar S, Konstantinou C, Mcdonald S, Baker A, Fardie A, Hill A, De Marchi J, O’Grady S, Faulkner G, Sekhar H, Martinez-Iglesias M, Alexander C, Lawrence E, Williams G, Bhasin S, The COVID: HAREM (Had Appendicitis REMCG. The management of adult appendicitis during the COVID-19 pandemic: an interim analysis of a UK cohort study. Tech Coloproctology [Internet]. 2021. Apr 1 [cited 2021 Aug 28];25(4):401–11. Available from: 10.1007/s10151-020-02297-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Kvasnovsky CL, Shi Y, Rich BS, Glick RD, Soffer SZ, Lipskar AM, Dolgin S, Bagrodia N, Hong A, Prince JM, James DE, Sathya C. Limiting hospital resources for acute appendicitis in children: Lessons learned from the U.S. epicenter of the COVID-19 pandemic. J Pediatr Surg [Internet]. 2021. May 1 [cited 2021 Aug 28];56(5):900–4. Available from: https://www.sciencedirect.com/science/article/pii/S0022346820304449 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The data that support the findings of this study are available from COVID-19 Research Database consortium, but restrictions apply to the availability of these data, which were used under license for the current study, and so are not publicly available. Data are however available from the authors upon reasonable request and with permission of COVID-19 Research Database consortium.