Abstract
The design of cancer immunotherapy drugs is essential for the continued investigation of novel drug regimens to improve responses and increase the survival of cancer patients. Methods to examine the interaction of effector immune cells with target cancer cells are limited by labor-intensive labeling that can be examined at specific time points. In this report, we examine an antigen-dependent model of effector cytotoxic (CD8+) T-cell-mediated cytotoxicity of target murine melanoma cells using a real-time cell impedance assay. The real-time monitoring allows measurement of viability and kinetics, allowing for a better understanding of effector/target cell interactions to support drug discovery.
Introduction
The clinical success of immune checkpoint inhibitors across many cancer types has reignited the interest in designing immune-based strategies for cancer treatment. Furthermore, while these cancer immunotherapies are promising, many patients fail to respond [1]. Therefore, it is important to understand the molecular rela-tionship between effector immune cells with target cancer cells to design translatable drug regimens to improve patient response. While immune checkpoint inhibitors can target proteins in distinct cell types, these drugs are primarily designed to halt inhibitory receptors on T cells or counter receptors in cancer cells that would result in inhibition of T-cell activation [2].
A variety of techniques exist to examine cytotoxic (CD8+) T cells and their ability to kill cancer cells for immunotherapy research. Among these, the chromium 51 (51Cr) release assay is considered a mainstay technique to monitor immune cell-mediated killing, as well as the use of lactate dehydrogenase (LDH) release as a surrogate for cancer cell cytotoxicity mediated by immune cells[3]. While these assays may be sensitive, they both are considered “endpoint assays” which limit examination of serial killing activity of effector cells interacting with tumor cells in vivo [3]. A useful technique involves the xCELLigence® Real-Time Cell Analysis (RTCA) instrument to acquire real-time measurements of T-cellmediated killing of cancer cells. Each well of the E-Plate® has gold biosensors that can record the growth and viability of adherent target cancer cells, which is reported as the cell index [3, 4]. Effector CD8+ T cells can be co-cultured with target cancer cells into the E-Plate®. Since effector CD8+ T cells are nonadherent, they do not interfere with the biosensors recording of the target cancer cells. Therefore, the cell index recorded by the biosensors is solely based on cancer cell viability and growth. If the effector CD8+ T cells have the ability to kill target cancer cells, the biosensors will detect and record the changes of cancer cell index in real time (Fig. 1). Aside from this T-cell-mediated killing of cancer cell assay, this instrument and technique have been used for immunotherapy research to examine the impact of other immune cells and their destruction of cancer cells [5, 6]. In this protocol, the CD8+ Tcells from Pmel-1 transgenic mice (B6.Cg-Thy1a/Cy Tg(TcraTcrb)8Rest) were co-cultured with target B16.F10 (B16) murine melanoma cells to examine T-cell-mediated cytolysis of melanoma cells [7, 8]. This method has been adapted from Ya Z, et al., where Pmel-1 CD8+ T cells were isolated and expanded from the spleen of C57Bl/6 Pmel-1 transgenic mice known to recognize gp100, a peptide fragment of the Pmel-1 antigen found on B16 cells [7]. This method could be modified to other antigen-specific effector target pairs, such as those based on ovalbumin to support immunomodulatory drug discovery.
Figure 1.
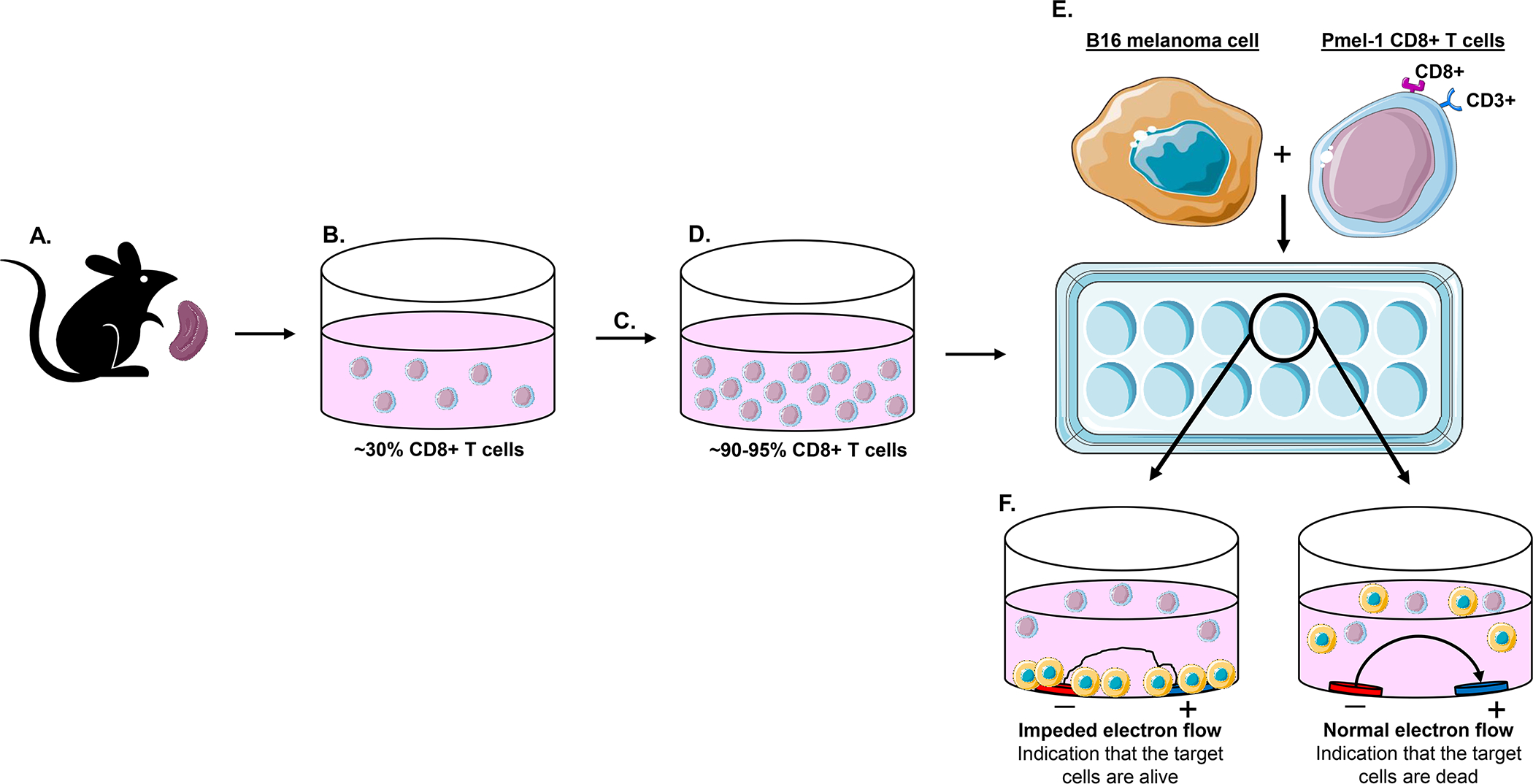
Experimental design for in vitro cell impedance assay in xCELLigence® Real-Time Cell Analysis (RTCA) instrument.
1. Materials
1.1. Growing and maintaining B16.F10 (B16) murine melanoma cells
B16 murine melanoma cells
Trypsin
1× PBS
B16 media consisting of RPMI 1640, 2mM L-glutamine, 10% FBS, 1% penicillin and streptomycin
2.2. Growing and maintaining Pmel-1 cytotoxic (CD8+) T cells
6–8 week old C57Bl/6 Pmel-1 transgenic mouse (B6.Cg-Thy1a/Cy Tg(TcraTcrb)8Rest/J, JAX stock #005023)
HBSS consisting of 1× PBS, 10 μM EDTA, 3% FBS
40μm cell strainer
Centrifuge
1× red blood cell (RBC) lysis buffer
T cell media consisting of RPMI 1640, 2 mM L-glutamine, 1% penicillin and streptomycin, 1 mM sodium pyruvate, 1% non-essential amino acids, 10 μM HEPES, 50 μM BME, 18% HyClone FBS
20–50 Units recombinant IL-2
1 μM gp100 peptide
2.3. T cell mediated killing of cancer cells with xCELLigence® Real-Time Cell Analysis (RTCA)
xCELLigence® RTCA instrument (Figure 2)
16 well E-Plate®
1μM gp100 peptide
20–50 Units recombinant IL-2
Figure 2.

xCELLigence® RTCA instrument (ACEA biosciences).
3. Methods
3.1. Growing and maintaining B16 murine melanoma cells
Culture and maintain adherent murine melanoma B16 cells with B16 media at 37°C with 5% CO2.
Once cells reach 60–80% confluence, cells are ready to split.
Aspirate media off cells and wash with 1× PBS.
Trypsinize cells with 1 mL trypsin to allow cells to detach from plate.
Resuspend cells in 4 mL B16 media.
Count cells and seed for experiment.
3.2. Growing and maintaining Pmel-1 CD8+ T cells
Euthanize 6–8 week old C57Bl/6 Pmel-1 transgenic mouse.
Harvest the spleen from mouse and place in 3 mL HBSS on ice (see Note 1, Figure 1A).
Acquire a single cell suspension by placing spleen through 40μm cell strainer with 7 mL HBSS (Figure 1B).
Centrifuge cells at 1000 RPM for 5 minutes at 4°C and remove supernatant.
Resuspend cells in 5 mL 1× RBC lysis buffer for 5 minutes on ice.
Place 45 mL of HBSS onto cells to dilute 1× RBC lysis buffer.
Centrifuge cells at 600g for 5 minutes at 4°C and remove supernatant.
Resuspend cells in T cell media at 1–2 million cells/mL.
Stimulate cells with 1 μM gp100 peptide and 20–50 Units of recombinant IL-2.
Let T cells differentiate and proliferate for 5–7 days at 37°C with 5% CO2 (see Note 2, Figure 1C–D).
3.3. T cell mediated killing of cancer cells with xCELLigence® Real-Time Cell Analysis (RTCA) instrument
Seed 10,000 target B16 cells in 200 μl B16 media per E-Plate® well (Figure 1E). Let cells adhere and incubate 24 hours at 37°C with 5% CO2 within xCELLigence® RTCA instrument.
Aspirate media off wells and co-culture 50,000 effector Pmel-1 CD8+ T cells in 200 μl T cell media with B16 cells into respective wells of E-Plate® (see Note 3, Figure 1E).
Activate CD8+ T cells with 1 μM gp100 peptide and 20–50 Units recombinant IL-2.
Incubate 24 hours at 37°C with 5% CO2 within xCELLigence® RTCA instrument (see Note 4).
3.4. Calculating target cell viability
Over 24 hours the xCELLigence® RTCA instrument will record B16 melanoma cell impedance measurements that are displayed as the cell index (Figure 1F).
To determine B16 cell viability, normalized the cell index to the time that the effector Pmel-1 CD8+ T cells were co-cultured with the target B16 melanoma cells.
This data can then be graphed to determine target B16 melanoma cell viability over and at 24 hours (see Note 5).
From the results displayed in Figure 3, Pmel-1 CD8+ T cells identified and killed target B16 melanoma cells as a decrease in target cell viability was observed over (Figure 3A) and at 24 hours (Figure 3B) compared to the control target B16 melanoma cells.
Figure 3. Pmel-1 CD8+ T cell mediated cytolysis of B16 murine melanoma cells with xCELLigence® RTCA instrument.
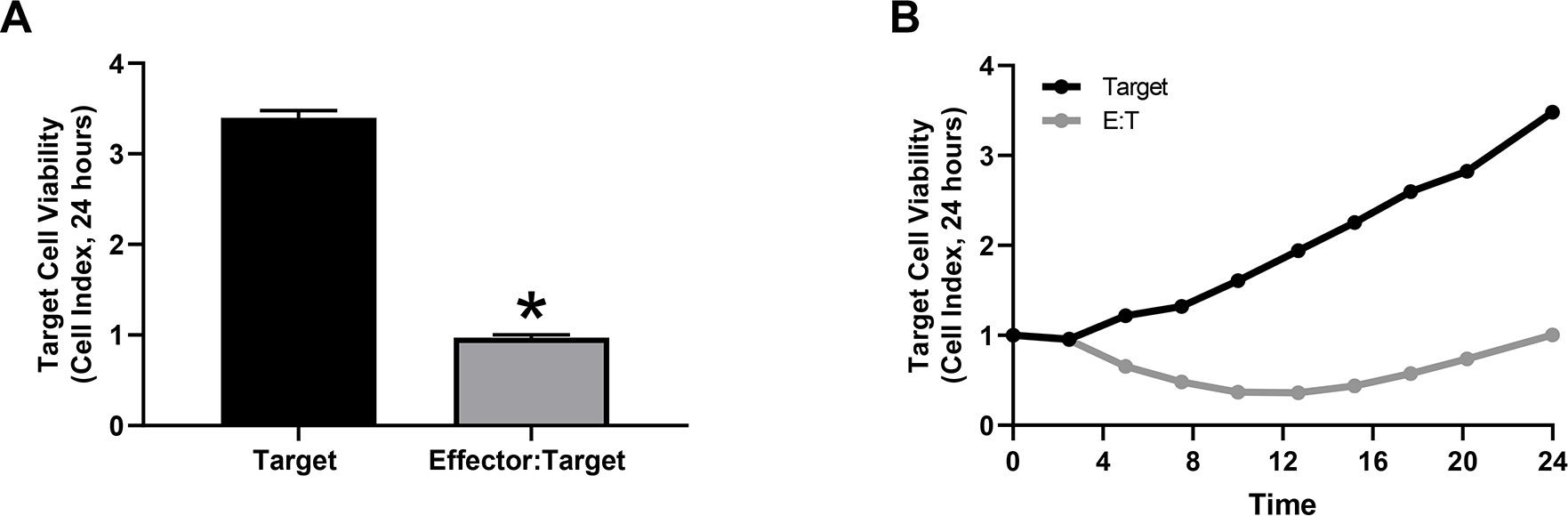
Pmel-1 CD8+ T cells were co-cultured with B16 murine melanoma cells to examine T cell mediated killing (A) over and (B) at 24 hours (*p< 0.05).
Acknowledgments
This work is supported by the NCI Cancer Center Support Grant award number P30CA012197, a V Foundation V Scholar in Cancer Research Award V2019–01(D.S.P) and a NIGMS NRSA T32 Fellowship T32GM127261 (E.R.S.).
Footnotes
5. Notes
The lymph nodes can also be harvested to derive and differentiate Pmel-1 CD8+ T cells.
Literature suggest ~95% of splenic cells of B6.Cg-Thy1a/Cy Tg(TcraTcrb)8Rest/J should be tumor reactive(9) this could be verified by flow cytometry.
The effector to target ratio should be tested depending on culture conditions a 5:1 ratio was used in Figure 3
The xCELLigence® RTCA instrument can run assays for greater or less than 24 hours.
Literature Cited
- 1.Havel JJ, Chowell D, Chan TA. The evolving landscape of biomarkers for checkpoint inhibitor immunotherapy. Nat Rev Cancer. 2019;19(3):133–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Sharma P, Allison JP. Dissecting the mechanisms of immune checkpoint therapy. Nat Rev Immunol. 2020;20(2):75–6. [DOI] [PubMed] [Google Scholar]
- 3.Cerignoli F, Abassi YA, Lamarche BJ, Guenther G, Santa Ana D, Guimet D, et al. In vitro immunotherapy potency assays using real-time cell analysis. PLoS One. 2018;13(3):e0193498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Xi B, Berahovich R, Zhou H, Xu S, Wei Y, Guan J, et al. A Real-time Potency Assay for Chimeric Antigen Receptor T Cells Targeting Solid and Hematological Cancer Cells. J Vis Exp. 2019(153). [DOI] [PubMed] [Google Scholar]
- 5.Soto-Pantoja DR, Terabe M, Ghosh A, Ridnour LA, DeGraff WG, Wink DA, et al. CD47 in the tumor microenvironment limits cooperation between antitumor T-cell immunity and radiotherapy. Cancer Res. 2014;74(23):6771–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Feliz-Mosquea YR, Christensen AA, Wilson AS, Westwood B, Varagic J, Melendez GC, et al. Combination of anthracyclines and anti-CD47 therapy inhibit invasive breast cancer growth while preventing cardiac toxicity by regulation of autophagy. Breast Cancer Res Treat. 2018;172(1):69–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ya Z, Hailemichael Y, Overwijk W, Restifo NP. Mouse model for pre-clinical study of human cancer immunotherapy. Curr Protoc Immunol. 2015;108:20 1 1–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hanada KI, Yu Z, Chappell GR, Park AS, Restifo NP. An effective mouse model for adoptive cancer immunotherapy targeting neoantigens. JCI Insight. 2019;4(10). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Overwijk WW, Tsung A, Irvine KR, Parkhurst MR, Goletz TJ, Tsung K, et al. gp100/pmel 17 is a murine tumor rejection antigen: induction of “self”-reactive, tumoricidal T cells using high-affinity, altered peptide ligand. J Exp Med. 1998;188(2):277–86. [DOI] [PMC free article] [PubMed] [Google Scholar]


