Abstract
Enrichment cultures obtained from soil exposed to benzene, toluene, and xylene (BTX) mineralized benzene and toluene but cometabolized only xylene isomers, forming polymeric residues. This observation prompted us to investigate the metabolism of 14C-labeled BTX hydrocarbons in soil, either individually or as mixtures. BTX-supplemented soil was incubated aerobically for up to 4 weeks in a sealed system that automatically replenished any O2 consumed. The decrease in solvent vapors and the production of 14CO2 were monitored. At the conclusion of each experiment, 14C distribution in solvent-extractable polymers, biomass, and humic material was determined, obtaining 14C mass balances of 85 to 98%. BTX compounds were extensively mineralized in soil, regardless of whether they were presented singly or in combinations. No evidence was obtained for the formation of solvent-extractable polymers from xylenes in soil, but 14C distribution in biomass (5 to 10%) and humus (12 to 32%) was unusual for all BTX compounds and especially for toluene and the xylenes. The results suggest that catechol intermediates of BTX degradation are preferentially polymerized into the soil humus and that the methyl substituents of the catechols derived from toluene and especially from xylenes enhance this incorporation. In contrast to inhibitory residues formed from xylene cometabolism in culture, the humus-incorporated xylene residues showed no significant toxicity in the Microtox assay.
Benzene, toluene, and xylene (BTX) isomers are major components of gasoline (22). They are also used extensively as solvents and feedstocks by the chemical industry (23). Compared to other hydrocarbons, BTX have relatively high water solubility; accidental gasoline spills and leaky service station tanks are prime sources of aquifer contamination (4, 28). Because of their water solubility and their acute toxicities and genotoxicities (10), BTX components are classified as priority pollutants by the U.S. Environmental Protection Agency (29, 31).
Under favorable conditions, all BTX components are biodegradable (14, 25), but when microorganisms were enriched from soil, using equal parts of benzene, toluene, and p-xylene, the resulting consortium mineralized benzene and toluene only. p-Xylene was cometabolized by this consortium to 3,6-dimethylcatechol that accumulated and polymerized (21). A similar observation was made by Chang et al. (9). Concerned that similar substrate interactions could also lead to an incomplete metabolism of xylene isomers in soil, we examined the metabolism of individual BTX components as well as BTX mixtures in the soil matrix. Specifically, we were testing the hypothesis that in the presence of benzene and toluene, xylene isomers would be metabolized in soil incompletely, yielding polymeric and/or soil-bound residues. To detect any polymers or soil-bound residues and to obtain a good mass balance, we applied all BTX components in 14C-labeled form. Since in culture (21) and in biofilters (20) the cometabolism of xylenes created inhibitory residues, Microtox toxicity assays were performed on soil exposed to p-xylene in the presence of benzene and toluene.
MATERIALS AND METHODS
Radiochemicals and chemicals.
[Ring-U-14C]benzene (19.34 mCi mmol−1) and [ring-U-14C]p-xylene (0.325 mCi mmol−1) were purchased from Sigma Chemical Co. (St. Louis, Mo.). The radiochemical purity of >95% was determined by the manufacturer using gas chromatography. [Ring-U-14C]toluene (10.9 mCi mmol−1) was purchased from Pathfinder Laboratories (St. Louis, Mo.). The purity as determined by the manufacturer using gas chromatography was >98%. [Ring-U-14C]o-xylene (1.4 μCi mmol−1; radiochemical purity of >98% as determined by gas chromatography) was synthesized in our laboratory from [ring-U-14C]o-phthalic acid (10.6 mCi mmol−1), which was purchased from Sigma Chemical Co. Synthesis was done by the procedures of Li et al. (17) and Benkeser et al. (8) for the reduction of aromatic carboxyl substituents to methyl. In brief, the carboxyl groups of o-phthalic acid were reduced by refluxing with trichlorosilane and tri-n-propylamine in acetonitrile and under a stream of dry N2. The product was diluted in anhydrous ether, precipitating the chlorosiloxanes, which were removed by filtration. The filtrate was concentrated by evaporating the solvents and subsequently treated with methanol and KOH. Finally, the o-xylene was extracted from the diluted aqueous mixture with n-pentane. o-Xylene was recovered from this solution by fractional distillation with a 36% overall yield. Unlabeled BTX solvents were of the highest available purity (99% or higher) and were purchased from J. T. Baker (Phillipsburg, N.J.).
Soil.
Nixon sandy loam (5) was freshly collected from a lawn area of the New Jersey Agricultural Experiment Station, New Brunswick. The soil had no known history of hydrocarbon exposure. The soil was sieved through a mesh with 2-mm-diameter holes, and its natural pH of 5.5 to 6.0 was raised to 7.0 by adding 10 mg of CaCO3 g of soil−1. Raising the soil pH was found to favor hydrocarbon degradation (11). The liming was performed at least 5 days prior to the experiment to avoid any interference with the measurement of microbial CO2 evolution.
Incubation.
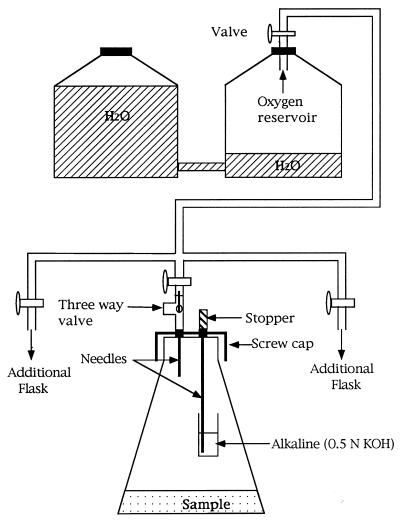
To obtain good mass balance with the highly volatile BTX solvents while maintaining aerobic incubation conditions, the experiments were conducted in the apparatus shown in Fig. 1. Soil samples, 50 g (dry weight), were incubated in one-liter Erlenmeyer flasks closed with Teflon-lined screw caps (Bellco Glass, Vineland, N.J.). The caps were modified by drilling two holes and sealing in, with epoxy resin, a short and a long 16-gauge syringe needle. The long needle was closed with a stopper, and attached to its lower end was a glass vial containing 10 ml of 0.5 N KOH. This KOH was periodically withdrawn by syringe and replaced. The withdrawn KOH was used for counting trapped 14CO2. The short needle was connected through a three-way valve (Popper & Sons, New Hyde Park, N.Y.) to an oxygen reservoir. By turning the valve to another position, the same port was used for sampling the flask headspace for solvent vapors, using an airtight syringe and gas chromatography. As soil respiration used up oxygen and the evolved CO2 was trapped, oxygen was automatically replaced from the reservoir.
FIG. 1.
Sealed aerobic incubation system for BTX compounds added to soil.
For comparison of single-substrate and multisubstrate utilization rates, the choice was to keep individual solvent concentrations or total solvent concentrations constant. Preliminary experiments (results not shown) indicated that doubling or tripling the 3-μl · g of soil−1 solvent addition level caused inhibitory effects. Consequently, the total solvent volume was kept constant. Whenever three solvents were added as mixtures, the concentration of each was 1 μl · g of soil−1. For easier comparisons, solvent depletion rates were recalculated as micromoles per gram of soil per day.
To minimize solvent loss during setup, the following process was followed. First, semidry soil was added to the flasks and the flasks were assembled, connected to the reservoir, and tested for leaks. BTX solvents, individually or in equal parts, but always in a total volume of 150 μl, were injected through the three-way valve and were distributed by quick shaking in the semidry soil. Thereafter, sufficient water was injected through the three-way valve to bring the soil moisture to 60% of its holding capacity. This water contained 0.3 mg of (NH4)2HPO4 g of soil−1 as inorganic fertilizer.
Abiotic soil (autoclaved and containing 0.5% [wt/wt] HgCl2) was included as a control. All flasks were incubated at 28°C with periodic monitoring of 14CO2 evolution and the decrease of solvent vapors in the headspace. Normally, incubation times were 3 to 4 weeks or until solvent vapor became undetectable in the headspace of the biologically active flasks.
Monitoring of solvent vapor depletion and 14CO2 evolution.
Periodically, a 100-μl sample of the flask headspace was withdrawn with a gas sampling syringe and injected into a Hewlett-Packard model 5890 A gas chromatograph with a fused silica column (inner diameter, 0.53 mm; length, 15 m) with a bonded 2.65-μm-thick polydimethylsiloxane stationary phase (Alltech, Deerfield, Ill.) and a flame ionization detector. Conditions were set as follows: injector temperature, 150°C; oven temperature, 50°C; detector temperature, 230°C; carrier (N2) flow, 8.57 ml min−1. Under these conditions, the retention times were 0.85 min for benzene, 1.79 min for toluene, 4.02 min for p-xylene, and 4.73 min for o-xylene. The peak areas were used for calculation of relative solvent depletion, assigning the maximal initial area as 100% of the added solvent.
Periodically, the KOH in the CO2 trap was withdrawn and replaced. One milliliter of the spent alkali was placed in a counting vial with 10 ml of Scintiverse BD scintillation fluid (Fisher Scientific, Springfield, N.J.), and the radioactivity was counted in a Beta Trac model 6895 instrument. As all four BTX compounds were 14C ring labeled, their percentages of 14CO2 evolved were directly comparable. Corrections for background and counting efficiency were made by the external standard ratio method.
Fractionation of the soil after incubation.
When gas chromatographic monitoring indicated that all BTX components were depleted, the experiments were terminated. The soil in each flask was divided into three equal portions based on wet weight. One portion was mixed with sufficient anhydrous Na2SO4 to take up all moisture and was Soxhlet extracted, using ethyl acetate as the solvent. The second portion was shaken for 7 h with 0.15 M Na4P2O7, and the solids were sedimented by centrifugation (27). Radioactivity in the supernatant was counted after wet combustion (1). This mild extraction procedure solubilized 28.6% of the humic compounds without solubilizing the soil biomass. Consequently, a correction factor of 3.5 was applied to the humus-associated radiocarbon extracted by 0.15 M Na4P2O7 from Nixon sandy loam (30). The third soil portion was divided again into two equal parts. One of these two parts was extracted immediately with 0.5 M K2SO4, and the other was first fumigated with chloroform and subsequently extracted with 0.5 M K2SO4 for selective extraction of biomass carbon (26). According to this reference, organic carbon in the postfumigation extract minus the amount of carbon in the prefumigation extract times three approximates the total biomass carbon in the soil. We have recalibrated this conversion factor specifically for Nixon sandy loam and found it to be 3.4 (30). We corrected the extracted biomass radiocarbon by this factor.
To verify the conversion factors and the overall recovery balance, occasionally the total radiocarbon bound in the soil was also determined by the wet combustion of 3.0-g soil samples immediately after opening the flasks (1). 14CO2 released during the combustion was trapped in Oxosol and counted.
Microtox toxicity assays.
These tests were conducted on extracts of soil samples incubated with benzene, toluene, and p-xylene mixtures and also on extracts of uncontaminated soil. Soil samples exposed to benzene, toluene, and p-xylene were extracted after 18 and 28 days of incubation. Aqueous extraction was conducted by the method of Matthews and Hastings (18). Ethyl acetate extracts, obtained as described above, were evaporated at room temperature under an air stream, and the residues were subsequently dissolved in dimethyl sulfoxide. Aqueous dilutions of the dimethyl sulfoxide solution and of the aqueous extracts were assayed as specified in the Microtox manual (3). Both 5- and 15-min 50% effective concentrations (EC50s) were measured and compared.
Replication and quality control.
Experiments were normally run in triplicate, and the standard deviations from the mean (1 standard deviation [SD]) are indicated by error bars. Abiotic controls were not replicated. Experiments with low mass balance due to leakage were rejected and repeated. Experiments on the efficiencies of biomass radiocarbon and humus radiocarbon extractions and on possible overlaps of these extractions were conducted and are described in a separate publication (30).
RESULTS
Depletion and mineralization kinetics of individual BTX compounds and their mixtures.
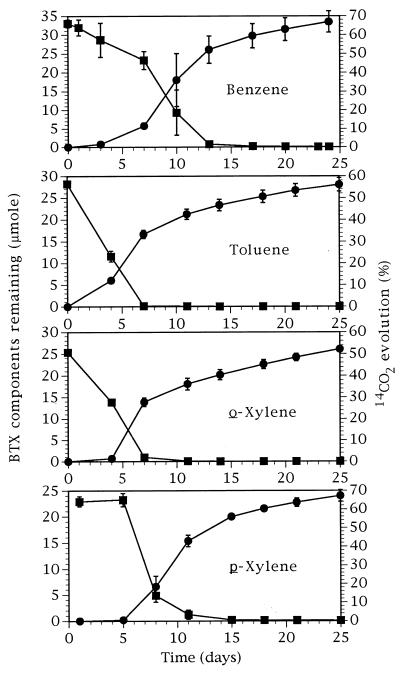
Toluene vapors became depleted in the soil samples without a discernible lag period, and depletion was near complete within the first week of the incubation (Fig. 2). Initial lag periods were apparent for benzene, o-xylene, and p-xylene depletion. Because additional evaporation compensated for its slow initial depletion rate, the vapor concentration of p-xylene failed to drop for the first 5 days. Because of the lag periods, vapor depletions for benzene, o-xylene, and p-xylene were near complete only by day 13 of the incubation. High proportions (52 to 68%) of BTX radiocarbon were mineralized, and the maximal rates of mineralization (6.1 to 8.0% g of soil−1 · day−1) coincided in time with the maximal rates of solvent depletion (Fig. 2). In abiotic controls, only negligible amounts of solvents were lost to the sampling process, and no measurable 14CO2 was evolved. Cumulatively in the course of an experiment, 2 to 3% of the radiolabeled solvents dissolved in the alkali trap without prior conversion to 14CO2 (data not shown). No corrections were made for these minor losses and interferences.
FIG. 2.
Substrate remaining in soil (■) and 14CO2 evolved from soil (•) treated with individual BTX compounds. Each compound was added at 3 μl · g of soil−1. Each error bar represents 1 SD of three samples.
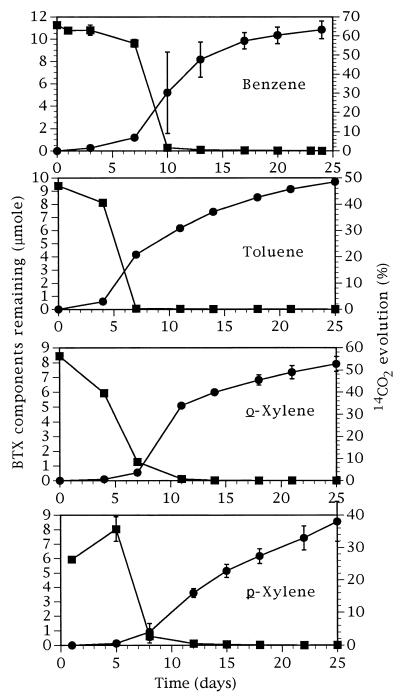
When applied as mixtures at the total concentration of 3 μl · g of soil−1 (1-μl · g−1 individual concentrations for three BTX compounds), the overall patterns of BTX vapor depletion (Fig. 3) were similar to the ones presented in Fig. 2. However, when added in a mixture, toluene depletion also showed an initial lag period, and the lag periods for the other three BTX compounds became more pronounced. Most significantly, while the maximal rates of benzene and toluene vapor depletion in mixtures continued to coincide in time with the maximal rates of their conversion to 14CO2, this was not the case for the xylene isomers (Fig. 3). For xylenes, a clear-cut time delay was evident between vapor depletion and 14CO2 evolution. The eventual conversion of solvent radiocarbon to 14CO2 remained high for benzene, toluene, and o-xylene, but it dropped to 38% for p-xylene.
FIG. 3.
Substrate remaining in soil (■) and 14CO2 evolved from soil (•) treated with BTX mixtures. Each component of a mixture was added at 1 μl · g of soil−1 (3 μl · g of soil−1 total). Only a single component of each BTX mixture (the one specified on the figure) was monitored. Benzene was measured in combination with toluene and p-xylene, toluene was measured in combination with benzene and o-xylene, o-xylene was measured in combination with benzene and toluene, and p-xylene was measured in combination with benzene and toluene. Each error bar represents 1 SD of three samples.
The maximal rates of BTX depletion and mineralization and the timing of these maximal rates are summarized in Table 1. When BTX components were applied to soil individually, benzene was depleted at the highest maximal rate, followed closely by o-xylene and toluene. p-Xylene was depleted at the lowest maximal rate. 14CO2 evolution followed a similar pattern. When applied as BTX mixtures, maximal vapor depletion rates dropped slightly. This was expected, since the individual components in the mixtures were at 1 μl · g of soil−1, while in the individual applications their concentration was 3 μl · g of soil−1. However, when the individual BTX component depletion rates are added up, the values are 5.5 μmol · g of soil−1 · day−1 for the benzene, toluene, and o-xylene mixture and 8.2 μmol · g of soil−1 · day−1 for the benzene, toluene, and p-xylene mixture. These rates were higher than any of the single BTX hydrocarbon depletion rates, suggesting that a greater diversity of microorganisms was involved in the depletion of mixtures than in the depletion of any single component. However, the increases in total rates compared to those of single-component rates were moderate, indicating that the majority of the BTX degraders utilized more than one of the BTX components at any one time. Notable also was the relatively high depletion and low mineralization rates of p-xylene in a mixture compared to those of p-xylene applied as a single compound. This indicates that at least some of the depletion was by a mechanism other than mineralization.
TABLE 1.
Maximal depletion rates of and maximal 14CO2 evolution rates from BTX compounds added to soil individually or in mixtures
| Addition(s)a | Maximal rate (days)b
|
|||||||
|---|---|---|---|---|---|---|---|---|
| Benzene
|
Toluene
|
o-Xylene
|
p-Xylene
|
|||||
| Depletion | 14CO2 evol. | Depletion | 14CO2 evol. | Depletion | 14CO2 evol. | Depletion | 14CO2 evol. | |
| Benzene | 4.7 (7–10) | 8.0 (7–10) | ||||||
| Toluene | 4.0 (0–7) | 7.0 (0–7) | ||||||
| o-Xylene | 4.3 (4–7) | 9.0 (4–7) | ||||||
| p-Xylene | 3.6 (5–11) | 6.1 (5–11) | ||||||
| B*Tp-X | 3.5 (7–10) | 7.8 (7–10) | 2.4 (7–10) | |||||
| BT*o-X | 2.2 (4–7) | 2.8 (4–7) | 6.0 (4–7) | 1.4 (4–7) | ND | |||
| BTo-X* | 1.9 (4–7) | 2.1 (4–7) | 1.6 (4–7) | 7.6 (7–11) | ||||
| BTp-X* | 3.3 (5–8) | 2.5 (5–8) | 2.5 (5–8) | 3.0 (8–12) | ||||
Individual compounds were added at 3 μl · g of soil−1; three-component mixtures were added at 1 μl · g of soil−1 each (3 μl · g−1 total). In mixtures, only the component followed by an asterisk was radiolabeled. Abbreviations: BTp-X, benzene, toluene, and p-xylene; BTo-X, benzene, toluene, and o-xylene.
Depletion rates expressed in micromoles per gram of soil per day and 14CO2 evolution (evol.) expressed in percent total radiolabel added. The numbers in parentheses give the time span (in days) when the specified maximal rate prevailed. ND, not determined.
Radiocarbon distribution of individual BTX compounds and their mixtures.
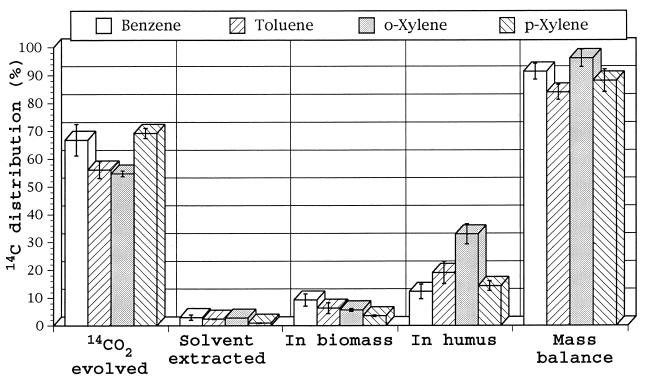
Radiocarbon distribution of individually applied BTX compounds is shown in Fig. 4. As in the previous experiments, conversions to 14CO2 were high (50 to 69%); incorporations into soil biomass were low (5 to 9%). Radiocarbon incorporation into soil humus was unusually high. Mineralization decreased in the order of benzene > toluene > o-xylene, with the opposite trend concerning humus incorporation. This trend correlated with the number of methyl substituents (none on benzene, one on toluene, and two on o-xylene). However, p-xylene did not follow the outlined trend. Mass balances were good (85 to 94%), considering the 4-week incubation periods and the highly volatile nature of the BTX compounds.
FIG. 4.
Radiocarbon distribution from uniformly 14C-ring-labeled benzene, toluene, o-xylene, and p-xylene incubated in soil for 4 weeks under aerobic conditions.
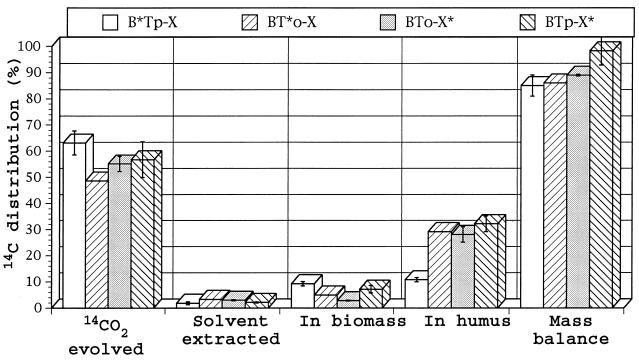
When added as three-component BTX mixtures containing only a single radiolabeled compound, radiocarbon distribution changed as shown in Fig. 5. The mineralization and humus incorporation of benzene changed little, but the mineralization of toluene and especially p-xylene decreased with corresponding increases in their humus incorporation. Mass balances were good, ranging from 85 to 100%. There was little change in biomass incorporation, and solvent-extractable polymers were very low in the incubations of both single compounds and their mixtures.
FIG. 5.
Radiocarbon distribution from uniformly 14C-ring-labeled benzene (B), toluene (T), o-xylene (o-X), and p-xylene (p-X) added to soil as BTX mixtures. Only the compound followed by an asterisk in each mixture was radiolabeled. The mixtures were incubated in soil under aerobic conditions for 4 weeks.
In Microtox tests, aqueous or ethyl acetate extracts of soils incubated with the benzene, toluene, and p-xylene mixture showed no higher toxicity than the extracts of control soil samples, which were not incubated with the benzene, toluene, and p-xylene mixture (data not shown).
DISCUSSION
Pathways and interactions in BTX biodegradation.
The two principal aerobic pathways leading to the mineralization of BTX hydrocarbons are a dioxygenase attack on the aromatic ring, referred to as the tod pathway, and a monooxygenase attack on the methyl substituents, referred to as the tol pathway (19). Benzene can be metabolized by the tod pathway only, but toluene and xylenes may be subject to oxidation by either the tod or tol pathway. It has been shown, however, that in the case of p-xylene, the tod pathway can lead to a dead-end product, 3,6-dimethylcatechol (13), and an analogous transformation was observed in the metabolism of p-xylene and o-xylene by Pseudomonas putida PPO1 (21, 30a). Although enrichments on individual BTX compounds produce cultures that can effectively mineralize all the compounds by either the tod or tol pathway, enrichments on mixtures of benzene, toluene, and p-xylene produced consortia and cultures that mineralized only benzene and toluene while cometabolizing p-xylene to inhibitory products (2, 9, 15, 21). The mineralization of BTX mixtures could be made complete by the genetic engineering of a P. putida strain that combined the tod and tol pathways (15, 16) or, in biofilters, by simply combining a tod strain and a tol strain of P. putida (20).
The great microbial diversity of natural soil appears to allow the simultaneous operation of the tol and tod pathways. The tol pathway results in extensive mineralization of the xylene isomers, a phenomenon not observed in enrichments and pure cultures obtained on BTX mixtures (20, 21). Although the presence of benzene in soil does not shut down the tol pathway, its induction of the tod pathway subjects some p-xylene and perhaps also o-xylene and toluene to dioxygenase attack. The resulting reactive monomethyl and dimethylcatechols are subject to spontaneous polymerization in culture solution (21), but in soil the same reactive intermediates are likely to be polymerized into humic compounds without prior incorporation into microbial biomass. This is a common fate of certain xenobiotic residues (7). The delay between the depletion of xylene vapors and the evolution of 14CO2 observed with BTX mixtures (Fig. 3) but absent when xylenes were applied alone (Fig. 2) hints that the substrate interactions so obvious in enrichments and pure cultures are also operational to some extent in soil. With reference to our hypothesis, the evidence supports the incomplete cometabolic transformation in soil of xylenes, when benzene is present. However, the simultaneous operation of the tol pathway assures that only a portion of the available xylenes is transformed in this manner.
BTX radiocarbon distribution in soil.
In radiocarbon distribution of individually applied BTX hydrocarbons (Fig. 4), the high conversion rates to 14CO2 and the small amounts of radiocarbon in biomass are striking. At least in part, this is a consequence of the long (4-week) incubation periods that were necessary for the complete depletion of all BTX vapors. During this period, some BTX radiocarbon originally incorporated into microbial biomass was mineralized and thus the remaining biomass radiocarbon does not represent a “yield” value. Nevertheless in soil, for aromatics such as benzoate, a high mineralization and a low biomass incorporation of radiocarbon are characteristic compared to glucose radiocarbon distribution (24, 30). The most likely reason for this phenomenon is the relatively low hydrogen content of aromatics compared to carbohydrates. The high humus incorporation of BTX radiocarbon reflects, in part, the turnover of microbial biomass during the long incubation periods. It is also likely to reflect some direct incorporation of catechol intermediates into soil humus. Methyl substituents were found to activate catechol for humus incorporation (6), possibly explaining the enhanced humification of toluene and of o-xylene over that of benzene. However, for unknown reasons, p-xylene failed to follow this expected pattern. Solvent-extractable compounds were low and appeared to represent a mixture of cell lipids and solvent-soluble humic components rather than a polymeric material. In cultures of P. putida PPO1 growing on benzene and toluene, up to 70% of o-xylene and p-xylene radiocarbon was converted to a water-soluble polymer with an acidic nature (30a). Clearly, this did not occur in soil.
The changes in radiocarbon distribution when BTX hydrocarbons were applied as parts of a mixture are of interest in relation to our hypothesis of direct xylene and perhaps toluene humification in soil (Fig. 5). Benzene radiocarbon distribution remained essentially unchanged. The mineralization of toluene and p-xylene decreased, and their humification increased substantially. There was little change in solvent-extractable or biomass-incorporated radiocarbon whether BTX compounds were applied to soil individually or as mixtures.
In chemostat competition studies using toluene as the sole substrate, Duetz et al. (12) observed that Pseudomonas strains with the tod pathway always outcompeted the tol pathway strains. We interpret our results to suggest that in BTX mixtures the presence of benzene and toluene causes the proliferation of hydrocarbon degraders with the tod pathway. Their activity increases the amount of p-xylene converted to 3,6-dimethylcatechol that polymerizes into soil humus instead of being mineralized and incorporated into biomass. Undoubtedly, hydrocarbon degraders with the tol pathway are also enriched, and they are responsible for the remaining mineralization and biomass incorporation of p-xylene. The humification of o-xylene is very extensive even if applied as the sole substrate and its humification did not increase in the presence of benzene and toluene. However, the presence of o-xylene appeared to enhance the humification of toluene radiocarbon. This could occur by some interaction of the more-reactive dimethylcatechol intermediates from o-xylene with the somewhat less-reactive monomethyl catechol intermediates from toluene.
Nature and toxicity of humified BTX residues.
The cometabolic transformations of p-xylene and o-xylene by P. putida PPO1 growing on benzene and toluene produced polymerized materials of substantial toxicity (21), and the main objective of this investigation was to ascertain whether similar transformations occur in soil. As discussed above, there is some evidence for xylenes being cometabolized in BTX mixtures applied to soil, but due to the great diversity of soil microorganisms, the bulk of the xylenes is nevertheless mineralized. The smaller cometabolized portion does not form extractable polymers but becomes part of soil humus. Considerable research has been devoted to soil-bound pesticide residues in terms of their chemical form, binding mechanism, and the possibility of their remobilization (7). Catechols and methylcatechols derived from BTX metabolism have no features such as halo- or nitro-substituents that would set them apart from natural humus precursors. This fact makes their tracking difficult and most likely also superfluous. For these reasons, we are satisfied that soil after complete metabolism of BTX mixtures is not more toxic in the Microtox assay than the same soil not exposed to BTX mixtures.
ACKNOWLEDGMENTS
This work was supported by the U.S. Department of Defense, Office of Naval Research and Advanced Research Projects Agency, University Research Initiative Program (grant N-0014-92-J-1888, R&T a40r41rnri), and state funds.
We are indebted to Tarek Mohamed for the radiochemical synthesis of [14C]o-xylene.
Footnotes
New Jersey Agricultural Experiment Station publication no. D-01512-02-98.
REFERENCES
- 1.Allison L E, Bollen W B, Moodie C D. Total carbon. In: Black C A, editor. Methods of soil analysis, part 2. Madison, Wis: American Society of Agronomy; 1965. pp. 1346–1366. [Google Scholar]
- 2.Alvarez P J J, Vogel T M. Substrate interactions of benzene, toluene, and para-xylene during microbial degradation by pure cultures and mixed culture aquifer slurries. Appl Environ Microbiol. 1991;57:2981–2985. doi: 10.1128/aem.57.10.2981-2985.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Azur Environmental Co. Microtox model 2055 system operational manual. Carlsbad, Calif: Azur Environmental Co.; 1992. [Google Scholar]
- 4.Barbee G C, Brown K W. Movement of xylene through unsaturated soils following simulated spills. Water Air Soil Pollut. 1986;29:321–331. [Google Scholar]
- 5.Bartha R, Bordeleau L. Cell-free peroxidases in soil. Soil Biol Biochem. 1969;1:139–143. [Google Scholar]
- 6.Bartha R, Hsu T S. Spectroscopic characterization of soil organic matter. ACS Symp Ser. 1976;29:258–271. [Google Scholar]
- 7.Bartha R, You I S, Saxena A. Humus-bound residues of phenylamide herbicides: their nature, persistence and monitoring. In: Miyamoto J, Kearney P C, editors. IUPAC pesticide chemistry. Oxford, England: Pergamon Press; 1983. pp. 345–350. [Google Scholar]
- 8.Benkeser R A, Foley K M, Gaul J M, Li G S. A new and convenient method for the reduction of an aromatic carboxyl to a methyl group. J Am Chem Soc. 1970;92:3232–3233. [Google Scholar]
- 9.Chang M K, Voice T C, Criddle C S. Kinetics of competitive inhibition and cometabolism in the biodegradation of benzene, toluene, and p-xylene by two Pseudomonas isolates. Biotechnol Bioeng. 1993;41:1057–1065. doi: 10.1002/bit.260411108. [DOI] [PubMed] [Google Scholar]
- 10.Dean B J. Recent findings on the genetic toxicity of benzene, toluene, xylenes, and phenol. Mutat Res. 1985;145:153–181. doi: 10.1016/0165-1110(85)90016-8. [DOI] [PubMed] [Google Scholar]
- 11.Dibble J T, Bartha R. Effect of environmental parameters on the biodegradation of oil sludge. Appl Environ Microbiol. 1979;37:729–739. doi: 10.1128/aem.37.4.729-739.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Duetz W A, De Jong C, Williams P A, Van Andel J G. Competition in a chemostat culture between Pseudomonas strains that use different pathways for the oxidation of toluene. Appl Environ Microbiol. 1994;60:2858–2863. doi: 10.1128/aem.60.8.2858-2863.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Gibson D T, Mahadevan V, Davey J F. Bacterial metabolism of para- and meta-xylene: oxidation of the aromatic ring. J Bacteriol. 1974;119:930–936. doi: 10.1128/jb.119.3.930-936.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Gibson D T, Subramanian V. Microbial degradation of aromatic hydrocarbons. In: Gibson D T, editor. Microbial degradation of organic compounds. New York, N.Y: Marcel Dekker; 1984. pp. 181–252. [Google Scholar]
- 15.Lee J Y, Roh J R, Kim H S. Metabolic engineering of Pseudomonas putida for the simultaneous biodegradation of benzene, toluene and p-xylene mixture. Biotechnol Bioeng. 1994;43:1146–1152. doi: 10.1002/bit.260431120. [DOI] [PubMed] [Google Scholar]
- 16.Lee J Y, Jung K H, Choi S H, Kim H S. Combination of the tod and the tol pathways in redesigning a metabolic route of Pseudomonas putida for the mineralization of a benzene, toluene, and p-xylene mixture. Appl Environ Microbiol. 1995;61:2211–2217. doi: 10.1128/aem.61.6.2211-2217.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Li G S, Ehler D F, Benkeser R A. Methyl groups by reduction of aromatic carboxylic acids with trichlorosilane-tri-n-propylamine: 2-methylbiphenyl. Org Synth. 1977;56:83–87. [Google Scholar]
- 18.Matthews E, Hastings L. Evaluation of toxicity procedure for screening treatability potential of waste in soil. Toxic Assess. 1987;2:265–281. [Google Scholar]
- 19.Mikesell M D, Kukor J J, Olsen R H. Metabolic diversity of aromatic hydrocarbon-degrading bacteria from a petroleum-contaminated aquifer. Biodegradation. 1993;4:249–259. doi: 10.1007/BF00695973. [DOI] [PubMed] [Google Scholar]
- 20.Oh Y S, Bartha R. Construction of a bacterial consortium for the biofiltration of benzene, toluene and xylene emissions. World J Microbiol Biotechnol. 1997;13:627–632. [Google Scholar]
- 21.Oh Y S, Shareefdeen Z, Baltzis B C, Bartha R. Interactions between benzene, toluene, and p-xylene (BTX) during their biodegradation. Biotechnol Bioeng. 1994;44:533–538. doi: 10.1002/bit.260440417. [DOI] [PubMed] [Google Scholar]
- 22.Potter T L. Fingerprinting petroleum products: unleaded gasolines. In: Kostecki P T, Calabrese E J, editors. Petroleum contaminated soils. Vol. 2. Chelsea, Mich: Lewis Publishers; 1992. pp. 83–92. [Google Scholar]
- 23.Reisch M S. Top 50 chemicals production stagnated last year. Chem Eng News. 1992;70:16–22. [Google Scholar]
- 24.Shen J K, Bartha R. Priming effect of substrate addition in soil-based biodegradation tests. Appl Environ Microbiol. 1996;62:1428–1430. doi: 10.1128/aem.62.4.1428-1430.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Smith R K. The biodegradation of aromatic hydrocarbons by bacteria. Biodegradation. 1990;1:191–206. doi: 10.1007/BF00058836. [DOI] [PubMed] [Google Scholar]
- 26.Sparling G P, West A W. A direct extraction method to estimate soil microbial C: calibration in situ using microbial respiration and 14C labeled cells. Soil Biol Biochem. 1988;20:337–343. [Google Scholar]
- 27.Stevenson F J. Extraction, fractionation, and general chemical composition of soil organic matter. In: Stevenson F J, editor. Humus chemistry—genesis, composition, reactions. New York, N.Y: John Wiley & Sons; 1994. pp. 24–58. [Google Scholar]
- 28.Stockman S. Proceedings of the conference on petroleum hydrocarbons and organic chemicals in ground water, prevention, detection and restoration. Houston, Tex: Association of Ground Water Scientists & Engineers; 1987. Estimates of concentrations of soluble petroleum hydrocarbons migrating into ground water from contaminated soil sources; pp. 541–558. [Google Scholar]
- 29.Thayer A M. Pollution reduction. Chem Eng News. 1992;70:22–52. [Google Scholar]
- 30.Tsao, C. W., and R. Bartha. Differential extraction of radiocarbon from soil biomass and humus. Soil Sci., in press.
- 30a.Tsao, C. W., and R. Bartha. Unpublished data.
- 31.U.S. Environmental Protection Agency. Test methods for evaluating solid waste. 3rd ed. I.A. SW-846. Washington, D.C: U.S. Environmental Protection Agency; 1986. [Google Scholar]