Background:
Apocrine sweat gland excision is a successful surgical treatment for bromhidrosis used in clinical practice due to its efficacy and unobtrusive postoperative scar. However, a small quantity of apocrine sweat gland residue is an unavoidable intraoperative concern to minimize losses of the dermal vascular network induced by extensive excision of the apocrine sweat glands. However, the relationship between the degree of remaining glands and clinical efficacy is yet unknown. This study looked at the histopathology of preexcision and postexcision specimens from bromhidrosis patients to see a connection between residual apocrine sweat glands and clinical efficacy following apocrine sweat gland excision.
Methods:
Twenty-one patients with bromhidrosis were recruited from April 2018 to December 2020. In this study, a description self-controlled case series was applied, with the patient preoperative sample as the control. The entire axillary skin was excised before and immediately after apocrine sweat gland excision, and skin tissue hemotoxylin-and-eosin staining was conducted to assess and compare the remnant apocrine sweat glands. Furthermore, preoperative and 6-month postoperative NRS-11 odor scores were analyzed, as well as patient satisfaction after surgery.
Results:
All patients had variable degrees of apocrine sweat gland excision residue, but they all passed clinical cure criteria and presented a high patient satisfaction rate.
Conclusions:
Apocrine sweat gland excision with a small quantity of apocrine sweat gland remnant can nevertheless result in a favorable clinical outcome and high patient satisfaction of bromhidrosis.
Takeaways
Question: Can apocrine sweat gland residue post apocrine sweat excision achieve satisfactory clinical efficacy?
Findings: A self-control case series study was performed on 21 bromhidrosis patients, by evaluating the apocrine gland portion before and just after the apocrine gland excision. Pathological evaluation demonstrated that a limited portion of apocrine sweat gland residue still achieves a good clinical outcome and high patient satisfaction.
Meaning: A limited portion of apocrine sweat gland residue post apocrine gland excision can still achieve satisfactory clinical efficacy.
INTRODUCTION
Bromhidrosis is a common dermatological issue that manifests as a unique unpleasant odor from the armpits. It has an incidence of around 2.8%, with adolescent patients being the most affected.1 This clinical issue has a negative impact on social and psychological activities, particularly in the young population. Bromhidrosis is caused by an abnormal increase in the size of the axillary apocrine sweat glands, and by the release of apocrine by the epithelial cells within the glands, which produces an unpleasant odor.2 Microbiological investigations have revealed that the breakdown of apocrine generated by particular bacteria from apocrine sweat glands is an essential pathogenic feature.3 The most often used therapies are nonsurgical and surgical techniques in clinical practice. Antiperspirants, iontophoresis, laser treatment, microwave therapy, and subcutaneous botulinum toxin type A injections are the mainstays of the nonsurgical strategy.4–7 Surgical procedures, such as fat aspiration, apocrine sweat gland scraping, suction curettage, and small endoscopic incisions, are primarily aimed at removing the apocrine sweat glands.1,3,8–13 Surgical therapy is generally successful and complete, with up to 90 percent efficiency14 and good clinical acceptability. However, these techniques bring up various complications (eg, gland scraping and suction curettage remove the apocrine sweat gland in an indirect vision), which induces extra injury on dermal blood vessel net and subcutaneous tissue,11,15 while small endoscopic incisions usually produce an unsatisfactory scar due to the repeated manipulation through the incision.16
Our department has been treating bromhidrosis with apocrine sweat gland excision since 2011, by removing the apocrine sweat gland precisely in a direct vision by turning over the axillary skin flap. This surgical technique significantly protected the cutaneous vascular network and attained an excellent clinical effect and high patient satisfaction. In comparison with surgical procedures such as scraping and suctioning, apocrine sweat gland excision gives specific benefits in terms of odor removal and aesthetic result. (See figure, Supplemental Digital Content 1, which displays the axillary area skin scar at 6 months postoperation. http://links.lww.com/PRSGO/C27.)
Nevertheless, during apocrine sweat gland excision, repeated trimming of the apocrine sweat glands with tissue scissors is performed, inducing a level of inevitable damage to the dermal vascular network and blood supply issues in various parts of the axillary region.17 Is it feasible to modestly preserve the apocrine sweat gland while assuring the treatment’s efficiency to prevent this complication? Few investigations have reported the impact of remaining apocrine sweat glands on clinical efficiency and patient satisfaction. We launched a self-controlled case series clinical study with bromhidrosis patients to elaborate on this problem.
MATERIALS AND METHODS
From April 2018 to December 2020, we recruited 21 bromhidrosis patients to investigate the effect of apocrine sweat gland residue. Also, we performed HE pathological examination of the skin tissue in the axillary area before and just after removing apocrine sweat glands during the operation.18 An investigation of clinical efficiency and patient satisfaction was carried out, as detailed below.7
1. Bromhidrosis diagnosis: Bromhidrosis is diagnosed when a patient exhibits visible sweating for more than 6 months and two of the following symptoms:
(1) Symmetrical perspiration on both sides; (2) daily activities harmed; (3) a positive family history; (4) onset before the age of 25; (5) at least one episode each week; (6) focal sweating that stops while sleeping.
2. Inclusion criteria: In this study, a descriptive self-controlled case series was used, with the patient preoperative sample serving as the control. Inclusion criteria were(1) bromhidrosis individuals between 18 and 35 years old who have not received laser or surgical therapy; (2) willingness to take part in the study; (3) agreement on follow-up visits.
3. Exclusion and elimination criteria: (1) patients who take medication prone to complications post operation, such as anticoagulant. (2) patients who did not finish the follow-up visit on time. (3) patients who did not finish both sides’ operations.
4. Adverse events: some adverse events of apocrine gland excision are observed postoperation, such as skin flap necrosis, subcutaneous hematoma, unsatisfactory skin scar, arm movement dysfunction.
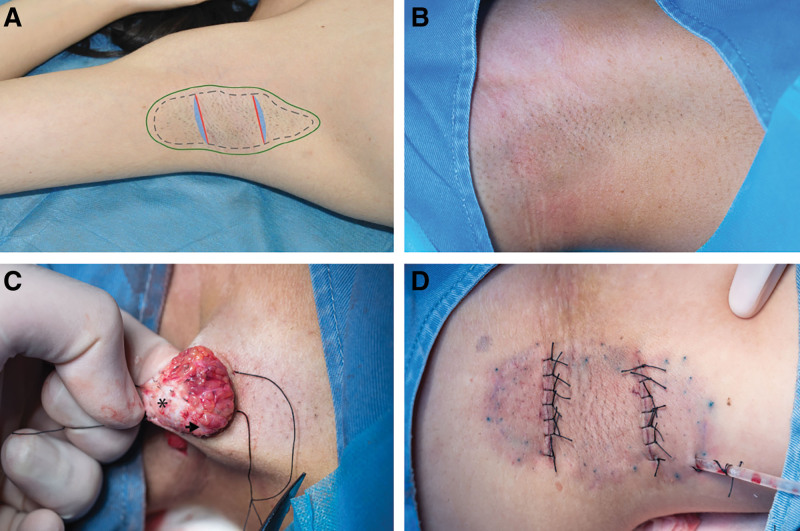
5. Surgical procedure: Preoperative skin preparation: supine posture with arms abducted about 150 degrees. Methylene blue was used to outline the axillary hair boundaries and the anticipated surgical incisions. A local swelling anesthetic (1% lidocaine with 1:100,000 epinephrine) was used during the operation. In the center of the axillary region, two parallel incisions were performed. The skin was appropriately incised, and the axillary area was dissected in the subcutaneous fat layer. As a preexcision control, a whole layer of skin (1.0 cm × 0.3 cm) was excised along the incision. The skin flap was then turned open, and the apocrine sweat glands were removed under direct observation (Fig. 1). As a postexcision sample, another full layer of skin (1.0 cm × 0.3 cm) was excised along the incision immediately after the apocrine sweat gland excision.
Fig. 1.
Diagram of apocrine sweat gland excision procedure. A, Incision design and pathological specimen excision site. Dotted area: axillary area. Solid area: region to be separated during the surgery. Red solid line: surgical incision. Blue area: region of specimen excision. B, Image of preoperative axillary area. C, Intraoperative image. Arrow: apocrine sweat gland. Star: region with apocrine sweat glands removed. D, Image immediately postoperation.
6. NRS-11 smell scoring: The NRS-11 numeric rating scale is a widely used index for self-report pain intensity that may also be used to assess odor. Bromhidrosis odor is graded from 0 to 10 based on intensity and whether it was noticed by a third party (eg, 0 for no odor noticeable by the patient and others, 1 for a slight odor noticeable by the patient but not by others, and 10 for a strong odor noticeable by the patient and others) and is evaluated by both the patient and the surgeon.
7. Pathological quantification evaluation of residual apocrine sweat glands:
The preexcision and postexcision skin specimens were fixed in 10% formalin, embedded in paraffin, cut into 4-μm slices, and stained with hematoxylin-eosin (HE). After staining, the slides were scanned using an Olympus VS110 scanning microscope (Olympus, Japan).
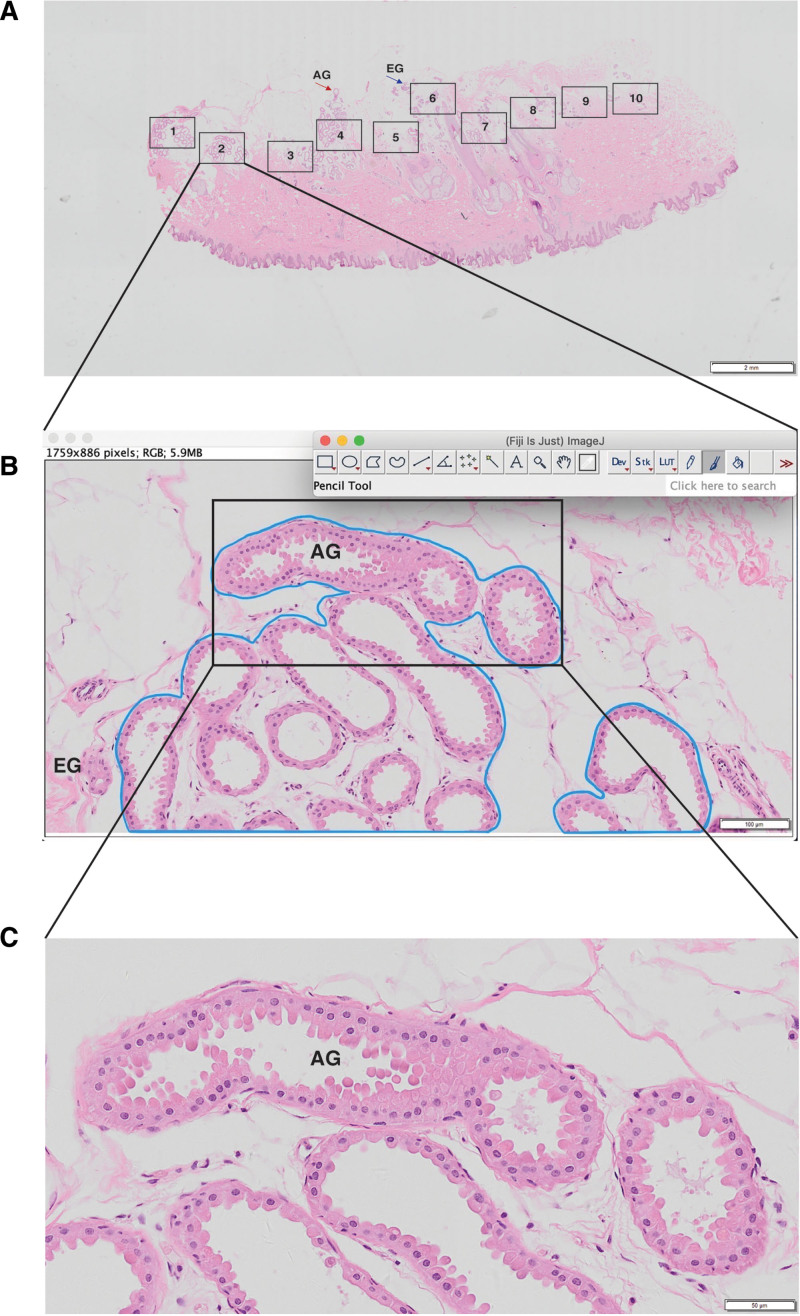
For the assessment, slices were examined and collected at various magnifications using Olyvia software, with ten high-magnification fields (100×) chosen at random in the subdermal sweat gland distribution area. [See figure, Supplemental Digital Content 2, which represents the Olyvia software capture (A: scale = 2 mm. B: scale = 200 μm. C: scale = 50 μm). http://links.lww.com/PRSGO/C28.] Two plastic surgeons independently examined the selected fields without knowing the specimen information. The density of apocrine sweat glands (D) was defined as the area occupied by apocrine sweat glands (G) divided by the entire area of the field of view (A) (Fig. 2), and the mean value was obtained for all findings in the same specimen. Based on this, the ratio of postexcision remaining apocrine sweat glands versus intraoperative excised apocrine sweat glands was estimated, as well as the density of intraoperative excised apocrine sweat glands. Based on this, the ratio of postexcision residual apocrine sweat glands versus intraoperative excised apocrine sweat glands was calculated as follows: intraoperative excised apocrine sweat gland density (ED) = preexcision apocrine sweat gland density (PRED) − postexcision apocrine sweat gland density (POSTD).
Fig. 2.
Sweat gland morphology in bromhidrosis patients. A, In the sweat gland distribution area, 10 high-magnification fields were chosen at random from a representative set of HE staining photographs from the surgical area (2×, numbered 1–10). B, Blue area: apocrine sweat gland extension (×100). C, High-magnification picture of a sweat gland preexcision specimen (×200), demonstrating the glandular composition and cellular morphology of the apocrine sweat gland and the epithelial cells of the sweat gland in the parietal pulp-secreting condition. EG: eccrine sweat gland; AG : apocrine sweat gland (small sweat gland).
8. Patient satisfaction evaluation: Patient satisfaction was evaluated by a questionnaire separated into three levels: note 1—dissatisfied; note 2—moderately satisfied; and note 3—extremely satisfied.
9. Statistic: For statistical analysis, Prism 8 (GraphPad Prism version 8.4.0 for Mac, GraphPad Software Inc, San Diego, Calif.) was used. All data were presented as mean ± SEM and compared across groups using an unpaired t-test, and a P value less than 0.05 was deemed statistically significant.
RESULTS
General Information
Twenty-one individuals with bromhidrosis, aged 18–25 years, were chosen from April 2018 to December 2020, comprising 14 female and seven male patients. The same surgeon performed apocrine sweat gland excision on all patients. Preoperative and 6-month postoperative NRS-11 odor rating evaluations were completed, as well as patient satisfaction evaluations. Two patients were omitted from this research because of not completing postoperative follow-up visits. Another patient was excluded due to not finishing both sides of the operation. Among the eligible patients, one patient had mild subcutaneous hematoma post operation; other adverse events were not found.
Apocrine Sweat Gland Excision Operation Achieves Satisfactory Clinical Efficacy
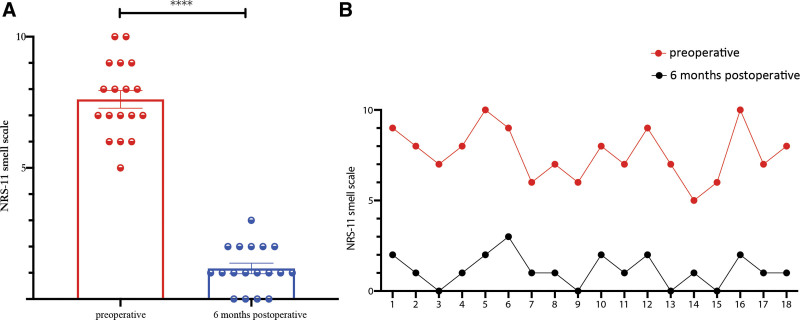
The NRS-11 smell scores revealed that all patients examined had a substantial drop in NRS-11 scores 6 months after surgery compared with presurgery (P < 0.0001). Furthermore, the NRS-11 smell score for each patient was significantly reduced (Fig. 3).
Fig. 3.
Evaluation of the NRS-11 smell score. A, Comparison of preoperative and 6 months postoperative NRS-11 smell scores. B, Comparison of each patient’s preoperative and six months postoperative NRS-11 smell scores. ****P < 0.0001.
Apocrine Sweat Gland Residue Post Operation Is Found in all Recruited Patients
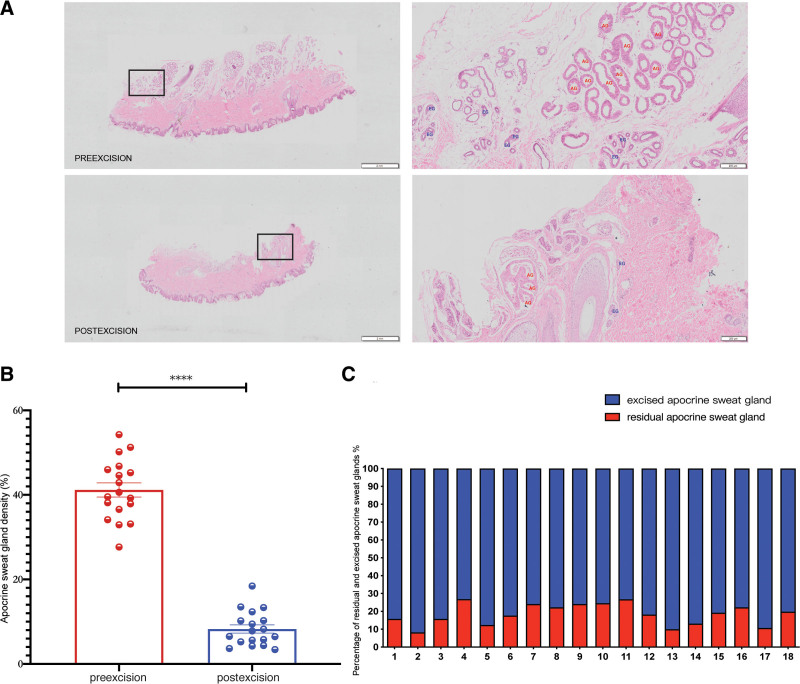
We performed a pathological analysis of HE-stained sections of the patient’s whole layer skin tissue in the operative area compared the apocrine sweat glands density preexcision and immediate postexcision (P < 0.0001). Furthermore, the pathological sections revealed a modest number of remaining apocrine sweat glands in the axillary area, with a mean value of 7.71% of residual apocrine sweat gland density. Among them, two patients had 12.35% (patient #4) and 13.34% (patient #11) remaining apocrine sweat glands, two patients had 10%, and the remaining had less than 10% postexcision apocrine sweat gland density (POSTD) (Fig. 4).
Fig. 4.
Analysis of residual apocrine sweat glands. A, Representative HE staining pictures before and after excision (left: 2×; right: 50×). B, Comparison of apocrine sweat gland density before and after excision. C, Ratio of residual sweat glands (POSTD) versus excised sweat glands (ED) in each patient. ****P < 0.0001.
Apocrine Sweat Gland Excision Operation Achieved a High Satisfaction with Partial Apocrine Sweat Gland Residue
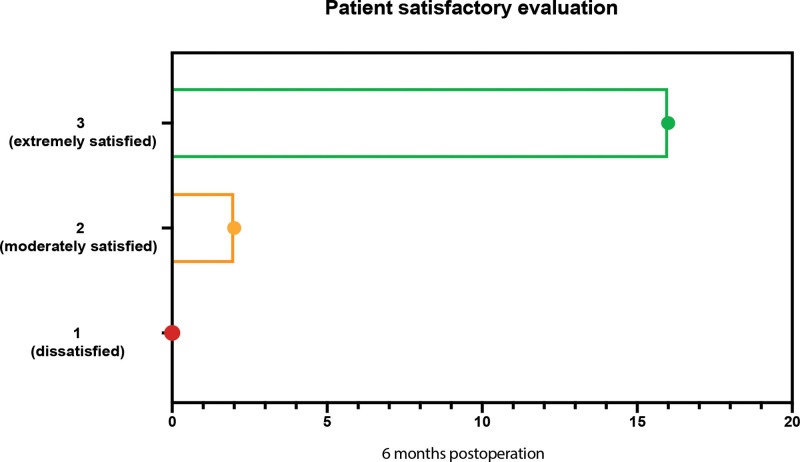
At the 6-month postoperative follow-up, most patients were satisfied with the surgical results (Fig. 5), with only two patients expressing moderate satisfaction. The POSTD in these two individuals was higher than that in other patients, as indicated in Figure 4 (Fig. 4C, patients #4 and #11). Consequently, we conclude there is a threshold of POSTD affecting the clinical effect after apocrine gland excision.
Fig. 5.
Patient satisfaction scores at 6 months after surgery (1: dissatisfied; 2: moderately satisfied; 3: extremely satisfied).
DISCUSSION
The unpleasant body odor of people with bromhidrosis has a significant impact on their lives and social activities. As a result, professionals and patients alike are becoming more aware of the significance of good clinical effectiveness and attractive appearance.
Because the surgical operation is carried out under direct vision, it is simple to establish the distribution of apocrine sweat glands and accomplish high precision manipulation. Therefore, surgical procedures present a long-term effect and a high clinical satisfactory rate compared with nonsurgical therapies. Regarding our surgical technique, removing apocrine sweat gland is much more precise by turning over the skin flap without extra tissue damage.11,15 Also, the paralleled double incision design better preserves the dermal vascular net than other incision shape manipulations.10 In summary, this procedure proposes a great combination of satisfactory clinical efficiency and cosmetic incision appearance.
However, owing to disruption to the dermal blood supply net, problems such as partial flap necrosis, delayed healing, or nonhealing of the incision margins are unavoidable. As a result, the excision of apocrine sweat glands becomes a significant issue in this procedure: excessive trimming may raise the risk of postoperative problems. Conservative trimming, on the other hand, may result in odor retention. We performed a pilot self-controlled case series study focusing on the association between the pathological evaluation of residual apocrine sweat glands and clinical effect. This study demonstrates that some remaining apocrine sweat glands may still be detected in the pathological assessment, even in patients with acceptable surgical outcomes. Although this study does not yet provide surgeons with a criterion for the extent of apocrine sweat gland excision trimming, one conclusion is clear: excessive intraoperative trimming of apocrine sweat gland excision glands does not improve surgical outcome; instead, it increases the likelihood of blood supply-related complications. In contrast, a small number of remnant apocrine sweat glands can still produce good clinical outcomes.
Despite the fact that bromhidrosis is a prevalent clinical illness, clinical therapies have been constantly improved. However, the molecular processes of bromhidrosis are not fully understood, particularly the role of apocrine sweat gland function. The most certain reason is an aberrant increase in the size of the apocrine sweat glands.1,19 The secretions of the apocrine sweat glands can be broken down by bacteria into ammonia and short-chain fatty acids, resulting in an unpleasant odor7. Furthermore, bromhidrosis has been linked to apocrine sweat gland malfunction, an aberrant microbiological environment in the axillary region, hormone receptor dysfunction, and hereditary inheritance.20 Research on hyperhidrosis and bromhidrosis has made some progress. A study by Bang et al3 confirmed that a small amount of apocrine sweat gland tissue is also present in the axillary skin of normal subjects, which has fewer and smaller apocrine sweat glands with a slightly atrophied shape compared with patients with bromhidrosis. Furthermore, glands in normal individuals are made up of flat epithelial cells grouped in a linear form, with no broken or constricted glandular structures visible. The apocrine sweat glands are more prominent in bromhidrosis patients, the epithelial cells of the apocrine sweat glands are grouped in a cuboidal or squamous linear form, and their secretion is more vigorous. Even in cases with acceptable clinical efficacy, some apocrine sweat gland tissue remained in the clipped axillary skin in our investigation. Thus, it is crucial to investigate the relationship between apocrine sweat gland subtypes and the existence of bromhidrosis odor. The molecular processes by which the remnant apocrine sweat glands do not contribute to the poor clinical result, on the other hand, remain unclear. One possible reason is that specific apocrine sweat gland subtypes may not generate clinical bromhidrosis because they are less secretory and may only be present at a superficial level. Of course, the secretory capacity of the apocrine sweat glands at different stages, the proportion of apocrine sweat glands creating body odor, and the secretory properties of the apocrine sweat glands in patients of all ages and genders are all issues that should be investigated further.
However, due to the limited number of recruited patients, this pilot study only represents the pathological observations of a small number of patient specimens. The next round of patient recruitment is already underway, and the issues mentioned above have been planned as new observation aims in a larger sample study.
CONCLUSIONS
Our findings show that apocrine sweat gland excision with a modest amount of remnant can nevertheless achieve good clinical results. This discovery might help clinical practitioners better utilize this approach and anticipate clinical outcomes.
Supplementary Material
Footnotes
Published online 11 May 2022.
Disclosure: The authors have no financial interest to declare in relation to the content of this article. This study is funded by National Natural Science Foundation of China–2021 Youth Foundation.
Related Digital Media are available in the full-text version of the article on www.PRSGlobalOpen.com.
REFERENCES
- 1.Murphrey MB, Safadi AO, Vaidya T. Histology, Apocrine Gland. Treasure Island, Fla.: StatPearls Publishing; 2021. [Google Scholar]
- 2.Semkova K, Gergovska M, Kazandjieva J, et al. Hyperhidrosis, bromhidrosis, and chromhidrosis: fold (intertriginous) dermatoses. Clin Dermatol. 2015;33:483–491. [DOI] [PubMed] [Google Scholar]
- 3.Bang YH, Kim JH, Paik SW, et al. Histopathology of apocrine bromhidrosis. Plast Reconstr Surg. 1996;98:288–292. [DOI] [PubMed] [Google Scholar]
- 4.Wu CJ, Chang CK, Wang CY, et al. Efficacy and safety of botulinum toxin A in axillary bromhidrosis and associated histological changes in sweat glands: a prospective randomized double-blind side-by-side comparison clinical study. Dermatol Surg. 2019;45:1605–1609. [DOI] [PubMed] [Google Scholar]
- 5.Wang T, Dong J, He J. Long-term safety and efficacy of botulinum toxin A treatment in adolescent patients with axillary bromhidrosis. Aesthetic Plast Surg. 2018;42:560–564. [DOI] [PubMed] [Google Scholar]
- 6.He J, Wang T, Dong J. Effectiveness of botulinum toxin A injection for the treatment of secondary axillary bromhidrosis. J Plast Reconstr Aesthet Surg. 2017;70:1641–1645. [DOI] [PubMed] [Google Scholar]
- 7.Jung SK, Jang HW, Kim HJ, et al. A prospective, long-term follow-up study of 1,444 nm Nd:YAG laser: a new modality for treating axillary bromhidrosis. Ann Dermatol. 2014;26:184–188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.He J, Wang T, Dong J. Excision of apocrine glands and axillary superficial fascia as a single entity for the treatment of axillary bromhidrosis. J Eur Acad Dermatol Venereol. 2012;26:704–709. [DOI] [PubMed] [Google Scholar]
- 9.He J, Wang T, Zhang Y, et al. Surgical treatment of axillary bromhidrosis by combining suction-curettage with subdermal undermining through a miniature incision. J Plast Reconstr Aesthet Surg. 2018;71:913–918. [DOI] [PubMed] [Google Scholar]
- 10.Malik AS, Porter CL, Feldman SR. Bromhidrosis treatment modalities: a literature review. J Am Acad Dermatol. 2021. [DOI] [PubMed] [Google Scholar]
- 11.Wang Y, Sun P, Leng X, et al. A new type of surgery for the treatment of bromhidrosis. Medicine (Baltimore). 2019;98:e15865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Chen W, Zhang X, Zhang L, et al. Treatment of axillary bromhidrosis in adolescents by combining electrocauterization with ultrasound-guided botulinum toxin type A injection. J Plast Reconstr Aesthet Surg. 2021;74:3114–3119.. [DOI] [PubMed] [Google Scholar]
- 13.Wang XW, Tang SJ, Xia Y, et al. The modified liposuction-curettage cannula for the treatment of secondary axillary bromhidrosis with subcutaneous scarring. Plast Reconstr Surg. 2015;135:1077e–1079e. [DOI] [PubMed] [Google Scholar]
- 14.Van TN, Manh TN, Minh PPT, et al. The effectiveness of local surgical technique in treatment of axillary bromhidrosis. Open Access Maced J Med Sci. 2019;7:187–191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Fang CL, Tsai CB, Chen MS, et al. Factors affecting the efficacy of suction curettage using an arthroscopic shaver for bromhidrosis. Dermatol Surg. 2021;47:245–249. [DOI] [PubMed] [Google Scholar]
- 16.Hu X, Zhang Y, Miao X, et al. Endoscopic surgical treatment of bromhidrosis: a report of 18 consecutive patients from 2010 to 2013. J Laparoendosc Adv Surg Tech A. 2014;24:323–327. [DOI] [PubMed] [Google Scholar]
- 17.Hsu KC, Wang KY. Sparing subcutaneous septa avoids skin necrosis in the treatment of axillary bromhidrosis with suction-curettage shaving. J Cosmet Dermatol. 2019;18:892–896. [DOI] [PubMed] [Google Scholar]
- 18.Kaminaka C, Mikita N, Inaba Y, et al. Clinical and histological evaluation of a single high energy microwave treatment for primary axillary hyperhidrosis in Asians: a prospective, randomized, controlled, split-area comparative trial. Lasers Surg Med. 2019;51:592–599. [DOI] [PubMed] [Google Scholar]
- 19.Lindsay SL, Holmes S, Corbett AD, et al. Innervation and receptor profiles of the human apocrine (epitrichial) sweat gland: routes for intervention in bromhidrosis. Br J Dermatol. 2008;159:653–660. [DOI] [PubMed] [Google Scholar]
- 20.Mao GY, Yang SL, Zheng JH. Etiology and management of axillary bromidrosis: a brief review. Int J Dermatol. 2008;47:1063–1068. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.