Abstract
Purpose:
Assess differences in outcome in an early and later time period in patients with hostile neck anatomy undergoing EVAR.
Materials and Methods:
Single center IRB approved retrospective study assessing patients who underwent EVAR between 2004 and 2013, dividing the two time periods, 2004–2008 and 2009–2013. 125 patients had at least 1 hostile neck parameter meeting inclusion criteria. 61 out of 216 (28%) patients in the early period, and 64 out of 144 (44%) patients in the late period. The patients in the late period were younger compared to the early group, (late period 74.5 ± 8.8 vs. early period 77.5 ± 7.5, p=0.046). There were no significant differences in hostile neck anatomic factors between early and late periods.
Results:
There was no statistical difference in periprocedural factors or outcome measures with the exception of AAA sac regression in the late period compared with the early period (late period 73.5 vs. early period 55.7%, p = 0.038). There was a statistically significant increase in type 1a endoleaks in patients with suprarenal fixation as compared to those with infrarenal fixation in the late group (suprarenal 27.0% vs infrarenal 7.9%, p =0.025) and in the overall time studied (suprarenal 20.3% vs. infrarenal 7.6%, p=0.045).
With the exception of AAA sac regression, there is no change in periprocedural factors and outcome measures over time in patients with hostile neck undergoing EVAR.
Introduction
The quality of the proximal aortic neck is probably the single most important factor in determining outcomes in EVAR. It is directly related to seal and fixation of an endograft, and therefore directly impacts both Type 1a endoleaks and graft migration.1
Instructions for use (IFUs) are based on both clinical and benchtop research, with the goal being to optimize outcomes of EVAR.2 However, over the years, boundaries set by these IFUs have been pushed to include more patients, and more challenging neck anatomy, with variable success.3–5 Today, up to 58% of EVAR are performed outside of the IFU6. In the literature, hostile neck parameters are defined by neck length ≤10 mm, focal bulge in the neck > 3 mm, > 2 mm reverse taper within 1 cm below the renal arteries, neck thrombus or calcification ≥ 50% of the circumference, and angulation ≥ 60% within 3 cm below the renal arteries.7
In the literature, there are retrospective observational trials that have demonstrated that over time, outcomes have improved in EVAR as a whole.8,9 However, trials assessing outcome trends in patients with hostile neck are largely lacking. It is the purpose of this study to assess differences in outcome in the early and late time periods in patients with hostile neck anatomy, as a potential marker for possible improvement in technique, equipment, and/or experience of the operator in challenging circumstances.
Materials and Methods
Study population
The study was developed in accordance with the Declaration of Helsinki and was approved by the Institutional Review Board. All consecutive patients admitted to a single institution for elective EVAR between January 2004 and December 2013, documented in a database, were evaluated for inclusion. Patients were suitable for inclusion if they had one or more parameters of a hostile neck (See Table 1 for parameters – neck length ≤10 mm, focal bulge in the neck > 3 mm, > 2 mm reverse taper within 1 cm below the renal arteries, neck thrombus or calcification ≥ 50% of the circumference, and angulation ≥ 60% within 3 cm below the renal arteries). Included patients had at least 12 months follow up and preprocedural and post procedural imaging available in PACS. Hostile neck anatomy was determined by a double independent retrospective review of the preprocedural imaging. Procedures included those done with infrarenal fixation or suprarenal fixation technique. Patients treated with a “Chimney”/’snorkel” technique or fenestrated EVAR technique were not included in this study. Patients without at least one hostile neck parameter and with unavailable, incomplete, or missing case notes, or who underwent imaging and clinical follow up at another institution were excluded from the study.
Table 1.
Hostile Neck Parameters
| Hostile neck parameter |
|---|
| Neck length ≤10 mm |
| Focal bulge in the neck > 3 mm |
| > 2 mm reverse taper within 1 cm below the renal arteries |
| Neck thrombus or calcification ≥ 50% of the circumference |
| Angulation ≥ 60% below within 3 cm the renal arteries |
Procedure details
The choice of EVAR versus open AAA repair for each patient with hostile neck anatomy was individualized for each patient but was ultimately made at the discretion of the board certified Interventionalist. There was no single treatment decision algorithm, but the patient’s surgical candidacy and the Interventionalists comfort level influenced the decision. Intervention was considered when the maximum AAA diameter was at least 50 mm and/or there was increase in maximum diameter of at least 5 mm in 6 months. EVAR was performed either percutaneously or with a surgical arteriotomy as dictated by the patient’s anatomy. The device was deployed as per the manufacturer’s instructions. By 2004, aortic endografting had been performed at the institution for over 10 years. In the first time period from 2004–2008, there were 5 main operators with at least 4–10 years of experience and in the period 2009–2013, there were again 5 main operators with at least 9–15 years of experience. The study was divided two 2 equal time periods, the first from 2004–2008 and the second, 2009–2013. Devices utilized from 2004–2008 were Endurant (1), Gore Excluder (28), and Zenith (32) and from 2009–2013, Endurant (10), Gore (38), Zenith (12), Endologix (3), and Ovation (1). All procedures were technically successful without a death within 30 days.
Outcome measures
Procedural and periprocedural factors were assessed such as fluoroscopy time, adjunctive procedure, suprarenal versus infrarenal fixation, and length of hospital stay. Outcome measures were stated as Type 1a endoleak, AAA sac expansion, and AAA sac regression.
An intraprocedural type 1a endoleak was treated with angioplasty initially and proximal extension with an aortic cuff or use of a Palmaz Stent if angioplasty did not resolve the type 1a endoleak. CT angiographic follow up visits were scheduled at 1 month, 6 months, 12 months after EVAR, and yearly thereafter to monitor AAA sac behavior. Patients with intraprocedural Type 1a endoleak who did not respond to angioplasty only and patients developing a Type 1a endoleak on subsequent imaging are categorized as having a Type 1a endoleak. In addition, AAA sac regression was defined as decrease in AAA sac size >5 mm from the preoperative study or between studies. Similarly, AAA sac expansion was defined as increase in AAA sac > 5 mm from the preoperative study or between studies.
Patient characteristics
In total, 415 patients underwent EVAR from 2004–2013. Of those, 360 patients (216 early period and 144 in late period) who underwent EVAR between 2004 and 2013 had complete records. Out of those, 125 had at least 1 hostile neck parameter. 61 out of 216 patients (28%) undergoing EVAR had a hostile neck parameter in the early time period, and 64 out of 144 (44%) patients in the late period. Age ranged from 52 to 94 with a mean age of 75.9. 99 patients were male and 26 were female. Follow up ranged from 12 to 91 months, with a mean follow up period of 47.3 months. In total, 66 patients underwent infrarenal fixation while 59 underwent suprarenal fixation. Demographic factors were assessed between both groups. There was no difference in the representation of male gender between both groups. However, there was a significant difference in age between both groups with a younger group in the late period (74.5 ± 8.8), compared to the early period (77.5 ± 7.5), p-value of 0.046. (Table 2)
Table 2.
Demographics
| Characteristic | Total (n=125) | Early Period (n=61) | Late Period (n=64) | P-value |
|---|---|---|---|---|
|
| ||||
| Age (years) | 75.9 ± 8.3 | 77.5 ± 7.5 | 74.5 ± 8.8 | 0.046 |
| Male gender (n, %) | 99 (79.2%) | 52 (85.2%) | 47 (73.4%) | 0.104 |
There were no significant differences in anatomic factors between early and late periods (Table 3). Short neck anatomy had borderline significance between both groups with 32 in the late period and 20 in the early period with a p value of 0.051.
Table 3.
Anatomic Factors
| Characteristic | Total (n=125) | Early Period (n=61) | Late Period (n=64) | P-value |
|---|---|---|---|---|
|
| ||||
| Maximum diameter of AAA, cm | 5.9 ± 1.1 | 5.9 ± 1.3 | 5.8 ± 0.8 | 0.410 |
| Short-Proximal Neck, (n, %) | 52 (41.6 %) | 20 (32.8 %) | 32 (50.0 %) | 0.051 |
| Proximal Neck Bulge, (n, %) | 9 (7.2 %) | 5 (8.2 %) | 4 (6.3 %) | 0.740 |
| Reverse Tapered type, (n, %) | 1 (0.8 %) | 0 (0 %) | 1 (1.6 %) | 1.000 |
| Thrombus or calcification, (n, %) | 15 (12.0 %) | 8 (13.1 %) | 7 (10.9 %) | 0.708 |
| Significant Angulation, (n, %) | 56 (44. 8%) | 29 (47.5 %) | 27 (42.2 %) | 0.547 |
There were 7 patients in the early period with more than 1 hostile neck parameter (2 hostile neck parameters) and there were 5 patients in the late period with more than 1 hostile neck parameter (4 patient with 2 hostile neck parameters and 1 patient with 4 hostile neck parameters).
Statistical analysis
Comparison of factors and outcomes between groups was performed with Chi-squared test and unpaired t-test as appropriate. In addition, univariate and multivariate analysis was performed to assess predictors for type 1a endoleak. The level of significance was set to <0.05.
Results
There was no statistical difference in procedural and periprocedural factors between the early period and late period. Specifically, between periods, there was no significant difference in fluoroscopy time, adjunctive procedure, suprarenal versus infrarenal fixation, or length of hospital stay. (Table 4)
Table 4.
Procedural/Periprocedural factors
| Characteristic | Total (n=125) | Early Period (n=61) | Late Period (n=64) | P-value |
|---|---|---|---|---|
|
| ||||
| Fluoroscopy time, min | 32.9 ± 18.1 | 35.1 ± 18.8 | 30.9 ± 17.4 | 0.202 |
| Supra-renal fixation, (n, %) | 59 (47.2 %) | 25 (41.0 %) | 34 (53.1 %) | 0.174 |
| Adjunctive procedure, (n, %) | 25 (20.0 %) | 15 (24.6 %) | 10 (15.6 %) | 0.210 |
| Length of hospital stay, day | 3.3 ± 2.9 | 3.7 ± 3.4 | 3.0 ± 2.2 | 0.148 |
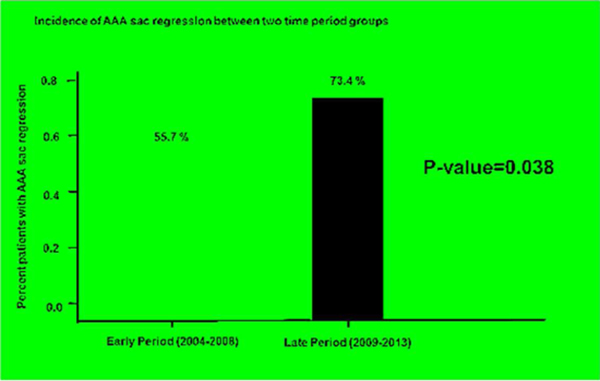
When comparing outcome measures, there was statistically greater percentage of patients with AAA sac regression in the late period (73.4%) compared with early period (55.7%), p = 0.038. There was no significant difference in other outcome measures however, including, percentage of patients with type 1a endoleak and patients with AAA sac expansion (See Table 5 and Figure 1).
Table 5.
Outcomes
| Characteristic | Total (n=125) | Early Period (n=61) | Late Period (n=64) | P-value |
|---|---|---|---|---|
|
| ||||
| Type 1a endoleak, (n, %) | 16 (13.6 %) | 10 (15.6 %) | 6 (9.8 %) | 0.337 |
| AAA sac regression, (n, %) | 81 (64. 8%) | 34 (55.7 %) | 47 (73.4 %) | 0.038 |
| AAA sac expansion, (n, %) | 28 (22.4 %) | 17 (27.9 %) | 11 (17.2 %) | 0.152 |
Figure 1.
Incidence of AAA sac regression between early and late time periods.
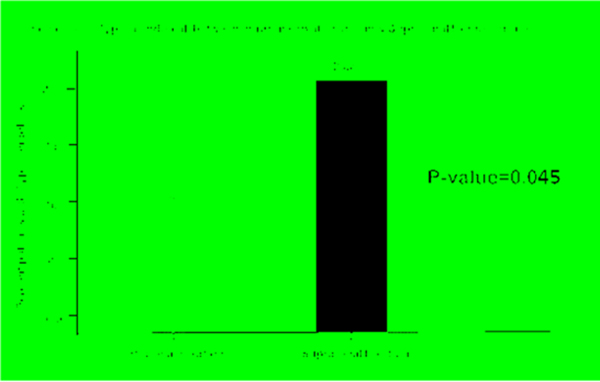
In the early group, there was a trend in an increase in type 1a endoleaks in patients with suprarenal fixation as compared to those with infrarenal fixation (suprarenal 15.2% vs infrarenal 3.6%, p =0.0529). This is compared to a statistically significant difference in type 1a endoleaks in late group in patients with suprarenal fixation as compared to those with infrarenal fixation (suprarenal 27.0% vs infrarenal 7.9%, p =0.0253). Overall, there was a significant increase in type 1a endoleaks in patients who underwent suprarenal fixation compared with those that underwent infrarenal fixation among the entire time period, (suprarenal 20.3% vs. infrarenal 7.6%, p=0.045). (See Table 6 and Figure 2). Overall, when comparing anatomic factors between infrarenal fixation and suprarenal fixation groups, there was only 1 significant anatomic factor. ≥50% thrombus/calcification was represented at a greater proportion in the infrarenal fixation group (18.2%) compared to the suprarenal fixation group (5.1%), p-value of 0.024. There was no difference in AAA sac diameter, short proximal neck, reverse taper, and significant angulation in either group (Table 7).
Table 6.
Outcomes between Infrarenal fixation and suprarenal fixation groups
| Characteristic | Total (n=125) | IF group (n=66) | SF group (n=59) | P - value |
|---|---|---|---|---|
|
| ||||
| Type 1a endoleak, (n, %) | 17 (13.6 %) | 5 (7.6 %) | 12 (20.3 %) | 0.045 |
| AAA sac regression, (n, %) | 81 (64. 8%) | 46 (69.7 %) | 35 (59.3 %) | 0.225 |
| AAA sac expansion, (n, %) | 28 (22.4 %) | 11 (16.7 %) | 17 (28.8 %) | 0.104 |
Figure 2.
Incidence of Type 1a endoleak between infrarenal and suprarenal fixation.
Table 7.
Anatomic factors between both groups
| Characteristic | Total (n=125) | IF group (n=66) | SF group (n=59) | P-value |
|---|---|---|---|---|
|
| ||||
| Maximum diameter of AAA, cm | 5.9 ± 1.1 | 6.0 ± 1.2 | 5.7 ± 0.8 | 0.202 |
| Short-Proximal Neck, (n, %) | 52 (41.6 %) | 23 (34.8 %) | 29 (49.2 %) | 0.105 |
| Proximal Neck Burge, (n, %) | 9 (7.2 %) | 7 (10.6 %) | 2 (3.4 %) | 0.119 |
| Reverse Tapered type, (n, %) | 1 (0.8 %) | 0 (0 %) | 1 (1.7 %) | 0.472 |
| Thrombus or calcification, (n, %) | 15 (12.0 %) | 12 (18.2 %) | 3 (5.1 %) | 0.024 |
| Significant Angulation, (n, %) | 56 (44. 8%) | 32 (48.5 %) | 24 (40.7 %) | 0.381 |
There was no significant difference in type 1a endoleak in patients with infrarenal fixation in the early period compared with infrarenal fixation in the late period (3.6% vs. 7.9%, p = 0.2203) or among suprarenal fixation in the early period compared with suprarenal fixation in the late period (15.2% vs. 27.0%, p=0.1358). In a Univariate analysis, the risk of type 1a endoleak was not different between time periods, but the risk of endoleak was higher in patients undergone suprarenal fixation than infrarenal fixation. In multivariable analysis controlling for time period, the risk of endoleak remained higher in suprarenal group (p=0.016) (See Table 8).
Table 8.
Associations of Type 1a endoleak with time period and fixation type.
| Univariate analysis | Multivariable analysis | |||||
|---|---|---|---|---|---|---|
| n | n of Endoleak | OR (95%CI) | p Value | OR (95%CI) | p Value | |
| Time Period | 0.500 | 0.329 | ||||
| 2004–2008 | 61 | 7 (11.5%) | 1 | 1 | ||
| 2009–2013 | 64 | 10 (15.6%) | 1.43 (0.51, 4.03) | 1.70 (0.58, 4.92) | ||
| Fixation Type | 0.045 | 0.035 | ||||
| Infrarenal | 66 | 5 (7.6%) | 1 | 1 | ||
| Suprarenal | 59 | 12 (20.3%) | 3.11 (1.03, 9.46) | 3.37 (1.09, 10.42) | ||
In this study, 1 patient in the early group and 3 patients in the late group required re-intervention due to neck anatomy. In the early group, a patient with Zenith stent graft developed a type 1a endoleak 33 months after the index procedure for which an endovascular repair was attempted. The patient ultimately required open repair to fix his type 1 endoleak. In the late group, 3 patients, all with Gore stent grafts, developed a type 1a endoleak with 2 of them at 1 month and one of them at 6 months. All 3 were successfully treated with endovascular techniques.
As can be presumed, patients with more hostile parameters had statistically more type 1 endoleaks. 2 of the 7 patients in the early period with more than 1 hostile neck parameter had a type 1a endoleak, and 3 of the 5 patients in the late period with more than 1 hostile neck parameter had a type 1a endoleak. Overall, there was a statistically significant increase in type 1a endoleak in patients with more than 1 hostile neck parameter, compared to those with only 1 hostile neck parameter (41.7% vs 10.6%, p=0.0029).
Discussion
The quality of the proximal aortic neck impacts EVAR outcome1, as was suggested in this study where there was an increase incidence of Type 1a endoleaks when a patient had more hostile neck parameters (more than 1 hostile neck parameter 41.7% vs only 1 hostile neck parameter 10.6%, p=0.0029). However, overtime, more patients outside the IFU, including those with hostile neck anatomy, are being treated with EVAR.6
In this study, a greater percentage of patients who underwent EVAR had a hostile neck in the late period (44%), compared with the early period (28%). This possibly indicates a growing experience and willingness to extend EVAR to more patients with hostile neck anatomy. However the experience did not translate to differences in fluoroscopy time, adjunctive procedure, or length of hospital stay. With regard to outcome measures, only sac regression emerged as a significant difference, perhaps due to better devices or operator technique, though this is difficult to ascertain in this retrospective trial. These findings are contrary to a study spanning the years 2000 to 2011, where the rates of in-hospital major morbidity, mortality, and procedural costs all decreased significantly over time (P < .0001), when adjusted for multiple demographics, comorbidities, and hospital-level factors.8 This study however obtained data from all patients undergoing EVAR and stressed staying within the IFU.
In addition, in this study, the patients treated were statistically younger in age in the late period compared with the early period. In a retrospective study including 721 patients spanning the dates of 1996 to 2008, the patients in the EVAR group were older than the open group (73.2 vs 70.6 years, P<0.01) in the earlier in the study, but that age-difference disappeared later on in the study.9 A similar trend is suggested in this study, where the treated population was statistically younger in the late treatment period compared to the early period. Justification for such practice is suggested in the study by Lee, K, et al. published in 2015 by Journal of Vascular Surgery where a substantial number of patients aged less than 60 years were included in the study. In this trial, EVAR offered, with decreased re-intervention rate (12% EVAR, 16% open repair; P = .80) and long-term survival (78% EVAR, 85% open repair) not significantly different than open repair.10 In the OVER trial comparing long term outcomes between EVAR and open AAA repair, survival was better with endovascular repair than with open repair among patients younger than 70 years of age (hazard ratio, 0.65; 95% CI, 0.43 to 0.98; P=0.04).11 Of note, these studies were not focused on patient with hostile neck anatomy.
Lastly, there was a trend towards an increased type 1a endoleak among patients with suprarenal fixation in the early period, and statistically increased type 1a endoleak with suprarenal fixation in both the late period and cumulatively overall. When assessing the cumulative data, the only anatomical difference between both groups was thrombus/calcification within the aneurysm neck of which the group with infrarenal fixation possessed the statistically higher number. Otherwise the groups were similar with respect to anatomical factors. The risk of type 1a endoleak was not different between time periods and controlling for time period, the risk of endoleak was higher in suprarenal group than in the infrarenal fixation group (p=0.016).
The idea of superior sealing and fixation with suprarenal fixation compared to infrarenal fixation has not been uniformly born out in the literature. In a retrospective study that examined 84 patients with short neck, there was no significant difference demonstrated in type 1a endoleak between patients who underwent suprarenal fixation as opposed to infrarenal fixation (suprarenal fixation, 8.3% vs infrarenal fixation, 4.2%, p=0.44).12 In a study by Oberhuber et al, where 103 patients underwent EVAR, patients undergoing EVAR with suprarenal fixation had a higher trend towards neck expansion, though not statistically significant. 7 of the 23 patients with a notable dilatation of the infrarenal neck required reintervention. All of them were stent-grafts with suprarenal fixation.13 As stated in the article by Rhee, et al, there has been no biomechanical or clinical studies to evaluate the metal structure across the suprarenal aorta, and in fact the device may disengage, at the very anatomic point conformation of the device to the aortic neck is needed.14
Many clinicians believe the answer lies in fenestrated EVAR (FEVAR) in patients with hostile neck, particularly short neck. However, in a study comparing infrarenal fixation to FEVAR, where 458 patients underwent FEVAR and 19,060 underwent EVAR patients, it was demonstrated that patients undergoing FEVAR had a statistically higher rate of overall complications (23.6% vs 14.3%; P < .001) and postoperative transfusions (15.3% vs 6.1%, P < .001).15 The quest for the ideal device and technique in patients with challenging neck anatomy is ongoing.
There are several limitations of this study. It is a single center, retrospective trial, with a relatively small study size. Some of the results, especially those with borderline results, may be due to under powering of the study. Moreover, selection bias is possible given the retrospective nature of the study. In addition, our study notably lacks patient factors such as cardiac, pulmonary, and renal co-morbidities, complications such as contrast allergy, groin complications, and infection, and long-term survival, as these were elements not collected in the database. Also, only suprarenal fixation and infrarenal fixation devices were explored in this study. Other techniques, such as using a fenestrated endograft or Snorkel or Chimney techniques may have elucidated additional findings. Moreover, because different numbers of endograft types were used at each time period it is impossible to draw general conclusions related to endograft type. Lastly, given it is an observational study, it is difficult to ascertain what caused any differences between early and late groups, and suprarenal and infrarenal fixation groups. A multicenter, prospective, large-size trial would be best to facilitate formulation of conclusions to affect practice.
In conclusion, at our institution, a larger percentage of patients with hostile neck are being treated with EVAR over time, however, with the exception of AAA sac regression, there is no difference in periprocedural factors and outcome measures between patients treated in an early time period compared with those in a later time period. Moreover, we found an increased rate of Type 1a endoleak with suprarenal fixation over infrarenal fixation, which was significant in the late time period and the overall time studied.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Contributor Information
Yolanda Bryce, Memorial Sloan Kettering Cancer Center, 300 E 66th Street, New York, NY 10065.
Wonho Kim, Miami Cardiac and Vascular Institute, 8900 North Kendall Drive, Miami, FL 33176.
Barry Katzen, Miami Cardiac and Vascular Institute, 8900 North Kendall Drive, Miami, FL 33176.
James F. Benenati, Miami Cardiac and Vascular Institute, 8900 North Kendall Drive, Miami, FL 33176.
Shaun Samuels, Miami Cardiac and Vascular Institute, 8900 North Kendall Drive, Miami, FL 33176.
References
- 1.Aburahma AF, Campbell JE, Mousa AY, et al. Clinical outcomes for hostile versus favorable aortic neck anatomy in endovascular aortic aneurysm repair using modular devices. J Vasc Surg. 2011;54:13–21. [DOI] [PubMed] [Google Scholar]
- 2.Walker J, Tucker L, Goodney P, Candell L, Hua H, Okuhn S, Hill B, Chang R. Adherence to endovascular aortic aneurysm repair device instructions for use guidelines has no impact on outcomes. J Vasc Surg. 2015. May; 61(5): 1151–1159 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Antoniou GA, Georgiadis GS, Antoniou SA, et al. A meta-analysis of outcomes of endovascular abdominal aortic aneurysm repair in patients with hostile and friendly neck anatomy. J Vasc Surg. 2013;57:527–538 [DOI] [PubMed] [Google Scholar]
- 4.Stather PW, Wild JB, Sayers RD, et al. Endovascular aortic aneurysm repair in patients with hostile neck anatomy. J Endovasc Ther. 2013;20:623–637 [DOI] [PubMed] [Google Scholar]
- 5.Stather PW, Sayers RD, Cheah A, et al. Outcomes of endovascular aneurysm repair in patients with hostile neck anatomy. Eur J Vasc Endovasc Surg. 2012;44:556–561 [DOI] [PubMed] [Google Scholar]
- 6.Schanzer A, Greenberg RK, Hevelone N, et al. Predictors of abdominal aortic aneurysm sac enlargement after endovascular repair. Circulation. 2011;123:2848–2855. [DOI] [PubMed] [Google Scholar]
- 7.Dillavou E, Muluk S, Rhee R, Tzeng E, Woody J, Gupta N, Makaroun M. Does hostile neck anatomy preclude successful endovascular aortic aneurysm repair. Journal of Vascular Surgery 2003. Oct; 38(4):657–663 [DOI] [PubMed] [Google Scholar]
- 8.Salzler G, Meltzer A, Mao J, Isaacs A, Connolly P, Schneider D, Sedrakyan A. Characterizing the evolution of perioperative outcomes and costs of endovascular abdominal aortic aneurysm repair. Journal of Vascular Surgery 2015. Nov;62(5):1134–9 [DOI] [PubMed] [Google Scholar]
- 9.Albuquerque F, Tonnessen B, Noll R, Cires G, Kim J, Sternberg III W. Paradigm shifts in the treatment of abdominal aortic aneurysm: Trends in 721 patients between 1996 and 2008. J of Vasc Surgery 2010. June; 51(6):1348–1353 [DOI] [PubMed] [Google Scholar]
- 10.Lee K, Tang T, Dubois L, Power A, DeRose G, Forbes T. Durability and survival are similar after elective endovascular and open repair of abdominal aortic aneurysms in younger patients. J of Vasc Surgery 2015. March; 61(3):636–641. [DOI] [PubMed] [Google Scholar]
- 11.Lederle F, Freischlag J, Kyriakides T, Matsumura J, Padberg F, Kohler T, Kougias P, Jean-Claude J, Cikrit D, Swanson K. Long-term comparison of endovascular and open repair of abdominal aortic aneurysm. New England Journal of Medicine 2012;367:1988–1997. [DOI] [PubMed] [Google Scholar]
- 12.Hager E, Cho J, Makaroun M, Park S, Chaer R, Marone L, Rhee R. Endografts with suprarenal fixation do not perform better than those with infrarenal fixation in treatment of patients with short straight proximal aortic neck. J of Vasc Surgery; 2012. May 55(5): 1242–1246. [DOI] [PubMed] [Google Scholar]
- 13.Oberhuber A, Schwarz A, Hoffmann M, Klass O, Schelzig H, Orend K, Mühling B. Influence of fixation mechanism on changes of the supra- and infrarenal segment of the aorta after endovascular treatment of infrarenal aortic aneurysm. Zentralbl Chir 2010. Oct; 135(5):433–7. [DOI] [PubMed] [Google Scholar]
- 14.Rhee R. Infrarenal fixation is all that is necessary. Endovascular Today 2012. March [Google Scholar]
- 15.Glebova N, Selvarajah S, Orion K, Black J III, Malas M, Perler B, Abularrage C. Fenestrated endovascular repair of abdominal aortic aneurysms is associated with increased morbidity but comparable mortality with infrarenal fixation endovascular aneurysm repair. J of Vasc Surgery 2015. March Vol 61(3): 604–610. [DOI] [PubMed] [Google Scholar]