Abstract
The environmental problems of synthetic plastics in food packaging have led researchers to synthesize biodegradable films. In this study, nanocomposite alginate‐based films containing TiO2 nanoparticles (1%) and cumin essential oil (CEO, 2%) were fabricated and the potential of these films to protect beef from chemical [pH, total volatile base nitrogen (TVBN), peroxide value, and thiobarbituric acid reactive substances (TBA)] and microbial [total viable count, Enterobacteriaceae, lactic acid bacteria, Listeria monocytogenes, and Pseudomonas spp.] spoilage was evaluated during 24 days of storage (4°C). The active films significantly induced the reduction in lipid oxidation, microbial growth, and TVBN values, improved the sensory attributes of treated samples, maintained the redness of meats for a longer time, and increased the shelf life of beef from 4 to 16 days. The results of this study showed that TiO2/CEO alginate‐based nanocomposite film has a great potential for application in meat and meat products.
Keywords: active packaging, cumin essential oil, meat quality, nanocomposite alginate‐based film, TiO2 nanoparticles
Cumin essential oil and TiO2 nanoparticles were incorporated into the alginate‐based film. The active packaging reduced the beef microbial growth and fat oxidation remarkably. The functional ingredients could quadruple the shelf life of fresh beef.

1. INTRODUCTION
In general, meat‐based foods have very low microbial and oxidative stability and are easily exposed to microbial and chemical spoilage during the production and storage chain (Mojaddar Langroodi et al., 2021). Therefore, improving the storage life of fresh meat is one of the important challenges of the meat industry which has attracted the attention of many researchers in recent years (Junior et al., 2020; Sepahvand et al., 2021). In this regard, the use of biopolymeric‐based coatings and films is proposed as an important solution due to their barrier, mechanical, optical, and biodegradability characteristics (Bagheri et al., 2019a; Molayi et al., 2018). Sodium alginate is a biopolymer with a high potential for being used as the basic component of films and coatings due to its unique properties such as biodegradability, low cost, availability, ease of use, nontoxicity, stability, and gel formation in the presence of polyvalent cations (Bagheri et al., 2019b; Karimi Khorrami et al., 2021; Nehchiri et al., 2021). The use of biopolymers in the forms of coating and film can restrict oxygen availability and prevent moisture loss, and thereby can increase the shelf life of many products in many researches (Bagheri et al., 2020; Martiny et al., 2020; Radi, Akhavan‐Darabi, et al., 2017; Radi, Firouzi, et al., 2017). The effectiveness of such coatings can be remarkably increased by incorporating antimicrobial and antioxidant compounds in their matrix to maintain high concentrations of these substances on the surface of coated products which are more susceptible to bacterial infestation (Akhavan et al., 2021). Different compounds like green and black tea extracts (Amiri et al., 2018; Radi, Firouzi, et al., 2017), Thymus vulgaris EO (Almasi, Radi, Amiri, 2020), polylactic acid (Guo et al., 2014), orange peel EO microemulsion (Radi, Akhavan‐Darabi, et al., 2017), (hot) acetic acid (Hosseini‐Farahi et al., 2018; Radi et al., 2010), calcium sulfate (Hosseini‐Farahi et al., 2016), and salycilic acid (Amiri et al., 2021; Hosseinifarahi et al., 2020), and treatments like UV irradiation (Abdipour et al., 2020) have been incorporated into the biopolymeric‐active packagings to enhance the antimicrobial activity of the films. In this regard, the use of essential oils (EOs) has received much attention due to their naturality and high antimicrobial activity (Najjaa et al., 2020; Radi, Akhavan‐Darabi, et al., 2017). Cumin is known for its antimicrobial, nutritional value, antioxidant, and pharmaceutical (antihypertensive, anticancer, etc.) properties, which are mainly associated with its EO (CEO) (Haghiroalsadat et al., 2010). The main components of CEO are cuminol, cumin aldehyde, menthon derivatives, and γ‐terpinene, which are responsible for its odor and biological effects. The antimicrobial effects of CEO on foodborne pathogens in different food products have been well demonstrated (Hyldgaard et al., 2012; Sharafati Chaleshtori et al., 2016; Taheri et al., 2018).
Titanium dioxide (TiO2), also known as titanium oxide, has many applications in today's modern world, and is mainly used as a pigment in different industries (Hur et al., 2005). The US Food and Drug Administration has approved the use of TiO2 as a harmless color additive in food, medicine, and cosmetic products (Alizadeh‐Sani et al., 2018). Extensive researches have been performed on the antimicrobial effect of TiO2 on a wide range of living organs including viruses, bacteria, fungi, algae, and cancer cells (Paspaltsis et al., 2006). The photocatalytic reaction of TiO2 is one of its known natural effects in reducing fungal contamination. Improving the application properties of biopolymer films by metal nanoparticles of TiO2 has been considered by researchers in recent years (Alizadeh‐Sani et al., 2018; Azizi‐Lalabadi et al., 2020; Marcous et al., 2017). In this study, the potential application of alginate‐based film containing TiO2 nanoparticles and CEO as functional ingredients on chemical, microbial, and physical properties of beef was evaluated during cold storage.
2. MATERIALS AND METHODS
2.1. Materials
TiO2 nanoparticles (anatase) with purity of more than 99% and particle size of 10–25 nm were obtained from US Research Nanomaterials, Inc. Na‐alginate was obtained from Behin Azma Co. Nutrient broth, Violet Red Bile Agar (VRBA), Cetrimide fucidin cephaloridine agar (CFC agar), De Man, Rogosa, Sharpe agar (MRS), Listeria Chrom agar, and plate count agar (PCA) were purchased from Merck Chemical Co.
2.2. The extraction of CEO
The CEO was extracted by the steam distillation method with the seed/water ratio of 1:5 (w/v) for 4 h by using a Clevenger apparatus. The yield and density of obtained CEO were 0.5% (v/w) and 0.878 (at 20°C), respectively (Sharafati Chaleshtori et al., 2016).
2.3. Chemical composition of CEO
A capillary gas chromatography (GC–MS; Hewlett Packard 6890) and a mass spectroscopy (Hewlett Packard 6890) were used to analyze the chemical composition of CEO. A capillary column with 0.32 mm diameter and length of 30 mm was used for the analysis. The analysis conditions were defined as follows: oven temperature (60°C), the column temperature (60°C—for the first 3 min after the injection, which was raised to 220°C with a rate of 6°C/min), the injection temperature (250°C), the flow rate of helium as the carrier gas (1 ml/min), and the MS ionization voltage (70 electronvolt; Amiri et al., 2013).
2.4. Alginate film preparation
To prepare the alginate film, 4 g of Na‐alginate was dissolved in 200 ml distilled water and stirred (200 rpm, for half an hour) at 60°C [to prevent lump formation, Na‐alginate powder was added gradually to the stirring water, and time was given for the Na‐alginate to be dissolved during the addition of powder (Radi & Amiri, 2013)]. Thereafter, 1 g glycerol was added to the film solution as a plasticizer. One percent of TiO2 nanoparticles (Al + TiO2), 2% CEO (Al + CEO), and a mixture of 1% TiO2 and 2% CEO (Al + TiO2 + CEO) were added, separately, as antimicrobial and antioxidant agents to prepare different treatments. Then, an ULTRA‐TURRAX® homogenizer (T18; IKA) was used to homogenize the solution (6000 rpm, 10 min). The prepared solutions were deaerated (at 40°C, 5 min) by a rotary vacuum evaporator (RV‐10 control; IKA). Then, 20 g of the solutions was poured into polystyrene Petri dishes (11 cm diameter) and dried in an incubator at 25°C with 40% relative humidity for 24 h (Nehchiri et al., 2021).
2.5. Samples preparation
After purchasing the beef from a local slaughterhouse, the meat was transferred to the laboratory under aseptic conditions (pH = 5.70 ± 0.04), cut into pieces with a thickness of 2 cm and a length of 20 cm, and then wrapped with the preprepared films. The treatments were defined as follows: Al, Al + TiO2, Al + CEO, and Al + TiO2 + CEO, as well as a control sample which was not wrapped within an Al‐based film. The wrapped meat samples were transferred to the polyethylene plastic bags and stored in a refrigerator (4 ± 1°C) for 24 days (Almasi et al., 2021). The sampling was performed at 4‐day intervals for microbiological, physical, chemical, and sensory experiments. Five Al‐based wraps containing meat samples were placed in a tray to be used as a replicate for each measurement time. Regarding the presence of seven measurement times (throughout the storage time), 35 plastic wraps were considered for the total measurement time of 24 days for one replicate and a total of three replicates were considered for each treatment.
2.6. Microbial analyses
To count the microbial population, 10 g of each sample was aseptically weighed, diluted, and homogenized (Seward Stomacher 400) with 50 ml of sterile saline solution (0.9%). After serial dilution, 0.1 ml of the desired dilutions prepared from the relevant treatments was cultured aerobically on selective media for determination of lactic acid bacteria (LAB, MRS), total mesophilic bacterial count (TVC, PCA), Enterobacteriaceae (VRBA), Pseudomonas spp. (CFC agar), and L. monocytogenes (Listeria Chrom agar) and incubated for 24 h at 37°C for PCA, VRBA, and Listeria Chrom agar, 24–72 h at 37°C for MRS agar, and 48 h at 20°C for CFC (CLSI, 1999).
2.7. The measurement of pH
Ten grams of meat samples was added to 90 ml of deionized water and homogenized (1000 rpm) for 1 min. After 10 min, the pH of the homogenate was measured using a pH meter (CG824, Schott pH meter; AOAC, 2000).
2.8. Chemical properties
The macrodistillation method was used to measure the total volatile base nitrogen (TVBN) content of the meat samples (Kirk & Sawyer, 1991). A colorimetric method was used for the measurement of thiobarbituric acid (TBA; Barbin, 1975). The measurement was performed at 538 nm using a spectrophotometer (T80+, PG Instruments). The results are reported as mg malonaldehyde (MDA)/kg sample. To measure the peroxide value (PV) of the beef samples, the iodometric method based on the titration of the samples with thiosulfate solution was performed (AOAC, 2000).
2.9. Color measurement
A digital colorimeter (CR‐400, Konica Minolta Sensing Inc.) was used to measure the color of meat samples. For this purpose, the colorimeter was first calibrated by a standard white plate (L* = 95.44, a* = −0.47, b* = 2.51). Then, the meat samples were placed on the standard white plate and the color coordinates values [L* (lightness), a* (green–red), and b* (blue–yellow)] were measured (Bagheri et al., 2014; Radi et al., 2010).
2.10. Sensory evaluation
The sensory evaluation was conducted according to the method described by Sharafati Chaleshtori et al. (2016) with some modifications. The taste panels consisted of 30 semi‐trained panelists (15 males and 15 females; aged 20–45 years; mean age, 36 years). Each panelist was asked to assess all of the samples at the same session. The meat odor, color, and overall acceptance were assessed using a 9‐point hedonic scale (dislike extremely [1], dislike very much [2], dislike moderately [3], dislike slightly [4], neither like nor dislike [5], like slightly [6], like moderately [7], like very much [8], and like extremely [9]). The whole process was performed at ambient temperature and standard lighting.
2.11. Statistical analysis
Statistical analyses were conducted by running one‐way analysis of variance (ANOVA), using MSTAT‐C. The comparison of means was performed by Duncan's multiple‐test range at p < .05.
3. RESULT AND DISCUSSIONS
3.1. Chemical composition of CEO
The main components of CEO, as it is shown in Table 1, are cuminal (43.4%), γ‐terpinene (32.62%), 1‐methyl‐2‐(1‐methylethyl)benzene (9.32%), pinocarveol (4.21%), copaene (3.86%), linalool (2.11%), and 1‐methyl‐4‐(1‐methylethyl)‐1,4‐cyclohexadiene (1.02%). The dominant compounds of CEO are the oxygenated monoterpenes, followed by the hydrocarbon monoterpenes (Abbdellaoui et al., 2019). The antimicrobial activity of CEO is largely attributed to its cuminal component (Wannera et al., 2010). The results of this study are in good agreement with the results of El‐Ghorab et al. (2010), who confirmed that cuminal (27.7%), γ‐terpinene (23.7%), pinocarveol (11.4%), and 1‐methyl‐2‐(1‐methylethyl)benzene (7.7%) are the major components of CEO. However, Abbdellaoui et al. (2019) demonstrated that cumin aldehyde (30%–33%), γ‐terpinen‐7‐al (20%–28%), α‐terpinen‐7‐al (13%), and γ‐terpinene (6%–12%) are the main components of CEO. The differences in components and quantities of different reports might refer to the different cultivation and climatic conditions as well as the environment which affects the chemical composition of the EO (El‐Ghorab et al., 2010).
TABLE 1.
Major chemical compositions of cumin essential oil
| Compound | RT (Min) a | Percent b |
|---|---|---|
| Cuminal | 17.238 | 43.4 |
| γ‐Terpinene | 22.325 | 32.62 |
| 1‐Methyl‐2‐(1‐methylethyl)benzene | 11.271 | 9.32 |
| Pinocarveol | 12.529 | 4.21 |
| Copaene | 13.422 | 3.86 |
| Linalool | 23.138 | 2.11 |
| 1‐Methyl‐4‐(1‐Methylethyl)‐1,4‐Cyclohexadiene | 14.261 | 1.02 |
| Sabinene | 17.239 | 0.72 |
| β‐Terpineol | 21.251 | 0.31 |
| Carotol | 12.427 | 0.24 |
| (5R)‐5‐Methyl‐2‐(1‐methylethylidene)cyclohexanone | 17.362 | 0.16 |
| α‐Pinene | 13.218 | 0.12 |
| 6,6‐Dimethyl‐2‐methylene‐bicyclo[2.2.1]heptan‐3‐one | 22.458 | 0.5 |
| Total identified | 98.59 |
Retention time.
Relative proportions as percent of the total peak area.
3.2. Microbial analyses
The meat samples wrapped with Al films were evaluated for total mesophilic bacteria, Enterobacteriaceae, LAB, pseudomonas spp., and L. monocytogenes. The results are shown in Figure 1. According to the results, in all samples, an increasing trend was observed for bacterial growth over time. Meanwhile, the control sample had the highest bacterial growth and the population of all studied bacteria in this sample increased from almost 2.37 to about 8.00 log CFU/g after 24 days of storage. The usage of all Al films slowed down the bacterial growth trend significantly throughout the storage time. In this regard, Al + TiO2 + CEO sample showed the greatest decrease in the microbial growth, followed by Al + TiO2 and Al + CEO samples, respectively (p <0.05). The lowest antimicrobial effect was related to the plain Al films. The Al + TiO2 + CEO films reduced the population of total mesophilic bacteria, Enterobacteriaceae, LAB, Pseudomonas spp., and L. monocytogenes by about 3.10, 2.44, 3.00, 3.36, and 3.41 logarithmic cycles in comparison with the control, respectively. The samples treated with Al + TiO2 reduced by about 2.93, 2.19, 2.53, 3.01, and 3.24 logarithmic cycles and the samples treated with Al + CEO reduced by 2.74, 2.05, 2.33, 2.93, and 3.15 logarithmic cycles the population of total mesophilic bacteria, Enterobacteriaceae, LAB, pseudomonas spp., and L. monocytogenes after 24 days of storage, respectively (p < .05). However, the samples covered with Al films reduced the population of total mesophilic bacteria, Enterobacteriaceae, LAB, pseudomonas spp., and L. monocytogenes by about 2.22, 1.94, 1.97, 2.40, and 3.03 logarithmic cycles compared to the control sample after 24 days of storage, respectively (p < .05).
FIGURE 1.
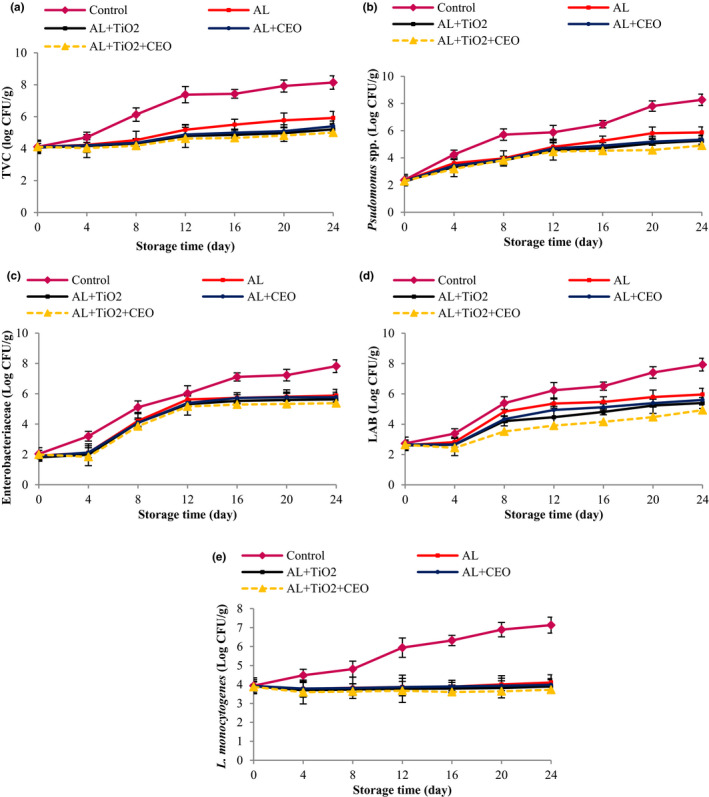
Total viable count (a), Pseudomonas spp. (b), Enterobacteriaceae (c), lactic acid bacteria (d), and Listeria monocytogenes (e) of beef packed in alginate‐based films containing TiO2 and CEO at 4°C during 24 days of storage
For L. monocytogenes, the use of plain Al or (nano)composite Al films effectively reduced the population of L. monocytogenes from 3.93 to 3.77 (in the Al and Al + CEO samples), 3.69 (in the Al + TiO2 sample), and 3.64 (in the GE + TiO2 + CEO sample) log CFU/g until the 4th day of storage, indicating that the plain and (nano)composite Al films were initially able to reduce the L. monocytogenes population (p < .05). After that, the L. monocytogenes population started to increase to 4.1, 3.98, 3.89, and 3.72 log CFU/g in the treated beef samples with Al, Al + CEO, Al + TiO2, and Al + TiO2 + CEO, respectively, while the increase in L. monocytogenes population was from 3.93 to 7.13 log CFU/g in the control sample (p < .05). Results showed that L. monocytogenes was the most sensitive bacteria against the used treatments, as the used films were able to inhibit the growth of L. monocytogenes remarkably.
According to the results, all treated samples, especially those treated with CEO, TiO2, and TiO2 + CEO, could effectively reduce the population of examined G+ and G− bacteria. The Al film can act as a semipermeable film and restrict the oxygen availability on the surface of the meat samples, decreasing the microbial growth trend in comparison with the control.
The antimicrobial activity of the CEO resulted from its phenolic compounds (Alizadeh Behbahani et al., 2019). The results of GC‐MS confirmed that CEO is a monoterpenoid EO and the monoterpenes in CEO can destroy the cellular respiratory mechanisms of bacteria and ruin the transmission system of ions, resulting in cellular death. Besides, it has been reported that the phenolic compounds of CEO inhibit the growth of microorganisms through the enzymatic inhibition of the oxidized compound or reacting with sulfhydryl groups of proteins via nonspecific modes, and modifying protein functionality (Alizadeh Behbahani et al., 2019). The antifungal and antimicrobial activities of CEO have been demonstrated in several studies (Alizadeh Behbahani et al., 2019; Sharafati Chaleshtori et al., 2016).
The antimicrobial of TiO2 is related to the ability of this compound to generate reactive oxygen species (ROS), which disrupt the bacteria membrane or react with the cytoplasmic membranes within the bacteria cell, as well as react with the key cellular enzymes (Alizadeh‐Sani et al., 2020). Othman et al. (2014) confirmed the antimicrobial activity of TiO2 nanoparticles against Escherichia coli in the LDPE films. Feng et al. (2019) found that the whey protein nanofibrils (WPNFs)/TiO2 nanoparticles films limit L. monocytogenes, Staphylococcus aureus, Salmonella Enteritidis, and E. coli growth. It has been reported that T. vulgaris‐loaded microemulsions in an alginate‐based film maintained the microbial quality of ground beef (Almasi et al., 2021; Almasi, Radi, Amiri, Torri, 2020). Noshad et al. (2021) reported that application of Plantago major seed mucilage containing Citrus limon essential oil reduced the microbial growth (total viable count, psychrotrophic bacteria, E. coli, S. aureus, and fungi) significantly in buffalo meat.
As it was demonstrated, TiO2 antimicrobial activity was greater than that of CEO and the presence of CEO and TiO2 together within the nanocomposite film had a synergistic effect as the greatest reduction in the bacteria counts was observed due to the presence of the phenolic compounds of CEO as well as the production of ROS as a result of TiO2 nanoparticles (Alizadeh‐Sani et al., 2020). Sani et al. (2017) reported a strong antimicrobial activity against L. monocytogenes and S. aureus by rosemary oil and TiO2. The results of this study were confirmed by previous studies (Ghaderi‐Ghahfarokhi et al., 2016; Raeisi et al., 2016).
3.3. pH
The initial pH of beef samples was 5.70 which increased significantly to 7.43 in the control, 6.36 in Al‐coated sample, 6.22 in Al–TiO2‐ and Al–CEO‐treated samples, and 5.64 in Al–TiO2–CEO sample after 24 days of storage (p < .05, Figure 2). According to the results, the pH increase rate in the control sample was considerably higher than in the treated samples. The higher pH in the control sample may be attributed to a higher bacterial growth in these samples, which was demonstrated in the microbial section results. As the bacterial population increases, the amount of bacterial enzymes increases in the meat tissue. The produced enzymes begin to decompose the meat proteins and produce nitrogenous compounds, resulting in an increase in the meat pH (Sayadi, Amiri, et al., 2021). The delay in the pH increase in nanocomposite films containing TiO2 and CEO might be attributed to the ability of the used antimicrobial and antioxidant agents to decrease microbial growth and to inhibit the decomposition of proteins and other nitrogenous compounds, such as ammonia and trimethylamine (Alizadeh‐Sani et al., 2020). The pH value of Al–TiO2–CEO did not exceed the upper limit value for pH (5.80) even on day 24.
FIGURE 2.
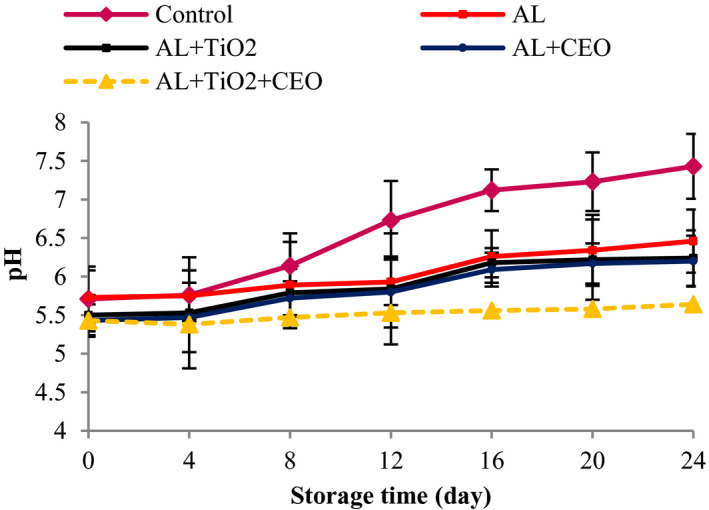
The pH changes in beef packed in alginate‐based films containing TiO2 and CEO at 4°C during 24 days of storage
In line with our findings, Alizadeh‐Sani et al. (2020) reported an increase in pH from 5.70 to 7.45 for the control and 5.70 to 6.18 for the lamb meats packed in whey protein/cellulose nanofiber matrix films containing rosemary oil–TiO2. In another study, Khezrian and Shahbazi (2018) reported an increase in pH values from 5.80 to 6.60 in the camel meat treated with nanocomposite films of chitosan and carboxymethyl cellulose (CMC). The higher pH values in the control sample were observed in other studies on chicken breast meat packaged by PET/chitosan/alginate films incorporating black cumin oil (Takma & Korel, 2019) and turkey breast meat coated with chitosan containing CEO (Taheri et al., 2018).
3.4. Total volatile base nitrogen
The activity of the endogenous enzymes of the meat as well as the bacteria enzymes produces nitrogenous compounds which are measured in the TVBN test. Therefore, the higher values of TVBN indicate the higher activity of endogenous enzymes as well as bacterial activity that in turn is an indication of the meat spoilage (Alizadeh‐Sani et al., 2020). On day 0, the TVBN value of the meat samples was 7.51 mg N/100 g meat, indicating the good hygienic quality of meat. The TVBN values of all samples increased significantly with time (Figure 3), which obeyed a faster trend for the control sample, followed by Al‐, Al–CEO‐, Al–TiO2‐, and Al–TiO2–CEO‐treated meats, respectively. After 12 and 24 days of storage, the TVBN values of control were 26.58 and 43.19 mg N/100 g meat, respectively. However, these values were 15.21 and 25.31 (for Al), 12.12 and 23.41 (for Al–CEO sample), 11.47 and 19.24 (for Al–TiO2), and 8.68 and 13.27 (for Al–TiO2–CEO) N/100 g meat after 12 and 24 days of storage, respectively (p < .05). The upper acceptable limit for TVBN is 25 mg N/100 g (Alizadeh‐Sani et al., 2020), and the TVBN content of the control sample was more than this value on day 12 (26.58 mg N/100 g). However, the Al sample reached 25.31 on the 24th day, and the other treated samples had considerably lower values for TVBN than 25 mg N/100 g even on the 24th day, especially the Al–TiO2–CEO samples which had the smallest value (about 13 mg N/100 g).
FIGURE 3.
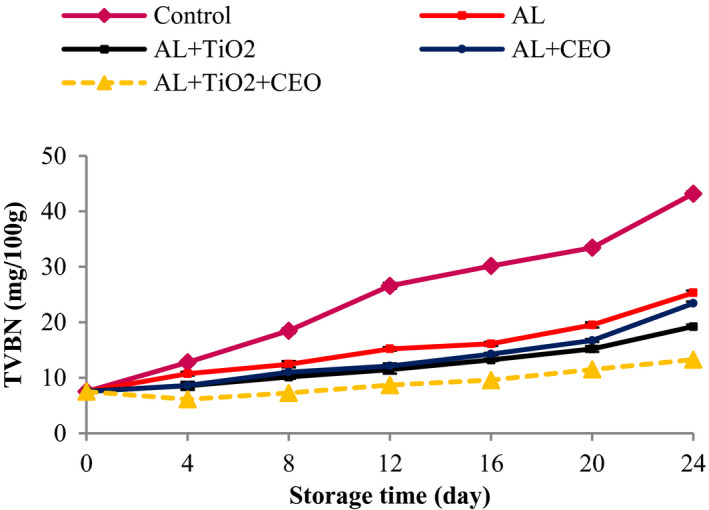
Changes in total volatile base nitrogen values of beef packed in alginate‐based films containing TiO2 and CEO at 4°C during 24 days of storage
Total volatile base nitrogen value in the meat samples is related to the decomposition of protein compounds to nonprotein nitrogen compounds as a result of bacteria activity and the proteolytic enzymes (Alizadeh‐Sani et al., 2020). In line with the microbial results, the antibacterial effect of CEO and TiO2 resulted in a lower activity of bacteria enzymes, and thereby, lower TVBN content of these samples. Similar results were obtained by Alizadeh‐Sani et al. (2020) on the addition of rosemary EO and TiO2 in refrigerated meat, Sayadi, Mojaddar Langroodi, Jafarpour (2021) on turkey meat coated with chitosan containing Berberis vulgaris extract and Mentha pulegium EO under MAP condition, Sayadi, Mojaddar Langroodi, Pourmohammadi et al. (2021) on using zein coating impregnated with ginger extract and Pimpinella anisum EO to prolong the shelf life of bovine meat, and Heydari‐Majd et al. (2019) on the application of poly lactic acid (PLA)/ZnO nanoparticles/Zataria multiflora Boiss. EO (ZEO) and Menthe piperita L. EO (MEO) on Otolithes ruber fillets.
3.5. Peroxide value
The PV is usually measured to evaluate the chemical health status of product fat. After the formation of peroxides, the formation of volatile compounds (ketones, aldehydes, and alcohols) is accelerated, resulting in rancidity of fat and production of off‐flavor (Khezrian & Shahbazi, 2018). For this reason, PV measurement is considered a very important parameter. The initial PV of the samples was 1 meq peroxide/1000 g lipid, indicating the good quality of the initial beef. But the PVs increased steadily and reached the highest value until the 12th day (3.94 meq peroxide/1000 g lipid for the control), and then decreased until the end of the 24th day (Figure 4). An increase in the formation of PV caused an increasing trend in the PV curve up to day 12 and the decomposition of peroxide compounds from day 12 onward caused the curve to start a declining trend. This reduction was accompanied by a sudden increase in secondary products. The PV of the control was significantly higher than those of treated samples during the storage time. Up to the 12th day, the Al samples showed the highest PV after control, followed by Al–TiO2 or Al–CEO, and Al–TiO2–CEO‐treated meats, respectively. But from day 16 until the end of the storage time, there was no statistically significant difference among the treated samples. It is noteworthy that the changes in PV of Al–TiO2–CEO sample were very small and it increased from 1.00 to 1.23 meq peroxide/1000 g lipid (until the 12th day). However, this increase was from 1.00 to 1.76 meq peroxide/1000 g lipid for Al–TiO2 and Al–CEO samples.
FIGURE 4.
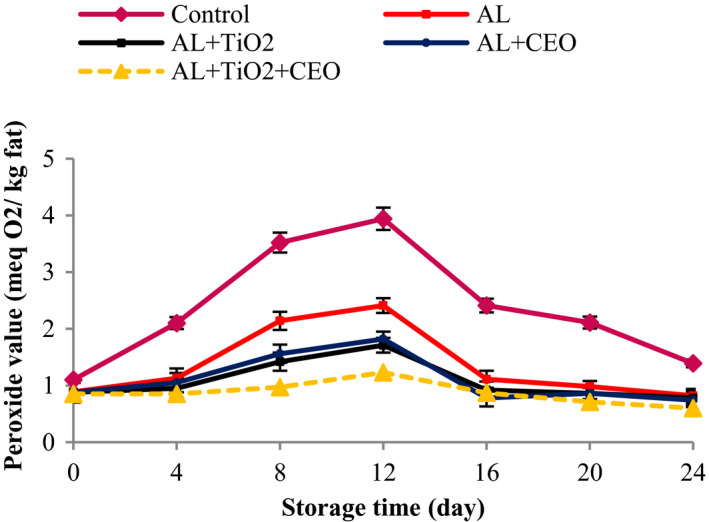
Changes in peroxide values of beef packed in alginate‐based films containing TiO2 and CEO at 4°C during 24 days of storage
According to the obtained results, the treatments of this study could effectively control the lipid oxidation of meat during 24 days of storage. The antioxidant activity of sodium alginate compound has been reported before (Sellimi et al., 2015). Sellimi et al. (2015) reported that sodium alginate extracted from Tunisian brown seaweed exerted 74% free radical scavenging activity at 0.5 mg/ml in DPPH radical scavenging activity test and demonstrated significant reducing activity (OD at 700 nm = 2) at 1.2 mg/ml in Ferric‐reducing activity test. These researchers showed that sodium alginate displayed a moderate ability to prevent bleaching of β‐carotene according to ß‐carotene–linoleic acid assay. In addition, sodium alginate, at 4 and 5 mg/ml, exerted potent scavenging activities (80% and 82%, respectively) according to hydroxyl radical scavenging activity test. Meanwhile, Al may act as an oxygen barrier (Nehchiri et al., 2021) between meat and its surroundings, resulting in the reduction in the lipid oxidation rate. On the other hand, it has been confirmed that CEO can reduce Fe3 ions effectively and scavenge the superoxide anion. This property comes from its polyphenolic compounds and good antioxidant activity of CEO (El‐Ghorab et al., 2010). The strong antioxidant activity of cuminal and γ‐terpinene has been reported. Meanwhile, monoterpene alcohols like linalool, terpineol, and pinocarveol have been indicated for CEO antioxidant activity (Abbdellaoui et al., 2019).
The antioxidant activity of TiO2 nanoparticles is confirmed by researchers (Alizadeh‐Sani et al., 2018). Alizadeh‐Sani et al. (2020) reported that TiO2 nanoparticles could turn the purple color of DPPH radical into yellow color of DPPH‐H, indicating that TiO2 has the ability to scavenge the free radical of DPPH, and therefore, can scavenge the produced free radicals during the oxidation reaction and therefore decrease the rate of oxidation. In our study, the synergistic effect of Al, CEO, and TiO2 in the prevention of lipid oxidation was evident. Similar results were also obtained on chilled meat coated with whey protein nanofibrils containing TiO2 nanotubes (Feng et al., 2019), fresh chicken packaged with gelatin film containing TiO2 nanoparticles and CEO (Sayadi, Amiri, et al., 2021), turkey breast meat coated with chitosan film containing 1% CEO (Taheri et al., 2018), and chicken breast fillet on application of silver nanoparticles in polyvinyl chloride films (Azlin‐Hasim et al., 2016).
3.6. Thiobarbituric acid
In the TBA test, MDA as an aldehyde compound is measured. Aldehydes are the secondary products of lipid oxidation, and their increase is an indication of lipid rancidity (Heydari‐Majd et al., 2019). The TBA of all samples increased significantly throughout 24 days of storage (Figure 5). The initial TBA value of beef was 0.45 mg MDA/kg, which was low and showed the freshness of the initial meat. The increasing trend in the TBA values of control was significantly much more than the treated samples, which reached 4.23 mg MDA/kg in the control samples after 24 days. However, the TBA values of Al, Al–TiO2, Al–CEO, and Al–TiO2–CEO reached 2.26, 1.61, 1.33, and 1.04 after 24 days (p < .05), respectively. The presence of strong antioxidant compounds in the CEO caused lower TBA values in Al–CEO sample than that of Al–TiO2. The physical barrier properties of Al to limit the oxygen penetration, the strong antioxidant properties of CEO due to its high phenolic content, and the free radical scavenging property of TiO2 are the reasons for the lower TBA values of the meat samples treated with Al, CEO, and TiO2 (Alizadeh‐Sani et al., 2020; Taheri et al., 2018). In line with PV results, from day 12 onward, with a decrease in the PVs, there was a large increase in the amount of TBA. Similar results were obtained by other researchers on turkey breast meat coated with chitosan containing 1% CEO (Taheri et al., 2018), O. ruber fillets packaged with nanocomposite film based on PLA/zinc oxide nanoparticle/ZEO and MEO (Heydari‐Majd et al., 2019), fresh pork and meat loins coated with alginate‐based film produced with turmeric (Bojorges et al., 2020), and beef wrapped with citric acid, cornstarch, and linear LDPE active films (Júnior et al., 2015).
FIGURE 5.
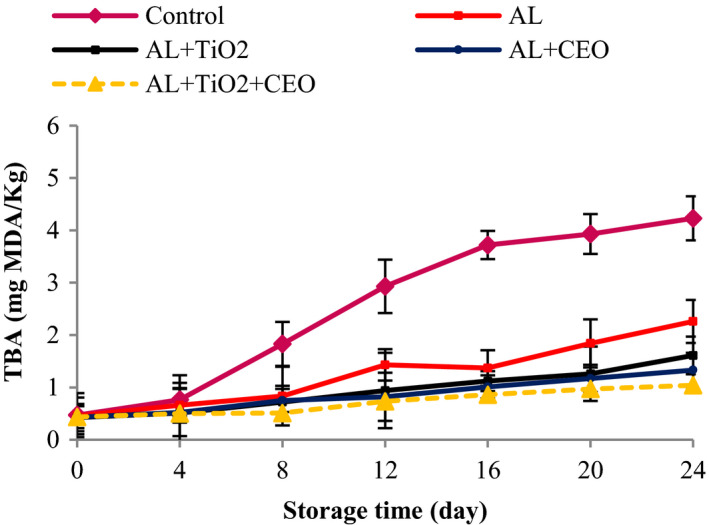
Changes in thiobarbituric acid values of beef packed in alginate‐based films containing TiO2 and CEO at 4°C during 24 days of storage
3.7. Color analysis
Table 2 shows the color coordinates (L*, a*, and b*) of beef samples. According to Table 2, lightness (L*) decreased throughout the storage time which is related to the oxidation phenomenon that turns the color of meat from red to dark brown. Therefore, the increase in oxidation during the storage time resulted in a decrease in L* (lightness) and a* (redness) values of beef samples. The a* value is an indication of redness and is considered an important color parameter for meat and meat products. The increase in a* values is attributed to the oxygenation of the myoglobin which causes the red color of meat, and the reduction in a* values is the indication of myoglobin oxidation and formation of metmyoglobin, which turns the color of meat to brown (El Adab & Hassouna, 2016).
TABLE 2.
Effect of alginate‐based films containing TiO2, CEO, and TiO2/CEO on color coordinates and sensory attributes of ground beef meat during 24 days of storage at refrigerated temperature
| Sensory attributes | Treatment | Storage time (day) | ||||||
|---|---|---|---|---|---|---|---|---|
| 0 | 4 | 8 | 12 | 16 | 20 | 24 | ||
| L* | Control | 36.20 ± 1.04a * | 35.20 ± 0.23a | 32.11 ± 0.12a | 30.18 ± 1.18a | 29.82 ± 0.98a | 27.71 ± 0.14a | 25.15 ± 0.55a |
| Al film | 36.20 ± 1.04a | 36.01 ± 0.42b | 34.02 ± 0.35b | 33.01 ± 0.15b | 31.11 ± 0.74b | 29.65 ± 0.31b | 28.12 ± 0.35b | |
| Composite Al‐based films | 36.20 ± 1.04a | 36.00 ± 0.55b | 35.02 ± 0.45c | 34.01 ± 0.25c | 33.11 ± 0.74c | 32.45 ± 0.41c | 31.87 ± 0.24c | |
| a* | Control | 23.87 ± 0.45a | 20.21 ± 0.87a | 16.10 ± 0.48a | 14.21 ± 0.95a | 12.21 ± 0.48a | 8.45 ± 0.98a | 6.12 ± 0.42a |
| Al film | 23.87 ± 0.45a | 22.22 ± 0.42b | 20.12 ± 0.31b | 19.00 ± 0.12b | 18.23 ± 0.34b | 16.42 ± 0.44b | 13.22 ± 0.55b | |
| Composite Al‐based films | 23.87 ± 0.45a | 22.10 ± 0.35b | 21.11 ± 0.45c | 20.32 ± 0.34c | 19.10 ± 0.25c | 17.52 ± 0.54c | 15.22 ± 0.65c | |
| b* | Control | 12.76 ± 0.45a | 10.93 ± 0.41a | 10.01 ± 0.21a | 9.22 ± 0.61a | 9.00 ± 0.25a | 8.86 ± 0.87a | 8.12 ± 0.12a |
| Al film | 12.76 ± 0.45a | 12.12 ± 0.24b | 11.00 ± 0.33b | 10.20 ± 0.42b | 9.81 ± 0.46b | 9.11 ± 0.42b | 9.00 ± 0.35b | |
| Composite Al‐based films | 12.76 ± 0.45a | 12.00 ± 0.34b | 11.50 ± 0.44c | 11.20 ± 0.24c | 10.82 ± 0.54c | 10.11 ± 0.42c | 9.54 ± 0.42c | |
| Control | 9 ± 0Aa | 7.6 ± 0.53Bc | 5.2 ± 0.36Cd | 3.1 ± 0.23Db | 1.7 ± 0.51Ec | 0 ± 0Fd | 0±0Fd | |
| AL | 8.9 ± 0.23Aa | 8.6 ± 0.68Aa | 8.1 ± 0.32Ba | 4.2 ± 0.36Ca | 3.2 ± 0.0Cb | 1.1 ± 0.42Dc | 0.3 ± 0.62Ec | |
| Color** | AL + TiO2 | 8.8 ± 0.33Aa | 8.8 ± 0.73Aa | 8.2 ± 0.34Ba | 4.3 ± 0.51Ca | 3.6 ± 0.33Da | 2.3 ± 0.21Eb | 1.6 ± 0.28Fb |
| AL + CEO | 8.9 ± 0.72Aa | 8.6 ± 0.56Ba | 8.0 ± 0.62Ca | 4.1 ± 0.39Da | 3.5 ± 0Ea | 2 ± 0Fb | 1.3 ± 0.62Gb | |
| AL + TiO2 + CEO | 8.9 ± 0.22Aa | 8.8 ± 0.29Ba | 8.2 ± 0.36Ca | 4.3 ± 0.44Da | 3.6 ± 0.63DEa | 2.9 ± 0.37Ea | 2.3 ± 0.48Ea | |
| Control | 9 ± 0Aa | 7.7 ± 0.52Bc | 6.3 ± 0.54Cc | 4.0 ± 0Dd | 1.4 ± 0.56Ec | 0 ± 0Fd | 0 ± 0Fd | |
| AL | 8.9 ± 0.23Aa | 8.5 ± 05Aa | 7.2 ± 0.51Bb | 6.2 ± 0.52Ccb | 5.3 ± 0.65Dab | 4.1 ± 0.43Eb | 2.6 ± 0.31Fc | |
| Odor | AL + TiO2 | 9 ± 0Aa | 8.7 ± 0.23Aa | 7.5 ± 0Ba | 6.4 ± 0.62Ca | 5.7 ± 0.28Da | 4.5 ± 0.36Ea | 3.8 ± 0.11Fb |
| AL + CEO | 8.5 ± 0Ab | 8.2 ± 0.68Ab | 7 ± 0Bb | 5.9 ± 0.51Cc | 4.9 ± 0.22Db | 3.7 ± 0.53Ec | 2.8 ± 0.24Fc | |
| AL + TiO2 + CEO | 8.5 ± 0.56Aa | 8.1 ± 0.58Ab | 7.2 ± 0Bb | 6 ± 0Cbc | 5.1 ± 0.82Dab | 4.2 ± 0.51Eb | 4 ± 0Ea | |
| Control | 9 ± 0Aa | 6.2 ± 0.56Bc | 3.6 ± 0.37Cc | 1.7 ± 0.43Dc | 1.2 ± 0.46ECc | 0 ± 0Fc | 0 ± 0Fd | |
| AL | 8.9 ± 0.25Aa | 8.4 ± 0.41Ba | 7.5 ± 0Cb | 5.9 ± 0.46Db | 5.4 ± 0.32Eb | 3.6 ± 0.25Fb | 3.5 ± 0.24Fc | |
| Overall acceptability | AL + TiO2 | 9 ± 0Aa | 8.7 ± 0.64Aa | 7.9 ± 0.36Ba | 6.5 ± 0.73Ca | 6 ± 0Ca | 3.9 ± 0.42Db | 3.7 ± 0.27Dc |
| AL + CEO | 8.8 ± 0.42Aa | 8.1 ± 0.62Bb | 8 ± 0Ba | 6.6 ± 0.26Ca | 6 ± 0Da | 3.8 ± 0.53Eb | 3.6 ± 0.51Ec | |
| AL + TiO2 + CEO | 8.9 ± 0.42Aa | 8.2 ± 0.32Bb | 8 ± 0Ba | 6.7 ± 0.72Ca | 6.1 ± 0.43Ca | 5.4 ± 0Da | 5 ± 0Db | |
Data with different small letters in each column are significantly different (p < .05) for color coordinates.
Data of sensory evaluation with different capital and small letters in each row and column are significantly different (p < .05).
The L* and a* values reduced from 36.20 to 25.15 and 23.87 to 6.12 at the end of the storage time for the control, respectively, while this reduction was to 31.87 for L* and 15.22 for a* value in the treated samples. This indicates the effect of active packaging and nanocomposite films containing antimicrobial and antioxidant compounds in retarding the formation of the undesirable metmyoglobin pigment and maintaining the color quality of meat for a longer time. The results of L* and a* values, in this study, were in line with the findings of Takma and Kore (2019) on the addition of chitosan and alginate layer to chicken breast meat, and Sirocchi et al. (2017) on the application of rosemary EO and modified atmosphere packaging to beef.
In relation to b* values, the indicator of “yellowness,” a significant decrease occurred for all the samples. The reduction in b* is attributed to a decrease in the oxymyoglobin content and an increase in the formation of metmyoglobin in the meat. The reduction in the oxymyoglobin content occurred due to the consumption of oxygen by microorganisms (El Adab & Hassouna, 2016). In this regard, the treated samples with nanocomposite films showed lower b* values than that of the control as a result of the antioxidant compounds of CEO and radical scavenging properties of TiO2 that retarded the oxidation phenomenon (El Adab & Hassouna, 2016). No significant difference was observed among the color coordinates of the Al + CEO, Al + TiO2, and Al + TiO2 + CEO samples; meanwhile, the color coordinates of Al samples were placed after the control. According to the results, the incorporation of TiO2 and CEO into the alginate‐based films could significantly enhance the stability of color in the meat samples. The results of color parameters variations in this study were in good agreement with the results of El Adab and Hassouna (2016) on the addition of oregano and thyme EOs in fermented poultry meat sausage, Heydari et al. (2020) on the application of Qodume Shirazi seed mucilage‐based edible coating containing lavender essential oil on the fresh ostrich meat, and Tosati et al. (2017) on the use of bovine gelatin and turmeric starch on frankfurter sausage.
3.8. Sensory properties
All sensory attributes of the meat samples (color, odor, and overall acceptability) declined throughout the storage time (Table 2). This reduction trend was much faster in the control sample than in the treated samples during the storage time, and hence, the obtained score for color, odor, and overall acceptance was zero for the control sample on day 20.
Regarding the color parameter, at the beginning of storage, there was no significant difference among the treated samples. However, by moving toward the end of the storage time, the Al sample obtained lower scores than the other treated samples and the Al + TiO2 + CEO sample received the highest score. Regarding the odor parameter, it seems that the treated samples without CEO obtained a higher score than the samples containing CEO. This can be due to the effect of the CEO smell. It is noteworthy that although the odor parameter score was significantly lower in the CEO‐containing samples over time, this difference was small. Another important point was that by moving toward the end of the storage time, this difference disappeared, which could be due to the decrease in CEO odor over time, and finally, the Al + TiO2 + CEO samples obtained the highest score. In terms of overall acceptance, it was observed that the score obtained by the treated samples throughout the storage time was significantly higher than that obtained by the control sample. Meanwhile, the scores obtained at the beginning of storage were not much different for all treatments, but toward the end of the storage time, the Al + TiO2 + CEO sample obtained the highest scores. The antimicrobial effect of Al, CEO, TiO2, and Al + TiO2 + CEO induced the enhancement of sensory attributes in the treated meats compared to the control. Our results are consistent with those of Alizadeh‐Sani et al. (2017) and Azizi‐Lalabadi et al. (2020). In these studies, it was demonstrated that the application of TiO2 nanoparticles effectively improved the sensory attributes of white shrimp and lamb, respectively. Sharafati Chaleshtori et al. (2016) and Taheri et al. (2018) declared that the application of chitosan containing CEO resulted in good overall acceptance in chicken meat and turkey breast meat, respectively. Similar results were obtained by other researchers (Chen et al., 2016; Diao et al., 2020; Sayadi, Amiri, et al., 2021).
3.9. The estimation of shelf life for beef samples
The shelf life of beef samples was estimated according to the results of microbial and chemical tests (Table 3). In terms of TVC, TBA, PV, a*, and TVBN results, all treated samples were acceptable until day 24, while this number was 8 for the control. However, the pH and sensory evaluation tests limited the shelf life of control to 4 days. Although the treated samples were sensually acceptable up to day 16, the pH test reduced the shelf life of the Al sample to 8 days and Al + CEO and Al + TiO2 samples to 12 days. However, the pH of Al + TiO2 + CEO sample did not exceed the upper limit until the 24th day. Overall, the shelf life of control, Al, and composite films was around 4, 8, and 16 days, respectively.
TABLE 3.
The estimation of beef shelf life according to data obtained from physicochemical, microbial, and sensorial experiments during 24 days of storage
| Shelf life (day) | |||
|---|---|---|---|
| Control | Alginate film | Composite alginate‐based films | |
| Total viable count a | 8 | 24 | Not achieved |
| Peroxide value | Not achieved (3.94 at day 12) | Not achieved (2.83 at day 12) | Not achieved [1.86 (in average) at day 12] |
| TBA value | ~8 | ~20 | Not achieved |
| TVBN value | 8 | 20 | Not achieved |
| a* c | 8 | 24 | 24 |
| Sensorial c | 4 | 16 | 16 |
| pH | 4 | 8 | 12 (and until 24 days for Al + TiO2 + CEO sample) |
According to Iran National Standards Organization, the upper limit value for TVC in fresh beef is 5 × 105 log CFU/g, 5 meq/kg fat is the upper limit value for peroxide value, 25 mg N/100 g is the upper limit value for TVBN, 5.8 is the upper limit value for pH, and no upper limit value is defined for TBA, but according to literature [Alizadeh Sani et al. (2020)], 2 mg MDA/1000 g is the upper limit value for TBA.
According to the color analysis results.
According to the sensory evaluation results and overall acceptance score more than 4.
4. CONCLUSION
In this study, nanocomposite alginate‐based films containing TiO2 nanoparticles and CEO (as antioxidant and antimicrobial agents), alone or in combination, were fabricated. The produced active packaging films could remarkably reduce the lipid oxidation and microbial spoilage, improve the color quality and sensory attributes, and increase the shelf life of fresh beef. In this regard, the combination use of TiO2 and CEO resulted in better results compared to the sole use of TiO2 and CEO. The results of this study showed that the use of Al + TiO2 + CEO as a novel nanocomposite film is greatly beneficial in preserving the quality parameters of fresh beef.
CONFLICT OF INTEREST
Mehran Sayadi, Ali Mojaddar Langroodi, Sedigheh Amiri, and Mohsen Radi declare that they have no conflict of interest.
AUTHOR CONTRIBUTIONS
Mehran Sayadi: Funding acquisition (lead); Investigation (equal); Methodology (equal). Ali Mojaddar Langroodi: Conceptualization (equal); Investigation (equal); Methodology (equal); Visualization (equal). Sedigheh Amiri: Investigation (supporting); Methodology (equal); Visualization (equal); Writing – original draft (lead); Writing – review & editing (equal). Mohsen Radi: Methodology (equal); Supervision (equal); Writing – review & editing (equal).
ETHICAL APPROVAL
This article does not contain any studies with human participants or animals performed by any of the authors.
ACKNOWLEDGMENT
The research was financially supported by the Fasa University of Medical Sciences.
Sayadi, M. , Mojaddar Langroodi, A. , Amiri, S. , & Radi, M. (2022). Effect of nanocomposite alginate‐based film incorporated with cumin essential oil and TiO2 nanoparticles on chemical, microbial, and sensory properties of fresh meat/beef. Food Science & Nutrition, 10, 1401–1413. 10.1002/fsn3.2724
Contributor Information
Ali Mojaddar Langroodi, Email: drali_ml2@yahoo.com.
Mohsen Radi, Email: m.radi@iauyasooj.ac.ir, Email: msnradi@gmail.com.
DATA AVAILABILITY STATEMENT
Data will be made available upon reasonable request.
REFERENCES
- Abbdellaoui, M. , Bouhlali, E. D. T. , & El Rhaffari, L. (2019). Chemical composition and antioxidant activities of the essential oils of cumin (Cuminum cyminum) conducted under organic production conditions. Journal of Essential Oil Bearing Plants, 22, 9. 10.1080/0972060X.2019.1699866 [DOI] [Google Scholar]
- Abdipour, M. , Sadat Malekhossini, P. , Hosseinifarahi, M. , & Radi, M. (2020). Integration of UV irradiation and chitosan coating: A powerful treatment for maintaining the postharvest quality of sweet cherry fruit. Scientia Horticulturae, 264, 109197. 10.1016/j.scienta.2020.109197 [DOI] [Google Scholar]
- Akhavan, H. R. , Hosseini, F. S. , Amiri, S. , & Radi, M. (2021). Cinnamaldehyde‐loaded nanostructured lipid carriers extend the shelf life of date palm fruit. Food and Bioprocess Technology, 14, 1478–1489. 10.1007/s11947-021-02645-8 [DOI] [Google Scholar]
- Alizadeh Behbahani, B. , Noshada, M. , & Falahb, F. (2019). Cumin essential oil: Phytochemical analysis, antimicrobial activity and investigation of its mechanism of action through scanning electron microscopy. Microbial Pathogenesis, 136, 103716. 10.1016/j.micpath.2019.103716 [DOI] [PubMed] [Google Scholar]
- Alizadeh‐Sani, M. , Ehsani, A. , & Hashemi, M. (2017). Whey protein isolate/cellulose nanofibre/TiO2 nanoparticle/rosemary essential oil nanocomposite film: Its effect on microbial and sensory quality of lamb meat and growth of common pathogenic bacteria during refrigeration. International Journal of Food Microbiology, 19, 8–14. [DOI] [PubMed] [Google Scholar]
- Alizadeh‐Sani, M. , Khezerlou, A. , & Ehsani, A. (2018). Fabrication and characterization of the bionanocomposite film based on whey protein biopolymer loaded with TiO2 nanoparticles, cellulose nanofibers and rosemary essential oil. Industrial Crops and Products, 124, 300–315. 10.1016/j.indcrop.2018.08.001 [DOI] [Google Scholar]
- Alizadeh‐Sani, M. , Mohammadian, E. , & McClements, D. J. (2020). Eco‐friendly active packaging consisting of nanostructured biopolymer matrix reinforced with TiO2 and essential oil: Application for preservation of refrigerated meat. Food Chemistry, 322, 126782. 10.1016/j.foodchem.2020.126782 [DOI] [PubMed] [Google Scholar]
- Almasi, L. , Radi, M. , & Amiri, S. (2020). The release rate and antimicrobial activity of calcium‐alginate films containing self‐microemulsifying Thymus vulgaris essential oil against Escherichia coli and Staphylococcus aureus . Journal of Food Safety, 40, e12828. 10.1111/jfs.12828 [DOI] [Google Scholar]
- Almasi, L. , Radi, M. , Amiri, S. , & McClements, D. J. (2021). Fabrication and characterization of antimicrobial biopolymer films containing essential oil‐loaded microemulsions or nanoemulsions. Food Hydrocolloids, 117, 106733. 10.1016/j.foodhyd.2021.106733 [DOI] [Google Scholar]
- Almasi, L. , Radi, M. , Amiri, S. , & Torri, L. (2020). Fully dilutable Thymus vulgaris essential oil: Acetic or propionic acid microemulsions are potent fruit disinfecting solutions. Food Chemistry, 343, 128411. 10.1016/j.foodchem.2020.128411 [DOI] [PubMed] [Google Scholar]
- Amiri, A. , Nicknam, Z. , Radi, M. , Sayadi, M. , Bagheri, F. , Karimi Khorrami, N. , & Abedi, E. (2021). Postharvest quality of orange fruit as influenced by salicylic acid, acetic acid, and carboxymethyl cellulose coating. Journal of Food Measurement and Characterization, 15, 3912–3930. 10.1007/s11694-021-00966-y [DOI] [Google Scholar]
- Amiri, S. , Abbasi, S. , Ezzatpanah, H. , & Hosseini, E. (2013). Nanocapsulation of orange peel oil using microemulsion technique. Agro Food Industry Hi Tech, 24(2), 72–75. [Google Scholar]
- Amiri, S. , Akhavan, H. R. , Zare, N. , & Radi, M. (2018). Effect of gelatin‐based edible coatings incorporated with Aloe vera and green tea extracts on the shelf‐life of fresh‐cut apple. Italian Journal of Food Science, 30(1), 61–74. 10.1155/2017/9764650 [DOI] [Google Scholar]
- AOAC (2000). Meat and meat products. In Soderberg D. L. (Ed.), Official methods of analysis (17th ed., pp. 931–948). Association of Official Analytical Chemists. [Google Scholar]
- Azizi‐Lalabadi, M. , Ehsani, A. , Ghanbarzadehc, B. , & Divbandd, B. (2020). Polyvinyl alcohol/gelatin nanocomposite containing ZnO, TiO2 or ZnO/TiO2 nanoparticles doped on 4A zeolite: Microbial and sensory qualities of packaged white shrimp during refrigeration. International Journal of Food Microbiology, 312, 108375. 10.1016/j.ijfoodmicro.2019.108375 [DOI] [PubMed] [Google Scholar]
- Azlin‐Hasim, S. , Cruz‐Romero, M. C. , Morris, M. A. , Padmanabhan, S. C. , Cummins, E. , & Kerry, J. P. (2016). The potential application of antimicrobial silver polyvinyl chloride nanocomposite films to extend the shelf‐life of chicken breast fillets. Food and Bioprocess Technology, 9, 1661–1673. 10.1007/s11947-016-1745-7 [DOI] [Google Scholar]
- Bagheri, F. , Nejatian, M. , Abbaszadeh, S. , & Taghdir, M. (2020). The effect of gelatin and thymol‐loaded nanostructured lipid carrier on physicochemical, rheological, and sensory properties of sesame paste/date syrup blends as a snack bar. Journal of Texture Studies, 51, 501–510. 10.1111/jtxs.12511 [DOI] [PubMed] [Google Scholar]
- Bagheri, F. , Radi, M. , & Amiri, S. (2014). Use of sweetener stevioside for produce dietary breakfast cream. Agriculture Science Developments, 3, 284–291. [Google Scholar]
- Bagheri, F. , Radi, M. , & Amiri, S. (2019a). Evaluating the physical, mechanical and morphological properties of sodium alginate nanocomposite film containing solid lipid nano‐particles. Iranian Journal of Food Science and Technology, 16, 263–271. [Google Scholar]
- Bagheri, F. , Radi, M. , & Amiri, S. (2019b). Drying conditions highly influence the characteristics of glycerol‐plasticized alginate films. Food Hydrocolloids, 90, 162–171. 10.1016/j.foodhyd.2018.12.001 [DOI] [Google Scholar]
- Barbin, W. (1975). Evaluation of the direct thiobarbituric acid extraction method for determining oxidative rancidity in Mackerel (Scomber scombrus L.). European Journal of Lipid Science and Technology, 77, 239–240. 10.1002/lipi.19750770610 [DOI] [Google Scholar]
- Bojorges, H. , Ríos‐Corripio, M. A. , Hernández‐Cázares, A. S. , Hidalgo‐Contreras, J. V. , & Contreras‐Oliva, A. (2020). Effect of the application of an edible film with turmeric (Curcuma longa L.) on the oxidative stability of meat. Food Science and Nutrition, 8(8), 4308–4319. 10.1002/fsn3.1728 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen, B. J. , Zhou, Y. J. , Wei, X. Y. , Xie, H. J. , Hider, R. C. , & Zhou, T. (2016). Edible antimicrobial coating incorporating a polymeric iron chelator and its application in the preservation of surimi product. Food and Bioprocess Technology, 9, 1031–1039. 10.1007/s11947-016-1693-2 [DOI] [Google Scholar]
- CLSI (1999). Methods for determining bactericidal activity of antimicrobial agents. Approved Guideline, CLSI document M26‐A, Volume 19, Number 18. Clinical and Laboratory Standards Institute, 950 West Valley Roadn Suite 2500, Wayne, Pennsylvania 19087, USA. [Google Scholar]
- Diao, X. , Huan, Y. , & Chitrakar, B. (2020). Extending the shelf life of ready‐to‐eat spiced chicken meat: Garlic aqueous extracts‐carboxymethyl chitosan ultrasonicated coating solution. Food and Bioprocess Technology, 13, 786–796. 10.1007/s11947-020-02428-7 [DOI] [Google Scholar]
- El Adab, S. , & Hassouna, M. (2016). Proteolysis, lipolysis and sensory characteristics of a Tunisian dry fermented poultry meat sausage with oregano and thyme essential oils. Journal of Food Safety, 36(1), 19–32. 10.1111/jfs.12209 [DOI] [Google Scholar]
- El‐Ghorab, A. H. , Nauman, M. , Anjum, F. M. , Hussain, S. H. , & Nadeem, M. (2010). A comparative study on chemical composition and antioxidant activity of ginger (Zingiber officinale) and cumin (Cuminum cyminum). Journal of Agricultural and Food Chemistry, 58, 8231–8237. 10.1021/jf101202x [DOI] [PubMed] [Google Scholar]
- Feng, Z. , Li, L. , Wang, Q. , Wu, G. , Liu, C. H. , Jiang, B. , & Xu, J. (2019). Effect of antioxidant and antimicrobial coating based on whey protein nanofibrils with TiO2 nanotubes on the quality and shelf life of chilled meat. International Journal of Molecular Science, 20, 1184. 10.3390/ijms20051184 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghaderi‐Ghahfarokhi, M. , Barzegar, M. , Sahari, M. A. , & Azizi, M. H. (2016). Nanoencapsulation approach to improve antimicrobial and antioxidant activity of thyme essential oil in beef burgers during refrigerated storage. Food and Bioprocess Technology, 9, 1187–1201. 10.1007/s11947-016-1708-z [DOI] [Google Scholar]
- Guo, M. , Jin, T. Z. , & Yang, R. (2014). Antimicrobial polylactic acid packaging films against Listeria and Salmonella in culture medium and on ready‐to‐eat meat. Food and Bioprocess Technology, 7, 3293–3307. 10.1007/s11947-014-1322-x [DOI] [Google Scholar]
- Haghiroalsadat, F. , Bernard, F. , Kalantar, S. M. , Sheikhha, M. H. , Hokmollahi, F. , & Azimzadeh, M. (2010). Bunium persicum (Black Caraway) of Yazd province: Chemical assessment and evaluation of its antioxidant effects. Journal of Shaheed Sadoughi University of Medical Science, 18(4), 284–291 [Persian]. [Google Scholar]
- Heydari, S. , Jooyandeh, H. , Alizadeh Behbahani, B. , & Noshad, M. (2020). The impact of Qodume Shirazi seed mucilage‐based edible coating containing lavender essential oil on the quality enhancement and shelf life improvement of fresh ostrich meat: An experimental and modeling study. Food Science and Nutrition, 8(12), 6497–6512. 10.1002/fsn3.1940 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heydari‐Majd, M. , Ghanbarzadeh, B. , Shahidi‐Noghabi, M. , Najafi, M. A. , & Hosseini, M. (2019). A new active nanocomposite film based on PLA/ZnO nanoparticle/essential oils for the preservation of refrigerated Otolithes ruber fillets. Food Packaging and Shelf Life, 19, 94–103. 10.1016/j.fpsl.2018.12.002 [DOI] [Google Scholar]
- Hosseinifarahi, M. , Jamshidi, E. , Amiri, S. , Kamyab, F. , & Radi, M. (2020). Quality, phenolic content, antioxidant activity, and the degradation kinetic of some quality parameters in strawberry fruit coated with salicylic acid and Aloe vera gel. Journal of Food Processing and Preservation, 44(9), e14647. 10.1111/jfpp.14647 [DOI] [Google Scholar]
- Hosseini‐Farahi, M. , Moradi Kohvare, M. , Rezaee, T. , Alahdadi, F. , & Bagheri, F. (2016). The influence of chitosan edible coatings and calcium treatments on quality indices of peach fruit cv'.Alberta'during cold storage. Agricultural Communications, 4, 7–13. [Google Scholar]
- Hosseini‐Farahi, M. H. , Radi, M. , Bagheri, F. , & Jamshidi, E. (2018). Evaluation of postharvest quality and organoleptic characteristics of strawberry with application of aloe vera gel, acetic acid and UV‐B irradiation. Iranian Journal of Horticultural Science and Technology, 19, 99–114. [Google Scholar]
- Hur, J. S. , Oh, S. O. , Lim, K. M. , Jung, J. S. , Kim, J. W. , & Koh, Y. J. (2005). Novel effects of TiO2 photocatalyticozonation on control of postharvest fungal spoilage of kiwifruit. Postharvest Biology and Technology, 35(1), 109–113. 10.1016/j.postharvbio.2004.03.013 [DOI] [Google Scholar]
- Hyldgaard, M. , Mygind, T. , & Meyer, R. L. (2012). Essential oils in food preservation: Mode of action, synergies and interactions with food matrix components. Frontiersin Microbiology, 3(12), 1–24. 10.3389/fmicb.2012.00012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Junior, A. C. S. , de Oliveira, R. F. , Henry, F. C. , Junior, J. A. M. , Moulin, M. M. , Lucia, S. M. D. , Quirino, C. R. , Martins, M. L. L. , & Rampe, M. C. C. (2020). Physicochemical composition, lipid oxidation, and microbiological quality of ram mortadella supplemented with Smallanthus sonchifolius meal. Food Science and Nutrition, 8, 5953–5961. 10.1002/fsn3.1880 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Júnior, A. V. , Fronza, N. , Foralosso, F. B. , Dezen, D. , Huber, E. , dos Santos, J. H. Z. , Machado, R. A. F. , & Quadri, M. G. N. (2015). Biodegradable duo‐functional active film: Antioxidant and antimicrobial actions for the conservation of beef. Food and Bioprocess Technology, 8, 75–87. 10.1007/s11947-014-1376-9 [DOI] [Google Scholar]
- Karimi Khorrami, N. , Radi, M. , Amiri, S. , & McClements, D. J. (2021). Fabrication and characterization of alginate‐based films functionalized with nanostructured lipid carriers. International Journal of Biological Macromolecules, 182, 373–384. 10.1016/j.ijbiomac.2021.03.159 [DOI] [PubMed] [Google Scholar]
- Khezrian, A. , & Shahbazi, Y. (2018). Application of nanocompostie chitosan and carboxymethyl cellulose films containing natural preservative compounds in minced camel’s meat. International Journal of Biological Macromolecules, 106, 1146–1158. 10.1016/j.ijbiomac.2017.08.117 [DOI] [PubMed] [Google Scholar]
- Kirk, R. S. , & Sawyer, R. (1991). Pearson’s composition and analysis of foods. Longman scientific and technical. Harllow. [Google Scholar]
- Marcous, B. A. , Rasouli, S. , & Ardestani, F. (2017). Low‐density polyethylene films loaded by titanium dioxide and zinc oxide nanoparticles as a new active packaging system against Escherichia coli O157:H7 in fresh calf minced meat. Packaging Technology and Science, 30, 693–701. 10.1002/pts.2312 [DOI] [Google Scholar]
- Martiny, T. R. , Pacheco, B. S. , Pereira, C. M. P. , Mansilla, A. , Astorga‐España, M. S. , Dotto, G. L. , Moraes, C. C. , & Rosa, G. S. (2020). A novel biodegradable film based on κ‐carrageenan activated with olive leaves extract. Food Science and Nutrition, 8, 3147–3156. 10.1002/fsn3.1554 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mojaddar Langroodi, A. , Nematollahi, A. , & Sayadi, M. (2021). Chitosan coating incorporated with grape seed extract and Origanum vulgare essential oil: An active packaging for turkey meat preservation. Journal of Food Measurement and Characterization, 15, 2790–2804. 10.1007/s11694-021-00867-0 [DOI] [Google Scholar]
- Molayi, R. , Ehsani, A. , & Yousefi, M. (2018). The antibacterial effect of whey protein–alginate coating incorporated with the lactoperoxidase system on chicken thigh meat. Food Science and Nutrition, 6, 878–883. 10.1002/fsn3.634 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Najjaa, H. , Chekki, R. , Elfalleh, W. , Tlili, H. , Jaballah, S. , & Bouzouita, N. (2020). Freeze‐dried, oven‐dried, and microencapsulation of essential oil from Allium sativum as potential preservative agents of minced meat. Food Science and Nutrition, 8, 1995–2003. 10.1002/fsn3.1487 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nehchiri, N. , Amiri, S. , & Radi, M. (2021). Improving the water barrier properties of alginate packaging films by submicron coating with drying linseed oil. Packaging Technology and Science, 34(5), 283–295. 10.1002/pts.2558 [DOI] [Google Scholar]
- Noshad, M. , Alizadeh Behbahani, B. , Jooyandeh, H. , Rahmati‐Joneidabad, M. , Ebrahimi, M. , Kaykha, H. , & Ghodsi Sheikhjan, M. (2021). Utilization of Plantago major seed mucilage containing Citrus limon essential oil as an edible coating to improve shelf‐life of buffalo meat under refrigeration conditions. Food Science and Nutrition, 9(3), 1625–1639. 10.1002/fsn3.2137 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Othman, S. H. , Abd Salam, N. R. , Zainal, N. , Kadir Basha, R. , & Talib, R. A. (2014). Antimicrobial activity of TiO2 nanoparticle‐coated film for potential food packaging applications. International Journal of Photoenergy, 2014, 1–6. 10.1155/2014/945930 [DOI] [Google Scholar]
- Paspaltsis, I. , Kotta, K. , Lagoudaki, R. , Grigoriadis, N. , Poulios, I. , & Sklaviadis, T. (2006). Titanium dioxide photocatalytic inactivation of prions. Journal of General Virology, 87(10), 3125–3130. 10.1099/vir.0.81746-0 [DOI] [PubMed] [Google Scholar]
- Radi, M. , Afshari Jouybari, H. , Mesbahi, G. , Farahnaky, H. , & Amiri, S. (2010). Effect of hot acetic acid solutions on postharvest decay caused by Penicillium expansum on Red Delicious apples. Scientia Horticulturae, 126, 421–425. 10.1016/j.scienta.2010.06.023 [DOI] [Google Scholar]
- Radi, M. , Akhavan‐Darabi, S. , Akhavan, H. R. , & Amiri, S. (2017). The use of orange peel essential oil microemulsion and nanoemulsion in pectin‐based coating to extend the shelf life of fresh‐cut orange. Journal of Food Processing and Preservation, 42, e13441. 10.1111/jfpp.13441 [DOI] [Google Scholar]
- Radi, M. , & Amiri, S. (2013). Comparison of the rheological behavior of solutions and formulated oil in water emulsions containing carboxymethylcellulose (CMC). Journal of Dispersion Science and Technology, 34(4), 582–589. 10.1080/01932691.2012.681607 [DOI] [Google Scholar]
- Radi, M. , Firouzi, E. , Akhavan, H. , & Amiri, S. (2017). Effect of gelatin based edible coatings incorporated with Aloe vera and black and green tea extracts on the shelf life of fresh‐cut oranges. Journal of Food Quality, 2017, 1–10. 10.1155/2017/9764650 [DOI] [Google Scholar]
- Raeisi, M. , Tabaraei, A. , Hashemi, M. , & Behnampour, N. (2016). Effect of sodium alginate coating incorporated with nisin, Cinnamomum zeylanicum, and rosemary essential oils on microbial quality of chicken meat and fate of Listeria monocytogenes during refrigeration. International Journal of Food Microbiology, 238, 139–145. 10.1016/j.ijfoodmicro.2016.08.042 [DOI] [PubMed] [Google Scholar]
- Sani, M. A. , Ehsani, A. , & Hashemi, M. (2017). Whey protein isolate/cellulose nanofibre/TiO2 nanoparticle/rosemary essential oil nanocomposite film: Its effect on microbial and sensory quality of lamb meat and growth of common foodborne pathogenic bacteria during refrigeration. International Journal of Food Microbiology, 251, 8–14. 10.1016/j.ijfoodmicro.2017.03.018 [DOI] [PubMed] [Google Scholar]
- Sayadi, M. , Amiri, S. , & Radi, M. (2021). Active packaging nanocomposite gelatin‐based films as a carrier of nano TiO2 and cumin essential oil: The effect on quality parameters of fresh chicken. Journal of Food Measurement and Characterization, 16, 420–430. 10.1007/s11694-021-01169-1 [DOI] [Google Scholar]
- Sayadi, M. , Mojaddar Langroodi, A. , & Jafarpour, D. (2021). Impact of zein coating impregnated with ginger extract and Pimpinella anisum essential oil on the shelf life of bovine meat packaged in modified atmosphere. Journal of Food Measurement and Characterization, 15, 5231–5244. 10.1007/s11694-021-01096-1 [DOI] [Google Scholar]
- Sayadi, M. , Mojaddar Langroodi, A. , & Pourmohammadi, K. (2021). Combined effects of chitosan coating incorporated with Berberis vulgaris extract and Mentha pulegium essential oil and MAP in the shelf life of turkey meat. Journal of Food Measurement and Characterization, 15(6), 5159–5169. 10.1007/s11694-021-01068-5 [DOI] [Google Scholar]
- Sellimi, S. , Younes, I. , Ayed, H. B. , Maalej, H. , Montero, V. , Rinaudo, M. , Dahia, M. , Mechichi, T. , Hajji, M. , & Nasri, M. (2015). Structural, physicochemical and antioxidant properties of sodium alginate isolated from a Tunisian brown seaweed. International Journal of Biological Macromolecules, 72, 1358–1367. 10.1016/j.ijbiomac.2014.10.016 [DOI] [PubMed] [Google Scholar]
- Sepahvand, S. , Amiri, S. , Radi, M. , & Akhavan, H. R. (2021). Antimicrobial activity of thymol and thymol‐nanoemulsion against three food‐borne pathogens inoculated in a sausage model. Food and Bioprocess Technology, 14(10), 1936–1945. 10.1007/s11947-021-02689-w [DOI] [Google Scholar]
- Sharafati Chaleshtori, F. , Taghizadeh, M. , Rafieian‐kopaei, M. , & Sharafati‐chaleshtori, R. (2016). Effect of chitosan incorporated with cumin and eucalyptus essential oils as antimicrobial agents on fresh chicken meat. Journal of Processing and Preservation, 40, 396–404. 10.1111/jfpp.12616 [DOI] [Google Scholar]
- Sirocchi, V. , Devlieghere, F. , Peelman, N. , Sagratini, G. , Maggi, F. , Vittori, S. , & Ragaert, P. (2017). Effect of Rosmarinus officinalis L. essential oil combined with different packaging conditions to extend the shelf life of refrigerated beef meat. Food Chemistry, 221, 1069–1076. 10.1016/j.foodchem.2016.11.054 [DOI] [PubMed] [Google Scholar]
- Taheri, T. , Fazlaraa, A. , Roomiani, L. , & Taheri, S. (2018). Effect of chitosan coating enriched with cumin (Cuminum cyminum L.) essential oil on the quality of refrigerated turkey breast meat. Italian Journal of Food Science, 30, 2018–2628. https://doi.org/ 10.14674/IJFS-1158 [DOI] [Google Scholar]
- Takma, D. K. , & Kore, F. (2019). Active packaging films as a carrier of black cumin essential oil: Development and effect on quality and shelf‐life of chicken breast meat. Food Packaging and Shelf Life, 19, 210–217. 10.1016/j.fpsl.2018.11.002 [DOI] [Google Scholar]
- Tosati, J. V. , Messias, V. C. , Carvalho, P. I. N. , Pollonio, M. A. R. , Meireles, M. A. A. , & Monteiro, A. R. (2017). Antimicrobial effect of edible coating blend based on turmeric starch residue and gelatin applied onto fresh frankfurter sausage. Food and Bioprocess Technology, 10, 2165–2175. 10.1007/s11947-017-1985-1 [DOI] [Google Scholar]
- Wannera, J. , Bail, S. , Jirovetz, L. , Buchbauer, G. , Schmidt, E. , Gochev, V. , Girova, T. , Atanasova, T. , & Stoyanova, A. (2010). Chemical composition and antimicrobial activity of cumin oil (Cuminum cyminum, Apiaceae). Natural Product Communications, 5(9), 1355–1358. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data will be made available upon reasonable request.


