Abstract
Introduction
To date, COVID‐19 has claimed 4.9 million lives. Diabetes has been identified as an independent risk factor of serious outcomes in people with COVID‐19 infection. Whether that holds true across world regions uniformly has not been previously assessed.
Methods
This study offers the first umbrella systematic review and meta‐analysis to analyse the collective and geographically stratified mortality, ICU admission, ventilation requirement, illness severity and discharge rate amongst patients with diabetes. Five databases (EMBASE, MEDLINE, CAB Abstracts, PsychInfo and Web of Science) and 3 additional sources (SSRN's eLibrary, Research Square and MedRxiv) were searched from inception to 30 August 2021. Prospective and retrospective cohort studies, reporting the association between diabetes and one or more COVID‐19 hospitalization outcomes, were included. This meta‐analysis was registered on PROSPERO, CRD42021278579. Abbreviated MeSH terms used for search were as follows: (Diabetes) AND (2019 Novel Coronavirus Disease), adapted per database requirements. Exclusion criteria exclusion criteria were as follows: (1) none of the primary or secondary outcomes of meta‐analysis reported, (2) no confirmed COVID‐19 infection (laboratory or clinical) and (3) no unexposed population (solely patients with diabetes included). Quality of the included studies were assessed using the Newcastle‐Ottawa Scale (NOS) whilst quality of evidence by the GRADE framework. Studies that were clinically homogeneous were pooled. Summative data and heterogeneity were generated by the Cochrane platform RevMan (V. 5.4).
Results
Overall, 158 observational studies were included, with a total of 270,212 of participants, median age 59 [53–65 IQR] of who 56.5% were male. A total of 22 studies originated from EU, 90 from Far East, 16 from Middle East and 30 from America. Data were synthesized with mixed heterogeneity across outcomes. Pooled results highlighted those patients with diabetes were at a higher risk of COVID‐19‐related mortality, OR 1.87 [95%CI 1.61, 2.17]. ICU admissions increased across all studies for patients with diabetes, OR 1.59 [95%CI 1.15, 2.18], a result that was mainly skewed by Far East‐originating studies, OR 1.94 [95%CI 1.51, 2.49]. Ventilation requirements were also increased amongst patients with diabetes worldwide, OR 1.44 [95%CI 1.20, 1.73] as well as their presentation with severe or critical condition, OR 2.88 [95%CI 2.29, 3.63]. HbA1C levels under <70 mmol and metformin use constituted protective factors in view of COVID‐19 mortality, whilst the inverse was true for concurrent insulin use.
Conclusions
Whilst diabetes constitutes a poor prognosticator for various COVID‐19 infection outcomes, variability across world regions is significant and may skew overall trends.
Keywords: COVID‐19, diabetes, discharge, disease severity, intensive care, mortality, ventilation
Whilst diabetes constitutes a poor prognosticator for various COVID‐19 infection outcomes, variability across world regions is significant and may skew overall trends.

1. INTRODUCTION
COVID‐19, a novel coronavirus identified in late 2019, has rapidly spread worldwide resulting in the first pandemic experienced in the modern world since 1918. 1 Currently, more than 220 million have been infected, with 4.9 million deaths as of 18 October 2021. Metabolic conditions, and primarily diabetes, have emerged since the beginning of the pandemic as significant risk factors for poor COVID‐19 outcomes. 2 A wealth of observational studies and consequently meta‐analyses have attempted to quantify the association of diabetes as an independent risk factor of poor COVID‐19 outcomes and consistently found that diabetes is associated with poorer outcomes across this patient group.
Until present and to the best of our knowledge, an umbrella systematic review and meta‐analysis has not been conducted to collectively assess available meta‐analyses. Furthermore, whilst patient ethnicity as well as global discrepancies of healthcare facilities and antidiabetic medication access are well‐established variables, 3 , 4 , 5 , 6 , 7 no previous work has factored in, study geographical origin to assess the potential impact of these parameters on COVID‐19 outcomes in patients with diabetes.
We primarily aim to quantify the overall impact of diabetes in COVID‐19 across three main outcomes: mortality, ICU admission and ventilation (invasive and non‐invasive). Secondary outcomes include illness severity, discharge rate, identification of putative geographical variability across outcomes and associated factors of poorer or improved prognosis, amongst patients with diabetes.
2. METHODS
2.1. Search strategy and selection criteria
A systematic literature review was conducted according to the guidelines of the Preferred Reporting Items for Systematic Reviews and Meta‐Analyses (PRISMA) (Figure 1). For the present study, a protocol was prospectively registered at the PROSPERO database (CRD42021278579), amended on the 14 October 2020 to extend date of expected submission. Independent literature search for relevant studies, restricted to systematic reviews and meta‐analyses, was performed up to 30 August 2021 on five databases: EMBASE, MEDLINE, CAB Abstracts, PsychInfo and Web of Science. Additional records were identified through other sources, including SSRN's eLibrary, Research Square and MedRxiv to reduce publication bias. The MedRxiv search was simplified according to database search functionality. The references of the included systematic review and meta‐analysis studies were scrutinized for additional relevant studies.
FIGURE 1.
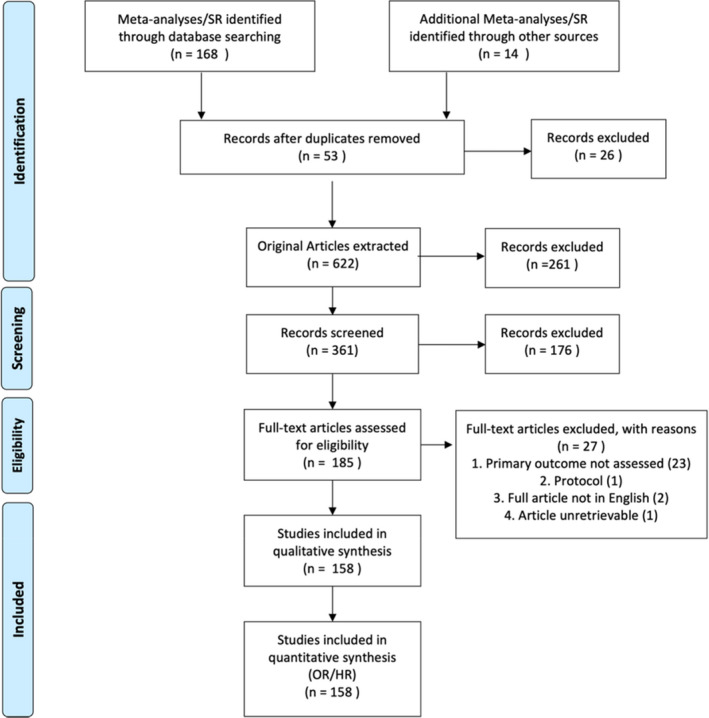
PRISMA 2009 flow diagram. Search strategy included and excluded studies 179
The following search term was used in OVID: (Diabetes OR T2DM OR T1DM OR Diabetes mellitus).mh,tw,ab,hw,kw. AND (2019 Novel Coronavirus Disease OR 2019 Novel Coronavirus Infection OR 2019‐nCoV Disease OR 2019‐nCoV Infection OR COVID‐19 Pandemic OR COVID‐19 Pandemics OR COVID‐19 Virus Disease OR COVID‐19 Virus Infection OR COVID19 OR Coronavirus Disease 2019 OR Coronavirus Disease‐19 OR SARS Coronavirus 2 Infection OR SARS‐CoV‐2 Infection).mp. limit to (English language and humans). The same search strategy was adapted for the remaining databases.
Prospective and retrospective cohort studies were extracted from eligible systematic reviews and meta‐analyses to enable umbrella systematic review of available data as described in Aromataris et al., 8 examining COVID‐19 mortality, ICU admission, ventilation requirement, disease severity and discharge in the context of diabetes (Table S1). Restrictions included English language and human. After removing duplicates (EndNote V.20), citations were screened by title and abstract; then, full texts were appraised to determine their eligibility by two authors (SK and MP) (Figure 2). Two authors (SK and MP) independently conducted the abstract and full‐text screening. Disagreements were resolved by a consensus meeting. Peer‐reviewed full‐text papers that reported one or more of the primary outcomes were selected. Full‐text exclusion criteria were as follows: (1) none of the primary or secondary outcomes of meta‐analysis reported, (2) no confirmed COVID‐19 infection (laboratory or clinical) and (3) no unexposed population (solely patients with diabetes included). Excluded studies and justifications are recorded in Table S1. Data from each article was extracted by two authors (SK and MP) and validated independently by author crossover: (1) Total number of participants, type of study, setting of study (hospital/community), sample size (total), patients with diabetes (total), Number of patients with T1DM or T2DM if available [N; %], mortality [N; %], ICU admission [N; %], Severity (mild, moderate, severe/critical [N;%], ventilation required included both non‐invasive [Continuous positive airway pressure (CPAP) Biphasic Positive Airway Pressure (BiPAP), High‐Flow Nasal Cannula (HFNC) and invasive ventilation application, positive end‐expiratory pressure (PEEP)] events [N; %], discharge rate [N; %], patient characteristics: age, gender method of COVID‐19 diagnosis.
FIGURE 2.
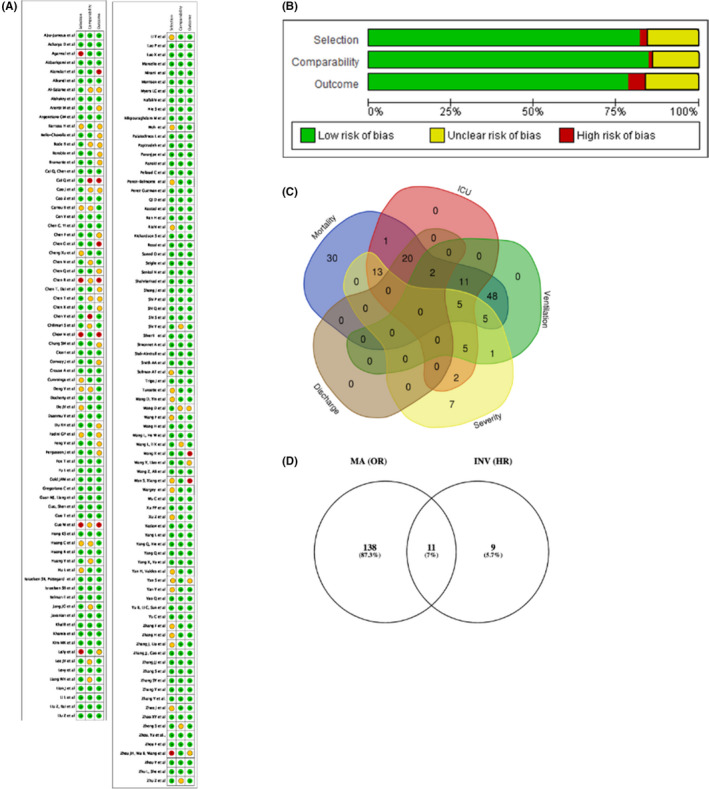
Risk of bias graphs and study data extraction strategy. (A) Review authors' judgements about each risk of bias item per included study. Review authors' judgements about each risk of bias item presented as percentages across all included studies (B). Outcomes addressed by total number of studies and overlap (C), Number of studies used for addressing primary and secondary outcomes (D)
Quality of the included studies were assessed by two independent reviewers (SK and MP) using the Newcastle‐Ottawa Scale (NOS) for observational studies. 9 Studies were of high quality if a NOS score ≥6 was achieved. 10 Adequate follow‐up was ≥30 days (Table S1). Overall grading the quality of evidence was assessed by the GRADE framework. 11 Heterogeneity was assessed using I 2.
2.2. Study outcomes
Study primary outcomes included mortality, ICU admission and ventilation requirement events. These were defined as the proportion of people with an event, of each respective outcome, in comparison to people without the event, in the same population. Secondary outcomes were disease severity [mild, moderate and severe/critical] (events) and discharge events amongst patients with diabetes vs. without. Stratified analysis was conducted by global geographical region to identify sources of heterogeneity amongst world regions.
Confounding factors of increased mortality were assessed using generic inverse variance model regression (IVR), adjusted with covariates consistent with the primary outcome and expressed as random effect (RE), hazard ratio (HR) measures. Variables assessed included age (continuous variable), gender (categorical variable), smoking status (categorical), alcohol misuse (categorical), HbA1C ≤ 70 mmol vs. >70 mmol (categorical), diabetes type (Type 1 vs. Type 2) (categorical), insulin use (categorical), metformin use (categorical), DPP4 inhibitor use (categorical), cardiovascular comorbidities including myocardial infarction, ischaemic cardiomyopathy, hypertension (categorical), acute and chronic kidney injury (categorical), immunocompromised (categorical), biochemical findings (including white blood count, C‐reactive protein) (continuous). Both crude (unadjusted) and adjusted HRs were presented with associated 95% confidence intervals (CI) (Figure S3). For crude HRs, antidiabetic medication brand, dose and duration of action were not possible to factor in, due to lack of data reporting in individual studies. Adjusted HR (95% CI) of mortality amongst patients with diabetes was adjusted for age, gender, cardiovascular comorbidities, biochemical findings, smoking/alcohol use, immunocompromised status and medications (Figure S4).
Mortality was measured as 28‐ or 30‐day death events or till the end of follow‐up of each individual study (Table S1). Illness severity was assessed by CURB‐65 stratification score; Guidance for Corona Virus Disease 2019 (6th edition) released by the National Health Commission of China, 12 , 13 modified version of the WHO/International Severe Acute Respiratory and Emerging infection Consortium case record form for severe acute respiratory infections, 12,13 or the necessity for the use of a high‐flow nasal cannula, mechanical ventilation, CRRT, or ECMO, or admission to an ICU, of as a respiratory rate > 30/min, oxygen saturation ≤ 93%, PaO2/FiO2 ≤ 300 mm Hg., with shock or respiratory failure, mechanical ventilation requirement, or combined with other organ failure, requiring admission to intensive care unit (ICU). Individual severity definition per study is presented in Table S1.
2.3. Data analysis
Clinical context and design were compared and where appraised as homogeneous, studies were considered as suitable for pooling. 14 The meta‐analysis was conducted by computing the pooled odds ratio (OR) as per Haensel–Mantel model or Hazard ratio (HR) as per inverse variance analysis, random effects (RE) with Review Manager (RevMan) V 5.4 software. Statistical heterogeneity was quantified using I 2 statistics and Cochrane Q tests.
2.3.1. Assessment of heterogeneity and subgroups to explain differences
Only studies that are clinically homogeneous were pooled. Heterogeneity was assessed using I 2, and I 2 greater than 70% was explored using subgroups. 14 The following subgroups were used to explain the heterogeneity: risk of bias; age, geography, study design (prospective). Asymmetry was assessed by funnel plot, and asymmetry was assessed formally by rank correlation test (Begg's test; RevMan V. 5.4). Sensitivity analyses were conducted to assess the impact of individual potential confounding variables. Publication bias was assessed visually by funnel plot, and asymmetry was formally assessed, by rank correlation test (Begg's test). 15
3. RESULTS
Following the PRISMA guidelines on systematic review search, we identified 53 eligible meta‐analyses studies for study extraction. Post‐individual study extraction and duplicate study removal, we identified 185 studies eligible for full‐text screening (Figure 2). Full‐text screening excluded 27 studies (Table S1). A total of 158 studies remained, 16 , 17 , 18 , 19 , 20 , 21 , 22 , 23 , 24 , 25 , 26 , 27 , 28 , 29 , 30 , 31 , 32 , 33 , 34 , 35 , 36 , 37 , 38 , 39 , 40 , 41 , 42 , 43 , 44 , 45 , 46 , 47 , 48 , 49 , 50 , 51 , 52 , 53 , 54 , 55 , 56 , 57 , 58 , 59 , 60 , 61 , 62 , 63 , 64 , 65 , 66 , 67 , 68 , 69 , 70 , 71 , 72 , 73 , 74 , 75 , 76 , 77 , 78 , 79 , 80 , 81 , 82 , 83 , 84 , 85 , 86 , 87 , 88 , 89 , 90 , 91 , 92 , 93 , 94 , 95 , 96 , 97 , 98 , 99 , 100 , 101 , 102 , 103 , 104 , 105 , 106 , 107 , 108 , 109 , 110 , 111 , 112 , 113 , 114 , 115 , 116 , 117 , 118 , 119 , 120 , 121 , 122 , 123 , 124 , 125 , 126 , 127 , 128 , 129 , 130 , 131 , 132 , 133 , 134 , 135 , 136 , 137 , 138 , 139 , 140 , 141 , 142 , 143 , 144 , 145 , 146 , 147 , 148 , 149 , 150 , 151 , 152 , 153 , 154 , 155 , 156 , 157 , 158 , 159 , 160 , 161 , 162 , 163 , 164 , 165 , 166 , 167 , 168 , 169 , 170 , 171 , 172 all of which were included in the systematic review and 149 were included in the meta‐analysis (Figures 1 and 2; Figure S2, Table S1). 16 , 17 , 18 , 19 , 20 , 21 , 22 , 23 , 24 , 25 , 26 , 27 , 28 , 29 , 30 , 31 , 32 , 33 , 34 , 35 , 36 , 37 , 38 , 39 , 40 , 41 , 42 , 43 , 44 , 45 , 46 , 47 , 48 , 49 , 50 , 51 , 52 , 53 , 54 , 55 , 56 , 57 , 58 , 59 , 60 , 61 , 62 , 63 , 64 , 65 , 66 , 67 , 68 , 69 , 70 , 71 , 72 , 73 , 74 , 75 , 76 , 77 , 78 , 79 , 80 , 81 , 82 , 83 , 84 , 85 , 86 , 87 , 88 , 89 , 90 , 91 , 92 , 93 , 94 , 95 , 96 , 97 , 98 , 99 , 100 , 101 , 102 , 103 , 104 , 105 , 106 , 107 , 108 , 109 , 110 , 111 , 112 , 113 , 114 , 115 , 116 , 117 , 118 , 119 , 120 , 121 , 122 , 123 , 124 , 125 , 126 , 127 , 128 , 129 , 130 , 131 , 132 , 133 , 134 , 135 , 136 , 137 , 138 , 139 , 140 , 141 , 142 , 143 , 144 , 145 , 146 , 147 , 148 , 149 , 150 , 151 , 152 , 153 , 154 , 155 , 156 , 157 , 158 , 159 , 160 , 161 , 162 , 163
3.1. Included study designs
Ten [N:10] studies were prospectivewhilst the remaining [N:148] retrospective observational (Table S1). A total of fifteen studies were preprints [N:15] (Table S1). All studies [N:157] included patients from a hospital setting, either ward level care or specialized COVID‐19 wards with the exception of one, which was conducted in a care home setting. 162 Total patient sample was comprising a total of 270,212 patients, of which 57,801 were diagnosed with diabetes. A total of 488 patients were diagnosed with Type 1 whilst the remaining patients [N:57313] with type 2 diabetes.
Median age of total patient sample was 59 [53–65 IQR25th–75th percentile] (Figure S1A). Over half (56.5%) [N: 105778/187253] of the COVID‐19‐positive patients were male (Figure S1B). Medians were calculated on percentage values to enable comparability across studies. Overall sample mortality was 13.45% (Median) [1.63–25.28 IQR25th–75th percentile] across of studies (Figure S1C), ventilation rate at 12.25% (median) [4.16–25 IQR25th–75th percentile], ICU admission 18.76% (median) [14.56–37.17 IQR25th–75th percentile] and discharge at 67.78% (median) [41.63–88.53 IQR25th–75th percentile] at end of study follow‐up, as per individual study (Table S1). Only mortality was found to be significantly different amongst patients with diabetes vs. without diabetes crude numbers (Figure S1C). A total of 22 studies were conducted in EU (Denmark, France, Italy, Spain, Switzerland and United Kingdom), 90 in Far East (China, Korea), 16 in Middle East (Iran, Iraq, Israel Kuweit, Oman, Qatar, Turkey) and 30 in America (29 from the United States and 1 from Mexico).
3.2. Risk of bias
We (SLK and MP) employed the NOS for quality assessment. 9 Ninety‐nine (99) studies were graded as good, forty‐two (42) as fair and eighteen (18) studies as poor according to independent grading as per NOS selection, comparability and outcome parameters (Table S1). Overall quality of evidence was assessed with the GRADE framework and was found to be high (Table S1). 11
3.3. Primary outcomes
3.3.1. Mortality
A total of 136 studies were included in the analysis of mortality as an outcome (Figure 3A; Figure S2A, Table S1). Overall, studies supported the previously reported increased mortality in patients with diabetes, OR 1.75 [95%CI 1.61, 2.17], p < .0001, I 2 = 91% (Figure 3A; Figure S2A). Heterogeneity was explored and explained by geographical region, with Far East studies (N: 77), indicating increased mortality with diabetes OR 2.40 [1.97, 2.91], I 2 = 56%, Middle East studies (N: 15), OR 1.71 [1.33, 2.19], p < .0001, I 2 = 41%, EU studies (N: 18), OR 1.47 [1.01, 2.13], p = .04, I 2 = 93% and American studies (N: 26), OR 1.42 [1.02, 1.97], p = .04, I 2 = 97% (Figure 3A, Table S1). Overall, mortality amongst the patients with diabetes was found to be higher in the Far East and Middle East world regions. Of note, prospective studies (N: 10) did not overall identify a significant increase of mortality amongst two patient groups, OR 1.32 [0.95, 1.83], p = .1, I 2 = 63% (Figure 3A, Table S1). Mortality was explored amongst patients with type 1 vs. type 2 diabetes. Only two studies 82 , 117 reported crude numbers of patients with type 1 or type 2 diabetes deaths, suggesting that patients with type 2 diabetes had worse outcomes in respect to mortality, OR 0.68 [95% CI 0.24, 1.87], I 2 = 0%. albeit the lack of statistical significance, possibly due to the limited sample size (p = .45, N: 308) (Figure S3D).
FIGURE 3.
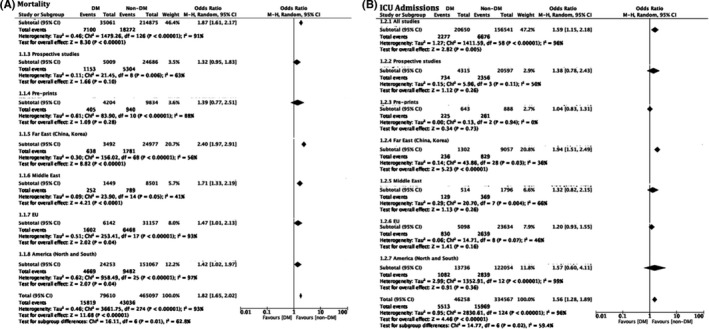
Odds associated with decreased mortality (A) or ICU admission requirement (B). Haensel–Mantel statistical method with odds ratio (random effects) as output only for included observational studies and subgroups as per subgroup title. Summative forest plots of included observational studies of the meta‐analysis (patients with Diabetes vs. without representing respective reduction in mortality (A) or ICU admission (B) rate as per patient population. Forrest and associated funnel plots (Figure S2A,B) were generated with Review Manager V. 5.4 Cochrane Tool for meta‐analysis
3.3.2. ICUadmission
A total of 59 studies were included in the analysis of ICU admission as an outcome (Figure 3B; Table S1). Overall, studies supported the previously reported increased requirement for ICU admission amongst patients with diabetes, OR 1.59 [1.15, 2.18], p = .005, I 2 = 96% (Figure 3B; Figure S2B). Heterogeneity was explored and explained by geographical region, with Far East studies (N: 29) indicating increased ICU admission requirement with diabetes, OR 1.94 [1.51, 2.49], p < .0001, I 2 = 36%, Middle East studies (N: 8), OR 1.32 [0.82, 2.15], p = .26, I 2 = 66%, EU studies (N: 9), OR 1.20 [0.93, 1.55], p = .16, I 2 = 46% and American studies (N: 13), OR 1.57 [0.60, 4.11], p = .36, I 2 = 99%. Of note, prospective (N = 4), 1.38 [0.78, 2.43], p = 0.26, I 2 = 50%, middle Eastern, European, and American studies did not reach statistical significance for this outcome (Figure 3B).
3.3.3. Ventilation requirement
A total of 83 studies were included in the analysis of ventilation requirement as an outcome amongst patients with diabetes vs. without (Figure 4A, Table S1). Overall, studies supported the previously reported increased requirement for ventilation with diabetes, OR 1.44 [1.20, 1.73], p < .0001, I 2 = 77% (Figure 4A; Figure S2C). Heterogeneity was explored and explained by geographical region, with Far East studies (N: 51) indicating increased ventilation requirements with diabetes, OR 1.61 [1.26, 2.05], p = .0001, I 2 = 41%, Middle East studies (N: 10), OR 2.02 [1.32, 3.09], p = .01, I 2 = 65%, EU studies (N: 8), OR 1.26 [1.12, 1.41], p < .0001, I 2 = 0% and American studies (N: 14), OR 0.71 [0.42, 1.18], p = .19, I 2 = 93% (Figure 4A, Table S1). Of note, American studies indicated a decrease of ventilation requirement in patients with diabetes, albeit the lack of statistical significance.
FIGURE 4.
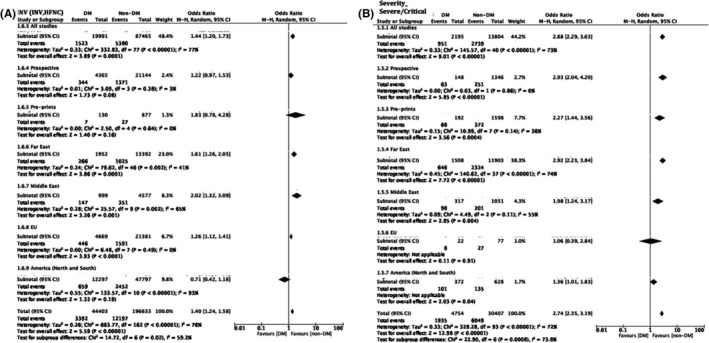
Odds associated with an increased ventilation (invasive and non‐invasive) requirement in patients with diabetes (A) and patients with diabetes presenting with severe or critical condition (B). Haensel–Mantel statistical method with odds ratio (random effects) as output only for included observational studies and subgroups as per subgroup title. Summative forest plots of included observational studies of the meta‐analysis (patients with Diabetes vs. without representing those with increased ventilation requirement (A) or those presenting with severe or critical illness (B) as per patient population. Illness severity definitions per included study are as presented in Table S1. Forrest and associated funnel plots (Figure S2C,D) were generated with Review Manager V. 5.4 Cochrane Tool for meta‐analysis
3.4. Secondary outcomes
3.4.1. Disease severity
A total of 43 studies were included in the analysis of disease severity (severe or critical) as an outcome amongst patients with diabetes vs. without (Figure 4B; Figure S2D, Table S1). Overall, studies indicated increased patient numbers with diabetes presenting in severe or critical condition, OR 2.88 [2.29, 3.63], p < .0001, I 2 = 73% (Figure 4B; Figure S2D). The reverse trend was observed for patients with diabetes presenting with mild disease severity, OR 0.45 [0.33, 0.61], p < .0001, I 2 = 83% (Figure S5). Heterogeneity was explored and explained by geographical region, with Far East studies (N: 38) indicating increased numbers of patients with diabetes presenting with severe condition, OR 2.92 [2.23, 3.84], p = .0001, I 2 = 74% and Middle East studies (N: 3), 27 , 32 , 105 OR 1.98 [1.24, 3.17], p = .004, I 2 = 55%. EU 24 and America 20 world region subgroupings were not effective given that only two studies reported patients in severe or critical condition for these world regions.
3.4.2. Discharge
A total of 22 studies reported patient discharge as an outcome amongst patients with diabetes vs. without (Figure 5, Figure S2E, Table S1). Summative results indicated decreased numbers of patients with diabetes being discharged by the end of each individual study follow‐up OR 0.59 [0.38, 0.93], p = .02, I 2 = 97% (Figure 5; Figure S2E). This finding was congruent across world regions, with Far East studies (N: 11) OR 0.40 [0.30, 0.53], p = .0001, I 2 = 53%, EU studies (N: 3), 110 , 115 , 117 OR 0.44 [0.25, 0.78], p = .004, I 2 = 81% and American studies (N: 7), OR 1.20 [0.52, 2.79] p = .0001, I 2 = 99%. Middle East world subgrouping was not feasible for this outcome given that only one study reported this outcome. 105
FIGURE 5.
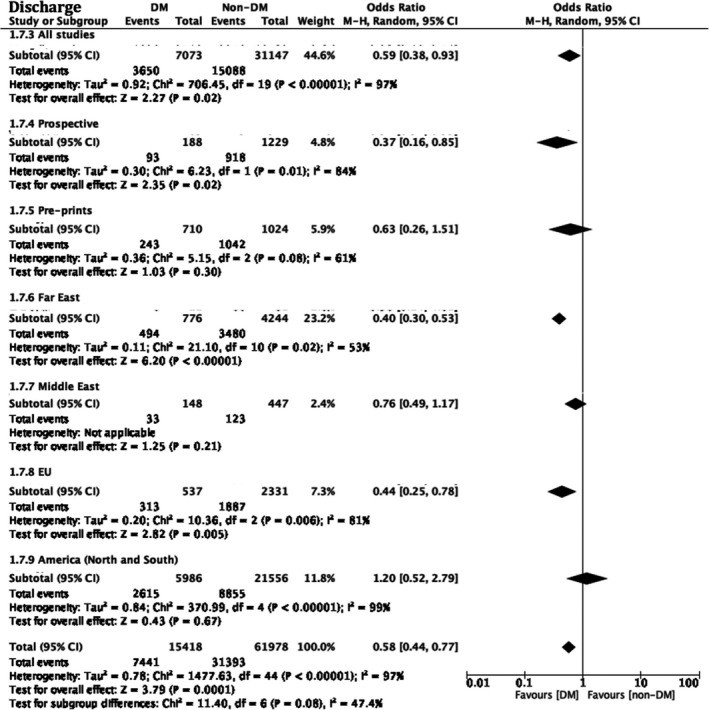
Odds associated with patient discharge at the end‐of study follow‐up. Haensel–Mantel statistical method with odds ratio (random effects) as output only for included observational studies and subgroups as per subgroup title. Summative forest plot of included observational studies of the meta‐analysis (patients with Diabetes vs. without) representing respective discharge odds between the two populations. Forrest and associated funnel plots (Figure S2E) were generated with Review Manager V. 5.4 Cochrane Tool for meta‐analysis
3.4.3. Sensitivity analysis
We sought to identify confounding factors that may correlate with COVID‐19 mortality across included studies (Figure S3). Overall, age over 65 years, HR 3.27 [2.83, 3.77], p < .0001 (Figure S3A), 58 , 157 , 172 HbA1C over 70 mmol, HR 2.75 [2.60, 2.91] p < .0001 (Figure S3C), insulin use HR 2.80 [2.29, 3.44], p < .0001 (Figure S3E), were found to increase the risk of mortality amongst patients with diabetes. The use of metformin was associated with decreased risk of mortality, HR 0.60 [0.54, 0.67], p < .0001 (Figure S3F)whilst smoking (Figure S3B), 75 , 166 diabetes type (Figure S3D) 82 , 117 and DPP4 inhibitor use (Figure S3G) were not identified as either risk or protective factors in the context of mortality of patients with diabetes with a COVID‐19 infection. Patients with diabetes had worse outcomes as displayed in the adjusted hazard ratio model, adjusted for age, gender and cardiovascular comorbidities, smoking status, alcohol abuse, immunocompromised, dementia and medications (HR 5.34 [95%CI 2.49, 11.45], p < .0001) (Figure S4).
4. DISCUSSION
Whilst overall patient mortality has decreased since the beginning of the pandemic, attributable to variable clinical and non‐clinical factors, metabolic conditions, amongst which diabetes, have emerged as significant risk factors for poor COVID‐19 outcomes. 2
The present work is the first systematic review to assess outcomes of patients with diabetes in the context of COVID‐19 infection whilst accounting for geographical location of outcome reports. Overall, our findings indicate that patients with diabetes are at a higher risk of poor hospitalization outcomes, and this is stratified by geographical region. Whilst studies originating from the Far and Middle East reported statistically significant, higher mortality across patients with diabetes, this finding was not the case for the EU, or America world regions (Table 1). Whether healthcare and affordable antidiabetic medication access inequalities or whether inherent non‐modifiable (such as genetic variants) and modifiable parameters (such as obesity) across ethnic groups are responsible for this data variability, should be considered. 3 , 4 , 5 , 6 , 7 Furthermore, whilst geographical stratification did not lead to significant differences amongst world regions regarding disease severity in patients with diabetes, the need for ventilation, here defined as either invasive or non‐invasive, was variable across the world. Studies from America, mostly reflecting USA trends, did not indicate higher ventilation requirements in this patient group. Whether this finding reflects overall healthcare system preparedness for catastrophic events, including pandemic emergence is not clear. 173
TABLE 1.
Summative results of geographical variation amongst study outcomes
| Outcome | America | EU | Far East | Middle East |
|---|---|---|---|---|
| Mortality [N total: 136] | 1.42 [1.02.1.97] [N:26] | 1.47 [1.01, 2.13] [N:18] | 2.4 [1.97, 2.91] [N:77] | 1.71 [1.33, 2.19] [N:15] |
| ICU Admission [N total: 59] | 1.57 [0.6, 4.11] [N:13] | 1.20 [0.93, 1.55] [N:9] | 1.94 [1.51, 2.49] [N:29] | 1.32 [0.82, 2.15] [N:8] |
| Ventilation requirement [N total: 83] | 0.71[0.42, 1.18] [N:14] | 1.26 [1.12, 1.41] [N:8] | 1.61 [1.26, 2.05] [N:51] | 2.02 [1.32, 3.09] [N:10] |
| Severity (Severe/Critical) [N total: 43] | 1.36 [1.01, 1.83] [N:1] | 1.06 [0.39, 2.84] [N:1] | 2.92 [2.23, 3.84] [N:38] | 1.98 [1.24, 3.17] [N:3] |
| Discharge [N total: 22] | 1.20 [0.52, 2.79] [N:7] | 0.44 [0.25, 0.78] [N:3] | 0.40 [0.30, 0.53] [N:11] | 0.76 [0.49, 1.17] [N:1] |
Note:OR 95% CI and number of studies [N] employed for the generation of each outcome depicted.
The present work has also highlighted those patients with overall better control of diabetes and on oral glucose‐lowering medications such as metformin, had significantly improved outcomes in terms of mortality. Intriguingly, insulin use has been identified as a risk factor in COVID‐19‐positive, patients with diabetes. As almost the entirety of the patients with diabetes included in the present study, were patients with type 2 diabetes and given that insulin use is the final step in the control of type 2 diabetes, this finding may signify an overall decreased patient physiological reserve or poorer all‐mortality outcomes, as shown in previous studies. 174 Whilst adjusted hazard ratios for medications amongst patients with diabetes still highlighted an increased risk of death in this patient group, biochemical variables including HbA1C where not consistently reported across studies to enable its inclusion in our adjusted model. Previous work has highlighted that hyperglycaemia in COVID‐19 patients is notable (reviewed in Accili, 2021). 175 Thus, the literature consensus, in agreement with our findings, supports that good glycaemic control is the best way prevent COVID‐19‐related admissions. 175 The lack of consistent evidence across studies did not allow for robust comparison of mortality outcomes amongst the patients with type 1 vs. with type 2 diabetes, albeit the clinical need for highlighting hospitalization outcomes in patients with type 1 diabetes. Overall, crude mortality rate for the patients with type 1 diabetes was found to be 18.5% in comparison to 20.1% in the patients with type 2 across the included studies. Whether control of diabetes, in the context of lifestyle and medical interventions rather that diabetes as a diagnosis, is a significant confounder of higher mortality rates across this patient group remains to be clarified and may pose a significant socioeconomic challenge worldwide in the light of the ongoing COVID‐19 pandemic.
4.1. Limitations
Our study suffers from the inherent limitations of the included observational studies and the evident lack of RCT studies, which whilst difficult to formulate in the context of a pandemic, would provide further insight in the delineation of diabetes effects upon COVID‐19 hospitalization outcomes. Additionally, whether patients without diabetes as reported per each study were truly representing an unaffected population from diabetes, given that approximately half of diabetes cases remain undiagnosed worldwide, remains obscure and a factor that was not feasible to be controlled in the present study. 177 Outcomes such as discharge rate which are directly affected by inadequate follow‐up periods may not be truly representative of the final discharge rates across the patients with diabetes, which may require longer hospitalization stays. 76 , 176 , 178 Inconsistent disease severity definitions as well as the consistent lack of BMI as a confounding variable of COVID‐19 mortality across patients with diabetes across studies increased overall reporting bias across the included studies. Lastly, temporal changes in COVID‐19 waves may present significant confounders of mortality reporting across world regions, albeit it should be mentioned that the majority of studies included in the present work have collected patient data during the year of 2020 with specific durations depicted in Table S1, Figure S6.
4.2. Strengths and implications for future research
To the best of our knowledge, the present work is the first umbrella meta‐analysis and systematic review, to assess patients with diabetes outcomes regarding COVID‐19 infection whilst accounting for geographical location of outcome reports. We have identified and addressed sources of heterogeneity by geographical and study design subgrouping sensitivity and IVR analysis. This study is the first to highlight major worldwide discrepancies and data variability worldwide in major clinical outcomes. Through this work, we highlight the overall healthcare system preparedness, medication availability and patient ethnicity‐related modifiable and non‐modifiable variables as putative risk factors of worldwide mortality, ICU and ventilation requirements, amongst the patients with diabetes.
5. CONCLUSION
Whilst diabetes is undoubtably a poor prognosticator of COVID‐19 infection outcomes, geographical variations across world regions are notable. Whether this finding comes as a result of the variability of healthcare provisions for control and management or patient ethnicity remains to be fully elucidated.
CONFLICT OF INTEREST
The authors have no conflict of interest to declare.
AUTHOR CONTRIBUTIONS
Stavroula Kastora:Conceptualization (lead); data curation (lead); formal analysis (lead); methodology (supporting); visualization (lead); writing – original draft (lead); writing – review and editing (lead). Manisha Patel: Data curation (supporting); methodology (supporting); writing – original draft (supporting). Ben Carter: Methodology (lead); writing – review and editing (equal). Mirela Delibegovic: Conceptualization (equal); supervision (equal); writing – review and editing (equal). Phyo Kyaw Myint: Conceptualization (equal); supervision (equal); writing – review and editing (lead).
Supporting information
Tables S1‐S2
Figures S1‐S6
Kastora S, Patel M, Carter B, Delibegovic M, Myint PK. Impact of diabetes on COVID‐19 mortality and hospital outcomes from a global perspective: An umbrella systematic review and meta‐analysis. Endocrinol Diab Metab. 2022;5:e00338. doi: 10.1002/edm2.338
DATA AVAILABILITY STATEMENT
The data used and analysed during the current study are available as online Supplementary Material.
REFERENCES
- 1. WHO Director‐General's remarks at the media briefing on 2019‐nCoV on 11 February 2020. Accessed January 25, 2021. http://www.who.int/dg/speeches/detail/who‐director‐general‐s‐remarks‐at‐the‐media‐briefing‐on‐2019‐ncov‐on‐11‐february‐2020
- 2. le Roux CW. COVID‐19 alters thinking and management in metabolic diseases. Nat Rev Endocrinol. 2021;17(2):71‐72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Kovács N, Nagy A, Dombrádi V, Bíró K. Inequalities in the global burden of chronic kidney disease due to type 2 diabetes mellitus: an analysis of trends from 1990 to 2019. Int J Environ Res Public Health. 2021;18(9):4723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Aizawa T. Inequality in the treatment of diabetes and hypertension across residency status in China. Ethn Health. 2021;26(4):512‐529. [DOI] [PubMed] [Google Scholar]
- 5. Beran D, Lazo‐Porras M, Mba CM, Mbanya JC. A global perspective on the issue of access to insulin. Diabetologia. 2021;64(5):954‐962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Sosa‐Rubí SG, Seiglie JA, Chivardi C, et al. Incremental risk of developing severe COVID‐19 among Mexican patients with diabetes attributed to social and health care access disadvantages. Diabetes Care. 2021;44(2):373‐380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. White A, Liburd LC, Coronado F. Addressing racial and ethnic disparities in COVID‐19 among school‐aged children: are we doing enough? Prev Chronic Dis. 2021;18:E55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Aromataris E, Fernandez R, Godfrey CM, Holly C, Khalil H, Tungpunkom P. Summarizing systematic reviews: methodological development, conduct and reporting of an umbrella review approach. Int J Evid Based Healthc. 2015;13(3):132‐140. [DOI] [PubMed] [Google Scholar]
- 9. Peterson J, Welch V, Losos M, Tugwell PJ. The Newcastle‐Ottawa Scale (NOS) for Assessing the Quality of Nonrandomised Studies in Meta‐Analyses. Ottawa Hospital Research Institute; 2011. [Google Scholar]
- 10. Lo CK, Mertz D, Loeb M. Newcastle‐Ottawa scale: comparing reviewers' to authors' assessments. BMC Med Res Methodol. 2014;14:45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Guyatt G, Oxman AD, Akl EA, et al. GRADE guidelines: 1. Introduction‐GRADE evidence profiles and summary of findings tables. J Clin Epidemiol. 2011;64(4):383‐394. [DOI] [PubMed] [Google Scholar]
- 12. National Health Commission . Diagnosis and treatment protocol for novel coronavirus pneumonia (trial version 7). Chin Med J (Engl). 2020;133(9):1087‐1095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. World Health Organization . WHO/2019‐nCoV/clinical/2021.1. Accessed January 25, 2021.
- 14. Chapter 24: Including non‐randomized studies on intervention effects. Accessed January 28, 2021. Training.cochrane.org. https://training.cochrane.org/handbook/current/chapter‐24
- 15. Begg CB, Mazumdar M. Operating characteristics of a rank correlation test for publication bias. Biometrics. 1994;50(4):1088‐1101. [PubMed] [Google Scholar]
- 16. Huang C, Wang Y, Li X, et al. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet. 2020;395(10223):497‐506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Chen N, Zhou M, Dong X, et al. Epidemiological and clinical characteristics of 99 cases of 2019 novel coronavirus pneumonia in Wuhan, China: a descriptive study. Lancet. 2020;395(10223):507‐513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Wang D, Hu B, Hu C, et al. Clinical characteristics of 138 hospitalized patients with 2019 novel coronavirus‐infected pneumonia in Wuhan, China. JAMA. 2020;323(11):1061‐1069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Zhang JJ, Dong X, Cao YY, et al. Clinical characteristics of 140 patients infected with SARS‐CoV‐2 in Wuhan, China. Allergy. 2020;75(7):1730‐1741. [DOI] [PubMed] [Google Scholar]
- 20. Argenziano MG, Bruce SL, Slater CL, et al. Characterization and clinical course of 1000 patients with coronavirus disease 2019 in New York: retrospective case series. BMJ. 2020;369:m1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Chilimuri S, Sun H, Alemam A, et al. Predictors of mortality in adults admitted with COVID‐19: retrospective cohort study from New York City. West J Emerg Med. 2020;21(4):779‐784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Chung SM, Lee YY, Ha E, et al. The risk of diabetes on clinical outcomes in patients with coronavirus disease 2019: a retrospective cohort study. Diabetes Metab J. 2020;44(3):405‐413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Du RH, Liang LR, Yang CQ, et al. Predictors of mortality for patients with COVID‐19 pneumonia caused by SARS‐CoV‐2: a prospective cohort study. Eur Respir J. 2020;55(5):2000524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Gregoriano C, Koch D, Haubitz S, et al. Characteristics, predictors and outcomes among 99 patients hospitalised with COVID‐19 in a tertiary care Centre in Switzerland: an observational analysis. Swiss Med Wkly. 2020;150:w20316. [DOI] [PubMed] [Google Scholar]
- 25. Hong KS, Lee KH, Chung JH, et al. Clinical features and outcomes of 98 patients hospitalized with SARS‐CoV‐2 infection in Daegu, South Korea: a brief descriptive study. Yonsei Med J. 2020;61(5):431‐437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Israelsen SB, Kristiansen KT, Hindsberger B, et al. Characteristics of patients with COVID‐19 pneumonia at Hvidovre Hospital, March‐April 2020. Dan Med J. 2020;67(6):A05200313. [PubMed] [Google Scholar]
- 27. Itelman E, Wasserstrum Y, Segev A, et al. Clinical characterization of 162 COVID‐19 patients in Israel: preliminary report from a large tertiary center. Isr Med Assoc J. 2020;22(5):271‐274. [PubMed] [Google Scholar]
- 28. Li L, Yang L, Gui S, et al. Association of clinical and radiographic findings with the outcomes of 93 patients with COVID‐19 in Wuhan, China. Theranostics. 2020;10(14):6113‐6121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Nikpouraghdam M, Jalali Farahani A, Alishiri G, et al. Epidemiological characteristics of coronavirus disease 2019 (COVID‐19) patients in IRAN: a single center study. J Clin Virol. 2020;127:104378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Pellaud C, Grandmaison G, Pham Huu Thien HP, et al. Characteristics, comorbidities, 30‐day outcome and in‐hospital mortality of patients hospitalised with COVID‐19 in a Swiss area ‐ a retrospective cohort study. Swiss Med Wkly. 2020;150:w20314. [DOI] [PubMed] [Google Scholar]
- 31. Ren H, Yang Y, Wang F, et al. Association of the insulin resistance marker TyG index with the severity and mortality of COVID‐19. Cardiovasc Diabetol. 2020;19(1):58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Şenkal N, Meral R, Medetalibeyoğlu A, Konyaoğlu H, Kose M, Tukek T. Association between chronic ACE inhibitor exposure and decreased odds of severe disease in patients with COVID‐19. Anatol J Cardiol. 2020;24(1):21‐29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Shi Y, Yu X, Zhao H, Wang H, Zhao R, Sheng J. Host susceptibility to severe COVID‐19 and establishment of a host risk score: findings of 487 cases outside Wuhan. Crit Care. 2020;24:108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Smith AA, Fridling J, Ibrahim D, Porter PS Jr. Identifying patients at greatest risk of mortality due to COVID‐19: a New England perspective. West J Emerg Med. 2020;21(4):785‐789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Trigo J, García‐Azorín D, Planchuelo‐Gómez Á, et al. Factors associated with the presence of headache in hospitalized COVID‐19 patients and impact on prognosis: a retrospective cohort study. J Headache Pain. 2020;21(1):94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Wang L, He W, Yu X, et al. Coronavirus disease 2019 in elderly patients: characteristics and prognostic factors based on 4‐week follow‐up. J Infect. 2020;80(6):639‐645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Xu PP, Tian RH, Luo S, et al. Risk factors for adverse clinical outcomes with COVID‐19 in China: a multicenter, retrospective, observational study. Theranostics. 2020;10(14):6372‐6383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Yan Y, Yang Y, Wang F, et al. Clinical characteristics and outcomes of patients with severe covid‐19 with diabetes. BMJ Open Diabetes Res Care. 2020;8(1):e001343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Yang Q, Zhou Y, Wang X, et al. Effect of hypertension on outcomes of adult inpatients with COVID‐19 in Wuhan, China: a propensity score–matching analysis. Respir Res. 2020;21(1):172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Zhang S, Guo M, Duan L, et al. Development and validation of a risk factor‐based system to predict short‐term survival in adult hospitalized patients with COVID‐19: a multicenter, retrospective, cohort study. Crit Care. 2020;24(1):438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Zhang SY, Lian JS, Hu JH, et al. Clinical characteristics of different subtypes and risk factors for the severity of illness in patients with COVID‐19 in Zhejiang, China. Infect Dis Poverty. 2020;9(1):85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Zhao XY, Xu XX, Yin HS, et al. Clinical characteristics of patients with 2019 coronavirus disease in a non‐Wuhan area of Hubei Province, China: a retrospective study. BMC Infect Dis. 2020;20(1):311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Zhou F, Yu T, Du R, et al. Clinical course and risk factors for mortality of adult inpatients with COVID‐19 in Wuhan, China: a retrospective cohort study. Lancet. 2020;395(10229):1054‐1062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Zhang J, Liu P, Wang M, et al. The clinical data from 19 critically ill patients with coronavirus disease 2019: a single‐centered, retrospective, observational study. Z Gesund Wiss. 2020:1‐4. Epub ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Guo T, Fan Y, Chen M, et al. Cardiovascular implications of fatal outcomes of patients with Coronavirus Disease 2019 (COVID‐19). JAMA Cardiol. 2020;5(7):811‐818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Wu C, Chen X, Cai Y, et al. Risk factors associated with acute respiratory distress syndrome and death in patients with coronavirus disease 2019 pneumonia in Wuhan, China. JAMA Intern Med. 2020;180(7):934‐943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Chen T, Wu D, Chen H, et al. Clinical characteristics of 113 deceased patients with coronavirus disease 2019: retrospective study. BMJ. 2020;368:m1091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Deng Y, Liu W, Liu K, et al. Clinical characteristics of fatal and recovered cases of coronavirus disease 2019 in Wuhan, China: a retrospective study. Chin Med J (Engl). 2020;133(11):1261‐1267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Yang X, Yu Y, Xu J, et al. Clinical course and outcomes of critically ill patients with SARS‐CoV‐2 pneumonia in Wuhan, China: a single‐centered, retrospective, observational study. Lancet Respir Med. 2020;8(5):475‐481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Guan WJ, Liang WH, Zhao Y, et al. Comorbidity and its impact on 1590 patients with COVID‐19 in China: a nationwide analysis. Eur Respir J. 2020;55(5):2000547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Simonnet A, Chetboun M, Poissy J, et al. High prevalence of obesity in Severe Acute Respiratory Syndrome Coronavirus‐2 (SARS‐CoV‐2) requiring invasive mechanical ventilation. Obesity. 2020;28(7):1195‐1199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Wan S, Xiang Y, Fang W, et al. Clinical features and treatment of COVID‐19 patients in northeast Chongqing. J Med Virol. 2020;92(7):797‐806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Huang R, Zhu L, Xue L, et al. Clinical findings of patients with coronavirus disease 2019 in Jiangsu province, China: a retrospective, multi‐center study. PLoS Negl Trop Dis. 2020;14(5):e0008280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Cai Q, Chen F, Wang T, et al. Obesity and COVID‐19 severity in a designated Hospital in Shenzhen, China. Diabetes Care. 2020;43(7):1392‐1398. [DOI] [PubMed] [Google Scholar]
- 55. Abu‐Jamous B, Anisimovich A, Baxter J, et al. Associations of comorbidities and medications with COVID‐19 outcome: a retrospective analysis of real‐world evidence data. medRxiv. 2020: 2020.08.20.20174169.
- 56. Chen Y, Yang D, Cheng B, et al. Clinical characteristics and outcomes of patients with diabetes and COVID‐19 in association with glucose‐lowering medication. Diabetes Care. 2020;43(7):1399‐1407. [DOI] [PubMed] [Google Scholar]
- 57. Cheng X, Liu YM, Li H, et al. Metformin is associated with higher incidence of acidosis, but not mortality, in individuals with COVID‐19 and pre‐existing type 2 diabetes. Cell Metab. 2020;32(4):537‐547.e3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Do JY, Kim SW, Park JW, Cho KH, Kang SH. Is there an association between metformin use and clinical outcomes in diabetes patients with COVID‐19? Diabetes Metab. 2021;47(4):101208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Kim MK, Jeon JH, Kim SW, et al. The clinical characteristics and outcomes of patients with moderate‐to‐severe coronavirus disease 2019 infection and diabetes in Daegu, South Korea. Diabetes Metab J. 2020;44(4):602‐613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Luo P, Qiu L, Liu Y, et al. Metformin treatment was associated with decreased mortality in COVID‐19 patients with diabetes in a retrospective analysis. Am J Trop Med Hyg. 2020;103(1):69‐72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Fadini GP, Morieri ML, Longato E, et al. Exposure to dipeptidyl‐peptidase‐4 inhibitors and COVID‐19 among people with type 2 diabetes: a case‐control study. Diabetes Obes Metab. 2020;22(10):1946‐1950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Arentz M, Yim E, Klaff L, et al. Characteristics and outcomes of 21 critically ill patients with COVID‐19 in Washington State. JAMA. 2020;323(16):1612‐1614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Barrasa H, Rello J, Tejada S, et al. SARS‐CoV‐2 in Spanish Intensive Care Units: early experience with 15‐day survival in Vitoria. Anaesth Crit Care Pain Med. 2020;39(5):553‐561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Cao J, Tu WJ, Cheng W, et al. Clinical features and short‐term outcomes of 102 patients with coronavirus disease 2019 in Wuhan, China. Clin Infect Dis. 2020;71(15):748‐755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Chen T, Dai Z, Mo P, et al. Clinical characteristics and outcomes of older patients with coronavirus disease 2019 (COVID‐19) in Wuhan, China: a single‐centered, retrospective study. J Gerontol A Biol Sci Med Sci. 2020;75(9):1788‐1795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. CDC COVID‐19 Response Team . Preliminary estimates of the prevalence of selected underlying health conditions among patients with coronavirus disease 2019 – United States, February 12–March 28, 2020. MMWR Morb Mortal Wkly Rep. 2020;69(13):382‐386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Feng Y, Ling Y, Bai T, et al. COVID‐19 with different severities: a multicenter study of clinical features. Am J Respir Crit Care Med. 2020;201(11):1380‐1388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Guo W, Li M, Dong Y, et al. Diabetes is a risk factor for the progression and prognosis of COVID‐19. Diabetes Metab Res Rev. 2020;36(7):e3319. doi: 10.1002/dmrr.3319. Epub ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Lian J, Jin X, Hao S, et al. Analysis of epidemiological and clinical features in older patients with Coronavirus Disease 2019 (COVID‐19) outside Wuhan. Clin Infect Dis. 2020;71(15):740‐747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Liang W‐H, Guan W‐J, Li C‐C, et al. Clinical characteristics and outcomes of hospitalised patients with COVID‐19 treated in Hubei (epicentre) and outside Hubei (non‐epicentre): a nationwide analysis of China. Eur Respir J. 2020;55(6):2000562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Richardson S, Hirsch JS, Narasimhan M, et al. Presenting characteristics, comorbidities, and outcomes among 5700 patients hospitalized with COVID‐19 in the New York City area. JAMA. 2020;323(20):2052‐2059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Wang L, Li X, Chen H, et al. Coronavirus Disease 19 infection does not result in acute kidney injury: an analysis of 116 hospitalized patients from Wuhan, China. Am J Nephrol. 2020;51(5):343‐348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Wang X, Fang J, Zhu Y, et al. Clinical characteristics of non‐critically ill patients with novel coronavirus infection (COVID‐19) in a Fangcang hospital. Clin Microbiol Infect. 2020;26(8):1063‐1068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Yao Q, Wang P, Wang X, et al. A retrospective study of risk factors for severe acute respiratory syndrome coronavirus 2 infections in hospitalized adult patients. Pol Arch Intern Med. 2020;130(5):390‐399. [DOI] [PubMed] [Google Scholar]
- 75. Acharya D, Lee K, Lee DS, Lee YS, Moon SS. Mortality rate and predictors of mortality in hospitalized COVID‐19 patients with diabetes. Healthcare (Basel). 2020;8(3):338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76. Al‐Salameh A, Lanoix JP, Bennis Y, et al. Characteristics and outcomes of COVID‐19 in hospitalized patients with and without diabetes. Diabetes Metab Res Rev. 2021;37(3):e3388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77. Conway J, Gould A, Westley R, et al. Characteristics of patients with diabetes hospitalised for COVID‐19 infection‐a brief case series report. Diabetes Res Clin Pract. 2020;169:108460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78. Fox T, Ruddiman K, Lo KB, et al. The relationship between diabetes and clinical outcomes in COVID‐19: a single‐center retrospective analysis. Acta Diabetol. 2021;58(1):33‐38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79. Shang J, Wang Q, Zhang H, et al. The relationship between diabetes mellitus and COVID‐19 prognosis: a retrospective cohort study in Wuhan, China. Am J Med. 2021;134(1):e6‐e14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80. Soliman AT, Prabhakaran Nair A, Al Masalamani MS, et al. Prevalence, clinical manifestations, and biochemical data of type 2 diabetes mellitus versus nondiabetic symptomatic patients with COVID‐19: a comparative study. Acta Biomed. 2020;91(3):e2020010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81. Xu Z, Wang Z, Wang S, et al. The impact of type 2 diabetes and its management on the prognosis of patients with severe COVID‐19. J Diabetes. 2020;12(12):909‐918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82. Crouse A, Grimes T, Li P, Might M, Ovalle F, Shalev A. Metformin use is associated with reduced mortality in a diverse popularion with Covid‐19 and diabetes. medRxiv. 2020. [DOI] [PMC free article] [PubMed]
- 83. Bode B, Garrett V, Messler J, et al. Glycemic characteristics and clinical outcomes of COVID‐19 patients hospitalized in the United States. J Diabetes Sci Technol. 2020;14(4):813‐821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84. Cai Q, Huang D, Ou P, et al. COVID‐19 in a designated infectious diseases hospital outside Hubei Province, China. Allergy. 2020;75(7):1742‐1752. [DOI] [PubMed] [Google Scholar]
- 85. Chen Q, Zheng Z, Zhang C, et al. Clinical characteristics of 145 patients with corona virus disease 2019 (COVID‐19) in Taizhou, Zhejiang, China. Infection. 2020;48(4):543‐551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86. Shi Q, Zhang X, Jiang F, et al. Clinical characteristics and risk factors for mortality of COVID‐19 patients with diabetes in Wuhan, China: a two‐center retrospective study. Diabetes Care. 2020;43(7):1382‐1391. [DOI] [PubMed] [Google Scholar]
- 87. Wang F, Yang Y, Dong K, et al. Clinical characteristics of 28 patients with diabetes and COVID‐19 in Wuhan, China. Endocr Pract. 2020;26(6):668‐674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88. Bello‐Chavolla OY, Bahena‐López JP, Antonio‐Villa NE, et al. Predicting mortality due to SARS‐CoV‐2: a mechanistic score relating obesity and diabetes to COVID‐19 outcomes in Mexico. J Clin Endocrinol Metab. 2020;105(8):2752‐2761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89. Li Y, Han X, Alwalid O, et al. Baseline characteristics and risk factors for short‐term outcomes in 132 COVID‐19 patients with diabetes in Wuhan China: a retrospective study. Diabetes Res Clin Pract. 2020;166:108299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90. Liu Z, Bai X, Han X, et al. The association of diabetes and the prognosis of COVID‐19 patients: a retrospective study. Diabetes Res Clin Pract. 2020;169:108386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91. Rastad H, Karim H, Ejtahed H‐S, et al. Risk and predictors of in‐hospital mortality from COVID‐19 in patients with diabetes and cardiovascular disease. Diabetol Metab Syndr. 2020;12(1):57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92. Seiglie J, Platt J, Cromer SJ, et al. Diabetes as a risk factor for poor early outcomes in patients hospitalized with COVID‐19. Diabetes Care. 2020;43(12):2938‐2944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93. Chen G, Wu D, Guo W, et al. Clinical and immunological features of severe and moderate coronavirus disease 2019. J Clin Invest. 2020;130(5):2620‐2629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94. Chen R, Liang W, Jiang M, et al. Risk factors of fatal outcome in hospitalized subjects with coronavirus disease 2019 from a Nationwide analysis in China. Chest. 2020;158(1):97‐105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95. Zhu Z, Cai T, Fan L, et al. Clinical value of immune‐inflammatory parameters to assess the severity of coronavirus disease 2019. Int J Infect Dis. 2020;95:332‐339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96. Zheng S, Fan J, Yu F, et al. Viral load dynamics and disease severity in patients infected with SARS‐CoV‐2 in Zhejiang province, China, January–March 2020: retrospective cohort study. BMJ. 2020;369:m1443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97. Myers LC, Parodi SM, Escobar GJ, Liu VX. Characteristics of hospitalized adults with COVID‐19 in an integrated health care system in California. JAMA. 2020;323(21):2195‐2198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98. Targher G, Mantovani A, Wang XB, et al. Patients with diabetes are at higher risk for severe illness from COVID‐19. Diabetes Metab. 2020;46(4):335‐337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99. Gold JAW, Wong KK, Szablewski CM, et al. Characteristics and clinical outcomes of adult patients hospitalized with Covid‐19 – Georgia, March 2020. Morb Mortal Wkly Rep. 2020;69(18):545‐550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100. Ferguson J, Rosser J, Quintero O, et al. Characteristics and outcomes of coronavirus disease patients under nonsurge conditions, Northern California, USA, March–April 2020. Emerg Infect Dis J. 2020;26(8):1679‐1685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101. Duanmu Y, Brown IP, Gibb WR, et al. Characteristics of Emergency Department patients with COVID‐19 at a single site in Northern California: clinical observations and public health implications. Acad Emerg Med. 2020;27:505‐509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102. Shi S, Qin M, Shen B, et al. Association of cardiac injury with mortality in hospitalized patients with COVID‐19 in Wuhan, China. JAMA Cardiol. 2020;5(7):802‐810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103. Shahriarirad R, Khodamoradi Z, Erfani A, et al. Epidemiological and clinical features of 2019 novel coronavirus diseases (COVID‐19) in the south of Iran. BMC Infect Dis. 2020;20(1):427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104. Javanian M, Bayani M, Shokri M, et al. Clinical and laboratory findings from patients with COVID‐19 pneumonia in Babol North of Iran: a retrospective cohort study. Rom J Intern Med. 2020;58(3):161‐167. [DOI] [PubMed] [Google Scholar]
- 105. Akbariqomi M, Hosseini MS, Rashidiani J, et al. Clinical characteristics and outcome of hospitalized COVID‐19 patients with diabetes: a single‐center, retrospective study in Iran. Diabetes Res Clin Pract. 2020;169:108467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106. Alamdari NM, Afaghi S, Rahimi FS, et al. Mortality risk factors among hospitalized COVID‐19 patients in a major referral Center in Iran. Tohoku J Exp Med. 2020;252(1):73‐84. [DOI] [PubMed] [Google Scholar]
- 107. Papizadeh S, Moradi P, Mehr MH, et al. Epidemiologic and clinical characteristics of 186 hospitalized patients with Covid‐19 in Tehran, Iran: a retrospective, single‐center case series. Preprints. 2020: 2020070060.
- 108. Pazoki M, Keykhaei M, Kafan S, et al. Risk indicators associated with in‐hospital mortality and severity in patients with diabetes mellitus and confirmed or clinically suspected COVID‐19. J Diabetes Metab Disord. 2021;20(1):1‐11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109. Rossi PG, Marino M, Formisano D, et al. Characteristics and outcomes of a cohort of SARS‐CoV‐2 patients in the Province of Reggio Emilia, Italy. PLoS One. 2020;15(8):e0238281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110. Borobia AM, Carcas AJ, Arnalich F, et al. A cohort of patients with COVID‐19 in a major teaching hospital in Europe. medRxiv. 2020: 2020.04.29.20080853. [DOI] [PMC free article] [PubMed]
- 111. Perez‐Guzman PN, Daunt A, Mukherjee S, et al. Clinical characteristics and predictors of outcomes of hospitalized patients with coronavirus disease 2019 in a multiethnic London National Health Service Trust: a retrospective cohort study. Clin Infect Dis. 2020;73:e4047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112. Levy TJ, Richardson S, Coppa K, et al. Development and validation of a survival calculator for hospitalized patients with COVID‐19. medRxiv. 2020: 2020.04.22.20075416.
- 113. Wang Z, Zheutlin AB, Kao Y‐H, et al. Analysis of hospitalized COVID‐19 patients in the Mount Sinai Health System using electronic medical records (EMR) reveals important prognostic factors for improved clinical outcomes. medRxiv. 2020: 2020.04.28.20075788.
- 114. Cummings MJ, Baldwin MR, Abrams D, et al. Epidemiology, clinical course, and outcomes of critically ill adults with COVID‐19 in New York City: a prospective cohort study. medRxiv. 2020. [DOI] [PMC free article] [PubMed]
- 115. Alkundi A, Mahmoud I, Musa A, Naveed S, Alshawwaf M. Clinical characteristics and outcomes of COVID‐19 hospitalized patients with diabetes in the United Kingdom: a retrospective single Centre study. Diabetes Res Clin Pract. 2020;165:108263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116. Docherty AB, Harrison EM, Green CA, et al. Features of 20 133 UKpatients in hospital with covid‐19 using the ISARIC WHO Clinical Characterisation Protocol: prospective observational cohort study. BMJ. 2020;369:m1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117. Ciceri F, Castagna A, Rovere‐Querini P, et al. Early predictors of clinical outcomes of COVID‐19 outbreak in Milan, Italy. Clin Immunol. 2020;217:108509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118. Lee JY, Kim HA, Huh K, et al. Risk factors for mortality and respiratory support in elderly patients hospitalized with COVID‐19 in Korea. J Korean Med Sci. 2020;35(23):e223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119. Zhao J, Gao HY, Feng ZY, Wu QJ. A retrospective analysis of the clinical and epidemiological characteristics of COVID‐19 patients in Henan provincial People's hospital, Zhengzhou, China. Front Med. 2020;7:286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120. Khamis F, Al‐Zakwani I, Al Naamani H, et al. Clinical characteristics and outcomes of the first 63 adult patients hospitalized with COVID‐19: an experience from Oman. J Infect Public Health. 2020;13(7):906‐913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121. Marcello RK, Dolle J, Grami S, et al. Characteristics and outcomes of COVID‐19 patients in New York City's Public Hospital System. medRxiv: 2020. [DOI] [PMC free article] [PubMed]
- 122. Cen Y, Chen X, Shen Y, et al. Risk factors for disease progression in patients with mild to moderate coronavirus disease 2019‐a multi‐Centre observational study. Clin Microbiol Infect. 2020;26(9):1242‐1247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123. Jang JG, Hur J, Choi EY, Hong KS, Lee W, Ahn JH. Prognostic factors for severe coronavirus disease 2019 in Daegu, Korea. J Korean Med Sci. 2020;35(23):e209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124. Wang D, Yin Y, Hu C, et al. Clinical course and outcome of 107 patients infected with the novel coronavirus, SARS‐CoV‐2, discharged from two hospitals in Wuhan, China. Crit Care. 2020;24(1):188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125. Hu L, Chen S, Fu Y, et al. Risk factors associated with clinical outcomes in 323 coronavirus disease 2019 (COVID‐19) hospitalized patients in Wuhan, China. Clin Infect Dis. 2020;71(16):2089‐2098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126. Yang Q, Xie L, Zhang W, et al. Analysis of the clinical characteristics, drug treatments and prognoses of 136 patients with coronavirus disease 2019. J Clin Pharm Ther. 2020;45(4):609‐616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127. Yang L, Liu J, Zhang R, et al. Epidemiological and clinical features of 200 hospitalized patients with corona virus disease 2019 outside Wuhan, China: a descriptive study. J Clin Virol. 2020;129:104475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128. Chen F, Sun W, Sun S, Li Z, Wang Z, Yu L. Clinical characteristics and risk factors for mortality among inpatients with COVID‐19 in Wuhan, China. Clin Transl Med. 2020;10:e40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129. Cao Z, Li T, Liang L, et al. Clinical characteristics of Coronavirus Disease 2019 patients in Beijing, China. PLoS One. 2020;15(6):e0234764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130. Wang Y, Liao B, Guo Y, et al. Clinical characteristics of patients infected with the Novel 2019 Coronavirus (SARS‐Cov‐2) in Guangzhou, China. Open Forum Infect Dis. 2020;7(6):ofaa187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131. Morrison AR, Johnson JM, Griebe KM, et al. Clinical characteristics and predictors of survival in adults with coronavirus disease 2019 receiving tocilizumab. J Autoimmun. 2020;114:102512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132. Huang Y, Lyu X, Li D, et al. A cohort study of 223 patients explores the clinical risk factors for the severity diagnosis of COVID‐19. medRxiv. 2020: 2020.04.18.20070656.
- 133. Zhou Y, Yang Z, Guo Y, et al. A new predictor of disease severity in patients with COVID‐19 in Wuhan, China. medRxiv. 2020: 2020.03.24.20042119.
- 134. Luo X, Xia H, Yang W, et al. Characteristics of patients with COVID‐19 during epidemic ongoing outbreak in Wuhan, China. medRxiv. 2020: 2020.03.19.20033175.
- 135. Alshukry A, Ali H, Ali Y, et al. Clinical characteristics of Coronavirus Disease 2019 (COVID‐19) patients in Kuwait. medRxiv. 2020: 2020.06.14.20131045. [DOI] [PMC free article] [PubMed]
- 136. Yan S, Song X, Lin F, et al. Clinical characteristics of Coronavirus Disease 2019 in Hainan, China. medRxiv. 2020: 2020.03.19.20038539.
- 137. Paranjpe I, Russak AJ, De Freitas JK, et al. Clinical characteristics of hospitalized Covid‐19 patients in New York City. medRxiv: the Preprint Server for Health Sciences. 2020: 2020.04.19.20062117.
- 138. Shi P, Ren G, Yang J, et al. Clinical characteristics of imported and second‐generation coronavirus disease 2019 (COVID‐19) cases in Shaanxi outside Wuhan, China: a multicentre retrospective study. Epidemiol Infect. 2020;148:e238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139. Yadaw AS, Li Y‐C, Bose S, Iyengar R, Bunyavanich S, Pandey G. Clinical predictors of COVID‐19 mortality. medRxiv: The Preprint Server for Health Sciences. 2020: 2020.05.19.20103036.
- 140. Chen X, Zheng F, Qing Y, et al. Epidemiological and clinical features of 291 cases with coronavirus disease 2019 in areas adjacent to Hubei, China: a double‐center observational study. medRxiv. 2020: 2020.03.03.20030353.
- 141. Qi D, Yan X, Tang X, et al. Epidemiological and clinical features of 2019‐nCoV acute respiratory disease cases in Chongqing municipality, China: a retrospective, descriptive, multiple‐center study. medRxiv. 2020: 2020.03.01.20029397.
- 142. Fu L, Fei J, Xiang H‐X, et al. Influence factors of death risk among COVID‐19 patients in Wuhan, China: a hospital‐based case‐cohort study. medRxiv. 2020: 2020.03.13.20035329.
- 143. Nie S, Zhao X, Zhao K, Zhang Z, Zhang Z, Zhang Z. Metabolic disturbances and inflammatory dysfunction predict severity of coronavirus disease 2019 (COVID‐19): a retrospective study. medRxiv. 2020: 2020.03.24.20042283.
- 144. Zhang F, Yang D, Li J, et al. Myocardial injury is associated with in‐hospital mortality of confirmed or suspected COVID‐19 in Wuhan, China: a single center retrospective cohort study. medRxiv. 2020: 2020.03.21.20040121.
- 145. Zhang H, Wang X, Fu Z, et al. Potential factors for prediction of disease severity of COVID‐19 patients. medRxiv. 2020: 2020.03.20.20039818.
- 146. Sisó‐Almirall A, Kostov B, Mas‐Heredia M, et al. Prognostic factors in spanish COVID‐19 patients: a case series from barcelona. medRxiv. 2020: 2020.06.18.20134510. [DOI] [PMC free article] [PubMed]
- 147. Wang H, Lu Y, Lv Q. Progression, recovery and fatality in patients with SARS‐CoV‐2 related pneumonia in Wuhan, China: a single‐centered, retrospective, observational study. Preprint at medRxiv. 2020. doi: 10.1101/2020.05.12.20099739 [DOI] [PMC free article] [PubMed]
- 148. Chen C, Zhang J, Li C, et al. The characteristics and death risk factors of 132 COVID‐19 pneumonia patients with comorbidities: a retrospective single center analysis in Wuhan, China. medRxiv. 2020: 2020.05.07.20092882.
- 149. Zhang Y, Cui Y, Shen M, et al. Comorbid diabetes mellitus was associated with poorer prognosis in patients with COVID‐19: a retrospective cohort study. medRxiv. 2020: 2020.03.24.20042358.
- 150. Israelsen SB, Pottegård A, Sandholdt H, Madsbad S, Thomsen RW, Benfield T. Comparable COVID‐19 outcomes with current use of GLP‐1 receptor agonists, DPP‐4 inhibitors or SGLT‐2 inhibitors among patients with diabetes who tested positive for SARS‐CoV‐2. Diabetes Obes Metab. 2021;23(6):1397‐1401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151. Noh Y, Oh IS, Jeong HE, Filion KB, Yu OHY, Shin JY. Association between DPP‐4 inhibitors and COVID‐19‐related outcomes among patients with type 2 diabetes. Diabetes Care. 2021;44:e64‐e66. [DOI] [PubMed] [Google Scholar]
- 152. Pérez‐Belmonte LM, Torres‐Peña JD, López‐Carmona MD, et al. Mortality and other adverse outcomes in patients with type 2 diabetes mellitus admitted for COVID‐19 in association with glucose‐lowering drugs: a nationwide cohort study. BMC Med. 2020;18(1):359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153. Silverii GA, Monami M, Cernigliaro A, et al. Are diabetes and its medications risk factors for the development of COVID‐19? Data from a population‐based study in Sicily. Nutr Metab Cardiovasc Dis. 2021;31(2):396‐398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154. Wargny M, Potier L, Gourdy P, et al. Predictors of hospital discharge and mortality in patients with diabetes and COVID‐19: updated results from the nationwide CORONADO study. Diabetologia. 2021;64(4):778‐794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155. Zhou J‐H, Wu B, Wang W‐X, et al. No significant association between dipeptidyl peptidase‐4 inhibitors and adverse outcomes of COVID‐19. World J Clin Cases. 2020;8(22):5576‐5588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156. Yu B, Li C, Sun Y, Wang DW. Insulin treatment is associated with increased mortality in patients with COVID‐19 and type 2 diabetes. Cell Metab. 2021;33(1):65‐77.e2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157. Riahi S, Sombra LRS, Lo KB, et al. Insulin use, diabetes control, and outcomes in patients with COVID‐19. Endocr Res. 2021;46(2):45‐50. [DOI] [PubMed] [Google Scholar]
- 158. Khalili S, Moradi O, Kharazmi AB, Raoufi M, Sistanizad M, Shariat M. Comparison of Mortality Rate and Severity of Pulmonary Involvement in Coronavirus Disease‐2019 Adult Patients With and Without Type 2 Diabetes: A Cohort Study. Can J Diabetes. 2021;45(6):524‐530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159. Turcotte JJ, Meisenberg BR, MacDonald JH, et al. Risk factors for severe illness in hospitalized Covid‐19 patients at a regional hospital. PLoS One. 2020;15(8):e0237558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160. Yan H, Valdes AM, Vijay A, et al. Role of drugs used for chronic disease management on susceptibility and severity of COVID‐19: a large case‐control study. Clin Pharmacol Ther. 2020;108(6):1185‐1194. [DOI] [PubMed] [Google Scholar]
- 161. Nafakhi H, Alareedh M, Al‐Buthabhak K, Shaghee F, Nafakhi A, Kasim S. Predictors of adverse in‐hospital outcome and recovery in patients with diabetes mellitus and COVID‐19 pneumonia in Iraq. Diabetes Metab Syndr. 2021;15(1):33‐38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162. Lally MA, Tsoukas P, Halladay CW, O'Neill E, Gravenstein S, Rudolph JL. Metformin is associated with decreased 30‐day mortality among nursing home residents infected with SARS‐CoV2. J Am Med Dir Assoc. 2021;22(1):193‐198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163. Guo T, Shen Q, Ouyang X, et al. Clinical findings in diabetes mellitus patients with COVID‐19. J Diabetes Res. 2021;2021:7830136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164. Zhu L, She ZG, Cheng X, et al. Association of blood glucose control and outcomes in patients with COVID‐19 and pre‐existing type 2 diabetes. Cell Metab. 2020;31(6):1068‐1077.e3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165. Bramante CT, Ingraham NE, Murray TA, et al. Observational study of metformin and risk of mortality in patients hospitalized with Covid‐19. medRxiv: The Preprint Server for Health Sciences. 2020: 2020.06.19.20135095.
- 166. Cariou B, Hadjadj S, Wargny M, et al. Phenotypic characteristics and prognosis of inpatients with COVID‐19 and diabetes: the CORONADO study. Diabetologia. 2020;63(8):1500‐1515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167. Liu Z, Li J, Huang J, et al. Association between diabetes and COVID‐19: a retrospective observational study with a large sample of 1,880 cases in Leishenshan Hospital, Wuhan. Front Endocrinol. 2020;11:478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168. Saeed O, Castagna F, Agalliu I, et al. Statin use and in‐hospital mortality in patients with diabetes mellitus and COVID‐19. J Am Heart Assoc. 2020;9(24):e018475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169. Palaiodimos L, Kokkinidis DG, Li W, et al. Severe obesity, increasing age and male sex are independently associated with worse in‐hospital outcomes, and higher in‐hospital mortality, in a cohort of patients with COVID‐19 in the Bronx, New York. Metabol Clin Experim. 2020;108:154262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170. Yu C, Lei Q, Li W, Wang X, Liu W, Fan X. Clinical characteristics, associated factors, and predicting COVID‐19 mortality risk: a retrospective study in Wuhan, China. Am J Prev Med. 2020;59(2):168‐175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171. Zhang Y, Cui Y, Shen M, et al. Association of diabetes mellitus with disease severity and prognosis in COVID‐19: a retrospective cohort study. Diabetes Res Clin Pract. 2020;165:108227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172. Mirani M, Favacchio G, Carrone F, et al. Impact of comorbidities and Glycemia at admission and Dipeptidyl peptidase 4 inhibitors in patients with type 2 diabetes with COVID‐19: a case series from an academic Hospital in Lombardy, Italy. Diabetes Care. 2020;43(12):3042‐3049. [DOI] [PubMed] [Google Scholar]
- 173. Global Health Security Index. Accessed September 3, 2021. https://www.ghsindex.org/#l‐section‐‐map
- 174. Nyström T, Bodegard J, Nathanson D, Thuresson M, Norhammar A, Eriksson JW. Novel oral glucose‐lowering drugs are associated with lower risk of all‐cause mortality, cardiovascular events and severe hypoglycaemia compared with insulin in patients with type 2 diabetes. Diabetes Obes Metab. 2017;19(6):831‐841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175. Accili D. Can COVID‐19 cause diabetes? Nat Metab. 2021;3(2): 123‐125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176. Marcolino MS, Ziegelmann PK, Souza‐Silva MVR, et al. Clinical characteristics and outcomes of patients hospitalized with COVID‐19 in Brazil: results from the Brazilian COVID‐19 registry. Int J Infect Dis. 2021;107:300‐310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177. International Diabetes Federation . IDF Diabetes Atlas, 9th edn. International Diabetes Federation. 2019. Available at: https://www.diabetesatlas.org [Google Scholar]
- 178. Radovanovic D, Coppola S, Franceschi E, et al. Mortality and clinical outcomes in patients with COVID‐19 pneumonia treated with non‐invasive respiratory support: a rapid review. J Crit Care. 2021;65:1‐8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179. Moher D, Liberati A, Tetzlaff J, Altman DG, Prisma Group . Preferred reporting items for systematic reviews and meta‐analyses: the PRISMA statement. PLoS Med. 2009;6(7):e1000097.179 (Referenced in Fig. S1). [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Tables S1‐S2
Figures S1‐S6
Data Availability Statement
The data used and analysed during the current study are available as online Supplementary Material.


