Abstract
Tanacetum balsamita (Asteraceae) is a medicinal plant previously used in human medicine to solve gastrointestinal problems such as abdominal pain. Anti-inflammatory, analgesic, immune-modulatory, and antioxidant effects of T. balsamita have been proven in previous studies. The present study investigated the effects of T. balsamita essential oil (TBEO) on ruminant smooth muscle contractions. The experiment was performed on the circular smooth muscle of ileum samples taken from slaughtered bulls in the organ bath. Nine cumulative concentrations of TBEO from 0.10 to 1000 µg mL-1 were added to tissue samples. The solution used was Tyrode’s solution aerated with a mixture of 95.00% oxygen and 5.00% carbon dioxide, and the temperature was set at 37.00 ˚C. The effect of TBEO on baseline contractions and three induced contractions with potassium chloride, barium chloride, and carbachol was investigated. In GC-MS analysis of TBEO, carvone was identified as a major ingredient. The effects of eight concentrations of 0.001 to 10.00 µM of carvone on all contractions were investigated under similar conditions. The effects of TBEO, carvone, and verapamil (standard calcium channel blocker) on calcium channels were assessed. The results revealed that TBEO and carvone significantly inhibit spontaneous contractions as well as all spasmogen-induced contractions. The TBEO and carvone exert their myorelaxant properties by inhibiting Ca++ channels in smooth muscle. The anti-spasmodic properties of T. balsamita can be employed for the treatment of intestinal spasms or hypermotility.
Key Words: Carvone, Costmary, Ethnoveterinary, Gastrointestinal motility, Medicinal plant
Introduction
Gastrointestinal dysmotility is an important part of alimentary problems in ruminants. Some stimulants may increase the intestinal motor activity and cause cramps, abdominal pain, and colic through causing problems such as diarrhea syndrome.1 Stressful situations can cause excessive motility in the gastrointestinal tract, and the hypermotility affects intestinal permeability and nutrient absorption.2,3 Moreover, constipation, intestinal obstruction, terminal ileitis, and intussusception are associated with intestinal movement disorders in ruminants.1 Spasm of intestinal smooth muscle in cattle is one of the reasons for abdominal pain;4 thus, anti-spasmodics can help relieve abdominal pain symptoms.
Medicinal plant products are economically viable in livestock that can be used as adjunctive or alternative therapies. The plants used in ethnoveterinary medicine are vary in different region, and different parts of plants such as roots, leaves, flowers, and seeds are used in powder, ointment, or decoction. Studies showed the beneficial effects of essential oils and extracts of medical plants on digestive problems of ruminants such as diarrhea, gastrointestinal inflammation, abdominal pain, colic, impaired digestion, and tympany. Some medical plants may stimulate salivation, restore rumination, promote growth, and improve liver function.5
Tanacetum balsamita (costmary) is an aromatic plant used in medicine from ancient times. Tanacetum genus, belongs to the Asteraceae family, is called “Shahesparam” in the local language. T. balsamita is one of the 26 species of Tanacetum genus in Iran6 widely grown in East and West Azarbaijan province in Iran.7 This plant has been used in traditional Iranian medicine for years as a pain reliever, tranquilizer, and heart tonic.8 Tanacetum species are used as stomach strengthen, food preservatives, food additives, cosmetics, insecticides, balsams and dyes.9,10 The T. balsamita, as one of the medicinal plants in human and animal medicine, has been considered by researchers worldwide. Interesting reports have been recorded about the medicinal effects of T. balsamita extracts and essential oils. Their antibacterial, antioxidant, anti-inflammatory, immune-modulatory, and analgesic effects have been confirmed.7,11-15 The T. balsamita, in the formulation of massage lotion, has shown analgesic, anti-congestion, anti-irritant, muscle relaxant properties, and blood circulation booster.16 Previous in vitro studies in organ bath have revealed the spasmolytic properties of T. parthenium and T. vulgare.17-19
Carvone belongs to Terpenoids and is a main component of T. balsamita, Mentha longifolia, and Mentha spicata.14,20,21 Carvone has potent antioxidant, anti-inflammatory, and anti-spasmodic properties.22,23 A previous study showed that carvone had an anti-spasmodic effect on the guinea pig ileum.23
Due to the history of successful use of T. balsamita in the treatment of human gastrointestinal problems6,9,10 and the various effects proven for this plant,7,11-16 this plant seems to have good potential for the treatment and prevention of gastrointestinal problems. However, the effects of plants on gastrointestinal motility were unknown. Dysmotility is an important part of the ruminant GI disease’s pathophysiology, and natural remedies of these problems is important in the production of organic food for human. This experiment was aimed at evaluating the in vitro effects of T. balsamita and its major compounds on contraction of small intestine in ruminant.
Materials and Methods
Plant material. The fresh T. balsamita plant was collected under the supervision of a botanist from Urmia, Iran, in July 2020. The plant was authenticated at the Department of Medicinal and Aromatic Plants of Urmia University. Hydro-distillation was performed to extract the T. balsamita essential oil (TBEO) from its aerial parts. TBEO was stored in a refrigerator at 4.00 ˚C until being used.
Gas chromatography-mass spectrometry (GC-MS). It was performed to identify the components of the plant’s essential oil. For chemical analysis, an Agilent 7890 (Agilent, Santa Clara, USA) A gas chromatograph coupled to a 5975 A mass spectrometer (Agilent) using a HP-5 MS capillary column (5.00% Phenyl Methylpolysiloxane, 30.00 m, 0.25 mm inner diameter, 0.25 μm film thickness) was used. The oven temperature was programmed as follows: 3 min at 80.00 ˚C, subsequently increased 8.00 ˚C per min to 180 ˚C, held for 10 min at 180 ˚C. Helium was used as carrier gas at a flow rate of 1.00 mL per min, and electron impact (EI) was 70.00 eV. The split mode injector was set in a split ratio of 1:500, and mass range acquisition was from 40.00 to 500 m/z. The Wiley 2007 (https://www. wiley.com/en-us) and NIST 2005 (https://www.nist.gov) libraries were used to identify the TBEO compounds. The GC-MS data processing was performed using Chemstation Software (Agilent).
Chemicals. Standard carvone, acetylcholine chloride (Ach), verapamil hydrochloride, and carbachol chloride (Cch) were obtained from Sigma Chemicals Co. (St. Louis, USA). Calcium chloride (CaCl2), potassium chloride (KCl), sodium chloride (NaCl), glucose, sodium dihydrogen phosphate (NaH2PO4), magnesium chloride (MgCl2), sodium bi-carbonate (NaHCO3), barium chloride (BaCl2), ethylene-diaminetetraacetic acid (EDTA) and dimethyl sulfoxide (DMSO) were purchased from Merck (Darmstadt, Germany).
Tissue samples collection. Ileum tissue samples were taken from slaughtered bulls between 2 and 4 years old from Urmia Industrial Slaughterhouse. Bulls were evaluated before sampling, and those with a history or symptoms of gastrointestinal problems were excluded from the sampling process. The ileum was excised from the gastrointestinal tract less than 20 min after slaughter. A complete 15.00 cm piece of ileum was cut, and a longitudinal incision was then made to open the inner (mucosal) surface of the ileum. Tyrode’s solution containing NaCl (136.90 mM), NaHCO3 (11.90 mM), glucose (5.60 mM), KCl (2.70 mM), CaCl2 (1.80 mM), MgCl2 (1.10 mM) and NaH2PO4 (0.40 mM) was used as the rinse, transportation and incubation medium. The mucosal and serous surface of the tissue samples were immediately rinsed with cooled (4.00 ˚C) and aerated Tyrode’s solution to remove the digestive contents.24 Specimens were immersed in 4.00 ˚C Tyrode’s solution and kept at this temperature until reaching the laboratory. The solution was exchanged 10 min after the initial collection to remove digestive contents and supply the vital material to the tissue. In the laboratory, whole pieces of ileum tissue were placed on the dissected board filled with Tyrode’s solution. The non-smooth muscle (mucosa and submucosa) was carefully separated from the ileum smooth muscle. The tissue strips (5.00 × 20.00 mm) were cut in the direction of the circular muscle layer.24
Recording of smooth muscle activity. To mount the samples in chambers, one end of each strip was jointed to the bottom hook of the chamber, and the other end was connected to an isometric transducer with suture thread (TRI 202P; PanLab, Barcelona, Spain). Six transducers connected to an amplifier (ML224; AD Instruments, Castle Hill, Australia) and Power Lab data acquisition system (ML870; AD Instruments) were used for data collecting. Lab chart software (version 8.0; AD Instruments) was employed to view and record data. The organ bath chambers filled with 25.00-mL Tyrode’s solution was set at 37.00 ˚C and gassed continuously with a mixture of 95.00% O2 and 5.00% CO2 .24
Design of experiments. First, the ileum muscle samples were rested in the Tyrode’s medium for 1 hr to adapt to the new environment. In this step, the solution was changed every 15 min, and two 1.20 g tensions were applied to the tissues at 15-min intervals. To evaluate the viability and contractile function, the samples were tested before the main experiment through adding 10.00 µM of acetylcholine. After the acetylcholine, tissues were washed to achieve basal contraction of muscles. This procedure was repeated three times and if the results were the same, the tissue was considered acceptable for testing.24 The effects of Tanacetum balsamita essential oils (TBEO) on the contractions of bovine ileal circular smooth muscle were investigated in four groups. The experiment groups were designed to observe the effects of TBEO on spontaneous, Cch, KCl, and BaCl2 induced contractions on bovine ilium muscles.23 In the first group, the cumulative concentrations of TBEOs were added to the chambers when the muscle tissue reached a stable spontaneous contraction. In the other groups, after the stable induced contraction of Cch (1.00 µM), KCl (20.00 and 60.00 mM), and BaCl2 (3.00 mM), TBEO concentrations added to the organ bath and its cumulative effects on contractions were recorded. TBEO dissolved in DMSO 5.00% and dilutions of 0.10, 0.30, 1.00, 3.00, 10.00, 30.00, 100, 300, and 1000 µg mL-1 were prepared by adding the Tyrode solution. All concentrations of 0.10 to 1000 µg mL-1 TBEO in separate groups were added to the medium at 2-minute intervals. Concentrations of 0.001, 0.003, 0.01, 0.03, 0.10, 0.30, 1.00, 3.00, and 10.00 µM of carvone were prepared and tested in the same TBEO method. At the end of the test, the tissues were washed with thyroid solution. The viability of muscles was confirmed by their response to the addition of 10.00 µM acetylcholine.23
Mechanism of action. Effect of TBEO and carvone on calcium channels was investigated in bovine ilium smooth muscle. Tissue strip samples were separately treated with concentrations of 30.00 and 100 µg mL-1 TBEO and 0.10 and 0.30 µM carvone, and the concentration-response curves (CRCs) were then obtained by adding calcium to the Ca++ free medium. Furthermore, CRCs of 0.10 and 0.30 µM verapamil concentrations as standard calcium channel blockers were evaluated in distinct groups.25 The mechanism test was performed by placing ileum samples in a Ca++ free medium containing: NaCl (136.90), NaHCO3 (11.90), glucose (5.60), KCl (2.70), MgCl2 (1.10), NaH2PO4 (0.40), and EDTA (0.10) mM) for 30 min and then replacing it with depolarizing solution (containing: NaCl (91.04), KCl (60.00), NaHCO3 (11.90), glucose (5.55), MgCl2 (1.05), NaH2PO4 (0.42), and EDTA (0.10) mM for 45 min. Before doing this, the samples passed 1 hr adaptation period in thyroid solution and their viability and contractive activity were evaluated with acetylcholine. Exchanging the solutions in each step was done every 15min. After the K+ rich and Ca++ free (depolarizing) solutions, two control CRCs were recorded with the addition of calcium concentrations. After each period, washing the tissues with the depolarizing solution restores the contractions to baseline. In the third round, the rates of Ca++ CRCs were recorded 10 min after adding the essential oil (30.00 and 100 µg mL-1), carvone (0.10 and 0.30 µM), or verapamil (0.10 and 0.30 µM).25 Cumulative concentrations of 0.003, 0.01, 0.03, 0.10, and 0.30 µM verapamil were added to the induced contractions of 60.00 mM KCl (K60) to investigate the effect of standard calcium channel blocker. Cumulative concentrations of TBEO and carvone were added to the KCl 20.00 mM (K20) induced contractions in separate groups to assess their effects on low dose KCl (K20) and compare it with high dose KCl (K60).23
Statistical analysis. The assumption of data normality was tested by the Shapiro-Wilkes test. Moreover, data were graphically assessed using histogram and box plots for assumptions of normal distribution and homogeneity of variance. Since the assumptions were not met, the nonparametric Friedman repeated measures analysis of variance on ranks was employed to compare the results. Pairwise caparison between each concentration and the control were identified by the use of the Dunnett’s test. The significance level was set at p < 0.05. Results were expressed as medians and interquartile ranges (25th-75th percentiles). IBM SPSS Statistics for Windows, (version 25.0; IBM Corp., Armonk, USA) was used for statistical analysis of data.
Results
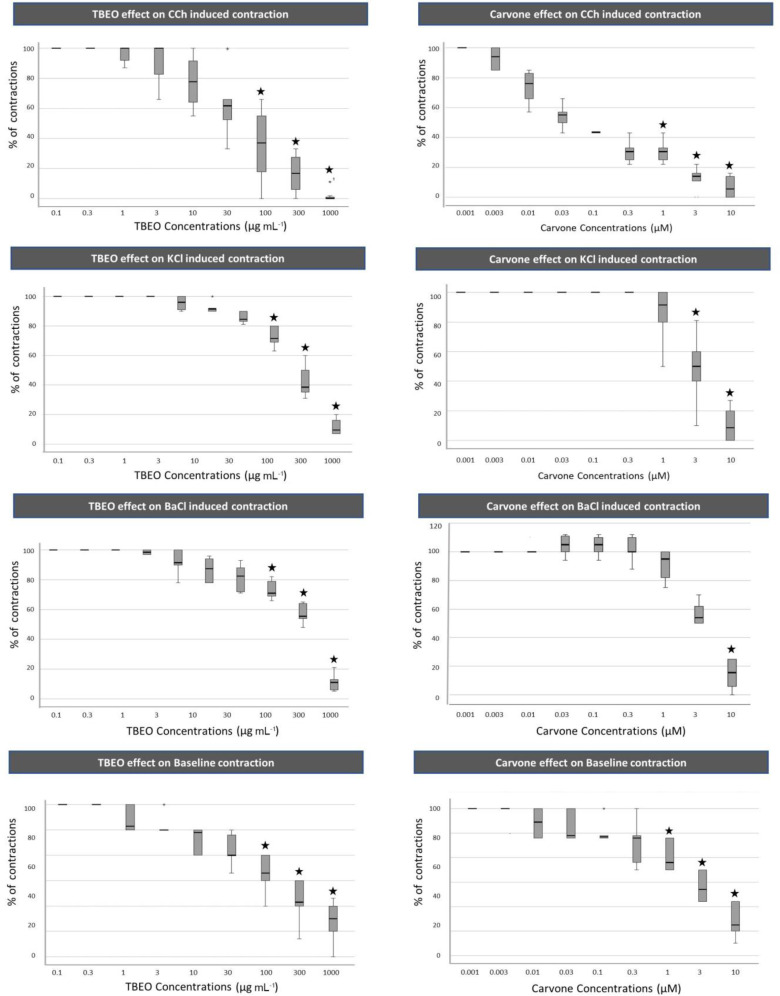
The overall result of this study was that TBEO has spasmolytic effects on bovine ileum smooth muscle. TBEO reduces both spontaneous and spasmogen-induced contractions. TBEO significantly reduced ileal circular smooth muscle baseline contractions at concentrations of 100 to 1000 µg mL-1. The cumulative addition of TBEO at concentrations of 100 to 1000 µg mL-1 significantly relaxed each of the contractions induced by BaCl2, KCl, and Cch (p < 0.05), (Fig. 1).
Fig. 1.
The spasmolytic effect of TBEO and carvone on spontaneous and induced contractions (by Cch, KCl, and BaCl2) on bovine ileum. Results are reported as the percentage of stable initial maximum contraction (SIMC) of each spasmogen and the percentage of stable initial basal contractions (SIBC) about spontaneous contractions. ★ Indicates significant reduction from SIBC or SIMC of each group at p < 0.05
The GC-MS analysis of TBEO revealed the main ingredient in this essential oil (Table 1). The results showed that in our essential oil sample, as in previous studies, carvone was the major (63.92%) ingredient. Cumulative concentrations of 1.00 to 10.00 µM carvone led to a significant reduction in spontaneous basal contraction of the ileum smooth muscle. The Cch-induced contractions were significantly relaxed at 1.00 to 10.00 µM concentrations of carvone. The spasmolytic effect of carvone on KCl induced contractions was significant at 3.00 and 10.00 µM concentrations of carvone. The BaCl2- induced contractions were significantly inhibited only at 10.00 µM concentration of carvone (p < 0.05), (Fig. 1).
Table 1.
The main components of Tanacetum balsamita essential oil (TBEO) based on GCMS findings
| Components | RI | RT | Percentage |
|---|---|---|---|
| 1,8-Cineole | 1032 | 7.21 | 3.39 |
| Beta thujone | 1099 | 8.95 | 11.53 |
| Alpha thujone | 1115 | 9.17 | 1.20 |
| Menthatriene | 1119 | 9.25 | 2.15 |
| Para menthatriene | 1133 | 9.58 | 1.42 |
| Pinocarvone | 1162 | 10.26 | 0.54 |
| Borneol | 1165 | 10.33 | 0.63 |
| Terpinen-4-ol | 1176 | 10.58 | 0.56 |
| p-Diethylbenzene | 1186 | 10.82 | 2.77 |
| trans-Dihydrocarvone | 1196 | 11.04 | 1.33 |
| trans-Piperitol | 1200 | 11.13 | 1.52 |
| Mentha-1,4,8-triene | 1219 | 11.58 | 2.19 |
| Nerol | 1229 | 11.81 | 2.04 |
| Carvone | 1249 | 12.25 | 63.92 |
RI: Retention index, RT: Retention time
The greatest amount of contraction reduction was in Cch-induced contractions, in which contractions average with TBEO reached 1.83% and with carvone 6.83% of the control contractions. The significant effect of TBEO in all spontaneous and induced contractions by Cch, KCl, and BaCl2 started at 100 µg mL-1 concentration, reaching the most amounts at 1000 µg mL-1 concentration. The maximum concentration of TBEO (1000 mg L-1) reduced spontaneous contractions less than induced contractions. The highest resistance to carvone’s myorelaxant effect was in BaCl2-induced contractions, significantly reducing the contraction with only one concentration of carvone (10.00 µM). The next resistance was in KCl contractions, significantly decreasing at 3.00 and 10.00 µM concentrations of carvone. Baseline and Cch-induced contractions were most impressible against carvone and showed a significant reduction at 1.00 to 10.00 µM of concentrations. Evaluation of the results revealed that the myorelaxant effect of TBEO and carvone is a concentration-dependent that increases with increasing concentrations. The most potent effect of TBEO was at a concentration of 1000 µg mL-1, and the most potent effect of carvone was in 10.00 µM concentration.
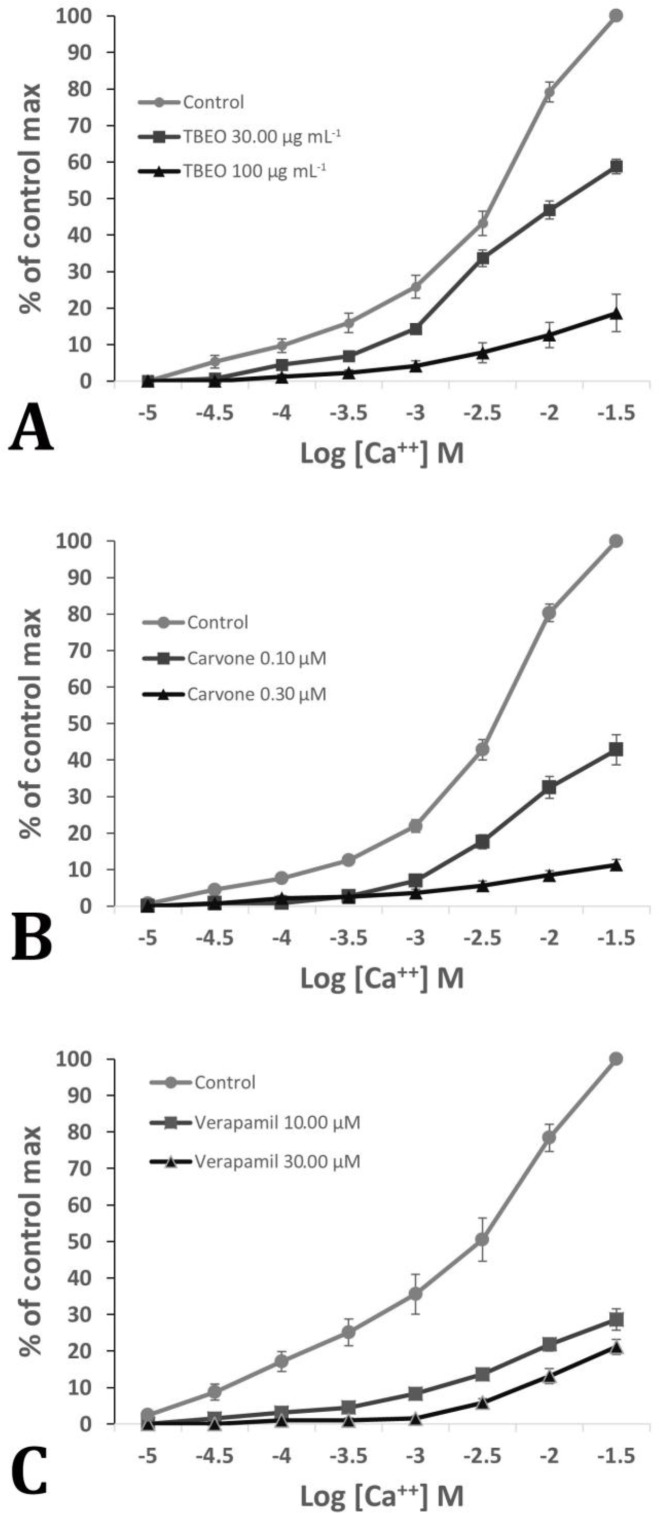
Two concentrations of TBEO reduced the contractions created by adding calcium to the depolarizing solution (K+ rich and Ca++ free). Besides, two different concentrations of carvone inhibited contractions due to the influx of calcium into the depolarizing solution. The effect of standard calcium channels blocker (verapamil) on bovine ileum smooth muscle contractions was shown, too. Comparison of the results obtained from the blockade of calcium channels by TBEO and carvone with verapamil proved that TBEO and carvone also have the property of inhibiting the calcium channels of smooth intestinal muscles (Fig. 2). The 0.10 and 0.30 µM concentrations of verapamil caused a significant reduction in K60 contractions. None of TBEO or carvone concentrations significantly reduced the K20 contractions.
Fig. 2.
Concentration-response curves (CRCs) of bovine ileal smooth muscle to increasing Ca++ dose in the absence and presence of two different concentrations of each of TBEO (A), carvone (B), and verapamil (C).
The control treatments showed that the contractions created with the three spasmogens Cch, KCl, and BaCl2, survived for 25 min. The primary solvent of essential oils and carvone (i.e. DMSO) had no significant effect on ileal contractions.
Discussion
This study investigated the effects of TBEO and its main ingredient (carvone) on contractions induced by several spasmogens in bovine ileum smooth muscle in an organ bath.
Due to the necessity of research ethics and animal rights rules, in vitro studies were preferred. Many in vitro (organ bath) studies have been performed on the gastrointestinal motility of animals, and it is believed that these experiments may reflect in vivo conditions.24-27 Although the ileum is used to evaluate bowel motilities in most species,24-31 there are few studies on ileal motilities in cattle.32,33 However, one of the sites involved in bovine gastrointestinal syndromes such as diarrhea syndrome is the ileum, which may play a significant role in the patho-physiology of GI diseases such as bovine viral diarrhea virus (BVDV) and Johne’s disease.34,35 The ileums of ruminants and non-ruminants have many anatomical and physiological similarities,36 making it possible to extend the results of this study to other animals and even humans.
The exact effect of T. balsamita and its mechanism of action on gastrointestinal motility are undefined. T. balsamita in the formulation of massage lotion has muscle relaxant properties,16 agreeing with its anti-spasmodic properties in the gastrointestinal muscles in the present study. In other members of Tanacetum genus, the antispasmodic effect on smooth muscles has been explained for T. parthenium and T. vulgare. The extract of T. parthenium reduced the spontaneous and induced contraction of the ear artery and aortic smooth muscle as well as the anococcygeal body in rabbits. The effects of T. parthenium were concentration-dependent and aortic irreversible.17,18 However, the myorelaxant effect of T. balsamita was concentration-dependent and reversible (the present study). The T. parthenium may inhibit migraine pain by the anti-spasmodic effect on cerebrovascular smooth muscles.37 The aqueous extract of T. vulgare (Tansy) relaxed the KCl induced contraction in the smooth muscle of the rat aorta.19 The significant effect of T. parthenium came from concentrations of 50.00 to 500 µg mL-1, and 1000 µg mL-1completely blocked the contractions. Significant effect of T. vulgare was observed from 100 to 800 µg mL-1. In the present study, the first significant effect of TBEO concentrations was 100 µg mL-1, peaking at 1000 µg mL-1, but complete inhibition occurred only in some of the Cch-induced contractions. These results showed that the spasmolytic potency of T. balsamita was similar to T. vulgare and slightly lower than T. parthenium.
The GC-MS analysis results revealed that, as in previous studies,7,14,38 carvone was the major ingredient in TBEO. In various TBEO samples, components other than carvone have been identified as major one, including quercetin,13 Beta-thujone,12 bornyl acetate,39 and Trans-chrysantheno.40 In various TBEO samples, carvone,7,14,38 beta-thujone,12 quercetin,13 bornyl acetate,39 and Trans-chrysantheno40 have been identified as major components. In previous studies, the authors identified carvone,7,14,38 beta-thujone,12 quercetin,13 bornyl acetate,39 and Trans-chrysantheno40 as major components of tested TBEO. Differences in the chemical composition of the TBEO in previous studies may be due to the different geographical origins of the plant.41 The most studies in Iran concluded carvone as the main composition of TBEO which was consistent with the present study results.7,14,38 Moreover, carvone had an anti-spasmodic effect on the smooth muscle of the bovine ileum (Fig. 1), justifying the hypothesis of carvone being responsible for TBEO anti-spasmodic activity.
Carvone could reduce Cch-induced contractions in the aorta of rats and guinea pig trachea smooth muscles.42 Different carvone reversals have the same effect on the relaxation of vascular and respiratory smooth muscles.42 However, in another study, the anti-spasmodic effect of carvone on guinea pig ileum smooth muscle was stronger than carvone.23 In an experiment, carvone inhibited the contractions of mercury and lead on rat aorta.22 In this experiment carvone had no significant effect on the passive tension of the aorta.22 The spasmolytic effects of carvone on contractions induced by Cch, KCl, and BaCl2 in guinea pig ileum smooth muscle have been proved.23 In this study, carvone had no significant effect on spontaneous contractions of the ileum. The Cch-induced contraction significantly reduced by carvone but did not decrease to baseline.23 In line with the study of de Sousa et al., in the present study the largest decrease of contractions due to carvone was observed in the Cch-induced contractions. Although the average of contractions did not reach the baseline, in half of the samples the contractions were completely inhibited (Fig. 1). Carvone had no significant effect on spontaneous smooth muscle contractions in guinea pig ileum42 and rat aorta,22 while in the present study antispasmodic effects of 0.01 to 0.001 µM carvone on bovine ileum spontaneous contractions were significant. Cumulative concentrations of 0.01 to 0.001 µM carvone showed a significant reduction in all contractions. This difference may be due to differences in the animal species being tested. The significant concentrations of carvone in previous studies were from 1.00 to 300 µM,22,23,42 which were significant from 0.01 to 0.001 µM in our study. These results could indicate that the bovine ileum is more sensitive to carvone than the guinea-pig ileum.
Antispasmodics can either block specific receptors or act in a non-specific way.43 Different pathways and receptors are involved in causing each of the spontaneous and induced (Cch, KCl, and BaCl2) contractions.23 In the present study, TBEO and carvone inhibited all types of these contractions; therefore, their mechanism of action is nonspecific. The Cch-induced contraction in smooth muscle is highly dependent on intracellular calcium stores,23 and in present study, the more significant effect of TBEO and carvone on Cch-induced contractions reinforced the hypothesis of their inhibitory effect on calcium channels.23 Comparing the effects of TBEO and carvone with the standard calcium channel blocker (verapamil) showed that the muscle relaxant effect in TBEO and carvone was exerted by blocking calcium channels.25 Other researches have not provided sufficient evidence to the mechanism of the muscle relaxant effect of T. balsamita. In addition, the impaired calcium transfer has been observed in other Tanacetums,44 and in carvone22,23 consistent with the results of the present study. Studies on carvone mechanism of action have been investigated by comparing the effects of carvone and verapamil on induced contractions in the aorta of rats and guinea pig ileum. 22,23 KCl works both at high doses (K60) and at low doses (K20) by opening calcium channels and opening potassium channels, respectively.23 The significant effect of TBEO and carvone on K60 contractions and their ineffectiveness on K20 confirm the CCB mechanism in smooth muscle.
Concentrations of carvone caused a significant decrease in all spontaneous and induced contractions. These concentrations were different for each spasmogen. On the other hand, significant concentrations of TBEO were equal in all groups. This difference may be due to the presence of non-carvone spasmolytic compounds in TBEO. These compounds (non-carvone) may have functional mechanisms other than CCB. In an experiment, the spectrophotometry method showed the inhibitory activity of T. balsamita extract on acetylcholinesterase.45 Thus, the components with contradictory properties in the composition of this plant are possible.
Muscle relaxants may have beneficial clinical uses. In-vitro myorelaxant effect of herbal extracts can be expected to reduce gastrointestinal transit and aim to diarrhea treatment in live animals.46 Using modulators of intestinal motility may lead to better results than using antibiotics alone for diarrhea.47 Intestinal smooth muscle spasm is one of the causes of the acute abdominal syndrome in ruminants. TBEO can be a natural alternative to antispasmodics such as atropine and hyoscine butyl bromide in this syndrome. TBEO products can also help relieve the physical obstruction of the intestinal lumen by relaxing the intestines. Anti-spasmodic action of TBEO may be useful in facilitating the passage of calculi and reducing the pain caused by uroliths in ruminants.1,48 The T. balsamita can concomitantly exert several beneficial medicinal (anti- inflammatory, antimicrobial, antioxidant, analgesic, and myorelaxant) effects in gastrointestinal disorders associated with hypermotility and/or spasm. A promising finding of the clinical use of T. balsamita is that TBEO consumption in animal diets had no adverse effects on carcasses and nutritional value of the meat.11
In conclusion, the ability of the TBEO to reduce spontaneous and induced contractions in vitro on bovine ileum reveals its ability to relieve spasms and control intestinal hypermotility in living ruminants. Carvone, as the major component in TBEO, has similar muscle relaxant effects on ileal smooth muscle contractions and it is probably responsible for an important part of TBEO’s antispasmodic effects. Both TBEO and carvone relax smooth muscles by calcium channel blocking. T. balsamita can be an excellent candidate for producing a gastrointestinal motility modulator in bovine medicine and even in human medicine. The products of this plant or its major ingredient (carvone) may be used to treat ruminant gastrointestinal disorders such as acute abdomen or intestinal hypermotility (such as diarrhea syndrome). However, further research should be done to evaluate the effects of T. balsamita on gastrointestinal motility in vivo.
Conflict of interest
The authors declare no competing financial interest.
Acknowledgments
The authors would like to express their sincere appreciation and gratitude to all the technical staff of the Veterinary Hospital of Urmia University and Urmia Industrial Slaughterhouse.
References
- 1.Constable PD, Hinchclif KW, Done SH, et al. Veterinary medicine: A textbook of the diseases of cattle, horses, sheep, pigs and goats. 11th ed. New York, USA: Elsevier Health Sciences ; 2017. pp. 176–908. [Google Scholar]
- 2.Szymaszkiewicz A , Włodarczyk J , Mazur M , et al Cyclic derivatives of morphiceptin possess anti-transit effect in the gastrointestinal tract and alleviate abdominal pain in mice. Pharmacol Rep. 2020;72(2):314–321. doi: 10.1007/s43440-020-00084-4. [DOI] [PubMed] [Google Scholar]
- 3.Graziani C , Talocco C , De Sire R , et al Intestinal permeability in physiological and pathological conditions: major determinants and assessment modalities. Eur Rev Med Pharmacol Sci. 2019;23(2):795–810. doi: 10.26355/eurrev_201901_16894. [DOI] [PubMed] [Google Scholar]
- 4.Fecteau G , Desrochers A , Francoz D , et al Diagnostic approach to the acute abdomen. Vet Clin North Am Food Anim Pract. 2018;34(1):19–33. doi: 10.1016/j.cvfa.2017.10.001. [DOI] [PubMed] [Google Scholar]
- 5.El Mahdy C , Popescu S , Borda C , et al Plants used in ethnoveterinary medicine in cows A Review. Bull UASVM Anim Sci Biotechnol. 2019;76(2):61–76. [Google Scholar]
- 6.Mozaffarian V. Dictionary of Iranian plant names. 2nd ed. Costa Mesa, USA: Mazda Publishers ; 1998. p. 244. [Google Scholar]
- 7.Sharif M, Najafizadeh P, Asgarpanah J, et al. In vivo analgesic and anti-inflammatory effects of the essential oil from Tanacetum balsamita L. Braz J Pharm Sci. 2020;56:e18357. [Google Scholar]
- 8.Nickavar B , Amin G , Mehregan N Quercetine, a major flavonol aglycon from Tanacetum balsamita L. Iran J Pharm Res. 2003;2(4):249–250. [Google Scholar]
- 9.Ivănescu B , Tuchiluș C , Corciovă A , et al Antioxidant, antimicrobial and cytotoxic activity of Tanacetum vulgare, Tanacetum corymbosum and Tanacetum macrophyllum extracts. Farmacia. 2018;66(2):282–288. [Google Scholar]
- 10.Latifian E, Arslanoğlu ŞF. Traditional medicinal plants of Azerbaijan province of Iran. Agric Sci. 2018;9:157–170. [Google Scholar]
- 11.Karami A , Khatibjoo A , Akbari Gharaei M , et al Considering the effect of Tanacetum balsamita essential oil on broiler chickens performance, meat quality and ileal microbial population. Iran J Anim Sci Res. 2019;11(1):73–84. [Google Scholar]
- 12.Bączek KB, Kosakowska O, Przybył JL, et al. Antibacterial and antioxidant activity of essential oils and extracts from costmary (Tanacetum balsamita L ) and tansy (Tanacetum vulgare L ) Ind Crops Prod. 2017;102:154–163. [Google Scholar]
- 13.Kazemzadeh M , Yaghmaei P , Mohammadi S Analgesic and anti-inflammatory effects of Tanacetum balsamita essential oil and one of its major constituents (quercetin) in male rats. Clin Neurol Neurosci. 2017;1(3):60–66. [Google Scholar]
- 14.Yousefzadi M , Ebrahimi SN , Sonboli A , et al Cytotoxicity, antimicrobial activity and composition of essential oil from Tanacetum balsamita L subsp balsamita. Nat Prod Commun. 2009;4(1):119–122. [PubMed] [Google Scholar]
- 15.Nobakht A , Hosseini Mansoub N , Nezhady MAM Effect of Melissa officinalis L Tanacetum balsamita and Ziziphora clinopodioides on performance, blood biochemical and immunity parameters of laying hens. Asian J Anim Vet Adv. 2012;7(1):74–79. [Google Scholar]
- 16.Mărculescu A Contribution to the clinical study of chemical taxa from the species Chrysanthemum balsamita L. [French]. Phytotherapie. 2013;11(4):219–224. [Google Scholar]
- 17.Barsby RW J , Knight DW , McFadzean I A chloroform extract of the herb feverfew blocks voltage‐ dependent potassium currents recorded from single smooth muscle cells. J Pharm Pharmacol. 1993;45(7):641–645. doi: 10.1111/j.2042-7158.1993.tb05669.x. [DOI] [PubMed] [Google Scholar]
- 18.Barsby RW , Salan U , Knight DW , et al Feverfew extracts and parthenolide irreversibly inhibit vascular responses of the rabbit aorta. J Pharm Pharmacol. 1992;44(9):737–740. doi: 10.1111/j.2042-7158.1992.tb05510.x. [DOI] [PubMed] [Google Scholar]
- 19.Lahlou S , Tangi KC , Lyoussi B , et al Vascular effects of Tanacetum vulgare L leaf extract: in vitro pharma-cological study. J Ethnopharmacol. 2008;120(1):98–102. doi: 10.1016/j.jep.2008.07.041. [DOI] [PubMed] [Google Scholar]
- 20.Younis YMH , Beshir SM Carvone-rich essential oils from Mentha longifolia ( ) Huds ssp schimperi Briq and Mentha spicata L Grown in Sudan. J Essent Oil Res. 2004;16(6):539–541. [Google Scholar]
- 21.Başer KHC , Demirci B , Tabanca N , et al Composition of the essential oils of Tanacetum armenum (DC.) Schultz Bip., T. balsamita L., T. chiliophyllum (Fisch. & Mey.) Schultz Bip. var. chiliophyllum and T. haradjani (Rech. fil.) Grierson and the enantiomeric distribution of camphor and carvone. Flavour Fragr J. 2001;16:195–200. [Google Scholar]
- 22.Kundu S, Shabir H, Basir SF, et al. Inhibition of As(III) and Hg(II) caused aortic hypercontraction by eugenol, linalool and carvone. J Smooth Muscle Res. 2014;50:93–102. doi: 10.1540/jsmr.50.93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Souza FVM, da Rocha MB, de Souza DP, et al. (-)-Carvone: antispasmodic effect and mode of action. Fitoterapia. 2013;85:20–24. doi: 10.1016/j.fitote.2012.10.012. [DOI] [PubMed] [Google Scholar]
- 24.Mendel M, Chłopecka M, Latek U, et al. Evaluation of the effects of Bidens tripartita extracts and their main constituents on intestinal motility – An ex vivo study. J Ethnopharmacol. 2020;259:112982. doi: 10.1016/j.jep.2020.112982. [DOI] [PubMed] [Google Scholar]
- 25.Gilani AH , Bukhari IA , Khan RA , et al Cholinomimetic and calcium channel blocking activities of Carthamus oxycantha. Phytother Res. 2005;19: 679–683. doi: 10.1002/ptr.1727. [DOI] [PubMed] [Google Scholar]
- 26.Jalilzadeh-Amin G , Maham M , Dalir-Naghadeh B , et al Effects of Mentha longifolia essential oil on ruminal and abomasal longitudinal smooth muscle in sheep. J Essent Oil Res. 2012;24(1):61–69. [Google Scholar]
- 27.Mohseni M , Maham M , Dalir-Naghadeh B , et al Does Achillea millefolium extracts possess prokinetic effects on the bovine abomasum thourgh M3 muscarinic receptors? Vet Res forum. 2017;8(2):115–120. [PMC free article] [PubMed] [Google Scholar]
- 28.Ching MOY , Chinnappan S , Rajagopal MS In vitro anti-motility and antispasmodic effects of Garcinia mangostana extracts in isolated chicken ileum preparation. Int J Res Pharm Sci. 2019;10(2):1444–1447. [Google Scholar]
- 29.Guerra DD , Bok R , Lorca RA , et al Protein kinase A facilitates relaxation of mouse ileum via phosphorylation of neuronal nitric oxide synthase. Br J Pharmacol. 2020;177(12):2765–2778. doi: 10.1111/bph.15001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Afzal A , Khan A , Ajmal K , et al Effect of fluoxetine and paroxetine on intestinal motility in presence of ondansetron in ex-vivo model. Life Sci. 2020;1(3) [Google Scholar]
- 31.Unterköfler MS , McGorum BC , Milne EM , et al Establishment of a model for equine small intestinal disease: effects of extracorporeal blood perfusion of equine ileum on metabolic variables and histological morphology-an experimental ex vivo study. BMC Vet Res. 2019;15(1) doi: 10.1186/s12917-019-2145-9. doi: 0.1186/s12917-019-2145-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Zanolari P, Meylan M, Marti M, et al. In vitro effects of bethanechol on intestinal smooth muscle preparations in presence and absence of M2 or M3 muscarinic receptor antagonists in healthy dairy cows. Dtsch Tierärztl Wschr. 2007;114:171–177. [Google Scholar]
- 33.Ansia I , Stein HH , Brøkner C , et al Short communication: A pilot study to describe duodenal and ileal flows of nutrients and to estimate small intestine endogenous protein losses in weaned calves. J Dairy Sci. 2020;103(10):9102–9109. doi: 10.3168/jds.2020-18281. [DOI] [PubMed] [Google Scholar]
- 34.Khodakaram-Tafti A , Farjanikish GH Persistent bovine viral diarrhea virus (BVDV) infection in cattle herds. Iran J Vet Res. 2017;18(3):154–163. [PMC free article] [PubMed] [Google Scholar]
- 35.Albarrak SM, Waters WR, Stabel JR, et al. Evaluating the cytokine profile of the WC1+ γδ T cell subset in the ileum of cattle with the subclinical and clinical forms of MAP infection. Vet Immunol Immunopathol. 2018;201:26–31. doi: 10.1016/j.vetimm.2018.05.003. [DOI] [PubMed] [Google Scholar]
- 36.Balemba OB , Mbassa GK , Semuguruka WD , et al The topography, architecture and structure of the enteric nervous system in the jejunum and ileum of cattle. J Anat. 1999;195(Pt 1):1–9. doi: 10.1046/j.1469-7580.1999.19510001.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Shahhoseini R , Azizi M , Asili J , et al Comprehensive assessment of phytochemical potential of Tanacetum parthenium (L phenolic compounds, antioxidant activity, essential oil and parthenolide. J Essent Oil-Bear Plants. 2019;22(3):614–629. [Google Scholar]
- 38.Monfared A , Hosseiny Davarani SS , Rustaiyan A , et al Composition of the essential oil of Tanacetum balsamita L ssp balsamitoides (Schultz Bip ) Grierson from Iran. J Essent Oil Res. 2002;14(1):1–2. [Google Scholar]
- 39.Jaimand K , Rezaee MB Chemical constituents of essential oils from Tanacetum balsamita L balsamitoides (Schultz-Bip ) Grierson from Iran. J Essent Oil Res. 2005;17(5):565–566. [Google Scholar]
- 40.Kumar V , Tyagi D Chemical composition and biological activities of essential oils of genus Tanacetum - a review. J Pharmacogn Phytochem. 2013;2(3):159–163. [Google Scholar]
- 41.Mohammedi H , Mecherara-Idjeri S , Hassani A Variability in essential oil composition, antioxidant and antimicrobial activities of Ruta montana collected from different geographical regions in Algeria. J Essent Oil Res. 2020;32(3) oi: 10.1080/10412905. 2019.1660238. [Google Scholar]
- 42.de Sousa DP , Mesquita RF , de Araújo Ribeiro LA , et al Spasmolytic activity of Carvone and Limonene Enantiomers. Nat Prod Commun. 2015;10(11):1893–1896. [PubMed] [Google Scholar]
- 43.Madeira SVF , Matos FJA , Leal-Cardoso JH , et al Relaxant effects of the essential oil of Ocimum gratissimum on isolated ileum of the guinea pig. J Ethnopharmacol. 2002;81(1):1–4. doi: 10.1016/s0378-8741(02)00049-1. [DOI] [PubMed] [Google Scholar]
- 44.Pareek A , Suthar M , Rathore GS , et al Feverfew (Tanacetum parthenium L ): A systematic review. Pharmacogn Rev. 2011;5(9):103–110. doi: 10.4103/0973-7847.79105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Orhan IE, Tosun F, Gülpinar AR, et al. LC-MS quantification of parthenolide and cholinesterase inhibitory potential of selected Tanacetum L. (Emend. Briq.) taxa. Phytochem Lett. 2015;11:347–352. [Google Scholar]
- 46.Aleem A, Janbaz KH. Dual mechanisms of anti-muscarinic and Ca++ antagonistic activities to validate the folkloric uses of Cyperus niveus Retz as antispasmodic and antidiarrheal. J Ethnopharmacol. 2018;213:138–148. doi: 10.1016/j.jep.2017.11.006. [DOI] [PubMed] [Google Scholar]
- 47.Mullowney PC , Patterson WH Therapeutic agents used in the treatment of calf diarrhea. Vet Clin North Am Food Anim Pract. 1985;1(3):563–579. doi: 10.1016/s0749-0720(15)31303-7. [DOI] [PubMed] [Google Scholar]
- 48.Smith BP. Large animal internal medicine. . 5th ed. St. Louis, USA: Mosby, Elsevier Inc; 2015. pp. 897–903. [Google Scholar]