Abstract
The potential rates and control of aerobic root-associated carbon monoxide (CO) consumption were assessed by using excised plant roots from five common freshwater macrophytes. Kinetic analyses indicated that the maximum potential uptake velocities for CO consumption ranged from 0.4 to 2.7 μmol of CO g (dry weight)−1 h−1 for the five species. The observed rates were comparable to previously reported rates of root-associated methane uptake. The apparent half-saturation constants for CO consumption ranged from 50 to 370 nM CO; these values are considerably lower than the values obtained for methane uptake. The CO consumption rates reached maximum values at temperatures between 27 and 32°C, and there was a transition to CO production at ≥44°C, most likely as a result of thermochemical organic matter decomposition. Incubation of roots with organic substrates (e.g., 5 mM syringic acid, glucose, alanine, and acetate) dramatically reduced the rate of CO consumption, perhaps reflecting a shift in metabolism by facultative CO oxidizers. Based on responses to a suite of antibiotics, most of the CO consumption (about 90%) was due to eubacteria rather than fungi or other eucaryotes. Based on the results of acetylene inhibition experiments, methanotrophs and ammonia oxidizers were not active CO consumers.
Carbon monoxide (CO) is one of the most important chemical reactants in the troposphere. It influences the fate of methane and ozone by removing the major atmospheric oxidizing agent, hydroxyl radical (29). The background CO mixing ratios range from about 50 to 300 ppb and vary considerably over time and space (33). Fossil fuel use, biomass burning, and oxidation of atmospheric hydrocarbons (methane and other compounds) account for most of the CO source strength (1,200 to 2,850 Tg year−1), and direct natural emissions from oceans and vegetation (80 to 360 Tg year−1 [34]) account for 10 to 15% of the total. However, each of these components of the CO budget is uncertain (34), thus limiting an accurate assessment of the effects of CO on atmospheric chemistry and climate change.
The role of wetlands in the global CO cycle has not been adequately investigated. Several limited studies have indicated that wetlands are not important. Conrad et al. (17) and Khalil and Rasmussen (23) measured CO emissions from rice paddies and extrapolated the results globally to predict that wetland CO emissions are a minor part of the CO budget at most. However, these results have not been validated with natural wetland data, and emissions may have been underestimated since the roles of photochemical reactions with dissolved organic carbon (9, 43, 46) and vegetation (17, 39, 42) were ignored. In addition, Funk et al. (20) showed that CO emissions were considerably higher than expected based on extrapolations made by Conrad et al. (17).
CO emissions from wetlands and their relative importance in the global CO budget may be determined in part by populations of root-associated CO oxidizers. As is the case for methane, CO produced in sediments may be transported through roots and stems to the atmosphere. Both aerobic and anaerobic CO oxidizers growing on or in plant roots could significantly attentuate such a flux. The aerobic CO oxidizers include carboxydotrophs that grow chemolithotrophically on CO or heterotrophically on various organic compounds (30–32, 37, 45); the significance of carboxydotrophs in situ is unknown but has been questioned (13). Methanotrophs, ammonia oxidizers, and certain fungi also consume CO (5, 39). Although each of these groups may contribute to CO oxidation in soil (10a, 12), the role of these groups in wetlands has not been addressed.
The objective of the study described here was to characterize aerobic root-associated CO consumption. Potential rates and controls of activity were assessed in vitro following using methods previously used to characterize root-associated methanotrophy (25). Kinetic analyses of CO consumption revealed that there were substantial variations among plant taxa and root types. Eubacteria were primarily responsible for CO consumption, but methanotrophs and ammonium oxidizers were probably not involved. Organic substrates limited CO consumption, and uptake had a mesophilic response to temperature.
MATERIALS AND METHODS
Sampling and root processing.
Plants were obtained from a wetland near Bristol, Maine (26). As described previously, intact plants (roots and sediment) were removed with a spade and transported to the laboratory for analysis (25). Sediment was washed from the plants and intact root systems with tap water. Roots were cut from rhizomes, carefully teased apart, rinsed again, and sorted. Subsamples of individual roots (length, up to 45 cm) were obtained after extraneous particulate organic matter was removed. The sampling procedure minimized damage to the roots and any associated loss of soluble organic compounds. Pontederia cordata roots were used for all temperature response, organic carbon, and antibiotic assays (which were conducted in August through October 1997). Wet roots were blotted gently with paper towels and sealed in 50-ml culture tubes containing 1 to 2 ml of deionized water and having neoprene stoppers. Root volume was determined after kinetic analyses by water displacement; the root volume was 4.0 ± 0.2 cm3 (mean ± standard error; n = 24). All experiments were initiated within 10 h of the time that plant samples were obtained, and clean latex gloves were worn when the roots were handled. The roots were incubated with ambient laboratory lighting during time course assays.
Differences in CO consumption as a function of root type were determined by separating P. cordata roots into four groups based on the following simple visual distinctions: color, form, thickness, fine root growth, and position on the plant. The root types distinguished were “feeder,” “shallow-anchor,” “deep-anchor,” and “miscellaneous” roots. The feeder roots had extensive lateral fine root growth (twofold more than anchor roots on a dry weight basis). Anchor roots had thicker tap roots. Deep-anchor roots were coated with brown iron oxide plaques, and shallow-anchor roots were white or purple. Miscellaneous roots had combinations of features. Feeder roots grew at the sediment-water interface, while shallow- and deep-anchor roots penetrated into the sediment matrix.
Differences in CO consumption among taxa were determined by using triplicate samples of five species (Table 1) that grew under similar conditions (i.e., relatively dense colonies within an approximately 30-m radius; Sagittaria latifolia occurred as solitary plants). The roots of each plant were sorted to ensure that samples were monospecific.
TABLE 1.
Kinetic constants for CO oxidation by macrophyte roots
| Source of roots | Vmaxp (μmol of CO g [dry wt] of root−1 h−1)a | Kapp (nM CO)a |
|---|---|---|
| Pontederia cordata | ||
| Feeder roots | 1.2 ± 0.2 | 50 ± 5 Ab |
| Shallow-anchor roots | 1.1 ± 0.1 | 150 ± 30 |
| Miscellaneous roots | 1.1 ± 0.2 | 180 ± 10 |
| Deep-anchor roots | 1.2 ± 0.4 | 260 ± 40 B |
| Sagittaria latifolia | 2.7 ± 0.1 B | 370 ± 60 A |
| Sparganium eurycarpum | 0.9 ± 0.2 A | 230 ± 20 |
| Elymus riparius | 0.4 ± 0.1 | 180 ± 60 |
| Typha latifolia | 0.5 ± 0.2 | 140 ± 40 |
Data are means ± standard deviations (n = 3). We assumed that the solubility coefficient was 0.018 cm3 of CO ml of H2O−1 at 20°C (36).
Values followed by different letters in the same column are significantly different as determined by Tukey’s test (P < 0.05); the data for P. cordata and the data for the four other taxa were treated as separate data sets for the statistical analysis.
Kinetic analyses.
Kinetic data were obtained by using triplicate samples consisting of approximately 3 g (fresh weight) of roots obtained during early autumn. CO consumption was assayed at headspace CO concentrations of <30 ppm in order to obtain a first-order rate constant (k), and this was followed immediately by an assay at a headspace CO concentration of approximately 1% to obtain the maximum potential uptake velocity (Vmaxp). Vmaxp and k were used to approximate the apparent half-saturation constant of the Michaelis-Menten equation (Kapp) from the relationship k = Vmaxp Kapp−1. This approach, which was based on the Michaelis-Menten model, was chosen in order to minimize the variability inherent in the use of replicate samples for a series of concentration-versus-rate assays and because use of individual roots for a complete concentration series (i.e., subsaturating to saturating concentrations) would have required excessively long incubations.
Temperature analysis.
k values were obtained at 23 to 25°C by using headspace CO concentrations of <20 ppm for triplicate 0.7-g (fresh weight) samples of P. cordata feeder roots. After this initial assay, which lasted about 7 h, the roots were vented with ambient air and incubated for 2 to 4 h at temperatures ranging from 2 to 44°C without CO. After CO (<20 ppm) was added, a second uptake assay was used to determine k as a function of temperature.
Response to added organic substrates.
Triplicate samples consisting of approximately 1.5 g (fresh weight) of P. cordata deep-anchor roots were incubated horizontally at 30°C on a rotary shaker (100 rpm) for 5 days with a headspace containing 2,000 ppm of CO and an aqueous phase containing 20 ml of either syringic acid, glucose, alanine, or acetate (5 mM). The controls were treated with deionized water and 2,000 ppm of CO. On day 5, the incubation medium was decanted and centrifuged (15,000 × g, 30 min), and the pellet was resuspended in 2 ml of fresh substrate, which was transferred to 30-ml tubes. The roots were blotted with paper towels and returned to the culture tubes. The solution and roots were assayed for CO consumption rates with a headspace containing approximately 1,000 ppm of CO. A linear function best described CO consumption at this mixing ratio.
Antibiotic assay.
Antibiotics at the following concentrations (in deionized water) were used to differentiate among fungi, bacteria, and plant cells: streptomycin, 100 μg ml−1; ampicillin, 50 μg ml−1; chlortetracycline, 20 μg ml−1; nystatin, 50 μg ml−1; and cycloheximide, 50 μg ml−1. Twenty milliliters of each fresh antibiotic solution and 500 ppm of CO were added to triplicate samples consisting of approximately 2 g (fresh weight) of P. cordata deep-anchor roots incubated horizontally at 30°C on a rotary shaker (100 rpm). The incubation medium was decanted after 24 h, and the excess liquid was blotted from the roots with paper towels. CO consumption rates were obtained by using a headspace CO concentration of approximately 1,000 ppm. Immediately after this initial assay, fresh antibiotic solution was added to the roots, and the preparations were incubated for an additional 4 days. On day 5, the CO consumption assay was repeated. The controls were treated with only deionized water and CO.
Acetylene inhibition.
The potential role of methanotrophic and ammonia-oxidizing bacteria was assessed by using acetylene, which is a well-known nonspecific irreversible inhibitor of methane and ammonia monooxygenases. Washed, sediment-free roots (about 1 g [fresh weight]) of Sparganium eurycarpum, S. latifolia, and Typha latifolia were incubated with an oxic headspace in triplicate in 40-cm3 tubes containing 1 ml of deionized water. The tubes were sealed with neoprene stoppers, and the headspaces of three tubes for each root type were amended with 1% acetylene; unamended tubes served as controls. All of the tubes received 0.5 μCi (18.5 kBq) of 14CO prepared by dehydrating [14C]formic acid (60 mCi mmol−1; 2.2 GBq mmol−1; New England Nuclear) as described by Bartholomew and Alexander (2). The total headspace CO concentrations were approximately 1 ppm. Headspace subsamples (0.5 cm3) were removed at intervals to assay for 14CO2 as an index of CO oxidation. The 14CO2 was trapped in 2 ml of 1 N KOH, and then the amount of 14CO2 was determined by liquid scintillation counting with an LKB model 1217 counter (LKB-Wallac Instruments, Inc.).
Gas chromatography.
Headspace CO concentrations less than 30 ppm were measured by using 1-cm3 gas samples injected into a model RGA3 instrument (Trace Analytical, Inc.) equipped with a mercury vapor detector. The instrument was fitted with a gas sampling valve and a 1-m Molecular Sieve type 5A column operated at 105°C with an airflow rate of 20 cm3 min−1. The detector was operated at 265°C. The detection limit was <10 ppb of CO. The retention times for hydrogen and CO were 0.5 and 1.5 min, respectively, and the peaks were well separated. The instrument was standardized by using a certified 91.9-ppb CO standard (National Oceanic and Atmospheric Administration) a 10.8-ppm CO standard (Maine Oxy Supply Co.), and laboratory dilutions (1 to 40 ppm) of certified 996-ppm CO (Maine Oxy Supply Co.). The instrument response at concentrations above about 2 to 5 ppm was nonlinear, and the relationship between detector response and concentration was established by nonlinear curve fitting.
Headspace samples (volume, 0.1 cm3) with CO concentrations of >0.01% were analyzed with a model 3700 gas chromatograph (Varian Instruments, Inc.) equipped with a methanizer (SRI Instruments, Inc.) and a flame ionization detector. The columns used for gas separation were a Poropak Q column (length, 1 m; outside diameter, 0.125 in.; inside diameter, 0.0625 in.) in series with a silica gel column (Davison Grade 12; 80/100; Supelco, Inc.) to retain hydrocarbons and a Molecular Sieve type 5A column (length, 1 m; outside diameter, 0.125 in.; inside diameter, 0.0625 in.) to retain CO2. The columns were operated at 60°C, and the air, H2, and N2 carrier gas flow rates were approximately 30, 300, and 30 cm3 min−1, respectively. The flame ionization detector, injector, and methanizer were operated at 200, 80, and 380°C, respectively. The instrument was standardized with a certified 996-ppm CO standard (Maine Oxy Supply Co.) and laboratory dilutions (0.25 to 1.5% CO) of pure 99.3% CO (Scott Specialty Gases, Inc.).
Statistical analyses.
Headspace CO mixing ratios were plotted as a function of time for individual replicates; rate constants (i.e., k and Vmaxp) were determined from linear and nonlinear regression analyses by using Kaleidagraph software (Synergy, Inc.). The significance of regression analyses and the significance of differences among means were determined by using analysis of variance (Systat); Tukey’s test for mean multiple comparisons was performed to define significant groups of samples.
RESULTS
Rate estimates.
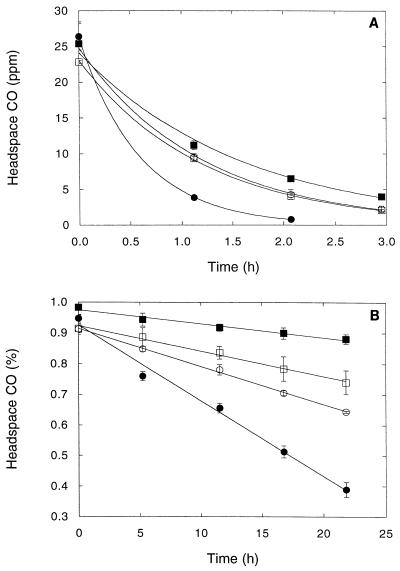
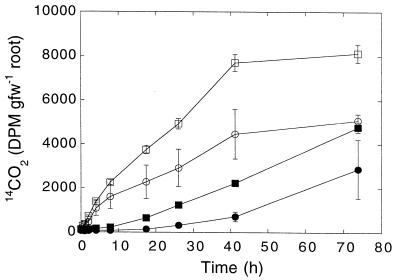
Roots under aerobic conditions (Fig. 1) rapidly and efficiently consumed CO. The kinetic constants for the various plant taxa differed significantly (Table 1). The Vmaxp for S. latifolia (2.7 μmol of CO g [dry weight]−1 h−1) exceeded by a factor of more than 2 the Vmaxp values for S. eurycarpum and P. cordata (0.9 to 1.1 μmol of CO g [dry weight]−1 h−1) and by a factor of 5 the Vmaxp values for Elymus riparius and T. latifolia, (0.4 to 0.5 μmol of CO g [dry weight]−1 h−1) (Table 1). In contrast, the Vmaxp values for the various P. cordata root types were very similar (Table 1).
FIG. 1.
Root-associated CO oxidation by different P. cordata root types (see text) (A) and different plant taxa (B). (A) Symbols: •, feeder roots; ○, shallow-anchor roots; □, miscellaneous roots; ■, deep-anchor roots. (B) Symbols: •, S. latifolia; ○, S. eurycarpum; □, T. latifolia; ■, E. riparius. The data are means ± 1 standard error (n = 3) fit by exponential (A) and linear (B) regression.
The Kapp values differed significantly as a function of root type and plant taxa. The feeder roots exhibited the highest affinity for CO, with a Kapp of 54 ± 5 nM CO (67 ± 6 ppm of CO) (mean ± standard deviation). The Kapp values for shallow and miscellaneous roots were intermediate, 154 ± 25 to 182 ± 13 nM CO (192 ± 32 to 226 ± 16 ppm of CO), while the Kapp values for deep-anchor roots were 258 ± 40 nM CO (321 ± 50 ppm of CO). The Kapp values for S. latifolia, 367 ± 63 nM CO (457 ± 78 ppm of CO), were significantly different from the Kapp values for T. latifolia, E. riparius, and S. eurycarpum, which were 137 ± 40 to 229 ± 21 nM CO (171 ± 50 to 285 ± 27 ppm of CO). In general, the Kapp values (0.1 to 0.4 μM CO) were less variable than the Vmaxp values (0.4 to 2.7 μmol of CO g [dry weight]−1 h−1).
The biomass of each of the four root types was measured, and most of the roots (45% ± 10% [mean ± standard deviation; n = 2]) were miscellaneous roots, which were roots that had combinations of features. The relative biomasses of the feeder, shallow-anchor, and deep-anchor roots were 6% ± 2%, 22% ± 9%, and 27% ± 1% (n = 2), respectively.
Temperature analysis.
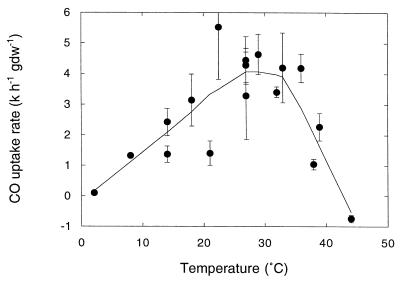
CO consumption by roots increased with temperature from 2°C, reaching a maximum at temperatures between 27 and 32°C (Fig. 2) and decreasing at higher temperatures. Net CO production was evident at temperatures of ≥44°C (Fig. 2). The activation energy (Ea) for CO oxidation determined from an Arrhenius plot for temperatures from 2 to 29°C was 59 kJ mol−1 (P < 0.0001) (data not shown). The Q10 value from 2 to 29°C was approximately 2.
FIG. 2.
Carbon monoxide consumption as a function of temperature for roots of P. cordata. The curve is a weighted fit, and the data are means ± 1 standard error (n = 3). gdw, gram (dry weight).
Response to added organic substrates.
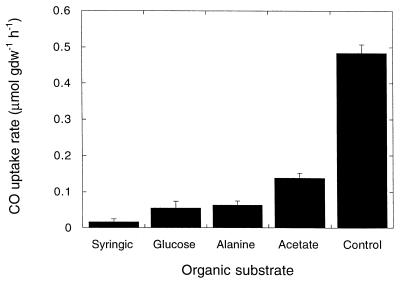
The rates of CO consumption decreased significantly compared to controls with roots incubated with a headspace containing 2,000 ppm of CO and solutions containing various simple organic compounds at a concentration of 5 mM (Fig. 3). All of the compounds assayed yielded similar results, which did not differ statistically. Negligible CO consumption was observed in the incubation medium after root tissue was removed.
FIG. 3.
Carbon monoxide consumption after 5-days of incubation with 5 mM organic substrate and a headspace CO concentration of 2,000 ppm. Organic compounds were not added to the control. Data are means ± 1 standard error (n = 3). gdw, gram (dry weight).
Antibiotic assay.
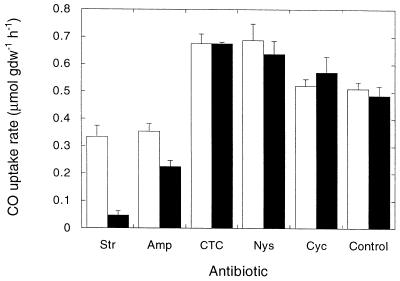
The antifungal agents nystatin and cycloheximide had no effect on CO consumption, while two of the antibacterial agents significantly reduced activity (Fig. 4). Streptomycin was the most effective inhibitor, decreasing the CO consumption rate by 90% compared to controls; ampicillin inhibited activity to a lesser extent. Inhibition by streptomycin and ampicillin increased over time and was not significant until day 5. Chlortetracycline, a general antimicrobial agent, had no effect on CO consumption. Although the antifungal agents and chlortetracycline appeared to slightly stimulate CO consumption, the difference was insignificant (P ≥ 0.052).
FIG. 4.
Carbon monoxide consumption after incubation with antibiotics and 500 ppm of CO for 1 day (open bars) and 5 days (solid bars). See the text for the concentrations of antibiotics used. Amp, ampicillin; CTC, chlorotetracycline; Cyc, cycloheximide; Nys, nystatin; Str, streptomycin. The following values were significantly different (P ≤ 0.007): day 5 control value and day 5 streptomycin value; day 5 control value and day 5 ampicillin value; and day 5 streptomycin value and day 1 streptomycin value. Data are means ± 1 standard error (n = 3). gdw, gram (dry weight).
Acetylene inhibition.
14CO was oxidized rapidly (Fig. 5), and the maximum relative extent of oxidation approached 70% during a 24-h incubation. In these assays, S. eurycarpum control roots exhibited the greatest rates of oxidation. The time course of 14CO2 accumulation in controls was consistent with exponential CO uptake since the data fit a model having the following form: [14CO2(t)/14CO(t0)] = 1 − 14CO(t0)e−kt. CO oxidation was inhibited completely immediately after 1% acetylene was added and for 15 to 25 h thereafter. However, during extended incubation in the presence of acetylene, the extent of oxidation increased significantly (Fig. 5).
FIG. 5.
Time course for accumulation of 14CO2 from 14CO oxidation by roots of S. eurycarpum (circles) and T. latifolia (squares). The open and solid symbols indicate the data for controls and 1% acetylene treatment, respectively. gfw, gram (fresh weight).
DISCUSSION
CO consumption by macrophyte roots apparently can contribute significantly to the fate of wetland CO. A comparison of CO and methane consumption rates is illustrative. The Vmaxp values for methane oxidation range from 0.3 to 27 μmol of CH4 g [dry weight] of root−1 h−1 for a variety of macrophytes (25), including rice (5 μmol of CH4 g [dry weight]−1 h−1 [8]). The Vmaxp values for CO oxidation, 0.5 to 2.7 μmol of CO g (dry weight) of root−1 h−1 (Table 1), are about 10-fold lower for the same taxa (10). However, the methane concentrations in pore water are more than 100 times greater than the CO concentrations (27). Thus, CO consumption may have an impact similar to that of CH4 consumption, which reduces potential emissions by 25 to 50% (10, 26).
The Vmaxp values for CO oxidation varied significantly among plant taxa but not as a function of root type for P. cordata. Differences among taxa may reflect differences in root aeration (10, 35, 38, 40) that affect the oxygen and CO concentrations in the rhizosphere, as well as the densities of CO oxidizers. The average Kapp values ranged from 50 to 370 nM CO among taxa and root types (Table 1). The differences may reflect variations in the root microenvironment. The Kapp values varied substantially and independently of the Vmaxp values in a comparison of P. cordata roots. In contrast, the Vmaxp values varied substantially more among taxa than the Kapp values varied.
Root Kapp values are greater than soil Kapp values (4 to 41 nM CO [3, 15, 18, 41]) and water column Kapp values (7 to 9 nM CO [13]) but less than carboxydotrophic culture Kapp values (370 to 890 nM CO [15]). This may indicate that distinct populations of CO oxidizers dominate roots. However, half-saturation constants are not fixed parameters for cultures (7), and differences among roots and other systems may simply reflect adaptation to different concentration regimes by similar populations.
The Ea for root CO consumption at temperatures from 2 to 29°C was 59 kJ mol−1 or about 60% of the value obtained for root-associated methane oxidation (97 kJ mol−1 [25]). The lower Ea suggests that CO consumption is less sensitive to temperature; alternatively, simultaneous CO production may reduce the influence of temperature. In support of the latter hypothesis, net CO production was observed at ≥44°C; this production may have been due to abiological processes similar to those that occur in soil (14). Humic substances, such as those associated with plant roots, contribute to CO formation in soils and may contribute to CO formation in wetlands, although the specific mechanisms involved remain unknown (14).
Preliminary estimates of pore water CO concentrations, which range from about 10 to 100 nM CO (27), are at the lower limit of root Kapp values (Table 1). This suggests that CO concentrations could limit the rate of CO oxidation in situ. In contrast, pore water CH4 is less of a limiting factor for root-associated methane oxidation since the Kapp for this process (3 to 6 μM CH4 [25]) is considerably less than the in situ concentrations (>100 μM CH4 [10]). For methane oxidation, oxygen (i.e., root aeration) is a more important control (10). Oxygen may also limit CO consumption but may be less important than other factors, such as the availability of alternative carbon sources.
The dramatic decrease in CO consumption rates in roots incubated with exogenous organic substrates (Fig. 3) may be attributed to the facultative nature of at least some CO-oxidizing bacteria. In general, carboxydotrophic bacteria express CO dehydrogenase only when CO is the primary growth substrate; heterotrophically grown cells either do not oxidize CO or exhibit a reduced rate of oxidation (15, 24, 30). In the root assays, exogenous organic substrates may have allowed opportunistic heterotrophs to outgrow CO oxidizers or may have decreased the expression of CO dehydrogenase. Abiological CO production from exogenous substrates probably does not explain the results since controls revealed no significant production from the compounds assayed (27).
Streptomycin was the most effective inhibitor of CO consumption, decreasing the rate of uptake by 90%. In contrast, the antifungal agents nystatin and cycloheximide had no effect (Fig. 4). Although the efficacies of the various antibiotics are uncertain, the results suggest that bacteria may be responsible for most of the root-associated CO oxidation and that neither root cells nor fungi are important. In contrast, Conrad and Seiler (12) showed that fungi consumed the majority of CO in certain soils, while bacteria dominated in other soils. Since only one root type was examined in this study, it is not known whether the observed response to antibiotics is typical.
The results of the assay performed with acetylene (Fig. 5) indicate that methanotrophs probably contribute little to root CO consumption despite the fact that they are active on most aquatic plant roots (10, 25, 26) and are capable of consuming CO (5). Acetylene effectively and irreversibly inhibits methane monooxygenase, the enzyme used by methanotrophs to oxidize CO. Thus, the initial inhibition of root CO consumption by acetylene is consistent with methanotrophic activity, but the subsequent reversal of inhibition in the continued presence of acetylene is not. Based on analogous reasoning, a significant role for ammonia oxidizers is also unlikely. Similar results were obtained in experiments in which CO consumption by soils was examined (27); in these experiments reversible inhibition by acetylene and the absence of any effect of methyl fluoride also suggested that methanotrophs and ammonia oxidizers played a negligible role. The results obtained with both roots and soils indicate that acetylene might be a short-term (albeit nonspecific) inhibitor of CO consumption. However, neither the mechanism of inhibition nor the reason for the reversal observed is clear.
In conclusion, preliminary kinetic and pore water data suggest that root-associated CO oxidizers have the potential to consume significant amounts of CO produced in sediments but may be limited in situ by the presence of organic substrates and the absence of oxygen. Assuming that Vmaxp is a surrogate for population size and Kapp is a marker for different microbial populations, the number of root-associated CO-oxidizing bacteria appears to vary among plant species, while specific populations may vary as a function of root type. Thus, multiple populations of CO oxidizers may be present on roots of individual plants. Differences in densities and types of root-associated CO oxidizers may contribute to differences among plant taxa in the extent to which CO is transported from peats or sediments through stems and to the atmosphere. Understanding the characteristics and control of root-associated CO consumption should not only help explain wetland CO emissions but also contribute to a greater understanding of the interactions among microbial populations, plant species, and root types.
ACKNOWLEDGMENTS
This work was supported in part by grant DEB-9528552 from the National Science Foundation.
We thank K. Hardy for technical assistance.
Footnotes
Contribution 321 from the Darling Marine Center.
REFERENCES
- 1.Armstrong W. Root aeration in the wetland condition. In: Hook D D, Crawford R M M, editors. Plant life in anaerobic environments. Ann Arbor, Mich: Ann Arbor Science; 1978. pp. 269–297. [Google Scholar]
- 2.Bartholomew G W, Alexander M. Microbial metabolism of carbon monoxide in culture and in soil. Appl Environ Microbiol. 1979;37:932–937. doi: 10.1128/aem.37.5.932-937.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bartholomew G W, Alexander M. Soil as a sink for atmospheric carbon monoxide. Science. 1981;212:1389–1391. doi: 10.1126/science.212.4501.1389. [DOI] [PubMed] [Google Scholar]
- 4.Bartholomew G W, Alexander M. Microorganisms responsible for the oxidation of carbon monoxide in soil. Environ Sci Technol. 1982;16:300–301. doi: 10.1021/es00099a013. [DOI] [PubMed] [Google Scholar]
- 5.Bedard C, Knowles R. Physiology, biochemistry, and specific inhibitors of CH4, NH4+, and CO oxidation by methanotrophs and nitrifiers. Microbiol Rev. 1989;53:68–84. doi: 10.1128/mr.53.1.68-84.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bender M, Conrad R. Microbial oxidation of methane, ammonium, and carbon monoxide, and turnover of nitrous oxide and nitric oxide in soils. Biogeochemistry. 1994;27:97–112. [Google Scholar]
- 7.Benstead J, King G M, Williams H G. Methanol promotes atmospheric methane oxidation by methanotrophic cultures and soils. Appl Environ Microbiol. 1998;64:1091–1098. doi: 10.1128/aem.64.3.1091-1098.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bosse U, Frenzel P. Activity and distribution of methane-oxidizing bacteria in flooded-rice soil microcosms and in rice plants (Oryza sativa) Appl Environ Microbiol. 1997;63:1199–1207. doi: 10.1128/aem.63.4.1199-1207.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bourbonniere R A, Miller W L, Zepp R G. Distribution, flux, and photochemical production of carbon monoxide in a boreal beaver impoundment. J Geophys Res. 1997;102(D):29321–29329. [Google Scholar]
- 10.Calhoun A, King G M. Regulation of root-associated methanotrophy by oxygen availability in the rhizosphere of two aquatic macrophytes. Appl Environ Microbiol. 1997;63:3051–3058. doi: 10.1128/aem.63.8.3051-3058.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10a.Castro M S, Peterjohn W T, Mellilo J M, Steudler P A, Gholz H L, Lewis D. Effects of nitrogen fertilization on the fluxes of N2O, CH4 and CO2 from soils in a Florida slash pine plantation. Can J For Res. 1994;24:9–13. [Google Scholar]
- 11.Conrad R. Biogeochemistry and ecophysiology of atmospheric CO and H2. Adv Microb Ecol. 1988;10:231–283. [Google Scholar]
- 12.Conrad R, Seiler W. Role of microorganisms in the consumption and production of atmospheric carbon monoxide by soil. Appl Environ Microbiol. 1980;40:437–445. doi: 10.1128/aem.40.3.437-445.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Conrad R, Seiler W. Utilization of traces of carbon monoxide by aerobic oligotrophic microorganisms in ocean, lake and soil. Arch Microbiol. 1982;132:41–46. [Google Scholar]
- 14.Conrad R, Seiler W. Characteristics of abiological carbon monoxide formation from soil organic matter, humic acids, and phenolic compounds. Environ Sci Technol. 1985;19:1165–1169. doi: 10.1021/es00142a004. [DOI] [PubMed] [Google Scholar]
- 15.Conrad R, Meyer O, Seiler W. Role of carboxydobacteria in the consumption of atmospheric carbon monoxide by soil. Appl Environ Microbiol. 1981;42:211–215. doi: 10.1128/aem.42.2.211-215.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Conrad R, Aragno M, Seiler W. Production and consumption of carbon monoxide in a eutrophic lake. Limnol Oceanogr. 1983;28:42–49. doi: 10.1128/aem.45.2.502-510.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Conrad R, Schütz H, Seiler W. Emission of carbon monoxide from submerged rice fields into the atmosphere. Atmos Environ. 1988;22:821–823. [Google Scholar]
- 18.Duggin J A, Cataldo D A. The rapid oxidation of atmospheric CO to CO2 by soils. Soil Biol Biochem. 1985;17:469–474. [Google Scholar]
- 19.Epp M A, Chanton J P. Rhizospheric methane oxidation determined via the methyl fluoride inhibition technique. J Geophys Res. 1993;98(D):18413–18422. [Google Scholar]
- 20.Funk D W, Pullman E R, Peterson K M, Crill P M, Billings W D. Influence of water table on carbon dioxide, carbon monoxide, and methane fluxes from taiga bog microcosms. Global Biogeochem Cycles. 1994;8:271–278. [Google Scholar]
- 21.Hino S, Tauchi H. Production of carbon monoxide from aromatic amino acids by Morganella morganii. Arch Microbiol. 1987;148:167–171. [Google Scholar]
- 22.Jones R D, Morita R Y. Carbon monoxide oxidation by chemolithotrophic ammonium oxidizers. Can J Microbiol. 1983;29:1545–1551. [Google Scholar]
- 23.Khalil M A K, Rasmussen R A. Emissions of trace gases from Chinese rice fields and biogas generators: CH4, N2O, CO, CO2, chlorocarbons, and hydrocarbons. Chemosphere. 1990;20:207–226. [Google Scholar]
- 24.Kiessling M, Meyer O. Profitable oxidation of carbon monoxide or hydrogen during heterotrophic growth of Pseudomonas carboxydoflava. FEMS Microbiol Lett. 1982;13:333–338. [Google Scholar]
- 25.King G M. Associations of methanotrophs with the roots and rhizomes of aquatic vegetation. Appl Environ Microbiol. 1994;60:3220–3227. doi: 10.1128/aem.60.9.3220-3227.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.King G M. In situ analyses of methane oxidation associated with the roots and rhizomes of a bur reed, Sparganium eurycarpum, in a Maine wetland. Appl Environ Microbiol. 1996;62:4548–4555. doi: 10.1128/aem.62.12.4548-4555.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.King, G. M. 1998. Unpublished results.
- 28.Loewus M W, Delwiche C C. Carbon monoxide production by algae. Plant Physiol. 1963;38:371–374. doi: 10.1104/pp.38.4.371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Logan J A, Prather M J, Wofsy S C, McElroy M B. Tropospheric chemistry: a global perspective. J Geophys Res. 1981;86(C):7210–7254. [Google Scholar]
- 30.Meyer O, Schlegel H G. Reisolation of the carbon monoxide utilizing hydrogen bacterium Pseudomonas carboxydovorans (Kistner), comb. nov. Arch Microbiol. 1978;118:35–43. doi: 10.1007/BF00406071. [DOI] [PubMed] [Google Scholar]
- 31.Meyer O, Frunzke K, Mörsdorf G. Biochemistry of the aerobic utilization of carbon monoxide. In: Murrell J C, Kelly D P, editors. Microbial growth on C1 compounds. Andover, United Kingdom: Intercept Limited; 1993. pp. 433–459. [Google Scholar]
- 32.Mörsdorf G, Frunzke K, Gadkari D, Meyer O. Microbial growth on carbon monoxide. Biodegradation. 1992;3:61–82. [Google Scholar]
- 33.Novelli P C, Masarie K A, Tans P P, Lang P M. Recent changes in atmospheric carbon monoxide. Science. 1994;263:1587–1590. doi: 10.1126/science.263.5153.1587. [DOI] [PubMed] [Google Scholar]
- 34.Prather M, Derwent R, Ehhalt D, Fraser P, Sanhueza E, Zhou X. Other trace gases and atmospheric chemistry. In: Houghton J T, et al., editors. Climate change 1994. Radiative forcing of climate change, Intergovernmental Panel on Climate Change. Cambridge, United Kingdom: University Press; 1995. pp. 73–126. [Google Scholar]
- 35.Sand-Jensen K, Prahl C, Stokholm H. Oxygen release from roots of submerged aquatic macrophytes. Oikos. 1982;38:349–354. [Google Scholar]
- 36.Schmidt U. The solubility of carbon monoxide and hydrogen in water and sea-water at partial pressures of about 10−5 atmospheres. Tellus. 1979;31:68–74. [Google Scholar]
- 37.Schübel U, Kraut M, Mörsdorf G, Meyer O. Molecular characterization of the gene cluster coxMSL encoding the molybdenum-containing carbon monoxide dehydrogenase of Oligotropha carboxidovorans. J Bacteriol. 1995;177:2197–2203. doi: 10.1128/jb.177.8.2197-2203.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Sebacher D L, Harris R C, Bartlett K B. Methane emissions to the atmosphere through aquatic plants. J Environ Qual. 1985;14:40–46. [Google Scholar]
- 39.Seiler W. The influence of the biosphere on the atmospheric CO and H2 cycles. In: Krumbein W E, editor. Environmental biogeochemistry and geomicrobiology. Ann Arbor, Mich: Ann Arbor Science; 1978. pp. 773–810. [Google Scholar]
- 40.Smits A J M, Laan P, Thier R H. Root aerenchyma, oxygen leakage patterns and alcoholic fermentation ability of the roots of some nymphaeid and isoetid macrophytes in relation to the sediment type of their habitat. Aquat Bot. 1990;38:3–17. [Google Scholar]
- 41.Spratt H G, Hubbard J S. Carbon monoxide metabolism in roadside soils. Appl Environ Microbiol. 1981;41:1192–1201. doi: 10.1128/aem.41.5.1192-1201.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Tarr M A, Miller W L, Zepp R G. Direct carbon monoxide production from plant matter. J Geophys Res. 1995;100(D):11403–11413. [Google Scholar]
- 43.Valentine R L, Zepp R G. Formation of carbon monoxide from the photodegradation of terrestrial dissolved organic carbon in natural waters. Environ Sci Technol. 1993;27:409–412. [Google Scholar]
- 44.Wolff D G, Bidlack W R. The formation of carbon monoxide during the peroxidation of microsomal lipids. Biochem Biophys Res Commun. 1976;73:850–857. doi: 10.1016/0006-291x(76)90199-6. [DOI] [PubMed] [Google Scholar]
- 45.Zavarzin G A, Nozhevnikova A N. Aerobic carboxydobacteria. Microbial Ecol. 1977;3:305–326. doi: 10.1007/BF02010738. [DOI] [PubMed] [Google Scholar]
- 46.Zuo Y, Jones R D. Photochemistry of natural dissolved organic matter in lakes and wetland waters—production of carbon monoxide. Water Res. 1997;31:850–858. [Google Scholar]