Several reports have described development of myopericarditis among individuals receiving the COVID-19 vaccines,1 , 2 but none have systematically assessed the postdischarge course in these patients. We performed cardiac magnetic resonance (CMR) with tissue characterization, including late gadolinium enhancement, in 11 patients presenting with chest pain and troponin elevation within 14 days after receiving a dose of COVID-19 vaccine, in whom there were no clinical suspicions of acute coronary syndrome (Table 1 ). Median age was 19 years (range: 16-53 years), and 10 were men. All patients had a preserved left ventricular systolic function and CMR findings suggestive of myocarditis and/or pericarditis (Figures 1 and 2 ). The length of hospital stay ranged from 1 to 8 days. There were no severe acute complications. At follow-up ranging from 1 to 4 months, 9 patients were asymptomatic. Two reported varying degrees of chest discomfort. CMR findings had generally improved, though not resolved completely (Figures 1 and 2).
Table 1.
Clinical and Imaging Characteristics at Admission and Follow-Up in 11 Patients With Post–COVID-19 Vaccine Associated Myopericarditis
| Case | Age, Race, and Sex | Vaccine | Time From Vaccine to Presentation | Symptoms at Admission | CMR Findings at Admission | LVEF and RVEF at Admissiona | Symptoms at Follow-Up | CMR Findings at Follow-Up | LVEF and RVEF at Follow-Up |
|---|---|---|---|---|---|---|---|---|---|
| 1 | 21-year-old White man | BNT162b2 | 24 h | Pericarditic substernal chest pain | Edema. Focal epicardial enhancement. Thickening and enhancement of the pericardium. | 53% and 44% | 4 mo: asymptomatic | Not available | Not available |
| 2 | 16-year-old Black man | BNT162b2 | 48 h | Fever, fatigue, headache, dyspnea, pericarditic substernal chest pain | Diffuse, patchy edema. Focal subepicardial to near-transmural enhancement. Hyperintense pericardial signal. | 57% and 47% | 3 mo: intermittent dyspnea and chest discomfort with exertion | 2 mo: no edema; continued enhancement, but with improved intensity and thickness; unchanged pericardial signal | 54% and 49% |
| 3 | 17-year-old White man | BNT162b2 | 72 h | Fever, headache, myalgias, pericarditic substernal chest pain | No edema. Focal epicardial delayed enhancement. Thickening and enhancement of the pericardium. | 58% and 50% | 4 mo: asymptomatic | 2 mo: no edema; continued enhanced, but improved thickness; unchanged pericardial findings | 62% and 51% |
| 4 | 28-year-old Black man | mRNA-1273 | 24 h | Fever, chills, nausea, lethargy, palpitations, chest pain, back pain | Patchy edema. Focal epicardial delayed enhancement. No pericardial abnormalities. | 55% and 48% | 1 mo: asymptomatic | Not available | Not available |
| 5 | 18-year-old White man | BNT162b2 | 24 h | Fever, headache, myalgias, chest pain radiating to left shoulder | Localized edema. Focal, linear delayed epicardial enhancement. Hyperintense pericardial signal and thickened pericardium. | 56% and 49% | 4 mo: asymptomatic | 4 mo: no edema; improved enhancement; mildly thickened pericardium | 56% and 50% |
| 6 | 38-year-old White woman | BNT162b2 | 24 h | Fever, myalgias, dyspnea, pericarditic substernal chest pain | No edema. Focal delayed mid-myocardial enhancement. No pericardial changes, but trace pericardial effusion. | 62% and 62% | 2 mo: asymptomatic | 3 mo: no edema; near-complete resolution of enhancement; resolution of pericardial effusion | 59% and 58% |
| 7 | 23-year-old Black man | BNT162b2 | 12 d | Epigastric pain, nausea, chest pain | No edema. Linear mid-myocardial delayed enhancement. No pericardial changes. | 62% and 53% | 2 mo: asymptomatic | Not available | Not available |
| 8 | 53-year-old White man | Ad26.COV2.S | 8 d | Weakness, myalgias, chest pain | No edema. Focal subepicardial enhancement. Diffuse pericardial enhancement and mild thickening. Small pericardial effusion. | 70% and 51% | 2 mo: asymptomatic | Not available | Not available |
| 9 | 19-year-old White man | BNT162b2 | 36 h | Fever, headache, pericarditic substernal chest pain | No edema. Negative enhancement. Bright and prominent pericardium with trivial pericardial effusion. | 60% and 53% | 2 mo: intermittent palpitations and sharp chest pain | 3 mo: focal subepicardial enhancement; unchanged pericardial findings | 56% and 49% |
| 10 | 17-year-old White man | BNT162b2 | 36 h | Fever, chills, fatigue, myalgias, pericarditic substernal chest pain | No edema. Focal mid-myocardial and epicardial delayed enhancement. No pericardial changes. | 61% and 59% | 4 mo: asymptomatic | 4 mo: no edema; mild improvement in enhancement; hyperintense pericardial signal and thickened pericardium; trivial pericardial effusion | 57% and 53% |
| 11 | 19-year-old White man | BNT162b2 | 72 h | Palpitations, pericarditic substernal chest pain | No edema. Focal mid-myocardial and near-transmural delayed enhancement. No pericardial changes. Hyperintense pericardial signal and thickened pericardium. | 59% and 54% | 4 mo: asymptomatic | 4 mo: no edema; mild focal improvement in enhancement; hyperintense pericardial signal and thickened pericardium; trivial pericardial effusion | 56% and 58% |
CMR = cardiac magnetic resonance; LVEF = left ventricular ejection fraction; RVEF = right ventricular ejection fraction.
LVEF and RVEF were measured via CMR.
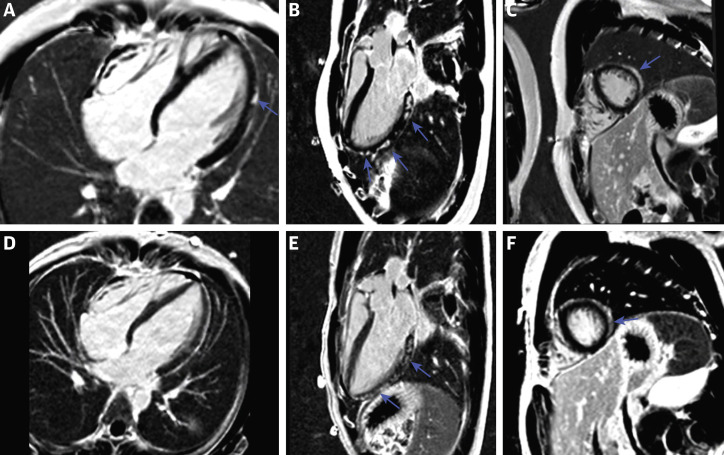
Figure 1.
Phase-Sensitive Inversion Recovery Images in a 19-Year-Old Man With Post–COVID-19 Vaccine Associated Myopericarditis
(A to C) 4-chamber, 3-chamber, and short-axis images at the time of diagnosis demonstrating remarkable subepicardial and midmyocardial late gadolinium enhancement (LGE) along the left ventricular lateral wall (arrows). (D to F) Follow-up images 3 months after diagnosis. There is mild improvement in the 4-chamber image (D) with minimal to no improvement noted in the 3-chamber and short-axis images (E and F, arrows).
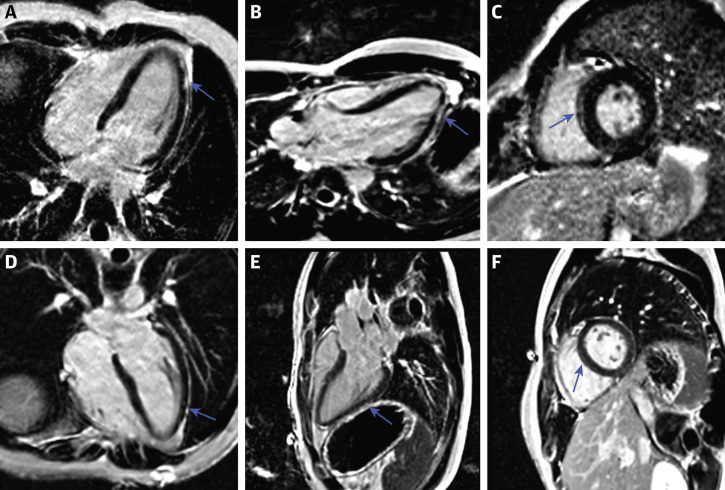
Figure 2.
Phase-Sensitive Inversion Recovery Images in an 18-Year-Old Man With Post–COVID-19 Vaccine Associated Myopericarditis
(A to C) 4-chamber, 3-chamber, and short-axis images at the time of diagnosis demonstrating subepicardial late gadolinium enhancement along the left ventricular lateral wall and mid-septal wall (arrows). (D to F) Follow-up images 6 months after diagnosis. There is notable improvement in the quantity and intensity of the late gadolinium enhancement (arrows).
Conclusions
The incidence of myocarditis or pericarditis after COVID-19 vaccination has been higher than expected when compared with background rates.1 , 2 Findings on CMR appear to mimic those seen in idiopathic myopericarditis. The course seems benign, but longer-term data are needed to fully clarify the reversibility of the myocardial changes.
Funding Support and Author Disclosures
Dr Pareek is on advisory boards for AstraZeneca and Janssen-Cilag; and has received speaker honoraria from AstraZeneca, Bayer, Boehringer Ingelheim, and Janssen-Cilag. Dr Khera has received support from the National Heart, Lung, and Blood Institute of the National Institutes of Health (grant K23HL153775) outside of the submitted work. Dr Miller is a consultant for Eidos, Pfizer, Siemens, Alnylam, and Roivant; and has received grant support from Eidos, Pfizer, and Argospect. All other authors have reported that they have no relationships relevant to the contents of this paper to disclose.
Footnotes
The authors attest they are in compliance with human studies committees and animal welfare regulations of the authors’ institutions and Food and Drug Administration guidelines, including patient consent where appropriate. For more information, visit the Author Center.
References
- 1.Wise J. Covid-19: Should we be worried about reports of myocarditis and pericarditis after mRNA vaccines? BMJ. 2021;373:n1635. doi: 10.1136/bmj.n1635. [DOI] [PubMed] [Google Scholar]
- 2.Montgomery J., Ryan M., Engler, et al. Myocarditis following immunization with mRNA COVID-19 vaccines in members of the US military. JAMA Cardiol. 2021 doi: 10.1001/jamacardio.2021.2833. [DOI] [PMC free article] [PubMed] [Google Scholar]