Abstract
Purpose
To characterize the association between the frequency of screening for diabetic retinopathy (DR) and the detection of DR in patients with newly diagnosed type 2 diabetes mellitus (T2DM).
Methods
This nationwide population-based cohort study used data from the National Health Insurance Research Database to identify adult patients who were newly diagnosed with T2DM between 2000 and 2004. Data from their follow-up Diabetic retinopathy (DR) treatments over the next 10 years following diagnosis were also analyzed.
Results
The 41,522 subjects were respectively assigned to a periodic screening group (n = 3850) and nonperiodic screening group (n = 37,672). Significant differences were observed between the two groups in terms of age, Charlson Comorbidity Index (CCI), sex, DR treatment, and the prevalence of DR. The association between periodic screening and DR treatment, only the elderly, female, and patient with severe CCI status showed the significance in the further stratified analysis.
Conclusion
Periodic screening (annual or biannual screening in the first 5 years) was more effective than nonperiodic screening in detecting instances of DR in the middle-to-advanced aged group but not among younger patients. Screening pattern did not have a significant effect on the likelihood of DR-related treatment during the 5-year follow-up. It appears that a tight screening schedule for the first 5 years after diagnosis with diabetes is not necessary.
Keywords: newly diagnosed diabetes, type 2 diabetes mellitus, diabetic retinopathy, screen, ophthalmology
Introduction
Diabetes mellitus (DM) is a growing public health problem. The International Diabetes Federation Diabetes Atlas reported that 463 million adults (9.3%) were living with diabetes in 2019, with estimates of 578 million (10.2%) by 2030 and 700 million (10.9%) by 2045 (1). The global economic burden of diabetes in 2015 was US$1.31 trillion or 1.8% of global gross domestic product (GDP), which was higher in middle-income countries (2). Diabetic retinopathy (DR) is the leading cause of visual impairment among working-age adults; however, increasing awareness and the early identification of DM has ameliorated the problem somewhat (3).
Most standard protocols for the screening of DR recommend annual or biannual dilated retinal examinations for all patients with diabetes (4). Nonetheless, several recent studies on screening and modeling have reported that increasing the screening interval may be more cost-effective for certain groups of patients (5–10).
This retrospective study used data from the Taiwan National Health Insurance Research Database (NHIRD) to assemble five 10-year cohorts by which to analyze the relationship between DR screening frequency and the corresponding incidence of treatment for DR.
Methodology
Data Sources
The National Health Insurance Research Database (NHIRD) contains anonymized linked data (e.g., demographic data and medical utilization records), which are available for clinical and epidemiologic research. The coding of diagnostic data is based on the International Classification of Diseases 9th revision Clinical Modification (ICD-9-CM). Note that this retrospective cohort study focused on the “NHIRD-2005 Million Beneficiary” database as the main source of data.
Research Enrollees
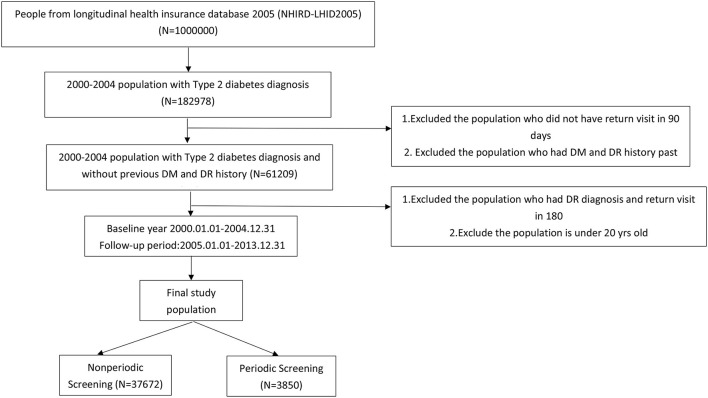
Enrollees included patients diagnosed for the 1st time with type 2 diabetes mellitus (DM) with at least one subsequent visit within 90 days. The study period was from January 1, 2000, to December 31, 2004. Exclusion criteria included individuals with a history of DM and/or DR in the previous 2 years (ICD-9-CM code 250.xx excluded 250.x1, 250.x3) and those with a DR diagnosis and a return visit within 180 days. The status of enrollees was tracked for 5 years (January 1, 2005, to December 31, 2013; Figure 1). The final study population was then divided into groups according to screening interval, as follows: periodic screening (N = 3850) and nonperiodic screening (N = 37,672).
Figure 1.
Flowchart.
Research Variables
The independent variable in this study was whether subjects underwent periodic screenings (procedure code: 23,501, 23,502, 23,702). The dependent variable was whether subjects diagnosed with DR in the following 5 years (ICD-9-CM code 362.0x) received treatment for DR in the form of laser photocoagulation (procedure code: 60001C−60004C), intravitreal injection (procedure code: 86201C), or/and vitrectomy (procedure code: 86206B−86208B, 86208C, 86212B, 86407B, 86408A, 86408B). Control variables included gender, age, and the Charlson Comorbidity Index (CCI).
Statistical Analysis
Continuous variables were according to the mean standard deviation, and independent samples t-tests were used to examine the mean difference between the two groups. Numbers and percentages were used to describe categorical variables, and simultaneously chi-square tests were performed to assess differences in proportion between the two groups. The Kaplan–Meier method was used to plot survival curves in order to analyze the probability distribution of DR in the two groups. The log-rank test was used to determine whether there was a difference between periodic screening and nonperiodic screening groups. Cox regression analysis was used to examine variables related to DR incidence and treatment with the significant level (α) set at 0.05 (two-sided). The sample size calculations were done, and the sample size needed is 384. SAS 9.4 was used for all statistical analysis.
Results
Sample Characteristics
Table 1 lists the demographic characteristics of the enrollees. The mean age of enrollees was as follows: overall (58.0 ± 713.42), periodic screening (60.4 ± 312.07), and nonperiodic screening (57.8 ± 313.53; P < 0.001). The baseline CCI scores were as follows: overall (3.5 ± 92.1), periodic screening group (3.9 ± 72.12), and nonperiodic screening group (3.5 ± 52.10; P < 0.001). The gender distribution was as follows: overall (20,739; 49.9% males and 20,783; 50.1% females), periodic screening group (1,831; 47.6% males and 2,019; 52.4% females), and nonperiodic screening group (18,908; 50.2% males and 18,764; 49.8% females; P = 0.002). The number of patients diagnosed with DR was as follows: overall, (282; 6.8%), periodic screening group (409; 10.6%), and nonperiodic screening group (2,420; 6.4%; P < 0.001). The number of patients that received treatment for DR was as follows: overall (1,691; 4.1%), periodic screening group (186; 4.8%), and nonperiodic screening group (1,505; 4.0%; P = 0.01).
Table 1.
Baseline characteristics.
| Variables | Total (N = 41,522) | Periodic screening (N = 3850) | Nonperiodic screening (N = 37,672) | P-value |
|---|---|---|---|---|
| Mean ±SD or N (%) | Mean ±SD or N (%) | Mean ±SD or N (%) | ||
| Age | 58.0 ± 713.42 | 60.4 ± 312.07 | 57.8 ± 313.53 | <0.001 |
| CCI | 3.5 ± 92.10 | 3.9 ± 72.12 | 3.5 ± 52.10 | <0.001 |
| Sex | ||||
| Male | 20,739 (49.9) | 1,831 (47.6) | 18,908 (50.2) | 0.002 |
| Female | 20,783 (50.1) | 2,019 (52.4) | 18,764 (49.8) | |
| DR treatment* | ||||
| Yes | 1,691 (4.1) | 186 (4.8) | 1,505 (4.0) | 0.01 |
| No | 39,831 (95.9) | 3,664 (95.2) | 36,167 (96.0) | |
| DR | ||||
| Yes | 2829 (6.8) | 409 (10.6) | 2,420 (6.4) | <0.001 |
| No | 38,228 (93.2) | 3,441 (89.4) | 35,252 (93.6) |
Diabetic retinopathy (DR) treatments.
Table 2 illustrates the correlation between screening interval and DR treatment as a function of age, gender, or baseline CCI. A significant correlation between periodic screening and DR treatment was observed only in the oldest age group (>65 years; P < 0.001). Within females, periodic screening showed a higher proportion in the total amount of DR treatments (P = 0.048). Baseline CCI was classified as mild, moderate, and severe, and only acquired significance within the periodic screening group (67,4.9%) which accepted DR treatment more than two times in comparison to the other groups.
Table 2.
Stratified analysis.
| Variables | Periodic screening (N = 3850) | Nonperiodic screening (N = 37,672) | P-value | |
|---|---|---|---|---|
| N (%) | N (%) | |||
| AGE | ||||
| 20–44 years (N = 6,647) | DR treatment* | 0.44 | ||
| Yes | 15 (3.6) | 272 (4.4) | ||
| No | 405 (96.4) | 5,955 (95.6) | ||
| 46–64 years (N = 20,544) | DR treatment* | 0.40 | ||
| Yes | 107 (5.8) | 994 (5.3) | ||
| No | 1,743 (94.2) | 17,700 (94.7) | ||
| Over 65 years (N = 14,331) | DR treatment* | <0.001 | ||
| Yes | 64 (4.1) | 239 (1.9) | ||
| No | 1,516 (95.9) | 12,512 (98.1) | ||
| SEX | ||||
| Female (N = 20,783) | DR treatment* | 0.048 | ||
| Yes | 101 (5.0) | 765 (4.1) | ||
| No | 1,918 (95.0) | 17,999 (95.9) | ||
| Male (N = 20,739) | DR treatment* | 0.13 | ||
| Yes | 85 (4.6) | 740 (3.9) | ||
| No | 1,746 (95.4) | 18,168 (96.1) | ||
| Baseline CCI | ||||
| Mild (0–2; N = 14,312) | DR treatment* | 0.34 | ||
| Yes | 47 (4.7) | 714 (5.4) | ||
| No | 960 (95.3) | 12,591 (94.6) | ||
| Moderate (3–4; N = 14,950) | DR treatment* | 0.14 | ||
| Yes | 72 (4.9) | 553 (4.1) | ||
| No | 1,396 (95.1) | 12,929 (95.9) | ||
| Severe (over 5; N = 12,260) | DR treatment* | <0.001 | ||
| Yes | 67 (4.9) | 238 (2.2) | ||
| No | 1,308 (95.1) | 10,647 (97.8) | ||
Diabetic retinopathy (DR) treatments.
Tables 3, 4 lists correlations between DR treatment and variables related to the incidence of DR. As shown in Table 3, after controlling for gender, age, and CCI, the risk of developing DR clearly differed as a function of screening pattern. Among older patients, the risk of DR was higher among patients who underwent periodic screening (46–64 years: HR: 1.53, 95% CI: 1.35–1.73; over 65 years: HR: 2.24, 95% CI: 1.89–2.65). Male subjects were shown to be at a lower risk of developing DR (46–64 years: HR: 0.88, 95% CI: 0.81–0.96; over 65 years: HR: 0.78, 95% CI: 0.68–0.90). The risk of developing DR was higher in the middle-aged group (46–64 years) than in the reference group (20–44 years), regardless of gender (male: HR: 1.47, 95% CI: 1.27–1.71; female: HR: 1.60, 95% CI: 1.36–1.88).
Table 3.
The correlations between Diabetic retinopathy (DR) treatments and variables related to the incidence.
| Variables | Hazard ratio | 95% CI | P-value | ||
|---|---|---|---|---|---|
| Lower | Upper | ||||
| Age | |||||
| 20–44 years | Sex (male vs. female) | 0.96 | 0.79 | 1.16 | 0.67 |
| Periodic screening (yes vs. no) | 1.38 | 0.99 | 1.92 | 0.06 | |
| 46–64 years | Sex (male vs. female) | 0.88 | 0.81 | 0.96 | 0.004 |
| Periodic screening (yes vs. no) | 1.53 | 1.35 | 1.73 | <0.001 | |
| Over 65 years | Sex (male vs. female) | 0.78 | 0.68 | 0.90 | 0.001 |
| Periodic screening (yes vs. no) | 2.24 | 1.89 | 2.65 | <0.001 | |
| Sex | |||||
| Male | 46–64 years (ref. 20–44) | 1.47 | 1.27 | 1.71 | <0.001 |
| Over 65 years (ref. 20–44) | 0.72 | 0.59 | 0.88 | 0.001 | |
| Periodic screening (yes vs. no) | 1.68 | 1.45 | 1.94 | <0.001 | |
| Female | 46–64 years (ref. 20–44) | 1.60 | 1.36 | 1.88 | <0.001 |
| Over 65 years (ref. 20–44) | 0.93 | 0.76 | 1.13 | 0.44 | |
| Periodic screening (yes vs. no) | 1.72 | 1.52 | 1.96 | <0.001 | |
Table 4.
Cox regression of diabetic retinopathy (DR) severity (N = 3294).
| Variables | Hazard ratio | 95% CI | P-value | ||
|---|---|---|---|---|---|
| Lower | Upper | ||||
| Age | |||||
| 20–44 years | Sex (male vs. female) | 1.04 | 0.94 | 1.14 | 0.45 |
| Periodic screening (yes vs. no) | 1.04 | 0.74 | 1.46 | 0.82 | |
| 46–64 years | Sex (male vs. female) | 1.01 | 0.96 | 1.05 | 0.81 |
| Periodic screening (yes vs. no) | 0.97 | 0.85 | 1.10 | 0.61 | |
| Over 65 years | Sex (male vs. female) | 1.02 | 0.95 | 1.10 | 0.52 |
| Periodic screening (yes vs. no) | 0.93 | 0.78 | 1.10 | 0.39 | |
| Sex | |||||
| Male | 46–64 years (ref. 20–44) | 1.13 | 0.98 | 1.31 | 0.09 |
| Over 65 years (ref. 20–44) | 0.95 | 0.78 | 1.15 | 0.59 | |
| Periodic screening (yes vs. no) | 0.86 | 0.74 | 1.00 | 0.05 | |
| Female | 46–64 years (ref. 20–44) | 1.06 | 0.90 | 1.24 | 0.51 |
| Over 65 years (ref. 20–44) | 0.90 | 0.75 | 1.09 | 0.29 | |
| Periodic screening (yes vs. no) | 1.03 | 0.91 | 1.18 | 0.61 | |
Overall, the risk of DR was higher among patients in the periodic screening group than among those in the nonperiodic screening group (male: HR: 1.68, 95% CI: 1.45–1.94; female: HR: 1.72, 95% CI: 1.52–1.96).
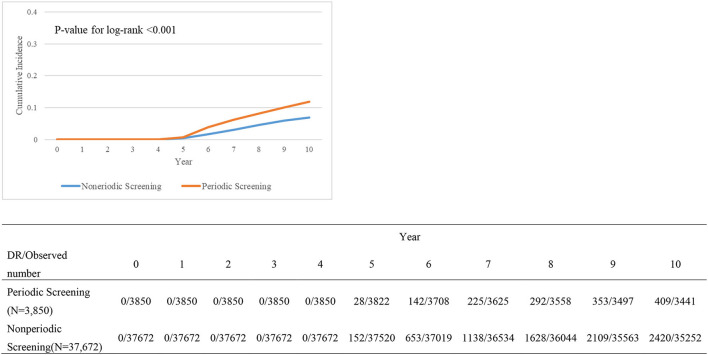
Table 4 presents the relationship between variables and DR treatments. DM patients with or without periodic screening also differed significantly. However, there was no visible significance when it refers to the included variables. Kaplan–Meier survival plot (Figure 2) shows the cumulative incidence differences between periodic screening and nonperiodic screening groups (P < 0.001).
Figure 2.
Kaplan–Meier survival plot comparing periodic screening and nonperiodic screening groups.
Discussion
Every year, roughly 1.5 million adults are newly diagnosed with diabetes. In 2018, the age-adjusted incidence was 6.7 per 1000 adults, which is close to the values reported in 2000 (11). Diabetic retinopathy (DR) is characterized by an inflammatory component especially in the early phases (12, 13). With the natural course of DR, patients suffer from nonproliferative stages to proliferative stages that led to extraretinal neovascularization, causing extensive hemorrhage and tractional retinal detachment, and subsequent visual loss. Given the tremendous number of new cases of diabetes yearly and the limited capacity of ophthalmology services, it is important to optimize the screening parameters for DR to prevent sight-threatening DR while balancing the need to control costs. This population-based study sought to characterize the effects of periodic fundus screening for DR among patients newly diagnosed with type 2 DM in terms of DR incidence and DR-related treatment over the first 5 years after diagnosis. We examined 41,522 patients from five longitudinal cohorts.
In the current study, only 9.2% of newly diagnosed diabetic patients underwent periodic fundus screening in the first 5 years after diagnosis. More than half of these patients (59%) were not subjected to ophthalmic screening, as reported in previous studies (14, 15). The overall incidence of DR in the 2nd 5-year period was 7.9%. Note that the incidence of DR in this study was close to that recorded in Chinese populations (16, 17) and other ethnic groups (18). Patients that underwent periodic screening presented a higher incidence of DR in the 2nd 5-year period. The Cox regression results in Table 3 revealed that periodic screening was the main variable contributing to the detection of DR in middle-to-advanced aged patients. Screening patterns did not have a significant effect among younger patients. Note that screening is meant to identify previously unknown cases of DR. Thus, it is reasonable to expect that periodic screening would result in a higher incidence of DR. Our data further narrow the group benefit from periodic screening to middle-to-advanced aged patients. Previous studies have identified a number of major risk factors for DR, including duration of diabetes, hyperglycemia (19), and hypertension (20). Advanced age has also been implicated in DR (18, 21, 22). In one previous hospital-based study of 127 patients newly diagnosed with diabetes, multivariate analysis revealed age and HbA1c levels as the only factors associated with DR incidence, wherein the likelihood of developing DR increased by 11% per year in the age of the patients (18).
Treatment for DR is required in 4.1% of patients newly diagnosed with diabetes (i.e., advanced DR). In the current study, the proportion of cases requiring treatment was significantly higher in the periodic screening group than in the nonperiodic group (Table 1, periodic: nonperiodic = 4.8%:4.0%, P = 0.01). Further analysis revealed that the major risk factors for treatment were advanced age (P < 0.001) and female gender (P = 0.048). We also examined the relationship between screening and the incidence of treatment in patients diagnosed with DR during the 2nd 5-year period. The Cox regression results in Table 4 revealed that none of the variables (age, gender, and pattern of screening) had a significant effect on the likelihood of receiving treatment for DR. In one previous study, the median time to DR progression was 7.6 years (23). One retrospective cohort study of 4513 patients reported that progression to advanced DR is infrequent within the first 10 years of diabetes (24). The Liverpool Diabetic Eye Study (4770 patients newly diagnosed with diabetes) reported on the annual incidence of sight-threatening DR among patients without retinopathy at baseline: first year (0.3%), fifth year (1.8%), and cumulative incidence at 5 years (3.9%) (10). Recent hospital-based and population-based studies have reported that only 0.3–5% of newly diagnosed diabetes patients required treatment for DR (25–27). The low overall incidence of treatment in our nonperiodic screening group can be attributed to volunteer bias, wherein a large proportion of the patients made no effort to undergo ophthalmic examinations during the study period. Table 5 lists the relevant studies related to DR screening.
Table 5.
Characteristics of the relevant studies.
| First author, year | Region | Study subject | Newly diagnosed diabetes | Follow up period | Age (Mean ±SD, years) | Sex N (%) | Results/Conclusion |
|---|---|---|---|---|---|---|---|
| Liu et al. (7) | Taiwan | 795 | No | January 1990–December 1992 | 59.7 ± 8.32 | Female: 441 (55.5); Male: 354 (44.5) | 1. An annual screening program, a biennial screening regime and a 4-yearly screening regime can lead to 54% (95% CI: 44–62%), 51% (95% CI: 41–59%), and 46% (95% CI: 36–54%) reductions in blindness, respectively |
| Younis et al. (10) | United Kingdom | 4,770 | Yes, but number not specified | 6 years | (median [IQR]) NDR: 63.4 (56.1–69.8); Background DR: 64.7 (57.9–71.1); Mild preproliferative DR: 65.0 (57.6–71.8) | Female: 2,116 (44.4); Male: 2,654 (55.6) | 1. Yearly incidence of sight-threatening DR in patients without DR at baseline was 0.3% (95% CI 0.1–0.5) in the first year, rising to 1.8% (1.2–2.5) in the fifth year; cumulative incidence at 5 years was 3.9% (2.8–5.0) 2. Rates of progression to sight-threatening DR in year 1 by baseline status were: background 5.0% (3.5–6.5), and mild preproliferative 15% (10.2–19.8) 3. Mean screening intervals by baseline status were: no DR 5.4 years (95% CI 4.7–6.3), background 1.0 years (0.7–1.3), and mild preproliferative 0.3 years (0.2–0.5) |
| Agarwal et al. (28) | India | 301 | Yes (n = 128) | June 2003–September 2004 | Group I (Targeted Screening): 54 ± 11; Group II (Newly Diagnosed): 52 ± 12 | Female: 148 (49.2); Male: 153 (50.8) | 1. The occurrence of DR was 6.35% (95% CI, 2.5–9.5) in Group I and 11.71% (95% CI, 5.6–16.4) in Group II. (P > 0.05), including sight-threatening retinopathy, in rural versus urban population and in Group I versus Group II 2. Group II with systolic blood pressure (BP) >140 were more likely to have retinopathy (P = 0.02) |
| Namperu malsamy et al. (22) |
India | 25,969 | Yes (n = 1478) | August 2005–March 2006 | N/A | Female: 13,525 (52.1%); Male: 12,444 (47.9%) | 1. Among the subjects screened for DM, 2802 (10.8%, 95% CI 9.3–12.2%) were found to have DM 2. DR was detected in 298 (1.2%) of included subjects. The age-gender-adjusted prevalence of DR is 0.05% (95% CI 0.04–0.06%) for rural and 1.03% (95% CI 0.89 to 1.12%) for urban areas 3. The overall age–gender-cluster adjusted prevalence of DR was 0.74% (95% CI 0.66–0.83%). DR was present in 12.2% (95% CI 10.4 to 14.1%) of the DM population |
| Agardh et al. (9) | Sweden | 1,322 | Not specified | 3 years | 55 ± 12 | N/A | 1.73% were still without retinopathy after 3 years, and 28% had developed mild or moderate retinopathy, but none developed severe nonproliferative or proliferative retinopathy |
| Lee et al. (29) | China | 3,510 | Yes (n = 3510) | 2006–2009 | 59.5 | Female: 1,811 (51.6); Male: 1,699 (48.4) | 1. The prevalence of DR was 18.2% (639 patients) among the recently diagnosed DM patients 2. In 639 patients with DR, 7% were with significant macular edema |
| Wang et al. (30) | China | 368 | Yes (n = 247) | 2006–2007 | N/A | Female: 233 (63.3); Male: 135 (36.7) | 1. The age-standardized prevalence of DR was 43.1%. In multivariable-adjusted logistic regression models for all DM participants, independent risk factors for DR were longer duration of diabetes (OR = 3.07, 95% CI 1.94–4.85), higher FPG levels (OR 1.17; 95% CI: 1.08–1.27) and higher systolic BP (OR 1.22; 95% CI: 1.08–1.37) 2. For newly diagnosed diabetes, the only significant factor of DR was higher FPG levels (OR 1.17; 95% CI 1.05–1.29, per mmol/l increase) |
| Looker et al. (27) | United Kingdom | 51,526 | Yes (n = 51,526) | Jan. 2005–May 2008 | 61.8 ± 12.8 | Female: 22,950 (45); Male: 28,576 (55) | 1. The prevalence at first screening of any retinopathy was 19.3%, and for referable retinopathy it was 1.9%. For individuals screened after a year the prevalence of any retinopathy was 20.5% and referable retinopathy was 2.3% |
| 2. Any retinopathy at screening was associated with male sex (OR 1.19, 95% CI 1.14–1.25), HbA1c (OR 1.07, 95% CI 1.06–1.08), systolic BP (OR 1.06, 95% CI 1.05, 1.08), time to screening (OR for screening >1 year post diagnosis = 1.12, 95% CI 1.07–1.17) and obesity (OR 0.87, 95% CI 0.82–0.93) | |||||||
| Hayat et al. (26) | Pakistan | 100 | Yes (n = 100) | Nov 2009–Jun 2010 | 45.1 ± 3.2 | Female: 60 (60.0); Male: 40 (40.0) | 1.17% of type 2 DM patients had retinopathy within 1 month of diagnosis 2. Background retinopathy was predominant (12%) followed by pre-proliferative (4%) and proliferative (1%) lesions |
| Jammal et al. (18) | Jordan | 127 | Yes (n = 127) | 6 months | 49.7 ± 10.0 | Female: 46 (36.2); Male: 81 (63.8) | 1.7.9% DR in the included subjects 2. Patients with DR, 40% with significant macular edema 3. The odds of DR increased by 11% for each 1 year increase in age (odds ratio [OR] 1.11; 95% confidence interval [CI] 1.02–1.20). For each 1% increase in HbAlc, the odds of DR increased by 43% (OR 1.43; 95% CI 1.09–1.88) |
| Xu et al. (17) | China | 2602 | Not specified | 10 years | 64.6 ± 9.7 | N/A | 1.109 subjects (39 men) developed new DR with an incidence of 4.2% (95% CI: 3.45–5.03) 2. In multiple logistic regression analysis, incident DR was associated with higher HbA1c value (P = 0.001; OR = 1.73 95% CI: 1.35–2.21), longer duration of DM (P = 0.001; OR: 1.16; 95% CI: 1.10,1.22), higher serum concentration of creatinine (P = 0.02; OR: 1.01; 95% CI: 1.002,1.022), lower educational level (P = 0.049; OR: 0.74; 95% CI: 0.55,0.99), higher estimated cerebrospinal fluid pressure (P = 0.038; OR: 1.10; 95% CI: 1.01,1.22), and shorter axial length (P, 0.001; OR: 0.48; 95% CI: 0.33, 0.71) |
| Ponto et al. (25) | Germany | 285 | Yes (n = 285) | N/A | N/A | Female: 114 (40.0); Male: 171 (60.0) | 1. The weighted prevalence of DR in screening-detected type 2 DM was 13.0%; 12% of participants had a mild non-proliferative DR and 0.6% had a moderate nonproliferative DR 2. DR was proliferative in 0.3%. No cases of severe non-proliferative DR or diabetic maculopathy were found |
| Tóth et al. (31) | Germany | 3,523 | Yes (n = 44) | April–July 2015 | N/A | Female: 2,250 (63.9); Male: 1,273 (36.1) | 1.20% of participants with known DM had a blood glucose level ≥200 mg/dL, and 27.4% had never had an ophthalmological examination for DR 2. Prevalence of DR and/or maculopathy was 20.7% and prevalence of sight-threatening DR was 4.3% in one or both eyes among participants with DM |
| Al-Zamil et al. (32) | Saudi Arabia | 112 | Yes (n = 112) | Jan. 2012–Jan. 2015 | 51.2 ± 5.3 (DR: 53.4 ± 6.4) | Female: 62 (55.4); Male: 50 (44.6) | 1. DR was in seven patients (6.25%) 2. Two patients (28.6%) presented with bilateral clinically significant macular edema requiring further treatments 3. At the time of type 2 DM diagnosis, uncontrolled HbA1C levels were significantly associated with the presence of retinopathy (P = 0.045) |
| Chatziralli et al. (33) | United Kingdom | 1,062 | Yes (n = 1,062) | 2 years | 56.0 ± 10.9 | Female: 477 (44.9); Male: 585 (55.1) | Risk factors that remained significantly associated with DR presence at the multivariate analysis were male sex, any cardiovascular event, HbA1c, and IL-1RA |
| Rudnisky et al. (23) | Canada | 980 | Not specified | 10 years | DR status Progressed: 54.9 ± 12.7; Stable: 53.6 ± 13.7 | Female: 829 (84.6); Male: 151 (15.4) | 1. At baseline, most patients had no DR (n = 777, 79.3%) whereas 203 people (20.7%) had either nonproliferative DR (n = 179, 18.3%) or proliferative DR (n = 24, 2.5%) 2. Two-step progression occurred in 163 patients (16.6%), with only a minority of these individuals progressing to proliferative DR (n = 23). The median time to progression was 7.6 years. Multivariate Cox regression demonstrated that elevated hemoglobin A1C (hazard ratio [HR] = 1.42; P < 0.0001) and systolic BP (HR = 1.24; P = 0.009) were independent predictors of progression of DR |
| Voigt et al. (24) | Germany | 2,272 | Not specified | 1987–2014 | 65.4 ± 12.6 | N/A | 1.25.8 % of the patients had DR (20.2 % nonproliferative, 4.7 % proliferative, 0.7 % were not classified, 0.1 % blindness) 2. The prevalence of DR in dependence on diabetes duration was 1.1 % at diagnosis, 6.6 % after 0 < 5 years, 12 % after 5 < 10 years, 24 % after 10 < 15 years, 39.9 % after 15 < 20 years, 52.7 % after 20 < 25 years, 58.7 % after 25 <30 years and 63 % after ≥ 30 years 3. In a subset of 586 (25.7 %) patients with retinal photography of 3 consecutive years 7.0 % showed deterioration after 1 and 12.2 % after 2 years; 2.6 % improved after 1 and 2.8 % after 2 years. 201 (34.3 %) of this group had < 10 years diabetes and lower deterioration (4.5 % worsened after one and 9.5 % after 2 years). Their retinopathy mainly transformed from no retinopathy to nonproliferative. Four patients (2.0 %) developed proliferative retinopathy |
| Cui et al. (34) | China | 1,500 | Yes (n = 936) | September 2011–February 2012 | 59.5 ± 11.1 | Female: 886 (59.1); Male: 614 (40.9) | 1. Standardized prevalence rate of DR was 18.2% for all patients with diabetes, 32.8% for the patients with previously diagnosed diabetes, and 12.6% for newly diagnosed patients with T2DM. The prevalence rate of male DR was significantly higher than that of female DR (23.0% vs 14.1%, P < 0.001) 2. The prevalence rates of vision-threatening DR, diabetic macular oedema, and clinically significant macular oedema were 2.5%, 2.8% and 0.9%, respectively. Male, higher education level, longer duration of DM, higher systolic BP and glycosylated hemoglobin were independent risk factors for DR development |
| Hao et al. (35) | China | 947 | Yes (n = 947) | December 2018–April 2019 | No DR: 53.3 ± 11.7; DR: 52.9 ± 11.1 | Female: 381 (40.2); Male: 566 (59.8) | 1. BMI was shown to be a related factor for DR in patients with newly diagnosed diabetes (OR = 0.592, P = 0.004). When BMI was ≥28 kg/m2, heavy smoking was associated with DR (OR = 2.219, P = 0.049) 2. There was a negative correlation between DR and the age of diagnosis of diabetes ≥60 years (OR = 0.289, P = 0.009) |
| Hwang et al. (36) | Korea | 380 | Yes (n = 380) | Jan. 2013–Jan. 2018 | No DR: 51.61 ± 12.48; DR: 50.78 ± 10.21 | Female: 159 (41.8); Male: 221 (58.2) | 1.40 (10.53%) patients had DR at the initial ophthalmologic examination |
| 2. Glycated hemoglobin, fasting plasma glucose, urine albumin to creatinine ratio, and urine microalbumin level were significantly higher in DR patients than in patients without DR 3. In the multivariate logistic regression analysis, high HbA1C was a significant risk factor for the presence of DR at new T2DM diagnosis (OR: 2.372; P < 0.001). HbA1C, FPG, UACR, and urine microalbumin levels showed significantly positive correlations with DR severity |
|||||||
| Shah et al. (37) | United Kingdom | 11,399 | Yes (n = 11,399) | 2005–2009 | Median (IQR); At baseline; No DR: 60 (51–69); DR: 61 (52–69) | Female: 5,116 (45) Male: 6,283 (55) | 1. Baseline retinopathy prevalence was 18% (n = 2048) versus 37% in UKPDS. At 7 years, 11.6% (n = 237) of those with baseline retinopathy had progression of retinopathy 2. In those without baseline retinopathy, 46.4% (n = 4337/9351) developed retinopathy by 7 years. Retinopathy development (OR: 1.05; 95%CI: 1.02–1.07) and progression (OR: 1.05 [1.04–1.06]) at 7 years was associated with higher HbA1c at diabetes diagnosis. Obesity (OR: 0.88 [0.79–0.98]) and high socioeconomic status (OR: 0.63; 0.53–0.74) were negatively associated with retinopathy development at 7 years |
The strength of this study lies in the evidence derived from a large population-based database. Note that DR treatment was selected as the main outcome measurement due to the fact that the coding for treatment in the NHIRD system is more precise than that for diagnosis. Furthermore, DR treatment reflects the actual economic burden on the healthcare system. Nonetheless, this study has several limitations. We did not examine the related parameters, such as glycemic index, insulin use, lipid levels, or blood pressure, hence the level of glucose control and the co-existence of other risk factors cannot be included in the analysis. Second, the follow-up for DR was limited to 5 years, thereby preventing analysis of long-term effects. Third, the limited availability of data imposed a number of difficulties in defining cases of severe DR and subgroup analysis. Fourth, we encountered potential overlaps in data pertaining to age-related macular degeneration and retinal vein occlusion. Fifth, the diagnosis of DM in this study may have been delayed, due to difficulties in defining the specific day of diagnosis using the NHRI database. Further analysis over a longer period will be needed to gain a more comprehensive understanding of the effects of periodic screening.
In summary, periodic screening of patients newly diagnosed with T2DM (annual to biannual screening in first 5 years) was shown to increase the detection of DR among middle-to-advanced aged patients; however, it was not correlated to the incidence of DR-related treatment. Periodic screening had no effect on detection rates among younger patients (<45 years).
We recommend a longer screening interval for younger patients newly diagnosed with diabetes. From the perspective of public health policy, this study suggests that a tight screening schedule probably is not necessary for all patients during the first 5 years after diagnosis, however the ophthalmologist should consider very careful the clinical history of each patient and anticipate the follow-up if necessary.
Data Availability Statement
The original contributions presented in the study are included in the article/supplementary materials, further inquiries can be directed to the corresponding author.
Author Contributions
Y-CC: writing manuscript. T-HT: supervise and professional statistical counseling. P-EC: data analysis and drafting manuscript. TX and MC participated and performed data synthesis of the revised stage. All authors contributed to the article and approved the submitted version.
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher's Note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
- 1.Saeedi P, Petersohn I, Salpea P, Malanda B, Karuranga S, Unwin N, et al. Global and regional diabetes prevalence estimates for 2019 and projections for 2030 and 2045: Results from the International Diabetes Federation Diabetes Atlas, 9th edition. Diabetes Res Clin Pract. (2019) 157:107843. 10.1016/j.diabres.2019.107843 [DOI] [PubMed] [Google Scholar]
- 2.Bommer C, Heesemann E, Sagalova V, Manne-Goehler J, Atun R, Bärnighausen T, et al. The global economic burden of diabetes in adults aged 20-79 years: a cost-of-illness study. Lancet Diabetes Endocrinol. (2017) 5:423–30. 10.1016/S2213-8587(17)30097-9 [DOI] [PubMed] [Google Scholar]
- 3.Wong TY, Mwamburi M, Klein R, Larsen M, Flynn H, Hernandez-Medina M, et al. Rates of progression in diabetic retinopathy during different time periods: a systematic review and meta-analysis. Diabetes Care. (2009) 32:2307–13. 10.2337/dc09-0615 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Flaxel CJ, Adelman RA, Bailey ST, Fawzi A, Lim JI, Vemulakonda GA, et al. Diabetic retinopathy preferred practice Pattern®. Ophthalmology. (2020) 127:P66–145. 10.1016/j.ophtha.2019.09.025 [DOI] [PubMed] [Google Scholar]
- 5.Bebu I, Lachin JM. Optimal screening schedules for disease progression with application to diabetic retinopathy. Biostatistics. (2018) 19:1–13. 10.1093/biostatistics/kxx009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Jones CD, Greenwood RH, Misra A, Bachmann MO. Incidence and progression of diabetic retinopathy during 17 years of a population-based screening program in England. Diabetes Care. (2012) 35:592–6. 10.2337/dc11-0943 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Liu WJ, Lee LT, Yen MF, Tung TH, Williams R, Duffy SW, et al. Assessing progression and efficacy of treatment for diabetic retinopathy following the proliferative pathway to blindness: implication for diabetic retinopathy screening in Taiwan. Diabet Med. (2003) 20:727–33. 10.1046/j.1464-5491.2003.01019.x [DOI] [PubMed] [Google Scholar]
- 8.Soto-Pedre E, Hernaez-Ortega MC, Vázquez JA. Six-year retrospective follow-up study of safe screening intervals for sight-threatening retinopathy in patients with diabetes mellitus. J Diabetes Sci Technol. (2009) 3:812–8. 10.1177/193229680900300430 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Agardh E, Tababat-Khani P. Adopting 3-year screening intervals for sight-threatening retinal vascular lesions in type 2 diabetic subjects without retinopathy. Diabetes Care. (2011) 34:1318–9. 10.2337/dc10-2308 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Younis N, Broadbent DM, Vora JP, Harding SP. Liverpool Diabetic Eye Study. Incidence of sight-threatening retinopathy in patients with type 2 diabetes in the Liverpool Diabetic Eye Study: a cohort study. Lancet. (2003) 361:195–200. 10.1016/S0140-6736(03)12267-2 [DOI] [PubMed] [Google Scholar]
- 11.Centers for Disease Control and Prevention . National Diabetes Statistics Report, 2020. Atlanta, GA: Centers for Disease Control and Prevention, U.S. Dept of Health and Human Services; (2020). [Google Scholar]
- 12.Bucolo C, Gozzo L, Longo L, Mansueto S, Vitale DC, Drago F. Long-term efficacy and safety profile of multiple injections of intravitreal dexamethasone implant to manage diabetic macular edema: a systematic review of real-world studies. J Pharmacol Sci. (2018) 138:219–32. 10.1016/j.jphs.2018.11.001 [DOI] [PubMed] [Google Scholar]
- 13.Tang J, Kern TS. Inflammation in diabetic retinopathy. Prog Retin Eye Res. (2011) 30:343–58. 10.1016/j.preteyeres.2011.05.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Gange WS, Xu BY, Lung K, Toy BC, Seabury SA. Rates of eye care and diabetic eye disease among insured patients with newly diagnosed type 2 diabetes. Ophthalmol Retina. (2021) 5:160–8. 10.1016/j.oret.2020.07.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kreft D, McGuinness MB, Doblhammer G, Finger RP. Diabetic retinopathy screening in incident diabetes mellitus type 2 in Germany between 2004 and 2013 - a prospective cohort study based on health claims data. PLoS ONE. (2018) 13:e0195426. 10.1371/journal.pone.0195426 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Song H, Liu L, Sum R, Fung M, Yap MK. Incidence of diabetic retinopathy in a Hong Kong Chinese population. Clin Exp Optom. (2011) 94:563–7. 10.1111/j.1444-0938.2011.00628.x [DOI] [PubMed] [Google Scholar]
- 17.Xu J, Xu L, Wang YX, You QS, Jonas JB, Wei WB. Ten-year cumulative incidence of diabetic retinopathy. The Beijing Eye Study 2001/2011. PLoS One. (2014) 9:e111320. 10.1371/journal.pone.0111320 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Jammal H, Khader Y, Alkhatib S, Abujbara M, Alomari M, Ajlouni K. Diabetic retinopathy in patients with newly diagnosed type 2 diabetes mellitus in Jordan: prevalence and associated factors. J Diabetes. (2013) 5:172–9. 10.1111/1753-0407.12015participated and performed data synthesis of the revised stage. [DOI] [PubMed] [Google Scholar]
- 19.Mohamed Q, Gillies MC, Wong TY. Management of diabetic retinopathy: a systematic review. JAMA. (2007) 298:902–16. 10.1001/jama.298.8.902 [DOI] [PubMed] [Google Scholar]
- 20.Klein R, Knudtson MD, Lee KE, Gangnon R, Klein BE. The Wisconsin epidemiologic study of diabetic retinopathy XXIII: the twenty-five-year incidence of macular edema in persons with type 1 diabetes. Ophthalmology. (2009) 116:497–503. 10.1016/j.ophtha.2008.10.016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Yin L, Zhang D, Ren Q, Su X, Sun Z. Prevalence and risk factors of diabetic retinopathy in diabetic patients: a community based cross-sectional study. Medicine (Baltimore). (2020) 99:e19236. 10.1097/MD.0000000000019236 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Namperumalsamy P, Kim R, Vignesh TP, Nithya N, Royes J, Gijo T, et al. Prevalence and risk factors for diabetic retinopathy: a population-based assessment from Theni District, south India. Br J Ophthalmol. (2009) 93:429–34. 10.1136/bjo.2008.147934 [DOI] [PubMed] [Google Scholar]
- 23.Rudnisky CJ, Wong BK, Virani H, Tennant MTS. Risk factors for progression of diabetic retinopathy in Alberta First Nations communities. Can J Ophthalmol. (2017) 52 Suppl 1:S19–29. 10.1016/j.jcjo.2017.09.023 [DOI] [PubMed] [Google Scholar]
- 24.Voigt M, Schmidt S, Lehmann T, Köhler B, Kloos C, Voigt UA, et al. Prevalence and progression rate of diabetic retinopathy in type 2 diabetes patients in correlation with the duration of diabetes. Exp Clin Endocrinol Diabetes. (2018) 126:570–6. 10.1055/s-0043-120570 [DOI] [PubMed] [Google Scholar]
- 25.Ponto KA, Koenig J, Peto T, Lamparter J, Raum P, Wild PS, et al. Prevalence of diabetic retinopathy in screening-detected diabetes mellitus: results from the Gutenberg Health Study (GHS). Diabetologia. (2016) 59:1913–9. 10.1007/s00125-016-4013-5 [DOI] [PubMed] [Google Scholar]
- 26.Hayat AS, Khan AH, Baloch GH, Shaikh N. Frequency and pattern of retinopathy in newly diagnosed type 2 diabetic patients at tertiary care settings in Abbottabad. J Ayub Med Coll Abbottabad. (2012) 24:87–9. Available online at: https://www.ayubmed.edu.pk/JAMC/24-2/Atif.pdf [PubMed] [Google Scholar]
- 27.Looker HC, Nyangoma SO, Cromie D, Olson JA, Leese GP, Black M, et al. Diabetic retinopathy at diagnosis of type 2 diabetes in Scotland. Diabetologia. (2012) 55:2335–42. 10.1007/s00125-012-2596-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Agarwal S, Raman R, Kumari RP, Deshmukh H, Paul PG, Gnanamoorthy P, et al. Diabetic retinopathy in type II diabetics detected by targeted screening versus newly diagnosed in general practice. Ann Acad Med Singap. (2006) 35:531–5. Available online at: https://annals.edu.sg/pdf/35VolNo8Aug2006/V35N8p531.pdf [PubMed] [Google Scholar]
- 29.Lee KM, Sum WM. Prevalence of diabetic retinopathy in patients with recently diagnosed diabetes mellitus. Clin Exp Optom. (2011) 94:371–5. 10.1111/j.1444-0938.2010.00574.x [DOI] [PubMed] [Google Scholar]
- 30.Wang FH, Liang YB, Peng XY, Wang JJ, Zhang F, Wei WB, et al. Risk factors for diabetic retinopathy in a rural Chinese population with type 2 diabetes: the Handan eye study. Acta Ophthalmol. (2011) 89:e336–43. 10.1111/j.1755-3768.2010.02062.x [DOI] [PubMed] [Google Scholar]
- 31.Tóth G, Szabó D, Sándor GL, Szalai I, Lukács R, Pék A, et al. Diabetes and diabetic retinopathy in people aged 50 years and older in Hungary. Br J Ophthalmol. (2017) 101:965–9. 10.1136/bjophthalmol-2016-309016 [DOI] [PubMed] [Google Scholar]
- 32.Al-Zamil WM. Hospital prevalence of retinopathy in patients with newly-diagnosed type 2 diabetes. Saudi J Med Med Sci. (2017) 5:26–30. 10.4103/1658-631X.194248 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Chatziralli I, Sergentanis TN, Crosby-Nwaobi R, Winkley K, Eleftheriadis H, Ismail K, et al. Model for risk-based screening of diabetic retinopathy in people with newly-diagnosed type 2 diabetes mellitus. Invest Ophthalmol Vis Sci. (2017) 58:BIO99–105. 10.1167/iovs.17-21713 [DOI] [PubMed] [Google Scholar]
- 34.Cui Y, Zhang M, Zhang L, Zhang L, Kuang J, Zhang G, et al. Prevalence and risk factors for diabetic retinopathy in a cross-sectional population-based study from rural southern China: Dongguan Eye Study. BMJ Open. (2019) 9:e023586. 10.1136/bmjopen-2018-023586 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Hao Z, Huang X, Qin Y, Li H, Tian F, Xu R, et al. Analysis of factors related to diabetic retinopathy in patients with newly diagnosed type 2 diabetes: a cross-sectional study. BMJ Open. (2020) 10:e032095. 10.1136/bmjopen-2019-032095 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Hwang H, Kim JY, Oh TK, Chae JB, Kim DY. Relationship between clinical features of diabetic retinopathy and systemic factors in patients with newly diagnosed type II diabetes mellitus. J Korean Med Sci. (2020) 35:e179. 10.3346/jkms.2020.35.e179 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Shah S, Feher M, McGovern A, Sherlock J, Whyte MB, Munro N, et al. Diabetic retinopathy in newly diagnosed Type 2 diabetes mellitus: prevalence and predictors of progression; a national primary network study. Diabetes Res Clin Pract. (2021) 175:108776. 10.1016/j.diabres.2021.108776 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The original contributions presented in the study are included in the article/supplementary materials, further inquiries can be directed to the corresponding author.