Abstract
Background
With the ongoing COVID-19 pandemic, growing evidence shows that a considerable proportion of people who have recovered from COVID-19 have long-term effects on multiple organs and systems. A few longitudinal studies have reported on the persistent health effects of COVID-19, but the follow-up was limited to 1 year after acute infection. The aim of our study was to characterise the longitudinal evolution of health outcomes in hospital survivors with different initial disease severity throughout 2 years after acute COVID-19 infection and to determine their recovery status.
Methods
We did an ambidirectional, longitudinal cohort study of individuals who had survived hospitalisation with COVID-19 and who had been discharged from Jin Yin-tan Hospital (Wuhan, China) between Jan 7 and May 29, 2020. We measured health outcomes 6 months (June 16–Sept 3, 2020), 12 months (Dec 16, 2020–Feb 7, 2021), and 2 years (Nov 16, 2021–Jan 10, 2022) after symptom onset with a 6-min walking distance (6MWD) test, laboratory tests, and a series of questionnaires on symptoms, mental health, health-related quality of life (HRQoL), return to work, and health-care use after discharge. A subset of COVID-19 survivors received pulmonary function tests and chest imaging at each visit. Age-matched, sex-matched, and comorbidities-matched participants without COVID-19 infection (controls) were introduced to determine the recovery status of COVID-19 survivors at 2 years. The primary outcomes included symptoms, modified British Medical Research Council (mMRC) dyspnoea scale, HRQoL, 6MWD, and return to work, and were assessed in all COVID-19 survivors who attended all three follow-up visits. Symptoms, mMRC dyspnoea scale, and HRQoL were also assessed in controls.
Findings
2469 patients with COVID-19 were discharged from Jin Yin-tan Hospital between Jan 7 and May 29, 2020. 1192 COVID-19 survivors completed assessments at the three follow-up visits and were included in the final analysis, 1119 (94%) of whom attended the face-to-face interview 2 years after infection. The median age at discharge was 57·0 years (48·0–65·0) and 551 (46%) were women. The median follow-up time after symptom onset was 185·0 days (IQR 175·0–197·0) for the visit at 6 months, 349·0 days (337·0–360·0) for the visit at 12 months, and 685·0 days (675·0–698·0) for the visit at 2 years. The proportion of COVID-19 survivors with at least one sequelae symptom decreased significantly from 777 (68%) of 1149 at 6 months to 650 (55%) of 1190 at 2 years (p<0·0001), with fatigue or muscle weakness always being the most frequent. The proportion of COVID-19 survivors with an mMRC score of at least 1 was 168 (14%) of 1191 at 2 years, significantly lower than the 288 (26%) of 1104 at 6 months (p<0·0001). HRQoL continued to improve in almost all domains, especially in terms of anxiety or depression: the proportion of individuals with symptoms of anxiety or depression decreased from 256 (23%) of 1105 at 6 months to 143 (12%) 1191 at 2 years (p<0·0001). The proportion of individuals with a 6MWD less than the lower limit of the normal range declined continuously in COVID-19 survivors overall and in the three subgroups of varying initial disease severity. 438 (89%) of 494 COVID-19 survivors had returned to their original work at 2 years. Survivors with long COVID symptoms at 2 years had lower HRQoL, worse exercise capacity, more mental health abnormality, and increased health-care use after discharge than survivors without long COVID symptoms. COVID-19 survivors still had more prevalent symptoms and more problems in pain or discomfort, as well as anxiety or depression, at 2 years than did controls. Additionally, a significantly higher proportion of survivors who had received higher-level respiratory support during hospitalisation had lung diffusion impairment (43 [65%] of 66 vs 24 [36%] of 66, p=0·0009), reduced residual volume (41 [62%] vs 13 [20%], p<0·0001), and total lung capacity (26 [39%] vs four [6%], p<0·0001) than did controls.
Interpretation
Regardless of initial disease severity, COVID-19 survivors had longitudinal improvements in physical and mental health, with most returning to their original work within 2 years; however, the burden of symptomatic sequelae remained fairly high. COVID-19 survivors had a remarkably lower health status than the general population at 2 years. The study findings indicate that there is an urgent need to explore the pathogenesis of long COVID and develop effective interventions to reduce the risk of long COVID.
Research in context.
Evidence before this study
We searched PubMed for follow-up studies regarding long-term consequences of COVID-19 published between Jan 1, 2020, and March 15, 2022, without applying any language restrictions. The search terms we used were (“COVID-19” OR “SARS-CoV-2” OR “Coronavirus disease 2019” OR “2019-ncov”) AND (“survivor*” OR “recover*” OR “persistent” OR “follow up” OR “discharge*” OR “long term” OR “sequelae”). To our knowledge, most follow-up studies of COVID-19 are cross-sectional surveys (∼200), and only a few longitudinal cohort studies (<10) described the dynamic recovery of health outcomes in people who had survived hospitalisation with COVID-19, of which the longest follow-up time was about 1 year after discharge; in addition, the sample sizes in these studies were generally small. Furthermore, most previous studies did not record baseline health status before COVID-19 and did not have the general population as a control group, making it difficult to establish how well COVID-19 survivors recovered. Longitudinal cohort studies with a longer follow-up time than that in the previous studies are urgently needed to fully characterise the natural history of long COVID.
Added value of this study
To the best of our knowledge, this is the longest longitudinal cohort study of individuals who had survived hospitalisation with COVID-19, including an age-matched, sex-matched, and comorbidity-matched control group of individuals who had never had COVID-19, to describe the dynamic recovery of health in the 2 years after symptom onset. The proportion of individuals with at least one sequelae symptom decreased significantly from 68% at 6 months to 55% at 2 years, with fatigue or muscle weakness being the most frequently reported symptom throughout follow-up. Long COVID symptoms at the 2-year follow-up were related to decreased health-related quality of life (HRQoL) and exercise capacity, psychological abnormality, and increased use of health care after discharge. HRQoL continued to improve in almost all domains, especially in terms of anxiety or depression, with the proportion of participants reporting symptoms of anxiety or depression dropping significantly from 23% at 6 months to 12% at 2 years. The proportion of individuals with reduced walking distance ability declined continuously to 8% at 2 years. 89% of COVID-19 survivors who had a job before COVID-19 have returned to their original work, regardless of initial disease severity. However, COVID-19 survivors still had more symptoms and lower HRQoL than controls did at 2 years.
Implications of all the available evidence
Long COVID could persistently last to 2 years after acute infection, indicating that ongoing longitudinal follow-up is urgently needed to better characterise the natural history of long COVID and to establish when COVID-19 survivors will fully recover. Future studies should further explore the pathogenesis of long COVID and develop effective intervention strategies to reduce the risk of long COVID. In addition, the increased proportion of restrictive ventilatory impairment during the late recovery period brings a concern of pulmonary interstitial abnormalities, especially for those COVID-19 survivors with acute respiratory distress syndrome. Simultaneous lung imaging and pulmonary function tests are required in this particular population.
Introduction
With the ongoing COVID-19 pandemic and the increasing number of patients recovered from the disease, growing evidence shows that a considerable proportion of those who have survived hospitalisation with COVID-19 have long-term effects on multiple organs and systems, a condition commonly termed long COVID or post-COVID-19 condition.1, 2 Due to the high heterogeneity in previous follow-up studies of COVID-19 survivors, in terms of case definitions, assessment tools, duration of follow-up, and selection of the study population, the true prevalence of the emerging condition after acute infection is largely unclear. Given the huge number of individuals who have recovered from COVID-19 up to now, the sequelae after recovery from acute COVID-19 are undoubtedly a great health concern and might cause a big medical and socioeconomic burden. Several cohort studies have highlighted that the health effects of COVID-19 could persist up to 1 year after acute infection,3, 4, 5, 6, 7, 8, 9, 10, 11, 12 most of which had no control groups of individuals who had not contracted COVID-19 and focused only on symptomatic sequelae or respiratory outcomes. Hence, long-term (ie, beyond 1 year) and overall health outcomes of COVID-19 are largely unknown. Additionally, few studies with large sample sizes have described the longitudinal evolution of health outcomes in COVID-19 survivors with differing severity;3, 6 thus, large longitudinal cohort studies are urgently needed to systematically describe the natural history of long COVID, especially in patients stratified by initial disease severity.
The primary objective of this study was to systematically and comprehensively characterise the longitudinal progression of health outcomes in COVID-19 survivors with different initial disease severity up to 2 years after acute infection, and to establish the health impact of long COVID. The secondary objective was to establish whether COVID-19 survivors had returned to a health and functional status similar to that of the general population 2 years after infection.
Methods
Study design and participants
We did an ambidirectional, longitudinal cohort study of individuals who had survived hospitalisation with COVID-19 and who had been discharged from Jin Yin-tan hospital in Wuhan, China between Jan 7 and May 29, 2020. We measured health outcomes at three timepoints: 6 months, 12 months, and 2 years after symptom onset. The inclusion and exclusion criteria, follow-up procedures, and the 6-month and 12-month health outcomes of COVID-19 survivors have been described previously (appendix pp 3–13).3, 13 To establish whether COVID-19 survivors in this cohort completely recovered at 12 months, a dataset of the health status of 3383 community-dwelling adults without previous SARS-CoV-2 infection was created, described in detail in the 12-month follow-up study (appendix pp 4–5).3 This dataset serves as a control group to establish the recovery status of COVID-19 survivors at 2 years. COVID-19 survivors who attended the three follow-up visits were matched (1:1) by age, sex, and comorbidities (including cardiovascular disease, chronic respiratory disease, chronic kidney disease, hypertension, and diabetes) to control participants. The maximum allowed age difference between COVID-19 survivors and their matched controls was 5 years. The study followed the Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) reporting guideline for cohort studies. The study was approved by the Research Ethics Commission of Jin Yin-tan Hospital (KY-2020-78.01, KY-2020-78.03, and KY-2020-78.05). Written informed consent was obtained from controls and COVID-19 survivors who attended the face-to-face interview at Jin Yin-tan hospital. Verbal informed consent was obtained from COVID-19 survivors willing to participate in the telephone survey.
Follow-up assessment of COVID-19 survivors
Eligible COVID-19 survivors were invited to participate in a face-to-face interview at the outpatient clinic of Jin Yin-tan Hospital 6 months, 12 months, and 2 years after symptom onset (appendix pp 6–8). A telephone survey was available for COVID-19 survivors at the 2-year follow-up visit as an alternative to the face-to-face interview, conducted by trained clinicians using the same questionnaires. At each visit, COVID-19 survivors underwent a detailed interview, a physical examination, a 6-min walking distance (6MWD) test, and routine laboratory tests, and completed a series of questionnaires, including a self-reported symptom questionnaire, the modified British Medical Research Council (mMRC) dyspnoea scale,14 the EQ-5D-5L questionnaire to assess health-related quality of life (HRQoL),15 the EuroQol Visual Analogue Scale (EQ-VAS; scores range from 0 to 100, with a higher score indicating a better health status),16 and an ischaemic stroke and cardiovascular event registration form.17 Additionally, at the 12-month and 2-year visits, health-care use after discharge and work status were also collected by a questionnaire.
Notably, at the 2-year visit, a series of psychiatric questionnaires in Chinese were used to evaluate mental health, including the Generalized Anxiety Disorder seven-item scale (GAD-7), the Patient Health Questionnaire 9 (PHQ-9), and the Post-Traumatic Stress Disorder (PTSD) Checklist, Civilian version (PCL-C). GAD-7 is a seven-item, self-rated scale that is used as a screening tool and severity indicator for generalised anxiety disorder in the past 2 weeks.18 Each item is scored from 0 (not at all) to 3 (nearly every day). Total scores of 5, 10, and 15 were taken as the cutoff points for mild, moderate, and severe anxiety, respectively.18 PHQ-9 was used to evaluate the severity of depressive symptoms during the previous 2 weeks through nine questions.19, 20 Each item was rated from 0 (not at all) to 3 (nearly every day). Cutoff scores of 5, 10, 15, and 20 indicate mild, moderate, moderately severe, and severe depression, respectively.20 The PCL-C is a 17-item self-report scale for examining post-traumatic stress symptoms, with each item scoring from 1 (not at all) to 5 (extremely) in three domains: intrusion, avoidance and numbing, and hyperarousal.21 PCL-C total scores of 38 or more reflected clinically relevant post-traumatic stress symptoms.22, 23
We used a stratified, disproportionate, random sampling procedure according to severity scale to select patients to receive high-resolution chest CT and pulmonary function tests at the 6-month follow-up visit.1 COVID-19 survivors with abnormal lung images at follow-up were arranged to receive another high-resolution CT scan in the next assessment. 353 COVID-19 survivors completed high-resolution chest CT at the 6-month visit,13 of whom 186 presented with abnormal CT and were further invited to receive another chest CT at the 12-month visit.3 At the 12-month visit, 65 of 118 survivors who had completed a chest CT scan presented with abnormal CT,3 and were invited to receive another high-resolution CT scan at the 2-year visit. 349 survivors had completed pulmonary function tests at the 6-month visit,13 and they were all invited to perform this test again at the 12-month and 2-year visits.
Pulmonary function tests of participants without COVID-19
To better evaluate the recovery of pulmonary function among COVID-19 survivors 2 years after acute infection, a subgroup from the non-COVID-19 cohort was invited to perform pulmonary function tests and a health check at Jin Yin-tan hospital during the 2-year follow-up visit, and they were matched by age, sex, and chronic lung disease with COVID-19 survivors who also completed pulmonary function tests at the 2-year visit.
Outcomes
The primary outcomes were sequelae symptoms, HRQoL, mental health, exercise capacity assessed by 6MWD, and return to work (appendix pp 9–12). These outcomes were assessed in all COVID-19 survivors who attended three follow-up visits; symptoms, mMRC dyspnoea scale, and HRQoL were also assessed in non-COVID-19 controls. Sequelae symptoms are defined as those that are newly occurring and persistent, or worse than the status before getting COVID-19, and that cannot be explained by an alternative disease. COVID-19 survivors with long COVID symptoms are defined as having at least one sequelae symptom, which is largely consistent with the case definition of post-COVID-19 condition.2 Prevalent symptoms are defined as the existing symptoms at follow-up (appendix p 13) The secondary outcomes were lung function, imaging, and health-care use after discharge.
Statistical analysis
Demographic characteristics and long-term health consequences of COVID-19 survivors were presented as median (IQR) for continuous variables and expressed as absolute values along with percentages for categorical variables. Participants were categorised into three groups according to their severity scale during their hospital stay (scale 3: not requiring supplemental oxygen; scale 4: requiring supplemental oxygen; scale 5–6: requiring high-flow nasal cannula, non-invasive mechanical ventilation, or invasive mechanical ventilation). Demographic and clinical characteristics and long-term consequences across participants with different severity scales are reported here. For the comparison of demographic and clinical characteristics among participants with different disease severities, we used the Kruskal-Wallis test, χ2 test, Fisher's exact test, or Mann-Whitney U test as appropriate. For the comparison of symptoms, HRQoL, exercise capacity, health-care use after discharge, work status, and lung function between different follow-up visits, we used the Wilcoxon signed-rank test or McNemar test as appropriate. For the comparison of symptoms, HRQoL, and lung function between COVID-19 survivors and their matched controls, we used the χ2 test, Fisher's exact test, or Mann-Whitney U test as appropriate. We estimated correlation coefficients between different symptoms in COVID-19 survivors at the 6-month, 12-month, and 2-year follow-up visits, and presented them as a heatmap.
To explore the association of long COVID symptoms with health outcomes and health-care use at the 2-year follow-up visit, we used multivariable adjusted logistic regression models for categorical outcomes and generalised linear regression models for continuous outcomes. We adjusted for age, sex, cigarette smoking (ie, never-smoker, current smoker, or former smoker), body-mass index, education (ie, college or higher vs high school or lower), self-reported comorbidities (ie, respiratory disease, hypertension, diabetes, coronary heart disease, cerebrovascular disease, tumour, chronic kidney disease, and neurological disease), and disease severity (based on the severity scale mentioned above).
According to the longitudinal design, we used mixed-effect regression models to explore fixed effects of risk factors associated with long COVID, fatigue or muscle weakness, anxiety or depression, and diffusion impairment. For the association of disease severity with the outcome, we adjusted for age, sex, cigarette smoking, education, comorbidity, corticosteroids, antivirals, and intravenous immunoglobulin. For the association of factors, including sex, corticosteroid, antivirals, and intravenous immunoglobulin, with the outcome, we included all the aforementioned variables in the model. When exploring associations of education with the outcome, we included the aforementioned variables except for comorbidity; for the association of smoking with the outcome, we included the aforementioned variables except for comorbidity and disease severity (due to the potential mediation). Only sex, smoking, and education were included for the association between age and the outcome due to the potential mediation of other factors. For the association of comorbidity with the outcome, we included the aforementioned variables except for disease severity.
All significance tests were two-sided, and a p value less than 0·05 was considered significant unless stated otherwise. To correct for multiple comparison of demographic and clinical characteristics between the two groups of study participants with different severity scales, we used a Bonferroni-corrected α threshold of 0·0167. To correct for multiple comparison of symptoms, HRQoL, exercise capacity, health-care use after discharge, and work status at the 6-month, 12-month, and 2-year follow-up visits, we used a Bonferroni-corrected α threshold of 4·17 × 10−3. A stringent Bonferroni correction was also used for comparing the lung function of COVID-19 survivors between different follow-up visits (with the α threshold set as 5·56 × 10−3) and between COVID-19 survivors and their matched controls (with the α threshold set as 0·0167) to determine statistical significance. The missing data were not imputed. All statistical analyses were done with SAS, version 9.4. The correlation plot and proportional Venn diagram was generated in R, version 4.1.2.
Role of the funding source
The funder of the study had no role in study design, data collection, data analysis, data interpretation, or writing of the report.
Results
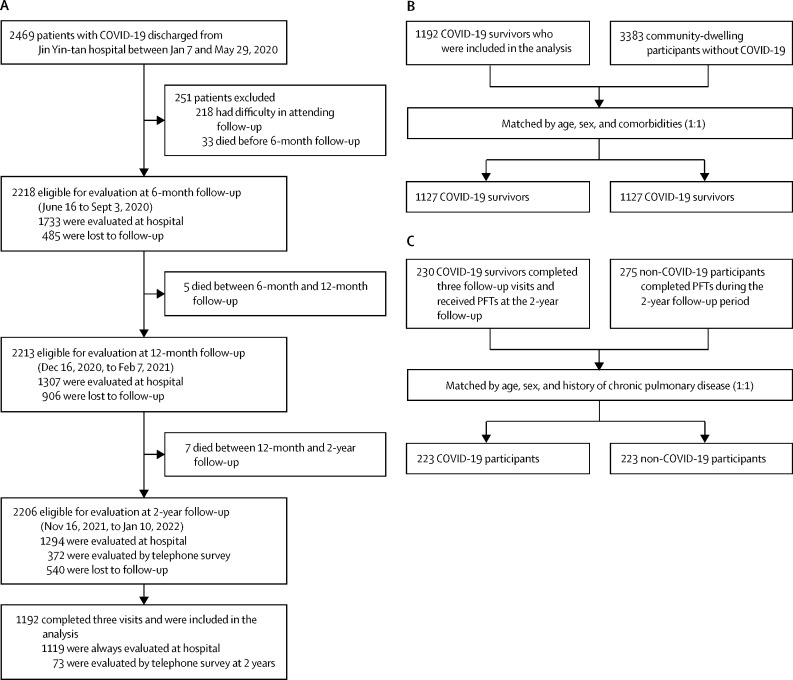
2469 patients with COVID-19 were discharged from Jin Yin-tan Hospital between Jan 7 and May 29, 2020, and 2218 were eligible for evaluation at the 6-month follow-up between June 16 and Sept 3, 2020. 1192 COVID-19 survivors completed assessments at the three follow-up visits and were included in the final analysis, 1119 (94%) of whom attended the face-to-face interview 2 years after infection, between Nov 16, 2021, and Jan 10, 2022 (figure 1A ; appendix p 25). COVID-19 survivors who completed pulmonary function tests and high-resolution chest CT at each visit are shown in the appendix (p 26–27). The baseline characteristics of COVID-19 survivors included in the final analysis were similar to those not included, except that the proportions of men, smokers, those receiving oxygen, and those receiving corticosteroid treatment during hospitalisation were slightly higher in those who were included in the final analysis than in those who were not (appendix p 16).
Figure 1.
Flow chart of the study
(A) Flow diagram of COVID-19 participants. (B) Matching process of COVID-19 survivors who attended all three visits and community-dwelling participants without COVID-19 (1:1). (C) Matching process of COVID-19 survivors and non-COVID-19 participants who completed PFTs at the 2-year follow-up visit (1:1). PFT=pulmonary function tests.
The demographic and clinical characteristics of the 1192 COVID-19 participants who attended three visits are shown in table 1 , grouped by initial disease severity. The median follow-up time after symptom onset was 185·0 days (IQR 175·0–197·0) for the visit at 6 months, 349·0 days (337·0–360·0) for the visit at 12 months, and 685·0 days (675·0–698·0) for the visit at 2 years. The median age at baseline was 57·0 years (48·0–65·0) and 551 (46%) were women. 806 (68%) participants received oxygen via nasal cannulas or mask during hospitalisation (scale 4), and 91 (8%) received higher-level respiratory support (scale 5–6). 51 (4%) participants had been admitted to an intensive care unit, with a median length of stay of 18·0 days (6·0–30·0).
Table 1.
Baseline characteristics of COVID-19 survivors who completed the 6-month, 12-month, and 2-year follow-up visits
| Total (n=1192) | Scale 3 (n=295) | Scale 4 (n=806) | Scale 5–6 (n=91) | p value | |||
|---|---|---|---|---|---|---|---|
| Age at discharge, years | 57·0 (48·0–65·0) | 57·0 (47·0–65·0) | 57·0 (48·0–65·0) | 56·0 (48·0–65·0) | 0·72 | ||
| Sex | .. | .. | .. | .. | 0·0091 | ||
| Men | 641 (54%) | 147 (50%) | 432 (54%) | 62 (68%)*† | .. | ||
| Women | 551 (46%) | 148 (50%) | 374 (46%) | 29 (32%)*† | .. | ||
| Education | .. | .. | .. | .. | 0·011 | ||
| College or higher | 326/1185 (28%) | 82 (28%) | 207/799 (26%) | 37 (41%)† | .. | ||
| High school or lower | 859/1185 (72%) | 213 (72%) | 592/799 (74%) | 54 (59%)† | .. | ||
| Work status before COVID-19 | .. | .. | .. | .. | 0·08 | ||
| Retired | 647/1187 (55%) | 161/294 (55%) | 446/803 (56%) | 40/90 (44%) | .. | ||
| Full-time or part-time job | 494/1187 (42%) | 124/294 (42%) | 321/803 (40%) | 49/90 (54%) | .. | ||
| Jobless | 42/1187 (4%) | 7/294 (2%) | 34/803 (4%) | 1/90 (1%) | .. | ||
| Homemaker | 4/1187 (0%) | 2/294 (1%) | 2/803 (0%) | 0/90 (0%) | .. | ||
| Cigarette smoking | .. | .. | .. | .. | 0·31 | ||
| Never-smoker | 976/1188 (82%) | 232 (79%) | 671/802 (84%) | 73 (80%) | .. | ||
| Current smoker | 88/1188 (7%) | 25 (8%) | 57/802 (7%) | 6 (7%) | .. | ||
| Former smoker | 124/1188 (10%) | 38 (13%) | 74/802 (9%) | 12 (13%) | .. | ||
| Comorbidity | |||||||
| Hypertension | 410/1191 (34%) | 106 (36%) | 265/805 (33%) | 39 (43%) | 0·14 | ||
| Diabetes | 164/1191 (14%) | 43 (15%) | 107/805 (13%) | 14 (15%) | 0·77 | ||
| Coronary heart diseases | 104/1190 (9%) | 25/294 (9%) | 67 (8%) | 12/90 (13%) | 0·27 | ||
| Cerebrovascular diseases | 66/1191 (6%) | 13 (4%) | 51/805 (6%) | 2 (2%) | 0·16 | ||
| Chronic kidney disease | 50 (4%) | 10 (3%) | 35 (4%) | 5 (5%) | 0·64 | ||
| Malignancy | 31 (3%) | 5 (2%) | 25 (3%) | 1 (1%) | 0·24 | ||
| COPD | 18 (2%) | 2 (1%) | 15 (2%) | 1 (1%) | 0·29 | ||
| Treatment received during hospital stay | |||||||
| Corticosteroids | 295 (25%) | 31 (11%) | 196 (24%)‡ | 68 (75%)*† | <0·0001 | ||
| Antivirals | 656 (55%) | 151 (51%) | 446 (55%) | 59 (65%) | 0·07 | ||
| Lopinavir–ritonavir | 166 (14%) | 26 (9%) | 114 (14%) | 26 (29%)*† | <0·0001 | ||
| Arbidol | 576 (48%) | 137 (46%) | 390 (48%) | 49 (54%) | 0·46 | ||
| Chloroquine phosphate | 4 (0%) | 0 (0%) | 3 (0%) | 1 (1%) | 0·21 | ||
| Hydroxychloroquine | 1 (0%) | 1 (0%) | 0 | 0 | 0·32 | ||
| Antibiotics | 924 (78%) | 170 (58%) | 665 (83%)‡ | 89 (98%)*† | <0·0001 | ||
| Thymosin | 191 (16%) | 39 (13%) | 137 (17%) | 15 (16%) | 0·32 | ||
| Intravenous immunoglobulin | 235 (20%) | 28 (9%) | 153 (19%)‡ | 54 (59%)*† | <0·0001 | ||
| Length of hospital stay, days | 14·0 (10·0–20·0) | 11·0 (8·0–16·0) | 14·0 (11·0–19·0)‡ | 39·5 (23·0–52·0)*† | <0·0001 | ||
| ICU admission | 51 (4%) | 0 | 18 (2%)‡ | 33 (36%)*† | <0·0001 | ||
| Length of ICU stay, days | 18·0 (6·0–30·0) | NA | 6·5 (2·0–18·0) | 23·0 (10·0–43·0)† | <0·0001 | ||
| Time from symptom onset to 6-month follow-up, days | 185·0 (175·0–197·0) | 187·0 (174·0–198·0) | 183·0 (175·0–195·0) | 203·0 (184·0–216·0)*† | <0·0001 | ||
| Time from symptom onset to 12-month follow-up, days | 349·0 (337·0–360·0) | 345·0 (335·0–356·0) | 349·0 (338·0–360·0)‡ | 360·0 (351·0–371·0)*† | <0·0001 | ||
| Time from symptom onset to 2-year follow-up, days | 685·0 (675·0–698·0) | 681·0 (671·0–695·5) | 687·0 (676·0–698·0)‡ | 685·0 (676·0–698·0) | 0·0009 | ||
Data are n (%), n/N (%), or median (IQR). Scale 3 indicates those who did not require supplemental oxygen during hospitalisation; scale 4 indicates those who required supplemental oxygen; and scale 5–6 indicates those who required high-flow nasal cannula, non-invasive mechanical ventilation, or invasive mechanical ventilation. The differing denominators used indicate missing data. Data on demographic characteristics, smoking history, and comorbidities were confirmed at the 12-month follow-up visit and self-reported by patients. COPD=chronic obstructive pulmonary disease. ICU=intensive care unit. NA=not applicable.
p<0·0167 for the comparison of scale 5–6 with scale 3.
p<0·0167 for the comparison of scale 5–6 with scale 4.
p<0·0167 for the comparison of scale 4 with scale 3.
The proportion of COVID-19 survivors with at least one sequelae symptom decreased from 777 (68%) of 1149 at 6 months to 583 (49%) of 1188 at 12 months (p<0·0001), but increased slightly to 650 (55%) of 1190 at 2 years (p=0·0010); this trend was also observed in the three subgroups with varying disease severity (table 2 ). Fatigue or muscle weakness and sleep difficulties were the most commonly reported symptomatic sequelae throughout the 2-year follow-up, regardless of disease severity. The proportion of dyspnoea, defined by an mMRC score of 1 or more, gradually decreased from 288 (26%) of 1104 at 6 months to 168 (14%) of 1191 at 2 years. Nearly all domains of HRQoL significantly improved by 2 years, especially the domain of anxiety or depression: the proportion of individuals with symptoms or anxiety or depression decreased from 256 (23%) of 1105 at 6 months to 143 (12%) of 1191 at 2 years (table 2; appendix pp 17–18). 156 (14%) of 1105 COVID-19 survivors had a reduced 6MWD (less than the lower limit of the normal range) at 6 months, and the proportion significantly dropped to 89 (8%) of 1065 at 2 years (p<0·0001). As for mental health assessed by psychiatry-specific questionnaires, 98 (8%) of 1187 had anxiety symptoms at 2 years, 75 (6%) of 1190 had depression symptoms, and 27 (2%) of 1189 had PTSD symptoms. 226 (19%) of 1187 participants had outpatient clinic visits and 159 (13%) of 1187 were admitted to hospital throughout the 2 years after discharge, mainly due to pre-existing illnesses (table 2; appendix pp 19–20). 438 (89%) of 494 participants who had a job before COVID-19 had returned to their original work at 2 years. Reported reasons for not returning to original work were as follows: decreased physical function, unwilling to return, and unemployment (table 2; appendix p 20). As for symptoms co-occurrence, taste disorder and smell disorder were moderately correlated at 6 months, but this correlation decreased over time (appendix pp 28–29).
Table 2.
Clinical outcomes of COVID-19 survivors who completed 6-month, 12-month, and 2-year follow-up
|
Total (n=1192) |
Scale 3 (n=295) |
Scale 4 (n=806) |
Scale 5–6 (n=91) |
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 6 months | 12 months | 2 years | 6 months | 12 months | 2 years | 6 months | 12 months | 2 years | 6 months | 12 months | 2 years | |
| Sequelae symptoms | ||||||||||||
| Any of the following symptoms | 777/1149 (68%) | 583/1188 (49%)* | 650/1190 (55%)†‡ | 194/286 (68%) | 141 (48%)* | 158/294 (54%)† | 509/774 (66%) | 395/802 (49%)* | 440/805 (55%)† | 74/89 (83%) | 47 (52%)* | 52 (57%)† |
| Fatigue or muscle weakness | 593/1151 (52%) | 240/1188 (20%)* | 357/1190 (30%)†‡ | 143/286 (50%) | 60 (20%)* | 89/294 (30%)†‡ | 385/776 (50%) | 161/802 (20%)* | 235/805 (29%)†‡ | 65/89 (73%) | 19 (21%)* | 33 (36%)† |
| Sleep difficulties | 313/1151 (27%) | 206/1188 (17%)* | 298/1190 (25%)‡ | 75/286 (26%) | 47 (16%)* | 70/294 (24%)‡ | 205/776 (26%) | 146/802 (18%)* | 203/805 (25%)‡ | 33/89 (37%) | 13 (14%)* | 25 (27%) |
| Hair loss | 252/1151 (22%) | 131/1188 (11%)* | 142/1190 (12%)† | 61/286 (21%) | 27 (9%)* | 41/294 (14%) | 169/776 (22%) | 97/802 (12%)* | 88/805 (11%)† | 22/89 (25%) | 7 (8%)* | 13 (14%) |
| Smell disorder | 128/1151 (11%) | 56/1188 (5%)* | 67/1190 (6%)† | 32/286 (11%) | 16 (5%) | 21/294 (7%) | 82/776 (11%) | 34/802 (4%)* | 42/805 (5%)† | 14/89 (16%) | 6 (7%) | 4 (4%)† |
| Joint pain | 126/1147 (11%) | 141/1188 (12%) | 117/1190 (10%) | 40/287 (14%) | 33 (11%) | 30/294 (10%) | 70/772 (9%) | 93/802 (12%) | 79/805 (10%) | 16/88 (18%) | 15 (16%) | 8 (9%) |
| Palpitations | 108/1151 (9%) | 110/1188 (9%) | 145/1190 (12%) | 28/286 (10%) | 19 (6%) | 41/294 (14%)‡ | 66/776 (9%) | 84/802 (10%) | 95/805 (12%) | 14/89 (16%) | 7 (8%) | 9 (10%) |
| Decreased appetite | 92/1151 (8%) | 34/1188 (3%)* | 33/1190 (3%)† | 25/286 (9%) | 6 (2%)* | 10/294 (3%) | 56/776 (7%) | 25/802 (3%)* | 21/805 (3%)† | 11/89 (12%) | 3 (3%) | 2 (2%) |
| Taste disorder | 87/1151 (8%) | 35/1188 (3%)* | 35/1190 (3%)† | 21/286 (7%) | 6 (2%)* | 11/294 (4%) | 58/776 (7%) | 29/802 (4%)* | 20/805 (2%)† | 8/89 (9%) | 0 (0%) | 4 (4%) |
| Dizziness | 64/1151 (6%) | 61/1188 (5%) | 131/1190 (11%)†‡ | 19/286 (7%) | 15 (5%) | 31/294 (11%) | 39/776 (5%) | 38/802 (5%) | 90/805 (11%)†‡ | 6/89 (7%) | 8 (9%) | 10 (11%) |
| Chest pain | 53/1147 (5%) | 86/1188 (7%)* | 83/1190 (7%) | 15/287 (5%) | 23 (8%) | 19/294 (6%) | 34/772 (4%) | 59/802 (7%) | 54/805 (7%) | 4/88 (5%) | 4 (4%) | 10 (11%) |
| Sore throat or difficult to swallow | 45/1151 (4%) | 40/1188 (3%) | 64/1190 (5%) | 18/286 (6%) | 11 (4%) | 20/294 (7%) | 23/776 (3%) | 26/802 (3%) | 40/805 (5%) | 4/89 (4%) | 3 (3%) | 4 (4%) |
| Skin rash | 36/1151 (3%) | 50/1188 (4%) | 34/1190 (3%) | 11/286 (4%) | 13 (4%) | 6/294 (2%) | 21/776 (3%) | 35/802 (4%) | 25/805 (3%) | 4/89 (4%) | 2 (2%) | 3 (3%) |
| Myalgia | 31/1147 (3%) | 50/1188 (4%) | 88/1190 (7%)†‡ | 9/287 (3%) | 11 (4%) | 22/294 (7%) | 19/772 (2%) | 34/802 (4%) | 59/805 (7%)†‡ | 3/88 (3%) | 5 (5%) | 7 (8%) |
| Headache | 20/1147 (2%) | 55/1188 (5%)* | 81/1190 (7%)† | 6/287 (2%) | 15 (5%) | 23/294 (8%)† | 11/772 (1%) | 36/802 (4%)* | 50/805 (6%)† | 3/88 (3%) | 4 (4%) | 8 (9%) |
| Nausea or vomiting | 17/1150 (1%) | 10/1188 (1%) | 27/1190 (2%)‡ | 8/286 (3%) | 4 (1%) | 8/294 (3%) | 9/775 (1%) | 4/802 (0%) | 18/805 (2%)‡ | 0/89 (0%) | 2 (2%) | 1 (1%) |
| mMRC dyspnoea score | ||||||||||||
| 0 | 816/1104 (74%) | 834/1187 (70%)* | 1023/1191 (86%)†‡ | 216/288 (75%) | 222/294 (76%) | 253 (86%)†‡ | 551/734 (75%) | 556/802 (69%) | 694/805 (86%)†‡ | 49/82 (60%) | 56 (62%) | 76 (84%)†‡ |
| ≥1 | 288/1104 (26%) | 353/1187 (30%)* | 168/1191 (14%)†‡ | 72/288 (25%) | 72/294 (24%) | 42 (14%)†‡ | 183/734 (25%) | 246/802 (31%) | 111/805 (14%)†‡ | 33/82 (40%) | 35 (38%) | 15 (16%)†‡ |
| EQ-5D-5L§ | ||||||||||||
| Pain or discomfort | 300/1104 (27%) | 348/1187 (29%) | 284/1191 (24%)‡ | 78/286 (27%) | 78/294 (27%) | 73 (25%) | 189/736 (26%) | 240/802 (30%) | 189/805 (23%)‡ | 33/82 (40%) | 30 (33%) | 22 (24%) |
| Anxiety or depression | 256/1105 (23%) | 312/1187 (26%) | 143/1191 (12%)†‡ | 70/288 (24%) | 73/294 (25%) | 34 (12%)†‡ | 158/736 (21%) | 213/802 (27%)* | 98/805 (12%)†‡ | 28/81 (35%) | 26 (29%) | 11 (12%)†‡ |
| Mobility problem | 68/1109 (6%) | 106/1187 (9%)* | 42/1191 (4%)†‡ | 15/289 (5%) | 21/294 (7%) | 10 (3%) | 40/738 (5%) | 78/802 (10%) | 27/805 (3%)‡ | 13/82 (16%) | 7 (8%) | 5 (5%) |
| Personal care problem | 8/1109 (1%) | 17/1187 (1%) | 14/1191 (1%) | 0/289 (0%) | 3/294 (1%) | 4 (1%) | 7/738 (1%) | 11/802 (1%) | 8/805 (1%) | 1/82 (1%) | 3 (3%) | 2 (2%) |
| Usual activity problem | 16/1100 (1%) | 14/1187 (1%) | 35/1191 (3%)†‡ | 3/288 (1%) | 2/294 (1%) | 8 (3%) | 10/731 (1%) | 10/802 (1%) | 20/805 (2%) | 3/81 (4%) | 2 (2%) | 7 (8%) |
| Utility index score¶ | 1·0 (0·9–1·0) | 1·0 (0·9–1·0)* | 1·0 (0·9–1·0)‡ | 1·0 (0·9–1·0) | 1·0 (0·9–1·0) | 1·0 (0·9–1·0) | 1·0 (0·9–1·0) | 1·0 (0·9–1·0)* | 1·0 (0·9–1·0)‡ | 1·0 (0·9–1·0) | 1·0 (0·9–1·0) | 1·0 (0·9–1·0) |
| EQ-VAS score | 80·0 (75·0–90·0) | 80·0 (70·0–90·0) | 80·0 (70·0–90·0)† | 80·0 (70·0–90·0) | 80·0 (70·0–90·0) | 80·0 (70·0–90·0) | 80·0 (75·0–90·0) | 80·0 (75·0–90·0)* | 80·0 (70·0–90·0)† | 80·0 (70·0–90·0) | 80·0 (70·0–85·0) | 80·0 (70·0–90·0) |
| Distance walked in 6 min, m | 495·0 (450·0–540·0) | 495·0 (445·0–545·0) | 512·0 (458·0–563·0)†‡ | 494·5 (450·0–540·0) | 495·0 (440·0–540·0) | 510·0 (455·0–564·0)†‡ | 496·0 (450·0–540·0) | 495·0 (445·0–545·0) | 510·0 (457·0–555·0)†‡ | 495·0 (430·0–528·0) | 496·5 (455·0–551·0) | 530·0 (480·0–600·0)†‡ |
| Percentage of predicted value‖ | 88·1 (79·7–96·2) | 90·2 (81·6–98·8) | 94·0 (84·7–104·1)†‡ | 86·9 (78·5–94·9) | 89·6 (81·0–96·7) | 93·8 (85·0–103·5)†‡ | 88·7 (80·5–97·1) | 90·7 (82·6–100·2) | 94·1 (84·6–104·0)†‡ | 83·6 (76·0–92·8) | 87·9 (80·4–98·0) | 95·0 (84·5–105·9)†‡ |
| Less than LLN** | 156/1105 (14%) | 132/1167 (11%) | 89/1065 (8%)† | 45/287 (16%) | 36/286 (13%) | 17/254 (7%)† | 91/738 (12%) | 81/793 (10%) | 65/726 (9%)† | 20/80 (25%) | 15/88 (17%) | 7/85 (8%)† |
| Mental health | ||||||||||||
| Anxiety symptom (GAD-7≥5) | NA | NA | 98/1187 (8%) | NA | NA | 26/294 (9%) | NA | NA | 66/802 (8%) | NA | NA | 6 (7%) |
| Depression symptom (PHQ-9≥5) | NA | NA | 75/1190 (6%) | NA | NA | 25 (8%) | NA | NA | 45/804 (6%) | NA | NA | 5 (5%) |
| PTSD symptom (PCL-C ≥38) | NA | NA | 27/1189 (2%) | NA | NA | 12 (4%) | NA | NA | 14/803 (2%) | NA | NA | 1 (1%) |
| Health-care use | ||||||||||||
| Outpatient clinic visit | NA | 215/1169 (18%) | 226/1187 (19%) | NA | 54/290 (19%) | 56/294 (19%) | NA | 149/790 (19%) | 150/803 (19%) | NA | 12/89 (13%) | 20/90 (22%) |
| Hospitalisation | NA | 152/1169 (13%) | 159/1187 (13%) | NA | 38/290 (13%) | 45/294 (15%) | NA | 100/790 (13%) | 95/803 (12%) | NA | 14/89 (16%) | 19/90 (21%) |
| Emergency department visit | NA | 12/1169 (1%) | 7/1187 (1%) | NA | 3/290 (1%) | 2/294 (1%) | NA | 8/790 (1%) | 5/803 (1%) | NA | 1/89 (1%) | 0/90 (0%) |
| Returned to original work†† | NA | 401/455 (88%) | 438/494 (89%) | NA | 99/115 (86%) | 112/124 (90%) | NA | 268/300 (89%) | 282/321 (88%) | NA | 34/40 (85%) | 44/49 (90%) |
Data are median (IQR), n (%), or n/N (%). Scale 3 indicates those who did not require supplemental oxygen during hospitalisation; scale 4 indicates those who required supplemental oxygen; and scale 5–6 indicates those who required high-flow nasal cannula, non-invasive mechanical ventilation, or invasive mechanical ventilation. The differing denominators used indicate missing data. mMRC=modified British Medical Research Council. EQ-VAS=EuroQol Visual Analogue Scale. LLN=lower limit of normal range. GAD-7=Generalized Anxiety Disorder 7. PHQ-9=Patient Health Questionnaire 9. PTSD=post-traumatic stress disorder. PCL-C=Post-Traumatic Stress Disorder Checklist, Civilian version. NA=not applicable.
p<4·17 × 10−3 for the comparison of 12-month with 6-month follow-up.
p<4·17 × 10−3 for the comparison of 2-year with 6-month follow-up.
p<4·17 × 10−3 for the comparison of 2-year with 12-month follow-up.
Detailed results of EQ-5D-5L questionnaire of COVID-19 survivors are shown in the appendix (p 17); EQ-VAS scores range from 0–100, with higher scores indicating better health status.
Utility index score: 1 indicates perfect health, 0 indicates death, and negative scores represent values as worse than death.
Predicted values were calculated according to the method of Enright and Sherrill.
The LLN was calculated by subtracting 153 m from the predicted value for men or by subtracting 139 m for women.
This category only includes those who had a full-time or part-time job before COVID-19.
After multivariable adjustment, COVID-19 participants with long COVID symptoms had an odds ratio (OR) of 3·81 (95% CI 1·62–8·93) for mobility problems, 4·42 (3·14–6·21) for pain or discomfort, and 7·46 (4·12–13·52) for anxiety or depression compared with participants without long COVID symptoms (appendix p 21). The median 6MWD of symptomatic participants was 12·8 m shorter than that of asymptomatic participants (p=0·01). As for mental health, symptomatic participants had an OR of 4·63 (2·53–8·50) for anxiety symptoms and 11·43 (4·55–28·72) for depression symptoms, as compared with asymptomatic participants. Moreover, participants with long COVID symptoms had a higher risk of an outpatient clinic visit (OR 2·82 [1·99–4·00]) and rehospitalisation (1·64 [1·12–2·41]) after discharge (appendix p 21).
1127 matched pairs of COVID-19 survivors and controls were derived by adopting the matching process shown in figure 1B. No significant differences were observed between the two groups in terms of age, sex, and comorbidities (appendix p 22). At the 2-year follow-up, 736 (65%) COVID-19 survivors had at least one prevalent symptom, significantly higher than 366 (32%) in the matched control population (p<0·0001; table 3 ). Furthermore, the proportions of all recorded prevalent symptoms were significantly higher in the COVID-19 survivor group than in the control group. Compared with controls, COVID-19 survivors had more problems with usual activity (27 [2%] vs five [<1%]), pain or discomfort (254 [23%] vs 57 [5%]), and anxiety or depression (131 [12%] vs 61 [5%]), and lower median self-assessment scores of quality of life (80·0 vs 85·0; all p<0·0001; table 3).
Table 3.
Prevalent symptoms and health-related quality of life of COVID-19 survivors at 2-year follow-up and matched non-COVID-19 controls
| COVID-19 survivors at 2-year follow-up visit (n=1127) | Matched non-COVID-19 controls (n=1127) | p value | ||
|---|---|---|---|---|
| Prevalent symptoms | ||||
| Any one of the following symptoms | 736 (65%) | 366 (32%) | <0·0001 | |
| Sleep difficulties | 354 (31%) | 153 (14%) | <0·0001 | |
| Fatigue or muscle weakness | 351 (31%) | 55 (5%) | <0·0001 | |
| Hair loss | 201 (18%) | 94 (8%) | <0·0001 | |
| Joint pain | 202 (18%) | 94 (8%) | <0·0001 | |
| Palpitations | 174 (15%) | 50 (4%) | <0·0001 | |
| Dizziness | 164 (15%) | 78 (7%) | <0·0001 | |
| Cough | 108 (10%) | 41 (4%) | <0·0001 | |
| Headache | 110 (10%) | 34 (3%) | <0·0001 | |
| Sore throat or difficult to swallow | 94 (8%) | 8 (1%) | <0·0001 | |
| Myalgia | 94 (8%) | 9 (1%) | <0·0001 | |
| Chest pain | 91 (8%) | 18 (2%) | <0·0001 | |
| Smell disorder | 68 (6%) | 4 (<1%) | <0·0001 | |
| Skin rash | 52 (5%) | 4 (<1%) | <0·0001 | |
| Decreased appetite | 35 (3%) | 11 (1%) | 0·0003 | |
| Taste disorder | 33 (3%) | 3 (<1%) | <0·0001 | |
| Nausea or vomiting | 29 (3%) | 4 (<1%) | <0·0001 | |
| mMRC score | .. | .. | 0·0004 | |
| 0 | 980 (87%) | 919 (82%) | .. | |
| ≥1 | 147 (13%) | 208 (18%) | .. | |
| EQ-5D-5L questionnaire | ||||
| Pain or discomfort | 254 (23%) | 57 (5%) | <0·0001 | |
| Anxiety or depression | 131 (12%) | 61 (5%) | <0·0001 | |
| Mobility problem | 34 (3%) | 41 (4%) | 0·41 | |
| Usual activity problem | 27 (2%) | 5 (<1%) | <0·0001 | |
| Personal care problem | 12 (1%) | 4 (<1%) | 0·045 | |
| EQ-VAS score* | 80·0 (70·0–90·0) | 85·0 (80·0–90·0) | <0·0001 | |
Data are median (IQR) or n (%). mMRC=modified British Medical Research Council. EQ-5D-5L=EuroQol five-dimension five-level questionnaire. EQ-VAS=EuroQol Visual Analogue Scale.
EQ-VAS was used to assess quality of life, ranging from 0 (worst imaginable health) to 100 (best imaginable health).
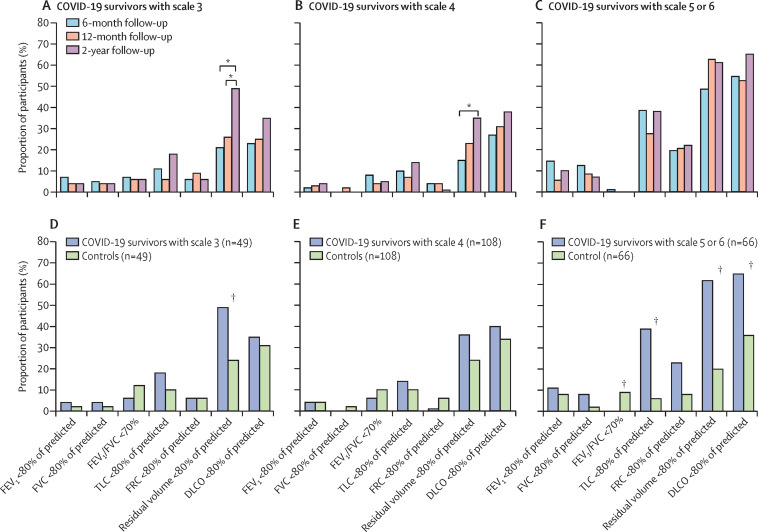
Of the 349 participants who completed pulmonary function tests at 6 months, 244 completed the test at 12 months and 230 completed the test at 2 years (appendix p 26). Among COVID-19 survivors who were included in the final analysis and had pulmonary function tests results, there was no significant difference in the proportion with lung diffusion impairment (ie, with a diffusion capacity of carbon monoxide less than 80% of the predicted capacity) over time in the three subgroups (figure 2A–C ). In subgroups with scale 3 or 4 disease severity, the proportion with reduced residual volume (ie, less than 80% of the predicted residual volume) increased significantly between the 6-month and 2-year follow-ups (p<0·0001); an increasing but non-significant trend was also seen in the proportion with reduced total lung capacity (ie, less than 80% of the predicted total lung capacity; figure 2A–B). Generally speaking, spirometry parameters did not differ significantly over time for all three subgroups (figure 2A–C). Of the 57 COVID-19 survivors with abnormal CT at 12 months who completed lung imaging at the 2-year follow-up, ten participants achieved complete imaging restoration (appendix pp 23 and 27). The most common remaining imaging abnormalities were ground glass opacity and irregular lines, mainly in the scale 5–6 subgroup (appendix p 23).
Figure 2.
Pulmonary function of COVID-19 survivors and matched non-COVID-19 controls
(A–C) Longitudinal evolution of lung function in COVID-19 survivors with different disease severity scales (scale 3: not requiring supplemental oxygen during hospitalisation; scale 4: requiring supplemental oxygen via nasal cannulae or mask during hospitalisation; scale 5–6: requiring high-flow nasal cannula, non-invasive mechanical ventilation, or invasive mechanical ventilation during hospitalisation). (D–F) Comparison of lung function between COVID-19 survivors with different disease severity and their controls at the 2-year follow-up visit. FEV1=forced expiratory volume in 1 s. FVC=forced vital capacity. TLC=total lung capacity. FRC=functional residual capacity. DLCO=diffusion capacity for carbon monoxide. *p<5·56 × 10−3 for the comparison of different time points in (A), (B), and (C). †p<0·0167 for the comparison of COVID-19 survivors with controls in (D), (E), and (F).
Of the COVID-19 survivors who participated in the three assessments, 230 completed pulmonary function tests at the 2-year follow-up. 275 participants from the non-COVID-19 cohort completed pulmonary function tests at the 2-year follow-up. 223 matched pairs were finally derived and were balanced in terms of age, sex, and chronic pulmonary disease (figure 1C; appendix p 24). Nearly all parameters of spirometry, lung volume, and diffusing capacity did not differ significantly between the scale 3 or 4 subgroups and their controls, except for a higher proportion with reduced residual volume in the scale 3 subgroup than in the matched control group (figure 2D–E). However, a significantly higher proportion of COVID-19 survivors with a scale of 5–6 than their matched controls had lung diffusion impairment (43 [65%] of 66 vs 24 [36%] of 66, p=0·0009), reduced residual volume (41 [62%] vs 13 [20%], p<0·0001), and total lung capacity (26 [39%] vs four [6%], p<0·0001; figure 2F).
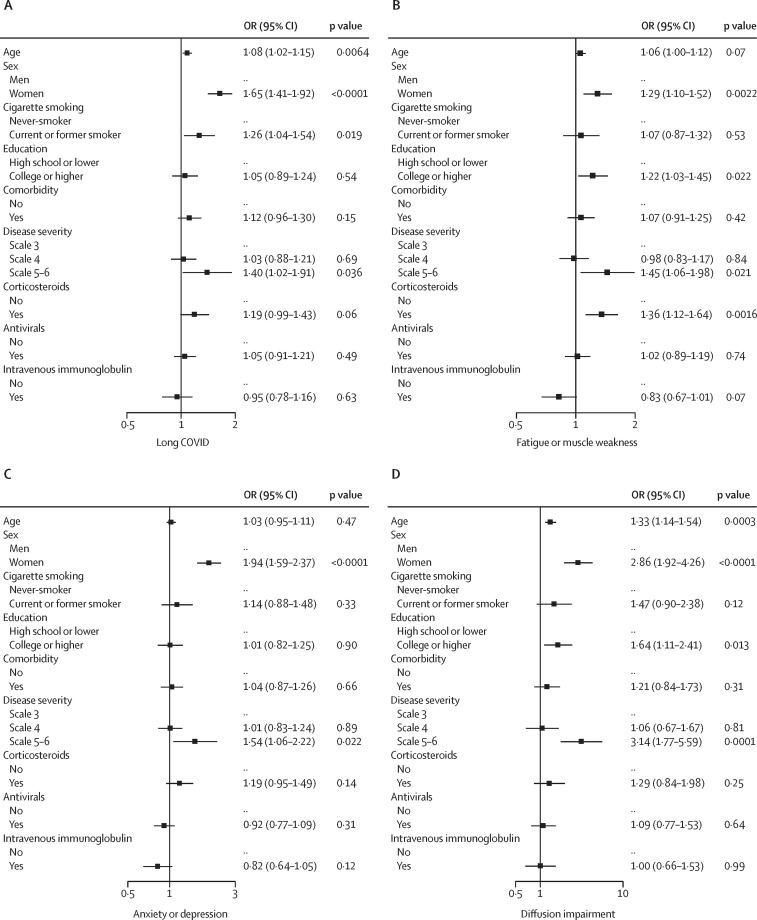
After multivariable adjustment, women had an OR of 1·65 (95% CI 1·41–1·92) for long COVID, 1·29 (1·10–1·52) for fatigue or muscle weakness, 1·94 (1·59–2·37) for anxiety or depression, and 2·86 (1·92–4·26) for lung diffusion impairment, compared with men, (figure 3 ). Participants with a scale of 5 or 6 had an OR of 1·40 (1·02–1·91) for long COVID, 1·45 (1·06–1·98) for fatigue or muscle weakness, 1·54 (1·06–2·22) for anxiety or depression, and 3·14 (1·77–5·59) for lung diffusion impairment, compared with participants with a scale of 3. Corticosteroid therapy at the acute phase was associated with an increased risk of fatigue or muscle weakness (OR 1·36 [1·12–1·64]). Age was positively associated with long COVID and diffusion impairment, with the risk of long COVID 8% higher (OR 1·08 [1·02–1·15]) and diffusion impairment 33% higher (OR 1·33 [1·14–1·54]) per 10-year increase of age (figure 3).
Figure 3.
Risk factors for long COVID, fatigue or muscle weakness, anxiety or depression, and lung diffusion impairment
OR (95% CI) for age indicates the risk of long COVID, fatigue or muscle weakness, anxiety or depression, and diffusion impairment per 10-year age increase. OR=odds ratio.
Discussion
To the best of our knowledge, this is the longest longitudinal follow-up study of individuals who have recovered from acute COVID-19, systematically and comprehensively describing the longitudinal evolution of health and functional outcomes among COVID-19 survivors with differing severity up to 2 years. We found that HRQoL, exercise capacity, and mental health continued to improve throughout the 2 years regardless of initial disease severity, but about half still had symptomatic sequelae at 2 years. Long COVID symptoms at 2 years were related to decreased quality of life, lower exercise capacity, abnormal mental health, and increased use of health care after discharge. Physical health and HRQoL of COVID-19 participants were still poorer than those of the control population 2 years after acute infection. Critically ill patients had a significantly higher burden of restrictive ventilatory impairment and lung diffusion impairment than controls at the 2-year follow-up.
Previous data showed that COVID-19 survivors had sustained recovery of symptoms, exercise capacity, and HRQoL throughout the 1 year after acute infection,3, 4, 6 and this trajectory was observed up to 2 years in our study. We found that fatigue was the most frequently reported symptom throughout the 2 years, regardless of initial disease severity. Consistent with our findings, a high prevalence of fatigue was also observed during the recovery phase of severe acute respiratory syndrome (SARS) and could persist for up to 4 years.24, 25 Notably, we also found that post-COVID-19 fatigue fluctuated or relapsed over time, but the mechanism was largely unclear, most probably due to a combination of central, peripheral, and psychological factors.26 Despite the fairly high burden of sequelae symptoms at 2 years, we found that the vast majority of COVID-19 survivors had returned to their original work, consistent with previous follow-up studies of SARS and COVID-19.24, 27 The negative effect on quality of life, exercise capacity, and health-care utilisation highlights the importance of studying the pathogenesis of long COVID and promoting the exploration of targeted treatment to manage or alleviate the condition.
Consistent with a previous follow-up study of COVID-19,6 we found that the proportion of COVID-19 survivors with restrictive ventilatory impairment was increased during the late recovery period. However, in the absence of concurrent lung imaging, it was difficult to establish whether this new-onset restrictive ventilatory impairment was due to new or worsening interstitial abnormalities. Previous studies of SARS and Middle East respiratory syndrome described the fibrotic abnormalities during convalescence,28, 29, 30, 31 and this sign could also be observed months or even years after COVID-19 infection,3, 12, 32 indicating that pulmonary fibrosis after COVID-19 might be a long-term outcome. Pulmonary fibrosis after COVID-19 might be explained by the key aspects of acute COVID-19 pathobiology, including monocyte or macrophage–T-cell circuits, profibrotic RNA transcriptomics, protracted increased concentrations of inflammatory cytokines, and duration of illness and mechanical ventilation.33 We also found that patients with COVID-19 receiving respiratory support for acute respiratory distress syndrome (ARDS) exhibited significantly more severe lung diffusion impairment, which was consistent with ARDS survivors unrelated to COVID-19.34, 35, 36 The natural history of pulmonary fibrosis after COVID-19, especially in those with ARDS, should be well described in longer longitudinal cohort studies.
Mental health disorders after acute COVID-19, including mainly anxiety, depression, and PTSD, have attracted widespread attention, but the prevalence varies widely among studies.7, 37, 38, 39, 40, 41, 42, 43 Mental health problems after COVID-19 might be attributed to the direct effects of SARS-CoV-2 infection, isolation, physical distancing, incomplete recovery of physical health, and financial difficulties.26, 44 An encouraging finding of our study was that the proportion of participants with anxiety or depression gradually decreased throughout the 2 years, regardless of initial disease severity. Additionally, differences in the prevalence of symptoms of mental health disorders, as assessed by the EQ-5D-5L and psychiatry-specific questionnaires, were observed in our study, suggesting that non-specific psychiatry questionnaires might overestimate the actual prevalence of mental health problems. Different assessment methods for mental health might partly explain the huge variation in the prevalence of mental health disorders after COVID-19 in previous studies.7, 37, 38, 39, 40, 41, 42, 43 In fact, due to the high heterogeneity of follow-up studies of COVID-19, differences were also observed in long COVID prevalence and obscured our understanding of it.26 Hence, there is an urgent need to develop norms to reduce heterogeneity among studies, such as core outcome sets (especially for symptoms requiring priority evaluation), validated assessment tools, broadly recognised symptom questionnaires, and specific follow-up timepoints. Additionally, according to our experience, good study design, detailed division of tasks, close collaboration among team members, timely progress meetings, strict quality control, and earning the trust of participants and their families are the keys to a successful COVID-19 follow-up study. Standardised and successful follow-up studies of COVID-19 will be undoubtedly invaluable in understanding the epidemiology and estimating the burden of long COVID.
The strengths of our study are the large sample size, the well defined longitudinal design with a long follow-up, recognised disease severity groupings, comprehensive in-person assessment, and the inclusion of a control group of participants without COVID-19 hospitalisation to help determine the recovery status of COVID-19 survivors. Our study has several limitations. First, without a control group of hospital survivors of respiratory infection other than COVID-19, it is hard to establish whether the observed abnormalities are specific to COVID-19. Second, the moderate response rate could introduce selection bias. However, most baseline characteristics were balanced between COVID-19 survivors who were included in the analysis and those who were not, except for the slightly increased proportion of participants receiving oxygen therapy among the survivors included in the analysis. It is possible that patients who did not participate had fewer symptoms than those who did, which might result in an overestimated prevalence of long COVID symptoms. Third, this is a single-centre study and COVID-19 survivors came from the early stages of the global pandemic, so the findings might not directly extend to the long-term health outcomes of patients infected with later SARS-CoV-2 variants. Moreover, the low proportion of patients who had been admitted to an intensive care unit in our cohort limits the generalisability of the study findings to this particular population. Fourth, similar to most follow-up studies of COVID-19, information bias was possible in self-reported comorbidities during the acute phase and several self-reported health outcomes during convalescence. Finally, several outcome measures were not collected in all three visits, including work status, health-care use after discharge, and mental health as assessed by psychiatry-specific questionnaires, so the longitudinal analysis of these outcomes is not possible.
Throughout the 2 years after acute infection, hospital survivors with COVID-19 continued to recover in terms of symptomatic sequelae, exercise capacity, mental health, and quality of life, regardless of initial disease severity, but a fairly high burden of symptoms was still seen at 2 years. The COVID-19 survivors had not returned to the same health status as the general population 2 years after acute infection, so ongoing follow-up is needed to characterise the protracted natural history of long COVID; we plan to conduct yearly follow-ups in this cohort. The value of rehabilitation programmes in mitigating the effects of long COVID and in accelerating recovery requires further exploration.
Data sharing
Restrictions apply to the availability of these data and they are not publicly available. However, data are available from the corresponding author upon reasonable request and with the permission of the institution.
Declaration of interests
We declare no competing interests.
Acknowledgments
Acknowledgments
This work was supported by the Chinese Academy of Medical Sciences Innovation Fund for Medical Sciences (CIFMS 2018-I2M-1-003 and 2020-I2M-CoV19-005); the National Natural Science Foundation of China (82041011/H0104); the National Key Research and Development Program of China (2018YFC1200102); the National Administration of Traditional Chinese Medicine (ZYYCXTD-D-202208); and Major Projects of National Science and Technology on New Drug Creation and Development of Pulmonary Tuberculosis (2020ZX09201001). This work was also supported by the China Evergrande Group, Jack Ma Foundation, Sino Biopharmaceutical, Ping An Insurance (Group), and New Sunshine Charity Foundation. We acknowledge all patients who participated in this study and their families. We would also like to thank all staff of this follow-up study team at Jin Yin-tan Hospital. We also thank Yutao Xiang from the University of Macau for his suggestion on the use of psychiatry-specific questionnaires to assess mental health.
Contributors
BC, JW, LH, XG, and YeW conceived of and designed the study. LH, XG, BC, HZ, XZ, and LS drafted the paper. XG did the analysis. HZ, LH, XL, LR, DC, LG, YiW, JZ, and XW collected and verified the data. ML evaluated the lung imaging at three follow-up visits. All authors had full access to the data in the study, critically revised the manuscript for important intellectual content, and had final responsibility for the decision to submit for publication. All authors agree to be accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Supplementary Material
References
- 1.National Institute for Health and Care Excellence. Scottish Intercollegiate Guidelines Network. Royal College of General Practitioners COVID-19 rapid guideline: managing the long-term effects of COVID-19. Dec 18, 2020. https://www.nice.org.uk/guidance/ng188 [PubMed]
- 2.Soriano JB, Murthy S, Marshall JC, Relan P, Diaz JV, on behalf of the WHO Clinical Case Definition Working Group on Post-COVID-19 Condition A clinical case definition of post-COVID-19 condition by a Delphi consensus. Lancet Infect Dis. 2021;22:e102–e107. doi: 10.1016/S1473-3099(21)00703-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Huang L, Yao Q, Gu X, et al. 1-year outcomes in hospital survivors with COVID-19: a longitudinal cohort study. Lancet. 2021;398:747–758. doi: 10.1016/S0140-6736(21)01755-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Wu X, Liu X, Zhou Y, et al. 3-month, 6-month, 9-month, and 12-month respiratory outcomes in patients following COVID-19-related hospitalisation: a prospective study. Lancet Respir Med. 2021;9:747–754. doi: 10.1016/S2213-2600(21)00174-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Zhang X, Wang F, Shen Y, et al. Symptoms and health outcomes among survivors of COVID-19 infection 1 year after discharge from hospitals in Wuhan, China. JAMA Netw Open. 2021;4 doi: 10.1001/jamanetworkopen.2021.27403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Liu T, Wu D, Yan W, et al. Twelve-month systemic consequences of COVID-19 in patients discharged from hospital: a prospective cohort study in Wuhan, China. Clin Infect Dis. 2021 doi: 10.1093/cid/ciab703. published online Aug 14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Zhou F, Tao M, Shang L, et al. Assessment of sequelae of COVID-19 nearly 1 year after diagnosis. Front Med (Lausanne) 2021;8 doi: 10.3389/fmed.2021.717194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Wynberg E, van Willigen HDG, Dijkstra M, et al. Evolution of COVID-19 symptoms during the first 12 months after illness onset. Clin Infect Dis. 2021 doi: 10.1093/cid/ciab759. published online Sept 2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Seessle J, Waterboer T, Hippchen T, et al. Persistent symptoms in adult patients one year after COVID-19: a prospective cohort study. Clin Infect Dis. 2021;74:1191–1198. doi: 10.1093/cid/ciab611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Fumagalli C, Zocchi C, Tassetti L, et al. Factors associated with persistence of symptoms 1 year after COVID-19: a longitudinal, prospective phone-based interview follow-up cohort study. Eur J Intern Med. 2022;97:36–41. doi: 10.1016/j.ejim.2021.11.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Liu YH, Chen Y, Wang QH, et al. One-year trajectory of cognitive changes in older survivors of COVID-19 in Wuhan, China: a longitudinal cohort study. JAMA Neurol. 2022 doi: 10.1001/jamaneurol.2022.0461. published online March 8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Pan F, Yang L, Liang B, et al. Chest CT patterns from diagnosis to 1 year of follow-up in patients with COVID-19. Radiology. 2022;302:709–719. doi: 10.1148/radiol.2021211199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Huang C, Huang L, Wang Y, et al. 6-month consequences of COVID-19 in patients discharged from hospital: a cohort study. Lancet. 2021;397:220–232. doi: 10.1016/S0140-6736(20)32656-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Mahler DA, Wells CK. Evaluation of clinical methods for rating dyspnea. Chest. 1988;93:580–586. doi: 10.1378/chest.93.3.580. [DOI] [PubMed] [Google Scholar]
- 15.Herdman M, Gudex C, Lloyd A, et al. Development and preliminary testing of the new five-level version of EQ-5D (EQ-5D-5L) Qual Life Res. 2011;20:1727–1736. doi: 10.1007/s11136-011-9903-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Rabin R, de Charro F. EQ-5D: a measure of health status from the EuroQol Group. Ann Med. 2001;33:337–343. doi: 10.3109/07853890109002087. [DOI] [PubMed] [Google Scholar]
- 17.Xie W, Wu Y, Wang W, et al. A longitudinal study of carotid plaque and risk of ischemic cardiovascular disease in the Chinese population. J Am Soc Echocardiogr. 2011;24:729–737. doi: 10.1016/j.echo.2011.02.011. [DOI] [PubMed] [Google Scholar]
- 18.Spitzer RL, Kroenke K, Williams JB, Löwe B. A brief measure for assessing generalized anxiety disorder: the GAD-7. Arch Intern Med. 2006;166:1092–1097. doi: 10.1001/archinte.166.10.1092. [DOI] [PubMed] [Google Scholar]
- 19.Spitzer RL, Kroenke K, Williams JB. Validation and utility of a self-report version of PRIME-MD: the PHQ primary care study. Primary Care Evaluation of Mental Disorders. Patient Health Questionnaire. JAMA. 1999;282:1737–1744. doi: 10.1001/jama.282.18.1737. [DOI] [PubMed] [Google Scholar]
- 20.Kroenke K, Spitzer RL, Williams JB. The PHQ-9: validity of a brief depression severity measure. J Gen Intern Med. 2001;16:606–613. doi: 10.1046/j.1525-1497.2001.016009606.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Weathers F, Litz B, Huska J, et al. National Center for PTSD, Behavioral Science Division; Boston, MA: 1994. PTSD checklist—civilian version. [Google Scholar]
- 22.Ning L, Guan S, Liu J. Impact of personality and social support on posttraumatic stress disorder after traffic accidents. Medicine (Baltimore) 2017;96 doi: 10.1097/MD.0000000000007815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Yeager DE, Magruder KM. PTSD checklist scoring rules for elderly Veterans Affairs outpatients. Am J Geriatr Psychiatry. 2014;22:545–550. doi: 10.1016/j.jagp.2013.03.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Tansey CM, Louie M, Loeb M, et al. One-year outcomes and health care utilization in survivors of severe acute respiratory syndrome. Arch Intern Med. 2007;167:1312–1320. doi: 10.1001/archinte.167.12.1312. [DOI] [PubMed] [Google Scholar]
- 25.Lam MH, Wing YK, Yu MW, et al. Mental morbidities and chronic fatigue in severe acute respiratory syndrome survivors: long-term follow-up. Arch Intern Med. 2009;169:2142–2147. doi: 10.1001/archinternmed.2009.384. [DOI] [PubMed] [Google Scholar]
- 26.Crook H, Raza S, Nowell J, Young M, Edison P. Long covid-mechanisms, risk factors, and management. BMJ. 2021;374 doi: 10.1136/bmj.n1648. [DOI] [PubMed] [Google Scholar]
- 27.Jacobsen PA, Andersen MP, Gislason G, et al. Return to work after COVID-19 infection—a Danish nationwide registry study. Public Health. 2022;203:116–122. doi: 10.1016/j.puhe.2021.12.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Xie L, Liu Y, Xiao Y, et al. Follow-up study on pulmonary function and lung radiographic changes in rehabilitating severe acute respiratory syndrome patients after discharge. Chest. 2005;127:2119–2124. doi: 10.1378/chest.127.6.2119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Hui DS, Wong KT, Ko FW, et al. The 1-year impact of severe acute respiratory syndrome on pulmonary function, exercise capacity, and quality of life in a cohort of survivors. Chest. 2005;128:2247–2261. doi: 10.1378/chest.128.4.2247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Xie L, Liu Y, Fan B, et al. Dynamic changes of serum SARS-coronavirus IgG, pulmonary function and radiography in patients recovering from SARS after hospital discharge. Respir Res. 2005;6:5. doi: 10.1186/1465-9921-6-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Das KM, Lee EY, Singh R, et al. Follow-up chest radiographic findings in patients with MERS-CoV after recovery. Indian J Radiol Imaging. 2017;27:342–349. doi: 10.4103/ijri.IJRI_469_16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Caruso D, Guido G, Zerunian M, et al. Post-acute sequelae of COVID-19 pneumonia: six-month chest CT follow-up. Radiology. 2021;301:e396–e405. doi: 10.1148/radiol.2021210834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Mylvaganam RJ, Bailey JI, Sznajder JI, Sala MA, Northwestern Comprehensive COVID Center Consortium Recovering from a pandemic: pulmonary fibrosis after SARS-CoV-2 infection. Eur Respir Rev. 2021;30 doi: 10.1183/16000617.0194-2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Herridge MS, Cheung AM, Tansey CM, et al. One-year outcomes in survivors of the acute respiratory distress syndrome. N Engl J Med. 2003;348:683–693. doi: 10.1056/NEJMoa022450. [DOI] [PubMed] [Google Scholar]
- 35.Herridge MS, Tansey CM, Matté A, et al. Functional disability 5 years after acute respiratory distress syndrome. N Engl J Med. 2011;364:1293–1304. doi: 10.1056/NEJMoa1011802. [DOI] [PubMed] [Google Scholar]
- 36.Cheung AM, Tansey CM, Tomlinson G, et al. Two-year outcomes, health care use, and costs of survivors of acute respiratory distress syndrome. Am J Respir Crit Care Med. 2006;174:538–544. doi: 10.1164/rccm.200505-693OC. [DOI] [PubMed] [Google Scholar]
- 37.Bellan M, Soddu D, Balbo PE, et al. Respiratory and psychophysical sequelae among patients with COVID-19 four months after hospital discharge. JAMA Netw Open. 2021;4 doi: 10.1001/jamanetworkopen.2020.36142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Latronico N, Peli E, Calza S, et al. Physical, cognitive and mental health outcomes in 1-year survivors of COVID-19-associated ARDS. Thorax. 2022;77:300–303. doi: 10.1136/thoraxjnl-2021-218064. [DOI] [PubMed] [Google Scholar]
- 39.Abel KM, Carr MJ, Ashcroft DM, et al. Association of SARS-CoV-2 infection with psychological distress, psychotropic prescribing, fatigue, and sleep problems among UK primary care patients. JAMA Netw Open. 2021;4 doi: 10.1001/jamanetworkopen.2021.34803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Staudt A, Jörres RA, Hinterberger T, Lehnen N, Loew T, Budweiser S. Associations of post-acute COVID syndrome with physiological and clinical measures 10 months after hospitalization in patients of the first wave. Eur J Intern Med. 2022;95:50–60. doi: 10.1016/j.ejim.2021.10.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Bai F, Tomasoni D, Falcinella C, et al. Female gender is associated with long COVID syndrome: a prospective cohort study. Clin Microbiol Infect. 2021;28:611. doi: 10.1016/j.cmi.2021.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Zhao YJ, Zhang SF, Li W, et al. Mental health status and quality of life in close contacts of COVID-19 patients in the post-COVID-19 era: a comparative study. Transl Psychiatry. 2021;11:505. doi: 10.1038/s41398-021-01623-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Hellemons ME, Huijts S, Bek LM, et al. Persistent health problems beyond pulmonary recovery up to 6 months after hospitalization for COVID-19: a longitudinal study of respiratory, physical, and psychological outcomes. Ann Am Thorac Soc. 2022;19:551–561. doi: 10.1513/AnnalsATS.202103-340OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Rogers JP, Chesney E, Oliver D, et al. Psychiatric and neuropsychiatric presentations associated with severe coronavirus infections: a systematic review and meta-analysis with comparison to the COVID-19 pandemic. Lancet Psychiatry. 2020;7:611–627. doi: 10.1016/S2215-0366(20)30203-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Restrictions apply to the availability of these data and they are not publicly available. However, data are available from the corresponding author upon reasonable request and with the permission of the institution.