Abstract
To understand the meaning of the lactate threshold (LT) and to test the hypothesis that endurance training augments lactate kinetics [i.e., rates of appearance and disposal (Ra and Rd, respectively, mg·kg−1·min−1) and metabolic clearance rate (MCR, ml·kg−1·min−1)], we studied six untrained (UT) and six trained (T) subjects during 60-min exercise bouts at power outputs (PO) eliciting the LT. Trained subjects performed two additional exercise bouts at a PO 10% lower (LT-10%), one of which involved a lactate clamp (LC) to match blood lactate concentration ([lactate]b) to that achieved during the LT trial. At LT, lactate Ra was higher in T (24.1 ± 2.7) than in UT (14.6 ± 2.4; P < 0.05) subjects, but Ra was not different between UT and T when relative exercise intensities were matched (UT-LT vs. T-LT-10%, 67% V̇o2max). At LT, MCR in T (62.5 ± 5.0) subjects was 34% higher than in UT (46.5 ± 7.0; P < 0.05), and a reduction in PO resulted in a significant increase in MCR by 46% (LT-10%, 91.5 ± 14.9, P < 0.05). At matched relative exercise intensities (67% V̇o2max), MCR in T subjects was 97% higher than in UT (P < 0.05). During the LC trial, MCR in T subjects was 64% higher than in UT (P < 0.05), in whom %V̇o2max and [lactate]b were similar. We conclude that 1) lactate MCR reaches an apex below the LT, 2) LT corresponds to a limitation in MCR, and 3) endurance training augments capacities for lactate production, disposal and clearance.
Keywords: exercise; maximal lactate steady state (MLSS), exertion; endurance training; lactate oxidation; gluconeogenesis from lactate; intermediary metabolism
blood lactate accumulation increases little with small increments in exercise intensity, but there occurs a power output (PO) above which blood lactate accumulation accelerates in response to further increments in exercise PO. This inflection point has been termed the lactate threshold (LT) (11). The LT has received much attention by exercise physiologists, sports medicine practitioners, coaches, and athletes. This interest comes from the fact that the LT is correlated to endurance exercise capability and can be used in the management of endurance training (18, 51, 69, 74). However, the LT remains unclear in its physiological significance, some interpreting the inflection point to indicate the onset of anaerobiosis (77, 78), whereas others interpret the LT to reflect an imbalance between lactate appearance and removal (12, 72). Moreover, lactate kinetics and its parameters [e.g., rates of appearance (Ra) and disposal (Rd) and metabolic clearance rate (MCR)], have never been investigated during sustained exercise at the LT.
Previous experiments have investigated lactate kinetics as functions of exercise intensity (7, 47, 48, 72). These studies have shown that lactate Ra is matched by Rd during rest, and that both rise with increases in exercise intensity as described by PO, metabolic rate [oxygen consumption (V̇o2) or percentage of maximal oxygen consumption (%V̇o2max)], and blood lactate concentration ([lactate]b). Those investigators also observed that during continual graded exercise, the increase in Rd lags behind the increase in Ra, resulting in rising [lactate]b (47, 72). Also, previous investigators observed that MCR, a measure of efficiency for lactate disposal (19), increases from rest to moderate intensity exercise, but then decreases from moderate to hard exercise (7, 47, 48, 72, 73). Because none of the earlier investigations studied lactate kinetics at the LT, the question of whether a limitation in lactate clearance occurs at the LT remains. Hence, our first aim of the present study was to interrogate the physiological significance of the LT by determining lactate kinetics in subjects exercising at and just below the LT to test the hypothesis that the LT represents a limitation in lactate clearance. Also, because endurance training has been observed to increase lactate clearance capacity in both laboratory rats (19) and humans (7), our second aim was to test the hypothesis that endurance training augments rates of lactate disposal and clearance at a given relative exercise intensity and blood lactate concentration.
MATERIALS AND METHODS
Many details of methodology have been presented elsewhere (21), but are repeated here for the convenience of the reader.
Subjects.
Six untrained men (UT) and six trained male cyclists (T) took part in the study. Recruitment occurred by word of mouth, posting of fliers, and e-mail notices within the University of California Berkeley campus and the surrounding community. Untrained subjects were healthy and recreationally active (maximal oxygen consumption, V̇o2max <50 ml·kg−1·min−1). Trained subjects were members of competitive cycling or triathlon teams, currently in the race phase of their training season (V̇o2max >55 ml·kg−1·min−1). Subjects were included in the study if they had a body mass index (BMI) of ≥18 and <26 kg/m2, were nonsmokers, were diet and weight stable, had a FEV1/FVC of >70%, and were injury and disease free as determined by physical examination. This study was approved by the University of California Berkeley Committee for the Protection of Human Subjects (CPHS 2010–4-1300) and conformed to the standards set by the Declaration of Helsinki. Eligible volunteers were verbally informed of the purposes, procedures, and associated risks, and gave written informed consent.
Experimental design.
The study consisted of a preliminary screening that included a medical examination and a maximal exercise stress test to determine V̇o2max, an incremental exercise test to determine the LT and associated parameters, and one (for UT) or three (for T) isotope tracer trials to measure glucose and lactate kinetics during rest and exercise. Isotope tracer trials took place in the morning after a 12-h fast; study participants rested for 90 min and then exercised for 60 min. During the first tracer trial, UT and T subjects exercised at the PO corresponding to their previously determined LT. Trained subjects completed two additional tracer trials at a PO 10% below that eliciting the LT (LT-10%), one of which included exogenous lactate infusion using the Lactate Clamp (LC) procedure to match blood lactate concentration ([lactate]b) obtained during the LT trial (LT-10%+LC). During rest and exercise, study participants were infused with stable, nonradioactive tracers of lactate, glucose, and bicarbonate. Exercise sessions were separated by at least 1 wk. For the day before each exercise session, study participants were instructed to abstain from structured physical exercise or hard physical activity, but to continue typical activities of daily living. This experimental design of the tracer trials aimed at assessing the effects of endurance training, [lactate]b, exercise intensity, and their combinations on lactate kinetics. Within the T subjects, we investigated 1) the effects of exercise intensity given the same [lactate]b (LT vs. LT-10%+LC); 2) the effects of [lactate]b given the same exercise intensity (LT-10% vs. LT-10%+LC); and 3) the combined effects of [lactate]b and exercise intensity (LT vs. LT-10%). To evaluate the effects of training on metabolic responses, we compared UT and T groups exercising 1) at the LT [i.e., same [lactate]b, but different absolute PO and relative intensities (UT-LT vs. T-LT)]; 2) at the same relative intensity but different [lactate]b (UT-LT vs. T-LT-10%); and 3) at the same relative intensity and [lactate]b (UT-LT vs. T-LT-10%+LC).
Dietary controls and standardized meals.
A 3-day diet record was collected prior to the study to verify that the subjects had normal eating patterns. Subjects also provided complete 24-h diet records of the day preceding each exercise session. Diet records were analyzed for macronutrient composition and energy intake by using Diet Analysis Plus, version 6.1 (ESHA Research, Salem, OR). Subjects were instructed to keep their dietary composition and amount consistent throughout the duration of the study. The day before each exercise session, subjects reported to the laboratory to pick up their dinner and evening snack. Dinner consisted of ∼60% carbohydrate (CHO), 25% fat, and 15% protein; the evening snack consisted of ∼55% CHO, 30% fat, and 15% protein. The snack was consumed between 9:00 and 9:15 P.M. Exercise commenced 12 h following completion of the evening snack, and no food or beverages were allowed except water (ad libitum) until the end of the exercise session. Standardized diets consisted on average of 2,400 and 3,200 kcal per day for the UT and T groups, respectively. Diets were individualized and determined for each participant considering a Physical Activity Level of 1.25 for UT and 1.48 for T according to the current dietary reference intake guidelines for Estimated Energy Requirements established by the Institute of Medicine (National Academies, Washington, DC).
Preliminary screening, V̇o2max test, and medical examination.
Subjects completed a health and exercise history questionnaire that had been developed in cooperation with the Student Health Service. Subsequently, anthropometric data including height, weight, and body composition [evaluated by BMI and skinfold measurements at seven sites (abdominal, triceps, chest/pectoral, midaxillary, subscapular, suprailiac, and thigh)], pulmonary function (FEV1/FVC), and resting blood pressure (determined by auscultation) were measured and recorded. A blood sample was taken for a metabolic panel screening, complete blood count, and serological evaluation for HIV infection. On a subsequent day, subjects underwent an exercise stress test in accordance with the American College of Sports Medicine Guidelines for Exercise Testing and Prescription (7th ed.). This test consisted of graded progressive cycle ergometry to volitional exhaustion. Subjects arrived in the laboratory at 8:45 A.M. having fasted. After a 10-min rest period during which resting electrocardiograph (ECG) (Quinton Q750 ECG, Seattle, WA) and pulmonary gas exchange parameters were recorded, exercise started for 3 min at 75 W for UT subjects; and at 120, 150, or 180 W for T subjects. Thereafter, resistance increased every 3 min by 25 W for UT subjects and 30 W for T subjects, until volitional exhaustion. ECG, minute ventilation (VE), oxygen consumption (V̇o2), carbon dioxide production (V̇co2), and respiratory exchange ratio (RER = V̇co2/V̇o2) were recorded continuously during exercise. VE, V̇o2, V̇co2, and RER were measured using a TrueOne 2400 apparatus (ParvoMedics, Sandy, UT), which was calibrated beforehand using precision-analyzed gas mixtures and a 3-l syringe. Blood pressure was measured in the mid stage, and rating of perceived exertion (RPE) was recorded during the last 30 s of each stage. Blood [lactate] was determined in 10-μl finger-prick samples taken at the end of each stage via a portable lactate analyzer (Nova Lactate Plus, Waltham, MA). This test was performed to assess the maximal heart rate (fHmax) and oxygen consumption (V̇o2max), and V̇o2max-associated power output (POmax), as well as to approximate the PO eliciting the LT. Finally, participants were subjected to a physical examination before final inclusion.
LT determination and confirmation.
Subjects arrived in the laboratory at 8:45 A.M. having fasted. A catheter was inserted into a warmed dorsal hand vein for arterialized blood sampling (38). Following a 10-min rest period, a 1-ml blood sample was collected, and blood pressure was measured. Exercise workload started at 75 W for UT and 120 or 150 W for T subjects, and progressively increased to reach in 3 min ∼50% and ∼60% of V̇o2max (i.e., approximately 40–50 W below the approximated PO at LT) for UT and T subjects, respectively. Thereafter, resistance increased every 3 min by 10 W until volitional exhaustion. Blood pressure was measured in the mid stage; RPE was recorded, and a 1-ml blood sample was drawn at the end of each exercise stage. ECG and pulmonary gas exchange parameters (VE, V̇o2, V̇co2, and RER) were recorded continuously during rest and exercise. This test was performed to determine the LT as defined previously (11) and associated V̇o2, heart rate (fH), PO, and [lactate]b. On a subsequent day, subjects arrived in the laboratory at 8:45 A.M. having fasted. After 10 min of rest, subjects exercised for 60 min at the PO corresponding to their LT to confirm the feasibility of the task and stabilization of [lactate]b over the 60-min exercise test. Every 10 min, 10-μl finger-prick blood samples were assayed for [lactate]b, and heart rate and RPE were monitored. When necessary, PO was adjusted by ∼5 W. The mean PO during the last 15 min of exercise was used for the isotope tracer trials.
Isotope tracer trials.
Subjects performed one (UT) or three (T) isotope tracer trials (see Experimental design). Subjects reported to the laboratory at 7:15 A.M. having fasted. ECG was monitored continuously during rest and exercise. Resting oxygen consumption was measured, and an aliquot of expired air was taken to determine the 13CO2 background enrichment. A catheter was then inserted into a warmed dorsal hand vein. Blood samples were collected for determination of background lactate and glucose isotopic enrichments, and metabolite and hormone concentrations. In the contralateral arm, a second catheter was placed in an antecubital vein for isotope [i.e., [3-13C]lactate, H13CO3−, and [6,6-2H2]glucose (D2-glucose), for all trials] and unlabeled lactate cocktail-saline (i.e., Na-lactate, for LC-10%+LC) infusions. Catheters were kept patent by a saline drip (0.9%, no heparin).
After background sampling, a priming bolus containing 57.5 mg of [3-13C]lactate (i.e., 23 times the resting lactate infusion rate), 250 mg of D2-glucose (i.e., 125 times the resting lactate infusion rate), and 136 mg of NaH13CO3− were injected. The resting infusion rate for [3-13C]lactate was 2.5 mg/min during LT and LT-10%, and 7.5 mg/min during LT-10%+LC. The resting infusion rate of D2-glucose was 2 mg/min in all trials. Subjects rested semi-supine for 90 min. During the resting period of the LT-10%+LC trial, unlabeled lactate infusion began at 104 ml/h, which delivered lactate at ∼200 mg/min. Infusion was then adjusted to match the [lactate]b observed during exercise of the LT trial. Results obtained using D2-glucose to assess glucose kinetics and gluconeogenesis via secondary labeling of blood glucose with 13C from infused [3-13C]lactate are reported separately (21).
At the onset of exercise, [3-13C]lactate infusion rate was increased to 11.25 mg/min and 15 mg/min for UT and T subjects, respectively. During exercise of the LT-10%+LC trial, unlabeled lactate infusion was adjusted to match [lactate]b observed during the LT trial.
Blood for metabolite concentrations and isotopic enrichments and hormonal analyses was sampled at 0, 60, 75, and 90 min of rest and at 10, 20, 30, 40, 50, and 60 min of exercise. During exercise of the LT-10%+LC trial, [lactate]b was also immediately analyzed (NOVA Lactate Plus; Nova Biomedical, Waltham, MA) and the information was used to adjust the exogenous lactate infusion rate to achieve the desired target level. Pulmonary gas exchange parameters were simultaneously sampled to determine VE, V̇o2, V̇co2, and RER. Blood pressure was determined by auscultation 5 min prior to the blood sampling. A sample of expired air was also collected in a 10-ml Vacutainer at each time point of blood sampling to determine 13CO2 isotopic enrichment in expired gases. Hematocrit was also measured at each time point using the microhematocrit method, and subjects indicated their RPE according to the Borg scale.
Isotope tracer and unlabeled lactate cocktail preparations.
Tracers were purchased from Sigma-Aldrich (St. Louis, MO). The individual components were United States Pharmacopeia–National Formulary certified. Tracer cocktails were prepared in 0.9% sterile saline. For the lactate clamp, unlabeled lactate cocktail was prepared in 0.9% sterile saline by mixing a 30% L(+)-lactic acid solution (Sigma-Aldrich) with 2N NaOH (Spectrum Chemicals, Auburn, WA) to a pH of 4.8 as previously described (53). The tracer and LC infusates were subsequently tested for sterility and pyrogenicity at the University of California San Francisco, School of Pharmacy, Pharmacy Drug Product Services Division. Infusates passed through a 0.2-mm Millipore filter (Nalgene, Rochester, NY) prior to infusion and were delivered with calibrated pumps (Baxter Colleague 3CX, Deerfield, IL).
Ergometry.
All exercises were performed on an electronic braked cycle ergometer (839E; Monark Exercise, Vansbro, Sweden). The instantaneous power output and the pedaling frequency were delivered online by a microprocessor. For all exercise tests, the subjects remained in the seated position.
Blood sampling.
Arterialized blood samples (∼3 ml) for analyses of lactate and glucose concentrations and isotopic enrichments (IE) were collected in 8% perchloric acid in a 1:2 ratio. Blood samples (∼6 ml) for hormone analyses were split in two aliquots of ∼3 ml each. Samples were centrifuged for 18 min at 3,000 g. Blood (for lactate and glucose IEs and concentrations) and plasma (for catecholamines, insulin, and glucagon) were transferred and stored at −80°C until analysis. Results for insulin and glucagon are reported separately (21).
Epinephrine and norepinephrine analyses.
Epinephrine and norepinephrine were analyzed as previously described (23). Briefly, catecholamines were extracted from the plasma using acid-washed WA-4 Alumina (Sigma) and 1.5 M Tris buffer containing 2% EGTA at a pH of 8.6. Perchloric acid (0.1 M) was used to elute the catecholamines. Finally, 100 μl of this eluent was injected in the HPLC system (Coulochem model LC/EC 5200A; ESA, Chelmsford, MA). The mobile phase was Cataphase 2 (ESA), and the electrodes were set at +350, +50, and −350 mV. Standard catecholamine solutions were purchased from ESA. Chromatographs were analyzed using an ESA 501 Data Chromatography System.
Isotopic enrichments and lactate and glucose concentration analyses.
Lactate and glucose were prepared for gas chromatography/mass spectrometry (GC/MS) analysis using the heptafluorobutyric anhydride and pentaacetate derivatives, respectively. Known amounts of uniformly labeled internal standards [U-13C]lactate and [U-13C]glucose were added to the supernatant samples collected in 8% perchloric acid. Samples were then neutralized with 2N KOH and transferred to ion exchange columns that were previously washed with double-deionized water (ddH2O) through a cation resin (Analytical Grade 50W-X8, 50–100 mesh H+ resin; Bio-Rad Laboratories, Hercules, CA) and with ddH2O followed by 2N formic acid through an anion resin (Analytical Grade 1-X8, 100–200 mesh formate resin). Glucose was eluted first with ddH2O followed by elution of lactate through the anion column with 2N formic acid. The samples were then transferred to a 2-ml gas chromatography vial and lyophilized. Glucose analyses and kinetics data are reported separately (21).
For derivatization, lactate samples were resuspended in 200 μl of 2,2-dimethoxypropane and transferred to a vial to which 20 μl of 10% HCl in methanol was added. After samples sat at room temperature for 60 min, 50 μl of N-propylamine was added. Samples were then heated for 30 min at 100°C and subsequently dried under a stream of N2 gas, resuspended in 200 μl ethyl acetate, transferred to a GCMS vial, and dried again under N2 gas, resuspended in 20 μl of heptafluorobutyric anhydride, left for 5 min at room temperature to react, and dried under N2 gas. Finally, the derivatized lactate was resuspended in 50 μl ethyl acetate.
Lactate IEs were determined by GC/MS (GC model 6890 series and MS model 5973N; Agilent Technologies). Methane was used for chemical ionization with selected ion monitoring of mass-to-charge ratios (m/z) 328 (nonlabeled lactate), 329 (M+1 isotopomer, [3-13C]lactate), and 331 (M+3 isotopomer, [U-13C]lactate internal standard). Whole blood lactate concentrations were determined by an abundance ratio of 328/331.
The expired air samples were stored at room temperature until analyzed via isotope ratio mass spectrometry (IRMS) by Metabolic Solutions (Nashua, NH) (data not shown).
Calculations.
Calculations of lactate kinetics were performed during the last 30 min of rest and 20 min of exercise. Lactate flux [i.e., rate of appearance (Ra, mg·kg−1·min−1), rate of disposal (Rd, mg·kg−1·min−1), and metabolic clearance rate (MCR, ml·kg−1·min−1) were calculated from the equations of Steele modified for use with stable isotopes (80):
| (1) |
| (2) |
| (3) |
where F represents isotope infusion rate (mg·kg−1·min−1); V is the volume of distribution for lactate (180 ml/kg); C1 and C2 are concentrations (mg/l) at sampling times t1 and t2, respectively; and IE1 and IE2 are the excess isotopic enrichments of lactate at these sampling times.
Statistical analyses.
Descriptive statistics are expressed as means ± SE. Significance of differences in subject characteristics between UT and T subjects were analyzed using a Mann-Whitney U-test. Differences in responses of parameters in the transition from rest to exercise were analyzed using paired t-tests. Differences in parameters between the UT and the three T conditions within rest and exercise were analyzed using a one-way ANOVA. Comparisons of results within T subjects across conditions were performed by repeated-measures ANOVA. Post hoc analyses to identify where significant differences occurred across conditions were made by Fisher's least significant difference multiple comparison tests. When called for by stated hypotheses of results of previous investigations (e.g., exercise increases lactate kinetics over rest), one-tailed comparisons were made. Relationships between the different variables were studied by means of linear, polynomial, or exponential regression techniques. Statistical significance was set at α = 0.05 (i.e., P < 0.05).
RESULTS
Anthropometric and dietary data of subjects and their cardio-ventilatory responses to graded tests and isotope tracer trials are reported separately (21). Some previously presented results are repeated here for the convenience of the reader or when used for novel calculations.
Physiological responses of subjects to V̇o2max and LT determination tests.
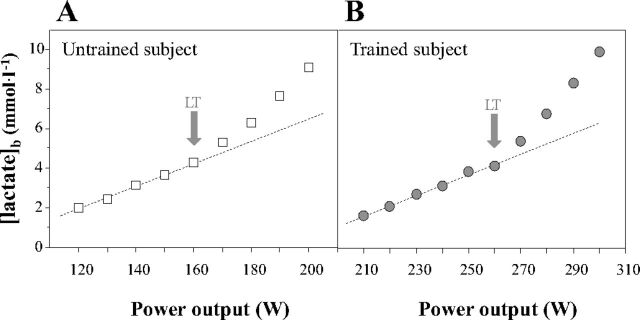
Compared with untrained subjects, cyclists had significantly higher V̇o2max (P < 0.05) and POmax (P < 0.05) (Table 1). Figure 1 shows typical blood lactate evolution curves during the incremental exercise used to determine the LT. LT-associated V̇o2 and percentage of V̇o2max (%V̇o2max) were significantly higher in trained cyclists compared with untrained subjects (P < 0.05).
Table 1.
Physiological responses to maximal oxygen consumption and lactate threshold determination tests for untrained and trained subjects
| Variable | Untrained | Trained |
|---|---|---|
| Maximal oxygen consumption test | ||
| fHmax (bpm) | 189 ± 5 | 189 ± 3 |
| VEmax (l/min) | 115 ± 8 | 144 ± 13† |
| V̇O2max (l/min) | 3.7 ± 0.1 | 5.0 ± 0.3* |
| POmax (W) | 248 ± 7 | 357 ± 12* |
| Lactate threshold test | ||
| fH at LT (bpm) | 158 ± 5 | 168 ± 3 |
| %fHmax at LT (%) | 83 ± 3 | 89 ± 1 |
| V̇O2 at LT (l/min) | 2.57 ± 0.09 | 3.85 ± 0.21* |
| %V̇O2max (%) | 69 ± 3 | 77 ± 2* |
| [lactate]b at LT (mmol/l) | 4.3 ± 0.6 | 3.6 ± 0.6 |
Values are means ± SE; n = 6 for untrained and trained groups. Differences between groups:
P < 0.05;
= 0.05 < P < 0.1. fHmax, maximal heart rate; bpm, beats per minute; VEmax, maximal pulmonary ventilation; V̇o2max, maximal oxygen consumption; POmax, V̇o2max-associated power output; fH at LT, heart rate at lactate threshold (LT); %fHmax at LT, percent of maximal heart rate at LT; V̇O2 at LT, oxygen consumption at LT; %V̇O2max, percent of maximal oxygen consumption; [lactate]b, blood lactate concentration.
Fig. 1.
Typical blood lactate evolution curves obtained in untrained (A) and trained (B) subjects during the lactate threshold (LT) determination test. Deviation from the dashed line is indicative of the acceleration in blood lactate accumulation (i.e., the LT). Of note, the dashed line should not be considered as demonstrating a linear relationship between blood lactate concentrations and power output.
Exercise power outputs and relative metabolic rates during isotope tracer trials.
Absolute POs during the LT trial in untrained subjects (161 ± 4 W) were lower than those during either LT (259 ± 10 W; P < 0.05) or LT-10% trials (234 ± 9 W; P < 0.05) in cyclists. Relative metabolic rates (%V̇o2max) were similar in the untrained LT trial compared with cyclists at LT-10% and LT-10%+LC (∼67% V̇o2max). However, relative exercise intensity was significantly higher during the LT trial in the cyclists (75% V̇o2max; P < 0.05).
Lactate, glucose, and hormone concentrations during isotope tracer trials.
Before tracer or LC infusions, resting [lactate]b values were lower (P < 0.05) in UT subjects (0.57 ± 0.09 mmol/l) than in T cyclists, no matter the trial (1.08 ± 0.09 mmol/l) (Fig. 2). Resting [lactate]b was similar among the trials in the T group (not significant; NS). To elevate [lactate]b to LT levels in T subjects during the LT-10%+LC trial, mean exogenous lactate infusion rates were 3.91 ± 0.26 mg·kg−1·min−1 and 3.16 ± 0.95 mg·kg−1·min−1 during rest and exercise, respectively. LC increased resting [lactate]b to 4.30 ± 0.39 mmol/l, which was significantly higher than all other conditions (P < 0.05). After 10 min of exercise, [lactate]b rose to reach 4.11 ± 1.01 mmol/l in UT subjects; and 3.71 ± 1.02, 2.57 ± 1.20, and 4.49 ± 1.36 mmol/l in T subjects during LT, LT-10%, and LT-10%+LC, respectively. Blood lactate concentrations remained stable during the entire period of exercise in all trials (Fig. 2A). During LT trials in UT and T subjects and during LT-10%+LC in T subjects, [lactate]b was not different (Fig. 2B). During LT-10% in T subjects, [lactate]b was significantly lower than the three other trials (P < 0.05).
Fig. 2.
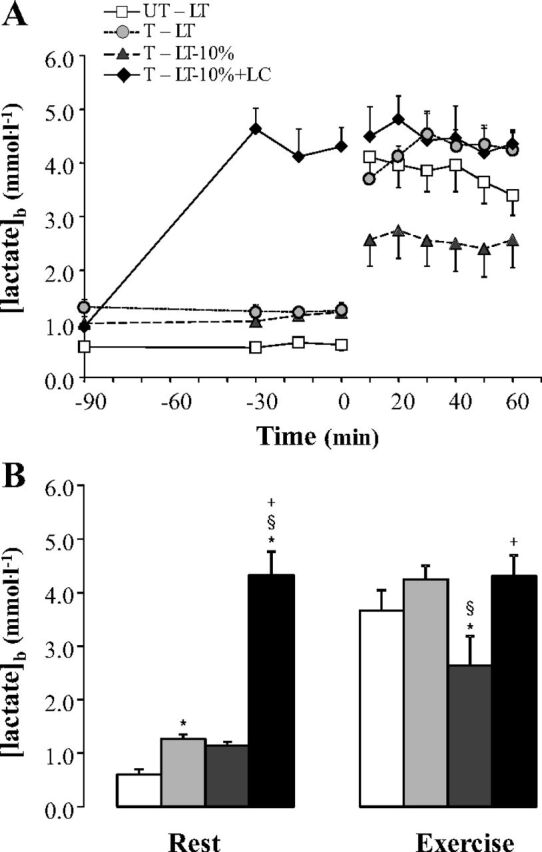
Time course (A) and mean values (B) of blood lactate concentrations at rest and during exercise at the lactate threshold (LT), 10% below the LT workload (LT-10%), and LT-10% with a lactate clamp (LT-10%+LC) in untrained (UT) and trained (T) subjects. Values are means ± SE; n = 6 for UT and T groups. White squares indicate LT trial in UT subjects (UT-LT). Dark grey circles, striated triangles, and black diamonds indicate LT, LT-10%, and LT-10%+LC trials in T subjects (T-LT, T-LT-10%, and T-LT-10%+LC, respectively). *Significantly different from UT-LT (P < 0.05). §Significantly different from T-LT (P < 0.05). +Significantly different from T-LT-10% (P < 0.05).
Resting blood [glucose] was elevated in UT subjects (5.4 ± 0.1 mmol/l) compared with T cyclists (5.0 ± 0.1 mmol/l) (21). During exercise at LT, blood [glucose] was significantly lower in the UT than T subjects (5.1 ± 0.2 vs. 5.9 ± 0.4 mmol/l; P < 0.05). Blood [glucose] during exercise in LT-10% and LT-10%+LC trials in T subjects was not different from that during LT in UT subjects (21).
At rest, plasma concentrations of epinephrine and norepinephrine ([epinephrine] and [norepinephrine]) were not different between UT and T subjects (21). During exercise, [epinephrine] and [norepinephrine] displayed striking changes, increasing by 3- to 8-fold and 6- to 13-fold, respectively, compared with their resting values (P < 0.05). Epinephrine levels were similar during the LT trials for UT and T subjects (NS), and were higher than they were during the LT-10% and LT-10%+LC trials (P < 0.05). Norepinephrine levels were lower during the LT trial in UT subjects than during the LT-10% trial in T subjects (P < 0.05); the latter was itself lower than [norepinephrine] during LT in T subjects (P < 0.05). The LC procedure dampened [epinephrine] and [norepinephrine] by 33% (NS) and 49% (P < 0.05), respectively, compared with the LT-10% values (21).
Lactate kinetics.
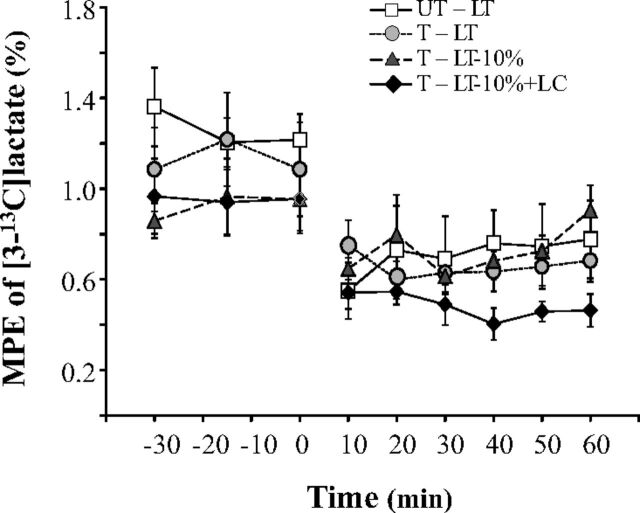
Arterialized blood isotopic enrichments achieved steady conditions during each experimental condition (Fig. 3), thus justifying use of steady-rate assumptions for computation of metabolic kinetics.
Fig. 3.
Mole percent excess (MPE, in %) of [3-13C]lactate (M+1 lactate). Values are means ± SE. See Fig. 2 for an explanation of symbols.
At rest, lactate rate of appearance (Ra, Fig. 4A) was significantly increased (P < 0.05) by exogenous lactate infusion (i.e., LT-10%+LC trial). Lactate Ra was not different between the three other resting trials. In all treatments, Ra increased from rest to exercise (P < 0.05). For the LT-10%+LC trial, Ra increased from rest to exercise by 4.4-fold, whereas for the three other trials, the increase from rest to exercise ranged between 7.3- and 9.3-fold. Lactate Ra at LT was 65% higher in T than in UT subjects (24.1 ± 2.7 vs. 14.6 ± 2.4 mg·kg−1·min−1; P < 0.05). Lactate Ra during LT-10% (18.2 ± 2.6 mg·kg−1·min−1) was not statistically different than Ra during LT in UT subjects (NS), but was 24% and 36% lower than Ra during LT (P = 0.06) and LT-10%+LC (P < 0.05) in T subjects. During the LT-10%+LC trial, Ra (28.4 ± 2.8 mg·kg−1·min−1) was higher than that during exercise at LT in UT subjects (P < 0.05) and not significantly different from Ra at LT in T subjects (NS).
Fig. 4.
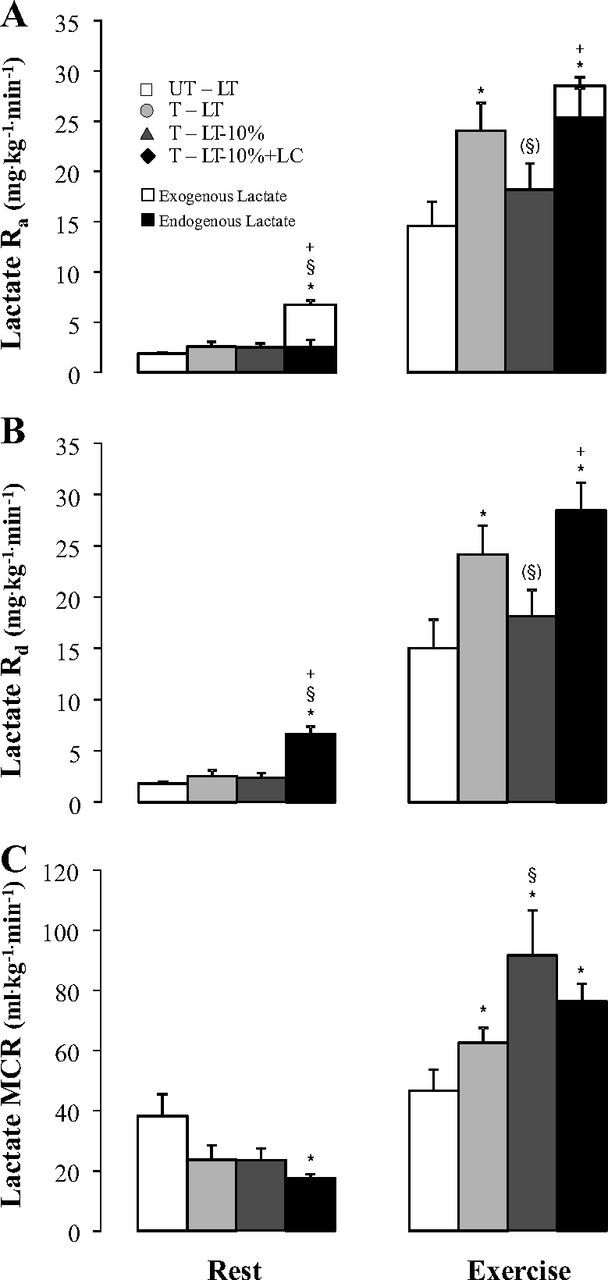
Lactate rates of appearance (Lactate Ra) (A) and disposal (Lactate Rd) (B), and metabolic clearance rate of lactate (Lactate MCR) (C) at rest and during 60 min of exercise at LT, LT-10%, and LT-10%+LC in untrained and trained subjects. Values are means ± SE. See Fig. 2 for an explanation of symbols. Parentheses mean a trend (P < 0.10).
At rest, lactate rates of disposal (Rd; Fig. 4B) were not significantly different among non-LC conditions (ranging from 1.8 ± 0.1 to 2.6 ± 0.6 mg·kg−1·min−1; NS). For the LT-10%+LC trial, lactate Rd was significantly higher than during the three other trials (6.6 ± 0.7 mg·kg−1·min−1; P < 0.05). Rd increased from rest to exercise in all treatments (P < 0.05). Rd during exercise at LT was 61% higher in T than in UT subjects (24.2 ± 2.8 vs. 15.0 ± 2.8 mg·kg−1·min−1; P < 0.05). Rd during LT-10% (18.1 ± 2.6 mg·kg−1·min−1) was not statistically different from Rd during LT in UT subjects (NS), but was 25% and 36% lower than lactate Rd during LT (P = 0.06) and LT-10%+LC (P < 0.05) in T subjects. During the LT-10%+LC trial, Rd (28.4 ± 2.8 mg·kg−1·min−1) was higher than that during exercise at LT in UT subjects (P < 0.05) and not significantly different from Rd at LT in T subjects (NS).
At rest, lactate metabolic clearance rate (MCR; Fig. 4C) was not different among non-LC conditions, but it was decreased in the LT-10%+LC condition compared with LT in UT subjects (P < 0.05). In all conditions for T subjects, MCR during exercise was higher than during rest (P < 0.05). In contrast, MCR in UT subjects did not change significantly from rest to exercise. During exercise at LT, MCR was 34% greater in T subjects compared with UT subjects (62.5 ± 5.0 vs. 46.5 ± 7.0, ml·kg−1·min−1, respectively; P < 0.05, one-tailed). In T subjects, MCR during the LT-10% trial (91.5 ± 14.9 ml·kg−1·min−1) was 46% greater than at LT, and 97% greater than in UT subjects who exercised at the same relative intensity (P < 0.05). When relative exercise intensity and [lactate]b were matched in UT and T groups (i.e., comparing UT-LT vs. T-LT-10%+LC), MCR was significantly higher by 64% due to endurance training (76.2 ± 5.9 ml·kg−1·min−1 in the LT-10%+LC trial).
Lactate Ra as functions of exercise intensity and catecholamine concentrations.
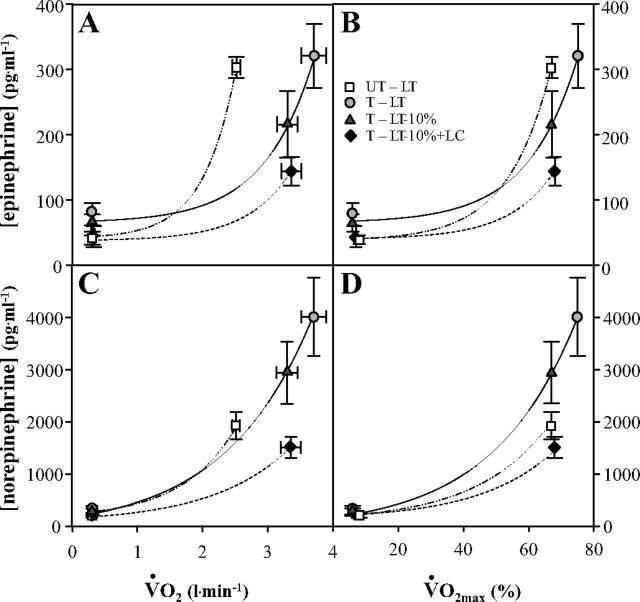
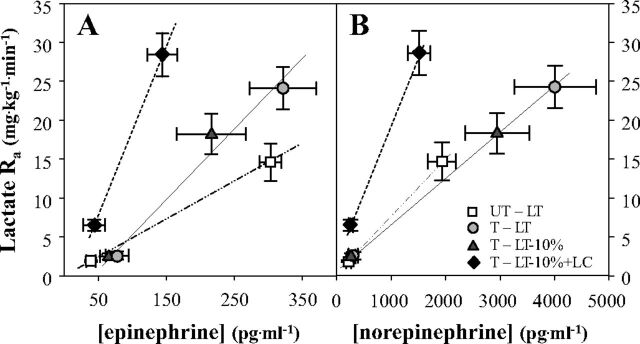
Lactate Ra rose exponentially as a function of metabolic rate during exercise, whether expressed as V̇o2 (l/min) (Fig. 5A), or as %V̇o2max (Fig. 5B). Similarly, plasma [epinephrine] and [norepinephrine] also rose exponentially as functions of metabolic rate whether expressed on absolute (V̇o2, l/min), or relative (%V̇o2max) bases (Fig. 6). Of note in Fig. 6 is that preexercise [epinephrine] is elevated in trained athletes, and that the catecholamine response to exercise is greater in athletes than untrained men. Lactate Ra rose linearly as functions of [epinephrine] and [norepinephrine] (Fig. 7, A and B, respectively). Of note in Fig. 7A, the slope of the regression between lactate Ra and [epinephrine] is greater in athletes than in untrained men. Also of note is that slopes of regression lines changed in response to the LC procedure because exogenous infusion both raised lactate Ra and suppressed sympathetic nervous system activity.
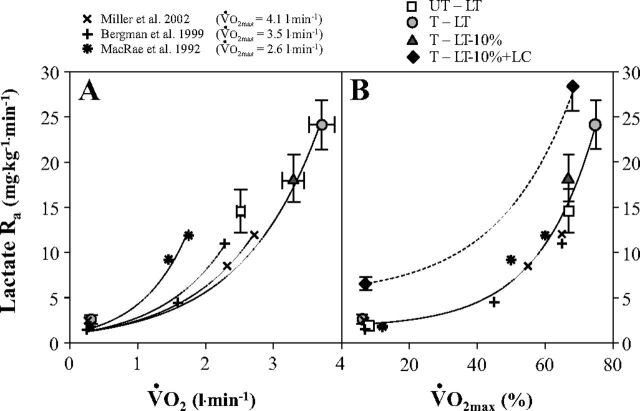
Fig. 5.
Lactate Ra as a function of absolute (V̇o2) (A) and relative (to V̇o2max) (B) metabolic rates elicited at rest and exercise in the present and previous studies involving subjects with different physical fitness status. V̇o2max values (means ± SE) of UT and T subjects are 3.7 ± 0.1 and 5.0 ± 0.3 liter/min, respectively.
Fig. 6.
Plasma epinephrine (A, B) and norepinephrine (C, D) concentrations as a function of absolute (V̇o2) and relative (to V̇o2max) metabolic rates elicited at rest and exercise. See Fig. 2 for an explanation of symbols.
Fig. 7.
Lactate Ra as a function of plasma [epinephrine] (A) and [norepinephrine] (B). See Fig. 2 for an explanation of symbols.
DISCUSSION
Here we report the first attempt to determine and interpret lactate kinetics in trained and untrained men exercising at the lactate threshold. Major findings are that lactate flux rates at the LT are much greater in trained cyclists than in untrained subjects, and a decline in MCR occurs as power outputs approach those that elicit the LT. As such, our conclusion is that although endurance training increases the capacities for lactate production, disposal, and clearance, regardless of training state, the LT represents the point at which clearance of lactate becomes limited.
Lactate kinetics at the lactate threshold in trained subjects: a function of exercise intensity.
No previous study specifically determined lactate kinetics during exercise at the LT. In our present study, lactate Ra, Rd, and MCR values obtained during exercise performed by trained cyclists at the LT were higher than the values previously reported in the literature during submaximal exercise performed by untrained, active, or short-term-trained men (7, 13, 46–48, 53, 58, 73), but were very close to those reported in highly trained cross-country skiers exercising approximately at the same relative exercise intensity (i.e., ∼75% of V̇o2max) (75). Because the LT very closely represents the highest absolute and relative workload for which lactate concentrations remain at steady state (35), the training status of the subjects and, consequently, the elevated absolute and relative (to V̇o2max) power outputs they achieved while exercising at the LT must be considered when attempting to understand the very high lactate flux values achieved by trained cyclists.
In the aggregate, present and past results (7, 47, 53, 72) indicate a direct, exponential, relationship between lactate Ra and metabolic rate (V̇o2) elicited by exercise (Fig. 5A). The apparent rightward shift in the lactate Ra vs. V̇o2 curve (Fig. 5A) is due to the greater exercise power outputs sustained by subjects in the present investigation. However, when normalized to relative exercise intensity, it is apparent from results of the several studies depicted that lactate Ra is closely related to %V̇o2max (Fig. 5B).
The relationships between lactate Ra and circulating catecholamines (Fig. 7) indicate a role for sympathetic nervous system (SNS) activation in determining lactate kinetics. Increased plasma catecholamine concentrations during exercise result from increased SNS activity and from spillover at terminal SNS nerve endings and SNS-stimulated secretions by the adrenal medullas (45, 67). High circulating levels of norepinephrine affect cardiodynamics and regional blood flow distribution, including splanchnic vasoconstriction, whereas elevated circulating epinephrine stimulates muscle glycogenolysis (24, 62, 79) and lactate production. Both vasoactive and metabolic effects of circulating catecholamines have the potential to affect lactate kinetics. The linear relationships between lactate Ra and circulating catecholamines (Fig. 7) have been previously observed (13).
Lactate clearance declines as the LT is approached.
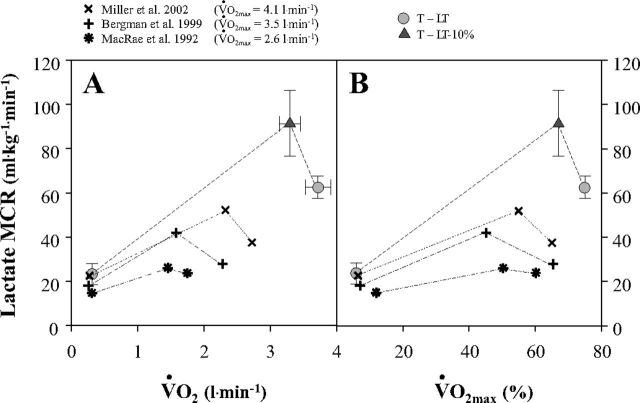
Initial studies of lactate kinetics using radiotracers in laboratory rats (19) showed that lactate MCR increases from rest to moderate intensity exercise, but then decreases from moderate to hard exercise. Similar results have been subsequently obtained on humans in previous investigations (7, 47, 48, 72, 73). Fig. 8 illustrates the relationship between lactate MCR and absolute and relative metabolic rates in the present and former studies involving healthy men of variable fitness levels. The top curve in Fig. 8 reflects the very high flux rates obtained in the present investigation on well-trained cyclists (V̇o2max = 5.0 l/min) compared with the lower curves obtained on healthy men of variable but lesser fitness levels (V̇o2max = 2.6, 3.5, and 4.1 l/min). In the present study, when PO was raised from LT-10% to LT workloads, trained cyclists experienced a 60% increase in [lactate]b, but only a 33% increase in Rd, resulting in a 30% decline in MCR. These results suggest that at workloads approaching the LT, lactate MCR declines rapidly.
Fig. 8.
Lactate metabolic clearance rate (MCR) as a function of absolute (V̇o2) (A) and relative (to V̇o2max) (B) metabolic rates elicited at rest and exercise in the present and previous studies involving subjects with different physical fitness status. See Fig. 2 for an explanation of symbols. V̇o2max values (means ± SE) for UT and T subjects are 3.7 ± 0.1 and 5.0 ± 0.3 l/min, respectively.
Oxidation (approximately 70–80%) and gluconeogenesis (approximately 20–30%) account for most whole-body lactate disposal during exercise (6, 7, 48, 54). Lactate uptake and subsequent utilization by consumer tissues (especially active oxidative muscle fibers, liver, kidney, heart, lungs, and brain) have been reported to be directly dependent on lactate delivery to these tissues as determined from blood lactate concentration and regional blood flow (1, 2, 5, 7, 15, 29, 40, 46–48, 55, 56, 61, 75, 76). Previous experiments underlined that when PO was increased, hepatic blood flow decreased (55, 56, 65, 66), counterbalancing the associated increase in blood lactate concentrations and curbing lactate uptake by the liver (56). The lack of increase in gluconeogenesis from lactate when exercise intensity increased despite a rise in blood lactate concentrations (21) may come from this leveling off in hepatic lactate uptake, likely attributable to reductions in splanchnic blood flow, and may contribute to the decline of lactate MCR as the LT is approached.
A limitation in lactate transport into consumer tissues, including working muscle, may also account for the decline in MCR when exercise intensity increases from moderate to high. Because approximately 60–80% of lactate Rd is accounted for by active-limb lactate uptake (7), of which approximately 90–95% is oxidized directly in muscle (15), a limitation of lactate transport into the active muscles during exercise is deleterious for lactate clearance. To be taken up and subsequently utilized by active muscles, lactate needs to first cross cell membranes. This transport occurs by facilitated diffusion via monocarboxylate transporters (i.e., the MCT family), which cotransport lactate and H+ in a 1:1 ratio (27, 28, 41, 63, 64). The transport of lactate is affected by sarcolemmal transporter content, but is driven by transmembrane lactate anion and H+ gradients, both of which change with exercise intensity. At low exercise intensities, muscle and blood lactate concentrations and their gradients (16, 44) are favorable for the entry of lactate into myocytes. However, as exercise intensity increases and more muscle and more type II muscle fibers are recruited, muscle and blood lactate concentrations, and their gradients, become less favorable for the influx into, but rather facilitate release (efflux) of lactate from active muscle, all effects detrimental for lactate clearance. Conversely, the LC, by increasing [lactate]b, alters the gradients, in effect pushing lactate into the tissues that are net lactate consumers, possibly accounting for the observed increase in Rd in the LT-10%+LC trial. Taken together, these results indicate that the transport of lactate across the muscle membrane might constitute a possible limiting factor for subsequent utilization. As well, tissues other than liver, kidneys, and active muscles such as the integument, might be involved in the decline in MCR when exercise intensity transitions from moderate to hard. Further studies would be necessary to specifically investigate the underlying mechanisms limiting MCR during exercises at LT.
Regardless of the mechanisms of lactate transport and uptake, and the limitations to those mechanisms, as shown in Figs. 4 and 8, the higher MCR during LT-10% compared with the LT trial strongly suggests that the LT is due to a limitation of MCR. Rephrased, any increase in power output above that which elicits the LT induces an increase in lactate Ra that cannot be accommodated by a corresponding rise in lactate Rd, thus causing a continuous rise in [lactate]b. Hence, results of the present study (Figs. 4 and 8) show that the optimal lactate MCR is below the LT. In that sense, it is interesting to note that in most endurance activities (e.g., cross-country skiing and rowing) athletes predominantly train at exercise intensities below the LT (22, 50).
Effects of endurance training on lactate kinetics for exercises performed at the LT and the same relative exercise intensities.
Exercise at the LT required higher absolute mechanical and metabolic power outputs to be performed by the trained subjects compared with their untrained counterparts. Accordingly, lactate Ra and Rd were higher by 65% in T men than in UT men even though exercise at the LT resulted in almost similar [lactate]b in both groups (Fig. 2). Consequently, MCR was higher in T than in UT men (Fig. 4), whereas T men exercised at higher relative workloads than UT men (75 vs. 67% of V̇o2max, respectively).
The comparison of data obtained during the LT trial in UT subjects and the LT-10% trial in T subjects (both at ∼67% V̇o2max) showed the effects of long-term endurance training on lactate kinetics during exercise of similar relative intensity. As has been already well described (7, 39, 52), [lactate]b was lower for a same relative exercise intensity in the trained subjects. Furthermore, Ra and Rd were not different, although MCR was 97% higher in the trained cyclists. Accordingly, our current results of a training-induced increase in lactate MCR agree with results of previous studies describing the effects of endurance training on lactate MCR (7, 47). An important distinction between results of this and previous studies lies in magnitude. Previously, 9 wk of endurance training increased lactate MCR by approximately 70–75% (7, 47); in contrast, the current results indicate that long-term endurance training can increase lactate MCR by 97%. This latter comparison underlines that long-term training may further improve MCR at a given relative workload.
Our experimental design allowed comparisons of UT and T individuals while exercising at specific blood lactate levels. Elevation of [lactate]b using the LC procedure allowed comparison of lactate kinetics between untrained and trained subjects at the same relative workload and similar [lactate]b. In other words, comparison of LT in UT subjects and LT-10%+LC in T subjects allowed determination of the effects of endurance training per se on lactate kinetics. As suspected, Rd was importantly improved in the trained cyclists subjected to the LC. Consequently, at the same relative workload and similar [lactate]b, Rd of the T cyclists was 89% higher than that of their UT counterparts (Fig. 4B). As a result, MCR remained significantly higher by 64% (P < 0.05) in T subjects than in UT subjects for the same relative (i.e., higher absolute) exercise intensity and similar [lactate]b.
In the aggregate, the present results (Figs. 4 and 8) extend the current knowledge and support the idea (7, 47) that endurance training improves lactate disposal and clearance, reinforcing the Lactate Shuttle Concept. Because active muscles account for the majority of lactate disposal during exercise (7, 15), improvements in lactate MCR after endurance training are likely due to adaptations involving greater lactate removal and oxidation in active muscles. Endurance training has been shown to alter lactate dehydrogenase (LDH) activity by shifting its distribution toward a higher proportion of its H-LDH isoenzyme (20, 52, 70), which is more favorable for lactate oxidation to pyruvate than the M-LDH isoenzyme. Endurance training has also been shown to increase muscle oxidative capacity and lactate oxidation via enhancements of mitochondrial mass and the expression of mitochondrial constituent proteins, including citrate synthase (CS) and cytochrome oxidase (COx) (4, 20, 30, 36, 37, 52, 57). Importantly, with regard to mitochondrial lactate oxidation, the first step is catalyzed by LDH, which has been found to be present in the mitochondria of skeletal muscle (3, 10, 14, 32, 43, 49, 68), and which is necessary for mitochondrial lactate oxidation (10, 14, 43). Furthermore, endurance training has been shown to enhance lactate transport capacity (59, 60) and muscle content of MCT1 (9, 20, 60), the isoform most abundant in the sarcolemmal and mitochondrial membranes of oxidative muscle (20, 32, 34). Of note, LDH, MCT1, and COx constitute a mitochondrial lactate oxidation complex (mLOC) (14, 31, 32, 34). Although the effect of training on the mLOC remains to be investigated, it is interesting to note that lactate, whose turnover is increased during exercise, acts as a hormone (i.e., a lactormone) that activates a cascade that upregulates MCT1 and COx gene and protein expression and mitochondrial biogenesis (31, 33).
Unsolved questions and limitations.
In the present study, UT subjects displayed lower resting [lactate]b than T cyclists (either at LT or LT-10%), for reasons that are not clearly known. Previous studies have not reported any differences in resting [lactate]b between (short- or long-term) trained and untrained men (7, 8, 17). However, the values we reported for our T and UT subjects were within the wide range of the literature for resting [lactate]b (approximately 0.3–1.7 mmol/l) [e.g., (7, 17, 25, 53)]. One possible explanation would be that the difference in resting [lactate]b was due to an anticipatory autonomic response in the trained athletes prior to exercise. In a previous report (21) we noted that preexercise heart rates were similar in the untrained men and athletes we studied. Knowing the training-induced bradycardia at rest (71), our suspicion of an anticipatory sympathetic response in athletes is justified on the basis of our observations of elevated epinephrine concentrations in trained subjects preexercise, and exaggerated epinephrine responses to exercise in trained athletes than in untrained men (Fig. 6A). Elevated catecholamine levels in trained men during exercise have been observed by others (42) and us (26).
On the basis of our experience, we expected that during the LC trial, endogenous lactate production for LT-10% and exogenous infusion would have been additive during exercise (53). Contrary to this expectation, endogenous lactate Ra was substantially augmented during LC (Fig. 4A). We also noted that glucagon concentrations, glucose Ra, and RER also responded differently to LC during exercise in the present study on athletes (21) compared with responses observed previously on nonathletes (53). Further studies are required to investigate specifically why LC induces different metabolic responses in trained and untrained subjects.
And finally, because of the cross-sectional design of the present study, we cannot exclude the effects of genetic factors to explain the differences in lactate flux rate, exercise power output, and response to the LC procedure of athletes compared with results obtained from nonathletes.
Summary and conclusions.
We used combinations of exercise intensity and a lactate clamp technique involving exogenous lactate infusion to interrogate the meaning of the LT in healthy young men and competitive male cyclists. Exercise and exogenous infusion resulted in significant increases in lactate kinetics. In trained cyclists exercising at the LT, we observed the greatest values of lactate flux ever reported. In both healthy controls and trained cyclists, lactate clearance rose during the transition from rest to moderate intensity exercise, but MCR fell when the exercise task came close to eliciting the LT, suggesting that LT would be determined by a limitation in MCR. In contrast to the comparison in trained men exercising at LT and a power output lesser by 10%, exogenous lactate infusion resulted in an increase in lactate disposal. That result was interpreted to mean that lactate MCR at the LT was limited by endogenous, intramuscular lactate production. We conclude that although endurance training increases the capacities for lactate production, and disposal and clearance for higher absolute and relative workload, the LT represents the point at which clearance of endogenous lactate becomes limited.
GRANTS
Support for this study was provided by the France-Berkeley Fund and by a gift from CytoSport Inc., Benicia, CA. L.A.M. was a Fulbright fellow.
DISCLOSURES
G.A.B. has a financial interest in CytoSport, Inc.
AUTHOR CONTRIBUTIONS
Author contributions: L.A.M. and G.A.B. conception and design of research; L.A.M., C.-A.W.E., J.A.F., and T.J.C. performed experiments; L.A.M., C.-A.W.E., J.A.F., M.A.H., and G.A.B. analyzed data; L.A.M., C.-A.W.E., M.A.H., and G.A.B. interpreted results of experiments; L.A.M. prepared figures; L.A.M. and G.A.B. drafted manuscript; L.A.M., C.-A.W.E., M.A.H., and G.A.B. edited and revised manuscript; L.A.M., C.-A.W.E., J.A.F., M.A.H., T.J.C., and G.A.B. approved final version of manuscript.
ACKNOWLEDGMENTS
We thank the study participants for their time and efforts. We thank Nick Hatamiya, Britt Marett, Mark Matusak, Nitish Nag, Yeon Park, and Gabriella Rios-Sotelo for their technical assistance.
REFERENCES
- 1. Ahlborg G , Hagenfeldt L , Wahren J. Substrate utilization by the inactive leg during one-leg or arm exercise. J Appl Physiol 39: 718–723, 1975. [DOI] [PubMed] [Google Scholar]
- 2. Ahlborg G , Wahren J , Felig P. Splanchnic and peripheral glucose and lactate metabolism during and after prolonged arm exercise. J Clin Invest 77: 690–699, 1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Baba N , Sharma HM. Histochemistry of lactic dehydrogenase in heart and pectoralis muscles of rat. J Cell Biol 51: 621–635, 1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Baldwin KM , Hooker AM , Herrick RE. Lactate oxidative capacity in different types of muscle. Biochem Biophys Res Commun 83: 151–157, 1978. [DOI] [PubMed] [Google Scholar]
- 5. Bangsbo J , Aagaard T , Olsen M , Kiens B , Turcotte LP , Richter EA. Lactate and H+ uptake in inactive muscles during intense exercise in man. J Physiol 488 (Pt 1): 219–229, 1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Bergman BC , Horning MA , Casazza GA , Wolfel EE , Butterfield GE , Brooks GA. Endurance training increases gluconeogenesis during rest and exercise in men. Am J Physiol Endocrinol Metab 278: E244–E251, 2000. [DOI] [PubMed] [Google Scholar]
- 7. Bergman BC , Wolfel EE , Butterfield GE , Lopaschuk GD , Casazza GA , Horning MA , Brooks GA. Active muscle and whole body lactate kinetics after endurance training in men. J Appl Physiol 87: 1684–1696, 1999. [DOI] [PubMed] [Google Scholar]
- 8. Bloom SR , Johnson RH , Park DM , Rennie MJ , Sulaiman WR. Differences in the metabolic and hormonal response to exercise between racing cyclists and untrained individuals. J Physiol 258: 1–18, 1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Bonen A , McCullagh JA , Putman CT , Hultman E , Jones NL , Heigenhauser GJ. Short-term training increase human muscle MCT1 and femoral venous lactate in relation to muscle lactate. Am J Physiol Endocrinol Metab 274: E102–E107, 1998. [DOI] [PubMed] [Google Scholar]
- 10. Brandt RB , Laux JE , Spainhour SE , Kline ES. Lactate dehydrogenase in rat mitochondria. Arch Biochem Biophys 259: 412–422, 1987. [DOI] [PubMed] [Google Scholar]
- 11. Brooks GA. Anaerobic threshold: review of the concept and directions for future research. Med Sci Sports Exerc 17: 22–34, 1985. [PubMed] [Google Scholar]
- 12. Brooks GA. The lactate shuttle during exercise and recovery. Med Sci Sports Exerc 18: 360–368, 1986. [DOI] [PubMed] [Google Scholar]
- 13. Brooks GA , Butterfield GE , Wolfe RR , Groves BM , Mazzeo RS , Sutton JR , Wolfel EE , Reeves JT. Decreased reliance on lactate during exercise after acclimatization to 4,300 m. J Appl Physiol 71: 333–341, 1991. [DOI] [PubMed] [Google Scholar]
- 14. Brooks GA , Dubouchaud H , Brown M , Sicurello JP , Butz CE. Role of mitochondrial lactate dehydrogenase and lactate oxidation in the intracellular lactate shuttle. Proc Natl Acad Sci USA 96: 1129–1134, 1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Brooks GA , Wolfel EE , Groves BM , Bender PR , Butterfield GE , Cymerman A , Mazzeo RS , Sutton JR , Wolfe RR , Reeves JT. Muscle accounts for glucose disposal but not blood lactate appearance during exercise after acclimatization to 4,300 m. J Appl Physiol 72: 2435–2445, 1992. [DOI] [PubMed] [Google Scholar]
- 16. Chwalbinska-Moneta J , Robergs RA , Costill DL , Fink WJ. Threshold for muscle lactate accumulation during progressive exercise. J Appl Physiol 66: 2710–2716, 1989. [DOI] [PubMed] [Google Scholar]
- 17. Cobb LA , Johnson WP. Hemodynamic relationships of anaerobic metabolism and plasma free fatty acids during prolonged, strenuous exercise in trained and untrained subjects. J Clin Invest 42: 800–810, 1963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Coyle EF , Coggan AR , Hopper MK , Walters TJ. Determinants of endurance in well-trained cyclists. J Appl Physiol 64: 2622–2630, 1988. [DOI] [PubMed] [Google Scholar]
- 19. Donovan CM , Brooks GA. Endurance training affects lactate clearance, not lactate production. Am J Physiol Endocrinol Metab 244: E83–E92, 1983. [DOI] [PubMed] [Google Scholar]
- 20. Dubouchaud H , Butterfield GE , Wolfel EE , Bergman BC , Brooks GA. Endurance training, expression, and physiology of LDH, MCT1, and MCT4 in human skeletal muscle. Am J Physiol Endocrinol Metab 278: E571–E579, 2000. [DOI] [PubMed] [Google Scholar]
- 21. Emhoff CW , Messonnier LA , Horning MA , Fattor JA , Carlson TJ , Brooks GA. Gluconeogenesis and hepatic glycogenolysis during exercise at the lactate threshold. J Appl Physiol 114: 297–306, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Evertsen F , Medbø JI , Bonen A. Effect of training on muscle lactate transporters and lactate threshold of cross-country skiers. Acta Physiol Scand 173: 195–205, 2001. [DOI] [PubMed] [Google Scholar]
- 23. Fattor JA , Miller BF , Jacobs KA , Brooks GA. Catecholamine response is attenuated during moderate-intensity exercise in response to the “lactate clamp”. Am J Physiol Endocrinol Metab 288: E143–E147, 2005. [DOI] [PubMed] [Google Scholar]
- 24. Febbraio MA , Lambert DL , Starkie RL , Proietto J , Hargreaves M. Effect of epinephrine on muscle glycolysis during exercise in trained men. J Appl Physiol 84: 465–470, 1998. [DOI] [PubMed] [Google Scholar]
- 25. Freund H , Oyono-Enguelle S , Heitz A , Marbach J , Ott C , Zouloumian P , Lampert E. Work rate-dependent lactate kinetics after exercise in humans. J Appl Physiol 61: 932–939, 1986. [DOI] [PubMed] [Google Scholar]
- 26. Friedlander AL , Casazza GA , Horning MA , Huie MJ , Brooks GA. Training-induced alterations of glucose flux in men. J Appl Physiol 82: 1360–1369, 1997. [DOI] [PubMed] [Google Scholar]
- 27. Garcia CK , Goldstein JL , Pathak RK , Anderson RG , Brown MS. Molecular characterization of a membrane transporter for lactate, pyruvate, and other monocarboxylates: implications for the Cori cycle. Cell 76: 865–873, 1994. [DOI] [PubMed] [Google Scholar]
- 28. Garcia CK , Li X , Luna J , Francke U. cDNA cloning of the human monocarboxylate transporter 1 and chromosomal localization of the SLC16A1 locus to 1p13.2-p12. Genomics 23: 500–503, 1994. [DOI] [PubMed] [Google Scholar]
- 29. Gertz EW , Wisneski JA , Neese R , Houser A , Korte R , Bristow JD. Myocardial lactate extraction: multi-determined metabolic function. Circulation 61: 256–261, 1980. [DOI] [PubMed] [Google Scholar]
- 30. Hambrecht R , Fiehn E , Yu J , Niebauer J , Weigl C , Hilbrich L , Adams V , Riede U , Schuler G. Effects of endurance training on mitochondrial ultrastructure and fiber type distribution in skeletal muscle of patients with stable chronic heart failure. J Am Coll Cardiol 29: 1067–1073, 1997. [DOI] [PubMed] [Google Scholar]
- 31. Hashimoto T , Brooks GA. Mitochondrial lactate oxidation complex and an adaptive role for lactate production. Med Sci Sports Exerc 40: 486–494, 2008. [DOI] [PubMed] [Google Scholar]
- 32. Hashimoto T , Hussien R , Brooks GA. Colocalization of MCT1, CD147, and LDH in mitochondrial inner membrane of L6 muscle cells: evidence of a mitochondrial lactate oxidation complex. Am J Physiol Endocrinol Metab 290: E1237–E1244, 2006. [DOI] [PubMed] [Google Scholar]
- 33. Hashimoto T , Hussien R , Oommen S , Gohil K , Brooks GA. Lactate sensitive transcription factor network in L6 cells: activation of MCT1 and mitochondrial biogenesis. FASEB J 21: 2602–2612, 2007. [DOI] [PubMed] [Google Scholar]
- 34. Hashimoto T , Masuda S , Taguchi S , Brooks GA. Immunohistochemical analysis of MCT1, MCT2 and MCT4 expression in rat plantaris muscle. J Physiol 567: 121–129, 2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Heck H , Mader A , Hess G , Mucke S , Muller R , Hollmann W. Justification of the 4-mmol/l lactate threshold. Int J Sports Med 6: 117–130, 1985. [DOI] [PubMed] [Google Scholar]
- 36. Hood MS , Little JP , Tarnopolsky MA , Myslik F , Gibala MJ. Low-volume interval training improves muscle oxidative capacity in sedentary adults. Med Sci Sports Exerc 43: 1849–1856, 2011. [DOI] [PubMed] [Google Scholar]
- 37. Hoppeler H , Howald H , Conely K , Lindstedt SL , Claassen H , Vock P , Weibel ER. Endurance training in humans: aerobic capacity and structure of skeletal muscle. J Appl Physiol 59: 320–327, 1985. [DOI] [PubMed] [Google Scholar]
- 38. Horning MA , Friedlander AL , Casazza GA , Huie MJ , Brooks GA. Arterial and ‘arterialized’ sampling sites do not change isotopic enrichment using [6,6-d-glucose] and [1,1,2,3,3,-d-glycerol]. FASEB J 12: A864, 1998. [Google Scholar]
- 39. Hurley BF , Hagberg JM , Allen WK , Seals DR , Young JC , Cuddihee RW , Holloszy JO. Effect of training on blood lactate levels during submaximal exercise. J Appl Physiol 56: 1260–1264, 1984. [DOI] [PubMed] [Google Scholar]
- 40. Johnson ML , Emhoff CW , Horning MA , Brooks GA. Transpulmonary lactate shuttle. Am J Physiol Regul Integr Comp Physiol 302: R143–R149, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Juel C. Lactate-proton cotransport in skeletal muscle. Physiol Rev 77: 321–358, 1997. [DOI] [PubMed] [Google Scholar]
- 42. Kjær M , Farrell PA , Christensen NJ , Galbo H. Increased epinephrine response and inaccurate glucoregulation in exercising athletes. J Appl Physiol 61: 1693–1700, 1986. [DOI] [PubMed] [Google Scholar]
- 43. Kline ES , Brandt RB , Laux JE , Spainhour SE , Higgins ES , Rogers KS , Tinsley SB , Waters MG. Localization of L-lactate dehydrogenase in mitochondria. Arch Biochem Biophys 246: 673–680, 1986. [DOI] [PubMed] [Google Scholar]
- 44. Knuttgen HG , Saltin B. Muscle metabolites and oxygen uptake in short-term submaximal exercise in man. J Appl Physiol 32: 690–694, 1972. [DOI] [PubMed] [Google Scholar]
- 45. Leuenberger U , Sinoway L , Gubin S , Gaul L , Davis D , Zelis R. Effects of exercise intensity and duration on norepinephrine spillover and clearance in humans. J Appl Physiol 75: 668–674, 1993. [DOI] [PubMed] [Google Scholar]
- 46. MacRae HH , Noakes TD , Dennis SC. Effects of endurance training on lactate removal by oxidation and gluconeogenesis during exercise. Pflugers Arch 430: 964–970, 1995. [DOI] [PubMed] [Google Scholar]
- 47. MacRae HS , Dennis SC , Bosch AN , Noakes TD. Effects of training on lactate production and removal during progressive exercise in humans. J Appl Physiol 72: 1649–1656, 1992. [DOI] [PubMed] [Google Scholar]
- 48. Mazzeo RS , Brooks GA , Schoeller DA , Budinger TF. Disposal of blood [l-13C]lactate in humans during rest and exercise. J Appl Physiol 60: 232–241, 1986. [DOI] [PubMed] [Google Scholar]
- 49. McClelland GB , Brooks GA. Changes in MCT 1, MCT 4, and LDH expression are tissue specific in rats after long-term hypobaric hypoxia. J Appl Physiol 92: 1573–1584, 2002. [DOI] [PubMed] [Google Scholar]
- 50. Messonnier L , Aranda-Berthouze SE , Bourdin M , Bredel Y , Lacour JR. Rowing performance and estimated training load. Int J Sports Med 26: 376–382, 2005. [DOI] [PubMed] [Google Scholar]
- 51. Messonnier L , Freund H , Bourdin M , Belli A , Lacour JR. Lactate exchange and removal abilities in rowing performance. Med Sci Sports Exerc 29: 396–401, 1997. [DOI] [PubMed] [Google Scholar]
- 52. Messonnier L , Freund H , Feasson L , Prieur F , Castells J , Denis C , Linossier MT , Geyssant A , Lacour JR. Blood lactate exchange and removal abilities after relative high-intensity exercise: effects of training in normoxia and hypoxia. Eur J Appl Physiol 84: 403–412, 2001. [DOI] [PubMed] [Google Scholar]
- 53. Miller BF , Fattor JA , Jacobs KA , Horning MA , Navazio F , Lindinger MI , Brooks GA. Lactate and glucose interactions during rest and exercise in men: effect of exogenous lactate infusion. J Physiol 544: 963–975, 2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Miller BF , Fattor JA , Jacobs KA , Horning MA , Suh SH , Navazio F , Brooks GA. Metabolic and cardiorespiratory responses to “the lactate clamp”. Am J Physiol Endocrinol Metab 283: E889–E898, 2002. [DOI] [PubMed] [Google Scholar]
- 55. Nielsen HB , Clemmesen JO , Skak C , Ott P , Secher NH. Attenuated hepatosplanchnic uptake of lactate during intense exercise in humans. J Appl Physiol 92: 1677–1683, 2002. [DOI] [PubMed] [Google Scholar]
- 56. Nielsen HB , Febbraio MA , Ott P , Krustrup P , Secher NH. Hepatic lactate uptake versus leg lactate output during exercise in humans. J Appl Physiol 103: 1227–1233, 2007. [DOI] [PubMed] [Google Scholar]
- 57. Pesta D , Hoppel F , Macek C , Messner H , Faulhaber M , Kobel C , Parson W , Burtscher M , Schocke M , Gnaiger E. Similar qualitative and quantitative changes of mitochondrial respiration following strength and endurance training in normoxia and hypoxia in sedentary humans. Am J Physiol Regul Integr Comp Physiol 301: R1078–R1087, 2011. [DOI] [PubMed] [Google Scholar]
- 58. Phillips SM , Green HJ , Tarnopolsky MA , Grant SM. Increased clearance of lactate after short-term training in men. J Appl Physiol 79: 1862–1869, 1995. [DOI] [PubMed] [Google Scholar]
- 59. Pilegaard H , Bangsbo J , Richter EA , Juel C. Lactate transport studied in sarcolemmal giant vesicles from human muscle biopsies: relation to training status. J Appl Physiol 77: 1858–1862, 1994. [DOI] [PubMed] [Google Scholar]
- 60. Pilegaard H , Domino K , Noland T , Juel C , Hellsten Y , Halestrap AP , Bangsbo J. Effect of high-intensity exercise training on lactate/H+ transport capacity in human skeletal muscle. Am J Physiol Endocrinol Metab 276: E255–E261, 1999. [DOI] [PubMed] [Google Scholar]
- 61. Quistorff B , Secher NH , Van Lieshout JJ. Lactate fuels the human brain during exercise. FASEB J 22: 3443–3449, 2008. [DOI] [PubMed] [Google Scholar]
- 62. Richter EA , Ruderman NB , Gavras H , Belur ER , Galbo H. Muscle glycogenolysis during exercise: dual control by epinephrine and contractions. Am J Physiol Endocrinol Metab 242: E25–E32, 1982. [DOI] [PubMed] [Google Scholar]
- 63. Roth DA , Brooks GA. Lactate and pyruvate transport is dominated by a pH gradient-sensitive carrier in rat skeletal muscle sarcolemmal vesicles. Arch Biochem Biophys 279: 386–394, 1990. [DOI] [PubMed] [Google Scholar]
- 64. Roth DA , Brooks GA. Lactate transport is mediated by a membrane-bound carrier in rat skeletal muscle sarcolemmal vesicles. Arch Biochem Biophys 279: 377–385, 1990. [DOI] [PubMed] [Google Scholar]
- 65. Rowell LB , Blackmon JR , Bruce RA. Indocyanine green clearance and estimated hepatic blood flow during mild to maximal exercise in upright man. J Clin Invest 43: 1677–1690, 1964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Rowell LB , Blackmon JR , Martin RH , Mazzarella JA , Bruce RA. Hepatic clearance of indocyanine green in man under thermal and exercise stresses. J Appl Physiol 20: 384–394, 1965. [DOI] [PubMed] [Google Scholar]
- 67. Shah SD , Tse TF , Clutter WE , Cryer PE. The human sympathochromaffin system. Am J Physiol Endocrinol Metab 247: E380–E384, 1984. [DOI] [PubMed] [Google Scholar]
- 68. Sjodin B. Lactate dehydrogenase in human skeletal muscle. Acta Physiol Scand 436: 1–32, 1976. [PubMed] [Google Scholar]
- 69. Sjodin B , Jacobs I. Onset of blood lactate accumulation and marathon running performance. Int J Sports Med 2: 23–26, 1981. [DOI] [PubMed] [Google Scholar]
- 70. Sjodin B , Thorstensson A , Frith K , Karlsson J. Effect of physical training on LDH activity and LDH isozyme pattern in human skeletal muscle. Acta Physiol Scand 97: 150–157, 1976. [DOI] [PubMed] [Google Scholar]
- 71. Smith ML , Hudson DL , Graitzer HM , Raven PB. Exercise training bradycardia: the role of autonomic balance. Med Sci Sports Exerc 21: 40–44, 1989. [DOI] [PubMed] [Google Scholar]
- 72. Stanley WC , Gertz EW , Wisneski JA , Morris DL , Neese RA , Brooks GA. Systemic lactate kinetics during graded exercise in man. Am J Physiol Endocrinol Metab 249: E595–E602, 1985. [DOI] [PubMed] [Google Scholar]
- 73. Stanley WC , Wisneski JA , Gertz EW , Neese RA , Brooks GA. Glucose and lactate interrelations during moderate-intensity exercise in humans. Metabolism 37: 850–858, 1988. [DOI] [PubMed] [Google Scholar]
- 74. Tanaka K , Matsuura Y. Marathon performance, anaerobic threshold, and onset of blood lactate accumulation. J Appl Physiol 57: 640–643, 1984. [DOI] [PubMed] [Google Scholar]
- 75. Van Hall G , Jensen-Urstad M , Rosdahl H , Holmberg HC , Saltin B , Calbet JA. Leg and arm lactate and substrate kinetics during exercise. Am J Physiol Endocrinol Metab 284: E193–E205, 2003. [DOI] [PubMed] [Google Scholar]
- 76. van Hall G , Stromstad M , Rasmussen P , Jans O , Zaar M , Gam C , Quistorff B , Secher NH , Nielsen HB. Blood lactate is an important energy source for the human brain. J Cereb Blood Flow Metab 29: 1121–1129, 2009. [DOI] [PubMed] [Google Scholar]
- 77. Wasserman K , Koike A. Is the anaerobic threshold truly anaerobic? Chest 101: 211S–218S, 1992. [DOI] [PubMed] [Google Scholar]
- 78. Wasserman K , McIlroy M. Detecting the threshold of anaerobic metabolism in cardiac patients during exercise. Am J Cardiol 14: 844–852, 1964. [DOI] [PubMed] [Google Scholar]
- 79. Watt MJ , Howlett KF , Febbraio MA , Spriet LL , Hargreaves M. Adrenaline increases skeletal muscle glycogenolysis, pyruvate dehydrogenase activation and carbohydrate oxidation during moderate exercise in humans. J Physiol 534: 269–278, 2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80. Wolfe RR. Radioactive and Stable Isotope Tracers in Biomedicine: Principles and Practice of Kinetic Analysis. New York: Wiley-Liss, 1992. [Google Scholar]