Abstract
Mexico is located in a transition zone between the Nearctic and Neotropical biogeographical regions and contains a rich and unique biodiversity. A total of 496 Bacillus thuringiensis strains were isolated from 503 soil samples collected from the five macroregions of the country. The characterization of the strain collection provided useful information on the ecological patterns of distribution of B. thuringiensis and opportunities for the selection of strains to develop novel bioinsecticidal products. The analysis of the strains was based on multiplex PCR with novel general and specific primers that could detect the cry1, cry3, cry5, cry7, cry8, cry9, cry11, cry12, cry13, cry14, cry21, and cyt genes. The proteins belonging to the Cry1 and Cry9 groups are toxic for lepidopteran insects. The Cry3, Cry7, and Cry8 proteins are active against coleopteran insects. The Cry5, Cry12, Cry13, and Cry14 proteins are nematocidal. The Cry11, Cry21, and Cyt proteins are toxic for dipteran insects. Six pairs of general primers are used in this method. Strains for which unique PCR product profiles were obtained with the general primers were further characterized by additional PCRs with specific primers. Strains containing cry1 genes were the most abundant in our collection (49.5%). Thirty-three different cry1-type profiles were identified. B. thuringiensis strains harboring cry3 genes represented 21.5% of the strains, and 7.9% of the strains contained cry11 and cyt genes. cry7, cry8, and cry9 genes were found in 0.6, 2.4, and 2.6% of the strains, respectively. No strains carrying cry5, cry12, cry13, cry14, or cry21 genes were found. Finally, 14% of the strains did not give any PCR product and did not react with any polyclonal antisera. Our results indicate the presence of strains that may harbor potentially novel Cry proteins as well as strains with combinations of less frequently observed cry genes.
Chemical insecticides may be toxic and may cause environmental problems when used improperly. This problem is increasing due to the selection of insect resistance to some pesticides. Consequently, interest has developed in the use of alternative strategies for insect control, such as Bacillus thuringiensis toxins (31).
The entomopathogenic activity of this bacterium is principally due to the presence of proteinaceous inclusions that can be distinguished as distinctively shaped crystals under phase-contrast microscopy. These inclusions are comprised of proteins known as insecticidal crystal proteins (Cry proteins) or δ endotoxins (20). Cry proteins have been used as biopesticide sprays on a significant scale for more than 30 years, and their safety has been demonstrated (27).
Currently, 45 different serotypes of B. thuringiensis have been classified as 58 serovars (23). Many Cry protein genes have been cloned, sequenced, and named cry and cyt genes. To date, over 100 cry gene sequences have been determined and classified in 22 groups and different subgroups with regard to their amino acid similarity (12). The proteins toxic for lepidopteran insects belong to the Cry1, Cry9, and Cry2 groups; toxins active against coleopteran insects are the Cry3, Cry7, and Cry8 proteins as well as the Cry1B and Cry1I proteins, which have dual activity. The Cry5, Cry12, Cry13, and Cry14 proteins are nematocidal, and the Cry2, Cry4, Cry10, Cry11, Cry16, Cry17, Cry19, and Cyt proteins are toxic for dipteran insects. The revised Cry toxin nomenclature is available on the World Wide Web at http://epunix.biols.susx.ac.uk/Home/Neil_Crickmore/Bt/index .html.
Intensive screening programs have identified B. thuringiensis strains from soil samples, plant surfaces, dead insects, and stored grains. The isolated strains show a wide range of specificity for different insect orders (Lepidoptera, Diptera, Coleoptera, Hymenoptera, Homoptera, and Mallophaga) and Acari (16). Furthermore, B. thuringiensis strains able to control other invertebrates, such as Nemathelminthes, Platyhelminthes, and Sarcomastigorphora, have been described (16).
Estruch et al. (14) have described a novel class of lepidopteran-specific toxic protein (Vip3A) produced by different B. thuringiensis strains. Vip3 proteins (88.5 kDa) have no homology with the Cry and Cyt toxins and are expressed and secreted during vegetative growth and sporulation.
Notwithstanding the variability of Cry proteins described up to now, it is still necessary to search for more toxins, since a significant number of pests are not controlled with the available Cry proteins. It is also important to provide alternatives for coping with the problem of insect resistance, especially with regard to the expression of B. thuringiensis genes encoding insecticidal proteins in transgenic plants (29).
The characterization of B. thuringiensis strain collections may help in the understanding of the role of B. thuringiensis in the environment and the distribution of cry genes. Several B. thuringiensis strain collections have been described (3, 4, 9, 11, 24, 25). The strains were from different countries, mainly Europe, Asia, Africa, New Zealand, and the United States. None of these collections have included samples from Latin America, with the exception of the collection reported by Bernhard et al. (4); only 5% of their samples came from South America. Mexico is located in a transition zone between the Nearctic and Neotropical biogeographical regions and contains a rich and unique biodiversity (19). Since it has been proposed that insect species and B. thuringiensis strains have coevolved (15), a high diversity of B. thuringiensis strains was expected for Mexican soils.
The information about the distribution of cry genes is limited. This type of analysis has been performed only for the collections from Israel (3) and Taiwan (9). The characterizations done for most of the collections described above were based on bioassays against different insect larvae without identification of the cry genes present in the B. thuringiensis strains. In the last few years, some PCR-based methodologies have been proposed to identify different cry genes in B. thuringiensis strains (3, 5–8, 18, 22). However, the cry gene list is increasing, and novel PCR primers are needed in order to identify some of the recently described cry genes.
In this paper, we present the characterization of a Mexican B. thuringiensis strain collection. The strategy used was based on multiplex PCR analysis with novel general and specific primers that could detect the cry1, cry3, cry5, cry7, cry8, cry9, cry11, cry12, cry13, cry14, cry21, and cyt genes. The PCR method could be highly efficient for the identification of the cry genes present in B. thuringiensis strains; however, is important to mention that this method cannot distinguish between expressed and silent genes. We found B. thuringiensis strains containing some of the known cry genes. In addition, we found B. thuringiensis strains harboring potentially novel Cry proteins, as well as strains with diverse profiles of cry genes not found in other regions. This information provided important data for the understanding of the ecology of B. thuringiensis strains.
(Preliminary findings of this study were communicated at the 29th Annual Meeting of the Society for Invertebrate Pathology, Cordoba, Spain, 1996, and the Second Pacific Rim Conference on Biotechnology of Bacillus thuringiensis and its Impact to the Environment, Chiang Mai, Thailand, 1996.)
MATERIALS AND METHODS
Bacterial strains.
Known B. thuringiensis strains were provided by the Bacillus Genetic Stock Center, Ohio State University, Columbus. B. thuringiensis strains that express the Cry3, Cry1Ga, Cry1Ha, or Cry7A toxins and an Escherichia coli strain expressing the Cry9Ca protein were kindly supplied by M. Peferoen and J. Van Rie of Plant Genetic Systems, Ghent, Belgium. A B. thuringiensis strain containing both Cry9Aa and Cry9Ba proteins was kindly supplied by A. Shevelev of the Institute of Genetics and Selection of Industrial Organisms, Moscow, Russia. B. thuringiensis strains with Cry1Ja and Cry1Ib were obtained from the U.S. Department of Agriculture, and B. thuringiensis strains with Cry11A and Cry11B were kindly supplied by Sarjeet S. Gill of the University of California, Riverside. Mexican B. thuringiensis strains were isolated from soil samples by the acetate selection method (28). Soil samples were collected from the surface to a depth of 10 cm. All bacterial strains were maintained in nutrient medium (Difco).
Oligonucleotide PCR primers.
Novel general primers (gral) used for the detection of cry1 genes, cry8 genes, cry11 genes, cyt genes, and the nematode-active cry5, cry12, and cry14 genes were selected from highly conserved regions by multiple alignment of all reported DNA sequences. This survey was done with the Gene Works 2.3 program (Intelligenetics, Inc.) and the GCG sequence analysis program PILEUP (13). Table 1 shows the sequences of these general primers, their locations within the respective gene sequences, and the expected sizes of their PCR products. Table 1 also shows the sequences of specific primers (spe) used for the identification of cry8, cry9, and cry13 genes that were selected from highly variable regions. Other primers used in this work for the detection of cry3, cry7, and cry1 genes were described in previous reports (7, 8). Oligonucleotides were synthesized by use of a DNA synthesizer (Microsyn 1450A; Systec Inc.) with the reagents and conditions specified by the manufacturer.
TABLE 1.
Characteristics of general and specific primers for cry1, cry5, cry8, cry9, cry11, cry12, cry13, cry14, cry21, and cyt genes
| Primer pair | Positionsa | Gene(s) recognized | Product size (bp) | Annealing temp (°C) | Sequenceb | Accession no.c |
|---|---|---|---|---|---|---|
| gral-cry1 | 1472–2029 | cry1Aa, cry1Ad | 558 | 52 | 5′ CTGGATTTACAGGTGGGGATAT (d) | M11250, M73250 |
| 1475–2032 | cry1Ab, cry1Ae | 558 | 5′ TGAGTCGCTTCGCATATTTGACT (r) | M13898, M65252 | ||
| 1472–2035 | cry1Ac | 564 | M11068 | |||
| 1636–2194 | cry1Af | 558 | U82003 | |||
| 1559–2116 | cry1Ba | 558 | X06711 | |||
| 1577–2131 | cry1Bb | 555 | L32020 | |||
| 2181–2723 | cry1Bc | 555 | Z46442 | |||
| 1463–2056 | cry1Ca | 594 | X07518 | |||
| 1463–2017 | cry1Cb | 555 | M97880 | |||
| 1442–1984 | cry1Da, cry1Db | 543 | X54160, | |||
| 1454–2011 | cry1Ea, cry1Fa | 558 | X53985, M63897 | |||
| 1451–2005 | cry1Eb | 555 | M73253 | |||
| 1936–2490 | cry1Fb | 555 | Z22512 | |||
| 1430–1987 | cry1G | 558 | Z22510 | |||
| 1457–2005 | cry1Ha | 549 | Z22513 | |||
| 2181–2723 | cry1Hb | 543 | U35780 | |||
| 1583–2140 | cry1Ia, cry1Ib | 558 | X62821, U07642 | |||
| 1439–1993 | cry1Ja | 555 | L32019 | |||
| 1615–2172 | cry1Jb | 558 | U31527 | |||
| 2012–2569 | cry1K | 558 | U28801 | |||
| gral-cry8 | 1–373 | cry8A | 373 | 49 | 5′ ATGAGTCCAAATAATCTAAATG (d) | U04364 |
| 1–376 | cry8B | 376 | 5′ TTTGATTAATGAGTTCTTCCACTCG (r) | U04365 | ||
| 1–376 | cry8C | 376 | U04366 | |||
| gral-cry11 | 1553–1857 | cry11A | 305 | 51 | 5′ TTAGAAGATACGCCAGATCAAGC (d) | M31737 |
| 1588–1892 | cry11B | 305 | 5′ CATTTGTACTTGAAGTTGTAATCCC (r) | X86902 | ||
| gral-nem | 2848–3321 | cry5Aa | 474 | 50 | 5′ TTACGTAAATTGGTCAATCAAGCAAA (d) | L07025 |
| 2560–3033 | cry5Ab | 474 | 5′ AAGACCAAATTCAATACCAGGGTT (r) | L07026 | ||
| 2353–2826 | cry5Ac | 474 | I34543 | |||
| 2428–2901 | cry5B | 474 | U19725 | |||
| 2401–2877 | cry12A | 477 | L07027 | |||
| 2326–2808 | cry14A | 483 | U13955 | |||
| 2263–2751 | cry21Aa | 489 | I32932 | |||
| gral-cyt | 193–714 | cyt1Aa | 522 | 51 | 5′ AACCCCTCAATCAACAGCAAGG (d) | X03182 |
| 81–605 | cyt1Ab | 525 | 5′ GGTACACAATACATAACGCCACC (r) | X98793 | ||
| spe-cry9A | 2103–2673 | cry9A | 571 | 51 | 5′ GTTGATACCCGAGGCACA (d) | X58120 |
| 5′ CCGCTTCCAATAACATCTTTT (r) | ||||||
| spe-cry9B | 1770–2171 | cry9B | 402 | 51 | 5′ TCATTGGTATAAGAGTTGGTGATAGAC (d) | X75019 |
| 5′ CCGCTTCCAATAACATCTTTT (r) | ||||||
| spe-cry9C | 1853–2158 | cry9C | 306 | 51 | 5′ CTGGTCCGTTCAATCC (d) | Z37527 |
| 5′ CCGCTTCCAATAACATCTTTT (r) | ||||||
| spe-cry8A | 1–338 | cry8A | 338 | 49 | 5′ ATGAGTCCAAATAATCTAAATG (d) | U04364 |
| 5′ TCTCCCCATATATCTACGCTC (r) | ||||||
| spe-cry8B | 1–510 | cry8B | 510 | 49 | 5′ ATGAGTCCAAATAATCTAAATG (d) | U04365 |
| 5′ GAACATCTCGTAAGGCTC (r) | ||||||
| spe-cry8C | 1–963 | cry8C | 963 | 49 | 5′ ATGAGTCCAAATAATCTAAATG (d) | U04366 |
| 5′ GGTACTCGATTGTCCAGT (r) | ||||||
| spe-cry13 | 225–537 | cry13 | 313 | 50 | 5′ CTTTGATTATTTAGGTTTAGTTCAA (d) | L07023 |
| 5′ TTGTAGTACAGGCTTGTGATTC (r) |
Positions at 5′ end of forward and reverse primers for each PCR primer pair.
d and r, direct and reverse primers, respectively.
GenBank database.
Sample preparation and PCR.
B. thuringiensis strains were grown for 12 h on a nutrient medium plate. A loop of cells was transferred to 0.1 ml of H2O, and the mixture was frozen at −70°C for 20 min and then transferred to boiling water for 10 min to lyse the cells. The resulting cell lysate was briefly centrifuged (10 s at 10,000 rpm [Eppendorf model 5415C centrifuge]), and 15 μl of supernatant was used as a DNA sample in the PCR. PCR mixtures were as previously described (8). The amplification was done by use of a DNA thermal cycler (Perkin-Elmer model 480). The conditions for the PCRs done with cry1 general primers (gral-cry1) were as follows: a single denaturation step of 2 min at 95°C, a step cycle program set for 30 cycles (with a cycle consisting of denaturation at 95°C for 1 min, annealing at 52°C for 1 min, and extension at 72°C for 1 min), and an extra step of extension at 72°C for 5 min. The conditions for the PCRs done with other primers were similar, except that the annealing temperatures were set at 49°C for cry8 general and specific primers (gral-cry8 and spe-cry8), 51°C for cry9 specific and cry11 and cyt general primers (spe-cry9, gral-cry11, and gral-cyt), and 50°C for cry13 specific and nematode general primers (spe-cry13 and gral-nem). Following amplification, a 15-μl sample of each PCR mixture was electrophoresed on a 2% agarose gel in Tris-borate buffer (45 mM Tris-borate, 1 mM EDTA [pH 8.0]) at 250 V for 30 to 35 min and stained with ethidium bromide.
Protein electrophoresis and immunodetection by ELISA.
Protein analysis was done by sodium dodecyl sulfate (SDS)-polyacrylamide gel electrophoresis (PAGE) with 10% gels. The enzyme-linked immunosorbent assay (ELISA) was done as previously described (7). Ten and 20 μg of solubilized crystals were detected by polyclonal antisera raised in rabbits against purified trypsin-activated toxic fragments of Cry1Ab; Cry2A and Cry2B; Cry3A; or a mixture of activated Cry4, Cry10, and Cry11 toxins. Horseradish peroxidase-conjugated goat anti-rabbit immunoglobulin (1/1,000; Sigma) was used as the secondary antibody. Diaminobenzidine (25 mg/100 ml) and H2O2 were used as peroxidase substrates.
Bioassays.
The activities of different B. thuringiensis strains were screened on neonate larvae of Spodoptera frugiperda and S. exigua and third-instar larvae of Trichoplusia ni as described by Aranda et al. (1). Serial dilutions of spore-crystal suspensions were applied onto the diet surface. The mortality was recorded after 7 days. The 50% lethal concentrations and confidence limits were obtained by probit analysis (17).
Electron microscopy.
Cells grown in nutrient medium for 36 h at 30°C were centrifuged for 5 min at 1,000 × g. Samples were fixed in Karnowsky’s fixative (4% paraformaldehyde and 5% glutaraldehyde in 0.1 M phosphate buffer [pH 7.4]) and postfixed in 1% OsO4. Samples were dehydrated in ascending solutions of ethanol (30 to 100%) and embedded in LR-White (Ted Pella). Thin sections were cut on a Nova ultramicrotome (LKB, Bromma, Sweden), stained with 2% uranyl acetate and lead citrate, and examined in a Zeiss EM900 electron microscope operating at 50 kV.
RESULTS
Construction of the Mexican strain collection.
The Mexican B. thuringiensis strain collection encompassed strains from 503 soil samples collected from the five macroregions of the country from 1991 to 1994 (7, 8). Soil samples came from cultivated fields (maize, sorghum, rice, sugarcane, bean, pea, coffee, cacao, walnut, alfalfa, poblano pepper, prickly pear, agave plant, peach, mango, papaya, melon, tomato, cabbage, squash, onion, broccoli, and carrot) or natural vegetation (pine woods, deciduous tropical forest, temperate forest, and grasslands) where B. thuringiensis toxins have never been applied. The elevations of the places from which the samples were collected were highly variable, ranging from sea level to 2,900 m above sea level. Fifty-five samples were collected from the Pacific-North region (Sonora, Sinaloa, and Nayarit states), and 44 samples were collected from the North region (Durango, Zacatecas, and San Luis Potosi states). These two regions correspond to semiarid steppes. One hundred eighty-eight soil samples were collected from the Central High Plateau region (Jalisco, Guanajuato, Morelos, and Puebla states), corresponding to temperate and cold climates. Fifty soil samples were collected from the Gulf of Mexico region (Veracruz and Tabasco states), and 166 samples were collected from the South-Pacific region (Chiapas and Oaxaca States). The last two regions correspond to tropical rainy climates.
We found B. thuringiensis strains in 456 of the 503 soil samples analyzed. After microscopic observation of 8,179 selected strains, a total of 1,948 strains which produced crystal inclusions were selected. This result suggested that the soil samples analyzed contained a high background level of other spore-forming bacteria. SDS-PAGE analysis was carried out on all the strains from the same soil sample to identify siblings. The exclusion of sibling strains was important for a more realistic estimation of B. thuringiensis genetic diversity. This analysis resulted in the selection of 496 B. thuringiensis strains.
Determination of the cry gene content of B. thuringiensis isolates.
The Mexican strain collection was characterized by different methods: (i) SDS-PAGE of spore-crystal suspensions to determine the number and size of the Cry proteins (data not shown); (ii) ELISA with different polyclonal antisera to identify toxins active against Lepidoptera (Cry1 and Cry2), Coleopteran (Cry3), or Diptera (Cry4, Cry10, and Cry11) (data not shown); and (iii) PCR to identify cry1, cry3, cry5, cry7, cry8, cry9, cry11, cry12, cry13, cry14, cry21, and cyt genes (7, 8) (Table 1). PCR was done with six pairs of general primers (gral-cry1, gral-cry3, gral-cry8, gral-cry11, gral-nem, and gral-cyt) that were selected from highly conserved regions among each group of genes (8) (Table 1).
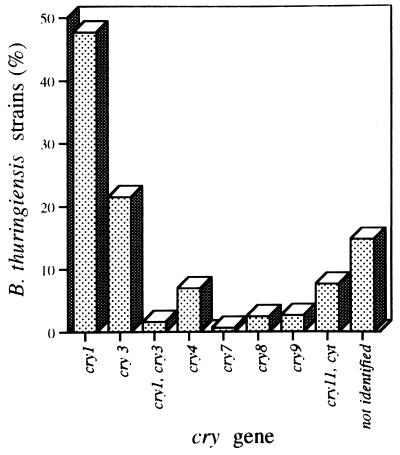
The cry gene content of the Mexican B. thuringiensis strains is shown in Fig. 1. Strains containing cry1 genes were the most abundant in our collection (246 strains, representing 49.5%). B. thuringiensis strains harboring cry3 genes were also highly abundant (21.7%), and 7.9% of the strains contained cry11 and cyt genes. cry7, cry8, and cry9 genes were less abundant, found in 0.6, 2.4, and 2.6% of the strains, respectively. No strains with cry5, cry12, cry13, cry14, or cry21 genes were found. Finally, 14% of the strains (73 strains) did not react with any polyclonal antisera and did not give any PCR product when assayed with the general primers (Fig. 1). However, these strains produced crystal inclusions, suggesting that they may contain potentially novel Cry toxins.
FIG. 1.
Distribution of cry-type genes obtained from 496 field-collected strains of B. thuringiensis and identified by PCR analysis with general primers and ELISAs with polyclonal antisera.
Identification of specific cry genes in B. thuringiensis isolates.
Strains with unique PCR product profiles obtained with the general primers were further characterized by additional PCRs with specific primers as previously reported (7, 8) and the novel specific primers described in Table 1. With the multiplex PCR method described here, 12 subgroups of cry1 genes (cry1Aa, cry1Ab, cry1Ac, cry1Ad, cry1Ae, cry1Ba, cry1Ca, cry1Da, cry1Ea, cry1Eb, cry1Fa, and cry1Fb), 3 subgroups of cry9 genes (cry9A, cry9B, and cry9C), 3 subgroups of cry8 genes (cry8A, cry8B, and cry8C), 4 subgroups of cry3 genes (cry3A, cry3Ba, cry3Bb, and cry3C), and the cry7A and cry13 genes could be identified when they were present in the analyzed strains. Each cry gene produces a PCR product with a unique molecular weight. Therefore, strains with PCR products of sizes other than those predicted are also candidates for harboring putative novel cry genes.
The 246 strains harboring cry1 genes (selected with gral-cry1, which was able to identify 25 of the 27 different cry1 genes) were analyzed with the cry1 specific primers (7, 8). We found 33 different cry1 gene profiles (Table 2). The most common profile of cry1 genes contained cry1Aa, cry1Ab, and cry1Ac genes (11.7%). Strains harboring the cry1Ba gene were frequently observed (7.7%). Strains harboring a combination of cry1A genes with cry1C and/or cry1D genes were also abundant (19%). In contrast, we found few strains harboring cry1F genes (4.8%) and only four strains containing cry1E genes, suggesting that the distribution of cry1E and cry1F genes is less abundant than that of cry1A, cry1B, cry1C, and cry1D genes. Some strains did not react with any cry1 specific primer (7.7%). These strains may have had some other cry1 gene not identified by the cry1 specific primers (cry1G, cry1H, cry1I, cry1J, and cry1K). We found strains with interesting combinations of cry genes, containing cry1 and cry3A, cry1 and cry3B, or cry1 and cry7A genes (Table 2). These profiles of cry genes indicate that these strains may be active against both lepidopteran and coleopteran insects.
TABLE 2.
Distribution of cry1 gene profiles present in the B. thuringiensis Mexican strain collection (n = 246)a
| No. of strains | cry gene profile | % |
|---|---|---|
| 5 | cry1Aa | 2.0 |
| 3 | cry1Ab | 1.2 |
| 3 | cry1Ac | 1.2 |
| 29 | cry1Aa, cry1Ab, cry1Ac | 11.7 |
| 4 | cry1Aa, cry1Ab, cry1B | 1.6 |
| 2 | cry1Ac, cry1B | 0.8 |
| 7 | cry1Aa, cry1B, cry1D | 2.8 |
| 9 | cry1Aa, cry1Ab, cry1Ac, cry1B, cry1D | 3.6 |
| 6 | cry1Aa, cry1C, cry1D | 2.4 |
| 15 | cry1Aa, cry1Ab, cry1C, cry1D | 6.1 |
| 2 | cry1Aa, cry1Ab, cry1Ac, cry1C, cry1D | 0.8 |
| 17 | cry1Aa, cry1D | 6.9 |
| 1 | cry1Ac, cry1D | 0.4 |
| 6 | cry1Aa, cry1Ab, cry1Ac, cry1D | 2.4 |
| 3 | cry1Ac, cry1E | 1.2 |
| 3 | cry1Aa, cry1D, cry1F | 1.2 |
| 2 | cry1Ab, cry1D, cry1F | 0.8 |
| 1 | cry1Aa, cry1Ab, cry1B, cry1F | 0.4 |
| 1 | cry1Aa, cry1C, cry1D, cry1F | 0.4 |
| 2 | cry1Ac, cry1F | 0.8 |
| 19 | cry1B | 7.7 |
| 1 | cry1B, cry1D | 0.4 |
| 1 | cry1B, cry1E | 0.4 |
| 4 | cry1C, cry1D | 1.6 |
| 6 | cry1D | 2.4 |
| 2 | cry1D, cry1F | 0.8 |
| 2 | cry1Aa, cry1Ab, cry1Ac, cry3Ba | 0.8 |
| 1 | cry1Ab, cry3Ba | 0.4 |
| 1 | cry1Ac, cry1D, cry1Bb | 0.4 |
| 1 | cry1Aa, cry1Ab, cry1Ac, cry3A | 0.4 |
| 1 | cry1Aa, cry1C, cry1D, cry1F, cry3A | 0.4 |
| 1 | cry1Ac, cry7A | 0.4 |
| 1 | cry1D, cry3Ba | 0.4 |
| 19 | Did not react with any cry1 specific primer | 7.7 |
| 65 | Produced different PCR products | 26.4 |
Bioassays were carried out with different native strains against S. exigua, S. frugiperda, and T. ni larvae. These insects are important agricultural pests in Mexico. The larval stage of S. frugiperda is an important pest of corn, but it is also a problem on cotton, alfalfa, clover, peanuts, and many garden crops. S. exigua is important in rice as well as in cotton, sugar beet, tomato, tobacco, and groundnut. T. ni is a serious pest in Brassica spp. and other Cruciferae. The bioassays showed that the strains harboring cry1C and cry1D genes were highly active against S. exigua and S. frugiperda larvae (Table 3). Strain IB126, containing both cry1C and cry1D genes, was the most active strain against S. frugiperda and S. exigua larvae. The most active strain against T. ni larvae was strain IB87, harboring cry1Aa, cry1Ab, cry1Ac, and cry1D genes (Table 3).
TABLE 3.
Dose-response activities of B. thuringiensis isolates against S. frugiperda, S. exigua, and T. ni larvaea
| Strain | cry gene profile identified by PCR analysis | LC50, ng/cm2 (95% confidence interval) for:
|
||
|---|---|---|---|---|
| S. frugiperda | S. exigua | T. ni | ||
| IB4 | cry1Aa, cry1D | 34 (23–52) | 32 (23–45) | 80 (50–112) |
| IB87 | cry1Aa, cry1Ab, cry1Ac, cry1D | 177 (119–261) | 72 (51–101) | 6 (4–9) |
| IB126 | cry1C, cry1D | 22 (15–30) | 22 (16–31) | 121 (80–190) |
| IB23 | cry1Aa, cry1C, cry1D | 106 (71–157) | 62 (44–86) | 62 (40–95) |
| IB33 | cry1Aa, cry1Ab, cry1Ac | 698 (480–977) | 579 (413–811) | 20 (14–32) |
| IB104 | cry1B | 843 (558–1,273) | 654 (436–981) | NDc |
| IB109 | cry1Aa, cry1Ab, cry1C, cry1D | 47 (30–71) | 24 (17–33) | 170 (121–255) |
| IB132 | cry1Aa, cry1Ab, cry1Ac, cry1B | 997 (651–1,525) | 656 (452–918) | 23 (16–32) |
| IB217 | cry1Aa, cry1C, cry1D, cry1F | 154 (100–235) | ND | ND |
| IB304 | cry1Aa, cry1Ac, cry1D | 31 (20–47) | ND | ND |
| HD1 | cry1Aa, cry1Ab, cry1Ac | 969 (651–1,363) | 1,330 (950–1, 862) | 25 (17–35) |
| HD73 | cry1Ac | 1,124 (802–1,573) | 1,202 (828–1, 742) | ND |
| HD137 | cry1Aa, cry1B, cry1C, cry1D | 43 (32–71) | 25 (17–35) | 350 (250–495) |
Bioassays were done with the spore-crystal complex. LC50, 50% lethal concentration; ND, not determined.
The 117 strains that gave PCR products with the cry3 general primers described by Cerón et al. (8) were further analyzed with specific primers (7) (Table 1). The most abundant strains were those harboring the cry3Ba gene (13.6% of the total coleopteran-active strains) (Table 4). However, 53 strains did not react with any cry3, cry7, or cry8 specific primers, and 25 strains gave PCR products of sizes other than those expected. Twelve strains gave PCR products when assayed with the cry8 general primers. When these strains were assayed with the cry8 specific primers, only three strains had the cry8C gene and three strains had the cry8B gene; the rest of them did not react with any cry8 specific primer. These data suggest that in our collection, there is great variability among cry3, cry7, and cry8 genes.
TABLE 4.
Distribution of cry3, cry7, and cry8 gene profiles present in the B. thuringiensis Mexican strain collection (n = 117)a
| No. of strains | cry gene profile | % |
|---|---|---|
| 6 | cry3A | 5.1 |
| 16 | cry3Ba | 13.6 |
| 6 | cry3Bb | 5.1 |
| 2 | cry3C | 1.7 |
| 3 | cry7A | 2.5 |
| 3 | cry8B | 2.5 |
| 3 | cry8C | 2.5 |
| 53 | Did not react with any cry3, cry8, or cry7 specific primer | 45.2 |
| 25 | Produced different PCR products | 21.3 |
A set of 13 strains reacted with the cry9 specific primers, and 38 strains contained cry11 and cyt genes. Some of these strains (containing cry11 and cyt) were found to be toxic against larvae of the mosquito Culex quinquefaciatus (data not shown).
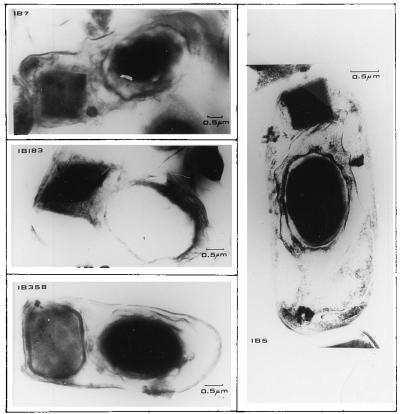
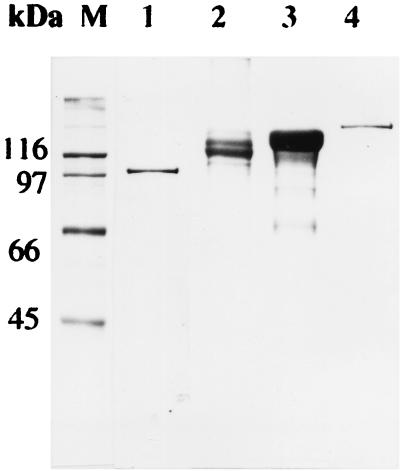
Finally, a significant number of strains (73 strains) did not react with any PCR primer. Within this group, we identified some strains which presented an unusual crystal morphology or a δ-endotoxin of a size different from that previously reported (16, 20). Figures 2 and 3 show an electron microscopic analysis and an SDS-PAGE analysis, respectively, of representative strains that did not react with any PCR primer. Strain IB5 produced a small bipyramidal crystal composed of 120- and 130-kDa proteins. Strain IB7 had a cuboidal crystal containing a single 100-kDa protein. Strain IB183 had a bipyramidal crystal composed of 130-kDa proteins, and strain IB358 had a round crystal made up of a 145-kDa protein enclosed in a multilayered envelope. This crystal morphology is similar to that previously reported for B. thuringiensis subsp. shandongiensis (26).
FIG. 2.
Transmission electron microscopy of representative Mexican B. thuringiensis strains that did not react with any PCR primers. Strain IB5 produced a small bipyramidal crystal. Strain IB7 had a square crystal. Strain IB183 had a bipyramidal crystal. Strain IB358 had a round crystal enclosed in a multilayered envelope.
FIG. 3.
SDS-PAGE of B. thuringiensis crystal proteins. Lane M, molecular mass markers; the numbers beside the gel indicate molecular masses of standard marker proteins. Lane 1, strain IB7; lane 2, strain IB5; lane 3, strain IB183; lane 4, strain IB358.
Distribution of cry genes in different geographical regions.
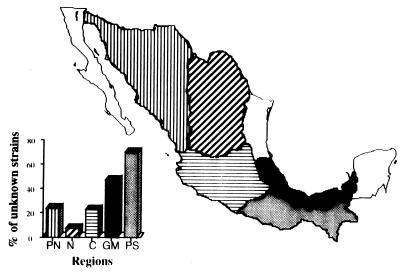
The distribution of cry genes in different geographical regions was analyzed. B. thuringiensis strains containing putative novel cry genes were most abundant in the tropical regions (Fig. 4). This pattern was judged by the production of PCR products of sizes different from those expected or a lack of reaction with any PCR primer or by the presence of Cry proteins of unusual sizes.
FIG. 4.
Distribution of putative novel cry genes in the different regions of Mexico. PN, Pacific-North region; N, North region. These two regions correspond to semiarid steppes. C, Central High Plateau region, corresponding to temperate and cold climates. GM, Gulf of Mexico region; PS, South-Pacific region. The last two regions correspond to tropical rainy climates.
In relation to the known cry genes, the distributions of cry1A, cry1B, cry1C, cry1D, cry7, cry8, and cry9 genes were similar in the five macroregions sampled. In contrast, the dipteran-specific cry11 and cyt genes were more frequently found in the tropical regions. The cry11 and cyt genes were present in 38% of the tropical strains containing known cry genes, compared with 12% of the strains obtained from both the semiarid steppe and the central regions. In contrast, the cry3 genes were more frequently found in the semiarid steppe regions than in the central regions and tropical rainy regions. The cry3 genes were present in 40% of the strains containing known cry genes and obtained from the semiarid steppes, in 15% of the strains obtained from the central regions, and in only 5% of the strains obtained from the tropical rainy regions. Finally, the cry1E and cry1F genes were found only in the tropical rainy regions.
DISCUSSION
The characterization of the Mexican B. thuringiensis strain collection is presented. This collection has great value, since Mexico has very different climatic regions with a high diversity of insects. We found a high diversity of B. thuringiensis strains.
We determined the presence of different cry genes within the collection (Fig. 1, Table 2, and Table 4). The cry1 genes were the most frequently found in the Mexican strain collection. A high frequency of cry1 genes seems to be common to all B. thuringiensis strain collections analyzed so far. The selection of B. thuringiensis isolates was based principally on phase-contrast microscopy. Isolates producing bipyramidal crystals (like the ones produced by Cry1 proteins) were more easily distinguished than isolates with the rhomboid, oval, or pointed crystal types. Therefore, it is possible that the high proportion of cry1 genes in all B. thuringiensis strain collections may have been biased because of the procedure used for strain selection. However, we cannot exclude the possibility that cry1 gene-containing strains may be more abundant.
The second most abundant genes in the Mexican strain collection were the cry3 genes and then the cry11, cry4, and cyt genes (Fig. 1). This distribution of cry genes was different from the distribution reported for other B. thuringiensis strain collections. Ben-Dov et al. (3) presented an interesting PCR analysis of 215 B. thuringiensis strains collected from soil samples from Israel, Kazakhstan, and Uzbekistan. They found that strains containing cry1 genes were the most abundant; however, strains harboring cry4 genes were the second most abundant, while strains with cry3 genes were absent. On the other hand, Chak et al. (9) presented a PCR characterization of 225 B. thuringiensis strains isolated from soil samples from Taiwan that showed a different cry gene distribution. They reported five different profiles of cry genes in their collection. The cry1A genes were the most abundant, followed by the cry1C and cry1D genes; only four strains harbored cry4 genes, and no strains harbored cry3, cry1B, cry1E, or cry1F genes. It is surprising that no cry3 genes were found in any of the Asian B. thuringiensis strain collections (3, 9). It will be interesting to analyze if the distribution of cry genes in B. thuringiensis strain correlates with the abundance and distribution of insects in these regions.
We did not find strains harboring any of the nematode-active cry genes. This finding could suggest that the corresponding proteins are not abundant in Mexico or that different kinds of samples (such as deeper soils or nematode populations themselves) should be tested.
Some strains containing combinations of cry genes that were less frequently observed, such as lepidopteran-active cry1 genes and coleopteran-active cry3A, cry3Ba, or cry7A genes, were identified (Table 2). These strains are good candidates in the search for biocontrol agents with a wider spectrum of action. Other groups have reported the presence of cry1 genes and cry3, cry8, or cry7 genes in the same B. thuringiensis strain (2, 3), suggesting that strains with dual activity are also present in other regions. Also, it is important to mention that many of the strains harbored more than one cry gene, suggesting that B. thuringiensis strains have a high frequency of genetic information exchange.
The identification of known cry genes in the B. thuringiensis strains is important, since the specificity of action is known for many of the Cry toxins. This fact allows the possibility of selecting native strains that could be used in the control of some targets and of selecting strains with the highest activity. As an example, we showed that native strains containing cry1C and cry1D genes were toxic for S. frugiperda and S. exigua larvae (Table 3) and that strain IB126 had twofold-higher activity against S. frugiperda than control strain HD137 (Table 3). Also, strain IB87 had fourfold-higher activity against T. ni than control strain HD1 (Table 3).
The characterization of the B. thuringiensis strain collection is also valuable because it may help in the understanding of the role of B. thuringiensis in the environment. The distribution of B. thuringiensis strains is ubiquitous, and their direct relationship with specific insects has been questioned (24). We found that some cry genes were distributed differently in the geographical regions analyzed. The dipteran-active cry11 and cyt genes were more frequently found in the tropical rainy regions than in the semiarid regions; this distribution correlates with the distribution of dipteran insects (21). Similarly, the cry1E and cry1F genes were found only in the tropical rainy regions. The Cry1E and Cry1F proteins were active against S. littoralis and S. exigua larvae (10, 30). However, there is not a clear difference in the distribution of Spodoptera spp. among the different macroregions. Finally, it is remarkable that B. thuringiensis strains containing putative novel cry genes were most abundant in the tropical rainy regions (Fig. 4), a finding which matches the pattern of insect diversity. We believe that our data support the idea that the distribution of some cry genes is correlated with the distribution of insects. A study aimed to find a correlation between the distribution of cry genes and specific targets is proposed, and in order to draw clear conclusions on a worldwide scale, it would be desirable to analyze the cry gene content of B. thuringiensis strains from other regions. A correlation between the frequency of active strains and the geographical origin of the samples was presented by Bernhard et al. (4). They reported a high number of B. thuringiensis strains active against Heliothis virescens in samples collected from North America, where Heliothis spp. are major agricultural pests.
Finally, 65 strains gave PCR products of different sizes (Table 2) when assayed with cry1 specific primers, and 25 strains gave PCR products of different sizes (Table 4) when assayed with the cry3 specific primers. Also, 73 strains did not react with any PCR primer or polyclonal antisera (Fig. 1). These strains are candidates for harboring putative novel cry genes. The identification of putative novel B. thuringiensis strains could be the first step in the sequence for finding novel toxicities, since novel toxins may be toxic for new targets. The isolation and sequencing of novel cry genes should be encouraged once the target insect is identified and more evidence on the potential of novel toxins as biological control agents is available.
ACKNOWLEDGMENTS
This work was supported in part by Consejo Nacional de Ciencia y Tecnología grants 4037P-B9608 and 400344-5-4311-N and Dirección General de Asuntos del Personal Académico/UNAM grant IN-217597.
We thank Paul Gaytan and Eugenio Lopez for primer synthesis and Mario Trejo for critical review of the manuscript.
REFERENCES
- 1.Aranda E, Sánchez J, Peferoen M, Güereca L, Bravo A. Interaction of Bacillus thuringiensis crystal protein with the midgut epithelial cells of Spodoptera frugiperda (Lepidoptera: Noctuidae) J Invertebr Pathol. 1996;68:203–212. doi: 10.1006/jipa.1996.0087. [DOI] [PubMed] [Google Scholar]
- 2.Aronson A I. Bacillus thuringiensis and its use as biological insecticide. Plant Breed Rev. 1994;12:19–45. [Google Scholar]
- 3.Ben-Dov E, Zaritsky A, Dahan E, Barak Z, Sinai R, Manasherob R, Khamraev A, Troitskaya E, Dubitsky A, Berezina N, Margalith Y. Extended screening by PCR for seven cry-group genes from field-collected strains of Bacillus thuringiensis. Appl Environ Microbiol. 1997;63:4883–4890. doi: 10.1128/aem.63.12.4883-4890.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bernhard K, Jarret P, Meadows M, Butt J, Ellis D J, Roberts G M, Pauli S, Rodgers P, Burges H D. Natural isolates of Bacillus thuringiensis: worldwide distribution, characterization and activity against insect pests. J Invertebr Pathol. 1997;70:59–68. [Google Scholar]
- 5.Bourque S N, Valero J R, Mercier J, Lavoie M C, Levesque R C. Multiplex polymerase chain reaction for detection and differentiation of the microbial insecticide Bacillus thuringiensis. Appl Environ Microbiol. 1993;59:523–527. doi: 10.1128/aem.59.2.523-527.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Carozzi N B, Kramer V C, Warren G W, Evola S, Koziel M G. Prediction of insecticidal activity of Bacillus thuringiensis strains by polymerase chain reaction product profiles. Appl Environ Microbiol. 1991;57:3057–3061. doi: 10.1128/aem.57.11.3057-3061.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Cerón J, Covarrubias L, Quintero R, Ortíz A, Ortíz M, Aranda E, Lina L, Bravo A. PCR analysis of the cryI insecticidal crystal family genes from Bacillus thuringiensis. Appl Environ Microbiol. 1994;60:353–356. doi: 10.1128/aem.60.1.353-356.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Cerón J, Ortíz A, Quintero R, Güereca L, Bravo A. Specific PCR primers directed to identify cryI and cryIII genes within a Bacillus thuringiensis strain collection. Appl Environ Microbiol. 1995;61:3826–3831. doi: 10.1128/aem.61.11.3826-3831.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Chak K F, Chao D C, Tseng M Y, Kao S S, Tuan S J, Feng T Y. Determination and distribution of cry-type genes of Bacillus thuringiensis isolates from Taiwan. Appl Environ Microbiol. 1994;60:2415–2420. doi: 10.1128/aem.60.7.2415-2420.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Chambers J A, Jelen A, Gilbert M P, Jany C S, Johnson T B, Gawron-Burke C. Isolation and characterization of a novel insecticidal crystal protein gene from Bacillus thuringiensis subsp. aizawai. J Bacteriol. 1991;173:3966–3976. doi: 10.1128/jb.173.13.3966-3976.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Chilcott C N, Wigley P J. Isolation and toxicity of Bacillus thuringiensis from soil and insect habitats in New Zealand. J Invertebr Pathol. 1993;61:244–247. [Google Scholar]
- 12.Crickmore N, Zeigler D R, Feitelson J, Schnepf E, Van Rie J, Lereclus D, Baum J, Dean D H. Bacillus thuringiensis toxin nomenclature. 1998. http://www.biols.susx.ac.uk/Home/Neil_Crickmore/Bt/index.html http://www.biols.susx.ac.uk/Home/Neil_Crickmore/Bt/index.html (date site accessed). (date site accessed). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Devereux J, Haeberli P, Smithies O. A comprehensive set of analysis programs for the VAX. Nucleic Acids Res. 1984;12:387–395. doi: 10.1093/nar/12.1part1.387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Estruch J J, Warren G W, Mullins M A, Nye G J, Craig J A, Kozoel M G. Vip3A, a novel Bacillus thuringiensis vegetative insecticidal protein with a wide spectrum of activities against lepidopteran insects. Proc Natl Acad Sci USA. 1996;93:5389–5394. doi: 10.1073/pnas.93.11.5389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Feitelson J S, Payne J, Kim L. Bacillus thuringiensis: insects and beyond. Bio/Technology. 1992;10:271–275. [Google Scholar]
- 16.Feitelson J S. The Bacillus thuringiensis family tree. In: Kim L, editor. Advanced engineered pesticides. New York, N.Y: Marcel Dekker, Inc.; 1993. pp. 63–72. [Google Scholar]
- 17.Finney D J. Probit analysis. Cambridge, England: Cambridge University Press; 1971. [Google Scholar]
- 18.Gleave A P, Williams R, Hedges R J. Screening by polymerase chain reaction of Bacillus thuringiensis serotypes for the presence of cryV-like insecticidal protein genes and characterization of a cryV gene cloned from B. thuringiensis subsp. kurstaki. Appl Environ Microbiol. 1993;59:1683–1687. doi: 10.1128/aem.59.5.1683-1687.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Halffter G. Biogeography of the Montane entamofauna of Mexico and Central America. Annu Rev Entomol. 1987;32:95–114. [Google Scholar]
- 20.Höfte H, Whiteley H R. Insecticidal crystal proteins of Bacillus thuringiensis. Microbiol Rev. 1989;53:242–255. doi: 10.1128/mr.53.2.242-255.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ibañez-Bernal S, Strickman D, Martínez-Campos C. Culidae (Diptera) In: Llorente Bousquets J, García Aldrete A N, González Soriano E, editors. Biodiversidad, taxonomía y biogeografía de artrópodos en México. México City, México: Universidad Nacional Autónoma de México; 1996. pp. 591–602. [Google Scholar]
- 22.Juárez-Pérez V M, Ferrandis M D, Frutos R. PCR-based approach for detection of novel Bacillus thuringiensis cry genes. Appl Environ Microbiol. 1997;63:2997–3002. doi: 10.1128/aem.63.8.2997-3002.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lecadet M M, Frachon E, Dumanoir V C, de Barjac H. Abstracts of the IInd International Conference on Bacillus thuringiensis. Montpellier, France: Society for Invertebrate Pathology; 1994. An updated version of the Bacillus thuringiensis strain classification according to H-serotypes; p. 345. [Google Scholar]
- 24.Martin P A W, Travers R S. Worldwide abundance and distribution of Bacillus thuringiensis isolates. Appl Environ Microbiol. 1989;55:2437–2442. doi: 10.1128/aem.55.10.2437-2442.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Meadows P M, Ellis D J, Butt J, Jarret P, Burges H D. Distribution, frequency, and diversity of Bacillus thuringiensis in animal feed mill. Appl Environ Microbiol. 1992;58:1344–1350. doi: 10.1128/aem.58.4.1344-1350.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Pietrantonio P, Gill S S. The parasporal inclusion of Bacillus thuringiensis subsp shandongiensis: characterization and screening for insecticidal activity. J Invertebr Pathol. 1992;59:295–302. doi: 10.1016/0022-2011(92)90136-r. [DOI] [PubMed] [Google Scholar]
- 27.Smits P H. Insect pathogens: their suitability as biopesticides. In: Evans H F, editor. Microbial insecticides: novelty or necessity? Nottingham, England: Major Design & Production Ltd.; 1997. pp. 21–28. [Google Scholar]
- 28.Travers R S, Martin P A W, Reichelderfer C F. Selective process for efficient isolation of soil Bacillus sp. Appl Environ Microbiol. 1987;53:1263–1266. doi: 10.1128/aem.53.6.1263-1266.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Van Rie J. Insect control with transgenic plants: resistance proof? Trends Biotechnol. 1991;9:177–179. [Google Scholar]
- 30.Visser B, Munsterman E, Stoker A, Dirkse W G. A novel Bacillus thuringiensis gene encoding a Spodoptera exigua-specific crystal protein. J Bacteriol. 1990;172:6783–6788. doi: 10.1128/jb.172.12.6783-6788.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Waage J K. Biopesticides at the crossroads: IPM products or chemical clones? In: Evans H F, editor. Microbial insecticides: novelty or necessity? Nottingham, England: Major Design & Production Ltd.; 1997. pp. 11–20. [Google Scholar]