Abstract
The hippocampal formation is needed to encode episodic memories, which may be consciously recalled at some future time. This review examines recent advances in understanding recollection in the context of spatiotemporally organized relational memory coding and discusses predictions and challenges for future research on conscious remembering.
We experience time in the present, with each fleeting moment slipping behind us into the past. A new electrophysiological study in humans suggests a plausible mechanism for recalling episodes and “reliving” the past: neurons within the hippocampus that were active during initial experience are reactivated on subsequent free recall of those experiences (Gelbard-Sagiv et al. 2008). A second study in rodents provides more detail about the nature of episodic encoding; the studies reveal that task-related sequential activity among a cohort of neurons in the hippocampus can be reliably generated in the absence of external input (Pastalkova et al. 2008). Taken together these studies indicate that sequential activation among neurons organized in an assembly may underlie the encoding and conscious retrieval of episodic memories.
Neuroscientists, psychologists, and philosophers have independently proposed the idea that conscious access to episodes is supported by sequential activation of neurons within an assembly (Crick and Koch 2004; Edelman 2003; Eichenbaum 2004). The three key elements of episodic memory—defined as our ability to consciously recall personal events and experiences—are spatiotemporal organization of events, a multimodal representation of past associations, and the binding of disparate percepts into a subjectively accessible construct. In the brain, episodic memory relies on information processing within the hippocampal formation (HPC, including regions dentate gyrus [DG], CA1–CA3, subiculum, and entorhinal cortex) (Eichenbaum 2004). Accordingly, lesions of the HPC disrupt rats' abilities to discriminate the correct sequence of sequentially presented novel odors, but do not affect odor recognition (Fortin et al. 2002). The physiology of the hippocampus reveals key mechanisms for encoding and retrieving sequential representations. Single hippocampal neurons fire in temporal sequences as rats move through sequential locations along a track (place fields) (Dragoi and Buzsáki 2006). Further, individual cells fire in phase relation to the local theta electroencephalogram—a 6- to 12-Hz brain rhythm present throughout the HPC—and demonstrate sequential firing across a compressed timescale during slow-wave sleep (Lee and Wilson 2002). There is also evidence that hippocampal neurons change firing rates in response to conscious memory recall. In humans, many cells in the medial temporal lobe respond to familiar faces or landmarks and exhibit all or no increases in firing rate that correlate with conscious awareness (i.e., by verbal report) (Quiroga et al. 2008).
Cell assembly theories, first proposed by Hebb (1949), provide a mechanistic framework for understanding memory coding and retrieval. The assembly, a network of many connected nodes, represents information as collections of features (Fig. 1). Network nodes may correspond to individual or collections of neurons in the brain. To be useful for memory, each node would represent a content-addressable element of a particular episode (a smell, location, image, or an abstract concept). In a direct test of the “cell assembly hypothesis,” it was found that hippocampal neuron populations are arranged into groups whose synchrony exceeds that predicted by spatial location alone (Harris et al. 2003). Rather, information about the simultaneous firing of other HPC cells increased accuracy in the prediction of the firing times of single target cells, suggesting that anatomically distributed neurons participate in unique cell assemblies.
FIG. 1.
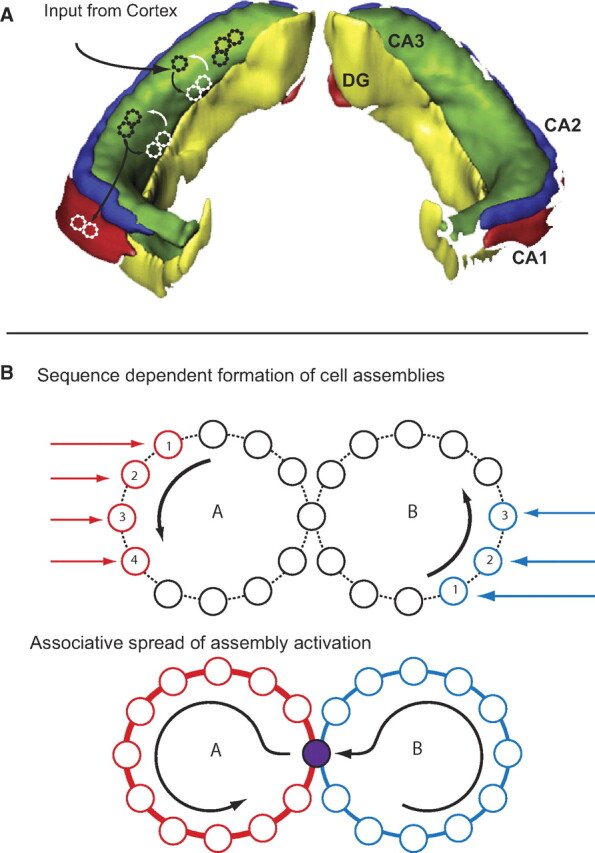
Schematic of hippocampal assemblies for episodic encoding. A: ventral view of human hippocampal formation (HPC) with color-coded HPC subfields (CA1–CA3, dentate gyrus [DG]; adapted from Thompson et al. 2008). Input from the cortex drives CA3 neural assemblies, which interact with other CA3 cells through recurrent collaterals (arrows). CA3 assemblies project to CA1 and may form associative networks to support complex episodes with distributed representations. B: sensory inputs (sounds, images, etc.: colored arrows) arrive sequentially and serially activate individual nodes (circles) of each assembly A and B, which are initially connected weakly (dashed lines). Active nodes are associated through potentiation and form strong connections with each other (colored lines) after simultaneous activation. Related assemblies share common nodes (purple circle) with overlapping representations. Activity spread from assembly B (bottom, curved arrow) could travel through the common node to activate the representation stored in assembly A and support pattern completion of episodic memories.
Networks that support related episodic memories would have to link these elements, i.e., by sharing common nodes that represent features that are common among different memories. In this way, single neurons may participate in multiple associated networks or assemblies. Episodic memories may thereby be linked by common features and can be internally reassembled and consciously “relived” at any time. The recollection of an episode would require sequential activation of the encoded representations.
How might neural assemblies account for recollection? Perhaps, subjective recall of episodes activates the same cohort of neurons that mediates their initial acquisition. We now know that single neurons in the human HPC have highly similar activation patterns during the acquisition and recall of episodes; the same neural assembly engaged by perception is reinstated during free recall of the same episodic sequences (Gelbard-Sagiv et al. 2008). Thirteen patients with intractable epilepsy were implanted with electrodes in the medial temporal lobe and anterior cingulate to identify seizure-generating foci. Individual cells in the HPC and anterior cingulate were then recorded while subjects viewed short audiovisual television clips in a repeated, pseudorandomized order. During viewing, nearly 55% of recorded cells increased their firing rates in response to at least one clip. Of these responsive neurons 10% showed sustained activation throughout the duration of at least one clip. By 1–5 min after the initial viewing (during which patients performed an intervening task) patients were asked to freely recall the movies that they had seen, in the absence of external stimuli. Neurons within the medial temporal lobe that preferentially responded to clips during initial viewing subsequently responded just prior to free recall of these same clips with an elevated firing rate that persisted for seconds even during a single-recall trial. For example, a single unit in the right entorhinal cortex with a sustained response to an episode of The Simpsons during initial viewing was later reactivated during free recall 1,500 ms before the subject reported thinking about the Simpson's scene and continued firing for >10 s. Although reactivation during free recall was observed in the population of all initially responsive HPC and entorhinal cortical cells, it was absent from units recorded from the anterior cingulate, amygdala, and parahippocampal gyrus. These observations offer the first strong evidence that individual neurons within the HPC code episodic representations (across short timescales) to support future conscious recall. Even though the existence of such neurons alone does not prove their requirement for conscious future recall, it does provide strong converging evidence that the same cells required for episodic memory support a neuronal mechanism for the emergence of past episodes into our present awareness.
Not only the selectivity of a neuron's sustained responses to particular scenes but also its transient firing in response to “nonpreferred” scenes are consistent with predictions of the Hebbian network assembly model. The transient versus sustained firing responses of single neurons may be interpreted as coding distinct representations between different episodes. Assembly theory predicts that a single episode is represented by the collective activity of many linked neurons that individually fire with different selectivities. By this logic, hippocampal assemblies should support pattern completion; sufficient activation among associated nonselective and selective nodes of the network could spread activity via recurrent collaterals to related cells and initiate activation of the entire episodic representation. If this reasoning is correct, then replaying only the audio or video from a particular clip would reactivate the same network activity that was recorded during initial encoding through associative activity spread (Fig. 1B).
Hebbian assembly models of episodic memory further predict that consistent and repeated conscious recollection of episodic memories is dependent on the reliable sequential activation of individual cells within an episodic network across multiple trials. Pastalkova et al. (2008) explored this hypothesis in rats running in a wheel during the delay of an HPC-dependent two-choice alternation task. If the place cells—that is, those cells with spatial receptive fields tuned to a particular location—that were active during maze exploration also reflect episodic coding, then similar patterns of activation should emerge during the wheel run (when spatial variables are constant). Single neurons recorded from the CA1 region of the HPC fired transiently and precisely at reliable places across repeated trials of maze runs. While rats were running in the wheel (with constant external landmarks and cues) between maze trials (10- to 20-s duration), the percentage of active cells increased more than threefold above levels observed in maze running, suggesting that the wheel run was accompanied by internally generated sequences (episode fields). Neural activity patterns were broadly similar during the maze and wheel-running tasks, so that the overall duration of activity for single neurons, average population autocorrelation functions, and the existence of phase precession (phase-specific firing to progressively earlier phases of theta cycles) along the local theta rhythm were indistinguishable. The authors interpret such similarities as evidence that episode cells in rats during the wheel run were generated by the same mechanism as place cells in rats during the maze run. Consistent with this hypothesis, reliable generation of episode fields during the wheel run crucially depended on memory demand on the maze.
When rats were required to spontaneously run in the wheel, without intervening HPC-dependent task performance, recorded neurons failed to respond in a transient and reliable pattern. Instead, the firing on the wheel was unreliable across trials, showing that wheel-running sequences were not generated by self-motion cues. Although the authors claim that head direction and speed of running were consistent throughout both tasks, these influences were not recorded as quantitative data.
Several neuronal assembly models have been postulated to underlie the emergence of thoughts and memories into conscious awareness. The theory of Neuronal Group Selection proposed by Edelman and colleagues proposes that each conscious experience is supported by a dynamic core of active neurons, generated through rapid “Darwinian” selection of neuronal groups that evolves with experience, and is selected through interaction with environment (Edelman 2004). The theory depends on integration of disparate events or items through recurrent collaterals, much like those present in the thalamocortical system and HPC, to maintain episodic mappings. Crick and Koch (2003) similarly describe one of the neural correlates of consciousness as a “maintained coalition of cells in high level” areas of the brain, such as the HPC. Both models propose that the medial temporal lobe is a critical locale for the correlation of neuronal activity with subjective perception. Because animals cannot verbalize their responses and indicate conscious accessibility, recordings derived from cognizant humans to clarify the nature of neural assemblies are invaluable.
Central to current models of episodic representation is an understanding of the stability and persistence of sequential activity within coalitions of neurons. We have yet to discover repeated patterns of activity among a population of human neurons that coincides with the unfurling of conscious perception. Such a discovery remains a gap in understanding how sequential activity contributes to conscious recall. Single human medial temporal lobe cell firing correlates with episodic recall that reaches awareness (Quiroga et al. 2008). However, those cells may be required only for subconscious access to information. Because hippocampal lesions do not abolish conscious experience, experiments that dissociate conscious accessibility from subconscious activation of episodic representations will be important to identify the role of neural assemblies in these processes.
Both articles discussed in the preceding text examine the neuronal substrates of recently acquired episodes and so represent recollection of short-term memory. Hippocampal-dependent memories undergo consolidation from a transient state into a stable, HPC-independent, long-lasting memory trace (Debiec et al. 2002) across days to weeks. Reactivation of these long-term memories returns the episodes to a “labile” state, dependent on protein transcription in the HPC. We experience the recalled long-term memories as similar, albeit less vivid, to recollection of recent memories. The HPC may represent episodic memories over long timescales in one of two ways: 1) it may maintain the identical physical mapping of sequential items and events to neurons as when the episode was initially internally constructed in the HPC or 2) it may reassign new physical mappings to neurons that had previously unrelated event and item preferences. Although the latter option limits the findings of the current studies to short timescales (minutes), it permits a more flexible and larger capacity short-term memory buffer for the encoding of new experiences. The long-term memory remapping hypothesis (2) can be tested using either paradigm described, but requires that individual HPC cells be stably recorded over periods of days to weeks. Because maintaining such recording stability is experimentally intractable at the present, future advances in recording technology will be necessary to test this hypothesis.
REFERENCES
- Debiec 2002.Debiec J, LeDoux JE, Nader K. Cellular and systems reconsolidation in the hippocampus. Neuron 36: 527–538, 2002. [DOI] [PubMed] [Google Scholar]
- Dragoi 2006.Dragoi G, Buzsáki G. Temporal encoding of place sequences by hippocampal cell assemblies. Neuron 50: 145–157, 2006. [DOI] [PubMed] [Google Scholar]
- Edelman 2004.Edelman G. Wider Than the Sky: The Phenomenal Gift of Consciousness. New Haven, CT: Yale Univ. Press, 2004, p. 148.
- Eichenbaum 2004.Eichenbaum H. Hippocampus: cognitive processes and neural representations that underlie declarative memory. Neuron 44: 109–120, 2004. [DOI] [PubMed] [Google Scholar]
- Fortin 2002.Fortin NJ, Agster KL, Eichenbaum HB. Critical role of the hippocampus in memory for sequences of events. Nat Neurosci 5: 458–462, 2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gelbard-Sagiv 2008.Gelbard-Sagiv H, Mukamel R, Harel M, Malach R, Fried I. Internally generated reactivation of single neurons in human hippocampus during free recall. Science 322: 96–101, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harris 2003.Harris KD, Csicsvari J, Hirase H, Dragoi G, Buzsáki G. Organization of cell assemblies in the hippocampus. Nature 424: 552–556, 2003. [DOI] [PubMed] [Google Scholar]
- Hebb 1949.Hebb DO. The Organization of Behavior. New York: Wiley, 1949.
- Lee 2002.Lee AK, Wilson MA. Memory of sequential experience in the hippocampus during slow wave sleep. Neuron 36: 1183–1194, 2002. [DOI] [PubMed] [Google Scholar]
- Pastalkova 2008.Pastalkova E, Itskov V, Amarasingham A, Buzsáki G. Internally generated cell assembly sequences in the rat hippocampus. Science 321: 1322–1327, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quiroga 2008.Quiroga RQ, Mukamel R, Isham EA, Malach R, Fried I. Human single-neuron responses at the threshold of conscious recognition. Proc Natl Acad Sci USA 105: 3599–3604, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thompson 2008.Thompson CL, Pathak SD, Jeromin A, Ng LL, MacPherson CR, Mortrud MT, Cusick A, Riley ZL, Sunkin SM, Bernard A, Puchalski RB, Gage FH, Jones AR, Bajic VB, Hawrylycz MJ, Lein ES. Genomic anatomy of the hippocampus. Neuron 60: 1010–1021, 2008. [DOI] [PubMed] [Google Scholar]


