Abstract
Background:
Depressive symptoms may increase risk for dementia, but findings are controversial because late-life depression may be a prodromal dementia symptom. Life course data on depression and dementia risk may clarify this association; however, data is limited.
Objective:
To impute adult depressive symptoms trajectories across adult life stages and estimate the association with cognitive impairment and decline.
Methods:
Using a pooled study of 4 prospective cohorts (ages 20–89), we imputed adult life course depressive symptoms trajectories based on Center for Epidemiologic Studies Depression Scale-10 (CESD-10) and calculated time-weighted averages for early adulthood (ages 20–49), mid-life (ages 50–69), and late-life (ages 70–89) for 6,122 older participants. Adjusted pooled logistic and mixed-effects models estimated associations of imputed depressive symptoms with two cognitive outcomes: cognitive impairment defined by established criteria and a composite cognitive score.
Results:
In separate models, elevated depressive symptoms in each life stage were associated with cognitive outcomes: early adulthood OR for cognitive impairment = 1.59 (95% CI: 1.35,1.87); mid-life OR = 1.94 (95%CI:1.16, 3.26); and late-life OR = 1.77 (95%CI:1.42, 2.21). When adjusted for depressive symptoms in the other life-stages, elevated depressive symptoms in early adulthood (OR = 1.73; 95%CI: 1.42,2.11) and late-life (OR = 1.43; 95%CI: 1.08,1.89) remained associated with cognitive impairment and were also associated with faster rates of cognitive decline (p < 0.05).
Conclusion:
Imputing depressive symptom trajectories from pooled cohorts may help expand data across the life course. Our findings suggest early adulthood depressive symptoms may be a risk factor for cognitive impairment independent of mid- or late-life depressive symptoms.
Keywords: Cognitive impairment, dementia, depression, imputation, life course
INTRODUCTION
Depressive symptoms are common, with up to 20% of adults experiencing a clinical depressive episode during their lifetime [1]. Numerous studies have found associations of late-life depression or higher depressive symptoms with dementia and cognitive decline [2–8] but some findings are conflicting [9–11]. Depression or high depressive symptoms could increase risk for cognitive impairment by affecting cognitive reserve or brain function [12]. Alternatively, depression could be a prodromal symptom of cognitive decline or dementia [13], a mimic of dementia [14], or a consequence of shared risk factors such as cardiovascular disease [3, 15]. Examining associations with depression across the life course could help rule out some of these alternative pathways and identify critical periods for possible intervention.
The relationships between depressive symptoms and dementia may depend on the life stages during which depression occurs [7, 16–18], which has important treatment and prevention implications. Depression can be a life-long illness with onset often occurring in young adulthood [19, 20]. Some studies suggest that early or mid-life depression may be associated with cognitive impairment [5, 17, 21, 22]. However, other studies find only later-onset depressive symptoms are associated with increased dementia risk and are more likely a prodrome for dementia [10, 23–28]. Additional life course data may help clarify whether and when depressive symptoms are associated with cognitive aging, but few studies span the whole adult life course. Synthetic or pooled cohort studies may help address this limitation and have been successfully implemented for cardiovascular risk factors [29–31]. Following this approach, we imputed long-term cumulative trajectories of depressive symptoms in a previously pooled cohort [29] and then examined their relationship with cognitive impairment and cognition in late life.
METHODS
Parent pooled cohort
We pooled data on 15,001 Black and White men and women aged 18 to 95 years old at baseline from four population-based cohorts: the Coronary Artery Risk Development in Young Adults (CARDIA) study of young to middle-aged adults [32], the Multi Ethnic Study of Atherosclerosis (MESA) [33] of middle to older-aged adults, the Health, Aging and Body Composition (HABC) [34] study of older adults and the Cardiovascular Health Study (CHS) of older adults [35]. The pooled cohort included: 4,632 Black and White adults aged 18–30 at enrollment (1985–86) and followed for 30 years from CARDIA; 4,238 Black and White adults aged 45–84 at enrollment (2000–2001) and followed for 10 years from MESA; 3,936 primarily white adults aged 65 and older at baseline (1989–1993) and followed for 10 years from CHS; and 2,195 Black and White adults ages 70–79 at enrollment (1997) and followed for 11 years from Health ABC. Measures were harmonized across cohorts. Details of the parent pooled cohort have been published elsewhere [29]. All participants provided written informed consent and each study was approved by local institutional review boards (IRBs), and this current analysis was approved by the Columbia University and University of California San Francisco IRBs approval.
Analytic sample
Primary analyses were conducted among the older participants in HABC and CHS, from the pooled parent cohort [29]. HABC is a recently completed prospective cohort study of 3,075 comprised of a random sample of Medicare-eligible community-dwelling older adults (52% women and 42% Black) aged 70–79 years at baseline (1997) who were living in Memphis and Pittsburgh [34]. Participants were followed annually or semi-annually for up to 11 years. CHS is an ongoing prospective study started in 1989; 5,888 community-dwelling adults (age 65 or older) were recruited from four US communities: Washington County in Maryland, Forsyth County in North Carolina, Sacramento County in California, and Allegheny County in Pennsylvania [35]. CHS participants were 58% women and 16% Black and were followed in-person annually for up to 10 years. Analyses were limited to participants from the pooled parent cohort [29] with complete data on depressive symptoms, dementia status, cognitive status, and covariates (n excluded HABC = 883, n excluded CHS = 1,958).
Depressive symptoms and imputation procedures
Depressive symptoms were measured in each study with the Center for Epidemiologic Studies Depression scale short form (CESD-10), which has 10 screening questions for depression (derived from the CESD-20 for CHS participants) [36]. We used previously established imputation methods based on the pooled parent cohort to estimate and impute trajectories of depressive symptoms over the adult life course for HABC and CHS participants, who were only observed in late-life [29]. Briefly, generalized linear mixed models (GLMMs) were used to estimate flexible trajectories of CESD-10 scores using data for all four cohorts (race-and-sex specific). The imputation model included splines for age and birth year, a four-level categorization of sex and race, as well as interactions of the age splines with sex/race, body mass index (BMI), diabetes, smoking status, cohort, and history and current use of anti-depressant use. The model also included random intercepts and random age splines, which account for deviations of the observed CESD score for each participant from the expected value determined by the fixed effects. Imputations on covariates were run sequentially prior to imputing CESD trajectories; the full imputation procedure is described in detail in the Supplementary Material. From the fitted GLMM, participant-specific trajectories from age 20 through the end of follow-up for each participant were estimated by best linear unbiased predictions (BLUPs) and were summarized for each participant as time-weighted averages (TWAs) within three life course periods: early adulthood (ages 20–49), mid-life (ages (50–69), and late-life (ages 70+). The CESD TWAs for each period were categorized as low (0–3), moderate (4–10), and high (> 10) levels of depressive symptoms.
Cognitive outcomes
Cognition was measured in both Health ABC (approximately biannually) and CHS (annually) by the Modified Mini-Mental State Exam (3MS), a test of global cognitive function [37], and the Digit Symbol Substitution Test (DSST), a measure of processing speed [38]. Incidence of cognitive impairment or dementia (subsequently referred to as cognitive impairment) was determined by each study protocol. In HABC, cognitive impairment was defined as meeting one or more of the following criteria through study Year 15: documented use of dementia medication; hospitalization with dementia as a primary or secondary diagnosis; or clinically meaningful global cognitive decline (1.5 SDs, racestratified) [39]. In CHS, a neuropsychological test battery was administered to selected participants at high risk for dementia; participants who failed the memory test or in at least two cognitive domains underwent a detailed neurological examination by a neurologist. Cognitive impairment (mild cognitive impairment or dementia) was diagnosed by an adjudication committee using all available data from each participant [40]. We also created a composite cognitive measure that combined the 3MS and DSST calculated as the average of the Z-scores for the two outcomes, after normalizing 3MS using -log (101–3MS). We used all observations to get the means and SDs used in Z-transformation of DSST and -log reflected 3MS. The resulting composite was standard normal, with mean 0.08 and SD 1, and a QQ-plot showed neither short nor long tails.
Covariates
We pooled and harmonized measures from each cohort including age at visit, calendar year, sex, race, educational attainment, cohort, BMI, history of diabetes, and smoking status. Anti-depressant medication use was defined based on a combination of participant interview, medical record review, and medication assessment. We used previously imputed cardiovascular risk factors TWAs for each life course period (BMI, systolic blood pressure, fasting low density lipoprotein cholesterol, fasting total cholesterol, and fasting glucose levels) [29], details on the imputation procedure for these variables is included in the Supplementary Material.
Statistical analysis
Pooled cohort imputation procedures were previously validated for cardiovascular risk factors [29], to further validate the imputation procedure for depressive symptoms we first examined the consistency of CESD trajectories across cohorts with imputed and observed trajectories, stratified by sex and race. Overlap in trajectories helps indicate that trends in depressive symptoms from the earlier cohorts (CARDIA and MESA) can be applied the late-life cohorts (HABC and CHS). Next, we ran a simulation study in which imputed BLUPs and TWAs were treated as the true values, and then used these as the basis for simulating new observations by adding random normal errors, with standard deviation (SD) determined by the initial GLMMs, to the imputed BLUPs at each observed age. We then obtained a new set of BLUPs and TWAs by refitting the GLMMs to these imputed outcomes; 25 imputations were used. We assessed agreement between the true and imputed TWAs using: 1) the bias of the imputed TWAs, scaled by the mean of the true values; 2) the mean absolute deviation (MAD) of the imputed from the true values, scaled by the MAD of the true measure from its sample mean; 3) correlation of the true and imputed continuous TWAs; and 4) agreement of the true and imputed TWAs, after categorization at established clinical cut-points.
We then examined the association between imputed depressive symptom TWAs and incident cognitive impairment in HABC and CHS participants. We used pooled logistic regression models to estimate the association of depressive symptoms with odds of subsequently developing cognitive impairment. Models allowed for left truncation by age at study entry, censoring, and time-varying covariates. Depressive symptom TWAs in early adulthood (ages 20–49) and in mid-life (ages 50–69) were fixed covariates as they were accrued prior to cohort entry. However, the depressive symptoms TWAs in late-life (ages 70–89) were time-varying. Among individuals with cognitive impairment, late-life TWAs were based on imputed depressive trajectories prior to onset of cognitive impairment. In primary analyses, we assessed depressive symptom TWAs by each life-period separately. In a secondary analysis, we included all life-periods in one model together. We calculated cognitive impairment incidence rates within periodspecific CESD-10 categories by sex and by race using Poisson regression models adjusting for age, sex (for estimates by race), race/ethnicity (for estimates by sex), education, and cohort; adjusted incidence rates were obtained using regression standardization. We plotted estimated adjusted incidence rates and 95% confidence intervals (CIs). We also examined whether associations between depressive symptoms and cognitive outcomes differed by sex or race.
Finally, as another secondary analysis we used linear mixed models to estimate the associations between depressive symptom TWAs and longitudinal change in composite cognition. Interaction terms between age and depressive symptom TWAs levels tested whether rate of change in cognition by age was associated with depressive symptom TWAs. All models adjusted for age, sex, race, education, cohort, and anti-depressant use. In a sensitivity analysis we re-ran models with additional adjustment for cardiovascular risk factor TWAs. Analyses were conducted using STATA version 16.
RESULTS
Imputation validation
There was generally good overlap between the individual cohort trajectories and imputed depressive symptom trajectories (Fig. 1). Depressive symptoms fit a U-shaped curve across age and increased especially in later ages; trends were relatively similar by sex and race. There were some differences in level of depressive symptoms between Health ABC and CHS, this supported inclusion of cohort-specific intercepts in the imputation models. The simulation study suggested high accuracy and agreement of imputed CESD trajectories across cohorts (Supplementary Table 1). There was a slight underestimation for imputations of earlier life periods for Health ABC and CHS (−5% bias) but high correlation and agreement for TWAs (at or above 0.90). Scatterplots for a 25% random sample of the true and imputed TWAs are presented in Supplementary Figure 1.
Fig. 1.
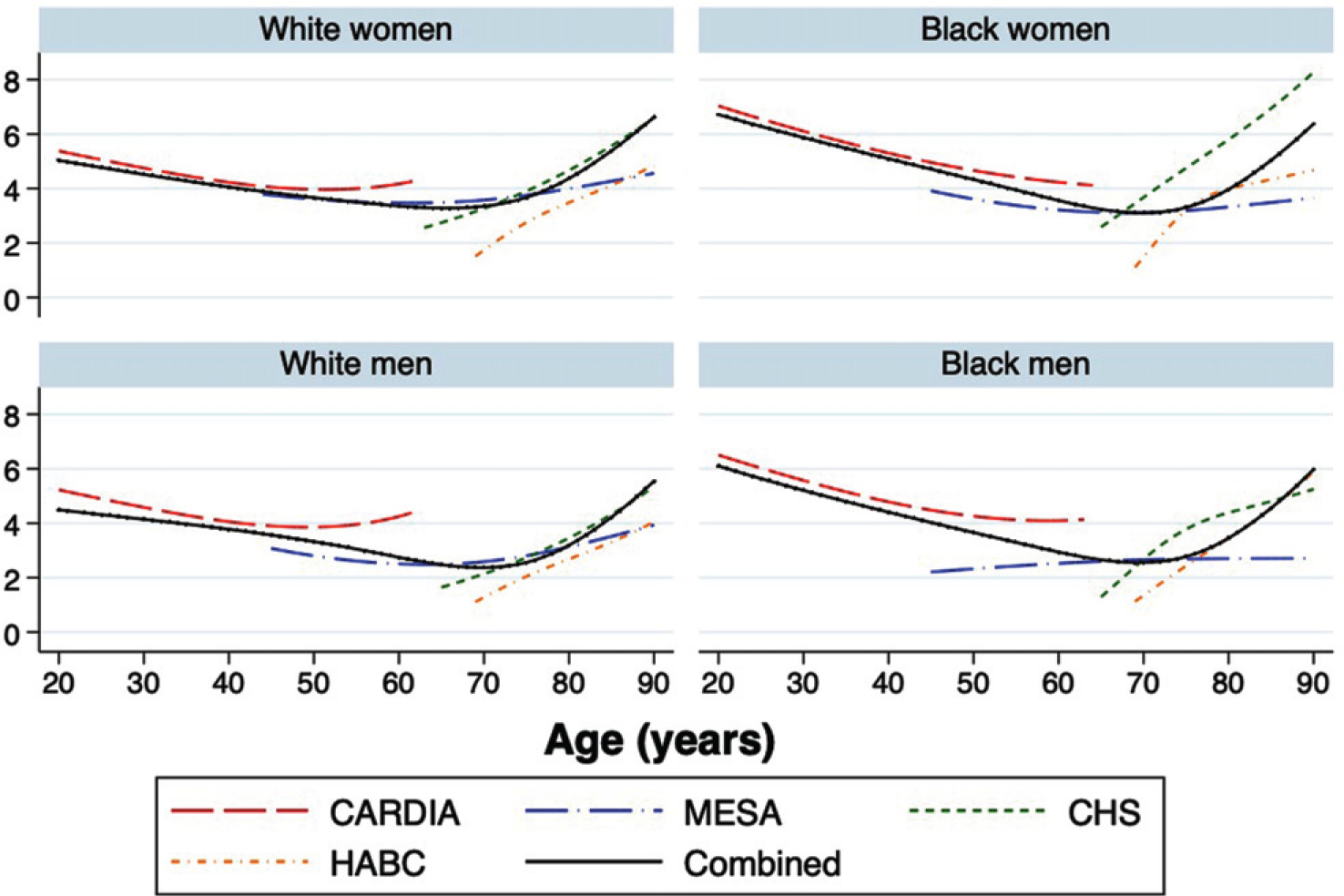
Imputed depressive symptom trajectories by cohort, sex, and race.
Analytic sample characteristics
6,122 participants in HABC and CHS were a median age of 72 (IQR 79–75) at baseline: 55.8% women and 16.1% Black (Table 1). Moderate or high estimated depressive symptom TWAs were relatively common and positively correlated with age (13% in early adulthood, 26% in mid-life and 34% in late-life); estimates were slightly higher for CHS participants compared to HABC participants. Early adulthood depressive symptom TWAs were not highly correlated with mid-life (0.06) and were slightly inversely correlated (−0.13) with late-life depressive symptom TWAs. Mid-life and late life depressive symptoms TWAs were more strongly correlated (0.39).
Table 1.
Depression TWAs by cohort, evaluated at study entry
| CHS | HABC | Total | |
|---|---|---|---|
|
| |||
| Characteristics | N = 3,930 | N = 2,192 N(%) | N = 6,122 |
| Age | |||
| <70 | 1,458 (37.1) | 85 (3.9) | 1,543 (25.2) |
| 70–79 | 2,072 (52.7) | 2,092 (95.4) | 4,164 (68.0) |
| >=80 | 400 (10.2) | 15 (0.7) | 415 (6.8) |
| Race/ethnicity and sex | |||
| White women | 2,150 (54.7) | 673 (30.7) | 2,823 (46.1) |
| Black women | 109 (2.8) | 488 (22.3) | 598 (9.8) |
| White men | 1,608 (40.9) | 704 (32.1) | 2,311 (37.7) |
| Black men | 63 (1.6) | 327 (14.9) | 390 (6.4) |
| Education | |||
| <HS | 960 (24.4) | 487 (22.2) | 1,449 (23.7) |
| Completed HS | 1,134 (28.9) | 704 (32.2) | 1,838 (30.0) |
| >HS | 1,836 (46.7) | 998 (45.6) | 2,835 (46.3) |
| CESD TWA for ages 20–49 | |||
| 0–3 | 3,197 (81.3) | 2,107 (96.1) | 5,304 (86.6) |
| 4–10 | 733 (18.7) | 85 (3.9) | 818 (13.4) |
| CESD TWA for ages 50–69 | |||
| 0–3 | 2,670 (67.9) | 1,834 (83.7) | 4,504 (73.6) |
| 4–10 | 1,221 (31.1) | 357 (16.3) | 1,959 (32.0) |
| >10 | 39 (1.0) | 1 (0.0) | 40 (0.7) |
| CESD TWA for ages 70–89 | |||
| 0–3 | 2,630 (66.9) | 1,412 (64.4) | 4,042 (66.0) |
| 4–10 | 1,211 (30.8) | 749 (34.2) | 1,959 (32.0) |
| >10 | 89 (2.3) | 31 (1.4) | 121 (2.0) |
Imputed depressive symptoms and incident cognitive impairment
There were 1,277 cases of incident cognitive impairment. In our primary analyses, when analyzed separately, higher depressive symptoms at each life course stage were associated with a higher risk of incident cognitive impairment (Table 2). In early adulthood, moderate depressive symptom TWAs were associated with a 59% (95% CI: 35%, 89%) higher odds of onset of cognitive impairment. Moderate and high depressive symptom TWAs in mid and late-life were associated with 33–94% higher odds (Table 2). In a secondary analysis, when we adjusted for the TWAs in other life course stages, moderate and higher depressive symptoms in early adulthood and late-life remained significantly associated with higher odds of dementia (Table 2). In this model, use of anti-depressants was also significantly associated with increased odds of dementia beyond effects of depressive symptoms (OR: 2.19; 95% CI: 1.82, 2.65). Adjustment for cardiovascular risk factor TWAs did not substantially change model estimates.
Table 2.
Odds of incident cognitive impairment by imputed depressive symptom time weighted averages adjusted for age, sex, race, cohort, and anti-depressant use (n = 5,098:2,906 CHS and 2,192 HABC participants)
| Separate models for each life course period (Primary Analysis) | |||
|
| |||
| Early Adulthood | Mid-life | Late-life | |
|
| |||
| CESD-10 | OR (95% CI) | OR (95% CI) | OR (95% CI) |
| Low (0–3) | ref | ref | ref |
| Moderate (4–10) | 1.59 (1.35, 1.89) | 1.33 (1.17, 1.51) | 1.34 (1.19, 1.50) |
| High (10+) | NA* | 1.94 (1.16, 3.26) | 1.77 (1.42, 2.21) |
|
| |||
| One model, each life course period adjusted for other periods (Secondary Analysis) | |||
|
| |||
| CESD-10 | Early Adulthood | Mid-life | Late-life |
|
| |||
| Low (0–3) | ref | ref | ref |
| Moderate (4–10) | 1.38 (1.14, 1.68) | 1.00 (0.85, 1.19) | 1.29 (1.14, 1.47) |
| High (10+) | NA* | 1.16 (0.65, 2.06) | 1.47 (1.12, 1.93) |
No participants had imputed young adulthood TWAs in this range.
Estimated incidence rates of cognitive impairment are showed in Fig. 2. In general, the incidence of cognitive impairment was higher for those with higher depressive symptom TWAs in early adulthood and late-life. Trends for late-life depressive symptoms in men were less consistent than other subgroups. Incidence rates and trends associated with higher depressive symptoms were higher overall for Black participants compared to White participants, although estimates had less precision.
Fig. 2.
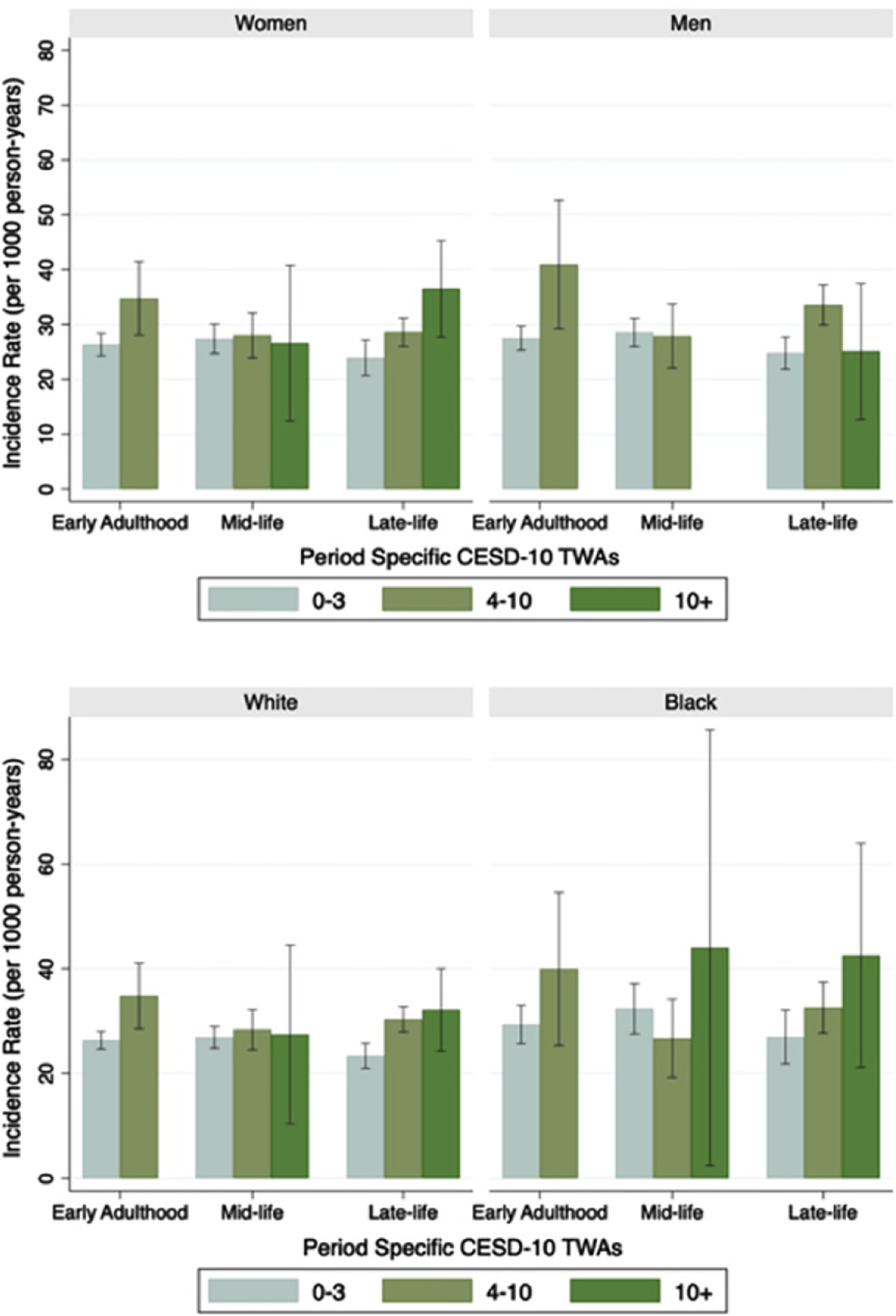
Adjusted incidence rates of cognitive impairment for depressive symptom time weighted averages (TWAs) by sex and race. Based on Poisson regression models adjusted for age, cohort, anti-depressant use and each other life course stage (n = 5,098). Early adulthood TWAs CESD-10 + and mid-life CESD-10 + for men are not shown due to no or few participants. Error bars represent 95% confidence intervals.
Imputed depressive symptoms and rate of cognitive decline
Higher depressive symptom TWAs were also generally associated with lower cognition and faster rates of decline, even when adjusted for other life course stages (Table 3). Early adulthood moderate depressive symptoms were associated with decreasing composite cognitive z-scores over time. Those with moderate depressive symptom TWAs in early adulthood (4–10 CESD-10 scores) had −0.07 SD of decline in composite cognition over 10 years (95% CI: −0.13, −0.01). Higher mid-life depressive symptoms were associated with lower cognition at baseline but not faster rates of decline (moderate depressive symptom TWAs were associated with a slower rate of decline than low depressive symptoms paradoxically). Higher late-life depressive symptoms were strongly associated with both baseline level and faster rates of decline. Those with high depressive symptom (CESD 10+) TWAs had −0.26 SD of decline in composite cognition over 10 years in late-life compared to those with low depressive symptoms TWAs (95% CI: −0.13, −0.01).
Table 3.
Model for association of CESD-10 TWAs with composite cognitive function outcome with inclusion of each life course stage age, sex, race, cohort, and anti-depressant use as predictors (45,811 observations, 6,122 [3,930 CHS, 2,192 HABC] participants)
| Early Adulthood β (95% CI) | Mid-life β (95% CI) | Late-life β (95% CI) | |
|---|---|---|---|
|
| |||
| Difference in cognition at age 80 versus CESD-10 low (0–3) scores | |||
|
| |||
| CESD-10 | |||
| Moderate (4–10) | 0.01 (−0.06, 0.08) | −0.04 (−0.09, 0.02) | −0.10 (−0.12, −0.07) |
| High (10+) | NA* | −0.28 (−0.55, −0.01) | −0.15 (−0.21, −0.10) |
|
| |||
| Difference in 10-year change in cognition versus CESD-10 low (0–3) scores | |||
|
| |||
| CESD-10 | |||
| Moderate (4–10) | −0.07 (−0.13, −0.01) | 0.08 (0.03,0.13) | −0.17 (−0.20, −0.14) |
| High (10+) | NA* | −0.07 (−0.29, 0.16) | −0.26 (−0.33, −0.20) |
Composite defined as average of Z-scores for DSST and −log(101-3MS).
DISCUSSION
We leveraged data from four population-based cohorts spanning the adult lifespan to impute depressive symptom trajectories across the adult life course and estimate associations with late-life cognitive decline and dementia. Depressive symptoms across the life course were associated with worse cognition, faster rates of cognitive decline and higher odds of developing cognitive impairment in late-life. In our primary analysis, early adulthood, mid-life, and late-life depressive symptoms were each separately associated with over 50% higher odds of cognitive impairment. With adjustment for depressive symptoms in other life course stages as well as imputed cardiovascular risk factor TWAs, early adulthood and late-life depression remained significantly associated with higher odds of cognitive impairment. Effects were relatively similar when looking at men, women, Black, and White participants. These associations were independent of anti-depressant use, which was also associated with increased odds of incident cognitive impairment. Although, the imputation procedure was extensive and rests on important assumptions, this approach offer insights into life course risk factors for dementia by pooling available cohort data. Our validation procedures suggest relatively good fit, accuracy, and agreement. There may be some underestimation of early adult and mid-life estimates, which would result in a conservative bias. These findings suggest that higher depressive symptoms in early adulthood are associated with cognitive impairment or dementia, independent of the effects of mid-life or late-life depressive symptoms.
Numerous studies have found associations between late-life depression, depressive symptoms, and future risk of cognitive impairment/dementia [2–7] but controversy remains as some studies have found no associations [9–11], and others have found differential effects by type and timing of depression [7, 17, 25, 27, 28]. It has remained difficult to distinguish whether depression is a cause of dementia or a prodromal symptom [41]. Several studies have found that earlier history of depression and recurrent or chronic depression is associated with increased risk of dementia and cognitive decline, suggesting depression is a risk factor for dementia [5, 42, 17, 8]. This is also supported by evidence that depression is associated with cognitive deficits and hippocampal volume in early life [21]. However, other studies have found that late-life and recent depression but not earlier depression is associated with dementia risk [10, 23–28], more consistent with depression as a prodrome rather than a true risk factor for dementia. We leveraged previously developed imputation methods and data from 4 U.S. cohorts to provide life course exposure estimates to examine depressive symptoms across the whole adult life span: early adulthood (ages 20–49), mid-life (50–69), and late-life (70–89). Although earlier depressive symptoms were imputed, and rest on assumptions, no longitudinal studies have been completed across the whole life course into late-life. Imputed depressive symptom trajectories fit a U-shaped curve, similar to agerelated trends in other research [19]; although further research is still needed to verify correlations between life course stages and trends in individual trajectories of depressive symptoms from early adulthood to late-life.
We found that higher depressive symptoms during each life course period was separately associated with cognitive impairment and cognitive decline, even after adjustment for concurrent vascular risk factors. Our finding that higher imputed depressive symptoms in early adulthood are independently associated with cognitive impairment apart from mid or late-life depressive symptoms is consistent with the hypothesis that early adulthood depression increases risk for dementia [12]. It is unclear how depression might increase dementia risk, although several mechanisms have been proposed [12, 41]. Patients with depression can show hyperactivity of the hypothalamic-pituitary-adrenal axis [43], which increases glucocorticoids and may lead to hippocampal damage and development of dementia. Depression has been associated with hippocampal atrophy [44–46] and faster rates of hippocampal volume loss in women [47]; although not all evidence is consistent or in support of glucocorticoids as the primary mediating mechanism [45, 48, 49]. Depression may also contribute to cognitive decline through other pathways such as vascular disease, inflammation, impact on nerve growth factors, or by increasing amyloid-beta accumulation [12]. Although we did not find significant independent association between mid-life depressive symptoms and cognitive impairment apart from depression in other life course periods, this may be due to the correlation between mid and late-life depressive symptom TWAs.
We also found independent associations of late-life depression with cognitive impairment apart from earlier depressive symptoms. Because of a potential overlap with late-life depression and neuropathology associated with dementia, this latter finding could be consistent either with depression as a prodrome or risk factor for dementia [41]. However, this suggests that individuals with late-life depressive symptoms, regardless of whether longstanding or incipient, are at higher risk of developing cognitive impairment. Depression, particularly in late-life, may be a symptom or marker for underlying Alzheimer’s disease (AD) or vascular neuropathology. In AD patients, those with depression have higher neurofibrillary tangle burden [50] and cognitively normal depressed patients had lower levels of cerebral spinal fluid amyloid-beta in one study [51]. Pathways linking cardiovascular disease, cerebrovascular disease, and depression (aka the vascular depression hypothesis [15]) also may explain a late-life depression-dementia association [52]. Lateonset depression is associated with cerebrovascular effects such as white matter hyperintensities [53], which also predict subsequent risk of depression [54] and some studies find stronger associations with depression and vascular dementia compared to AD [16, 17]. However, not all studies find evidence for depression or depressive symptoms as a prodromal symptom of dementia [55, 56]. Depression can mimic symptoms of cognitive impairment or it may be that depression affects cognitive function through impact on cognitive reserve, whereby depressed individuals have resilience to underlying pathology [56]. We also found increased odds of cognitive impairment associated with anti-depressant use independent of depressive symptom TWAs, similar to findings from some [57] but not all studies [58]. However, potential confounding by indication makes it difficult to disentangle whether there are effects of anti-depressant use or whether these indicate effects of more severe depression [58]. Future studies will be needed to determine underlying mechanisms and whether treatment of depression in early adulthood may be a strategy to reduce dementia burden.
This study has several important limitations. The most important assumption underlying the use of the pooled data for this purpose is that data from the early cohorts can be used to impute depressive symptom trajectories for the older cohorts. This was generally supported by our validation procedures, but error cannot be ruled out for instance if depression affected selection into early cohorts differently than older cohorts. Trajectories of depressive symptoms generally overlapped between cohorts, and fit a U-shaped curve as seen in other data [19] but data diverged a bit at the tail ends and in later ages. Imputation models were based on observed CESD-10 scores and covariates, however, error in the imputation model is possible and there may have been shrinkage in the early adulthood and mid-life TWAs towards the overall mean. Results from our simulation analysis further suggest there was good agreement and accuracy but a slight underestimation of depressive symptoms for earlier life course periods. This may have led to some misclassification of TWAs, especially in early adulthood. However, these errors are likely non-differential and would result in an underestimate of the true effect. It is difficult to tease apart the effects of depression from anti-depressant use, thus we could not fully address the impact of anti-depressant use apart from depressive symptoms. We examined depressive symptoms not diagnosed depression, and the sample population may not reflect the typical population of a psychiatric outpatient clinic, thus findings may not be generalizabile to clinical settings. HABC participants did not all have clinical dementia diagnoses, so we grouped mild cognitive impairment and dementia together to reduced potential misclassification of cognitive impairment status. These issues would tend to attenuate effect estimates, suggesting the true associations may be even stronger than estimated in this study. This study also has a number of strengths. We modeled life course risk factor trajectories of depressive symptoms from age 20 in Black and White men and women by pooling and harmonizing data from multiple prospective cohort studies with longitudinal follow-up using a previously developed imputation method [29]. We examined two outcomes: incident cognitive impairment and cognitive trajectories over time, which showed similar findings.
We found that higher depressive symptoms in early adulthood and late-life were independently associated with cognitive impairment/dementia and faster rates of cognitive decline. Our findings highlight the importance of life course exposures and suggest that early adulthood may be a critical time for modifying risk factors such as depression, for dementia. Future studies will be needed to verify findings of early adulthood depressive symptoms as well as to identify the primary mechanisms of the association between different life course stages of depression and dementia.
Supplementary Material
ACKNOWLEDGMENTS
This work was supported by grants from the National Institutes of Health, National Institute on Aging (1RF1AG054443).
CARDIA is supported by contracts HHSN2682 01800003I, HHSN268201800004I, HHSN2682018 00005I, HHSN268201800006I, and HHSN2682018 00007I from the National Heart, Lung, and Blood Institute (NHLBI).
MESA is supported by contracts 75N92020D00 001, HHSN268201500003I, N01-HC-95159, 75N9 2020D00005, N01-HC-95160, 75N92020D00002, N01-HC-95161, 75N92020D00003, N01-HC-951 62, 75N92020D00006, N01-HC-95163, 75N9202 0D00004, N01-HC-95164, 75N92020D00007, N01-HC-95165, N01-HC-95166, N01-HC-95167, N01-HC-95168 and N01-HC-95169 from the National Heart, Lung, and Blood Institute, and by grants UL1-TR-000040, UL1-TR-001079, and UL1-TR-001420 from the National Center for Advancing Translational Sciences (NCATS). The authors thank the other investigators, the staff, and the participants of the MESA study for their valuable contributions. A full list of participating MESA investigators and institutions can be found at http://www.mesa-nhlbi.org.
CHS is supported by contracts HHSN2682012 00036C, HHSN268200800007C, HHSN26820180 0001C, N01HC55222, N01HC85079, N01HC85 080, N01HC85081, N01HC85082, N01HC85083, N01HC85086, 75N92021D00006, and grants U01 HL080295 and U01HL130114 from the National Heart, Lung, and Blood Institute (NHLBI), with additional contribution from the National Institute of Neurological Disorders and Stroke (NINDS). Additional support was provided by R01AG023629 from the National Institute on Aging (NIA). A full list of principal CHS investigators and institutions can be found at CHS-NHLBI.org.
Health ABC study is supported by National Institute on Aging (NIA) Contracts N01-AG-6–2101; N01-AG-6–2103; NO 1-AG-6–2106; NIA grant R01-AG028050, and NINR grant R01-NR012459. Health ABC was funded in part by the Intramural Research Program of the NIH, National Institute on Aging.
The funding organization or sponsor had no role in the design and conduct of the study; collection, management, analysis, and interpretation of the data; preparation, review, or approval of the manuscript; and decision to submit the manuscript for publication.
Footnotes
Authors’ disclosures available online (https://www.j-alz.com/manuscript-disclosures/21-0588r1).
SUPPLEMENTARY MATERIAL
The supplementary material is available in the electronic version of this article: https://dx.doi.org/10.3233/JAD-210588.
REFERENCES
- [1].Kessler RC, Berglund P, Dernier O, Jin R, Merikangas KR, Walters EE (2005) Lifetime prevalence and age-of-onset distributions of DSM-IV disorders in the National Comorbidity Survey Replication. Arch Gen Psychiatry 62, 593–602. [DOI] [PubMed] [Google Scholar]
- [2].Yaffe K, Blackwell T, Gore R, Sands L, Reus V, Browner WS (1999) Depressive symptoms and cognitive decline in nondemented elderly women: A prospective study. Arch Gen Psychiatry 56, 425–430. [DOI] [PubMed] [Google Scholar]
- [3].Barnes DE, Alexopoulos GS, Lopez OL, Williamson JD, Yaffe K (2006) Depressive symptoms, vascular disease, and mild cognitive impairment: Findings from the Cardiovascular Health Study. Arch Gen Psychiatry 63, 273–279. [DOI] [PubMed] [Google Scholar]
- [4].Wilson R, Mendes d, Bennett D, Bienias J, Evans D (2004) Depressive symptoms and cognitive decline in a community population of older persons. J Neurol Neurosurg Psychiatry 75, 126–129. [PMC free article] [PubMed] [Google Scholar]
- [5].Ownby RL, Crocco E, Acevedo A, John V, Loewenstein D (2006) Depression and risk for Alzheimer disease: Systematic review, meta-analysis, and metaregression analysis. Arch Gen Psychiatry 63, 530–538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Saczynski JS, Beiser A, Seshadri S, Auerbach S, Wolf PA, Au R (2010) Depressive symptoms and risk of dementia: The Framingham Heart Study. Neurology 75, 35–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Kaup AR, Byers AL, Falvey C, Simonsick EM, Satterfield S, Ayonayon HN, Smagula SF, Rubin SM, Yaffe K (2016) Trajectories of depressive symptoms in older adults and risk of dementia. JAMA Psychiatry 73, 525–531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Zeki Al Hazzouri A, Vittinghoff E, Byers A, Covinsky K, Blazer D, Diem S, Ensrud KE, Yaffe K (2014) Long-term cumulative depressive symptom burden and risk of cognitive decline and dementia among very old women. J Gerontol A Biol Sci Med Sci 69, 595–601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Ganguli M, Du Y, Dodge HH, Ratcliff GG, Chang C-CH (2006) Depressive symptoms and cognitive decline in late life: A prospective epidemiological study. Arch Gen Psychiatry 63, 153–160. [DOI] [PubMed] [Google Scholar]
- [10].Vinkers DJ, Gussekloo J, Stek ML, Westendorp RGJ, van der Mast RC (2004) Temporal relation between depression and cognitive impairment in old age: Prospective population based study. BMJ 329, 881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Becker JT, Chang Y-F, Lopez OL, Dew MA, Sweet RA, Barnes D, Yaffe K, Young J, Kuller L, Reynolds CF (2009) Depressed mood is not a risk factor for incident dementia in a community-based cohort. Am J Geriatr Psychiatry 17, 653–663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Byers AL, Yaffe K (2011) Depression and risk of developing dementia. Nat Rev Neurol 7, 323–331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Lyketsos CG, Lopez O, Jones B, Fitzpatrick AL, Breitner J, DeKosky S (2002) Prevalence of neuropsychiatric symptoms in dementia and mild cognitive impairment. JAMA 288, 1475–1483. [DOI] [PubMed] [Google Scholar]
- [14].Alexopoulos GS, Meyers BS, Young RC, Mattis S, Kakuma T (1993) The course of geriatric depression with “reversible dementia”: A controlled study. Am J Psychiatry 150, 1693–1699. [DOI] [PubMed] [Google Scholar]
- [15].Alexopoulos GS, Meyers BS, Young RC, Campbell S, Silbersweig D, Charlson M (1997) “Vascular depression” hypothesis. Arch Gen Psychiatry 54, 915–922. [DOI] [PubMed] [Google Scholar]
- [16].Lenoir H, Dufouil C, Auriacombe S, Lacombe J-M, Dartigues J-F, Ritchie K, Tzourio C (2011) Depression history, depressive symptoms, and incident dementia: The 3C Study. J Alzheimers Dis 26, 27–38. [DOI] [PubMed] [Google Scholar]
- [17].Barnes DE, Yaffe K, Byers AL, McCormick M, Schaefer C, Whitmer RA (2012) Midlife vs late-life depressive symptoms and risk of dementia: Differential effects for Alzheimer disease and vascular dementia. Arch Gen Psychiatry 69, 493–498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Mirza SS, Wolters FJ, Swanson SA, Koudstaal PJ, Hofman A, Tiemeier H, Ikram MA (2016) 10-year trajectories of depressive symptoms and risk of dementia: A population-based study. Lancet Psychiatry 3, 628–635. [DOI] [PubMed] [Google Scholar]
- [19].Sutin AR, Terracciano A, Milaneschi Y, An Y, Ferrucci L, Zonderman AB (2013) The trajectory of depressive symptoms across the adult lifespan. JAMA Psychiatry 70, 803–811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Byers AL, Vittinghoff E, Lui L-Y, Hoang T, Blazer DG, Covinsky KE, Ensrud KE, Cauley JA, Hillier TA, Fredman L, Yaffe K (2012) Twenty-year depressive trajectories among older women. Arch Gen Psychiatry 69, 1073–1079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Barch DM, Harms MP, Tillman R, Hawkey E, Luby JL (2019) Early childhood depression, emotion regulation, episodic memory, and hippocampal development. J Abnorm Psychol 128, 81–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Holmquist S, Nordstrom A, Nordström P (2020) The association of depression with subsequent dementia diagnosis: A Swedish nationwide cohort study from 1964 to 2016. PLoS Med 17, el003016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Brommelhoff JA, Gatz M, Johansson B, McArdle JJ, Fratiglioni L, Pedersen NL (2009) Depression as a risk factor or prodromal feature for dementia? Findings in a population-based sample of Swedish twins. Psychol Aging 24, 373–384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Panza F, Frisardi V, Capurso C, D’ Introno A, Colacicco AM, Imbimbo BP, Santamato A, Vendemiale G, Seripa D, Pilotto A, Capurso A, Solfrizzi V (2010) Late-life depression, mild cognitive impairment, and dementia: Possible continuum? Am J Geriatr Psychiatry 18, 98–116. [DOI] [PubMed] [Google Scholar]
- [25].Li G, Wang LY, Shofer JB, Thompson ML, Peskind ER, McCormick W, Bowen JD, Crane PK, Larson EB (2011) Temporal relationship between depression and dementia: Findings from a large community-based 15-year follow-up study. Arch Gen Psychiatry 68, 970–977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Richard E, Reitz C, Honig LS, Schupf N, Tang MX, Manly JJ, Mayeux R, Devanand D, Luchsinger JA (2013) Late life depression, mild cognitive impairment and dementia. JAMA Neurol 70, 374–382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Singh-Manoux A, Dugravot A, Fournier A, Abell J, Ebmeier K, Kivimaki M, Sabia S (2017) Trajectories of depressive symptoms before diagnosis of dementia: A 28-year follow-up study. JAMA Psychiatry 74, 712–718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Lee ATC, Fung AWT, Richards M, Chan WC, Chiu HFK, Lee RSY, Lam LCW (2021) Risk of incident dementia varies with different onset and courses of depression. J Affect Disord 282, 915–920. [DOI] [PubMed] [Google Scholar]
- [29].Zeki Al Hazzouri A, Vittinghoff E, Zhang Y, Pletcher MJ, Moran AE, Bibbins-Domingo K, Golden SH, Yaffe K (2019) Use of a pooled cohort to impute cardiovascular disease risk factors across the adult life course. Int J Epidemiol 48, 1004–1013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Zeki Al Hazzouri A, Vittinghoff E, Hoang T, Golden SH, Fitzpatrick AL, Zhang A, Grasset L, Yaffe K (2021) Body mass index in early adulthood and dementia in late life: Findings from a pooled cohort. Alzheimers Dement, doi: 10.1002/alz.12367 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].Yaffe K, Vittinghoff E, Hoang T, Matthews K, Golden SH, Zeki Al Hazzouri A (2021) Cardiovascular risk factors across the life course and cognitive decline: A pooled cohort study. Neurology 96, e2212–e2219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32].Friedman GD, Cutter GR, Donahue RP, Hughes GH, Hulley SB, Jacobs DR, Fiu K, Savage PJ (1988) CARDIA: Study design, recruitment, and some characteristics of the examined subjects. J Clin Epidemiol 41, 1105–1116. [DOI] [PubMed] [Google Scholar]
- [33].Bild DE, Bluemke DA, Burke GE, Detrano R, Diez Roux AV, Folsom AR, Greenland P, Jacob DR, Kronmal R, Liu K, Nelson JC, O’Leary D, Saad MF, Shea S, Szklo M, Tracy RP (2002) Multi-Ethnic Study of Atherosclerosis: Objectives and design. Am J Epidemiol 156, 871–881. [DOI] [PubMed] [Google Scholar]
- [34].Harris TB, Visser M, Everhart J, Cauley J, Tylavsky F, Fuerst T, Zamboni M, Taaffe D, Resnick HE, Scherzinger A, Nevitt M (2000) Waist circumference and sagittal diameter reflect total body fat better than visceral fat in older men and women. The Health, Aging and Body Composition Study. Ann N Y Acad Sci 904, 462–473. [DOI] [PubMed] [Google Scholar]
- [35].Fried LP, Borhani NO, Enright P, Furberg CD, Gardin JM, Kronmal RA, Kuller LH, Manolio TA, Mittelmark MB, Newman A (1991) The Cardiovascular Health Study: Design and rationale. Ann Epidemiol 1, 263–276. [DOI] [PubMed] [Google Scholar]
- [36].Andresen EM, Malmgren JA, Carter WB, Patrick DL (1994) Screening for depression in well older adults: Evaluation of a short form of the CES-D (Center for Epidemiologic Studies Depression Scale). Am J Prev Med 10, 77–84. [PubMed] [Google Scholar]
- [37].Teng EL, Chui HC (1987) The Modified Mini-Mental State (3MS) examination. J Clin Psychiatry 48, 314–318. [PubMed] [Google Scholar]
- [38].Wechsler D (1987) Wechsler Memory Scale-Revised Manual, The Psychological Corporation, San Antonio, TX. [Google Scholar]
- [39].Hong CH, Falvey C, Harris TB, Simonsick EM, Satterfield S, Ferrucci L, Metti AL, Patel KV, Yaffe K (2013) Anemia and risk of dementia in older adults: Findings from the Health ABC study. Neurology 81, 528–533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [40].Lopez OL, Kuller LH, Fitzpatrick A, Ives D, Becker JT, Beauchamp N (2003) Evaluation of dementia in the cardiovascular health cognition study. Neuroepidemiology 22, 1–12. [DOI] [PubMed] [Google Scholar]
- [41].Bennett S, Thomas AJ (2014) Depression and dementia: Cause, consequence or coincidence? Maturitas 79, 184–190. [DOI] [PubMed] [Google Scholar]
- [42].Dotson VM, Beydoun MA, Zonderman AB (2010) Recurrent depressive symptoms and the incidence of dementia and mild cognitive impairment. Neurology 75, 27–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [43].Vreeburg SA, Hoogendijk WJG, van Pelt J, DeRijk RH, Verhagen JCM, van Dyck R, Smit JH, Zitman FG, Penninx BWJH (2009) Major depressive disorder and hypothalamicpituitary-adrenal axis activity: Results from a large cohort study. Arch Gen Psychiatry 66, 617–626. [DOI] [PubMed] [Google Scholar]
- [44].Taylor WD, McQuoid DR, Payne ME, Zannas AS, MacFall JR, Steffens DC (2014) Hippocampus atrophy and the longitudinal course of late-life depression. Am J Geriatr Psychiatry 22, 1504–1512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [45].O’Brien JT, Lloyd A, Me Keith I, Gholkar A, Ferrier N (2004) A longitudinal study of hippocampal volume, cortisol levels, and cognition in older depressed subjects. Am J Psychiatry 161, 2081–2090. [DOI] [PubMed] [Google Scholar]
- [46].Janssen J, Hulshoff Pol HE, Lampe IK, Schnack HG, de Leeuw F-E, Kahn RS, Heeren TJ (2004) Hippocampal changes and white matter lesions in early-onset depression. Biol Psychiatry 56, 825–831. [DOI] [PubMed] [Google Scholar]
- [47].Elbejjani M, Fuhrer R, Abrahamowicz M, Mazoyer B, Crivello F, Tzourio C, Dufouil C (2015) Depression, depressive symptoms, and rate of hippocampal atrophy in a longitudinal cohort of older men and women. Psychol Med 45, 1931–1944. [DOI] [PubMed] [Google Scholar]
- [48].Geerlings MI, den Heijer T, Koudstaal PJ, Hofman A, Breteler MMB (2008) History of depression, depressive symptoms, and medial temporal lobe atrophy and the risk of Alzheimer disease. Neurology 70, 1258–1264. [DOI] [PubMed] [Google Scholar]
- [49].Geerlings MI, Gerritsen L (2017) Fate-life depression, hippocampal volumes, and hypothalamic-pituitary-adrenal axis regulation: A systematic review and meta-analysis. Biol Psychiatry 82, 339–350. [DOI] [PubMed] [Google Scholar]
- [50].Rapp MA, Schnaider-Beeri M, Purohit DP, Perl DP, Haroutunian V, Sano M (2008) Increased neurofibrillary tangles in patients with Alzheimer disease with comorbid depression. Am J Geriatr Psychiatry 16, 168–174. [DOI] [PubMed] [Google Scholar]
- [51].Pomara N, Bruno D, Sarreal AS, Hernando RT, Nierenberg J, Petkova E, Sidtis JJ, Wisniewski TM, Mehta PD, Pratico D, Zetterberg H, Blennow K (2012) Lower CSF amyloid beta peptides and higher F2-isoprostanes in cognitively intact elderly individuals with major depressive disorder. Am J Psychiatry 169, 523–530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [52].Armstrong NM, Carlson MC, Schrack J, Xue Q-L, Carnethon MR, Rosano C, Chaves PHM, Gross AL (2018) Late-life depressive symptoms as partial mediators in the associations between subclinical cardiovascular disease with onset of mild cognitive impairment and dementia. Am J Geriatr Psychiatry 26, 559–568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [53].Demnitz N, Anatürk M, Allan CL, Filippini N, Griffanti L, Mackay CE, Mahmood A, Sexton CE, Suri S, Topiwala AG, Zsoldos E, Kivimaki M, Singh-Manoux A, Ebmeier KP (2020) Association of trajectories of depressive symptoms with vascular risk, cognitive function and adverse brain outcomes: The Whitehall IIMRI sub-study. J Psychiatr Res 131, 85–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [54].Teodorczuk A, O’Brien JT, Firbank MJ, Pantoni L, Poggesi A, Erkinjuntti T, Wallin A, Wahlund L-O, Gouw A, Waldemar G, Schmidt R, Ferro JM, Chabriat H, Bäzner H, Inzitari D, LADIS Group (2007) White matter changes and late-life depressive symptoms: Longitudinal study. Br J Psychiatry J Ment Sci 191, 212–217. [DOI] [PubMed] [Google Scholar]
- [55].Wilson RS, Arnold SE, Beck TL, Bienias JL, Bennett DA (2008) Change in depressive symptoms during the prodromal phase of Alzheimer disease. Arch Gen Psychiatry 65, 439–445. [DOI] [PubMed] [Google Scholar]
- [56].Wilson RS, Boyle PA, Capuano AW, Shah RC, Hoganson GM, Nag S, Bennett DA (2016) Late-life depression is not associated with dementia-related pathology. Neuropsychology 30, 135–142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [57].Leng Y, Diem SJ, Stone KL, Yaffe K (2018) Antidepressant use and cognitive outcomes in very old women. J Gerontol A Biol Sci Med Sci 73, 1390–1395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [58].Han F, Bonnett T, Brenowitz WD, Teylan MA, Besser LM, Chen Y-C, Chan G, Cao K-G, Gao Y, Zhou X-H (2020) Estimating associations between antidepressant use and incident mild cognitive impairment in older adults with depression. PloS One 15, e0227924. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


