Abstract
The COVID-19 pandemic spurred a broad interest in antiviral drug discovery. The SARS-CoV-2 main protease (Mpro) and papain-like protease (PLpro) are attractive antiviral drug targets given their vital roles in viral replication and modulation of host immune response. Structurally disparate compounds were reported as Mpro and PLpro inhibitors from either drug repurposing or rational design. Two polyphenols dieckol and 1,2,3,4,6-pentagalloylglucose (PGG) were recently reported as SARS-CoV-2 Mpro inhibitors. With our continuous interest in studying the mechanism of inhibition and resistance of Mpro inhibitors, we report herein our independent validation/invalidation of these two natural products. Our FRET-based enzymatic assay showed that neither dieckol nor PGG inhibited SARS-CoV-2 Mpro (IC50 > 20 µM), which is in contrary to previous reports. Serendipitously, PGG was found to inhibit the SARS-CoV-2 PLpro with an IC50 of 3.90 µM. The binding of PGG to PLpro was further confirmed in the thermal shift assay. However, PGG was cytotoxic in 293T-ACE2 cells (CC50 = 7.7 µM), so its intracellular PLpro inhibitory activity could not be quantified by the cell-based Flip-GFP PLpro assay. In addition, we also invalidated ebselen, disulfiram, carmofur, PX12, and tideglusib as SARS-CoV-2 PLpro inhibitors using the Flip-GFP assay. Overall, our results call for stringent hit validation, and the serendipitous discovery of PGG as a putative PLpro inhibitor might worth further pursuing.
Graphical abstract
Keywords: SARS-CoV-2, Main protease, Papain-like protease, Antiviral, Coronavirus
Introduction
COVID-19 is caused by the SARS-CoV-2, an enveloped, single-stranded, and positive-sense RNA virus [1]. Seven coronaviruses are known to infect humans including four common human coronaviruses OC43, 229E, NL63, and HKU1, and three highly pathogenic coronaviruses SARS-CoV, SARS-CoV-2 and MERS-CoV [2]. The COVID-19 pandemic is a timely call for the urgent need of orally bioavailable antivirals. Drug repurposing plays a pivotal role in combating emerging diseases such as COVID19 [3]. For example, the first FDA-approved COVID drug, remdesivir, was originally developed for Ebola virus [4], and was later found to have broad-spectrum antiviral activity against several viruses including SARS-CoV, MERS-CoV, and SARS-CoV-2 [5, 6]. Similarly, molnupiravir was a clinical candidate for the influenza virus before being repurposed for SARS-CoV-2 [7, 8]. The SARS-CoV-2 main protease (Mpro) and papain-like protease (PLpro) are also high-profile viral proteins for target-based drug repurposing. Numerous virtual screenings and high-throughput screenings have been conducted, revealing structurally disparate inhibitors that are at different stages of preclinical and clinical development [9]. For example, boceprevir [10, 11], calpain inhibitors [10], GC-376 [10, 12], and masitinib [13] were among the first hits reported as Mpro inhibitors. GRL0617 [14, 15], YM155 [16], 6-thioguanine [17], SJB2-043 [18], and others were identified as PLpro inhibitors. Natural products have always been a rich source of modern medicine [19], and multiple natural products have been reported as Mpro and PLpro inhibitors [20]. For example, two polyphenols dieckol and 1,2,3,4,6-pentagalloylglucose (PGG) were recently reported as SARS-CoV-2 Mpro inhibitors [21, 22]. With our continuous interest in validation/invalidation of literature reported SARS-CoV-2 Mpro and PLpro inhibitors [23–26], we report herein our independent validation of these two compounds using the established FRET enzymatic assay and cell-based Flip-GFP assay. In addition, we further confirmed that the previously reported promiscuous cysteine modifiers ebselen, disulfiram, carmofur, PX12, and tideglusib [27] are not PLpro inhibitors, despite the claim from several publications that they act as PLpro inhibitors [28, 29]. Interestingly, we serendipitously discovered PGG as a PLpro inhibitor and showed that PGG binds to PLpro and inhibited the enzymatic activity of PLpro in the FRET assay. Taken together, our results call for stringent hit validation, and the serendipitous discovery of PGG as a putative PLpro inhibitor might worth further investigation.
Results and discussion
Invalidation of dieckol and PGG as SARS-CoV-2 Mpro inhibitors and validation of PGG as a PLpro inhibitor
Dieckol was reported as a SARS-CoV-2 Mpro inhibitor through a fluorescence polarization-based high-throughput screening [21]. In the assay design, the biotin-labeled Mpro substrate was conjugated with a fluorescein isocyanate (FITC) fluorophore, resulting in a bifunctional probe FITC-AVLQ ↓ SGFRKK-Biotin (FITC-S-Biotin). Binding of this probe to avidin led to increased fluorescence polarization. Upon Mpro digestion, the fluorophore-peptide conjugate FITC-AVLQ was released, resulting in reduced millipolarization unit (mP) signal. Screening of a natural product library of 5,000 compounds identified dieckol as a potent Mpro inhibitor with IC50 values of 4.5 µM (no DTT) and 2.9 µM (1 mM DTT). The mechanism of action was characterized using the FRET assay and surface plasmon resonance binding assay, both of which showed consistent results with the FP assay. Enzymatic kinetic studies demonstrated that dieckol is a competitive Mpro inhibitor. It is noted that dieckol was also previously reported as a SARS-CoV Mpro inhibitor [30].
PGG was reported as an inhibitor for both SARS-CoV and SARS-CoV-2 Mpro with IC50 values of 6.89 and 3.66 µM, respectively [22]. In another study, PGG was found to bind to the SARS-CoV-2 spike protein receptor binding domain (RBD) with a KD of 6.69 µM in the bio-layer interferometry assay, while the binding of PGG to the ACE2 receptor was weaker with a KD of 22.2 µM [31]. PGG was further shown to block the RBD-ACE2 interactions in the ELISA assay with an IC50 of 46.9 µM. In the SARS-CoV-2 pseudovirus assay, PGG dose-dependently inhibited the viral entry and replication.
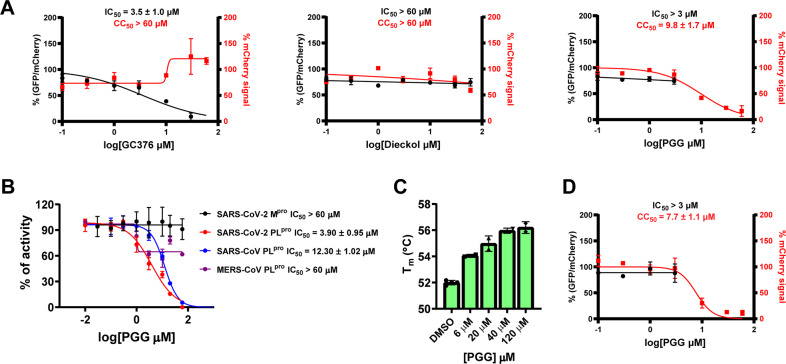
To validate whether dieckol and PGG are Mpro inhibitors, we repeated the FRET enzymatic assay using our standard FRET assay condition (20 mM HEPES, pH 6.5, 120 mM NaCl, 0.4 mM EDTA, 4 mM DTT, and 20% glycerol). Both dieckol and PGG were inactive (IC50 > 20 µM) (Table 1). To examine whether dieckol and PGG inhibited the intracellular protease activity of Mpro, we characterized both compounds in the cell-based Flip-GFP Mpro assay. Our previous results showed that there is generally a positive correlation between the Flip-GFP and antiviral assay results, while the correlation between the FRET enzymatic assay results and antiviral assay results is compound dependent [15]. In the Flip-GFP assay, the GFP is reconstituted upon cleavage of the engineered linker by Mpro, and the normalized GFP/mCherry signal ratio is proportional to the Mpro activity (mCherry serves as an internal control for the protein expression level or compound toxicity) [32, 33]. GC-376 was included as a positive control and it showed an EC50 of 3.5 µM (Fig. 1A). The results showed that both dieckol and PGG lacked the cellular Mpro inhibitory activity at non-toxic drug concentrations (Fig. 1A). Dieckol was not active (IC50 > 60 µM), while PGG was cytotoxic (CC50 = 9.8 µM) (Fig. 1A), therefore the result was not conclusive. Taken together, dieckol and PGG were both invalidated as Mpro inhibitors.
Table 1.
Validation and invalidation of SARS-CoV-2 Mpro and PLpro inhibitors
| Compound | Reported SARS-CoV-2 Mpro inhibition IC50 (µM) |
Reported SARS-CoV-2 PLpro inhibition IC50 (µM) |
Validation results IC50 (µM) |
|---|---|---|---|
| Dieckol |
IC50 = 4.5 ± 0.4 (1 mM DTT) IC50 = 2.9 ± 0.2 (no DTT) Competitive inhibitor Ki = 3.3 µM [21] SPR KD = 0.22 µM |
N.A. |
FRET assay: Mpro IC50 > 20 (4 mM DTT) PLpro IC50 > 20 (4 mM DTT) Flip-GFP Mpro assay: IC50 > 60 µM |
| PGG |
SARS-CoV-2 IC50 = 3.66 ± 0.02 SARS-CoV IC50 = 6.89 ± 0.15 [22] |
N. A. |
FRET assay: Mpro IC50 > 20 (4 mM DTT) PLpro IC50 = 3.90 ± 1.10 (4 mM DTT) Thermal shift assay: ΔTm = 3.91 oC Flip-GFP Mpro assay: IC50 > 3 µM Flip-GFP PLpro assay: IC50 > 3 µM |
| Ebselen |
IC50 = 3.7 ± 2.4 (4 mM DTT) IC50 > 60 (4 mM DTT) [25] |
IC50 = 10.3 ± 8.9 (4 mM DTT) IC50 > 60 (4 mM DTT) [25] |
Flip-GFP PLpro assay: IC50 > 30 µM |
| Disulfiram |
IC50 = 2.1 ± 0.3 (4 mM DTT) IC50 > 60 (4 mM DTT) [25] |
IC50 = 6.9 ± 4.2 (4 mM DTT) IC50 > 60 (4 mM DTT) [25] |
Flip-GFP PLpro assay: IC50 > 10 µM |
| Carmofur |
IC50 = 0.2 ± 0.1 (4 mM DTT) IC50 = 28.2 ± 9.5 (4 mM DTT) [25] |
IC50 = 0.7 ± 0.1 (4 mM DTT) IC50 > 60 (4 mM DTT) [25] |
Flip-GFP PLpro assay: IC50 > 50 µM |
| PX-12 |
IC50 = 0.9 ± 0.2 (4 mM DTT) IC50 > 60 (4 mM DTT) [25] |
IC50 = 18.7 ± 2.6 (4 mM DTT) IC50 > 60 (4 mM DTT) [25] |
Flip-GFP PLpro assay: IC50 > 50 µM |
| Tideglusib |
IC50 = 2.1 ± 0.3 (4 mM DTT) IC50 > 60 (4 mM DTT) [25] |
IC50 = 7.1 ± 1.4 (4 mM DTT) IC50 = 30.4 ± 17.1 (4 mM DTT) [25] |
Flip-GFP PLpro assay: IC50 > 60 µM |
N.A. not available
Fig. 1.
Invalidation of dieckol and PGG as SARS-CoV-2 Mpro inhibitors and validation of PGG as a PLpro inhibitor. A Flip-GFP Mpro assay results of dieckol and PGG. GC376 was included as a positive control. B FRET assay results of PGG against SARS-CoV-2 Mpro, SARS-CoV-2 PLpro, SARS-CoV PLpro, and MERS-CoV PLpro. C Thermal shift assay characterization of the binding of PGG to SARS-CoV-2 PLpro. D Flip-GFP PLpro assay result of PGG. The results are mean ± standard deviation of two repeats
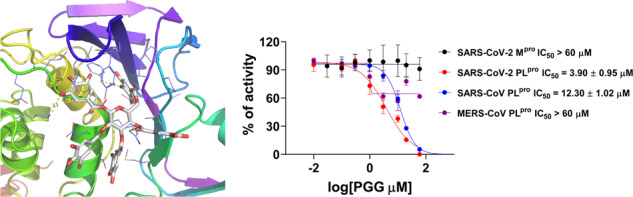
In parallel, we tested dieckol and PGG against SARS-CoV-2 PLpro in the FRET assay. While dieckol was not active (IC50 > 20 µM), PGG was serendipitously found to inhibit SARS-CoV-2 PLpro with an IC50 of 3.9 µM (Fig. 1B and Table 1). To profile the broad-spectrum activity, PGG was tested against SARS-CoV and MERS-CoV PLpro. PGG showed weak activity against SARS-CoV PLpro with an IC50 of 12.3 µM, while it was inactive against the MERS-CoV (IC50 > 60 µM) (Fig. 1B). These results suggest that the inhibition of SARS-CoV-2 PLpro by PGG might be specific. We further characterized the binding of PGG to SARS-CoV-2 PLpro in the thermal shift assay and found that PGG increased the thermal stability of PLpro in a dose dependent manner (Fig. 1C). To determine whether PGG inhibits the intracellular protease activity of SARS-CoV-2 PLpro, we performed the Flip-GFP PLpro assay. Unfortunately, PGG was cytotoxic to the 293 T cells used in the Flip-GFP PLpro assay (CC50 = 7.7 µM), resulting in inconclusive results (Fig. 1D).
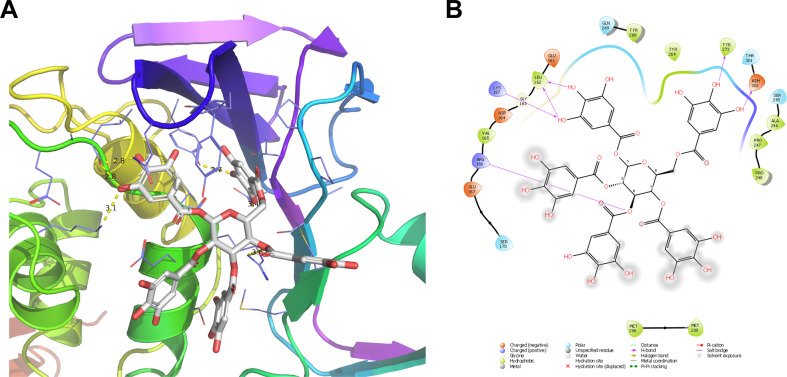
To gain insights of the binding mode, we performed molecular docking of PGG with SARS-CoV-2 PLpro (PDB: 7JRN) [15] using the Schrödinger Glide extra-precision. The binding sites in PLpro were determined by the sitemap, which revealed the BL2 loop region as the highest-ranking binding site, therefore it was selected for PGG docking. The BL2 loop region is also the drug binding site of the known PLpro inhibitors GRL0617 [15]. Docking results showed that PGG fits snugly in the binding site with a Glide score of −10.024 kcal/mol (Fig. 2A). PGG formed multiple hydrogen bonds with PLpro residues including the side chains of Tyr273, Asp302, Arg166, Lys157 and the main chain of Leu162 (Fig. 2B).
Fig. 2.
Docking model of PGG in SARS-CoV-2 PLpro. A Docking pose of PGG in the BL2 loop binding site of PLpro. B 2D ligand-protein interaction plot of PGG with SARS-CoV-2 PLpro. Docking was performed using the X-ray crystal structure of SARS-CoV-2 PLpro (PDB; 7JRN). The Glide score was −10.024 kcal/mol from the Schrödinger Glide extra-precision docking
Invalidation of disulfiram, ebselen, carmofur, PX-12, and tideglusib as SARS-CoV-2 PLpro inhibitors
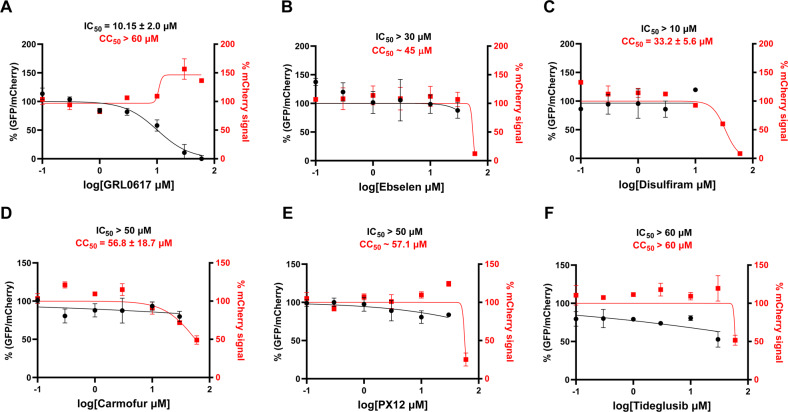
Disulfiram was previously reported as an inhibitor of both SARS-CoV and MERS-CoV PLpros [28]. Enzymatic kinetic studies showed that disulfiram acts as an allosteric inhibitor of MERS-CoV PLpro and a competitive inhibitor of the SARS-CoV PLpro. In contrary, our previous study revealed that the inhibition of SARS-CoV-2 PLpro by disulfiram in the FRET-based enzymatic assay is reducing reagent dependent [25]. Disulfiram inhibited SARS-CoV-2 PLpro with an IC50 of 6.9 µM in the absence of DTT but was not active in the presence of DTT (IC50 > 60 µM) (Table 1). Likewise, ebselen, carmofur, PX-12, and tideglusib all showed various degrees of inhibition against the SARS-CoV-2 PLpro in the absence of DTT, while the inhibition was abolished in the presence of DTT (Table 1) [25]. In contrary, Weglarz-Tomczak et al. reported that ebselen inhibited SARS-CoV and SARS-CoV-2 PLpros with IC50 values of 8.45 and 2.26 µM, respectively, in the presence of 2 mM DTT [29]. Disulfiram and ebselen were also proposed to inhibit SARS-CoV-2 PLpro through ejecting zinc from the zinc-binding domain [34]. Given the debate whether reducing reagent should be added to the cysteine protease assay buffer, coupled with the controversial FRET assay results of ebselen in the presence of DTT, we were interested in further characterizing the inhibition of SARS-CoV-2 PLpro by these compounds in a native cellular environment. For this, we employed our recently established cellular Flip-GFP PLpro assay [15] to test the intracellular activity of these compounds. It was found that none of the compounds tested reduced the GFP/mCherry ratio at non-cytotoxic concentrations (Fig. 3A–F), suggesting that they lack the intracellular target engagement and PLpro inhibition. Collectively, our data suggest that disulfiram, ebselen, carmofur, PX-12, and tideglusib should not be classified as PLpro inhibitors.
Fig. 3.
Invalidation of disulfiram, ebselen, carmofur, PX-12, and tideglusib as SARS-CoV-2 PLpro inhibitors using the Flip-GFP PLpro assay. GRL0617 (A) was included as a positive control. % (GFP/mCherry) ratio correlates with intracellular PLpro activity, and % mCherry signal correlates with compound toxicity or transfection efficiency. The results are mean ± standard deviation of two repeats
Conclusion
In conclusion, our data suggested that dieckol and PGG are not Mpro inhibitors as shown from the FRET and Flip-GFP Mpro assays. Furthermore, the previous reported promiscuous cysteine modifiers ebselen, disulfiram, carmofur, PX-12, and tideglusib were also invalidated as PLpro inhibitors by the Flip-GFP PLpro assay. Taken together with our previous efforts in invalidating these compounds as Mpro inhibitors, it can be concluded that Mpro and PLpro enzymatic assay IC50 results obtained in the absence of reducing reagents have no correlation with their cellular activity. Among the list of compounds examined, ebselen was previously shown to inhibit SARS-CoV-2 viral replication in cell culture [27, 35]. Coupled with the results presented here, it appears that the antiviral mechanism of action of ebselen is independent of either Mpro or PLpro inhibition.
Since the FRET assay conditions used in different labs vary, it might be challenging to directly compare the results. Nonetheless, the cell-based Flip-GFP assay is a valuable tool in evaluating the intracellular protease activity and is a close mimetic of virus-infected cells.
In summary, the results presented herein call for stringent hit validation before investing resources for lead optimization and translational antiviral development. The discovery of PGG as a PLpro inhibitor provides another starting point for further optimization.
Experimental
Materials and methods
All compounds were purchased from commercial source without further purification. PGG was ordered from Toronto Research Chemical with the Cat # P270450.
SARS-CoV-2 Mpro and PLpro expression and purification
SARS-CoV-2 main protease (Mpro) gene from strain BetaCoV/Wuhan/WIV04/2019 (GenBank: MN996528.1) was purchased from GenScript (Piscataway, NJ) with E. coli codon optimization and was inserted into pET29a(+) plasmid. The Mpro genes were then subcloned into the pE-SUMO plasmid as previously described [10, 36]. The expression and purification procedures were previously described [10]. SARS-CoV-2 papain-like protease (PLpro) gene (ORF 1ab 1564–1876) from strain BetaCoV/Wuhan/WIV04/2019 with E. coli codon optimization was ordered from GenScript in the pET28b(+) vector. The detailed expression and purification procedures were previously described [15].
FRET-based enzymatic assay
For the IC50 measurement with the FRET-based assay, the reaction was carried out in 96-well format with 100 μL of 200 nM PLpro protein in a PLpro reaction buffer (50 mM HEPES (pH 7.5), 5 mM DTT, and 0.01% Triton X-100); 1 μL of testing compounds at various concentrations was added to each well and was incubated at 30 °C for 30 min. The reaction was initiated by adding 1 μL of 1 mM FRET substrate and was monitored in a Cytation 5 image reader with filters for excitation at 360/40 nm and emission at 460/40 nm at 30 °C for 1 h. The initial velocity of the enzymatic reaction was calculated from the initial 10 min enzymatic reaction. The IC50 was calculated by plotting the initial velocity against various concentrations of testing compounds using a four-parameter variable slope dose–response curve in Prism 8 software. IC50 values for the testing compounds against SARS-CoV-2 Mpro was determined as previously described [10].
Flip-GFP Mpro and PLpro assay
Plasmid pcDNA3-TEV-FlipGFP-T2A-mCherry was ordered from Addgene (catalog No.124429). pcDNA3 FlipGFP-Mpro plasmid and pcDNA3 FlipGFP-PLpro plasmid were constructed by introducing SARS-CoV-2 Mpro cleavage site AVLQSGFR and SARS-CoV-2 PLpro cleavage site LRGGAPTK, respectively, via overlapping PCRs. pLVX SARS-CoV-2 Mpro and pcDNA3.1 SARS-CoV-2 PLpro plasmids was ordered from Genescript (Piscataway NJ) with codon optimization.
The Flip-GFP Mpro and PLpro assays were performed as previous reported [15, 23, 24, 37]. Briefly, the assay started with seeding 293T-ACE2 in 96-well, black, clear bottomed plate (Greiner, catalog No. 655090) and incubating overnight to allow cells to reach 70–80% confluency. 50 ng of pLVX SARS-CoV-2 Mpro (or pcDNA3.1 SARS-CoV-2 PLpro) and 50 ng of pcDNA3 FlipGFP- Mpro (or pcDNA3 FlipGFP- PLpro) reporter plasmid was mixed with transfection reagent TransIT-293 (Mirus, catalog No. MIR 2700). The mixture was then transfected to each well according to manufacturer’s instructions. After 2.5–3 hours of incubation in 37 °C, 1 μL of testing compound was added into each well directly and mixed by gentle plate shaking. 48 h post transfection, fluorescence was quantified using SpectraMax iD3 plate reader (Molecular Devices) and images were taken using BZ-X800E fluorescence microscope (Keyence) in GFP and mCherry channels at 4X objective lens.
Differential scanning fluorimetry (DSF)
The thermal shift binding assay (TSA) was carried out using a Thermo Fisher QuantStudio 5 Real-Time PCR system as described previously [10].
Molecular docking
Docking of PGG in SARS-CoV-2 PLpro was performed using the Schrödinger Glide extra precision program. The X-ray crystal structure of SARS-CoV-2 PLpro in complex with GRL0617 (PDB: 7JRN) was chosen for the docking. The gride box was centered on GRL0617. The docking poses were visualized using Pymol.
Acknowledgements
This research was supported by the National Institutes of Health (NIH) (grants AI147325, AI157046, and AI158775) to J.W.
Compliance with ethical standards
Conflict of interest
The authors declare no competing interests.
Footnotes
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Hu B, Guo H, Zhou P, Shi ZL. Characteristics of SARS-CoV-2 and COVID-19. Nat Rev Microbiol. 2021;19:141–54. doi: 10.1038/s41579-020-00459-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.V’kovski P, Kratzel A, Steiner S, Stalder H, Thiel V. Coronavirus biology and replication: implications for SARS-CoV-2. Nat Rev Microbiol. 2021;19:155–70. doi: 10.1038/s41579-020-00468-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Gordon DE, Jang GM, Bouhaddou M, Xu J, Obernier K, White KM, et al. A SARS-CoV-2 protein interaction map reveals targets for drug repurposing. Nature. 2020;583:459–68. doi: 10.1038/s41586-020-2286-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Warren TK, Jordan R, Lo MK, Ray AS, Mackman RL, Soloveva V, et al. Therapeutic efficacy of the small molecule GS-5734 against Ebola virus in rhesus monkeys. Nature. 2016;531:381–5. doi: 10.1038/nature17180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Sheahan TP, Sims AC, Graham RL, Menachery VD, Gralinski LE, Case JB, et al. Broad-spectrum antiviral GS-5734 inhibits both epidemic and zoonotic coronaviruses. Sci Transl Med. 2017;9:eaal3653. doi: 10.1126/scitranslmed.aal3653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Eastman RT, Roth JS, Brimacombe KR, Simeonov A, Shen M, Patnaik S, et al. Remdesivir: a review of its discovery and development leading to emergency use authorization for treatment of COVID-19. ACS Cent Sci. 2020;6:672–83. doi: 10.1021/acscentsci.0c00489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Cox RM, Wolf JD, Plemper RK. Therapeutically administered ribonucleoside analogue MK-4482/EIDD-2801 blocks SARS-CoV-2 transmission in ferrets. Nat Microbiol. 2021;6:11–8. doi: 10.1038/s41564-020-00835-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Toots M, Yoon J-J, Cox RM, Hart M, Sticher ZM, Makhsous N, et al. Characterization of orally efficacious influenza drug with high resistance barrier in ferrets and human airway epithelia. Sci Transl Med. 2019;11:eaax5866. doi: 10.1126/scitranslmed.aax5866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ghosh AK, Brindisi M, Shahabi D, Chapman ME, Mesecar AD. Drug development and medicinal chemistry efforts toward SARS-coronavirus and Covid-19 therapeutics. ChemMedChem. 2020;15:907–32. doi: 10.1002/cmdc.202000223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ma C, Sacco MD, Hurst B, Townsend JA, Hu Y, Szeto T, et al. Boceprevir, GC-376, and calpain inhibitors II, XII inhibit SARS-CoV-2 viral replication by targeting the viral main protease. Cell Res. 2020;30:678–92. doi: 10.1038/s41422-020-0356-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Fu L, Ye F, Feng Y, Yu F, Wang Q, Wu Y, et al. Both Boceprevir and GC376 efficaciously inhibit SARS-CoV-2 by targeting its main protease. Nat Commun. 2020;11:4417. doi: 10.1038/s41467-020-18233-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Vuong W, Khan MB, Fischer C, Arutyunova E, Lamer T, Shields J, et al. Feline coronavirus drug inhibits the main protease of SARS-CoV-2 and blocks virus replication. Nat Commun. 2020;11:4282. doi: 10.1038/s41467-020-18096-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Drayman N, DeMarco JK, Jones KA, Azizi S-A, Froggatt HM, Tan K, et al. Masitinib is a broad coronavirus 3CL inhibitor that blocks replication of SARS-CoV-2. Science. 2021;373:931–6. doi: 10.1126/science.abg5827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Osipiuk J, Azizi SA, Dvorkin S, Endres M, Jedrzejczak R, Jones KA, et al. Structure of papain-like protease from SARS-CoV-2 and its complexes with non-covalent inhibitors. Nat Commun. 2021;12:743. doi: 10.1038/s41467-021-21060-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ma C, Sacco MD, Xia Z, Lambrinidis G, Townsend JA, Hu Y, et al. Discovery of SARS-CoV-2 papain-like protease inhibitors through a combination of high-throughput screening and a FlipGFP-based reporter assay. ACS Cent Sci. 2021;7:1245–60. doi: 10.1021/acscentsci.1c00519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Zhao Y, Du X, Duan Y, Pan X, Sun Y, You T, et al. High-throughput screening identifies established drugs as SARS-CoV-2 PLpro inhibitors. Protein Cell. 2021;12:877–88. doi: 10.1007/s13238-021-00836-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Swaim CD, Dwivedi V, Perng YC, Zhao X, Canadeo LA, Harastani HH, et al. 6-Thioguanine blocks SARS-CoV-2 replication by inhibition of PLpro. iScience. 2021;24:103213. doi: 10.1016/j.isci.2021.103213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Cho CC, Li SG, Lalonde TJ, Yang KS, Yu G, Qiao Y, et al. Drug repurposing for the SARS-CoV-2 papain-like protease. ChemMedChem. 2022;17:e202100455. doi: 10.1002/cmdc.202100455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Christy MP, Uekusa Y, Gerwick L, Gerwick WH. Natural products with potential to treat RNA virus pathogens including SARS-CoV-2. J Nat Prod. 2021;84:161–82. doi: 10.1021/acs.jnatprod.0c00968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Chakravarti R, Singh R, Ghosh A, Dey D, Sharma P, Velayutham R, et al. A review on potential of natural products in the management of COVID-19. RSC Adv. 2021;11:16711–35. doi: 10.1039/D1RA00644D. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Yan G, Li D, Lin Y, Fu Z, Qi H, Liu X, et al. Development of a simple and miniaturized sandwich-like fluorescence polarization assay for rapid screening of SARS-CoV-2 main protease inhibitors. Cell Biosci. 2021;11:199. doi: 10.1186/s13578-021-00720-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Chiou W-C, Chen J-C, Chen Y-T, Yang J-M, Hwang L-H, Lyu Y-S, et al. The inhibitory effects of PGG and EGCG against the SARS-CoV-2 3C-like protease. Biochem Biophys Res Commun. 2022;591:130–6. doi: 10.1016/j.bbrc.2020.12.106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ma C, Wang J. Validation and invalidation of SARS-CoV-2 papain-like protease inhibitors. ACS Pharm Transl Sci. 2022;5:102–9. doi: 10.1021/acsptsci.1c00240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ma C, Tan H, Choza J, Wang Y, Wang J. Validation and invalidation of SARS-CoV-2 main protease inhibitors using the Flip-GFP and Protease-Glo luciferase assays. Acta Pharm Sin B. 2022;12:1636–51. doi: 10.1016/j.apsb.2021.10.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ma C, Hu Y, Townsend JA, Lagarias PI, Marty MT, Kolocouris A, et al. Ebselen, Disulfiram, Carmofur, PX-12, Tideglusib, and Shikonin are nonspecific promiscuous SARS-CoV-2 main protease inhibitors. ACS Pharm Transl Sci. 2020;3:1265–77. doi: 10.1021/acsptsci.0c00130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ma C, Wang J. Dipyridamole, chloroquine, montelukast sodium, candesartan, oxytetracycline, and atazanavir are not SARS-CoV-2 main protease inhibitors. Proc Natl Acad Sci USA. 2021;118:e2024420118. doi: 10.1073/pnas.2024420118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Jin Z, Du X, Xu Y, Deng Y, Liu M, Zhao Y, et al. Structure of M(pro) from SARS-CoV-2 and discovery of its inhibitors. Nature. 2020;582:289–93. doi: 10.1038/s41586-020-2223-y. [DOI] [PubMed] [Google Scholar]
- 28.Lin MH, Moses DC, Hsieh CH, Cheng SC, Chen YH, Sun CY, et al. Disulfiram can inhibit MERS and SARS coronavirus papain-like proteases via different modes. Antivir Res. 2018;150:155–63. doi: 10.1016/j.antiviral.2017.12.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Weglarz-Tomczak E, Tomczak JM, Talma M, Burda-Grabowska M, Giurg M, Brul S. Identification of ebselen and its analogues as potent covalent inhibitors of papain-like protease from SARS-CoV-2. Sci Rep. 2021;11:3640. doi: 10.1038/s41598-021-83229-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Park J-Y, Kim JH, Kwon JM, Kwon H-J, Jeong HJ, Kim YM, et al. Dieckol, a SARS-CoV 3CLpro inhibitor, isolated from the edible brown algae Ecklonia cava. Bioorg Med Chem. 2013;21:3730–7. doi: 10.1016/j.bmc.2013.04.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Chen RH, Yang LJ, Hamdoun S, Chung SK, Lam CW-K, Zhang KX et al. 1,2,3,4,6-pentagalloyl glucose, a RBD-ACE2 binding inhibitor to prevent SARS-CoV-2 infection. Front Pharmacol. 2021;12. 10.3389/fphar.2021.634176. [DOI] [PMC free article] [PubMed]
- 32.Froggatt HM, Heaton BE, Heaton NS. Development of a fluorescence-based, high-throughput SARS-CoV-2 3CL(pro) reporter assay. J Virol. 2020;94:e01265–20. doi: 10.1128/JVI.01265-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Li X, Lidsky PV, Xiao Y, Wu CT, Garcia-Knight M, Yang J, et al. Ethacridine inhibits SARS-CoV-2 by inactivating viral particles. PLoS Pathog. 2021;17:e1009898. doi: 10.1371/journal.ppat.1009898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Sargsyan K, Lin CC, Chen T, Grauffel C, Chen YP, Yang WZ, et al. Multi-targeting of functional cysteines in multiple conserved SARS-CoV-2 domains by clinically safe Zn-ejectors. Chem Sci. 2020;11:9904–9. doi: 10.1039/d0sc02646h. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Amporndanai K, Meng X, Shang W, Jin Z, Rogers M, Zhao Y, et al. Inhibition mechanism of SARS-CoV-2 main protease by ebselen and its derivatives. Nat Commun. 2021;12:3061. doi: 10.1038/s41467-021-23313-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Sacco MD, Ma C, Lagarias P, Gao A, Townsend JA, Meng X, et al. Structure and inhibition of the SARS-CoV-2 main protease reveal strategy for developing dual inhibitors against M(pro) and cathepsin L. Sci Adv. 2020;6:eabe0751. doi: 10.1126/sciadv.abe0751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Ma C, Xia Z, Sacco MD, Hu Y, Townsend JA, Meng X, et al. Discovery of di- and trihaloacetamides as covalent SARS-CoV-2 main protease inhibitors with high target specificity. J Am Chem Soc. 2021;143:20697–709. doi: 10.1021/jacs.1c08060. [DOI] [PMC free article] [PubMed] [Google Scholar]