Abstract
Abstract
Fungi produce several bioactive metabolites, pigments, dyes, antioxidants, polysaccharides, and industrial enzymes. Fungal products are also the primary sources of functional food and nutrition, and their pharmacological products are used for healthy aging. Their molecular properties are validated through the use of recent high-throughput genomic, transcriptomic, and metabolomic tools and techniques. Together, these updated multi-omic tools have been used to study fungal metabolites structure and their mode of action on biological and cellular processes. Diverse groups of fungi produce different proteins and secondary metabolites, which possess tremendous biotechnological and pharmaceutical applications. Furthermore, its use and acceptability can be accelerated by adopting multi-omics, bioinformatics, and machine learning tools that generate a huge amount of molecular data. The integration of artificial intelligence and machine learning tools in the era of omics and big data has opened up a new outlook in both basic and applied researches in the area of nutraceuticals and functional food and nutrition.
Key Points
• Multi-omic tool helps in the identification of novel fungal metabolites
• Intra-omic data from genomics to bioinformatics
• Novel metabolites and application in human health
Keywords: Fungal metabolites, Multi-omics, Transcriptomic, Metabolomic, Machine learning
Introduction
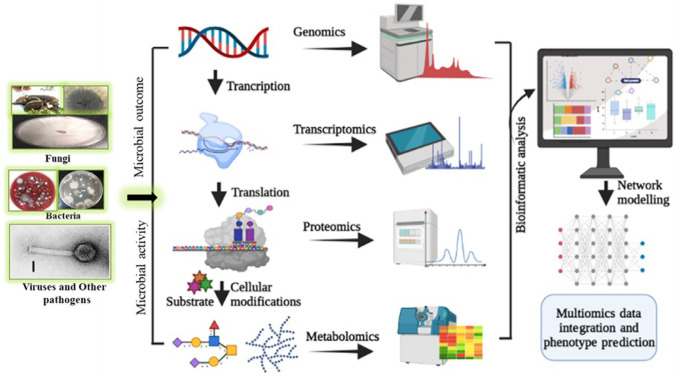
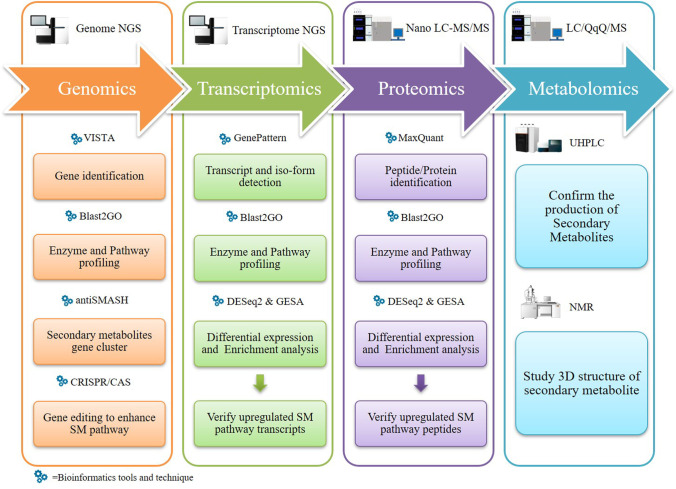
The evolution in multi-omic tools, like genomics, meta-genomics, transcriptomics, proteomics, and metabolomics, have explored many avenues in analyzing complex biological data (big data) to reveal the structure and function of fungal secondary metabolites. Nowadays, a researcher can sequence and study the whole genome (using next-generation sequencing approaches), proteome for both the extra and intracellular proteins study, and metabolome for secondary metabolite (SM) study from basidiomycetous fungi which has tremendous commercial potential (Jain et al. 2020). SMs are differentially structured organic compounds mainly extracted from plants, fungi, and bacteria. These metabolites not only are essential for the growth of the organism, but it has also been used in pharmacological as well as in the development of functional food. To date, ~35% of the naturally derived product contribute to drug discovery and are approved by the US Food and Drug Administration (FDA). Among the approved SMs, ~ 15% belongs to microbial origin, 25% plants, and approx 5% is extracted from animal source (Calixto 2019). In microbial groups of fungi and their products, for example, enzymes, fermented foods, animal feed, antibiotics, pharmacy products, and pigments have contributed significantly to humans and many biotechnological industries (Sahu et al. 2021). Furthermore, many flavoring compounds, flavored coffee, and cosmeceutical products have also been produced from a basidiomycetous fungus, for example, Ganoderma lucidum and Trametes versicolor (Sahu et al. 2021). Multi-omic tool refers to many advanced technologies which are used to collect the data of biological molecules for their functions as well as an action mechanism on the differentially affected cells (Bogale 2020). Multi-omic research is not new; it has been updated chronologically in the research field and accelerated its popularity in the scientific universe in the past few years. Prior to the advent of multi-omics, the scientists were unable to suggest or predict the structure, function, 3-D model, and mode of action of the fungal SMs. Earlier, single genomic or proteomic data were used to provide inconclusive results due to the absence of single pipeline for metabolite study. After the genesis of high-throughput omic technology, there is a quest for the accurate information about single-gene analysis and complex global investigation of the whole (fungal) organism like fungal metabolites and their evolution, fungal interaction with their substrates, fungal role in pharmaceutics, and their SM study. It also provides the functional information of genes and compare the genes with other organism’s genome (functional genomics and comparative genomics), the expression profile of mRNA of the metabolites producing fungi (transcriptomics), protein profiling (proteomics) and complete study of metabolites like the weight of compound, and functional properties in the cell (metabolomics) that assist in the rapid identification of novel SMs (Fig. 1) (Amer and Baidoo 2021). According to an old estimate, 1.5 million fungal species are present on the Earth, and only 10% have been investigated for their secretory products (Palazzotto and Weber 2018). Therefore, new and advanced omic approaches are needed to screen newer fungi and increase the pool of novel metabolites with their molecular interaction and exploit the fungal cultures with their metabolic capacity.
Fig. 1.
Flow chart depicts multi-omic tool with data integration and artificial intelligence modeling used for fungal metabolite studies
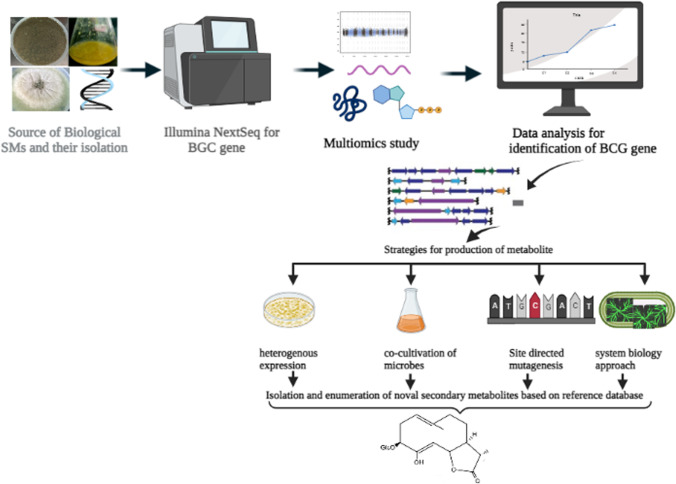
Initially, the whole-genome sequencing was used to provide limited data related to genomic features like annotated genome and enzymatic pathways (Sharma 2016). After comparing from the earlier estimation-based procedure, whole-genome sequencing tools were found to predict more SM production from fungal strains than plant or bacterial species (Kumar et al. 2019). Furthermore, the genetic makeup of a fungus provides useful information to improve the enzyme production from a culture through different culture conditions or media engineering. The data generated by the multi-omic sciences (such as comparative genomics, transcriptomics and deep sequencing, proteomics, and metabolomics) and bioinformatics (massive data analysis) can majorly contribute to improving the production methods and the use of metabolites (Amer and Baidoo 2021). In system biology research, data collected from the multi-omic tools are analyzed through statistical, mathematical, and computational models. Furthermore, the results obtained through these techniques explain the actual behavior of the fungal cells by providing data on the biological, cellular, and molecular functions, which help in the better understanding of interactions between environmental factors, genetic variants, genetic expression patterns, and changes in the concentration of metabolites (Fig. 2).
Fig. 2.
Scheme to screen and isolate novel fungal secondary metabolites
Finally, there is an urgent need to update the knowledge in the area of artificial intelligence (AI) and machine learning and its integration with multi-omic technologies to predict microbial metabolites for abovementioned applications. In this review, we had tried to explain multi-omic technologies used to facilitate the analysis of data on metabolites from basidiomycetous fungi and also updated their application in many biotechnology-based industries involved in the production of customized food, nutraceuticals, and functional foods.
White-rot basidiomycete fungi and their metabolites
White-rot basidiomycetes are lignocellulose degrading filamentous fungi that can grow in a lignin-rich environment with high humidity. They have an enormous role in lignin, cellulose, and hemicellulose degradation, which reflect a significant function in the carbon cycle. Fungi are one of the most important sources of many biological and chemical entities which may be beneficial or harmful (Hyde et al. 2019). Furthermore, most filamentous fungi synthesize SMs that have rich sources of compounds for the biotechnological and pharmaceutical industries, such as antibiotics, medicinal products, organic acids, food processing enzymes, and many other metabolites used in feed and food industry. Furthermore, lignocellulose degrading and polysaccharide secreting white and brown-rot fungi have greater diversity for lignin-degrading peroxidases, multi-copper oxidases, and glycoside hydrolase. In sequential pattern, these enzymes help in the progressive decay of lignin-rich substrates (Jain et al. 2020; Saini et al. 2020).
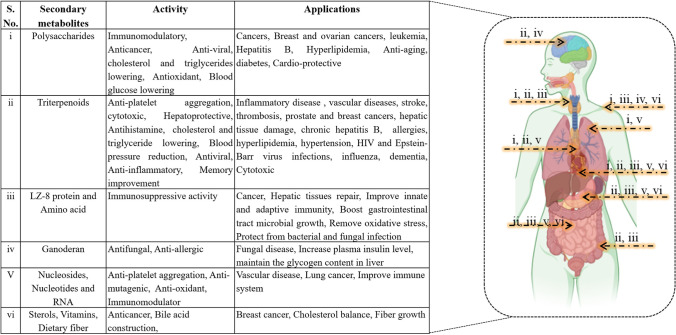
Due to biological demand of the SM-based products, milti-omic tool becomes an integral part in the detection and characterization of novel metabolites. After the genome sequencing, advance techniques such as transcriptomics, DNA microarrays, proteomics, and metablomics were explored in differential induction and production of SMs from fungi (Palazzotto and Weber 2018; Shankar et al. 2019). Basidiomycete fungi have the prime ability to degrade complex lignin structure and xenobiotic compound by sensing their environment and regulating the secretome and proteome profile (Téllez-Téllez and Diaz-Godinez 2019). From the past few years, SM studies are done by using the omic tools to find out the novel compounds and their important biological effects which can be used for human health, such as anticancer activity, antidiabetic effect, antimicrobial effect, and cytotoxicity studies (Fig. 3) (Ball et al. 2020). Many industrial products composing of fungal metabolites from basidiomycete source are directly or indirectly benefited to the human health (Table 1). People have been using many types of mushroom in their diet since long time, but their functions were not known. Nowadays, due to the upgraded and integrated multi-omic technology, their detection process and properties of SMs are known to us. Hence, the demand of fungal mushroom and their SMs has significantly increased (Table 1). Furthermore, most of the white-rot basidiomycetes produce different types of SMs with different structures and functions (Table 2).
Fig. 3.
Action mechanism of fungal secondary metabolites and their effect on human health
Table 1.
Multi-omic tools used to study basidiomycetous SMs and their products in food and pharmaceutical industries
| Product name/fungal sources | Key component of SMs | Mode of action and applications | Omic tool | References |
|---|---|---|---|---|
| SM of Pleurotus ostreatus | Resveratrol | Antioxidant | Genomics and metabolomics | Takahashi et al. (2020) |
| Nutrient of Dictyophora indusiata | Vitamin C | Antioxidant activity | Nuclear magnetic resonance spectroscopy | Liu et al. (2019) |
| Bitter tea powder from G. lucidum & L. edodes | Lentinan | Antitumor properties or immunostimulants | 2-DE coupled with mass spectrometry | Chuang et al. (2009) |
| Low-density lipoprotein (LDL) drug from A. bisporus, C. cibarius, I. abadia, and L. edodes | Lovastatin | Lowers cholesterol level | RP-HPLC | Kała et al. (2020) |
| MycoNutri Cordyceps Organic from C. militaris | Cordycepin | Reducing the LDL level, total cholesterol, triglycerides, and hyperlipidemia | Genomics and NMR | Ashraf et al. (2020) |
| Sun mushroom from A. subrufescens | Polyphenols and polysaccharides | Decrease oxidative stress and prevent non-transmissible chronic diseases (NTCD) | HPLC-MS | Navegantes-Lima et al. (2020) |
| Niu-Chang-Chih from A. cinnamomea | Antcins | Treat various diseases such as diarrhea, abdominal pain, hypertension, and reduced prostate cancer | NMR, electron ionization mass spectrometry (EI-MS) | Kumar et al. (2020) |
| Caterpillar as a H. sinensis | Polysaccharides | Prebiotic and diabetic control | Next-generation sequencing technology | Takahashi et al. (2020) |
| Edible mushroom V. volvacea, P. geesteranum, H. erinaceus, and P. sapidus in China, East and Southeast Asia | Ergosterol class, ergosterol peroxide | Immunoregulation and antitumor effect | NMR-based metabolomics | Chen et al. (2020); Takahashi et al. (2020) |
| Fusarium venenatum by Quorn™ industry | Food supplements | Immunological action, antioxidant | Fermentation and transcriptomics | Meyer et al. (2020) |
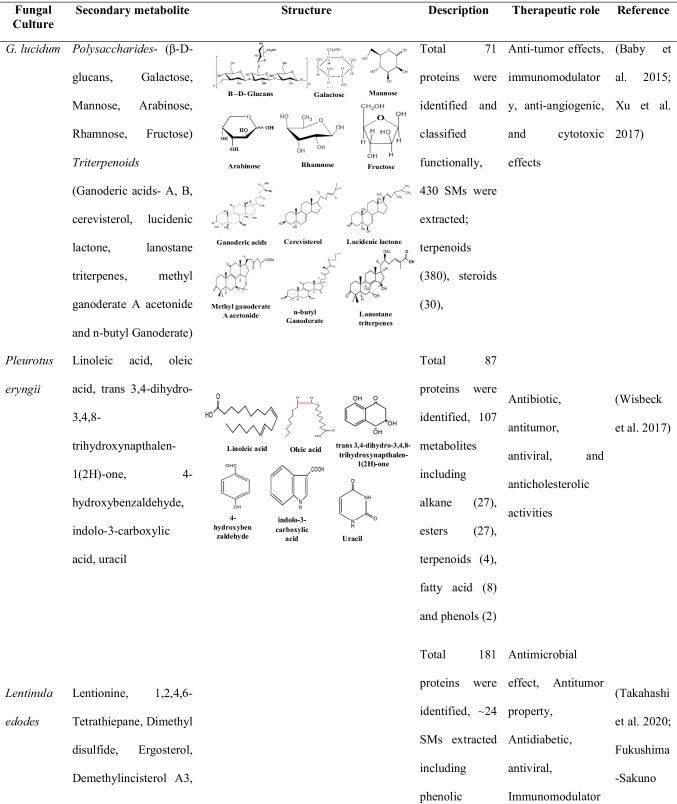
Table 2.
Different basidiomycete fungi and secondary metabolite with their application in human health
All the studies were done using LC-MS/MS and GC-MS studies
Ganoderma lucidum: Polyporales group of medicinal mushroom
Ganoderma is a filamentous wood-decaying fungus that belongs to the phyla Basidiomycota and has the unique ability to degrade the xylem cell wall components such as cellulose hemicelluloses and lignin. According to taxonomic reports, the genus Ganoderma consists of more than 300 species, and most of them are spread in tropical regions (Baby et al. 2015). G. lucidum belongs to a family of medicinal mushroom, and it is non-edible due to its thick, corky, and tough fruiting bodies; also, they do not have the fleshy texture as well as good taste. Till now, 431 secondary metabolites have been isolated particularly from the species G. lucidum (Baby et al. 2015); few of the examples are triterpenoids polysaccharides, steroids, ganoderic acid, alkaloids, ganomycins, fornicins, and ganocins. Among all, triterpenoids are important metabolites of G. lucidum, and it has beneficial biological effects. These major groups of SMs are extracted from many Ganoderma species like G. lucidum, G. applanatum, G. lipsense, G. colossum, G. orbiform, G. sinense, G. cochlear, G. amboinense, G. resinaceum, G. hainanense, G. pfeifferi, G. austral, G. tsugae, G. carnosum, G. capense, G. fornicatum, G. neo-japonicum, G. theaecolum, G. boninense, G. annulare, G. concinna, G. sinense, and G. mastoporum (Baby et al. 2015). The triterpenoids are comprised from six isoprene units (C30) which are found in a cyclic form like mono-, di-, tri-, tetra-, or pentacyclic carbon subunit, where the pentacyclic triterpenoids are well known group (Noji et al. 2021). Many volatile oils are also extracted from Ganoderma species. Baby et al. (2015) described the essential volatile oil from the fruiting material of G. lucidum by hydro-distillation, followed by GC with flame ionization detector (GC-FID) and GC-MS that analyzed their functional properties.
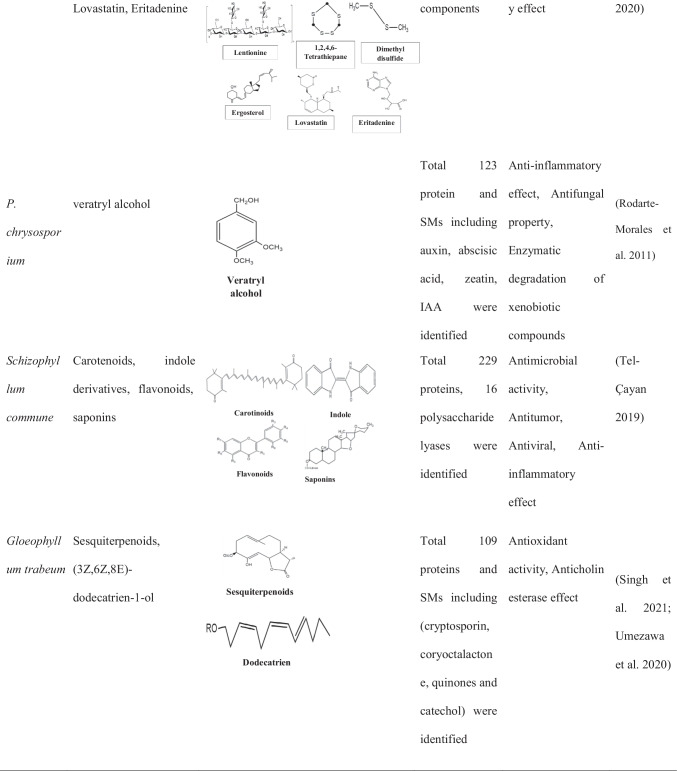
Phanerochaete chrysosporium: model in white-rot fungus of order Polyporales
Phanerochaete chrysosporium is a white-rot filamentous basidiomycete with ability to decompose lignin-rich plant material composing of lignocelluloses and other hemicelluloses. It produces two important SM compounds, i.e., veratryl glycerol and veratryl alcohol, in their extracellular culture fraction (Rodarte-Morales et al. 2011). Furthermore, P. chrysosporium has been considered as a model wood-decaying basidiomycete that has been used since long time in SM pathway studies. Additionally, it has also been used to study lignin metabolism through the omic tool of the whole-genome sequencing (WGS) (Ozsolak and Milos 2011), proteomic secretome analyses (Shankar et al. 2019), transcriptome studies (Ozsolak and Milos 2011), and gene transformation studies (Sharma et al. 2006). The first whole-genome sequencing of this model organism was published for wood decay and lignin metabolism to establish the significant enzymatic pathway and their role in degradation. The 32.5-Mb genome was sequenced and a total of 10,048 genes were screened, which is online available on public domain (Martinez-Gomez et al. 2012). Furthermore, in silico tool confirms the presence of genes for lignin peroxidases (LiP) and manganese peroxidases (MnP) in P. chrysosporium that has role in secondary metabolite production (Martinez-Gomez et al. 2012).
Trametes: a Polyporales
Trametes versicolor (also known as Coriolus versicolor) is a medicinal mushroom that refers to the family Polyporales and class Agaricomycetes of the white-rot basidiomycetes and grows on the dead decaying wood and deciduous trees. The pigment forming T. versicolor secretes a high amount of laccase enzyme, and also, its genomic as well as WGS updates confirm the lignin peroxidases (LiPs), dye-decolorizing peroxidases (DyPs), manganese peroxidases (MnPs), and versatile peroxidases (VPs) (Wang et al. 2015). Leliebre-Lara et al. (2015) extracted different types of metabolites like flavonoids, alkaloids, triterpenes, a small amount of cardenolides, and steroids through the ethanol extraction procedure. Xu et al. (2017) worked on the co-cultural fungal interaction of T. versicolor and G. applanatum, which confirm the series of carboxylic acid and novel xylosides with medicinal and industrial applications. In different countries like Japan and Europe, a chemical derivative of polysaccharide-K from T. versicolor has been approved for cancer treatment; also, different strains were screened for polysaccharide production having different structures and properties. Many other compounds were isolated from Trametes sp., namely spiroaxane sesquiterpenes, rosenonolactone, tramspiroins, and funatrol D with beneficial role in human health (Wang et al. 2015).
Pleurotus ostreatus: oyster mushroom of order Agaricales
Pleurotus is a white-rot basidiomycete group of fungi, which belongs to the family Pleurotaceae and order Agaricales group, formed as an oyster type of fruiting bodies. This edible mushroom has high nutritional value; also, its compounds are highly used in tumor treatment, antibacterial effect, and diabetes patients (Younis et al. 2015). These edible fungi secrete many SMs that have diverse structure of polysaccharides and terpenoids. These complex structures of polysaccharides activate many essential pathways which help in the defense mechanism of an organism. Berovic and Podgornik (2019) worked on a submerged culture of P. ostreatus and identified important SMs, for example, oleic acid, palmitic acid, linoleic acid, stearic acid, and their methyl esters. Many other species of Pleurotus contain various compounds like polysaccharides FII, eryngiolide A, β-glucan, ubiquinone-9, and some terpenoids like eryngiolide A, pleuroton A and B, and pleuroton B. These compounds have an essential role in disease treatment due to its antioxidant, antiviral, and antimicrobial properties, and it also boosts host immune system (Wisbeck et al. 2017).
Pycnoporus cinnabarinus: a cinnabar Polyporales
White-rot basidiomycete, Pycnoporus, is an edible mushroom that belongs to the Polyporaceae family member. These groups of fungi degrade cellulose, hemicelluloses, and lignin by secreting enzymes (redox and CAZy family) and pigments. P. sanguineus releases very effective metabolites like cinnabarinic acid which inhibits the growth of Gram-positive and Gram-negative bacteria and antimicrobial agents of many food products with other common pathogens (Evana et al. 2021). Many species of Pycnoporus release flavoring compounds like methyl anthranilate and phenoxazinone-type compounds like cinnabarinic acid and tramesanguin. Recent studies suggest that the molecule of cinnabarinic acid could play a beneficial role in neuro-inflammation disease, improve immune system, and have beneficial role in the disease of the central nervous system (Fukushima-Sakuno 2020).
Different fungal products and their health benefits
Fungi secrete different types of enzymes and metabolites for their survival in both favorable and unfavorable environmental conditions. The products of all fungal enzymes and metabolites are beneficial for human health due to its immunomodulatory (Fukushima-Sakuno 2020), anticancer (Ball et al. 2020), anti-inflammatory (Xu et al. 2017), antiviral (Wisbeck et al. 2017), antiatherosclerosis (Hyde et al. 2019), antidiabetic (Zhou et al. 2021), antioxidant (Kumar et al. 2015), and antiaging properties (Jue et al. 2017) (Table 1). Mushroom-producing white-rot fungi are mostly used for the extraction of the metabolites and their products that are beneficial for human health and fitness. These fungi are exploited for pharmaceutical and other medicinal products used for human health purposes.
There are six important classes of SM compounds secreted from white-rot fungi that are identified by using different multi-omic tools (Ijoma et al. 2021):
Terpenoids
Terpenes are a major class of bioactive metabolites that are produced from a higher class of Polyporales group of basidiomycetes, such as Pycnoporus sanguineus (Gauna et al. 2021), Pleurotus spp. (Wisbeck et al 2017), Ganoderma spp. (Baby et al. 2015), and Agaricus blazei (Shimizu et al. 2016), and other fungi from class ascomycetes, like Fusarium spp. (Nazari et al. 2016) and Trichoderma virens (Xu et al. 2017). These compounds are structural constituents of another group of metabolites that are formed through the linkage of isoprene units (hemiterpenes C5, monotertepenes C10, diterpenes C20, sesterterpenes C25, triterpenes C30, tetraterpenes C40). Different NMR and other omic tool confirm the member of the terpenoid class of compounds such as steroids, menthol, and taxol, which have key components for anticancer drugs and β-carotene properties. These bioactive compounds possess anti-infection, anti-inflammatory, anticancer, antiproliferative, and antiangiogenic medicinal properties (Ijoma et al. 2021).
Polyketides and fatty acids
Fatty acid and fungal polyketides are common metabolites present in large groups of ascomycete and basidiomycete fungi. These SMs are present in different species of mushroom-like Agaricus bisporus, Lentinus edodes, beefsteak fungus (Fistulina hepatica), and many other molds of ascomycetes like Aspergillus oryzae (Shimizu et al. 2016). All groups of microbial polyketides are synthesized by multi-domain polyketide synthases (PKSs), which are structurally diverse. It ranges from pharmaceutically modified drugs like lovastatin and griseofulvin to the most potent carcinogenic compound “aflatoxin B1” (Cox et al. 2018). PKSs are broadly categorized into three classes:
Type I polyketide synthases: They are large, multi-modular, and different functional proteins consisting of enzymatic domains that perform a direct reaction in polyketide chain assembly.
Type II PKSs are an assembly of regular proteins that catalyze the formation of aromatic polyketides like antibiotic actinorhodin.
Type III PKSs are a group of small aromatic compounds that produce different structures of SMs with many biological activities and antimicrobial properties (Navarro-Muñoz and Collemare 2020). Genomic study of A. oryzae has revealed the presence of four type III PKS genes, namely CsyA, CsyB, CsyC, and CsyD. Similar kind of genes has also been reported in the basidiomycete group of fungi (Navarro-Muñoz and Collemare 2020).
These secondary metabolites are formed through the acetate pathway within the cell by coupling of acetate group. In the fatty acid group, crude fat is considered under all categories of lipid compounds, including fatty acids, monoglycerides, diglycerides, triglycerides, sterols, and phospholipids. These groups of metabolites include antibiotics, statins (lowers the cholesterol levels), and antidepressant drugs (Ijoma et al. 2021).
Phenylpropanoids and aromatic amino acids
Secondary metabolites consist of phenolic compounds and are secreted from the fungal and plant metabolic pathways called the shikimate pathway. Metabolite of this group includes compounds like folic acid and salicylic acid, which help in reducing inflammation and pain; it is also used in antioxidant drug resveratrol (Singh et al. 2021). Phenolic compounds have many functions in plant, in which some have defense role against herbivores and other insects. Due to the high antioxidant effect and free radical scavenging property, phenylpropanoid compound has major attention in the area of human health, and nowadays, their functions are updated by the use of multi-omic tool (Singh et al. 2021).
Alkaloids
Alkaloids are nitrogen-containing compounds that have an important class of SMs, such as caffeine, cocaine, nicotine, morphine, and strychnine. These compounds were originated from one or many linked amino acids that can be synthesized from many biosynthetic pathways (Krause et al. 2020). It can regulate sodium ion channels and other microbial activity, which enhances their property to induce the immune system, causing cell death. The alkaloid products have been used to treat human diseases like cancer, lung disease, and acquired immune deficiency syndrome (AIDS) (Dey et al. 2020). Secondary metabolites of many endophytic fungi are composed of alkaloids, which have diverse biological and clinical properties like antifungal, antiviral, and antibacterial properties (Zhang et al. 2012). Fusarium chlamydosporum isolated from the root nodule, having indole alkaloids, shows phytotoxic activity against the bacterial species. Many other fungi, such as Mucor sp., A. terreus, and P. janthinellum, have been isolated from plant material. They are known to secrete different antimicrobial alkaloids like terezine E, indole derivative CC50, asperimides A–D, brasiliamide, and peniciolidone, respectively, that are proportionally beneficial to human health (Deshmukh et al. 2022; Nazari et al. 2016).
Proteins, peptides, and derivatives of amino acids
Peptides and proteins are significant component of SMs, where the products of metabolites have similarity between the compounds secreted from a large number of organisms. The protein contents are 20–30% by dry weight in most of the edible mushrooms (Pleurotus spp., Agaricus bisporus, Volvariella volvacea, and Lentinus edodes), which helps the vegetarians to maintain the protein content, vitamins, and fiber in their diet (Du et al. 2021).
Specialized carbohydrates
All fungal organisms are the primary source of carbohydrates, which can be described as primary metabolites. Those fungal metabolites have tremendous applications in the pharmaceutical industry. The polysaccharide of Pholiota microspora has a significant property to decrease low-density lipoprotein and increase high-density cholesterol. After comparison between different basidiomycete fungi, varying amounts of polysaccharide may be found, for example, polysaccharides present in Pleurotus species range from 46.6 to 81.8%, whereas A. bisporus has 60% on a dry weight basis (Berovic and Podgornik 2019). Nowadays, upgradation of many extractions and characterization tools help to discover novel carbohydrates and other metabolites that are key components of food and feed industry.
Multi-omic tools in basidiomycete metabolite regulation studies
Multi-omics composes of the most advance tools and techniques of the system biology. Many big pharma companies are using multi-omic tools for their basic research, finding natural products and novel molecules from the basidiomycetous fungi (Zhou et al. 2021; Amer and Baidoo 2021). In the past few years, many researchers have explored multi-omic technologies for clinical trials and biotechnological industries for screening and characterization of novel fungal SMs (Fig. 2). During COVID-19 pandemic condition, the researcher also worked in the area of SMs from endophytic fungi and predicted their inhibitory effect against single RNA-dependent RNA polymerase of coronavirus using molecular docking and simulation tools. The whole-genome data of the viruses and the plants helped in the prediction of two metabolites (namely dankasterone B and pyrrocidine A), which cause inhibition of viral RdRp (mutation associated in SARS-COV-2 genome) (Ebrahimi et al. 2021). Applying these high-throughput tools, researchers get access to the structure of the metabolite. Therefore, their functions can be predicted; furthermore, they can improve the production of the specific metabolite to confirm their role. In the recent past, the bioinformatic tools including transcriptomics and proteomics have assisted in gene ontology (GO) studies, pathway predictions, structure prediction, and genetic expression, and they have also been instrumental in the protein-protein interaction (PPI) studies (Jain et al. 2020).
Multi-omics can be used to study the complex process of life that learned through the various protocols and timeline for all sets of data and standards. Earlier, a single omic tool (genomics) was used for the analysis of the research query with many sets of samples. Due to the limitations in validating the data using genomics, like low computational power, less data storage capacity, and scarcity of data analysis software, lots of effort were needed to standardize the data, pipeline development, and report preparation for single data analysis (Brazma et al. 2001).
Nowadays, the research background has a suite of omic tools (multi-omics) which are in demand due to their technological advancement. The use of multi-omics has improved the ease of doing research due to the result clarity, cost-effectiveness, and high-throughput result analysis of biological entities (Subramanian et al. 2020). It offers a comprehensive, structured, and interactive overview of a biological mechanism and provides better opportunities with some challenges to the biologists, bioinformaticians, biostatisticians, and biomathematicians (Pavlopoulos et al. 2015). The major multi-omic tools and technologies are metagenomics, transcriptomics, proteomics, secretomics, and metabolomics. These new omic tools are high throughput and rapid in identifying the fungal genomes and their metabolites. Many other specialized fields which are included under the line of omic tools are epigenomics, ionomics (total elemental composition of an organism), lipidomics, fluxomics (analysis metabolic profiling), toxicogenomics (study change in the global gene expression profile and toxicological endpoints), nutrigenomics (study the relationship between human genome, nutrition, and health), and foodomics (omic approach to food science) (Capozzi and Bordoni 2012; Özdemir et al. 2017). By integration of these multi-omic techniques, production of targeted SM can be achieved and verified at various levels (Fig. 4). Furthermore, it can be helpful for the industries to identify the new commercial products and also to improve their metabolic fluxes.
Fig. 4.
Integration and prediction of SM from gene to metabolite level using multi-omic tool
Many recent reviewers had enlisted the omic techniques as genome-based molecular biology tools and envisaged their applications in sequence alignment (Cairns and Meyer 2017), nucleotide assembly (Keller 2019), RNA sequence analysis, metabolic pathway construction (Pavlopoulos et al. 2015), microarray analysis (Cary et al. 2018), and network analysis (Jain et al. 2020), for comparative genomics and transcriptomics study (Pavlopoulos et al. 2015). By the advancement in system biology approach in the genomic era, gene clusters and the conserved sequence of a SM-producing gene have been predicted. Furthermore, it can be edited through the molecular approach, whereas comparative genome analyses were used to know the SM biosynthetic pathway (Cairns and Meyer 2017). Proteomic timescale has provided an updated tool to estimate the production capability, quality of fungal enzyme, structure of SM, and their biosynthetic pathway at whole proteomic scale using LC-MS/MS technique (Jain et al. 2020).
Genomic tools for fungal metabolite study
Genomics is one of the initial omic tools that have been applied in high-throughput research field, which cover from comparative genetics to biotechnological and pharmaceutical industry like diagnostic, pharmacogenomics, and evolutionary genetic measurement (Sharma 2016). Earlier in genomics, SNP-ChIP was used to measure up to 5 million single nucleotide polymorphisms (SNPs), which were time-taking and costly, but next-generation sequencing (NGS) has come in limelight and has replaced the chip technology with reduced cost of sequencing by $0.10 per million bp. The NGS technologies like Roche 454, Illumina Solexa GA, and SOLiD (MacLean et al. 2009) and NextSeq 2000 (www.illumina.com) have many suitable platforms for recovering hundreds of millions of genomic sequences, single-cell gene expression, shotgun metagenomics of environmental sample, and discovering major novel genes of fungi at low cost. Nowadays, the cost of genome sequencing is estimated to be $600–$1000/genome when performed at a commercial scale using third-generation Oxford Nanopore PromethION (https://nanoporetech.com/products). Therefore, reducing costs has expanded the reach of the technology into the identification of microbial (fungal) metabolites.
The abovementioned NGS tools are explored in new commercial products and many metabolic pathways by the use of biological pathway analysis database and software (KEGG pathway, PANTHER, Ingenuity Pathway (IPA)) (Garber et al. 2011). Fungal SM identification and characterization have been done by using advanced technologies of NGS through mass spectrometry which rely on the freely available fungal genomes that accelerate the fungal proteome analysis (Jain et al. 2020). Genome mining tools have also been used to identify the uncharacterized natural product by estimating the genetic potential of strain through scanning the gene of interest (Ziemert et al. 2012). In genome sequencing of Aspergillus niger, a total of 200 new proteases have been checked during annotation; among them, two have been commercialized as prevention of haze in beer production and others applied for the production of sports drinks (van Schaick et al. 2021). The application of whole-genome sequencing technology in the SM secreting fungi or its gene clusters bypasses the need of DNA hybridization, library construction, screening, and chromosome editing techniques (Cacho et al. 2015).
Evidently, the connecting link between secondary metabolite and genes of organism is contributing majorly (a) in the exact prediction of secondary metabolite structure based on the DNA sequences and (b) in the designing of biosynthetic pathways for the synthesis of valuable products. Overall, the connecting gap between the genes and molecules using comparative genomics and transcriptomic tools helps in understanding of the natural function in SM study (Table 3) (Cacho et al. 2015). Many other established in silico tools like antiSMASH (Antibiotics and Secondary Metabolite Analysis Shell) (Blin et al. 2013) and PRISM 3 (Skinnider et al. 2017) have been upgraded with many features to know the function of enzymes found in the biosynthesis of SMs as well as to build the connection between biosynthetic gene clusters (BGCs) with their consistent natural products. Fungal genome sequencing had revealed that SMs represents <3% BGC, where the most common enzymes which are backbone for their gene cluster includes polyketide synthases (PKSs), non-ribosomal peptide synthetases (NRPSs), dimethylallyl transferases (DMATs), and terpene synthases (Li et al. 2016). BGC prediction in fungal gene cluster was explored by doing fungal genome sequencing of ~ 1037 species by antiSMASH (including Ascomycota, Basidiomycota, and other fungal taxa), and 36,399 BGCs were found to be organized in 12,067 network of gene cluster families (Robey et al. 2021). Thousands of identified or predicted fungal BGC data were uploaded and stored on public domain, like antiSMASH, MIBiG (Minimum Information about a Biosynthetic Gene Cluster), ClusterFinder, and IMG-ABC Database.
Table 3.
Genomics and transcriptomic tool with their uses in SM study of white rot fungi
| Tool and techniques | Mechanism | Application | References |
|---|---|---|---|
| Genomic techniques | |||
| DNA microarray | Hybridization based genomic technology identified gene from gene clusters | Prediction of specific protein and metabolite sequences by comparing with another genome | Özdemir et al. (2017) |
| Genome mining | Locate the genes into the genome | Search the location of genes that helps in metabolite formation | Narayanan et al. (2010) |
| Screening of unknown genes from whole-genome sequence | To know the biosynthetic potential of fungal SM BGC | Palazzotto and Weber (2018) | |
| Global Natural Product Social Molecular Networking (GNPS) | Added with MS/MS spectrum coupled with GC/LC to know the natural products | Perform MS searches using MS/MS spectrum as a query search, help in the quantification of metabolites or other drug components into the sample | Mao et al. (2021) |
| CRISPR/Cas9 | Based on genome editing technology | Highly efficient genetic manipulation technique with enabled the taking advantage and discovery of new bioactive compounds | Hadjithomas et al. (2017) |
| Transcriptomic approach | |||
| cDNA-AFLP | Sequence needed for cluster and alignment | Discover novel genes for metabolite production | Garber et al. (2011) |
| Shotgun sequencing | RNA identification by forming cDNA fragments | Detect, quantify, and annotate the coding/non-coding RNA | Fondi and Liò (2015) |
| Probe-based arrays | mRNA analysis with labeled sample and connect with cap analysis of gene expression tool (CAGE) | Explore gene expression at global level, screening of SM BGC cluster using Southern blots | Hasin et al. (2017) |
| Deep-sequencing technologies | RNA-sequencing for SM gene prediction in fungi | Determine RNA expression level, capture transcriptome dynamics | Ozsolak and Milos (2011) |
| Next-generation sequence (NGS) | RNA sequence identification by alter the DNA sequencing | Biomarker, therapeutic targets, SM gene cluster verification | Liu et al. (2021); Hasin et al. (2017) |
Similarly, hexaricins are a family of natural polyketide products identified through the genomic tool, and the presence of gene clusters was predicted in the rare marine actinomycete Streptosporangium sp. (Tian et al. 2016). Also, some of the natural metabolites, like bikaverin, immunomodulator endocrocin, and antibacterial compounds, were identified from the BGC group of Fusarium spp. (Keller 2019). Novel metabolite cyclic tetrapeptide, viz., cyclo-(l-leucyl-trans-4-hydroxy-l-prolyl-d-leucyl-trans-4-hydroxy-l-proline), was identified through co-culturing of Phomopsis sp. K38 and Alternaria sp. E33 (Rashmi and Venkateswara 2019). In recent times, unique secondary metabolites like lovastatin and swainsonine have been identified from filamentous fungi. However, an integration of clustered regularly interspaced short palindromic repeats-Cas9 (CRISPR/Cas9) technique in omic tool has improved the production of biosynthesized SMs (Jiang et al. 2021). With the help of the CRISPR/Cas9 tool, Wei et al. (2020) significantly enhanced the accumulation of pneumocandin B0 from Glarea lozoyensis. Thus, by predicting the SM gene clusters and its pathway from genomic analysis, researcher can find their gene of interest in a wild fungal strain and modify the pathway using CRISPR/Cas9 tool for enhanced SM production. The said pathway modifications can be verified by transcriptomic and proteomic tools.
Transcriptomics
Arguably, transcriptomics is the most advanced tool to study the metabolic update of molecular changes (like transcription, translation, and post-translation modifications), natural metabolite biosynthesis, and gene regulation and to validate the results of gene modification (CRISPR/Cas9) in wild genes at the transcriptional level (Palazzotto and Weber 2018). Compared to the traditional protein-coding transcriptome, modern non-coding RNA methods have shown high research output due to less complexity. Several RNA-based tools are used from the past few years (Table 3), where three main strategies have played a key role in the advancement of transcriptomic studies: (a) knowledge of microarray, (b) serial analysis of gene expression (SAGE) and suppression subtractive hybridization (SSH) (Brazma et al. 2001), and (c) de novo transcript sequence assembly (Jain et al. 2020). These instrumental techniques of the transcriptomic tool can increase the sequencing quality with their quantitative results at the average cost increment.
The transcriptomic study is deeply involved to detect the differentially expressed gene sets and their pathways, which are majorly responsible for the production of the metabolites using statistical tools, such as limma (Ritchie et al. 2015), DESeq2 (Love et al. 2014), Gene Set Enrichment Analysis (GSEA) (Zito et al. 2021), and DAVID (Ziemert et al. 2012). Researchers have developed pipelines such as GenePattern and UTAP (User-friendly Transcriptome Analysis Pipeline) for the transcriptomic data analysis and their evaluations. Among these models, probabilistic graphical models are most popular, which explains the distribution of transcripts data that is the key of transcriptomic tools (Palazzotto and Weber 2018; Hasin et al. 2017).
Earlier, transcriptomic studies suggest that ~ 3% of the genes code for the translational products, whereas up to 80% of the genome is transcribed into RNA transcripts (The ENCODE Project Consortium: An integrated encyclopedia of DNA elements in the human genome 2012). Additionally, this technique is helpful to reveal the link between the genes and pathways for the production of SMs. A differential gene regulation study by Jain and co-workers reveals that G. lucidum MDU-7, a white-rot basidiomycete has several up- and downregulated transcripts which eventually trigger the SM pathway like terpenoids and polyketides. These polyketide synthase transcripts elongate polyketide products, which include secondary metabolites (Jain et al. 2020). Using similar transcriptomic techniques, CRISPR-modified SM-producing pathway can be analyzed for required change in expression at transcript level.
Proteomic and secretomic tools
The proteomic tools play a pivotal role in functional analysis, as they give the knowledge at the peptide and protein level, which is not indeed equivalent to the transcriptomic information. Over a decade, proteomic tools have emerged because of the huge transcriptome and proteome cloud data availability (Jain et al. 2020). Also, bioinformatic advances have access to the detailed analysis of fungal biochemistry (Kumar et al. 2021). Proteomic studies are required for the analytical process of some techniques such as mass spectrometry (MS), 2-D mass spectrometry, and matrix-assisted laser desorption ionization-time of flight mass spectrometry (MALDI-TOF MS) that are involved in the SM identification and their analysis (Téllez-Téllez and Diaz-Godinez 2019). The proteomic study of P. chrysosporium revealed more than 1100 intracellular and 300 mitochondrial proteins which concluded in the 2-D gel electrophoresis method, and their metabolic flux shift was checked by comparative proteomic tool (Rodarte-Morales et al. 2011).
Recently, the experimental methods have been updated from 2-D gel electrophoresis, mass spectrometry (MS), MALDI to high-throughput tools like LC-MS/MS and iTRAQ to study fungal metabolites, with improvement in their analysis software and tools (Table 4) (Kumar et al. 2017; Shankar et al. 2019; Jain et al. 2020). Till date, more than hundreds of fungal genomes are publicly available (http://genome.jgi.doe.gov/programs/fungi/index; http://jgi.doe.gov/our-science/science-programs/fungal-genomics/recent-fungal-genome-eleases/) by 1000 Fungal Genome (1KFG) project (Sharma 2016). The LC-MS/MS tool provides data in spectral format, from which peptides and proteins can be identified using tools such as Andromeda and X!Tandem by utilizing referenced databases, i.e., 1KFG (Sharma 2016), UniProt (Apweiler et al. 2004), and Integrated Microbial Genomes (IMG) (Hadjithomas et al. 2017). Andromeda comes as a stand-alone tool or integrated into MaxQuant open source software; it can handle many labeling strategies and label-free quantification and 3D graphic visualization. The resulted peptides can be used for gene ontology (GO) analysis by Blast2Go and GOnet that provide profile of present proteins, enzymes, and pathways (Conesa et al. 2005; Pomaznoy et al. 2018). Differential expression and pathway enrichment analysis can be performed on peptide data using limma (Ritchie et al. 2015), DESeq2 (Love et al. 2014), GSEA (Zito et al. 2021), and DAVID (Ziemert et al. 2012).
Table 4.
Proteomic and secretomic tools used in secondary metabolite study
| Tool and techniques | Mechanism | Applications | References |
|---|---|---|---|
| 2-D PAGE and Isoelectric focusing (IEF) | Separation and identification of proteins by their charge-to-mass ratio | Helps in the identification of posttranslational modified natural protein and SM | Shankar et al. (2019) |
| MALDI-ToF/ToF | Ionize biological molecule to identify their mass and sequences | Ionized biological sample gives all about the molecular weight, protein sequence, and three-dimensional structure of metabolites | Shankar et al. (2019) |
| Electrospray ionization-mass spectrometry (ESI-MS) | Elucidation of biological mass, amino acid sequences, and modified structure of peptides and proteins | Ionized biological sample gives all about the mass, sequence and three-dimensional structure | Tsuchiya et al. (2020) |
| Systematic Evolution of Ligands by Exponential Enrichment (SELEX) | Isolate aptamers from different microbial targets, toxins, and chemical compound | Deciphering the protein-DNA sequence specificity to address the fundamental biological query | Liu et al. (2020) |
|
Microchip Capillary Electrophoresis |
Identification and separation of metabolites and their profiling | Highly applicable due to the requirement of less sample, short analysis time, high-throughput capability, low waste generation, and portability | Shankar et al. (2019) |
| 2-D fluorescence difference gel electrophoresis (2D-DIGE) | Labeling and separation of one or more proteins by isoelectric focusing | Done with comparative proteomics study by separating complex protein into simple component | Vilasi et al. (2013) |
| Exponentially modified protein abundance index (emPAI) | Comparative proteomic analysis performed to estimate protein abundance | Easy to applied in multi-dimensional proteomic separation-MS/MS | Shinoda et al. (2010) |
| iTRAQ/LC-MS/MS | Identify total protein with their differential regulation | Widely used for quantitative proteomics, prediction of SM production | Martinez-Gomez et al. (2012) |
| Nano-flow liquid chromatography tandem mass spectrometry (nano-flow LC-MS/MS) | Proteolytically digested proteins isolated by 2-D gel electrophoresis | Robust tool in the qualitative and quantitative protein identification | Laatsch (2011) |
| Stable isotope labeling by amino acids in cell culture (Absolute SILAC) | Identify targeted labeled protein from a large set of samples | Applied for targeted protein quantification in various biomedical research and clinical practices | Wang et al. (2016) |
|
LTQ-Orbitrap LC MS/MS |
Identify and structurally characterize peptides in a highly complex sample mixture | More sensitive with higher accuracy then other proteomic tools | Narayanan et al. (2010) |
| Label-free LCMS/MS | Total protein profiling in a single run with their quantification | Fast, low-cost, rigorous, powerful tools for analyzing protein changes in large-scale proteomics studies | Jain et al. (2020) |
Fungal secretomic study reveals many novel and functional SMs that help in the better understanding of their mechanism and enzymatic pathways. Due to the advances in these proteomic and secretomic tools, the research output of metabolic processes gives significant knowledge and beneficial product in biotechnological and pharmaceutical industries. The protein fractions of G. lucidum at developmental (16 days of mycelial grown stage) and fruiting bodies stages (60 and 90 days of mycelial stage) revealed 803 proteins after LC-MS/MS analysis and explore against genome database (Yu et al. 2015). Furthermore, proteomic and sequence alignment studies suggested a unique immunomodulatory protein (GL18769) with significantly high bioactivity. The proteomic and secretomic tools are important nowadays in the study of secondary metabolites due to regular up-gradation in mass spectrometry data on the cloud is going on. Implementation of shotgun proteomic approach in Aspergillus fumigatus detected 414 mycelial proteins which were represented in 2-D protein interaction maps (Owens et al. 2014). Quantitative proteomic analysis of different filamentous groups of Aspergillus spp. was checked and analyzed for various purposes, i.e., better production level of SMs, comparison to the metabolic process, and to know the involvement of the protein in SM production (Ma et al. 2021). However, recent secretome profiling of G. lucidum by Jain and co-workers revealed 83 proteins in control and 142 proteins in the copper-induced supernatant. They used the transcriptomic data as a referenced database for the unknown/ unreviewed peptide data in the protein identification. Thus, the function of unknown peptides can be predicted (Jain et al. 2020). In secretome analysis, different white-rot fungi and other ascomycetes are analyzed as well as their secretory protein and SMs; also, their functions were checked through gene ontology. They produce different lignin-, cellulose-, and hemicellulose-degrading enzymes like laccases, cellulases, peroxidases, LiP, MnP, and other related enzymes used by different biotechnologists and in pharmaceutical industries (Jain et al. 2020; Amer and Baidoo 2021; Kumar et al. 2021). In comparison to transcriptomics, better validation can be obtained from proteomics by upregulation of required proteins in SM pathway.
Metabolomic tool
Metabolomics analyzes metabolites, like small molecules of cells, tissues or organisms, and biofluids. This tiny fraction of molecules and their interaction with the biological system is called the metabolome. This tool is becoming very important for the researcher to collect information regarding fungal SM characterization and their production. Moreover, it also provides opportunities to explore the new fungal ecological niches with many unknown SM diversities and their functional, as well as production properties. The metabolomics makes an ideal tool for the biotechnological industry, agricultural industries, and pharmaceutical and healthcare products; therefore, biomarker products and drug safety issues are two examples where metabolomics has started making products and other testing kits. In the metabolome study, two complementary processes are mainly used for SM profiling, i.e., (a) targeted and (b) untargeted approaches. In targeted approaches, a fixed set of known metabolite compound is analyzed which mainly confirms the absolute quantification and, in untargeted profiling, it detects all the compounds and shows the potential of total metabolic profile, including unknown substance. Therefore, untargeted approaches are mainly preferred to detect the change in metabolome, and it is leading in the scientific field. The MS/MS-based tools are used under the untargeted approaches where GC-MS and LC-HRMS are the best techniques for the analysis of complex SMs produced by filamentous fungi (Klitgaard et al. 2014).
Many technologies like high-resolution mass spectrometry, liquid chromatography with tandem mass spectrometry (LC-MS/MS), nuclear magnetic resonance (NMR), and metabolite mass spectrometry imaging (MSI) have increased the scope of metabolomic tool to detect many unknown compounds. Liquid chromatography-ultraviolet (LC-UV), ultra-high performance liquid chromatography (UHPLC), LC-MS/MS, and GC-MS are highly sensitive detector machines with tremendous selectivity (like physical compound separation by chromatographic technique and co-eluting separation of analytes through m/z ratio of an ionized molecule), which determine hundreds of metabolic compounds in a single run (Klitgaard et al. 2014; El-Elimat et al. 2021). For targeted metabolite analysis, tool such as liquid chromatography triple quadrupole tandem mass spectrometry (LC/QqQ/MS) gives result by applying triple-stage quadrupole mass spectrometers (QqQ/MS) by operating in selected or multiple reaction monitoring (S/MRM) mode (Thadhani et al. 2021).
Earlier, the most accessed and frequently used technique for untargeted metabolomics was liquid chromatography with high-resolution mass spectrometer (LC-HRMS) which annotates the compound and confirms the function with similarly structured compound data from the respective database. Furthermore, it also predicts the molecular formula by comparing accurately measured masses with the well-known databases like Chemical Entities of Biological Interest (ChEBI) (Degtyarenkoet al. 2009), PubChem (Kim et al. 2016), and AntiBase 2014, which have comprehensive database of more than 40,000 natural compounds from bacteria and fungi (Laatsch 2011). In silico software, Global Natural Products Social Molecular Networking (GNPS) system, is a general web-based mass spectrometry ecosystem that has an open access library which shares approx. 220,000 MS/MS spectra that confirm more than 18,000 natural products from the Mass Bank (Horai et al. 2010), ReSpect (Sawada et al. 2012), and NIST (http://www.nist.gov/srd/nist1a.cfm) databases that also provide tools for metabolite identification, quantification, and analysis.
As mentioned earlier, genes of SM pathway identified in wild strain through genomic study and edited using CRISPR/Cas9 technology can be further verified at transcriptome level for successful transcription of pathway genes (i.e., no random splicing) and at proteomic level for scrupulous translation into peptides (i.e., no silencing at translational level). Finally, successful production of targeted SM can be identified using LC/QqQ/MS, and structure validation can be done by NMR (Fig. 4).
Bioinformatic tools: integration of artificial intelligence and machine learning
In the past, many analytical and chemical processes gave access to natural products; nowadays, genomics, proteomics, and metabolomic tools provide a complementary approach to identify and characterize the molecules. These complimentary tools come alone with several computational tools that together form system biology. Several important computational challenges have appeared after the emergence of next-generation sequencing tools including 454, Illumina, SOLiD, and ion semiconductor. Upgrading of this tool for secondary metabolite and the natural product study has generated large datasets with hundreds and thousands of MS and MS/MS spectra (Table 5). In practice, online data bank has been updated with microbial data that are related to metabolite studies like MIBiG, a BGC repository that has a database for 206 fungi and 1196 bacteria BGCs (Kautsar et al. 2020), and ClusterMine 360 is a peptide database that contains gene cluster domains for microbial polyketide synthase (PKS) and non-ribosomal peptide synthetase (NRPS) biosyntheses (http://www.clustermine360.ca/) (Cruz-Morales et al. 2016). Furthermore, many web accesses portal and database search engines have played a key role in the bioinformatic tool (Table 5). Bioinformaticians have designed tools using biological data, data mining approaches, mathematics, statistics, and machine learning. Many machine learning algorithms are designed to run the online database of bacterial, fungal, and other human genome data to predict the results used in the biotechnological and pharma fields (Fig. 1). The most frequently used machine learning algorithms applied for biological data analysis include artificial neural networks (ANNs) (Almeida et al. 2019), Naive Bayes (Quinlan 1993), support vector machine (SVM) (Huang et al. 2018), C4.5 decision tree (Quinlan 1993), k-nearest neighbors (KNN) (McKinney et al. 2013), and regression (Zeng and Lumley 2018). Majorly, two different strategies are used in the in silico tool, where best approaches are used to identify the biosynthetic gene clusters (BGCs), in which the data mining process identifies genes with their conserved enzymes that are linked with secondary metabolism, whereas the second strategy is used for the identified hits with different classes of natural product confirmation.
Table 5.
Bioinformatic tools and databases for SM study
| S. no. | Name | Functions | URL | References |
|---|---|---|---|---|
| 1. | Secondary Metabolite Bioinformatics Portal (SMBP) | Database catalog and links of bioinformatic tool for the secondary metabolite study | http://www.secondarymetabolites.org | Palazzotto and Weber (2018) |
| 2. | Antibiotics and Secondary Metabolite Analysis SHell (antiSMASH) | Web application and autonomous tool (LINUX, MacOS and MS Windows) to mine and analyze BGCs; include genomic tools and a homology-based metabolic modeling pipeline | http://antismash.secondarymetabolites.org. | Blin et al. (2013) |
| 3. | Natural Product Domain Seeker (NaPDoS) | Tool for the rapid detection and analysis of secondary metabolite genes to detect and classify KS and C domains | http://napdos.ucsd.edu/run_analysis.html | Ziemert et al. (2012) |
| 4. | NP.searcher | Web application search tool (LINUX) to mine for PKS/NRPS BGCs | http://dna.sherman.lsi.umich.edu/ | Chavali and Rhee (2018) |
| 5. | Genes to natural products/prediction informatics for secondary metabolomes (GNP/PRISM) | Cluster mining tool and analyze the pathway for PKS and NRPS | Skinnider et al. (2017) | |
| 6. | Secondary Metabolite Unknown Region Finder (SMURF) | Tool to mine PKS/NRPS/terpenoid gene clusters in fungal genome | www.jcvi.org/smurf/ | Wang et al. (2016) |
| 7. | Cluster Finder | Stand-alone tool (LINUX and MacOS) to detect BGCs with secondary metabolite gene clusters in genomic and metagenomic data | https://github.com/petercim/ClusterFinder | Chavali and Rhee (2018) |
| 8. | HMMER web server | Identify ketosynthase domain and condensation domain encoding genes in genomic and metagenomic datasets | http://hmmer.org/ | Potter et al. (2018) |
| 9. | XCMS | Metabolomic data processing algorithm tool to extract metabolic features from raw MS data and perform statistical analysis. | https://xcmsonline.scripps.edu. | Domingo-Almenara et al. (2018) |
| 10. | iSNAP | Web tool which automatically identify metabolites in MS/MS data based on genomic data | http://snap.cifr.ncsu.edu | Baptista et al. (2022) |
| 11. | CLUsterSEquenceANalyzer (CLUSEAN) | Web accessible database of PKS/NRPS BGCs and annotation pipeline for secondary metabolite biosynthetic gene clusters. | Chavali and Rhee (2018) | |
| 12. | Minimum Information about a Biosynthetic Gene cluster (MIBiG) | Web tool for annotations and metadata on biosynthetic gene clusters and their molecular products. | https://mibig.secondarymetabolites.org/ | Bian et al. (2020) |
| 13. | EvoMining approach | Stand-alone tool for phylogenomic approach of cluster identification | (https://github.com/nselem/evomining/wiki) | Sélem-Mojica et al. (2019) |
| 14. | Integrative meta-analysis of expression data (INMEX) | Web-based tool to support meta-analysis of multiple gene expression datasets | http://www.inmex.ca | Xia et al. (2013) |
| 15. | Metscape 2 | Web tool for the analysis and visualization of metabolomics and gene expression data and visualize changes in the gene/metabolite data. | http://metscape.ncibi.org | Rosato et al. (2018) |
| 16. | Crux | MS analysis toolkit that combine computational, machine learning and statistical methods for proteomics analysis | https://crux.ms/ | McIlwain et al. (2014) |
Comparative interactomic outlook is a robust alternative approach for discovering modules and their pathways across all cellular and metabolomic networks using SVM and ANN. Genomic and proteomic sequences of fungal species are used to integrate protein annotation into network analysis, potential signals, and different metabolic regulatory pathways. These sequences are modeled as complex network patterns that help in metabolite production and their application in many medical as well as industrial areas. Probabilistic models have integrated network and expression data from gene knockout expression studies to predict cell-signaling cascades (Palazzotto and Weber 2018). Many pipelines have been developed earlier for the study of the microbial products like Antibiotic Resistance Target Seeker (ARTS) using deep learning and Fungal Resistance Gene-directed Genome mining (FRIGG), and Crux (https://www.cruxinformatics.com/) is a mass spectrometry analysis toolkit that provides multiple machine learning based tool for tandem mass spectra analysis, statistics, and visualization (McIlwain et al. 2014; Stahlecker et al. 2021). These tools represent recent progress in many microbial aspects, including RNA sequencing analysis, comparative proteomics, metagenomics, and developments of new computational workflows in fungal physiology and biology (Xiao et al. 2017). Capecchi and Reymond (2021) classified the natural product as a resource of new drug discovery where they reported the Collection of Open Natural Products (COCONUT) database, a database assigned for bacteria, fungi, and plant SM products, and trained machine learning tool (MAP4 SVM -https://np-svm-map4.gdb.tools/) that predicts the presence of plant, bacterial, or fungal SMs.
Artificial intelligence is a complex network and layer of multiple machine learning algorithms resembling brain neurons. The deep neural network (DNN) implements in a machine learning tool. It is a robust approach for the omic tool’s classification, regression, and other statistical disciplines. Another emerging concept, meta-learning, i.e., “one’s learning and learning processes,” is fundamental in the transfer of learning from one situation to another. Meta-learning enables an automated way to optimize the DNN and save incredible human effort and time. A significant number of machine learning methodologies and system biology prediction tools are used in novel pharmaceutical product development. AlphaFold software, using artificial intelligence, predicts 3D protein structure only based on its genetic sequence; provided whole genome as input, it can predict all the proteins in genome (Jumper et al. 2021). Furthermore, definite metabolism site has also been reported using this bioinformatic tools (Chavali and Rhee 2018). Currently, in metabolite prediction study, data reflected with high false-positive results with low reliability while studying specific enzyme system. To overcome this issue, a method was designed by Wang and their co-workers that established a broad coverage of the SMARTS-coded metabolic reaction rule database. This intelligence tool could suggest the optimal metabolic reaction with accuracy rate of 70% (Wang et al. 2016). The use of metabolomics and AI learning tool in the mycotoxins study proposed the best linkage of aflatoxins and their biomarkers which is an example of potentially emerging mycotoxins. Recently, Xie and co-worker introduced the XGBoost algorithm tool into the global food safety risk management. It will be used in the prevention and control of mycotoxins by analyzing novel biomarkers associated with aflatoxigenic Aspergillus species using population metabolomics and machine learning tools (Xie et al. 2022).
Future prospects and conclusions
The multi-omic concept is still rooting in the area of fungal biology. The beauty of the fungal omic tools is to clarify the cellular, molecular, and biological processes and give a response at different levels of Crick’s central dogma of molecular biology (DNA, RNA, and protein). The application of modern highly sophisticated tools mentioned in this review highlights deep analysis, and many other essential metabolic engineering approaches help to enhance the knowledge in the improvement of industrial as well as pharmaceutical bioproducts. Multi-omic research tool gives a better way for self-accepting and validating the results through the use of combined or parallel tools that accelerate the understanding of complex raw data and help in the development of engineered metabolites of biotechnological importance.
Bioinformatic-assisted fusion of different omic tools which make a pipeline to form machine learning program needs to accept the challenges posed from different datasets of sample size, sample quality, small sample analysis pipelines, and data formats for many fungal or other selected raw data. The recent trend is followed by an updated multi-omic tool which forms a bridge between wet-lab work and computational tools in the basic and applied research area. In this review, many web servers are mentioned, which can explore diverse tools and databases in one stop for SM study. Overall, the development of multi-omics and big data, and many other integrated tools such as CRISPR/Cas9, artificial intelligence, and machine learning tools, are involved in the genomic, transcriptional, as well as translational study of fungal primary and secondary metabolomes, which will lead to the advancement in the understanding of fungal biology and its products. In the era of multi-omics, it is time to shift our focus on the precise prediction of fungal metabolites; therefore, the structural spectral fingerprints are collected for their identification and characterization using machine learning NMR tool. Extensive data management and processing require state-of-art super computational infrastructure and cloud computing resources to efficiently analyze and share fungal multi-omic data in a short time period to achieve coherence with multiple organizations, like universities, pharmaceutical industries, data resource centers, and government-funded research institutes.
Acknowledgements
The authors acknowledge the Maharshi Dayanand University, Rohtak, Haryana, India, for infrastructural support. They also acknowledge Biorender.com for the figure presentation. AS also acknowledges Maharshi Dayanand University, Rohtak, Haryana, for University Research Fellowship.
Author contribution
KKS conceived and designed the manuscript. AS wrote and formatted the manuscript. KKS read and approved the manuscript.
Funding
This work was supported by DST-FIST grant, Government of India.
Data availability
No data is associated with the manuscript.
Code availability
Not applicable.
Declarations
Ethics approval
Not applicable.
Consent to participate
Not applicable.
Consent for publication
Not applicable.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- Almeida H, Tsang A, Diallo AB (2019) Supporting supervised learning in fungal Biosynthetic Gene Cluster discovery: new benchmark datasets, Proceedings of the IEEE International Conference on Bioinformatics and Biomedicine (BIBM). PiscatawayIEEE1280–1287. 10.1109/BIBM47256.2019.8983041.
- Amer B, Baidoo EE. Omics-driven biotechnology for industrial applications. Front. Bioeng. Biotechnol. 2021;9:613307–613307. doi: 10.3389/FBIOE.2021.613307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Apweiler R, Bairoch A, Wu CH, Barker WC, Boeckmann B, Ferro S, Gasteiger E, Huang H, Lopez R, Magrane M, Martin MJ, Natale DA, O’Donovan C, Redaschi N, Yeh LSL. UniProt: the Universal Protein knowledgebase. Nucleic Acids Res. 2004;32:D115. doi: 10.1093/nar/gkh131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ashraf SA, Elkhalifa AEO, Siddiqui AJ, Patel M, Awadelkareem AM, Snoussi M, Ashraf MS, Adnan M, Hadi S. Cordycepin for health and wellbeing: a potent bioactive metabolite of an entomopathogenic Cordyceps medicinal fungus and its nutraceutical and therapeutic potential. Molecules. 2020;25:2735. doi: 10.3390/molecules25122735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baby S, Johnson AJ, Govindan B. Secondary metabolites from Ganoderma. Phytochemistry. 2015;114:66–101. doi: 10.1016/j.phytochem.2015.03.010. [DOI] [PubMed] [Google Scholar]
- Ball B, Langille M, Geddes-Mcalister J. Fun(Gi)omics: advanced and diverse technologies to explore emerging fungal pathogens and define mechanisms of antifungal resistance. MBio. 2020;11(5):1–18. doi: 10.1128/MBIO.01020-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baptista RP, Li Y, Sateriale A, Sanders MJ, Brooks KL, Tracey A, Ansell Brendan R.E., Jex AR, Cooper GW, Smith ED, Xiao R, Dumaine JE, Georgeson P, Pope BJ, Berriman M, Striepen B, Cotton JA, Kissinger JC. Long-read assembly and comparative evidence-based reanalysis of Cryptosporidium genome sequences reveal expanded transporter repertoire and duplication of entire chromosome ends including subtelomeric regions. Genome Res. 2022;32(1):203–213. doi: 10.1101/2021.01.29.428682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berovic M, Podgornik BB (2019) Engineering aspects in production of various medicinal mushrooms biomass in submerged bioreactors. Int J Med Mushrooms 21(8) 10.3390/molecules17032714 [DOI] [PubMed]
- Bian Y, Zheng R, Bayer FP, Wong C, Chang YC, Meng C, Zolg DP, Reinecke M, Zecha J, Wiechmann S, Heinzlmeir S, Scherr J, Hemmer B, Baynham M, Gingras AC, Boychenko O, Kuster B. Robust, reproducible and quantitative analysis of thousands of proteomes by micro-flow LC–MS/MS. Nat Commun. 2020;11(1):1–12. doi: 10.1038/s41467-019-13973-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blin K, Medema MH, Kazempour D, Fischbach MA, Breitling R, Takanoet E. antiSMASH 2.0–a versatile platform for genome mining of secondary metabolite producers. Nucleic Acids Res. 2013;41:W204–12. doi: 10.1093/NAR/GKT449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bogale TT. Biotechnological applications of white rot fungi: a review. GSC Adv Res Rev. 2020;52:097–103. doi: 10.30574/gscarr.2020.5.2.0043. [DOI] [Google Scholar]
- Brazma A, Hingamp P, Quackenbush J, Sherlock G, Spellman P, Stoeckert C, Aach J, Ansorge W, Ball CA, Causton HC, Gaasterland T, Glenisson P, Holstege FCP, Kim IF, Markowitz V, Matese JC, Parkinson H, Robinson A, Sarkans U, Schulze-Kremer S, Stewart J, Taylor R, Vilo J, Vingron M. Minimum information about a microarray experiment (MIAME)-toward standards for microarray data. Nat Genet. 2001;29(4):365–371. doi: 10.1038/NG1201-365. [DOI] [PubMed] [Google Scholar]
- Cacho RA, Tang Y, Chooi YH. Next-generation sequencing approach for connecting secondary metabolites to biosynthetic gene clusters in fungi. Front Microbiol. 2015;5:774. doi: 10.3389/FMICB.2014.00774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cairns T, Meyer V (2017) In silico prediction and characterization of secondary metabolite biosynthetic gene clusters in the wheat pathogen Zymoseptoria tritici. BMC Genomics 18:1, 18(1), 1–16 10.1186/S12864-017-3969-Y [DOI] [PMC free article] [PubMed]
- Calixto JB (2019) The role of natural products in modern drug discovery. An Acad Bras Cienc 91. 10.1590/0001-3765201920190105 [DOI] [PubMed]
- Capecchi A, Reymond JL (2021) Classifying natural products from plants, fungi or bacteria using the COCONUT database and machine learning. J Cheminform 13(1). 10.1186/s13321-021-00559-3 [DOI] [PMC free article] [PubMed]
- Capozzi F, Bordoni A. Foodomics: a new comprehensive approach to food and nutrition. Genes Nutr. 2012;8(1):1–4. doi: 10.1007/S12263-012-0310-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cary JW, Gilbert MK, Lebar MD, Majumdar R, Calvo AM. Aspergillus flavus secondary metabolites: more than just aflatoxins. Food Saf. 2018;61:7–32. doi: 10.14252/foodsafetyfscj.2017024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chavali AK, Rhee SY. Bioinformatics tools for the identification of gene clusters that biosynthesize specialized metabolites. Brief Bioinform. 2018;19(5):1022–1034. doi: 10.1093/BIB/BBX020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen P, Qin HJ, Li YW, Ma GX, Yang JS, Wang Q (2020) Study on chemical constituents of an edible mushroom Volvariella volvacea and their antitumor activity in vitro. Nat Prod Res 34(10):1417–1422 10.1080/14786419.2018.1509324 [DOI] [PubMed]
- Chuang MH, Chiou SH, Huang CH, Yang WB, Wong CH (2009) The lifespan-promoting effect of acetic acid and Reishi polysaccharide. Bioorg Med Chem 17(22):7831–7840 10.1016/j.bmc.2009.09.002 [DOI] [PubMed]
- Conesa A, Götz S, García-Gómez JM, Terol J, Talón M, Robles M. Blast2GO: a universal tool for annotation, visualization and analysis in functional genomics research. Bioinformatics. 2005;21(18):3674–6. doi: 10.1093/bioinformatics/bti610. [DOI] [PubMed] [Google Scholar]
- Cox RJ, Skellam E, Williams K (2018) Biosynthesis of fungal polyketides. In: Anke T, Schüffler A. (eds) Physiology and genetics. The mycota (a comprehensive treatise on fungi as experimental systems for basic and applied research), vol 15. Springer, Cham 10.1007/978-3-319-71740-1_13
- Cruz-Morales P, Kopp JF, Martínez-Guerrero C, Yáñez-Guerra LA, Selem-Mojica N, Ramos-Aboites H, Cruz-Morales P, Kopp JF, Martínez-Guerrero C, Yáñez-Guerra LA, Selem-Mojica N, Ramos-Aboites H, Feldmann J, Barona-Gómez F. Phylogenomic analysis of natural products biosynthetic gene clusters allows discovery of arseno-organic metabolites in model streptomycetes. Genome Biol Evol. 2016;8(6):1906–1916. doi: 10.1093/gbe/evw125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Degtyarenko K, Hastings J, de Matos P, Ennis M (2009) ChEBI: an open bioinformatics and cheminformatics resource. Curr Protoc Bioinform, Chapter 14(SUPPL. 26), Unit 14.9-Unit 14.9 10.1002/0471250953.BI1409S26 [DOI] [PubMed]
- Deshmukh SK, Dufossé L, Chhipa H, Saxena S, Mahajan GB, Gupta MK (2022) Fungal Endophytes: a potential source of antibacterial compounds. Journal of Fungi, Fungal Endophytes: A Potential Source of Antibacterial Compounds 8(2):164.10.3390/jof8020164 [DOI] [PMC free article] [PubMed]
- Dey P, Kundu A, Kumar A, Gupta M, Lee BM, Bhakta T, dash S, Kim HS (2020) Analysis of alkaloids indole alkaloids, isoquinoline alkaloids, tropane alkaloids. In Recent advances in natural products analysis. 505–567. Elsevier10.1016/B978-0-12-816455-6.00015-9
- Domingo-Almenara X, Montenegro-Burke JR, Ivanisevic J, Thomas A, Sidibé J, Teav T. Guijas C, Aisporna AE, Rinehart D, Hoang L, Nordström A, Gómez-Romero M, Whiley L, Lewis MR, Nicholson JK, Benton HP, Siuzdak G (2018) XCMS-MRM and METLIN-MRM: a cloud library and public resource for targeted analysis of small molecules. Nat Methods 15(9):681–68410.1038/S41592-018-0110-3 [DOI] [PMC free article] [PubMed]
- Du X, Muniz A, Davila M, Juma S. Egg white partially substituted with mushroom: taste impartment with mushroom amino acids, 5′-nucleotides, soluble sugars, and organic acids, and impact factors. Food Sci Technol. 2021;1(7):1333–1348. doi: 10.1021/acsfoodscitech.1c00229. [DOI] [Google Scholar]
- Ebrahimi KS, Ansari M, Moghaddam MSH, Ebrahimi Z, Shahlaei M, Moradi S. In silico investigation on the inhibitory effect of fungal secondary metabolites on RNA dependent RNA polymerase of SARS-CoV-II: a docking and molecular dynamic simulationstudy. Comput Biol Med. 2021;135:104613. doi: 10.1016/j.compbiomed.2021.104613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- El-Elimat T, Figueroa M, Ehrmann BM, Cech NB, Pearce CJ, Oberlies N. High-resolution MS, MS/MS, and UV database of fungal secondary metabolites as a dereplication protocol for bioactive natural products. J Nat Prod. 2021;76(9):1709–1716. doi: 10.1021/np4004307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Evana E, Palupi KD, Oktavia L, Fathoni A (2021) Bioprospection of Enggano macroscopic fungi as antibacterial and antioxidant agents. Berita Biologi, 20(2):201–210 10.14203/beritabiologi.v20i2.4110
- Fazio F, Lionetto L, CurtoM Iacovelli L, Copeland CS, Neale SA, Bruno V, Battaglia G, Salt TE, Nicoletti F. Cinnabarinic acid and xanthurenic acid: two kynurenine metabolites that interact with metabotropic glutamate receptors. Neuropharmacology. 2017;112:365–372. doi: 10.1016/J.NEUROPHARM.2016.06.020. [DOI] [PubMed] [Google Scholar]
- Fondi M, Liò P (2015) Genome-scale metabolic network reconstruction. Bacterial Pangenomics, In: Mengoni A., Galardini M., Fondi M. (eds), pp 233–256. Humana Press, New York, NY. 10.1007/978-1-4939-1720-4_15
- Fukushima-Sakuno E. Bioactive small secondary metabolites from the mushrooms Lentinula edodes and Flammulina velutipes. J Antibiot. 2020;73(10):687–696. doi: 10.1038/s41429-020-0354-x. [DOI] [PubMed] [Google Scholar]
- Garber M, Grabherr MG, Guttman M, Trapnell C. Computational methods for transcriptome annotation and quantification using RNA-seq. Nat Methods. 2011;8(6):469–477. doi: 10.1038/NMETH.1613. [DOI] [PubMed] [Google Scholar]
- Gauna A, Larran AS, Feldman SR, Permingeat HR, Perotti VE. Secretome characterization of the lignocellulose-degrading fungi Pycnoporus sanguineus and Ganoderma resinaceum growing on Panicum prionitis biomass. Mycologia. 2021;113(5):877–890. doi: 10.1080/00275514.2021.1922249. [DOI] [PubMed] [Google Scholar]
- Greco C, Keller NP, Rokas A. Unearthing fungal chemodiversity and prospects for drug discovery. Curr Opin Microbiol. 2019;51:22–29. doi: 10.1016/J.MIB.2019.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hadjithomas M, Chen IMA, Chu K, Huang J, Ratner A, Palaniappan K, Andersen E, Markowitz V, Kyrpides NC, Ivanova NN. IMG-ABC: new features for bacterial secondary metabolism analysis and targeted biosynthetic gene cluster discovery in thousands of microbial genomes. Nucleic Acids Res. 2017;45(D1):D560–D565. doi: 10.1093/NAR/GKW1103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hasin Y, Seldin M, Lusis A (2017) Multi-omics approaches to disease. Genome Biol 18, 83. 10.1186/s13059-017-1215-1 [DOI] [PMC free article] [PubMed]
- Hautbergue T, Jamin EL, Debrauwer L, Puel O, Oswald IP (2018) From genomics to metabolomics, moving toward an integrated strategy for the discovery of fungal secondary metabolites. Nat Prod Rep. 35(2):147–173. 10.1039/c7np00032d [DOI] [PubMed]
- Van Der Hooft JJ, Mohimani H, Bauermeister A, Dorrestein PC, Duncan KR, Medema MH. Linking genomics and metabolomics to chart specialized metabolic diversity. Chem Soc Rev. 2020;49(11):3297–3314. doi: 10.1039/D0CS00162G. [DOI] [PubMed] [Google Scholar]
- Horai H, Arita M, Kanaya S, Nihei Y, Ikeda T, Suwa K, Ojima Y, Tanaka K, Tanaka S, Aoshima K, Oda Y, Kakazu Y, Kusano M, Tohge T, Matsuda F, Sawada Y, Hirai MY, Nakanishi H, Ikeda K, Akimoto N, Maoka T, Takahashi H, Ara T, Sakurai N, Suzuki H, Shibata D, Neumann S, Iida T, Tanaka K, Funatsu K, Matsuura F, Soga T, Taguchi R, Saito K, Nishioka T. MassBank: a public repository for sharing mass spectral data for life sciences. J Mass Spectrom. 2010;45(7):703–714. doi: 10.1002/JMS.1777. [DOI] [PubMed] [Google Scholar]
- Huang S, Cai N, Pacheco PP, Narrandes S, Wang Y, and Xu W (2018) Applications of support vector machine (SVM) learning in cancer genomics. Cancer Genomics Proteomics, 15(1):41–51. 10.21873/cgp.20063. Accessed Jan-Feb 2018 [DOI] [PMC free article] [PubMed]
- Hyde KD, Xu J, Rapior S, Jeewon R, Lumyong S, Niego AGT, Abeywickrama PD, Aluthmuhandiram JVS, Brahamanage RS, Brooks S, Chaiyasen A, Chethana KWT, Chomnunti P, Chepkirui C, Chuankid B, de Silva NI, Doilom M, Faulds C, Gentekaki E, Gopalan V, Kakumyan P, Harishchandra D, Hemachandran H, Hongsanan S, Karunarathna A, Karunarathna SC, Khan S, Kumla J, Jayawardena RS, Liu JK, Liu N, Luangharn T, Macabeo APG, Marasinghe DS, Meeks D, Mortimer PE, Mueller P, Nadir S, Nataraja KN, Nontachaiyapoom S, O’Brien M, Penkhrue W, Phukhamsakda C, Ramanan US, Rathnayaka AR, Sadaba RB, Sandargo B, Samarakoon BC, Tennakoon DS, Siva R, Sriprom W, Suryanarayanan TS, Sujarit K, Suwannarach N, Suwunwong T, Thongbai B, Thongklang N, Wei D, Wijesinghe SN, Winiski J, Yan J, Yasanthika E, Stadler M. The amazing potential of fungi: 50 ways we can exploit fungi industrially. Fungal Divers. 2019;97(1):1–136. doi: 10.1007/s13225-019-00430-9. [DOI] [Google Scholar]
- Ijoma GN, Heri SM, Matambo TS, Tekere M. Trends and applications of omics technologies to functional characterisation of enzymes and protein metabolites produced by fungi. J Fungus. 2021;7(9):700. doi: 10.3390/jof7090700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jain KK, Kumar A, Shankar A, Pandey D, Chaudhary B, Sharma KK (2020) De novo transcriptome assembly and protein profiling of copper-induced lignocellulolytic fungus Ganoderma lucidum MDU-7 reveals genes involved in lignocellulose degradation and terpenoid biosynthetic pathways. Genomics 112(1) 10.1016/j.ygeno.2019.01.012 [DOI] [PubMed]
- Jiang C, Lv G, Tu Y, Cheng X, Duan Y, Zeng B, He B (2021) Applications of CRISPR/Cas9 in the synthesis of secondary metabolites in filamentous fungi. Front Microbiol 12 10.3389/FMICB.2021.638096 [DOI] [PMC free article] [PubMed]
- Jue W, Bin C, Haiping Z, Juan F, Jue W, Bin C, Haiping Z, Juan F (2017) Emerging roles of Ganoderma Lucidum in anti-aging. Aging Dis 8(6):691–707 10.14336/AD.2017.0410 [DOI] [PMC free article] [PubMed]
- Jumper J, Evans R, Pritzel A, Green T, Figurnov M, Ronneberger O, Tunyasuvunakool K, Bates R, Žídek A, Potapenko A, Bridgland A. Highly accurate protein structure prediction with AlphaFold. Nature. 2021;596(7873):583–589. doi: 10.1038/s41586-021-03819-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kała K, Kryczyk-Poprawa A, Rzewińska A, Muszyńska B. Fruiting bodies of selected edible mushrooms as a potential source of lovastatin. Eur Food Res Technol. 2020;246(4):713–722. doi: 10.1007/s00217-020-03435-w. [DOI] [Google Scholar]
- Kautsar SA, Blin K, Shaw S, Navarro-Muñoz JC, Terlouw BR, van der Hooft JJJ, van Santen JA, Tracanna V, Suarez Duran HG, Pascal Andreu V, Selem-Mojica N, Alanjary M, Robinson SL, Lund G, Epstein SC, Sisto AC, Charkoudian LK, Collemare J, Linington RG, Weber T, Medema MH. MIBiG 2.0: a repository for biosynthetic gene clusters of known function. Nucleic Acids Res. 2020;48(D1):D454–D458. doi: 10.1093/NAR/GKZ882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keller, NP (2019) Fungal secondary metabolism: regulation, function and drug discovery. Nat Rev Microbiol 17, 167–180. 10.1038/s41579-018-0121-1 [DOI] [PMC free article] [PubMed]
- Khaldi N, Seifuddin FT, Turner G, Haft D, Nierman WC, Wolfe KH, Fedorova ND. SMURF: genomic mapping of fungal secondary metabolite clusters. Fungal Genet Biol. 2010;47(9):736–741. doi: 10.1016/j.fgb.2010.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim S, Thiessen PA, Bolton EE, Chen J, Fu G, Gindulyte A, Han L, He J, He S, Shoemaker BA, Wang J, Yu B, Zhang J, Bryant SH. PubChem substance and compound databases. Nucleic Acids Res. 2016;44(D1):D1202–D1213. doi: 10.1093/nar/gkv951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klitgaard A, Iversen A, Andersen M, Larsen T, Frisvad J, Nielsen K. Aggressive dereplication using UHPLC-DAD-QTOF: screening extracts for up to 3000 fungal secondary metabolites. Anal Bioanal Chem. 2014;406(7):1933–1943. doi: 10.1007/S00216-013-7582-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krause K, Jung EM, Lindner J, Hardiman I, Poetschner J, Madhavan S,Matthäus C, Kai M, Menezes RC, Popp J, Svatoš A (2020) Response of the wood-decay fungus Schizophyllum commune to co-occurring microorganisms. PloS One 15(4) 10.1371/JOURNAL.PONE.0232145 [DOI] [PMC free article] [PubMed]
- Kumar A, Ahlawat S, Mohan H, Sharma KK. Stabilization–destabilization and redox properties of laccases from medicinal mushroom Ganoderma lucidum and human pathogen Yersinia enterocolitica. Int J Bio Macromol. 2021;167:369–381. doi: 10.1016/j.ijbiomac.2020.11.169. [DOI] [PubMed] [Google Scholar]
- Kumar A, Arora S, Jain KK, Sharma KK. Metabolic coupling in the co-cultured fungal-yeast suite of Trametes ljubarskyi and Rhodotorula mucilaginosa leads to hypersecretion of laccase isozymes. Fungal Biol. 2019;123(12):913–926. doi: 10.1016/j.funbio.2019.09.013. [DOI] [PubMed] [Google Scholar]
- Kumar A, Sharma KK, Kumar P, Ramchiary N. Laccase isozymes from Ganoderma lucidum MDU-7: isolation, characterization, catalytic properties and differential role during oxidative stress. J Mol Catal B Enzym. 2015;113:68–75. doi: 10.1016/j.molcatb.2015.01.010. [DOI] [Google Scholar]
- Kumar A, Singh D, Sharma KK, Arora S, Singh AK, Gill SS, Singhal B. Gel-based purification and biochemical study of laccase isozymes from Ganoderma sp. and its role in enhanced cotton callogenesis. Front Microbio. 2017;8:1–15. doi: 10.3389/fmicb.2017.00674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumar KS, Vani MG, Chen CY, Hsiao WW, Li J, Lin ZX, Chu FH, Yen GC, Wang SY. A mechanistic and empirical review of antcins, a new class of phytosterols of formosan fungi origin. J Food Drug Anal. 2020;28(1):38–59. doi: 10.1016/j.jfda.2019.09.001. [DOI] [PubMed] [Google Scholar]
- Laatsch H (2011) AntiBase 2014: The natural compound identifier. (Vol. 313). Weinheim, Germany: Wiley-Vch
- Leliebre-Lara V, García M, Nogueiras C, Monzote L. Qualitative analysis of an ethanolic extract from Trametes versicolor and biological screening against Leishmania amazonensis. Emir J Food Agric. 2015;27(7):592–595. doi: 10.9755/ejfa.2015.05.194. [DOI] [Google Scholar]
- Li YF, Tsai KJ, Harvey CJ, Li JJ, Ary BE, Berlew EE, Boehman BL, Findley MD, Friant AG, Gardner CA, Gould MP, Ha JH, Lilley BL, McKinstry EL, Nawal S, Parry RC, Rothchild KW, Silbert SD, Tentilucci MD, Thurston AM, Wai RB, Yoon Y, Aiyar RS, Medema MH, Hillenmeyer ME, Charkoudian LK. Comprehensive curation and analysis of fungal biosynthetic gene clusters of published natural products. Fungal Genet Biol. 2016;89:18–28. doi: 10.1016/j.fgb.2016.01.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu D, Chen YQ, Xiao XW, Zhong RT, Yang CF, Liu B, Zhao C (2019) Nutrient properties and nuclear magnetic resonance-based metabonomic analysis of macrofungi. Foods 8(9):397. 10.3390/foods8090397. Accessed Sep 2019 [DOI] [PMC free article] [PubMed]
- Liu Q, Zhang W, Chen S, Zhuang Z, Zhang Y, Jiang L, Lin JS. SELEX tool: a novel and convenient gel-based diffusion method for monitoring of aptamer-target binding. J Biol Eng. 2020;14(1):1–13. doi: 10.1186/s13036-019-0223-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu R, Wang Y, Li P, Sun L, Jiang J, Fan X, Liu C, Zhang Y. Genome assembly and transcriptome analysis of the fungus Coniella diplodiella during infection on grapevine Vitis vinifera L. Front Microbiol. 2021;11:3470. doi: 10.3389/fmicb.2020.599150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Love MI, Huber W, Anders S (2014) Moderated estimation of fold change and dispersion for RNA-seq data with DESeq2. Genome Biol 15(12) 10.1186/S13059-014-0550-8 [DOI] [PMC free article] [PubMed]
- Ma Y, Ling TJ, Su XQ, Jiang B, Nian B, Chen LJ, Liu M, Zhang ZY, Wang DP, Mu YY, Jiao WW, Liu QT, Pan YH, Zhao M. Integrated proteomics and metabolomics analysis of tea leaves fermented by Aspergillus niger, Aspergillus tamarii and Aspergillus fumigatus. Food Chem. 2021;334:127560. doi: 10.1016/j.foodchem.2020.127560. [DOI] [PubMed] [Google Scholar]
- MacLean D, Jones JDG, Studholme DJ. Application of “next-generation” sequencing technologies to microbial genetics. Nat Rev Microbiol. 2009;7(4):96–97. doi: 10.1038/nrmicro2088. [DOI] [PubMed] [Google Scholar]
- Mao L, van Arkel J, Hendriks WH, Cone JW, de Vos RCH, Sonnenberg ASM. Assessing the nutritional quality of fungal treated wheat straw: compounds formed after treatment with Ceriporiopsis subvermispora and Lentinula edodes. Anim Feed Sci Technol. 2021;1(276):114924. doi: 10.1016/j.anifeedsci.2021.114924. [DOI] [Google Scholar]
- Martinez-Gomez P, Sánchez-Pérez R, Rubio M. Clarifying omics concepts, challenges, and opportunities for Prunus breeding in the postgenomic era. OMICS J Integr Biol. 2012;16(5):268–283. doi: 10.1089/OMI.2011.0133. [DOI] [PubMed] [Google Scholar]
- McIlwain S, Tamura K, Kertesz-Farkas A, Grant CE, Diament B, Frewen B, Howbert JJ, Hoopmann MR, Käll L, Eng JK, MacCoss MJ, Noble WS. Crux: rapid open source protein tandem mass spectrometry analysis. J. Proteome Res. 2014;13(10):4488–4491. doi: 10.1021/pr500741y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McKinney BA, White BC, Grill DE, Li PW, Kennedy RB, Poland GA, Oberg AL. ReliefSeq: a gene-wise adaptive-K nearest-neighbor feature selection tool for finding gene-gene interactions and main effects in mRNA-Seq gene expression data. Plos One. 2013;8(12):81527. doi: 10.1371/JOURNAL.PONE.0081527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meyer V, Basenko EY, Benz JP, Braus GH, Caddick MX, Csukai M, de Vries RP, Endy D, Frisvad JC, Cimerman NG, Haarmann T, Hadar Y, Hansen K, Johnson RI, Keller NP, Kraševec N, Mortensen UH, Perez R, Ram AFJ, Record E, Ross P, Shapaval V, Steiniger C, Brink HVD, Munster JV, Yarden O, Wösten HAB. Growing a circular economy with fungal biotechnology: a white paper. Fungal Biol Biotechnol. 2020;7(1):1–23. doi: 10.1186/s40694-020-00095-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Narayanan M, Vetta A, Schadt EE, Zhu J (2010) Simultaneous clustering of multiple gene expression and physical interaction datasets. PLoS Comput Biol 6(4) 10.1371/JOURNAL.PCBI.1000742 [DOI] [PMC free article] [PubMed]
- Navarro-Muñoz JC, Collemare J. Evolutionary histories of type III polyketide synthases in fungi. Front Microbiol. 2020;10:3018. doi: 10.3389/fmicb.2019.03018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Navegantes-Lima KC, Monteiro VVS, Gaspar SLF, Oliveira ALB, de Oliveira JP, Reis JF, Gomes RS, Rodrigues CA, Stutz H, Sovrani V, Peres A, Romão PRT, Marta Chagas Monteiro MC. Agaricus brasiliensis mushroom protects against sepsis by alleviating oxidative and inflammatory response. Front. Immunol. 2020;11:1238. doi: 10.3389/fimmu.2020.01238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nazari L, Manstretta V, Rossi V. A non-linear model for temperature-dependent sporulation and T-2 and HT-2 production of Fusarium langsethiae and Fusarium sporotrichioides. Fungal Biol. 2016;120(4):562–571. doi: 10.1016/J.FUNBIO.2016.01.010. [DOI] [PubMed] [Google Scholar]
- Neelam Khatkar A, Sharma KK. Phenylpropanoids and its derivatives: biological activities and its role in food, pharmaceutical and cosmetic industries. Crit Rev Food Sci Nutr. 2020;60(16):2655–2675. doi: 10.1080/10408398.2019.1653822. [DOI] [PubMed] [Google Scholar]
- Noji M, Yoneyama T, Nishihama K, Elshamy AI, Hashimoto T, Umeyama A. Pentacyclic triterpenoids, fuscotorunones A and B, with ε-caprolactone in ring E from Fuscoporia torulosa. Phytochemistry. 2021;187:112748. doi: 10.1016/j.phytochem.2021.112748. [DOI] [PubMed] [Google Scholar]
- Owens RA, Hammel S, Sheridan KJ, Jones GW, Doyle S. A proteomic approach to investigating gene cluster expression and secondary metabolite functionality in Aspergillus fumigatus. Plos One. 2014;9(9):e106942. doi: 10.1371/journal.pone.0106942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Özdemir V, Dove ES, Gürsoy UK, Şardaş Yıldırım A, Yılmaz SG, Barlas IO, Güngör K, Mete A, Srivastava S (2017) Personalized medicine beyond genomics: alternative futures in big data—proteomics, environtome and the social proteome. J Neural Transm 124(1):25–32. 10.1007/s00702-015-1489-y [DOI] [PubMed]
- Ozsolak F, Milos PM. RNA sequencing: advances, challenges and opportunities. Nat Rev Genet. 2011;12(2):87–98. doi: 10.1038/nrg2934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palazzotto E, Weber T (2018) Omics and multi-omics approaches to study the biosynthesis of secondary metabolites in microorganisms. Curr Opin Microbiol 45:109–116. Elsevier Ltd. 10.1016/j.mib.2018.03.004 [DOI] [PubMed]
- Pavlopoulos GA, Malliarakis D, Papanikolaou N, Theodosiou T, Enright AJ, Iliopoulos I. Visualizing genome and systems biology: technologies, tools, implementation techniques and trends, past, present and future. Gigascience. 2015;4(1):38–38. doi: 10.1186/S13742-015-0077-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pomaznoy M, Ha B, Peters B. GOnet: a tool for interactive Gene Ontology analysis. BMC Bioinformatics. 2018;19(1):1–8. doi: 10.1186/s12859-018-2533-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Potter SC, Luciani A, Eddy SR, Park Y, Lopez R, Finn RD. HMMER web server: 2018 update. Nucleic Acids Res. 2018;46(W1):W200–W204. doi: 10.1093/NAR/GKY448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quinlan JR (1993) C4.5: programs for machine learning. Morgan kaufmann publisher, California. https://books.google.com/books/about/C4_5.html?id=b3ujBQAAQBAJ
- Rashmi M, Venkateswara SV (2019) Secondary metabolite oroduction by endophytic fungi: The gene clusters, nature, and expression. In: Jha S. (eds) Endophytes and Secondary Metabolites. Reference Series in Phytochemistry. Springer, Cham. 10.1007/978-3-319-90484-9_20
- Reich M, Liefeld T, Gould J, Lerner J, Tamayo P, Mesirov JP. GenePattern 2.0. Nat Genet. 2006;38(5):500–501. doi: 10.1038/ng0506-500. [DOI] [PubMed] [Google Scholar]
- Ritchie ME, Phipson B, Wu DI, Hu Y, Law CW, Shi W, Smyth GK (2015) limma powers differential expression analyses for RNA sequencing and microarray studies, Nucleic Acids Research, Vol 43(7): P e47. 10.1093/nar/gkv007. Accessed 20 April 2015 [DOI] [PMC free article] [PubMed]
- Robey MT, Caesar LK,Drott MT, Keller NP, Kelleher NL (2021) An interpreted atlas of biosynthetic gene clusters from 1,000 fungal genomes. Proc Natl Acad Sci 118(19) 10.1073/pnas.2020230118 [DOI] [PMC free article] [PubMed]
- Rodarte-Morales AI, Feijoo G, Moreira MT, Lema JM. Biotransformation of three pharmaceutical active compounds by the fungus Phanerochaete chrysosporium in a fed batch stirred reactor under air and oxygen supply. Biodegradation. 2011;23(1):145–156. doi: 10.1007/S10532-011-9494-9. [DOI] [PubMed] [Google Scholar]
- Rosato A, Tenori L, Cascante M, Carulla PRDA, Dos Santos VAM, Saccenti E. From correlation to causation: analysis of metabolomics data using systems biology approaches. Metabolomics. 2018;14(4):1–20. doi: 10.1007/s11306-018-1335-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sahu N, Merényi Z, Bálint B, Kiss B, Sipos G, Owens RA, Nagy LG. Hallmarks of basidiomycete soft- and white-rot in wood-decay-omics data of two Armillaria species. Microorganisms. 2021;91:149. doi: 10.4014/jmb.1608.08008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saini S, Chutani P, Kumar P, Sharma KK. Development of an eco-friendly deinking process for the production of bioethanol using diverse hazardous paper wastes. Renew Energy. 2020;146:2362–2373. doi: 10.1016/J.RENENE.2019.08.087. [DOI] [Google Scholar]
- Sawada Y, Nakabayashi R, Yamada Y, Suzuki M, Sato M, Sakata A, Akiyama K, Sakurai T, Matsuda F, Aoki T, Hirai YM, Saito K. RIKEN tandem mass spectral database (ReSpect) for phytochemicals: a plant-specific MS/MS-based data resource and database. Phytochemistry. 2012;82:38–45. doi: 10.1016/J.PHYTOCHEM.2012.07.007. [DOI] [PubMed] [Google Scholar]
- Schmid J, Stahl U, Meyer V (2009) Genetic and Metabolic Engineering in Filamentous Fungi. In: Anke, T., Weber, D. (eds) Physiology and Genetics. The Mycota, vol 15. Springer, Berlin, Heidelberg. 10.1007/978-3-642-00286-1_18
- Sélem-Mojica N, Aguilar C, Gutiérrez-García K, Martínez-Guerrero CE, and Barona-Gómez F. (2019) EvoMining reveals the origin and fate of natural product biosynthetic enzymes. Microb Genom 5(12) 10.1099/MGEN.0.000260 [DOI] [PMC free article] [PubMed]
- Shankar A, Ahlawat S, Sharma, KK (2019) Exploring fungi-associated lignocellulose degradation: secretomic and proteomic approaches. Advanc Front Mycol Mycotechnol 251–27710.1007/978-981-13-9349-5_10
- Sharma KK. Fungal genome sequencing: basic biology to biotechnology. Crit Rev Biotechnol. 2016;36(4):743–759. doi: 10.3109/07388551.2015.1015959. [DOI] [PubMed] [Google Scholar]
- Sharma KK, Gupta S, Kuhad RC. Agrobacterium-mediated delivery of marker genes to Phanerochaete chrysosporium mycelial pellets: a model transformation system for white-rot fungi. Biotechnol Appl Biochem. 2006;43(3):181–186. doi: 10.1042/BA20050160. [DOI] [PubMed] [Google Scholar]
- Shimizu T, Kawai J, Ouchi K, Kikuchi H, Osima Y, Hidemi R. Agarol, an ergosterol derivative from Agaricus blazei, induces caspase-independent apoptosis in human cancer cells. Int J Oncol. 2016;48(4):1670–1678. doi: 10.3892/IJO.2016.3391. [DOI] [PubMed] [Google Scholar]
- Shinoda K, Tomita M, Ishihama Y. emPAI Calc—for the estimation of protein abundance from large-scale identification data by liquid chromatography-tandem mass spectrometry. Bioinformatics. 2010;26(4):576–577. doi: 10.1093/bioinformatics/btp700. [DOI] [PubMed] [Google Scholar]
- Singh A, Singh DK, Kharwar RN, White JF, Gond SK. Fungal endophytes as efficient sources of plant-derived bioactive compounds and their prospective applications in natural product drug discovery: insights, avenues, and challenges. Microorganisms. 2021;9(1):197. doi: 10.3390/MICROORGANISMS9010197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Skinnider MA, Merwin NJ, Johnston CW, Magarvey NA. PRISM 3: expanded prediction of natural product chemical structures from microbial genomes. Nucleic Acids Res. 2017;45(W1):W49–W54. doi: 10.1093/NAR/GKX320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stahlecker J, Mingyar E, Ziemert N, Mungan MD. SYN-View: a phylogeny-based synteny exploration tool for the identification of gene clusters linked to antibiotic resistance. Molecules. 2021;26(1):144. doi: 10.3390/molecules26010144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Subramanian I, Verma S, Kumar S, Jere A, Anamika K. Multi-omics data integration, interpretation, and its application. Bioinform Biol Insights. 2020;14:7–9. doi: 10.1177/1177932219899051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takahashi JA, Barbosa BVR, Martins B de A, Guirlanda CP, Moura MAF (2020) Use of the versatility of fungal metabolism to meet modern demands for healthy aging, functional foods, and sustainability. J Fungi 6(4):1–27 10.3390/jof6040223 [DOI] [PMC free article] [PubMed]
- Tel-Çayan G. Phenolic profiles, antioxidant, and anticholinesterase activities of three Gloeophyllum species with chemometric approach. J Food Biochem. 2019;43(4):e12790. doi: 10.1111/JFBC.12790. [DOI] [PubMed] [Google Scholar]
- Téllez-Téllez M, Diaz-Godinez G. Omic tools to study enzyme production from fungi in the Pleurotus genus. BioResources. 2019;14(1):2420–2457. doi: 10.15376/biores.14.1.2420-2457. [DOI] [Google Scholar]
- Thadhani VM, Musharraf SG, Ali A. Sensitive analysis of secondary metabolites in different lichen species using liquid chromatography–mass spectrometry: a review. Stud Nat Prod Chem. 2021;70:23–49. doi: 10.1016/B978-0-12-819489-8.00007-7. [DOI] [PubMed] [Google Scholar]
- ENCODE Project Consortium (2012) An integrated encyclopedia of DNA elements in the human genome. Nature 489(7414):57–74. 10.1038/nature11247. Accessed 6 Sep 2012 [DOI] [PMC free article] [PubMed]
- Tian J, Chen H, Guo Z, Liu N, Li J, Huang Y, Xiang W, Chen Y (2016) Discovery of pentangular polyphenols hexaricins A–C from marine Streptosporangium sp. CGMCC 4.7309 by genome mining. Appl Microbiol Biotechnol 100(9):4189–4199 10.1007/S00253-015-7248-Z [DOI] [PubMed]
- Tsuchiya T, Nakayama A, Kawamura T, Sasaki K. Capillary electrophoresis electrospray ionization-mass spectrometry for peptidomics-based processing site determination. Biochem Biophys Res Commun. 2020;533(4):872–878. doi: 10.1016/j.bbrc.2020.09.056. [DOI] [PubMed] [Google Scholar]
- Umezawa K, Niikura M, Kojima Y, Goodell B, Yoshida M (2020) Transcriptome analysis of the brown rot fungus Gloeophyllum trabeum during lignocellulose degradation. PLoS One 15(12):e0243984. 10.1371/journal.pone.0243984. Accessed 14 Dec 2020 [DOI] [PMC free article] [PubMed]
- van Schaick G, Domínguez-Vega E, Gstöttner C, van den Berg-Verleg JH, Schouten O, Akeroyd M, Olsthoorn MMA, Wuhrer M, Heck AJR, Abello N, Franc V. Native structural and functional proteoform characterization of the prolyl-alanyl-specific endoprotease EndoPro from Aspergillus niger. J Proteome Res. 2021;20(10):4875. doi: 10.1021/acs.jproteome.1c00663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vilasi A, Monti MC, Tosco A, Marino SD, Margarucci L, Riccio R, Casapullo A. Differential in gel electrophoresis (DIGE) comparative proteomic analysis of macrophages cell cultures in response to perthamide C treatment. Mar Drugs. 2013;11(4):1288–1299. doi: 10.3390/md11041288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vural Ö, Eugene K (2016) Precision Nutrition 4.0: a big data and ethics foresight analysis—convergence of agrigenomics, nutrigenomics, nutriproteomics, and nutrimetabolomics. OMICS J Integr 20(2):69–75 10.1089/OMI.2015.0193 [DOI] [PubMed]
- Wang J, Zhang Y, Xu Y, Fang W, Wang X, Fang Z, Xiao Y. Genome sequence of a laccase producing fungus Trametes sp. AH28-2. J Biotechnol. 2015;216:167–168. doi: 10.1016/J.JBIOTEC.2015.11.001. [DOI] [PubMed] [Google Scholar]
- Wang X, Liang Y, Liu L, Shi J, Zhu HJ. Targeted absolute quantitative proteomics with SILAC internal standards and unlabeled full-length protein calibrators (TAQSI) Rapid Commun Mass Spectrom. 2016;30(5):553–561. doi: 10.1002/RCM.7482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wei TY, Wu YJ, Xie QP, Tang JW, Yu ZT, Yang SB, Chen SX. CRISPR/Cas9-based genome editing in the filamentous fungus Glarea lozoyensis and its application in manipulating gloF. ACS Synth Biol. 2020;9(8):1968–1977. doi: 10.1021/ACSSYNBIO.9B00491. [DOI] [PubMed] [Google Scholar]
- Wisbeck E, Facchini JM, Alves EP, Silveira MLL, Gern RMM, Ninow JL, Furlan SA. A polysaccharide fraction extracted from Pleurotus ostreatus mycelial biomass inhibit Sarcoma 180 tumor. An Acad Bras Cienc. 2017;89(3):2013–2020. doi: 10.1590/0001-3765201720150635. [DOI] [PubMed] [Google Scholar]
- Xia J, Fjell CD, Mayer ML, Pena OM, Wishart DS, Hancock REW. INMEX—a web-based tool for integrative meta-analysis of expression data. Nucleic Acids Res. 2013;41(W1):W63–W70. doi: 10.1093/NAR/GKT338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xiao G, Zhang X, Gao Q (2017) Bioinformatic approaches for fungal omics. Biomed Res Int 201710.1155/2017/7270485 [DOI] [PMC free article] [PubMed]
- Xie H, Wang X, van der Hooft JJ, Medema MH, Chen ZY, Yue X, Zhang Q, Li P. Fungi population metabolomics and molecular network study reveal novel biomarkers for early detection of aflatoxigenic Aspergillus species. J Hazard Mater. 2022;424:127173. doi: 10.1016/j.jhazmat.2021.127173. [DOI] [PubMed] [Google Scholar]
- Xu XY, Shen XT, Yuan XJ, Zhou YM, Fan H, Zhu LP, Du FY, Sadilek M, Yang J, Qiao B, Yang S. Metabolomics investigation of an association of induced features and corresponding fungus during the co-culture of Trametes versicolor and Ganoderma applanatum. Front Microbiol. 2017;8:2647–2647. doi: 10.3389/FMICB.2017.02647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Younis AM, Wu FS, El Shikh HH. Antimicrobial activity of extracts of the oyster culinary medicinal mushroom Pleurotus ostreatus (higher Basidiomycetes) and identification of a new antimicrobial compound. Int J Med Mushrooms. 2015;17(6):579–590. doi: 10.1615/INTJMEDMUSHROOMS.V17.I6.80. [DOI] [PubMed] [Google Scholar]
- Yu GJ, Yin YL, Yu WH, Liu W, Jin YX, Shrestha A. Yang Q,Ye XD, Sun H (2015) Proteome exploration to provide a resource for the investigation of Ganoderma lucidum. Plos One 10(3):e0119439–e011943910.1371/JOURNAL.PONE.0119439 [DOI] [PMC free article] [PubMed]
- Zeng IS, Lumley T. Review of statistical learning methods in integrated omics studies (an integrated information science) Bioinform Biol Insights. 2018;20(12):1177932218759292. doi: 10.1177/1177932218759292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Y, Han T, Ming Q, Wu L, Rahman K, Qin L (2012) Alkaloids produced by endophytic fungi: a review. Nat Prod Commun 7(7):1934578X1200700742 10.1177/1934578X1200700742 [PubMed]
- Zhou Q, Wang J, Jiang H, Wang G, Wang Y. Deep sequencing of the Sanghuangporus vaninii transcriptome reveals dynamic landscapes of candidate genes involved in the biosynthesis of active compounds. Arch Microbiol. 2021;203(5):2315–2324. doi: 10.1007/S00203-021-02225-6. [DOI] [PubMed] [Google Scholar]
- Ziemert N, Podell S, Penn K, Badger JH, Allen E, Jensen PR (2012) The natural product domain seeker NaPDoS: a phylogeny based bioinformatic tool to classify secondary metabolite gene diversity. PloS One 7(3) 10.1371/JOURNAL.PONE.0034064 [DOI] [PMC free article] [PubMed]
- Zito A, Lualdi M, Granata P, Cocciadiferro D, Novelli A, Alberio T, Casalone R, Fasano M. Gene set enrichment analysis of interaction networks weighted by node centrality. Front Genet. 2021;12:221. doi: 10.3389/fgene.2021.577623. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
No data is associated with the manuscript.
Not applicable.