Abstract
The clinical progression of the severe acute respiratory syndrome coronavirus-2 (SARS-CoV-2) to critical illness is associated with a systemic and uncontrolled inflammatory response of the innate and adaptive immunity with the release of a plethora of proinflammatory cytokines termed “cytokine storm”. In the absence of an effective treatment, many off-label agents from the armamentarium of rheumatology are used. Here, from the perspective of a rheumatologist, we will discuss the current therapeutic strategies in critically ill patients with SARS-CoV-2 pneumonia. Thus, we will discuss the agents that aim to target viral entry and its replication into the host cell and those focusing and targeting the inflammatory response. In this setting, many agents have been used with promising results but, not all have been approved by the International Authorities and Institutions. In the first step (viral entry), SARS-CoV-2 monoclonal antibodies and remdesivir have been approved to be used and, in the second step, corticosteroids along with interleukin-6 inhibitors, or Janus Kinase inhibitors are currently used.
Keywords: COVID-19, SARS-CoV-2 mAbs, Remdesivir, Colchicine, DMARDs, Dexamethasone
1. Introduction
Severe acute respiratory syndrome coronavirus-2 (SARS-COV-2), the cause of coronavirus disease 2019 (COVID-19), emerged in China at the end of 2019 and has developed into a pandemic. The disease has a variety of clinical manifestations ranging from asymptomatic, or flu like syndrome (low grade fever, sore throat, myalgias, arthralgias, fatigue), but also to the development of bilateral pneumonia that can progress to hypoxia, dyspnea, respiratory failure, thrombotic diathesis, multiorgan failure and death. The host's immune response is thought to play a cardinal role in the disease pathophysiology and multiorgan dysfunction. Indeed, the clinical progression of the infection to critical illness is associated with a systemic uncontrolled inflammatory response of the innate and adaptive immunity leading to exaggerated inflammation named cytokine release syndrome (CRS) [1,2]. There is an initial weak response to interferon (IFN) α, β and macrophage (MΦs) activation, that results in delayed polymorphonuclear (PMN) cell recruitment leading to diminished viral clearance [3]. This causes prolonged immune cell stimulation and the release of proinflammatory cytokines such as, tumor necrosis factor-alpha (TNFα), interleukin (IL)-1, IL-6, IL-12, IL-18, chemokines and many others. As a result, high levels of inflammatory markers such as D-dimers, C-reactive protein (CRP), ferritin and fibrinogen are produced. Subsequently, a dysregulation of the adaptive immunity with decrease of lymphocytes, mainly CD+ 4 and CD+ 8 may occur. All the above may contribute to the pathological features of severe COVID-19 pneumonia expressed with inflammatory infiltrations, diffuse alveolar damage and microvascular thrombosis [2,4].
Treatment decisions and therapeutic strategies in critically ill patients with viral pneumonia is a matter of concern worldwide and a challenge for physicians. A large number of drugs has been proposed for the treatment of COVID-19 [5,6]. Therapeutic strategies can be approached following the COVID-19 pathophysiology and are divided into those that aim to target viral entry and its life cycle into the host cell, and to those that are focused on the host response, targeting the inflammatory reaction [7]. To this end, let's review in brief the SARS-CoV-2 infection, the viral life cycle and host cell activation.
2. SARS-CoV-2 infection
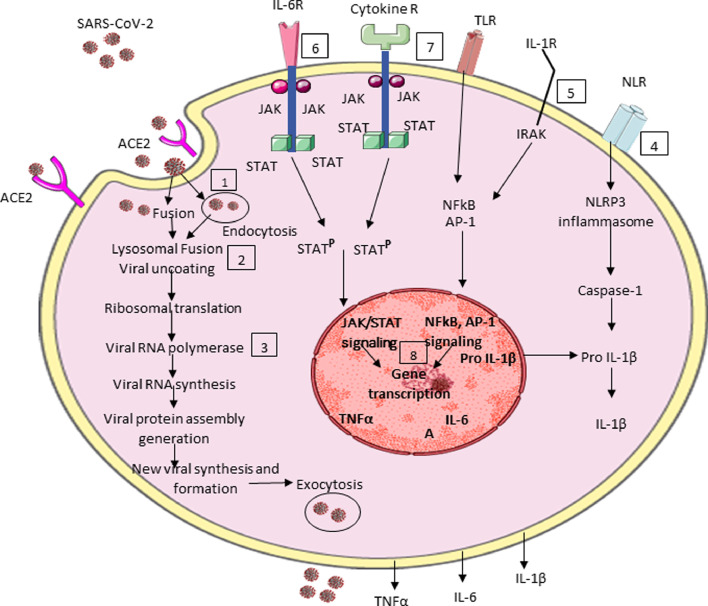
SARS-CoV-2 is an enveloped virus with a spherical morphology and a single-stranded RNA (ssRNA) genome. The SARS-CoV-2 genome encodes four structural proteins: spike (S), envelope (E), membrane (M), and nucleocapside (N), as well as non-structure and accessory proteins. The spike protein contains two subunits S1 and S2 that mediate host cell attachment and invasion. Through its receptor binding domain (RBD), S1 attaches to angiotensin converting enzyme receptor-2 (ACE2) on the host cell. This initiates a conformational change in S2 subunit that results in virus host cell membrane fusion and viral entry. The viral entry can also happen through endocytosis. Once inside, the virus particles are uncoated and its genome enters into the host cell cytoplasm. Through its ssRNA the virus can directly produce its proteins and new genome in the cytoplasm by attaching to the host's ribosomes, which translates the viral RNA to make proteins that will make RNA polymerase. Through the RNA polymerase, small RNA strands are made, which will be read by the host's ribosomes in the endoplasmic reticulum to help make up new structural components of the virus. Thus, new viral forms are made which are released from the host cells by exocytosis and can infect other cells (Fig. 1 ) [2,4]. Then the virus propagation causes tissue injury and activation of the immune system. Thus, signals driven by the SARS-CoV-2 (viral RNAs), pathogen associated molecular patterns (PAMPs) and damage associated molecular patterns (DAMPs) (cellular debris), act on resident tissue cells. Indeed, the virus through ACE2, Toll-Like Receptors (TLRs) and Node-Like Receptors (NLRs) leads to host cell activation and production of pro-inflammatory cytokines, like IL-1, IL-6, TNFα and others. These cytokines act on their own receptors in adjacent cells, with further cell activation and an exaggerated production of pro-inflammatory cytokines takes place, resulting in disease progression and deterioration (Fig. 1) [2,4].
Fig. 1.
Schematic representation of SARS-CoV-2 infection, its replication, host cell activation and potential points to be considered for targeting the viral infection. The first step requires agents interfering with viral entry and its replication. Thus, during viral entry, SARS-CoV-2 mAbs may be useful (1). HCQ acts by altering the lysosomal activity (2), while remdesivir inhibitis RNA polymerase (3). The second step focuses on targeting host cell activation. In this setting, colchicine acts by inhibiting NLRP3 inflammasome and caspace-1 (4). Anakinra is an IL-1 receptor antagonist (5), while TCZ and SAR are IL-6 receptor antagonists (6). BARI and TOFA inhibit JAKs (7). Finally, DX acts by inhibiting cytokine receptor signaling, gene transcription, cell differentiation, proliferation and survival (7), (8). SARS-CoV-2: severe acute respiratory syndrome coronavirus-2; ACE2: angiotensin converting enzyme 2; IL-6R: interleukin-6 receptor; Cytokine R: cytokine receptor; TLR: toll-like receptor; IL-1R: interleukin-1 receptor; NLR: nod-like receptor; JAK: Janus Kinase; STAT: signal transducer and activator of transcription; IRAK: interleukin-1 receptor-associated kinase; STATP: signal transducer and activator transcription phosphorylation; NFkB: nuclear factor kB; AP-1: activated protein-1; NLRP3: nod-like receptor protein-3; Pro-IL-β: pro interleukin-1β; TNFα: tumor necrosis factor α; IL-6: interleukin-6; IL-1β: interleukin-1β.
3. COVID-19 treatment
COVID-19 is a novel pandemic disease with fatal outcomes, in some patients, and significant worldwide health consequences. To treat COVID-19 is a challenging task for physicians and rheumatologists, since there are no specific drugs to combat SARS-CoV-2 infection. Thus, many off-label drugs, from the armamentarium of rheumatic diseases, are now used in COVID-19 and a large number of trials have been published and many others are in progress so far [8], [9], [10], [11]. As depicted in Fig. 1, following the viral entry into the host cell and its steps of viral lifecycle, as well as the cell activation, there are several points, as potential targets of SARS-CoV-2 infection. More specifically: Steps 1 to 3 comprise antiviral therapies aiming to reduce the viral replication and its load. In steps 4 to 8, the drugs used are focused to inhibit cytokine cell receptors, its signaling and finally cell activation, leading to reduced cytokine production. In this setting, biological drugs and corticosteroids, play a cardinal role to diminish the inflammatory response [8], [9], [10], [11].
The question which arises here is why to use these biological and other anti-inflammatory agents in an infectious disease? The answer comes from other well-known disorders like viral hepatitis B and C associated with vasculitis, especially polyarteritis nodosa and mixed cryoglobulineamia respectively [12,13]. In this setting, except of antiviral agents, anti-inflammatory and immunomodulatory therapies are used [12,14,15]. On the other hand, patients with autoimmune rheumatic diseases (ARDs) are also characterized by a dysregulation of the immune system, where several pro-inflammatory cytokines, such as TNFα, IL-1, IL-6, IL-17 and others play a significant pathogenetic role in patients with rheumatoid arthritis (RA), spondyloarthopathies (SpA), and inflammatory bowel disease (IBD) [16]. Treatment wise, a large number of drugs has been developed and great progress has been achieved over the last two decades with the use of targeted therapies. The introduction of conventional synthetic (cs) disease-modifying anti-rheumatic drugs (DMARDs), biologic (b) and targeted synthetic (ts) DMARDs have received great success to treat ARD patients [17], [18], [19], [20], [21]. The use of TNFα inhibitors, IL-6 and Janus kinase (JAK) inhibitors has revolutionized the treatment of these diseases. Furthermore, patients with ARDs treated with cs, b and/or tsDMARDs, appear not to have an increased risk of COVID-19, compared to the general population [22], [23], [24], [25]. Additionally, patients with ARDs treated with the above agents, when contract SARS-CoV-2 infection, the disease is expressed with less hospitalization, good outcomes and seems that these drugs may mitigate the clinical course of COVID-19 [22], [23], [24], [25].
-
upperLetter(%1)
Antiviral therapies
-
(1)
Anti SARS-CoV-2 monoclonal antibodies (mAbs).
Anti SARS-CoV-2 mAbs that target the RBD of the spike protein have been approved for emergency use from the Food and Drug Administration (FDA) for the treatment of mild to moderate COVID-19 in non-hospitalized patients, who are at high risk for severe COVID-19 outcome (Table 1 ). These mAbs comprise: (a) bamlanivimab plus etesevimab, which are neautralizing mAbs binding to different, but overlapping epitopes in the spike protein RBD of SARS-CoV-2, (b) casirivimab plus imdevimab, which are recombinant human mAbs that bind to nonoverlaping epitopes of the spike RBD, (c) sotrovimab is in old mAb discovered in 2003. It targets an epitope in the RBD of the spike protein that is conserved between SARS-CoV and SARS-CoV-2. Many studies evaluated the effectiveness of the above mAbs showed promising results in non-hospitalized patients [26], [27], [28], [29].
-
(2)
Chloroquine and hydroxychloroquine (HCQ).
Table 1.
Patients at high risk of developing severe COVID-19. Candidates to receive SARS-CoV-2 mAbs.
| P a t i e n t s’ a g e s | ||
|---|---|---|
| Any age | Aged ≥ 55 years | Aged 12–17 years |
| Obesity, BMI ≥ 35 g/m2 | Cardiovascular diseases | Single cell disease |
| Chronic kidney disease | Hypertension | Congenital or acquired heart disease |
| Diabetes mellitus | COPD | Neurological disorders |
| Immunosuppressive diseases | Other chronic respiratory diseases | Chronic respiratory diseases |
| Current immunosuppressive therapies | ||
BMI: body mass index; COPD: chronic obstructive pulmonary disease.
Both drugs are used in rheumatic diseases, especially in patients with RA and systemic lupus erythematosus (SLE) since 5 decades. Both are quinine derivatives used also to treat malaria. In vitro studies showed that HCQ alter viral replication by inhibiting the SARS-CoV-2 entry and interacting with glycosylation of ACE2 receptor and it's binding with RBD [10,30]. HCQ may help ΜΦs to destroy the virus into the cytoplasm, through the action of lysosomes and blocks its presentation through the major histocompatibility complex (MHC) to T-cells [31]. Both drugs were used widely in the first days of the pandemic, but the results of the trials were inconclusive [11,32].
-
(3)
Remdesivir
It is an adenosine analog that binds to viral RNA polymerase and inhibits viral replication of SARS-CoV-2, as it has been shown in in vitro studies [33]. It is approved by the FDA for the treatment of adult hospitalized COVID-19 patients and in pediatric population aged > 12 years. It has been studied in many clinical trials with promising results in some patients [33], [34], [35], [36]. Other antiviral agents have been proposed and used in hospitalized COVID-19 patients such as, lopinavir and/or ritonavir in combination with ribavirin.
-
(4)
Lopinavir-Ritonavir
In a randomized controlled open-label trial involving hospitalized adult COVID-19 patients, Cao et al. evaluated the effectiveness of lopinavir-ritonavir (400 mg and 100 mg) in addition to standard of care (SC), twice daily for 14 days versus SC alone. A total of 199 patients were randomized in which 99 were assigned to lopinavir-ritonavir group, while 100 to the SC group. No benefit was observed with lopinavir-ritonavir treatment beyond SC [37].
-
(5)
Triple therapy
An open-label randomized, phase-2 trial using triple combination of interferon beta-1b, lopinavir-ritonavir and ribavirin conducted by Hung et al. in hospitalized patients with mild to moderate COVID-19 disease. At that time, 86 patients were assigned to receive combination triple therapy, while 41 were assigned to receive lopinavir-ritonavir alone. Triple antiviral therapy was superior to lopinavir-ritonavir in alleviating symptoms and shortening the duration of viral shedding and hospital stay in patients with mild to moderate COVID-19 [38].
Other antiviral agents have been investigated in several clinical trials for the treatment of COVID-19 and some of them have received emergency use authorization from the FDA for the treatment of non-hospitalized patients.
-
(6)
Ritonavir-boosted nirmatrevil
Ritonavir-boosted nirmatrevil, is an orally used drug for high risk non-hospitalized adults with COVID-19. Treatment of symptomatic COVID-19 ritonavir-boosted nirmatrevil resulted in high risk progression to severe COVID-19 that was 89% lower than placebo [39].
-
(7)
Molnupiravir
Molnupiravir, is an orally used drug for several RNA viruses, including SARS-CoV-2. It is used in high risk non-hospitalized patients. Indeed, early treatment with molnupiravir reduced the risk of hospitalization or death in unvaccinated COVID-19 adult patients [40].
-
(B)
Targeting cell membrane receptors, sensors and signaling
-
(1)
Colchicine
It is a tricyclic alkaloid, a potent anti-inflammatory agent used in rheumatology for many years to treat gout, pseudogout and Familial Mediterranean Fever (FMF) as well as some heart disorders [41]. Colchicine is a potent inhibitor of tubulin polymerization. Microtubules are the main tools of cytoskeleton involved in many cellular processes [42]. Furthermore, colchicine inhibits neutrophil and monocyte chemotaxis and the expression of adhesion molecules in the setting of inflammation. It inhibits also Nod-Like Receptor (NLR) protein-3 inflammasome (NLRP3) suppressing Caspase-1 activation, resulting in inhibition of IL-1β [41,42]. Colchicine used in patients with gout and FMF, who contracted SARS-CoV-2 infection, may mitigate the COVID-19 virus and its outcome [43,44]. Given the above mechanism of action, colchicine was studied in non-hospitalized and hospitalized patients with COVID-19, and the results showed some beneficial effects on disease outcome [45]. In a large placebo-controlled trial (COLCORONA), Tardif et al. evaluated the use of colchicine (0,5 mg twice daily) in patients with positive test for SARS-CoV-2 infection at the time of inclusion, regardless of symptoms, with at least one risk factor for COVID-19. The study included 4488 patients of which 2235 received colchicine, while 2253 placebo. It was shown that patients on colchicine group had lower rates of death or hospital admission than those in the placebo group [46].
-
(2)
Interleukin-1 inhibitors
IL-1 is one of the proinflammatory cytokines which is elevated in COVID-19 and plays a pivotal role in the inflammatory process. Anakinra, is a recombinant human IL-1 receptor antagonist, while canakinumab is a human mAb that blocks IL-1 signaling. Both drugs can potentially interrupt the inflammatory process. Anakinra, is used to treat RA and cryopyrin associated period syndrome (CAPS), while canakinumab is used to treat juvenile idiopathic arthritis (JIA) and Still's disease. Several studies using IL-1 inhibitors showed promising results with a decreased mortality in some patients [47], [48], [49], [50], [51].
-
(3)
Corticosteroids
Patients with severe COVID-19 who developed lung injury and multiorgan dysfunction, may respond to the treatment with anti-inflammatory agents. It has been suggested that the potent anti-inflammatory effects of corticosteroids might prevent, or mitigate the detrimental consequences of COVID-19. Thus, systemic corticosteroids have been studied in several trials in hospitalized patients with severe disease. Corticosteroids act by inhibiting cytokine receptor signaling as well as cell proliferation, activation and cytokine production. Indeed, in a multicenter open-label trial, dexamethasone (DX) versus placebo showed lower mortality ratio in patients who were mechanically ventilated, or required supplemental oxygen. Indeed, in a multicenter open-label collaborative trial (RECOVERY), 2014 patients were assigned to receive DX, while 4321 received SC. Patients on DX group receiving invasive mechanical ventilation or oxygen showed lower rates on 28-day mortality compared to SC patients [52]. These results have been confirmed by other investigators [53,54]. If DX is not available, other corticosteroids can be used like prednisone, methylprednisolone or hydrocortisone in equivalent doses [55].
-
(4)
Interleukin-6 inhibitors
IL-6, is a pleiotropic pro-inflammatory cytokine which is elevated in SARS-CoV-2 infection and is associated with the production of inflammatory markers, such as CRP, ferritin, fibrinogen, D-dimers, all elevated in the sera of COVID-19 and were considered risk factors for fatal outcomes. It is postulated that modulating IL-6 may reduce COVID-19 severity and mortality. Thus, many studies have been published so far, using IL-6 inhibitors. IL-6 inhibitors comprise, tocilizumab (TCZ) and sarilumab (SAR), which are human mAbs targeting IL-6 receptor, while siltuximab is a human mAb against IL-6. TCZ and SAR were used to treat RA. while TCZ is used also in temporal arteritis with excellent results. Many studies using TCZ or SAR, showed beneficial effects in severely ill patients with COVID-19 and reduction in mortality. TCZ has been approved from International Authorities and should be used in combination with DX in hospitalized patients who required supplemental oxygen, high-flow oxygen, non-invasive ventilation, or mechanical ventilation [55], [56], [57], [58], [59], [60], [61]. The results of RECOVERY and REMAP-CAP trials provide consistent evidence that TCZ when combined with corticosteroids offers a modest mortality benefit in COVID-19 patients. More specifically, in the RECOVERY study the authors evaluated the effectiveness of TCZ in hospitalized COVID-19 patients versus placebo plus SC. Inclusion criteria were hypoxia, oxygen saturation (OX-SAT < 92%) on air, or requiring oxygen therapy, and systemic inflammation (CRP ≥ 75 mg/L). Thus, 2022 patients allocated to TCZ (400–800 mg/IV), plus SC while, 2044 allocated to placebo plus SC. A total of 3385 (82%) patients received systemic corticosteroids. The mortality rate was lower in the TCZ group vs placebo and patients allocated to TCZ were more likely to be discarded within 28 days [62].
In the REMAP-CAP trial investigators evaluated the efficacy of IL-6R antagonists in critically ill patients with COVID-19. Patients with COVID-19, within 24 h after starting organ support in the intensive care unit (ICU) were randomly assigned to receive TCZ (8 mg/kg) IV, SAR 400 mg IV, or SC (control group). At that time 353 patients received TCZ, 48 SAR and 402 were allocated as control group. It was shown that in critically ill patients receiving support in ICUs, treatment with TCZ and SAR improved outcomes, including survival [63].
Thus, both TCZ and SAR are equally suitable for treating adult patients with severe COVID-19 pneumonia and high levels of IL-6 and CRP. However, there are some differences between these two agents regarding the mode of action, receptor affinity and dose of administration. SAR binds IL-6Ra subunit in vitro, with a 15–22-fold higher affinity than TCZ and inhibits IL-6 mediated classical and trans signaling via membrane-bound and soluble IL-6Ra. Furthermore, receptor's occupancy was higher for SAR and was associated with greater CRP reduction as well as disease activity in RA patients [64]. Another difference is that TCZ is administered at a dose of 8 mg/kg IV, with a maximum of 800 mg, while the recommended dose of SAR is 400 mg (one dose of 400 mg iv) since several side effects have been reported like neutropenia, superinfections, reactivation of latent infections, hepatitis and cardiac abnormalities that necessitate proper monitoring and appropriate therapy [65].
5. Janus-Kinase inhibitors
JAK and the Signal Transducer and Activator of Transcription (STAT) system, consists of three components: (1) a receptor which penetrates the cell membrane, (2) the JAK which bounds to the receptor, and (3) the STAT which carries the signal into the nucleus (Fig. 1). JAK inhibitors are drugs which interfere with phosphorylation of STAT proteins involved in the signal transduction of many cytokines, especially IL-6, thus reducing the inflammatory process [66]. Furthermore, JAK inhibitors, may have antiviral activities through interference with viral endocytosis, preventing SARS-CoV-2 entry into the cell [66,67]. JAK inhibitors comprise tofacitinib (TOFA), which is a JAK 1, 3 inhibitor, baricitinib (BARI) which inhibits JAK 1, 2 as well as upadacitinib and filgotinib which inhibit JAK 1. JAK inhibitors are used to treat RA, SpA and IBD. The clinical trials using BARI and TOFA in patients with severe COVID-19 have shown beneficial effects of both drugs in reducing mortality in these patients. Thus, both drugs have been approved by the FDA authorities to be used in hospitalized patients in combination with DX in those requiring supplemental oxygen, high flow oxygen, non-invasive, or mechanical ventilation [68,69].
More specifically, Kalil et al. in a double-blind randomized control trial of hospitalized COVID-19 adult patients evaluated the efficacy of combination treatment of BARI plus remdesivir vs placebo. All received remdesivir (≤10 days) and either BARI 4 mg (≤14 days) or placebo. A total of 1075 were examined in which 515 were assigned to the combination group, while 518 were assigned to the control group. It was shown that BARI plus remdesivir was superior to remdesivir alone in terms of reducing morality and recovery time whilst accelerating improvement in clinical status [70]. Furthermore, higher doses of BARI have been tried in severe COVID-19 patients. More specifically, in a prospective cohort study 238 patients with severe COVID-19 pneumonia were evaluated. Of these, 122 received 8 mg/day of BARI, while 116 received 4 mg/day, for 14 days. It was shown that 8 mg of BARI on a daily basis (vs 4 mg) resulted in early normalization of respiratory function, reduced need of ICU and intubation, decrease of mortality rate and hospitalization [71]. In another prospective case control study an additional loading dose of BARI was administered in patients with moderate to severe COVID-19 pneumonia. The study included 37 patients. 17 received 4 mg BARI daily, while 20 patients received an additional single 8 mg of BARI oral loading dose. It was shown that an additional loading dose of BARI resulted in a better clinical outcome, with reduced requirement of ICU and ventilation support, length of hospitalization and mortality rate than those receiving 4 mg of BARI once daily [72].
In another study Guimareas et al. in a placebo control study examined the efficacy and safety of TOFA in hospitalized patients with COVID-19 pneumonia. A total of 289 patients underwent randomization, of which 144 allocated to TOFA (10 mg twice/day) and 145 to placebo for up to 14 days, or until discharged. In addition, 89,3% of all patients received glucocorticoids. It has been demonstrated that TOFA had lower risk of death or respiratory failure, through day 28 than placebo [73].
4. Treatment decisions and therapeutic strategies in COVID-19
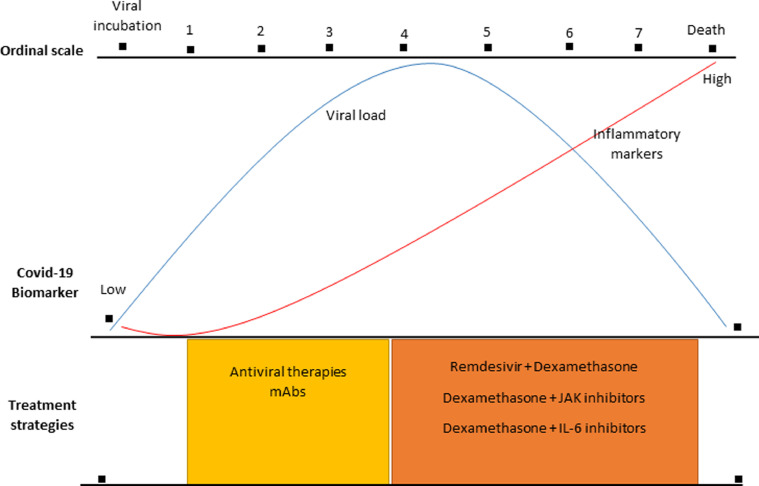
As we mentioned above, therapeutic strategies can be categorized to those that target viral entry and viral replication, such as SARS-CoV-2 targeted mAb therapies and antiviral drugs like remdesivir, and to those that are focused on targeting the inflammatory host response using corticosteroids and immune-modulators. More specifically: the clinical course of COVID-19 initially is characterized by mild constitutional symptoms affecting mainly the upper respiratory tract. During this stage, the viral load is very high, which after 7–10 days slowly decreases, while the levels of inflammatory markers CRP, ferritin and others are low and slowly increase. In this early stage, antiviral agents are likely to be most effective during the first week after symptoms onset, where the disease is mild or moderate. After 7–10 days of SARS-CoV-2 infection, approximately in 10–15% of patients, the disease progresses to bilateral pneumonia, severe dyspnea, low oxygen saturation, and there is need for supplementary oxygen therapy. This stage is characterized by exaggerated immune responses with high levels of inflammatory markers such as CRP, ferritin, fibrinogen, while the viral load decreases. In these critically ill patients the use of immunomodulatory agents is mandatory (Fig. 2 ). These drugs should be used with caution and from expert personnel since these compounds may cause immunosuppression. In this direction, the National Institute of Allergy and Infection Diseases (NIAID) had developed the ordinal scale of COVID-19 severity which is depicted in Fig. 2. Furthermore, the National Institute of Health (NIH) on December 2021 released guidelines from an expert recommendation panel, suggesting that in non-hospitalized patients with mild, or moderate COVID-19, only anti SARS-CoV-2 mAbs should be used, in those who have risk factors for developing severe disease (Table 1). However, in hospitalized patients who require supplemental oxygen, high-flow oxygen, non-invasive or mechanical ventilation, remdesivir plus DX and TCZ, or JAK inhibitors should be used (Fig. 2). The panel recommends not to use HCQ in non-hospitalized and hospitalized COVID-19 patients, while regarding the use of colchicine, the panel recommends that there is insufficient data for, or against the use of this agent in non-hospitalized patients, but recommends not to use colchicine in hospitalized patients. Furthermore, the panel recommends that there is insufficient data for, or against the use of anakinra in hospitalized patients, while suggests not to use canakimumab for the treatment of COVID-19. Finally, the panel suggests that no other JAK inhibitors, except for BARI and TOFA, should be used [55]. Recently, the European League Against Rheumatism (EULAR) has provided relevant and updated guidelines on immunomodulatory therapies utilization of COVID-19 from rheumatology perspective, which are similar to the NIH recommendations [74].
Fig. 2.
Current therapeutic strategies of COVID-19. In the early stages of COVID-19 and in non-hospitalized patients there is no evidence to support the use of any antiviral or immunomodulatory agent, except for SARS-mAbs in selected subgroups of patients. In hospitalized patients requiring supplemental oxygen, non-invasive or mechanical ventilation the use of remdesivir in combination with dexamethasone, with or without the use of IL-6 or JAK inhibitors are considered. The National Institute of Allergy and Infectious Diseases (NIAID), Ordinal scale: 1= Not hospitalized, no limitations on activities; 2= Not hospitalized, limitations on activities or, receiving home oxygen, or both; 3= Hospitalized, no supplemental oxygen, no ongoing medical care; 4= Hospitalized, no supplemental oxygen but should receive ongoing medical care; 5= Hospitalized, receiving supplemental oxygen through low-flow devices; 6= Hospitalized, receiving oxygen through non-invasive ventilation or high-flow oxygen devices; 7= Hospitalized, invasive mechanical ventilation or extracorporeal membrane oxygenation (ECMO).
5. Conclusions
In the last two years there has been a significant progress of scientific knowledge, as regards to COVID-19 immunopathology and its treatment. Thus, in the early stages of COVID-19 and in non-hospitalized patients there is no evidence to use any antiviral, or immunomodulatory agent. However, in selected sub groups of patients with risk factors of developing severe COVID-19, anti-SARS-CoV-2 mAbs may be considered. In hospitalized patients with SARS-CoV-2 infection, who do not need oxygen therapy, no immunomodulatory, or antiviral therapy is required. In contrary, in hospitalized patients requiring supplemental oxygen, non-invasive, or mechanical ventilation the use of remdesivir in combination with DX, with or without the use of TCZ, or JAK inhibitors, especially BARI is mandatory. The utilization of the above immunomodulatory therapies from rheumatology perspective, opens new ways of how to treat severe acute infection diseases, which may benefit from these immunomodulatory treatments. On the other hand, immune response to SARS-CoV-2 differs among individuals, with different clinical phenotypes. Thus, it is an imperative to elucidate better the immune response against SARS-CoV-2 in order to further define new therapeutic ways and strategies.
Funding
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Declaration of Competing Interest
The authors declare that they have no conflict of interest.
Acknowledgment
We would like to thank Ms. Chrysa Arvaniti for her excellent secretarial assistance.
References
- 1.Huang C., Wang Y., Li X., et al. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet. 2020;395:497–506. doi: 10.1016/S0140-6736(20)30183-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Fajgenbaum D.C., June C.H. Cytokine storm. N Engl J Med. 2020;383(23):2255–2273. doi: 10.1056/NEJMra2026131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Channappanavar R., Fehr A.R., Vijay R., et al. Dysregulated type I interferon and inflammatory monocyte-macrophage responses cause lethal pneumonia in SARS-CoV-infected mice. Cell Host Microbe. 2016;19:181–193. doi: 10.1016/j.chom.2016.01.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Vabret N., Britton G.J., Gruber C., et al. Immunology of COVID-19: current state of the science. Immunity. 2020;52:910–941. doi: 10.1016/j.immuni.2020.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Zhang W., Zhao Y., Zhang F., et al. The use of anti-inflammatory drugs in the treatment of people with severe coronavirus disease 2019 (COVID-19): the perspectives of clinical immunologists from China. Clin Immunol. 2020;214 doi: 10.1016/j.clim.2020.108393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Mehta P., McAuley D.F., Brown M., et al. COVID-19: consider cytokine storm syndromes and immunosuppression. Lancet. 2020;395:1033–1034. doi: 10.1016/S0140-6736(20)30628-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Chau A.S., Weber A.G., Maria N.I., et al. The longitudinal immune response to coronavirus disease 2019: chasing the cytokine storm. Arthritis Rheumatol. 2021;73:23–35. doi: 10.1002/art.41526. [DOI] [PubMed] [Google Scholar]
- 8.Alunno A., Najm A., Machado P.M., et al. EULAR points to consider on pathophysiology and use of immunomodulatory therapies in COVID-19. Ann Rheum Dis. 2021;80:698–706. doi: 10.1136/annrheumdis-2020-219724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Zhang W., Zhao Y., Zhang F., et al. The use of anti-inflammatory drugs in the treatment of people with severe coronavirus disease 2019 (COVID-19): the perspectives of clinical immunologists from China. Clin Immunol. 2020;214 doi: 10.1016/j.clim.2020.108393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Pelechas E., Drossou V., Voulgari P.V., et al. Anti-rheumatic drugs for the fight against the novel coronavirus infection (SARSCoV-2): what is the evidence? Mediterr J Rheumatol. 2020;31:259–267. doi: 10.31138/mjr.31.3.259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Putman M., Chock Y.P.E., Tam H., et al. Antirheumatic disease therapies for the treatment of COVID-19: a systematic review and meta-analysis. Arthritis Rheumatol. 2021;73:36–47. doi: 10.1002/art.41469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Guillevin L., Mahr A., Callard P., et al. Hepatitis B virus-associated polyarteritis nodosa: clinical characteristics, outcome, and impact of treatment in 115 patients. Medicine. 2005;84:313–322. doi: 10.1097/01.md.0000180792.80212.5e. (Baltimore) [DOI] [PubMed] [Google Scholar]
- 13.Agnello V., Chung R.T., Kaplan L.M. A role for hepatitis C virus infection in type II cryoglobulinemia. N Engl J Med. 1992;327:1490–1495. doi: 10.1056/NEJM199211193272104. [DOI] [PubMed] [Google Scholar]
- 14.Dammacco F., Sansonno D. Therapy for hepatitis C virus-related cryoglobulinemic vasculitis. N Engl J Med. 2013;369:1035–1045. doi: 10.1056/NEJMra1208642. [DOI] [PubMed] [Google Scholar]
- 15.Boleto G., Ghillani-Dalbin P., Musset L., et al. Cryoglobulinemia after the era of chronic hepatitis C infection. Semin Arthritis Rheum. 2020;50:695. doi: 10.1016/j.semarthrit.2020.05.004. [DOI] [PubMed] [Google Scholar]
- 16.Davidson A., Diamond B. Autoimmune diseases. N Engl J Med. 2001;345:340–350. doi: 10.1056/NEJM200108023450506. [DOI] [PubMed] [Google Scholar]
- 17.Rosman Z., Shoenfeld Y., Zandman-Goddard G. Biologic therapy for autoimmune diseases: an update. BMC Med. 2013;11:88. doi: 10.1186/1741-7015-11-88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Burmester G.R., Feist E., Dörner T. Emerging cell and cytokine targets in rheumatoid arthritis. Nat Rev Rheumatol. 2014;10:77–88. doi: 10.1038/nrrheum.2013.168. [DOI] [PubMed] [Google Scholar]
- 19.Smolen J.S., Landewé R.B.M., Bijlsma J.W.J., et al. EULAR recommendations for the management of rheumatoid arthritis with synthetic and biological disease-modifying antirheumatic drugs: 2019 update. Ann Rheum Dis. 2020;79:685–699. doi: 10.1136/annrheumdis-2019-216655. [DOI] [PubMed] [Google Scholar]
- 20.Gossec L., Baraliakos X., Kerschbaumer A., et al. EULAR recommendations for the management of psoriatic arthritis with pharmacological therapies: 2019 update. Ann Rheum Dis. 2020;79:700–712. doi: 10.1136/annrheumdis-2020-217159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lamb C.A., Kennedy N.A., Raine T., et al. British Society of gastroenterology consensus guidelines on the management of inflammatory bowel disease in adults. Gut. 2019;68:s1–s106. doi: 10.1136/gutjnl-2019-318484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Bakasis A.D., Mavragani C.P., Boki K.A., et al. COVID-19 infection among autoimmune rheumatic disease patients: data from an observational study and literature review. J Autoimmun. 2021;123 doi: 10.1016/j.jaut.2021.102687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Migkos M.P., Kaltsonoudis E., Pelechas E., et al. Use of conventional synthetic and biologic disease-modifying anti-rheumatic drugs in patients with rheumatic diseases contracting COVID-19: a single-center experience. Rheumatol Int. 2021;41:903–909. doi: 10.1007/s00296-021-04818-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Mateus E.F., Lawson-Tovey S., Trupin L., et al. Associations of baseline use of biologic or targeted synthetic DMARDs with COVID-19 severity in rheumatoid arthritis: results from the COVID-19 global rheumatology alliance physician registry. Ann Rheum Dis. 2021;80:1137–1146. doi: 10.1136/annrheumdis-2021-220418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Izadi Z., Brenner E.J., Mahil S.K., et al. Association between tumor necrosis factor inhibitors and the risk of hospitalization or death among patients with immune-mediated inflammatory disease and COVID-19. JAMA Netw Open. 2021;4 doi: 10.1001/jamanetworkopen.2021.29639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Jiang S., Hillyer C., Du L. Neutralizing antibodies against SARS-CoV-2 and other human coronaviruses. Trends Immunol. 2020;41:355–359. doi: 10.1016/j.it.2020.03.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Cohen M.S., Nirula A., Mulligan M.J., et al. Effect of bamlanivimab vs placebo on incidence of COVID-19 among residents and staff of skilled nursing and assisted living facilities: a randomized clinical trial. JAMA. 2021;326:46–55. doi: 10.1001/jama.2021.8828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Gupta A., Gonzalez-Rojas Y., Juarez E., et al. Early treatment for COVID-19 with SARS-CoV-2 neutralizing antibody sotrovimab. N Engl J Med. 2021;385:1941–1950. doi: 10.1056/NEJMoa2107934. [DOI] [PubMed] [Google Scholar]
- 29.ACTIV-3/TICO LY-CoV555 Study Group. Lundgren J.D., Grund B., Barkauskas C.E., et al. A neutralizing monoclonal antibody for hospitalized patients with COVID-19. N Engl J Med. 2021;384:905–914. doi: 10.1056/NEJMoa2033130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Yao X., Ye F., Zhang M., et al. In vitro antiviral activity and projection of optimized dosing design of hydroxychloroquine for the treatment of severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) Clin Infect Dis. 2020;71:732–739. doi: 10.1093/cid/ciaa237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Schrezenmeier E., Dörner T. Mechanisms of action of hydroxychloroquine and chloroquine: implications for rheumatology. Nat Rev Rheumatol. 2020;16:155–166. doi: 10.1038/s41584-020-0372-x. [DOI] [PubMed] [Google Scholar]
- 32.Salvarani C., Mancuso P., Gradellini F., et al. Susceptibility to COVID-19 in patients treated with antimalarials: a population-based study in Emilia-Romagna, Northern Italy. Arthritis Rheumatol. 2021;73:48–52. doi: 10.1002/art.41475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Beigel J.H., Tomashek K.M., Dodd L.E., et al. Remdesivir for the treatment of COVID-19 - final report. N Engl J Med. 2020;383:1813–1826. doi: 10.1056/NEJMoa2007764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Ader F., Bouscambert-Duchamp M., Hites M., et al. Remdesivir plus standard of care versus standard of care alone for the treatment of patients admitted to hospital with COVID-19 (DisCoVeRy): a phase 3, randomised, controlled, open-label trial. Lancet Infect Dis. 2022;22:209–221. doi: 10.1016/S1473-3099(21)00485-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Spinner C.D., Gottlieb R.L., Criner G.J., et al. Effect of remdesivir vs standard care on clinical status at 11 days in patients with moderate COVID-19: a randomized clinical trial. JAMA. 2020;324:1048–1057. doi: 10.1001/jama.2020.16349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Goldman J.D., Lye D.C.B., Hui D.S., et al. Remdesivir for 5 or 10 days in patients with severe COVID-19. N Engl J Med. 2020;383:1827–1837. doi: 10.1056/NEJMoa2015301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Cao B., Wang Y., Wen D., et al. A trial of lopinavir-ritonavir in adults hospitalized with severe COVID-19. N Engl J Med. 2020;382:1787–1799. doi: 10.1056/NEJMoa2001282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Hung I.F., Lung K.C., Tso E.Y., et al. Triple combination of interferon beta-1b, lopinavir-ritonavir, and ribavirin in the treatment of patients admitted to hospital with COVID-19: an open-label, randomised, phase 2 trial. Lancet. 2020;395:1695–1704. doi: 10.1016/S0140-6736(20)31042-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Hammond J., Leister-Tebbe H., Gardner A., Abreu P., Bao W., Wisemandle W., Baniecki M., Hendrick V.M., Damle B., Simón-Campos A., Pypstra R., Rusnak J.M., EPIC-HR Investigators Oral nirmatrelvir for high-risk, nonhospitalized adults with COVID-19. N Engl J Med. 2022;386:1397–1408. doi: 10.1056/NEJMoa2118542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Jayk Bernal A., Gomes da Silva M.M., Musungaie D.B., et al. Molnupiravir for oral treatment of COVID-19 in nonhospitalized patients. N Engl J Med. 2022;386:509–520. doi: 10.1056/NEJMoa2116044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Slobodnick A., Shah B., Krasnokutsky S., et al. Update on colchicine, 2017. Rheumatology. 2018;57:i4–i11. doi: 10.1093/rheumatology/kex453. (Oxford) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Leung Y.Y., Yao Hui L.L., Kraus V.B. Colchicine–update on mechanisms of action and therapeutic uses. Semin Arthritis Rheum. 2015;45:341–350. doi: 10.1016/j.semarthrit.2015.06.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Pelechas E., Drossou V., Voulgari P.V., et al. COVID-19 in patients with gout on colchicine. Rheumatol Int. 2021;41:1503–1507. doi: 10.1007/s00296-021-04902-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Bourguiba R., Delplanque M., Vinit C., et al. Clinical course of COVID-19 in a cohort of 342 familial Mediterranean fever patients with a long-term treatment by colchicine in a French endemic area. Ann Rheum Dis. 2020 doi: 10.1136/annrheumdis-2020-218707. annrheumdis-2020-218707. [DOI] [PubMed] [Google Scholar]
- 45.Drosos A.A., Pelechas E., Drossou V., et al. Colchicine against SARS-CoV-2 infection: what is the evidence? Rheumatol Ther. 2022 doi: 10.1007/s40744-022-00425-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Tardif J.C., Bouabdallaoui N., L'Allier P.L., et al. Colchicine for community-treated patients with COVID-19 (COLCORONA): a phase 3, randomised, double-blinded, adaptive, placebo-controlled, multicentre trial. Lancet Respir Med. 2021;9:924–932. doi: 10.1016/S2213-2600(21)00222-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Cavalli G., De Luca G., Campochiaro C., et al. Interleukin-1 blockade with high-dose anakinra in patients with COVID-19, acute respiratory distress syndrome, and hyperinflammation: a retrospective cohort study. Lancet Rheumatol. 2020;2:e325–e331. doi: 10.1016/S2665-9913(20)30127-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Huet T., Beaussier H., Voisin O., et al. Anakinra for severe forms of COVID-19: a cohort study. Lancet Rheumatol. 2020;2:e393–e400. doi: 10.1016/S2665-9913(20)30164-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.CORIMUNO-19 Collaborative group Effect of anakinra versus usual care in adults in hospital with COVID-19 and mild-to-moderate pneumonia (CORIMUNO-ANA-1): a randomised controlled trial. Lancet Respir Med. 2021;9:295–304. doi: 10.1016/S2213-2600(20)30556-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Kyriazopoulou E., Poulakou G., Milionis H., et al. Early treatment of COVID-19 with anakinra guided by soluble urokinase plasminogen receptor plasma levels: a double-blind, randomized controlled phase 3 trial. Nat Med. 2021;27:1752–1760. doi: 10.1038/s41591-021-01499-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Caricchio R., Abbate A., Gordeev I., et al. Effect of canakinumab vs placebo on survival without invasive mechanical ventilation in patients hospitalized with severe COVID-19: a randomized clinical trial. JAMA. 2021;326:230–239. doi: 10.1001/jama.2021.9508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.RECOVERY Collaborative Group. Horby P., Lim W.S., et al. Dexamethasone in hospitalized patients with COVID-19. N Engl J Med. 2021;384:693–704. doi: 10.1056/NEJMoa2021436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Shang L., Zhao J., Hu Y., et al. On the use of corticosteroids for 2019-nCoV pneumonia. Lancet. 2020;395:683–684. doi: 10.1016/S0140-6736(20)30361-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Villar J., Ferrando C., Martínez D., et al. Dexamethasone treatment for the acute respiratory distress syndrome: a multicentre, randomised controlled trial. Lancet Respir Med. 2020;8:267–276. doi: 10.1016/S2213-2600(19)30417-5. [DOI] [PubMed] [Google Scholar]
- 55.National Institute of Health . National Institute of Health; 2022. Treatment guidelines for COVID.https://www.covid19treatmentguidelines.nih.gov Accessed 28 January. [Google Scholar]
- 56.Luo P., Liu Y., Qiu L., et al. Tocilizumab treatment in COVID-19: a single center experience. J Med Virol. 2020;92:814–818. doi: 10.1002/jmv.25801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Gupta S., Wang W., Hayek S.S., et al. Association between early treatment with tocilizumab and mortality among critically ill patients with COVID-19. JAMA. 2021;181:41–51. doi: 10.1001/jamainternmed.2020.6252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Salvarani C., Dolci G., Massari M., et al. Effect of tocilizumab vs standard care on clinical worsening in patients hospitalized with COVID-19 pneumonia: a randomized clinical trial. JAMA Intern Med. 2021;181:24–31. doi: 10.1001/jamainternmed.2020.6615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Huang E., Jordan S.C. Tocilizumab for COVID-19 - the ongoing search for effective therapies. N Engl J Med. 2020;383:2387–2388. doi: 10.1056/NEJMe2032071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Stone J.H., Frigault M.J., Serling-Boyd N.J., et al. Efficacy of tocilizumab in patients hospitalized with COVID-19. N Engl J Med. 2020;383:2333–2344. doi: 10.1056/NEJMoa2028836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Salama C., Han J., Yau L., et al. Tocilizumab in patients hospitalized with COVID-19 pneumonia. N Engl J Med. 2021;384:20–30. doi: 10.1056/NEJMoa2030340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.RECOVERY Collaborative Group Tocilizumab in patients admitted to hospital with COVID-19 (RECOVERY): a randomised, controlled, open-label, platform trial. Lancet. 2021;397:1637–1645. doi: 10.1016/S0140-6736(21)00676-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.REMAP-CAP Investigators. Gordon A.C., Mouncey P.R., Al-Beidh F., et al. Interleukin-6 receptor antagonists in critically ill patients with COVID-19. N Engl J Med. 2021;384:1491–1502. doi: 10.1056/NEJMoa2100433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Xu C., Rafique A., Potocky T., et al. Differential binding of sarilumab and tocilizumab to IL-6Rα and effects of receptor occupancy on clinical parameters. J Clin Pharmacol. 2021;61:714–724. doi: 10.1002/jcph.1795. [DOI] [PubMed] [Google Scholar]
- 65.Charan J., Dutta S., Kaur R., et al. Tocilizumab in COVID-19: a study of adverse drug events reported in the WHO database. Expert Opin Drug Saf. 2021;20:1125–1136. doi: 10.1080/14740338.2021.1946513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Nash P., Kerschbaumer A., Dörner T., et al. Points to consider for the treatment of immune-mediated inflammatory diseases with Janus kinase inhibitors: a consensus statement. Ann Rheum Dis. 2021;80:71–87. doi: 10.1136/annrheumdis-2020-218398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Choy E.H.S., Miceli-Richard C., MA González-Gay, et al. The effect of JAK1/JAK2 inhibition in rheumatoid arthritis: efficacy and safety of baricitinib. Clin Exp Rheumatol. 2019;37:694–704. [PubMed] [Google Scholar]
- 68.Goletti D., Cantini F. Baricitinib therapy in COVID-19 pneumonia - an unmet need fulfilled. N Engl J Med. 2021;384:867–869. doi: 10.1056/NEJMe2034982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Stebbing J., Sánchez Nievas G., Falcone M., et al. JAK inhibition reduces SARS-CoV-2 liver infectivity and modulates inflammatory responses to reduce morbidity and mortality. Sci Adv. 2021;7:eabe4724. doi: 10.1126/sciadv.abe4724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Kalil A.C., Patterson T.F., Mehta A.K., et al. Baricitinib plus remdesivir for hospitalized adults with COVID-19. N Engl J Med. 2021;384:795–807. doi: 10.1056/NEJMoa2031994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Hasan M.J., Rabbani R., Anam A.M., et al. Impact of high dose of baricitinib in severe COVID-19 pneumonia: a prospective cohort study in Bangladesh. BMC Infect Dis. 2021;21:427. doi: 10.1186/s12879-021-06119-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Hasan M.J., Rabbani R., Anam A.M., et al. Additional baricitinib loading dose improves clinical outcome in COVID-19. Open Med. 2021;16:41–46. doi: 10.1515/med-2021-0010. (Wars) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Guimarães P.O., Quirk D., Furtado R.H., et al. Tofacitinib in patients hospitalized with COVID-19 pneumonia. N Engl J Med. 2021;385:406–415. doi: 10.1056/NEJMoa2101643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Alunno A., Najm A., Machado P.M., et al. 2021 update of the EULAR points to consider on the use of immunomodulatory therapies in COVID-19. Ann Rheum Dis. 2022;81:34–40. doi: 10.1136/annrheumdis-2021-221366. [DOI] [PubMed] [Google Scholar]