Abstract
PURPOSE
This in-vitro analysis aimed to compare the intaglio trueness, the antagonist’s wear volume loss, and fracture load of various single-unit zirconia prostheses fabricated by different manufacturing techniques.
MATERIALS AND METHODS
Zirconia crowns were prepared into four different groups (n = 14 per group) according to the manufacturing techniques and generations of the materials. The intaglio surface trueness (root-mean-square estimates, RMS) of the crown was measured at the marginal, axial, occlusal, and inner surface areas. Half of the specimens were artificially aged in the chewing simulator with 120,000 cycles, and the antagonist’s volume loss after aging was calculated. The fracture load for each crown group was measured before and after hydrothermal aging. The intaglio trueness was evaluated with Welch’s ANOVA and the antagonist’s volume loss was assessed by the Kruskal-Wallis tests. The effects of manufacturing and aging on the fracture resistance of the tested zirconia crowns were determined by two-way ANOVA.
RESULTS
The trueness analysis of the crown intaglio surfaces showed surface deviation (RMS) within 50 µm, regardless of the manufacturing methods (P = .053). After simulated mastication, no significant differences in the volume loss of the antagonists were observed among the zirconia groups (P = .946). The manufacturing methods and simulated chewing had statistically significant effects on the fracture resistance (P < .001).
CONCLUSION
The intaglio surface trueness, fracture resistance, and antagonist’s wear volume of the additively manufactured 3Y-TZP crown were clinically acceptable, as compared with those of the 4Y- or 5Y-PSZ crowns produced by subtractive milling.
Keywords: Additive manufacturing, Subtractive manufacturing, Zirconia, Intaglio surface trueness, Fracture resistance, Antagonist wear, Simulated mastication
INTRODUCTION
With the advances of the computer-aided design and computer-aided manufacturing (CAD-CAM) technologies, zirconia ceramic has been the choice of materials for dental prostheses, with their excellent biocompatibility and outstanding mechanical characteristics due to the transformation toughening mechanism.1 The first and second generations of zirconia generations (3 mol% yttria-stabilized tetragonal zirconia polycrystal, 3Y-TZP) have high fracture toughness and excellent flexural strength but limited translucency with relatively compromised esthetics compared to glass-ceramics.2,3 To enhance the optical characteristics, the third generation of dental zirconia was developed including 4 mol% or 5 mol% yttria partially stabilized zirconia (4Y- or 5Y-PSZ), as a larger cubic content can result in improved translucency and reduced fracture resistance.3,4 These newest generations of zirconia ceramics can also be applied to the esthetically demanding areas as monolithic and fully anatomic form, reproducing accurate shapes of natural teeth.3,4,5
In fabricating CAD-CAM zirconia dental prostheses, the 4- or 5-axis milling techniques are extensively utilized to fully or partially-sintered zirconia blocks because of their reliability and accuracy.6,7 The subtractive machining approach, however, has a number of manufacturing limitations including the difficulty of reproducing complex geometries,8 the wear of the milling tools, and microscopic cracks on the ceramic surface.9 In addition, the remaining part of the zirconia block after milling may be discarded after the fabrication process.10 As an alternative approach, many attempts have been made to additively produce elaborate ceramic objects with the required surface finish, manufacturing accuracy, and mechanical strength.11 The additive manufacturing (AM) of zirconia has been studied to overcome the shortcomings and limitations of subtractive manufacturing.11 The AM method is expected to take less production time and make less waste as well as allow the fabrication of complex geometries with almost no limitation.12,13,14 Among the various AM methods for ceramics, vat polymerization methods such as stereolithography (SLA) and digital light processing (DLP) are known for their high accuracy, which makes them suitable for the digital dental workflow.15 So far, the feasibility of zirconia-based objects produced by additive manufacturing as dental prostheses was mainly tested for 3Y-TZP, which was almost the same as the first generation of the machinable dental zirconia.15,16,17 It has been reported that the additively manufactured high-strength ceramic crowns were comparable to crowns manufactured using the subtractive technique and within the clinically acceptable range.15,16,17,18,19 In terms of the flexural strength values reported from the manufacturers, those of the AM-generated 3Y-TZP objects were in the range from 700 to 800 MPa, which was similar to the those of the third generation zirconia ceramics for milling (4Y-PSZ), with the values between 700 to 800 MPa.3,4,5 However, the differences in the slurry composition, heat treatment schedules, layer thicknesses, light strategies, and other parameters may affect the characteristics of AM-generated ceramic objects intended for dental prosthesis.20,21
The purpose of this study is to evaluate the intaglio trueness, antagonist’s wear volume loss, and fracture resistance of monolithic full-contoured single-unit restorations fabricated by subtractive and additive manufacturing techniques, using the latest generations of zirconia materials for each method (3Y-TZP for additive and 4Y- or 5Y-PSZ for subtractive). The first null hypothesis was that no difference would be found in the trueness between the fabrication techniques. The second null hypothesis was that no different antagonist’s wear volume would be found between the fabrication techniques. The third null hypothesis was that no difference would be found in the fracture load between the fabrication techniques regardless of artificial aging.
MATERIALS AND METHODS
The standardized acrylic resin teeth (Simple Root Tooth Model; Nissin Dental Products, Kyoto, Japan) of the mandibular right first molars were used for this study. The teeth were prepared with 1.5-mm occlusal reduction, 1 - 1.5-mm axial reduction with rounded internal line angles, and a 1-mm circumferential chamfer finish line. The prepared resin tooth was digitized using a laboratory scanner (T500; Medit, Seoul, Korea) and a total of 56 metal abutments were fabricated with layers of powdered Co-Cr alloys (SP2 CoCr; Eos GmbH, Krailling, Germany) by direct metal laser sintering technique (EOSINT M270; Eos GmbH, Krailling, Germany). A full-contour monolithic crown was virtually designed on the prepared die using a dental CAD software (Dental Designer; 3Shape, Copenhagen, Denmark), with the cementation space of 25-µm, starting from 1-mm distance to margin line.
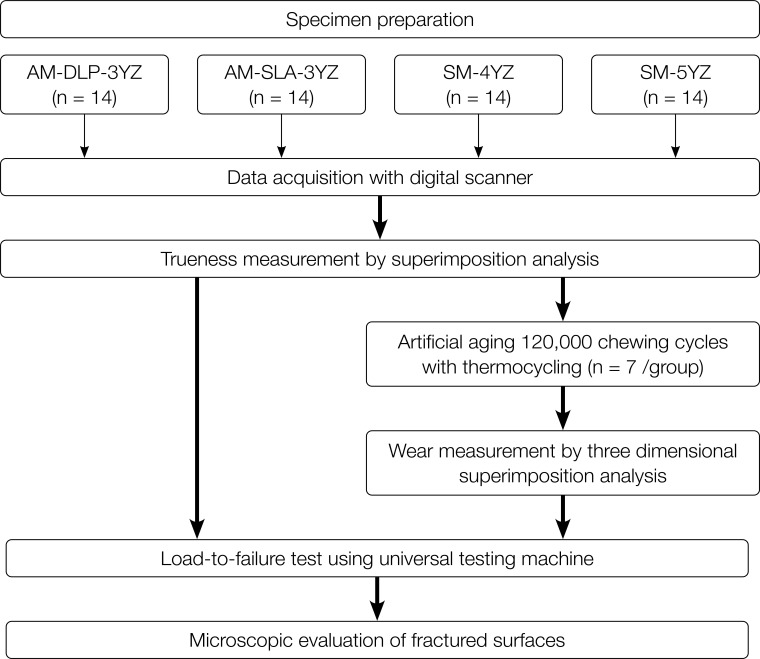
A total of 56 monolithic zirconia crowns with four testing groups, which were the latest generations for each manufacturing technology, were fabricated by 3Y-TZP-based additive (AM-DLP-3YZ and AM-SLA-3YZ groups) or subtractive (third generations of dental zirconia, SM-4YZ and SM-5YZ groups) manufacturing (n= 14 per each group) technique. First, for the AM-DLP-3YZ group, the printable zirconia suspension (3Y-TZP; M.O.P, Seoul, Korea) and the DLP format three-dimensional (3D) printer (Octave Light R1; Octave Light Limited, Shatin, N.T., Hong Kong) were used to produce full-contour monolithic zirconia crowns. For the AM-SLA-3YZ group, the printable zirconia suspension (3Y-TZP; 3D Ceram, Limoges, France) was used to fabricate full-contour crowns using the SLA format 3D printer (C100 EASY FAB; 3D Ceram, Limoges, France). For both AM groups, all the layering, post-processing, and sintering of the printed specimens were conducted by the manufacturer itself (3D Ceram) or according to the manufacturer’s instructions (M.O.P). For the SM-4YZ group, the partially sintered 4-mol% yttria-stabilized zirconia (4Y-PSZ; KATANA STML, Kuraray Noritake Dental Inc., Tokyo, Japan) was used to make full-contour crowns using a 5-axis milling machine (Arum 5X-300; Doowon, Daejeon, Korea). Likewise, for the SM-5YZ group, the partially sintered 5-mol% yttria-stabilized zirconia (5Y-PSZ; KATANA UTML, Kuraray Noritake Dental Inc., Tokyo, Japan) was used to mill the crowns by the same laboratory system. For both SM groups, all the sintering schedules were done according to the manufacturer’s instructions. The blocks used for SM groups were produced from the same company, to minimize possible difference in terms of raw material production. The process of subsequent experiments is summarized in the flow chart (Fig. 1). For all the specimens, no additional adjustment (grinding) or polishing were performed on their intaglio surfaces after the sintering.
Fig. 1. Flowchart of this research. AM: additive manufacturing; DLP: digital light processing; SM: subtractive manufacturing; SLA: stereolithography; 3YZ: 3 mol% yttria-stabilized tetragonal zirconia; 4YZ: 4 mol% yttria-stabilized partially sintered zirconia; 5YZ: 5 mol% yttria-stabilized partially sintered zirconia.
For the trueness measurement, the intaglio surfaces of all zirconia crowns were scanned with an intraoral scanner (i500; Medit, Seoul, Korea). Since the digitizing process was slightly limited for the laboratory scanner due to the scan angle and distance to the small intaglio surfaces of the crown specimens, the intraoral scanner was used for the experiment under meticulous calibration and manipulation according to the manufacturer’s instructions. The scanning procedure was conducted by a board-certified prosthodontist (H.I.Y) with a 15-year clinical experience, under no ambient light condition. The calibration was performed based on the manufacturer’s instructions, at each scanning process of the specimens. With the 3D inspection software (Geomagic Control X; Geomagic Inc., Morrisville, NC, USA), each scan data of the intaglio surface of the crown specimen was superimposed on the internal surface of the crown from the CAD file. After setting the internal area of the virtual crown as a region of interest, initial and best-fit alignment were conducted sequentially. The best-fit alignment used the iterative closest point algorithm based on point-to-point distance or linear distance measurement. The root-mean-square (RMS) value between the intaglio scan data and reference data was calculated in terms of the four different regions of inspection: inner surface, occlusal, margin, axial area. The color deviation map was also displayed for each group. All the scanning and superimposition of each zirconia crown specimen were performed by a single investigator (Y.K.K.).
For the thermal cycling and mechanical loading, half of the specimens of each group (n = 7 for each group) were cemented onto 3D-printed metal abutment, with a self-curing resin cement (RelyX-U200; 3M-ESPE, St. Paul, MN, USA). After cementation, the crown-abutment specimens were artificially aged in a chewing simulator (Chewing Simulator CS-4.8; SD Mechatronik, Feldkirchen-Westerham, Germany). The disto-palatal cusps of human maxillary molars were used as enamel antagonists after the cusp tips were adjusted to spherical shapes with a fine-grit diamond bur (Dia-Burs EX-21F; MANI, Tochigi, Japan) and fixed in a self-cure acrylic resin (Vertex Self-Curing; Vertex Dental, Soesterberg, Netherlands). The crown-abutment specimens were loaded with 50 N for 1.2 million cycles at 1.5 Hz. Simultaneous thermal cycling was performed in water by changing the temperature every 60 s from 5 to 55°C. All the testing parameters used in this thermal cycling and mechanical loading are presented in Table 1. The artificial aging of 1.2-million cycle dynamic loading with thermal cycling between 5 - 55°C was applied to simulate physiologic mastication of 5-year clinical service.22,23 To evaluate the surface wear, the occlusal surface of each antagonist tooth including disto-palatal cusp was digitized with the laboratory scanner (T500; Medit, Seoul, Korea) before and after each chewing cycle. The scan data were superimposed to calculate the amount of volume loss (mm3) using a 3D inspection software (Geomagic Control X; Geomagic Inc., Morrisville, NC, USA).
Table 1. Parameters for chewing simulation used in this study.
| Transverse path | Speed (mm/s) | Weight | Thermal cycling | Cycle |
|---|---|---|---|---|
| Downward: 2 mm | Up: 55 | 5 kg | Dwell time: 60 s | Number: 120,000 |
| Lateral: 0.7 mm | Down: 30 | Temperature: 5°C - 55°C | Frequency: 1.5 Hz | |
| Forward: 30 | ||||
| Back: 55 |
The artificially aged crown-abutment specimen was then fixed with a test jig on the universal testing machine (Instron 8871; Instron, Norwood, MA, USA) to test its fracture resistance, using a 10 kN-load cell. A stainless-steel spherical indenter with a diameter of 5 mm was used to contact the central fossa of each crown-abutment specimen. A 2-mm urethane sheet was placed on the occlusal surface of the crown to equally distribute the loading and prevent excessive local force. Compressive loading was performed at a crosshead speed of 0.5 mm/min until fracture occurred, at which time the load (N) at failure was recorded. After the load-to-failure test, the fractured surfaces of the specimens were compared between subtractive and additive manufacturing groups, examined by the scanning electron microscopy (Apreo S; Thermo Fisher Scientific, MA, USA) with magnifications of 500× and 5,000×.
For the statistical analysis, the means and standard deviations of the trueness measurements (RMS value, µm), the fracture load values (N), and the amount (mm3) of volume loss of the antagonists for all the groups were calculated. Since the RMS data satisfy the assumption of normality but violate the equality of variances, Welch’s analysis of variance (ANOVA) was performed, and post-validation was carried out with the Games-Howell test. The Kruskal-Wallis test was employed to assess the statistical significance of the amount of volume loss of the antagonists. For the fracture resistance analysis, a two-way ANOVA was performed to determine the effects of two factors, the manufacturing methods of the zirconia crowns and the artificial aging (thermo-cycling and mechanical loading), and their interactions. The one-way ANOVA was performed to find out whether the fracture load values before aging were different for each group, and the values after aging were also performed in the same way. A post-hoc pairwise comparison was conducted and adjusted using Bonferroni’s correction. A paired t-test was used to confirm whether there was a significant difference before and after aging for each group. For all the analysis, statistical software (IBM SPSS Statistics v25.0; IBM Corp., Armonk, NY, USA) was used with the level of statistical significance (P) set as 0.05.
RESULTS
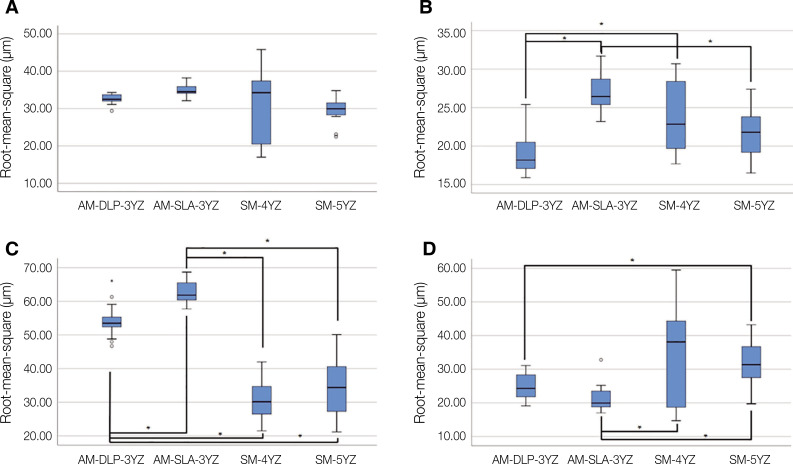
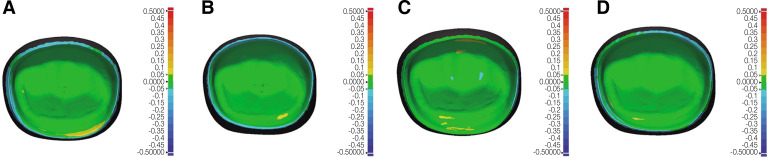
The mean RMS values (µm) of four zirconia crown groups in four different areas of inspection are presented in Table 2 and Figure 2. No statistically significant differences were detected among all the AM and SM groups at the inner surface area, meaning similar trueness of intaglio crown surface, regardless of the manufacturing methods (P = .053). At the occlusal area, however, the AM-DLP-3YZ group showed the lowest mean RMS value and had statistically significant differences compared to the AM-SLA-3YZ (P < .001) and SM-4YZ groups (P = .042). At the margin area, both AM groups showed significantly higher mean RMS values than both SM groups (all, P < .001). At the axial area, the mean RMS value of the AM-DLP-3YZ group was significantly different from those of the SM-5YZ groups (P = .027). The AM-SLA-3YZ group showed a significantly lower mean RMS value in the axial area than SM-4YZ (P = .019) and SM-5YZ groups (P < .001). The color deviation maps (Fig. 3) reported that most of the inner surfaces of the evaluated crowns showed green areas, meaning surface deviation within 50 µm, regardless of the manufacturing methods.
Table 2. Intaglio trueness (µm, root-mean-square estimates, mean ± standard deviation) of four zirconia crown groups measured in four different areas of inspection.
| Area | Groups | P | |||
|---|---|---|---|---|---|
| AM-DLP-3YZ | AM-SLA-3YZ | SM-4YZ | SM-5YZ | ||
| Inner surface (total) | 32.57 ± 1.30 | 34.81 ± 1.80 | 30.76 ± 9.11 | 29.76 ± 3.63 | > .05 |
| Occlusal | 19.46 ± 3.33 | 27.05 ± 2.49 | 23.87 ± 4.75 | 21.59 ± 3.12 | < .05 |
| Margin | 54.20 ± 5.18 | 62.39 ± 3.08 | 30.91 ± 6.09 | 34.80 ± 8.85 | < .05 |
| Axial | 25.11 ± 3.90 | 21.51 ± 4.19 | 35.41 ± 14.78 | 32.07 ± 7.46 | < .05 |
AM: additive manufacturing; DLP: digital light processing; SM: subtractive manufacturing; SLA: stereolithography; 3YZ: 3 mol% yttria-stabilized tetragonal zirconia; 4YZ: 4 mol% yttria-stabilized partially sintered zirconia; 5YZ: 5 mol% yttria-stabilized partially sintered zirconia. P: statistically significance level.
Fig. 2. Intaglio trueness (µm, root-mean-square estimates, RMS) of four zirconia crown groups measured in four different areas of inspection. (A) Inner surface, (B) Occlusal area, (C) Margin area, (D) Axial area. Statistically significant difference (P < .05) was marked with asterisk (*). AM: additive manufacturing; DLP: digital light processing; SM: subtractive manufacturing; SLA: stereolithography; 3YZ: 3 mol% yttria-stabilized tetragonal zirconia; 4YZ: 4 mol% yttria-stabilized partially sintered zirconia; 5YZ: 5 mol% yttria-stabilized partially sintered zirconia.
Fig. 3. Representative color deviation maps of intaglio trueness of four zirconia crown groups (positive deviation, yellow to red, negative deviation, cyan to blue, deviation below 50 µm, green). (A) AM-DLP-3YZ, (B) AM-SLA-3YZ, (C) SM-4YZ, (D) SM-5YZ. AM: additive manufacturing; DLP: digital light processing; SM: subtractive manufacturing; SLA: stereolithography; 3YZ: 3 mol% yttria-stabilized tetragonal zirconia; 4YZ: 4 mol% yttria-stabilized partially sintered zirconia; 5YZ: 5 mol% yttria-stabilized partially sintered zirconia.
None of the tested crowns were fractured during simulated chewing cycles. The amount of material wear on the occluding surfaces of zirconia crowns was almost negligible, hard to detect by 3D superimposition analysis. The measured volume loss (mm3, mean ± SD) of the enamel antagonist teeth after simulated chewing was calculated as 2.06 ± 1.24, 1.74± 1.20, 2.51 ± 2.13, and 2.40 ± 1.66 for AM-DLP-3YZ, AM-SLA-3YZ, SM-4YZ, and SM-5YZ groups, respectively. In terms of mean volume loss, no statistically significant differences were detected among the groups (P = .946).
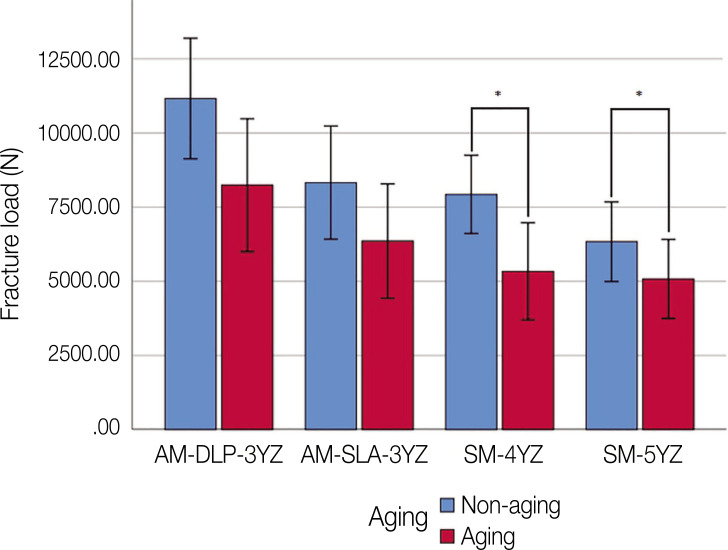
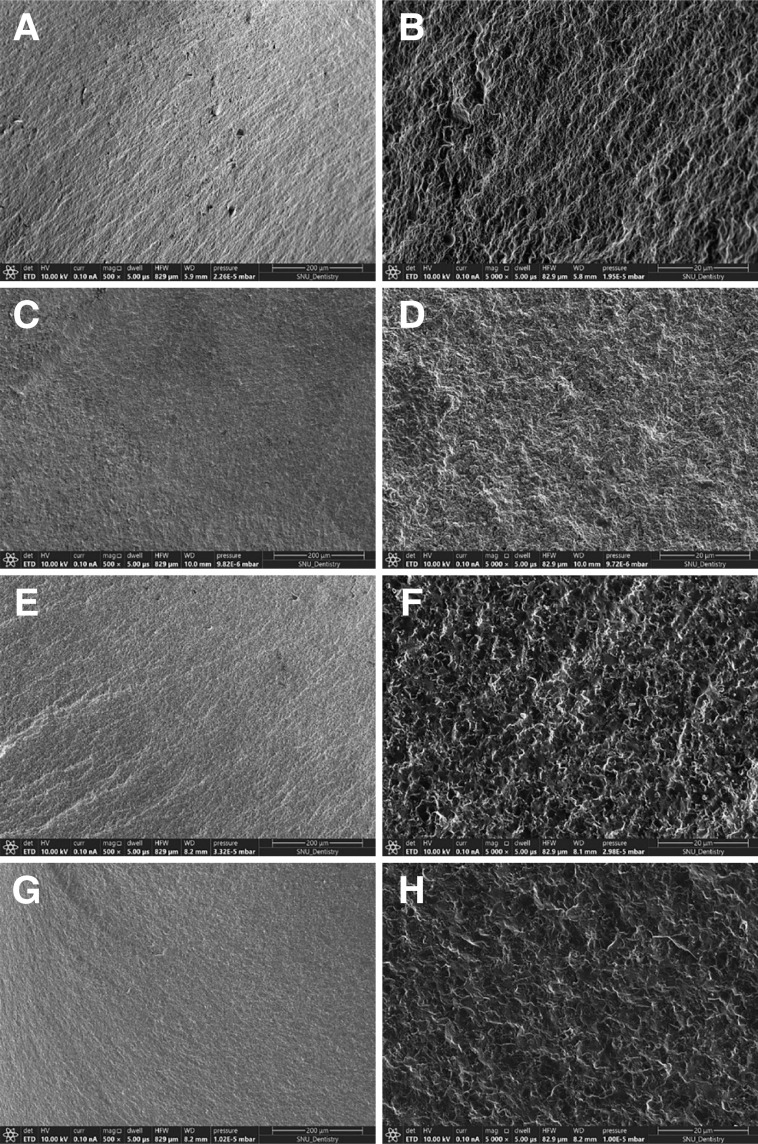
The means and standard deviations of the fracture load values (N) for all the groups were shown in Figure 4. Two-way ANOVA showed that both zirconia groups and thermo-mechanical aging had statistically significant effects on the value of fracture load (both, P < .001). No interaction between groups and aging was observed (P = .368). According to the one-way ANOVA results, the AM-DLP-3YZ group had significantly higher fracture resistance compared with the AM-SLA-3YZ (P = .026), SM-4YZ (P = .009), and SM-5YZ groups (P < .001), before thermo-mechanical aging. Also after aging, the AM-DLP-3YZ group had significantly higher fracture resistance compared with the SM-4YZ (P = .038), and SM-5YZ groups (P = .020). Paired t-test showed the significant decrease of fracture resistance after aging in SM-4YZ (P = .028) and SM-5YZ (P = .011) groups. The microscopic observation of fractured zirconia crowns revealed that both AM and SM groups showed highly dense structures with no pores or other manufacturing defects, showing similar morphologies of fractured surfaces for all the zirconia groups, at both ×500 and ×5,000 magnifications (Fig. 5).
Fig. 4. Fracture load of the AM and SM groups.
*Statistically significant difference (P < .05). AM: additive manufacturing; DLP: digital light processing; SM: subtractive manufacturing; SLA: stereolithography; 3YZ: 3 mol% yttria-stabilized tetragonal zirconia; 4YZ: 4 mol% yttria-stabilized partially sintered zirconia; 5YZ: 5 mol% yttria-stabilized partially sintered zirconia.
Fig. 5. Representative SEM images of the fractured surfaces of the specimens from AM and SM groups. (A, B) AM-DLP-3YZ (×500 and ×5000, respectively), (C, D) AM-SLA-3YZ (×500 and ×5000, respectively), (E, F) SM-4YZ (×500 and ×5000, respectively), (G, H) SM-5YZ (×500 and ×5000, respectively). AM: additive manufacturing; DLP: digital light processing; SM: subtractive manufacturing; SLA: stereolithography; 3YZ: 3 mol% yttria-stabilized tetragonal zirconia: 4YZ: 4 mol% yttria-stabilized partially sintered zirconia; 5YZ: 5 mol% yttria-stabilized partially sintered zirconia.
DISCUSSION
Based on the results of this study, the null hypotheses were partially rejected. When it comes to trueness, AM groups showed a significantly higher RMS value than SM groups in the margin areas. The accuracy of additively manufactured zirconia crowns reported in previous studies was in the range of 23 µm to 38 µm for the intaglio surface as well as the marginal area, which was similar to those manufactured by the milling method.15,16,17,18,19 The trueness of the AM groups in this study was slightly different from the measurements of previous studies, which may result from the difference in crown forms, inner surface parameters, and tooth preparation geometries.15,16,17,18,19 Due to the surface stepping phenomenon of AM, it may have influenced the trueness of the crown in the line angle or marginal area rather than the axial plane.15,19 The green structure manufactured by the additive method can be damaged by physical handling, post-processing, debinding, and sintering, so a large RMS value was observed.17 At the axial and occlusal areas, the RMS value of the SM group was significantly higher than that of the AM groups, and the deviation was also higher. This reflects the limitation of the subtractive milling process, which is the lack of manufacturing ability of details due to the limitations of the size and shape of the milling bur.24 In the group made with DLP, the RMS value was significantly smaller than that of the SLA group in occlusal and margin. Compared to the SLA method, which is cured in units of voxels, the DLP method is cured simultaneously in units of laminated cross-sections.25 The differences in the accuracy of printed objects in this study may have originated from the differences in ceramic slurries, light sources, printing mechanism, and polymerization methods.16,19
All specimens survived thermo-mechanical aging, demonstrating their applicability in clinical situations of approximately 5 years.22,23 The measured volume loss of the antagonist was approximately similar to that reported in previous studies.26 Although the Kruskal-Wallis test confirmed no significant difference between groups, the AM groups showed smaller wear of enamel antagonists than the SM groups. Ceramic-induced enamel wear is more related to surface roughness and microstructure than hardness.27 Since no additional polishing was done for all the sintered specimens from both SM and AM groups, this appears to be owing to the increased roughness caused by the staircase shape of the specimen surface of AM groups.16,28
In this study, the fracture loads of zirconia crowns of AM groups were higher than those of SM groups, possibly because the fracture resistance decreases as the yttria content increases.3,4 The crowns of SM groups were made from the dental zirconia classified as its third generation with increased yttria content, which showed lower fracture resistance than the earlier generations based on 3Y-TZP.3,4 Since the specimens of AM groups were made from the slurries containing 3Y-TZP powders, their physical properties should be better than those of the SM groups. In addition, the fracture load values of two AM groups were different. Differences in slurry composition and debinding rates may have affected the fracture resistance of the printed objects.20,21 Furthermore, differences in layer thickness, light source, and polymerization strategies may have led to differences in formation or distribution of micro-level pores in the sintered objects.20,21
In terms of hydrothermal aging, the presence of water and changes in surrounding temperature may induce micro-cracks of the zirconia ceramics by the penetration of water molecules and the phase transformations and, eventually, the degradation of mechanical properties.29,30,31 In this study, the SM groups with 4Y- and 5Y-PSZ were more affected by hydrothermal aging than the AM group that had 3Y-TZP. The aging process decreased the fracture resistance of the zirconia crowns in both AM and SM groups, but only SM groups showed statistically significant differences. The effects of aging of zirconia crowns produced by either SM or AM could be different in each group because the content of alumina capable of stabilizing oxygen vacancies in the polycrystalline structure was different for each group of the tested zirconia materials.32
There are some limitations of this in vitro study that the different variables in the SLA and DLP methods could not be controlled because of the differences in the printing mechanism, printing conditions, parameters, and slurry composition. Since the AM technique, as well as SM, has been reported to have different shrinkage rates depending on the geometric shape, further studies comparing different types of prostheses are needed.19 In addition, the comparison in this study was performed among the zirconia ceramics for subtractive and additive manufacturing, considering the flexural strength values reported from the manufacturers. However, the differences in the composition (3Y-TZP for AM, 4Y-PSZ and 5Y-PSZ for SM) may have influenced the findings of the analysis. Lastly, the specimens of SM groups of this study were from the same manufacturer, and it was not able to generalize the findings and identify the effects of the materials from various manufacturers and milling conditions.
CONCLUSION
Within the limitation of this study, the additive manufacturing produced an accurate 3Y-TZP single-unit prosthesis that was clinically acceptable, with possibilities of compensating the shortcomings of subtractive technique. As well as the intaglio surface trueness, the additively manufactured zirconia crowns showed comparable or better properties in terms of fracture resistance and antagonist’s wear after simulated mastication, compared with the crowns produced by subtractive manufacturing using the latest generations of dental zirconia.
ACKNOWLEDGEMENTS
The biospecimens and data used for this study were provided by the Biobank of Seoul National University Dental Hospital, a member of the Korea Biobank Network (KBN4_A04).
Footnotes
This work was supported by the Korea Medical Device Development Fund grant funded by the Korea Government (the Ministry of Science and ICT, the Ministry of Trade, Industry and Energy, the Ministry of Health & Welfare, the Ministry of Food and Drug Safety) (KMDF_PR_20200901_0002) and by Creative-Pioneering Researchers Program through Seoul National University (SNU).
References
- 1.Gautam C, Joyner J, Gautam A, Rao J, Vajtai R. Zirconia based dental ceramics: structure, mechanical properties, biocompatibility and applications. Dalton Trans. 2016;45:19194–19215. doi: 10.1039/c6dt03484e. [DOI] [PubMed] [Google Scholar]
- 2.Denry I, Kelly JR. State of the art of zirconia for dental applications. Dent Mater. 2008;24:299–307. doi: 10.1016/j.dental.2007.05.007. [DOI] [PubMed] [Google Scholar]
- 3.Stawarczyk B, Keul C, Eichberger M, Figge D, Edelhoff D, Lümkemann N. Three generations of zirconia: From veneered to monolithic. Part II. Quintessence Int. 2017;48:441–450. doi: 10.3290/j.qi.a38157. [DOI] [PubMed] [Google Scholar]
- 4.Kolakarnprasert N, Kaizer MR, Kim DK, Zhang Y. New multi-layered zirconias: Composition, microstructure and translucency. Dent Mater. 2019;35:797–806. doi: 10.1016/j.dental.2019.02.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Malkondu Ö, Tinastepe N, Akan E, Kazazoğlu E. An overview of monolithic zirconia in dentistry. Biotechnol Biotechnol Equip. 2016;30:644–652. [Google Scholar]
- 6.Abduo J, Lyons K, Swain M. Fit of zirconia fixed partial denture: a systematic review. J Oral Rehabil. 2010;37:866–876. doi: 10.1111/j.1365-2842.2010.02113.x. [DOI] [PubMed] [Google Scholar]
- 7.Att W, Komine F, Gerds T, Strub JR. Marginal adaptation of three different zirconium dioxide three-unit fixed dental prostheses. J Prosthet Dent. 2009;101:239–247. doi: 10.1016/S0022-3913(09)60047-0. [DOI] [PubMed] [Google Scholar]
- 8.van Noort R. The future of dental devices is digital. Dent Mater. 2012;28:3–12. doi: 10.1016/j.dental.2011.10.014. [DOI] [PubMed] [Google Scholar]
- 9.Schriwer C, Skjold A, Gjerdet NR, Øilo M. Monolithic zirconia dental crowns. Internal fit, margin quality, fracture mode and load at fracture. Dent Mater. 2017;33:1012–1020. doi: 10.1016/j.dental.2017.06.009. [DOI] [PubMed] [Google Scholar]
- 10.Strub JR, Rekow ED, Witkowski S. Computer-aided design and fabrication of dental restorations: current systems and future possibilities. J Am Dent Assoc. 2006;137:1289–1296. doi: 10.14219/jada.archive.2006.0389. [DOI] [PubMed] [Google Scholar]
- 11.Khanlar LN, Salazar Rios A, Tahmaseb A, Zandinejad A. Additive manufacturing of zirconia ceramic and its application in clinical dentistry: a review. Dent J (Basel) 2021;9:104. doi: 10.3390/dj9090104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Torabi K, Farjood E, Hamedani S. Rapid prototyping technologies and their applications in prosthodontics, a review of literature. J Dent (Shiraz) 2015;16:1–9. [PMC free article] [PubMed] [Google Scholar]
- 13.Schwentenwein M, Homa J. Additive manufacturing of dense alumina ceramics. Int J Appl Ceram Technol. 2014;12:1–7. [Google Scholar]
- 14.Ebert J, Ozkol E, Zeichner A, Uibel K, Weiss O, Koops U, Telle R, Fischer H. Direct inkjet printing of dental prostheses made of zirconia. J Dent Res. 2009;88:673–676. doi: 10.1177/0022034509339988. [DOI] [PubMed] [Google Scholar]
- 15.Wang W, Sun J. Dimensional accuracy and clinical adaptation of ceramic crowns fabricated with the stereolithography technique. J Prosthet Dent. 2021;125:657–663. doi: 10.1016/j.prosdent.2020.02.032. [DOI] [PubMed] [Google Scholar]
- 16.Wang W, Yu H, Liu Y, Jiang X, Gao B. Trueness analysis of zirconia crowns fabricated with 3-dimensional printing. J Prosthet Dent. 2019;121:285–291. doi: 10.1016/j.prosdent.2018.04.012. [DOI] [PubMed] [Google Scholar]
- 17.Li R, Chen H, Wang Y, Sun Y. Performance of stereolithography and milling in fabricating monolithic zirconia crowns with different finish line designs. J Mech Behav Biomed Mater. 2021;115:104255. doi: 10.1016/j.jmbbm.2020.104255. [DOI] [PubMed] [Google Scholar]
- 18.Lerner H, Nagy K, Pranno N, Zarone F, Admakin O, Mangano F. Trueness and precision of 3D-printed versus milled monolithic zirconia crowns: An in vitro study. J Dent. 2021;113:103792. doi: 10.1016/j.jdent.2021.103792. [DOI] [PubMed] [Google Scholar]
- 19.Revilla-León M, Methani MM, Morton D, Zandinejad A. Internal and marginal discrepancies associated with stereolithography (SLA) additively manufactured zirconia crowns. J Prosthet Dent. 2020;124:730–737. doi: 10.1016/j.prosdent.2019.09.018. [DOI] [PubMed] [Google Scholar]
- 20.Revilla-León M, Al-Haj Husain N, Ceballos L, Özcan M. Flexural strength and Weibull characteristics of stereolithography additive manufactured versus milled zirconia. J Prosthet Dent. 2021;125:685–690. doi: 10.1016/j.prosdent.2020.01.019. [DOI] [PubMed] [Google Scholar]
- 21.Ioannidis A, Bomze D, Hämmerle CHF, Hüsler J, Birrer O, Mühlemann S. Load-bearing capacity of CAD/CAM 3D-printed zirconia, CAD/CAM milled zirconia, and heat-pressed lithium disilicate ultra-thin occlusal veneers on molars. Dent Mater. 2020;36:109–116. doi: 10.1016/j.dental.2020.01.016. [DOI] [PubMed] [Google Scholar]
- 22.DeLong R, Douglas WH. An artificial oral environment for testing dental materials. IEEE Trans Biomed Eng. 1991;38:339–345. doi: 10.1109/10.133228. [DOI] [PubMed] [Google Scholar]
- 23.Steiner M, Mitsias ME, Ludwig K, Kern M. In vitro evaluation of a mechanical testing chewing simulator. Dent Mater. 2009;25:494–499. doi: 10.1016/j.dental.2008.09.010. [DOI] [PubMed] [Google Scholar]
- 24.Kirsch C, Ender A, Attin T, Mehl A. Trueness of four different milling procedures used in dental CAD/CAM systems. Clin Oral Investig. 2017;21:551–558. doi: 10.1007/s00784-016-1916-y. [DOI] [PubMed] [Google Scholar]
- 25.Dikova T, Dzhendov DA, Ivanov D, Bliznakova K. Dimensional accuracy and surface roughness of polymeric dental bridges produced by different 3D printing processes. Arch Mater Sci Eng. 2018;94:65–75. [Google Scholar]
- 26.Choi JW, Bae IH, Noh TH, Ju SW, Lee TK, Ahn JS, Jeong TS, Huh JB. Wear of primary teeth caused by opposed all-ceramic or stainless steel crowns. J Adv Prosthodont. 2016;8:43–52. doi: 10.4047/jap.2016.8.1.43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Oh WS, Delong R, Anusavice KJ. Factors affecting enamel and ceramic wear: a literature review. J Prosthet Dent. 2002;87:451–459. doi: 10.1067/mpr.2002.123851. [DOI] [PubMed] [Google Scholar]
- 28.Janyavula S, Lawson N, Cakir D, Beck P, Ramp LC, Burgess JO. The wear of polished and glazed zirconia against enamel. J Prosthet Dent. 2013;109:22–29. doi: 10.1016/S0022-3913(13)60005-0. [DOI] [PubMed] [Google Scholar]
- 29.Kohorst P, Borchers L, Strempel J, Stiesch M, Hassel T, Bach FW, Hübsch C. Low-temperature degradation of different zirconia ceramics for dental applications. Acta Biomater. 2012;8:1213–1220. doi: 10.1016/j.actbio.2011.11.016. [DOI] [PubMed] [Google Scholar]
- 30.Lümkemann N, Stawarczyk B. Impact of hydrothermal aging on the light transmittance and flexural strength of colored yttria-stabilized zirconia materials of different formulations. J Prosthet Dent. 2021;125:518–526. doi: 10.1016/j.prosdent.2020.01.016. [DOI] [PubMed] [Google Scholar]
- 31.Camposilvan E, Leone R, Gremillard L, Sorrentino R, Zarone F, Ferrari M, Chevalier J. Aging resistance, mechanical properties and translucency of different yttria-stabilized zirconia ceramics for monolithic dental crown applications. Dent Mater. 2018;34:879–890. doi: 10.1016/j.dental.2018.03.006. [DOI] [PubMed] [Google Scholar]
- 32.Wei C, Gong X, Xie C, Chen ZX, Li SB, Gremillard L. In vitro cyclic fatigue and hydro-thermal aging lifetime assessment of yttria-stabilized zirconia dental ceramics. J Eur Ceram Soc. 2020;40:4647–4654. [Google Scholar]