Abstract
Frontotemporal lobar degeneration (FTLD) comprises a heterogenous group of fatal neurodegenerative diseases and, to date, no validated diagnostic or prognostic biomarkers or effective disease-modifying therapies exist for the different clinical or genetic subtypes of FTLD. Current treatment strategies rely on the off-label use of medications for symptomatic treatment. Changes in several neurotransmitter systems including the glutamatergic, GABAergic, dopaminergic, and serotonergic systems have been reported in FTLD spectrum disease patients. Many FTLD-related clinical and neuropsychiatric symptoms such as aggressive and compulsive behaviour, agitation, as well as altered eating habits and hyperorality can be explained by disturbances in these neurotransmitter systems, suggesting that their targeting might possibly offer new therapeutic options for treating patients with FTLD. This review summarizes the present knowledge on neurotransmitter system deficits and synaptic dysfunction in model systems and patients harbouring the most common genetic causes of FTLD, the hexanucleotide repeat expansion in C9orf72 and mutations in the granulin (GRN) and microtubule-associated protein tau (MAPT) genes. We also describe the current pharmacological treatment options for FLTD that target different neurotransmitter systems.
Subject terms: Diseases, Neuroscience
Introduction
Frontotemporal lobar degeneration (FTLD) comprises a heterogenous group of neurodegenerative syndromes characterized by progressive atrophy in the frontal and temporal lobes. Neurodegeneration in these brain areas is clinically associated with deficits in behaviour, progressive personality changes, executive dysfunction and/or understanding or producing speech. The clinical subtypes of FTLD include behavioural variant FTD (bvFTD) [1], nonfluent variant of primary progressive aphasia (nfvPPA), semantic variant of PPA (svPPA), logopenic variant of PPA (lvPPA) [2] as well as progressive supranuclear palsy (PSP) and corticobasal syndrome (CBS) [3]. FTLD is commonly associated with parkinsonism [4] and a great number of FTLD patients also present neuropsychiatric symptoms [5] or motor neuron dysfunction meeting the criteria of amyotrophic lateral sclerosis (ALS) [6, 7]. Several genetic mutations are associated with FTLD such as those in the granulin (GRN) and microtubule-associated protein tau (MAPT) genes [2]. However, the major genetic cause underlying both FTLD and ALS is a GGGGCC(n) hexanucleotide repeat expansion in the C9orf72 gene (C9-HRE) [8–10]. Unlike in the other types of FTLD, genetics do not play a major role in CBS or PSP [11].
Currently, no specific validated diagnostic or prognostic biomarkers or effective disease-modifying therapies are available for FTLD which complicates the correct and timely diagnosis and treatment. However, FTLD is accompanied by changes in several neurotransmitter systems including the glutamatergic, GABAergic, dopaminergic, and serotonergic systems [12] which are linked with specific neuropsychiatric symptoms such as aggressive and compulsive behaviour, agitation, and altered eating habits and hyperorality [12]. Due to the lack of officially approved pharmacological therapies for FTLD in Europe and the US, off-label medications targeting neurotransmitter systems to alleviate the clinical symptoms are commonly used. Nevertheless, these treatments do not target the pathogenic mechanisms of FLTD, and there is limited evidence for their ability to slow disease progression.
Molecular mechanistic evidence from genetic model systems supporting synaptic dysfunction in FTLD
FTLD is suggested to have a strong genetic background and approximately 40% of patients have a family history with at least one affected family member [13]. Heritability varies between the different FTLD clinical subtypes. It is suggested that heritability plays a role in 48% of patients with bvFTD but only in 12% of svPPA patients [14]. Overall, the C9-HRE accounts for ~25% familial FTLD and ~6% of sporadic FTLD cases, although the frequency largely varies in geographically different populations [8]. Different MAPT mutations can be the underlying cause of up to 50% of familial and ~10% of sporadic FTLD patients and ~10% of FTLD cases are caused by loss of function mutations in GRN, but also the variant frequencies in these genes may differ between different geographical areas [14, 15]. Other genetic mutations have also been found to associate with FTLD, but the C9-HRE and mutations GRN and MAPT represent the three most common ones [14, 15].
C9-HRE
The C9-HRE, identified in 2011, is a major cause of both FTLD and ALS [9, 10]. C9-HRE most commonly associates with bvFTD and bvFTD with ALS clinical phenotypes [9, 10, 16]. Suggested pathological mechanisms of the C9-HRE are C9orf72 loss-of-function due to haploinsufficiency that leads to a decreased expression of the C9orf72 protein, and toxic gain-of-function caused by the accumulation of pathological RNA foci and dipeptide repeat-containing (DPR) proteins (poly-GA, poly-GP, poly-GR, poly-PA and poly-PR) [17–19].
Even though the C9orf72 protein is important in the regulation of key cellular and membrane trafficking events and actin cytoskeleton dynamics, C9orf72 loss-of-function does not appear to be a primary disease-driving mechanism in FTLD or ALS [20]. The C9orf72 knockout mouse models do not exhibit neurodegeneration, but display immune system dysfunction and develop autoimmune disease-like phenotypes, suggesting that C9orf72 is a central regulator in the immune system [21–23]. However, loss of C9orf72 has been shown to lead to the accumulation of DPR proteins through impaired lysosomal function and vesicle trafficking, which might cause neurotoxicity [24]. In addition, loss of C9orf72 function leads to the accumulation of glutamate receptors and excitotoxicity [24], which alone or in combination with e.g., DPR proteins could underlie synaptic alterations.
Deficits in neurotransmission have been detected in the CNS and neuromuscular junctions of C9-HRE carriers [25]. Alterations in neuronal excitability, morphology, and excitotoxicity have also been reported in different C9-HRE model systems [26]. Regulation of RNA metabolism is crucial for local synaptic protein synthesis and synaptic function, and dysregulation of this system leads to neuronal hyperexcitability [27]. Interestingly, the GGGGCC(n) repeat-containing RNA was actively transported into neurites, which resulted in decreased number of dendritic branches in Drosophila or rat spinal cord neurons [28]. In addition, overexpression of the GGGGCC(n) repeat expansion caused synaptic and neuromuscular junction deficits [29]. In line with these findings, overexpression of the poly-GA DPR protein in primary mouse cortical neurons led to deficits in dendritic spine formation [30], reduced synaptic vesicle-associated protein 2 (SV2) levels, and altered Ca2+ influx and release of synaptic vesicles in mice [31]. Similar defects have been detected in other C9-HRE models and models having alterations in certain RNA-binding protein species [32, 33].
Excitotoxicity is associated with synaptic dysfunction in ALS and probably also in FTLD [26]. The excitotoxic mechanisms include dysfunction of the α-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid (AMPA) or N-methyl-D-aspartate (NMDA) glutamate receptors, leading to excess Ca2+ influx and cell death. The research on excitotoxicity has mainly focused on AMPA receptors, which regulate fast synaptic transmission and synaptic plasticity [34]. The AMPA receptor consists of four subunits termed GluA1-4. GluA2 is responsible for regulating Ca2+ permeability and AMPA receptor trafficking. Usually, GluA2-containing AMPA receptors are impermeable to Ca2+, but insufficient editing of GluA2 RNA can lead to increased Ca2+ permeability and cell death [35]. Double-stranded RNA-specific editase B2 (ADARB2) modifies the GluA2 subunit, and its absence leads to neuronal death. In line with this, ADARB2 has been shown to interact with the GGGGCC(n)-containing RNA and sequester within the RNA foci in C9-HRE-carrying iPSC-derived neurons that are susceptible to glutamate-mediated excitotoxicity [36]. Contribution of the NMDA receptors in C9-HRE FTLD and ALS is understudied but findings in different genetic ALS models suggest a role for NMDA receptors in neurodegeneration [26]. Also, blockade of NMDA receptors in presynaptic glutamatergic motor neurons rescued the motor deficits and the short life span caused by poly-GR/PR expression in a Drosophila model [37]. Furthermore, C9-HRE-carrying iPSC-derived motor neurons display upregulated levels of Ca2+-permeable AMPA and NMDA receptor subunits and significantly increased Ca2+ transients upon depolarization, repeated firing in response to glutamate, and sustained high concentrations of cytosolic Ca2+ in association with increased susceptibility to cell death [32].
Neuronal hyperexcitability and subsequent hypoexcitability during disease progression is characteristic for ALS [26]. The hyperexcitability can be caused by altered GABAergic signalling in inhibitory neurons together with alterations of Na+ and K+ channel functions and extracellular K+ levels [33]. Human iPSC-derived neurons carrying the C9-HRE show initial hyperexcitability followed by declined synaptic function [29]. Furthermore, overexpression of poly-GR led to the suppression of neuronal excitability [26]. The hyper- and hypoexcitability in ALS and FTLD patients vary greatly in different neurons (cortical vs. motor neurons) and disease states (presymptomatic vs. active disease). Finally, supporting synaptic dysfunction at the neuronal network level, which could contribute to the neurodegenerative processes in patients, a recent study showed significant alterations in the network activity of iPSC-derived cortical neurons from C9-HRE carriers. This included enhanced network burst activity due to increased synaptic contacts and impaired presynaptic synaptic vesicle dynamics and synaptic plasticity [38]. All in all, these studies in different model systems collectively suggest that the C9-HRE is associated with alterations at synapses at the molecular, functional, and neuronal network activity levels.
GRN
Another major underlying cause of FTLD are the GRN loss-of-function mutations and the resulting haploinsufficiency leading to decreased levels of progranulin proteins. FTLD associated with GRN mutations is clinically heterogenous and neuropathologically characterized by ubiquitin and TDP-43-immunoreactive cytoplasmic inclusions [39]. Of the clinical phenotypes, bvFTD most commonly associates with GRN mutations and the patients usually present a combination of behavioural abnormality and language disturbance that is most often nfvPPA or PPA with a mixed phenotype [14]. The progranulin protein is involved in development, wound repair, and modulation of inflammation. In the CNS, progranulin expression is the highest in the cerebral cortex, hippocampus, and cerebellum, suggesting that reduced levels could affect neuronal survival as well as CNS inflammatory processes [40]. The GRN mutations have also been shown to alter synaptic function. Grn knockout mice show altered synaptic connectivity and impaired synaptic plasticity. In addition, pyramidal neurons in the hippocampus of the Grn knockout mice display altered neuronal morphology and synaptic transmission as well as significantly decreased dendric spine density compared to wild-type mice [41]. Grn depletion leads to diminished NMDA receptor density and NMDA-dependent tau phosphorylation, both leading to reduced neuronal arborization [42]. Moreover, studies in Grn-deficient mice showed that lysosomal dysregulation leads to neuroinflammation, synaptic loss, and decreased number for markers of oligodendrocytes, myelin, and neurons. Interestingly, upregulation of the lysosomal transmembrane glycoprotein NMB (GPNMB) levels, observed in the Grn-deficient mice in the same study, was also specifically detected in the brain and cerebrospinal fluid of GRN mutation carriers, implicating that it could be a potential discriminative biomarker for the differentiation of GRN mutation-carrying FTLD patients from the MAPT mutation or C9-HRE carriers [43]. Grn knockout mice have also been reported to show profound microglia infiltration and elimination of inhibitory synapses, which leads to hyperexcitability [44]. Moreover, knockdown of Grn in primary rat hippocampal neurons resulted in reduced neuronal connectivity because of decreased neuronal branching and branch length as well as diminished synaptic density [45]. Concomitantly increased synaptophysin, VGAT, and vGlut-1 puncta, associated with an increase in the number of synaptic vesicles per synapse, suggested an increase in the probability of release at the remaining synapses. Similar findings have been made in post-mortem brain samples of FTLD patients with GRN haploinsufficiency, possibly reflecting a compensatory mechanism related to synaptic loss [45].
MAPT
The first mutation in the MAPT gene, encoding the microtubule-associated tau protein, was identified in 1997 and linked to FTLD with parkinsonism [46]. Patients with MAPT mutations present atrophy in the frontal and temporal lobes and basal ganglia and are neuropathologically characterized by tau-positive inclusions, consisting of toxic intracellular aggregates of hyperphosphorylated tau, in the brain (FTLD‐tau). Alternative splicing of the MAPT gene generates six different tau protein isoforms. Pathological inclusions of tau containing either three (3 R) or four (4 R) microtubule-binding domains are characteristic in the brains of MAPT mutation carriers, depending on the clinical subtype [47]. MAPT mutation-carrying patients may show behavioural changes, semantic impairment, and memory decline accompanied by parkinsonism [48]. MAPT mutations most often associate with bvFTD but rarely with different types of PPA [14]. Tau expression is the highest in neurons compared to other cell types throughout the CNS. Healthy neurons maintain a spatial gradient of tau, whose concentration is greater in axons than in the somatodendritic compartment [49]. Inversion of this gradient leads to disturbed axonal transport [50]. In addition, changes in the tau gradient alters the actin cytoskeleton and the direct interaction between tau and actin is suggested to mediate tau-induced neurotoxicity [51]. A disturbed gradient also leads to the accumulation of tau in the dendritic spines and synapses, which causes synaptic dysfunction and degeneration of axons and neurons [52]. Expression of human mutant tau in mice revealed that early tau-related symptoms such as early memory deficits and disrupted synaptic plasticity develop due to the synaptic abnormalities caused by the missorting and accumulation of hyperphosphorylated tau within dendritic spines rather than due to the loss of synapses or neurons. Mutant tau expression also disrupts synaptic function by impairing AMPA and NMDA receptor trafficking and synaptic anchoring and AMPA receptor-mediated synaptic responses [53].
Tau pathology is a central pathological hallmark of Alzheimer’s disease (AD), the most common tauopathy. It has been suggested that tau can spread from cell to cell through neuronal connections in the AD brain, and this process is accelerated in the presence of β-amyloid (Aβ) in both animal models and humans [54]. This propagation of tau pathology throughout the brain using a prion-like mechanism is thought to involve several steps, including cellular uptake, seeding, secretion, and intercellular transfer through synaptic and non-synaptic pathways [55]. Interestingly, emerging evidence supports the theory that misfolded tau exhibits prion-like properties, including the ability to seed and self-propagate along the axons, also in the brains of patients with other tauopathies, such as FTLD [56], suggesting partially similar neurodegenerative processes between AD and FTLD.
Neurotransmitter system deficits in FTLD patients
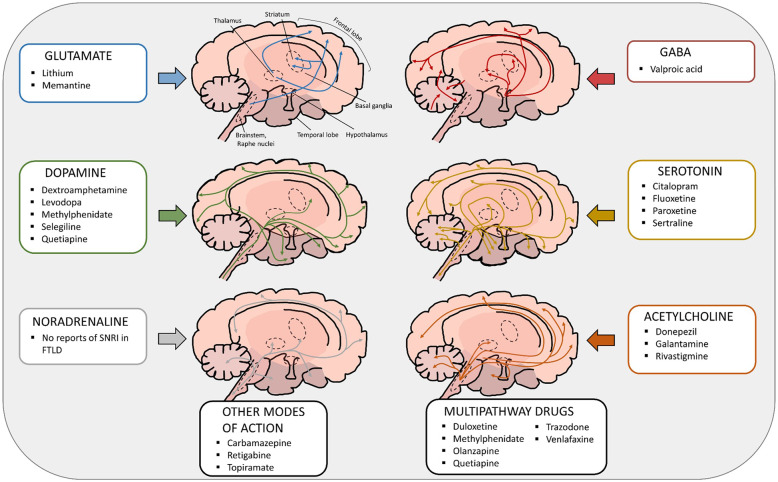
Patients with FTLD spectrum diseases have been reported to display changes in several principal neurotransmitter systems such as the glutamatergic, GABAergic, dopaminergic and serotonergic systems [57]. The different neurotransmitter system pathways are depicted in Fig. 1. The effects of individual neurotransmitter system deficits in the CNS of patients with FTLD spectrum diseases are described in more detail in the following chapters and summarized in Table 1.
Fig. 1. Neurotransmitter pathways involved in FTLD and current therapeutics affecting the different neurotransmitter systems.
FTLD has been linked to changes in several principal neurotransmitter systems such as the glutamatergic, GABAergic, dopaminergic and serotonergic systems. Due to the lack of officially validated and approved pharmacological therapies for FTLD, off-label medications targeting different neurotransmitter systems, indicated here, to alleviate the clinical symptoms are commonly used. Drugs with other modes of action are also in use. For instance, retigabine acts as a positive allosteric modulator of the neuronal potassium channels KNCQ (Kv2 to 5), carbamazepine preferentially binds to voltage-gated sodium channels in their inactive conformation, which prevents repetitive and sustained firing of an action potential, and topiramate blocks voltage-dependent sodium and calcium channels. There are several drugs (listed in the multipathway drug box) that act simultaneously on different pathways. For example, methylphenidate and quetiapine affect both dopaminergic and noradrenergic pathways. Also, antidepressants such as duloxetine and venlafaxine affect the noradrenergic system but have also other targets. Some drugs have multiple targets such as trazodone (serotonin, histamine, and α1-adrenergic receptors, serotonin reuptake) and olanzapine (dopamine, serotonin, α1-adrenergic, muscarinic receptors). The mode of action of olanzapine is not completely clear and it may possibly act through the dopaminergic, serotonergic or cholinergic pathways. Currently there are no selective serotonin-noradrenaline re-uptake inhibitors (SNRI) targeting the noradrenaline system on the market.
Table 1.
Summary of neurotransmitter system deficits in FTLD.
| Neurotransmitter pathway | Deficit | References |
|---|---|---|
| Dopamine | ||
| Dopaminergic neurons | Reduced number of neurons in nigrostriatal pathway, low dopamine levels | [67] |
| Dopaminergic receptors | Reduced levels of D2 dopamine receptors | [12] |
| Serotonin | ||
| Serotonergic neurons | Loss of neurons and tau deposition in raphe nucleus | [77] |
| Serotonergic receptors | Number of receptors reduced in midbrain, frontal and temporal lobes | [75] |
| Glutamate | ||
| Glutamatergic neurons | Neurons lost in the thalamus, frontal and temporal cortex, reduced glutamate levels in frontal and temporal lobes | [60] |
| Glutamatergic receptors | AMPA and NMDA receptor levels decreased in frontal and temporal lobes, metabotropic glutamate receptors reduced in cerebral cortex, basal ganglia and thalamus | [56, 119] |
| GABA | ||
| GABAergic neurons | Neurons reduced in frontal and temporal lobes | [57, 60] |
| GABAergic receptors | No evidence available of deficits in receptors | |
| Noradrenaline | ||
| Noradrenergic neurons | No clear evidence for neuronal loss, minimal loss observed in locus coeruleus | [77] |
| Noradrenergic receptors | No evidence for chance in noradrenergic receptors | [76] |
| Acetylcholine | ||
| Cholinergic neurons | Reduced cholinergic neurons in the nucleus basalis, no evidence of neuronal loss in cerebral cortex | [83] |
| Cholinergic receptors | Conflicting results | [130, 131] |
Glutamatergic system
Glutamate is the main excitatory neurotransmitter that acts through ionotropic glutamate receptors (NMDA, AMPA and kainate) and G protein-coupled metabotropic glutamate receptors [58]. Glutamate is crucial for memory formation, higher cognitive functions and regulation of NMDA receptor-mediated hippocampal long-term potentiation. Several NMDA receptor antagonists impair cognitive functions and may cause hallucinations. In contrast, excess glutamate may cause excitotoxic neuronal death and enhanced neurodegeneration. There are preclinical and clinical evidence for glutamatergic dysfunction in FTLD. Glutamate-mediated excitotoxicity is a possible disease-driving mechanism in AD and might also contribute to FTLD pathogenesis including that related to e.g., C9-HRE [59]. The number of glutamatergic neurons is decreased in the frontotemporal area and thalamus [60], and both ionotropic (NMDA and AMPA) and metabotropic glutamate receptors are reduced in FTLD spectrum patients. In addition, impairment of the glutamatergic neurotransmitter system is a typical finding in transcranial magnetic stimulation (TMS) measurements in FTLD patients [57, 61]. FTLD patients may display an abnormal composition of the AMPA receptor, as a reduction of GluA3 subunit-containing AMPA receptors has been detected in FTLD patients due to the formation of anti-GluA3 antibodies [62]. These patients often showed the clinical phenotype of bvFTD and an earlier onset of the disease. This is interesting because the lack of these AMPA receptor subtypes is known to result in increased dopamine levels in the striatum, reduced serotonin turnover in the olfactory bulb and aggressive behaviour in mice [63]. These results point to an association between AMPA receptors and the dopaminergic and serotonergic networks, which may be mechanistically important in FTLD pathogenesis.
GABAergic system
Gamma-aminobutyric acid (GABA) is the main inhibitory neurotransmitter, which acts through two receptor subclasses: ionotropic (GABAA) and G protein-coupled GABAergic receptors (GABAB) [64]. GABAergic neurons are important in balancing neuronal circuit function. Thus, dysfunction in the GABAergic system might lead to poor information processing and altered behaviour, such as schizophrenia-like behaviour, motor dysfunction function, altered reproduction, aggressive-defensive behaviours and changes in working memory [65]. There is evidence for GABAergic neuron loss in the frontal and temporal cortex of FTLD patients [60], and the detection of impairment of the GABAergic system has been used in FTLD diagnostics using TMS [57, 61].
Dopaminergic system
Dopamine is synthesized in dopaminergic neurons, which project throughout the brain. Dopamine acts through five known G protein-coupled receptors in the CNS and peripheral neurons [66]. Defects in the dopaminergic system are common in FTLD as there is an evident loss of presynaptic dopaminergic and nigrostriatal neurons [12]. The nigrostriatal pathway is important for movement and motor control and this pathway is degenerated for instance in Parkinson’s disease (PD). In line with this, extrapyramidal symptoms similar to PD are reported in up to 40 % in patients of FTLD spectrum on their first physician consultation. Parkinsonism is common among bvFTD patients, but it can be detected in all FTLD subtypes [67], and in general, up to 50 % of patients show parkinsonism symptoms [68, 69]. It has been postulated that the loss of mesocortical dopaminergic tracts and dopamine receptors in the frontal lobes could contribute to the behavioural symptoms in FTLD [12]. Moreover, dopamine release is involved in eating disorders and responsible for food reward [70]. Binge eating and increased alcohol consumption is a common clinical feature bvFTD, and therefore makes it distinguishable from other forms of dementia [71]. Many patients change their eating habits during disease progression, leading to compulsive overeating.
Serotonergic system
Serotonin (5-HT) is synthesized in the raphe nuclei, which project widely to the brain and spinal cord [72]. It regulates several higher cognitive functions including feeding behaviour, metabolism and energy balance [73]. Seven different serotonin receptor families are known, but due to the subtype-specific genetic polymorphisms and complexity of the serotonergic networks, research in FTLD has mainly focused on 5-HT1A and 5-HT2A receptors [12]. Furthermore, serotonin is an important neuromodulator of other neurotransmitters, and it regulates synaptic plasticity. Clinical signs of serotonergic dysfunction may include behavioural disturbances, apathy, depression, and anxiety [74]. Indeed, reduced serotonergic activity is especially associated with several behavioural symptoms detected among bvFTD patients. For example, post-mortem studies have indicated a reduction of 5-HT1A and 5-HT2A receptors in the frontotemporal area and hypothalamus [75]. Furthermore, despite normal or elevated serotonin levels in the post-mortem analysis, serotonin turnover appears disturbed in bvFTD patients [76]. There is also evidence for the loss of ascending serotonergic pathways and a 40 % reduction of neurons in the raphe nuclei in FTLD [77]. These differences between reduced receptor densities and normal neurotransmitter levels might refer to a functional compensation for the loss of serotonergic neurons at different stages of the disease. Thus, dysfunction of the serotonergic system might contribute to the clinical symptoms of FTLD spectrum diseases by disturbing the neuromodulatory effects of the serotonin network and subsequently affecting other neurotransmitter systems.
Noradrenergic system
Noradrenaline is synthesised in the locus coeruleus in the pons [78]. Noradrenergic pathways originating from locus coeruleus project especially to the forebrain and regulate, for instance, memory, attention, and decision-making but especially alertness and inactivity phase. Noradrenaline acts via G protein-coupled α- and β-adrenergic receptors. These receptors have excitatory or inhibitory effects depending on their synaptic location. For example, presynaptic α2-receptors can enhance the noradrenergic stimulus [79, 80]. Elevated noradrenaline levels decrease impulsivity and reduced noradrenergic transmission is associated with impaired executive function in rats [81]. Thus, due to intricacies of the noradrenaline signalling network, pharmacological interventions might result in complex behavioural outcomes. Despite the likely decreased metabolism and turnover of noradrenaline, there is no clear evidence for the loss of noradrenergic neurons or receptors in bvFTD patients [76]. However, the differences in research methods, brain regions investigated and the interplay among neurotransmitter systems might affect the findings. For example, the loss of serotonergic neurons might result in increased noradrenergic signalling [80].
Cholinergic system
Acetylcholine is mainly synthesized in the nucleus basalis of Meynert and regulates several cognitive functions such as memory, emotion, speech production, attention and motor control [82]. The two main acetylcholine receptor classes, the ion channel-gated nicotinic receptors and the G protein-coupled muscarinic receptors, mediate excitatory or inhibitory inputs depending on the receptor type and its pre- or postsynaptic localisation. The cholinergic pathways do not play a major role in bvFTD [57, 61], but especially in PPA, varying results on the cholinergic receptor loss are reported [83, 84]. However, cholinesterase inhibitors do not improve cognitive function or alleviate other symptoms of FTLD patients [3], and may even reinforce behavioural symptoms [85]. However, the AD drug galantamine has been reported to alleviate language deficits in PPA patients [86].
Functional neuronal network deficits in FTLD patients
Synaptic dysfunction may also be linked to deficits in neuronal network activity and dysconnectivity in core FTLD brain regions. However, such studies in FTLD are so far relatively rare. Intrinsic connectivity networks are temporally synchronous, low frequency fluctuations in the blood oxygen level-dependent signal, which correspond to spatial neural sets activated in task-activation functional magnetic resonance imaging (fMRI) studies [87, 88]. In various neurodegenerative diseases, functional intrinsic connectivity network deficits linked to clinical symptoms have been detected even in the absence of atrophy in the corresponding structural brain regions [89, 90]. Deficits in several intrinsic connectivity networks have also been indicated in FTLD spectrum diseases. Salience network, which has an essential role in the processing of consecutive responses to emotional and sensory stimuli, and which primarily adheres to the anterior insula, dorsal anterior cingulate cortex, amygdala, ventral striatum, and medial thalamus, has been shown to present deficits in different forms of FTLD [91–94]. A study by Lee and colleagues indicated that the behavioural symptoms in both C9-HRE-carrying and sporadic bvFTD patients correlated with deficits in the connectivity in the salience network [88]. Interestingly, the same study reported that sporadic FTLD patients had increased connectivity in the default mode network. This network is responsible for functions that are activated when an individual does not actively concentrate on the surrounding world. Default mode network alterations have also been observed in GRN mutation carriers who have a mood disorder [95]. Moreover, activity of the frontoparietal network, which is important in executive functions, appears to highly differ between GRN mutation-carrying FTLD-patients and healthy controls [96]. Finally, impairment of frontolimbic network, which is pivotal in emotion processing and moderating motivated behaviours, has been detected both in bvFTD and PPA patients and correlate with apathy [92]. Taken together, the observed deficits in the different neurotransmitter systems appear to be generally linked to wide dysfunction of the different functional networks in the brains of FTLD patients and underlie the altered behaviour or other clinical symptoms in these patients.
Current pharmacological treatment strategies for patients suffering from FTLD
Currently, there are no officially approved medicinal therapies for FTLD spectrum diseases in Europe or the US. The treatment of FTLD clinical syndromes is mostly symptomatic and the use of off-label medications is common. These off-label therapies are mostly targeted to neurotransmitter systems to normalize the brain function and unwanted behaviour, but do not target the underlying pathogenic mechanisms nor appreciably affect disease progression. Antidepressants, antipsychotics, antiepileptics, dopaminergic therapies or medications used to treat AD are commonly used to alleviate the behavioural and cognitive symptoms or motor deficits in FTLD spectrum diseases [3]. For ALS patients, riluzole and edaravone are currently the only approved drugs in Europe and the USA. However, they only increase the lifespan of the patients on average by three months [97, 98]. A summary of the current main pharmacological treatment options for patients within the FTLD spectrum is shown in Table 2 and these are discussed in the next chapters in more detail. Furthermore, the different drugs affecting specific neurotransmitter systems are indicated also in Fig. 1.
Table 2.
Current main pharmacological treatment options of FTLD.
| Pharmacological class | Therapeutic intervention | Drug | Effect | Evidence | References |
|---|---|---|---|---|---|
| Antidepressants | Behavioural symptoms | Citalopram Sertraline Fluoxetine Paroxetine Trazodone | May improve behavioural symptoms such as agitation and depression | Case reports and series, open label trials, randomised double-blind trial | [100–104, 132] |
| Antipsychotics | Behavioural symptoms | Quetiapine Risperidone Aripiprazole Olanzapine | May improve behavioural symptoms | Case reports and series, randomised double-blinded cross-over trial, follow-up study | [104–109] |
| Antiepileptics and lithium | Behavioural symptoms | Topiramate Valproic acid Carbamazepine | May improve behavioural symptoms | Case reports and series | [81, 102, 110, 112, 133, 134] |
| Acetylcholinesterase inhibitors | Cognitive symptoms | Donepezil Galantamine Rivastigmine | No improvement, may exacerbate behavioural symptoms | Open label trials, randomised double-blind placebo-controlled trials | [86, 117, 118] |
| NMDA antagonists | Cognitive symptoms, behavioural symptoms | Memantine | No improvement, may exacerbate cognition | Case series, open label trials, randomised double-blind placebo-controlled trials | [120–122, 135] |
| Dopaminergic therapies | Behavioural symptoms, psychotic symptoms | Dextroamphetamine Methylphenidate Selegiline Levodopa | Reduce agitation, may reduce psychotic and depressive symptoms | Randomised double-blinded cross-over trials, open label studies, case reports | [104, 105, 123, 124] |
Antidepressants
Because serotonin deficiency might contribute to the behavioural symptoms in bvFTD, increasing its levels in the brain might alleviate these symptoms. Thus, selective serotonin reuptake inhibitors (SSRI), which increase serotonin levels in the synaptic cleft, could correct the serotonergic imbalance and deficits in bvFTD patients. A recent report also showed that some antidepressants including SSRI mediate their actions by directly binding to the TrkB neurotrophin receptor. This facilitates the synaptic localization of TrkB and activation by its ligand, the brain-derived neurotrophic factor, which promotes neuronal plasticity [99]. Sertraline has shown beneficial effects in alleviating behavioural symptoms in an ALS-FTD patient [100]. In a randomised double-blind controlled crossover trial in 12 bvFTD patients, a 30-mg daily dose of citalopram alleviated the impulsivity and dysfunction of the cortical serotonergic network [101]. Furthermore, citalopram decreased irritability, depression and disinhibition in bvFTD patients in a six-week open-label study [101]. There are also positive reports from the use of fluoxetine and sertraline [76, 77]. Paroxetine has shown good efficacy in small-scale studies but no improvement of the behavioural symptoms of bvFTD patients has been observed in larger controlled studies [102]. Furthermore, trazodone, an atypical antidepressant with antagonism to multiple receptors, was effective in the treatment of irritability, agitation, depression and hyperorality of bvFTD patients in a randomised double-blind placebo-controlled study [103]. However, these potentially beneficial effects would need confirmation in larger randomized clinical trials.
Antipsychotics
Evidence for treating the behavioural symptoms, especially agitation and violent behaviour, of bvFTD patients with antipsychotics is limited. Quetiapine has been shown to decrease agitation in a small-scale study [104], but a double-blinded study with eight bvFTD patients showed no improvement of cognitive nor behavioural symptoms [105]. In a 24-month follow-up study with 17 bvFTD patients, olanzapine decreased the overall behavioural symptoms, delusions and caregiver burden [106]. However, using atypical antipsychotics to treat the behavioural symptoms related to dementia is associated with a higher mortality risk [107]. Although increased mortality is associated with antipsychotics use in older AD patients, there is no or little evidence of this in bvFTD patients. Other severe adverse effects and the overall risk-benefit ratio should be carefully considered before using antipsychotics for the behavioural symptoms of bvFTD. Patients with FTLD spectrum diseases are also known to display extrapyramidal symptoms [67]. Especially patients with bvFTD may show symptoms of parkinsonism [108]. Extrapyramidal symptoms are common adverse effects of antipsychotics, to which the FTLD patients are vulnerable due to the dopaminergic network deficits [109]. Thus, the newer generation of antipsychotics, having less severe extrapyramidal adverse effects, should be preferred and the dosage should be the lowest possible.
Antiepileptics and lithium
Antiepileptics to treat chronic pain and mood stabilisers in bipolar disorder patients have been successfully used [110]. They could be beneficial also for treating FTLD because they stabilise excess neuronal excitation [32, 43, 111]. One of these agents is retigabine, which activates Kv7-potassium channels and reduces neuronal excitation. Retigabine has been shown to increase the survival of human iPSC-induced motor neurons carrying the C9-HRE [24]. Other antiepileptics such as valproic acid and carbamazepine have been reported to reduce behavioural symptoms. Carbamazepine decreased inappropriate sexual behaviour in one bvFTD patient [112] and valproic acid has been shown to decrease agitation [104]. Topiramate is an antiepileptic with a mood-stabilising action but its adverse effects include depression, suicidal behaviour, weight loss and anorexia [113, 114]. It is an AMPA and kainate receptor antagonist and has been used for binge eating disorder. Case reports have shown normalised drinking and eating behaviour and remarkable weight loss with topiramate treatment [113, 114]. As mentioned earlier, the AMPA receptor composition is altered in FTLD and especially bvFTD patients show abnormal eating behaviour [59]. Thus, antagonising AMPA receptor function might alleviate disease symptoms. Finally, lithium is used for the treatment of bipolar disorder and has multiple effects on neuronal function. Lithium might restore the deficits in autophagy in FTLD [115] but there is no clinical evidence for its use in FTLD spectrum diseases.
Medications used in Alzheimer’s disease
AD is the most common neurodegenerative disorder characterised by loss of cholinergic neurons, and subsequent decline of memory and other cognitive functions [116]. Cholinesterase inhibitors rivastigmine, galantamine and donepezil, which increase acetylcholine levels in the brain, are widely used to treat AD patients. Rivastigmine has been reported to alleviate the behavioural symptoms to some extent but without an effect on cognitive abilities of bvFTD patients [117]. Galantamine has not shown any benefit in bvFTD patients but might have positive effects in aphasic PPA patients [86]. Donepezil in fact worsened the behavioural symptoms in a subset of FTLD patients [118], suggesting that these drugs may not be efficacious or suitable in general for symptomatic management of FTLD spectrum diseases. Moreover, theses medicines may not show efficacy since there are no cholinergic neurotransmitter system deficits in FTLD spectrum diseases [61].
The NMDA receptor-mediated excitotoxicity may be linked to FTLD. Memantine, an NMDA receptor antagonist, is used in the treatment of AD [119] but the results in bvFTD patients have not been promising. A six-month open-label study with memantine revealed no benefit for patients with FTLD-related syndromes [120]. A case series and one open-label study showed some benefits of memantine in relieving the behavioural symptoms of bvFTD patients [121] but a randomised placebo-controlled study indicated no clear benefit. In contrast, cognitive performance of bvFTD patients was decreased [122].
Dopaminergic therapies
Because dysfunction of the neuronal dopaminergic network has been reported in FTLD, elevating dopamine levels in specific brain areas might relieve disease symptoms. Dextroamphetamine, an inhibitor of serotonin and dopamine transporters, together with the antipsychotic quetiapine improved the behavioural symptoms and decreased apathy in bvFTD patients compared to control subjects. However, quetiapine alone showed even better efficacy in alleviating these symptoms [105]. Treatment of bvFTD patients with methylphenidate, an inhibitor of dopamine and noradrenaline reuptake, was reported to improve decision-making behaviour in a double-blinded placebo-controlled study [123]. Selegiline, an inhibitor of monoamine oxidase B, which increases dopamine levels in the brain, has been shown to improve decision-making and reduce risk-taking behaviour in bvFTD patients [124] in small randomized, double-blinded cross-over trials. Finally, the dopaminergic drug levodopa might restore the altered dopamine levels and alleviate the behavioural and motor symptoms of bvFTD. However, conclusive evidence favouring the use of levodopa for FTLD spectrum diseases is still lacking and only patients with parkinsonism have been shown to benefit from dopaminergic pharmacological intervention [104].
Limitations of current drugs and future directions
In summary, current therapies available for the treatment of FTLD spectrum diseases are mainly off-label therapies, aimed especially for symptomatic treatment of the behavioural disturbances associated with bvFTD. There are currently no pharmacological agents for the treatment of the speech deficits in PPA patients to complement speech therapy and counselling. Furthermore, there are no specific therapies for different genetic forms of FTLD. The efficacy of current pharmacological treatments is often unclear and limited due to the small scale and lack of randomisation in clinical studies. Moreover, the relative rarity of the FTLD spectrum diseases that limits the number of participating patients, the use of heterogeneous patient cohorts with different subtypes of FTLD, and variable clinical classification criteria might affect the study outcomes. However, the patients carrying C9-HRE, MAPT or GRN mutations can be identified by genetic testing. Thus, the genetic or clinical FTLD subtype should be taken into account when selecting patients for future clinical trials.
Even though FTLD comprises a group of neurodegenerative disorders partially affecting the same brain areas and neurotransmitter systems as AD, medications used for AD patients have shown few benefits or even have worsened the outcomes in FTLD clinical syndromes. Moreover, these studies have often been under-powered and used open-label drugs. Some SSRI have shown promising effects in small-scale randomised double-blind controlled studies, open-label studies and case reports, prompting further randomised placebo-controlled trials in clinically and genetically defined bvFTD patient groups. In addition, well-controlled studies are needed to assess the risk-benefit ratio of the initial promising effects of antiepileptics in the treatment of behavioural symptoms. The extrapyramidal adverse effects of antipsychotics can be difficult, and their pharmacokinetic interactions are common, which may complicate their potential. The age-dependent physiological changes affecting the pharmacokinetic properties and active metabolite formation of these drugs might contribute to the treatment efficacy, but so far these have not been studied in FTLD spectrum diseases [125].
In addition to therapeutics targeting the neurotransmitter systems, emerging therapeutic options directly targeting the fundamental molecular mechanisms of the disease such as those related to the C9-HRE or other genetic alterations by antisense oligonucleotides, micro-RNAs or small molecules have been reported [126–129]. These have been discussed in several recent reviews and will therefore not be considered here. However, an approach directly targeting the molecular mechanisms of the genetic forms of FTLD would be a major breakthrough if the treatment could be started early enough, e.g., in presymptomatic patients. Moreover, such disease-modifying therapies in combination with current symptomatic treatments that target specific neurotransmitter systems, might show benefits later during the disease. On the other hand, specific molecular mechanisms underpinning the disease may be more difficult to identify in patients who do not carry any known disease-causing genetic mutations. All in all, on the way to potential personalised treatment options at different phases of the disease, further studies are needed to verify the safety and the therapeutic outcomes of the current and future therapies in FTLD with different genetic backgrounds and clinical manifestations.
Acknowledgements
This study has been supported by the grants of Academy of Finland [nos. 315459 (AH) and 315460 (AMR)]; Sigrid Jusélius Foundation (AH and ES), Finnish Brain Foundation (ES), Instrumentarium Science Foundation (ES), Orion Research Foundation (ES), ALS tutkimuksen tuki ry. registered association (NH), and Esko Laukala and Eeva Kehus-Laukala fund to support well-performing pharmacy students (SK). NH, SL, and HR are or have been PhD students in the Doctoral Program of Molecular Medicine (DPMM) and GenomMed (HR) of the University of Eastern Finland (Kuopio, Finland). This publication is part of a project that has received funding from the European Union’s Horizon 2020 research and innovation programme under the Marie Skłodowska-Curie grant agreement No 740264. This study is also part of the research activities of the Finnish FTD Research Network (FinFTD).
Author contributions
NH, conceptualization, writing original draft, visualization, review, editing, revision; SK, conceptualization, writing original draft; DH, review, editing; SL, review, editing; HR, review, editing; AMR, review, editing, funding acquisition; PH, conceptualization, review, editing; ES conceptualization, writing original draft, review, editing, revision; AH, conceptualization, writing original draft, review, editing, revision, supervision, funding acquisition.
Funding
Open access funding provided by University of Eastern Finland (UEF) including Kuopio University Hospital.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
These authors contributed equally: Nadine Huber, Sonja Korhonen.
References
- 1.Rascovsky K, Hodges JR, Knopman D, Mendez MF, Kramer JH, Neuhaus J, et al. Sensitivity of revised diagnostic criteria for the behavioural variant of frontotemporal dementia. A J Neurol. 2011;143:2456–77. doi: 10.1093/brain/awr179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Gorno-Tempini ML, Hillis AE, Weintraub S, Kertesz A, Mendez M, Cappa SF, et al. Classification of primary progressive aphasia and its variants. Neurology. 2011;76:1006–14. doi: 10.1212/WNL.0b013e31821103e6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Tsai RM, Boxer AL. Treatment of frontotemporal dementia. Curr Treat Options Neurol. 2014;16:1–14.. doi: 10.1007/s11940-014-0319-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Espay AJ, Litvan I. Parkinsonism and frontotemporal dementia: the clinical overlap. J Mol Neurosci. 2011;45:343–9. doi: 10.1007/s12031-011-9632-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Solje E, Hartikainen P, Valori M, Vanninen R, Tiihonen J, Hakola P, et al. The C9ORF72 expansion does not affect the phenotype in Nasu-Hakola disease with the DAP12 mutation. Neurobiol Aging. 2014;35:1780.e13–1780.e17. doi: 10.1016/j.neurobiolaging.2014.01.149. [DOI] [PubMed] [Google Scholar]
- 6.Bang J, Spina S, Miller BL. Frontotemporal dementia. Lancet. 2015;386:1672–82. doi: 10.1016/S0140-6736(15)00461-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Irwin DJ, Cairns NJ, Grossman M, McMillan CT, Lee EB, Van Deerlin VM, et al. Frontotemporal lobar degeneration: defining phenotypic diversity through personalized medicine. Acta Neuropathol. 2015;129:469–91. doi: 10.1007/s00401-014-1380-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Majounie E, Renton AE, Mok K, Dopper EGP, Waite A, Rollinson S, et al. Frequency of the C9orf72 hexanucleotide repeat expansion in patients with amyotrophic lateral sclerosis and frontotemporal dementia: a cross-sectional study. Lancet Neurol. 2012;11:323–30. doi: 10.1016/S1474-4422(12)70043-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Renton AE, Majounie E, Waite A, Simón-Sánchez J, Rollinson S, Gibbs JR, et al. A hexanucleotide repeat expansion in C9ORF72 is the cause of chromosome 9p21-linked ALS-FTD. Neuron. 2011;72:257–68. doi: 10.1016/j.neuron.2011.09.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.DeJesus-Hernandez M, Mackenzie IR, Boeve BF, Boxer AL, Baker M, Rutherford NJ, et al. Expanded GGGGCC hexanucleotide repeat in non-coding region of C9ORF72 causes chromosome 9p-linked frontotemporal dementia and amyotrophic lateral sclerosis. Neuron. 2011;72:245–56. doi: 10.1016/j.neuron.2011.09.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Murray ME, Kouri N, Lin WL, Jack CR, Dickson DW, Vemuri P. Clinicopathologic assessment and imaging of tauopathies in neurodegenerative dementias. Alzheimer’s Res Ther. 2014;6:1. doi: 10.1186/alzrt231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Murley AG, Rowe JB. Neurotransmitter deficits from fronto temporal lobar degeneration. Brain. 2018;141:1263–85. doi: 10.1093/brain/awx327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Pottier C, Ravenscroft TA, Sanchez-Contreras M, Rademakers R. Genetics of FTLD: overview and what else we can expect from genetic studies. J Neurochem. 2016;138(Suppl 1):32–53. doi: 10.1111/jnc.13622. [DOI] [PubMed] [Google Scholar]
- 14.Greaves CV, Rohrer JD. An update on genetic frontotemporal dementia. J Neurol. 2019;266:2075–86. doi: 10.1007/s00415-019-09363-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Haapasalo A, Remes AM. Genetic and molecular aspects of frontotemporal lobar degeneration. Curr Genet Med Rep. 2015;3:8–18. doi: 10.1007/s40142-014-0063-5. [DOI] [Google Scholar]
- 16.Kaivorinne A-L, Bode MK, Paavola L, Tuominen H, Kallio M, Renton AE, et al. Clinical characteristics of C9ORF72-linked frontotemporal lobar degeneration. Dement Geriatr Cogn Dis Extra. 2013;3:251–62. doi: 10.1159/000351859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Mori K, Weng SM, Arzberger T, May S, Rentzsch K, Kremmer E, et al. The C9orf72 GGGGCC repeat is translated into aggregating dipeptide-repeat proteins in FTLD/ALS. Science (80-) 2013;339:1335–8. doi: 10.1126/science.1232927. [DOI] [PubMed] [Google Scholar]
- 18.Gendron TF, Bieniek KF, Zhang YJ, Jansen-West K, Ash PEA, Caulfield T, et al. Antisense transcripts of the expanded C9ORF72 hexanucleotide repeat form nuclear RNA foci and undergo repeat-associated non-ATG translation in c9FTD/ALS. Acta Neuropathol. 2013;126:829–44. doi: 10.1007/s00401-013-1192-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Frick P, Sellier C, Mackenzie IRA, Cheng CY, Tahraoui-Bories J, Martinat C, et al. Novel antibodies reveal presynaptic localization of C9orf72 protein and reduced protein levels in C9orf72 mutation carriers. Acta Neuropathol Commun. 2018;6:72. doi: 10.1186/s40478-018-0579-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Fratta P, Poulter M, Lashley T, Rohrer JD, Polke JM, Beck J, et al. Homozygosity for the C9orf72 GGGGCC repeat expansion in frontotemporal dementia. Acta Neuropathol. 2013;126:401–9. doi: 10.1007/s00401-013-1147-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.O’Rourke JG, Bogdanik L, Yáñez A, Lall D, Wolf AJ, Muhammad AKMG, et al. C9orf72 is required for proper macrophage and microglial function in mice. Science (80-) 2016;351:1324–9. doi: 10.1126/science.aaf1064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Atanasio A, Decman V, White D, Ramos M, Ikiz B, Lee HC, et al. C9orf72 ablation causes immune dysregulation characterized by leukocyte expansion, autoantibody production, and glomerulonephropathy in mice. Sci Rep. 2016;6:23204. doi: 10.1038/srep23204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Koppers M, Blokhuis AM, Westeneng HJ, Terpstra ML, Zundel CAC, Vieira De Sá R, et al. C9orf72 ablation in mice does not cause motor neuron degeneration or motor deficits. Ann Neurol. 2015;78:426–38. doi: 10.1002/ana.24453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Shi Y, Lin S, Staats KA, Li Y, Chang WH, Hung ST, et al. Haploinsufficiency leads to neurodegeneration in C9ORF72 ALS/FTD human induced motor neurons. Nat Med. 2018;24:313–25. doi: 10.1038/nm.4490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Sellier C, Campanari M, Julie Corbier C, Gaucherot A, Kolb‐Cheynel I, Oulad‐Abdelghani M, et al. Loss of C9ORF72 impairs autophagy and synergizes with polyQ Ataxin‐2 to induce motor neuron dysfunction and cell death. EMBO J. 2016;35:1276–97. doi: 10.15252/embj.201593350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Starr A, Sattler R. Synaptic dysfunction and altered excitability in C9ORF72 ALS/FTD. Brain Res. 2018;1693:98–108. doi: 10.1016/j.brainres.2018.02.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Iacoangeli A, Tiedge H. Translational control at the synapse: role of RNA regulators. Trends Biochem Sci. 2013;38:47–55. doi: 10.1016/j.tibs.2012.11.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Burguete AS, Almeida S, Gao F-B, Kalb R, Akins MR, Bonini NM. GGGGCC microsatellite RNA is neuritically localized, induces branching defects, and perturbs transport granule function. Elife. 2015;4:e08881. doi: 10.7554/eLife.08881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Perry S, Han Y, Das A, Dickman D. Homeostatic plasticity can be induced and expressed to restore synaptic strength at neuromuscular junctions undergoing ALS-related degeneration. Hum Mol Genet. 2017;26:4153–67. doi: 10.1093/hmg/ddx304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.May S, Hornburg D, Schludi MH, Arzberger T, Rentzsch K, Schwenk BM, et al. C9orf72 FTLD/ALS-associated Gly-Ala dipeptide repeat proteins cause neuronal toxicity and Unc119 sequestration. Acta Neuropathol. 2014;128:485–503. doi: 10.1007/s00401-014-1329-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Jensen BK, Schuldi MH, McAvoy K, Russell KA, Boehringer A, Curran BM, et al. Synaptic dysfunction induced by glycine‐alanine dipeptides in C9orf72‐ ALS / FTD is rescued by SV 2 replenishment. EMBO Mol Med. 2020;12:e10722. doi: 10.15252/emmm.201910722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Dafinca R, Barbagallo P, Farrimond L, Candalija A, Scaber J, Ababneh NA, et al. Impairment of mitochondrial calcium buffering links mutations in C9ORF72 and TARDBP in iPS-derived motor neurons from patients with ALS/FTD. Stem Cell Rep. 2020;14:892–908. doi: 10.1016/j.stemcr.2020.03.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Devlin A-C, Burr K, Borooah S, Foster JD, Cleary EM, Geti I, et al. Human iPSC-derived motoneurons harbouring TARDBP or C9ORF72 ALS mutations are dysfunctional despite maintaining viability. Nat Commun. 2015;6:1–12.. doi: 10.1038/ncomms6999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Henley JM, Wilkinson KA. Synaptic AMPA receptor composition in development, plasticity and disease. Nat Rev Neurosci. 2016;17:337–50. doi: 10.1038/nrn.2016.37. [DOI] [PubMed] [Google Scholar]
- 35.Hideyama T, Yamashita T, Suzuki T, Tsuji S, Higuchi M, Seeburg PH, et al. Induced loss of ADAR2 engenders slow death of motor neurons from Q/R site-unedited GluR2. J Neurosci. 2010;30:11917–25. doi: 10.1523/JNEUROSCI.2021-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Donnelly CJ, Zhang P-W, Pham JT, Haeusler AR, Heusler AR, Mistry NA, et al. RNA toxicity from the ALS/FTD C9ORF72 expansion is mitigated by antisense intervention. Neuron. 2013;80:415–28. doi: 10.1016/j.neuron.2013.10.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Xu W, Xu J. C9orf72 dipeptide repeats cause selective neurodegeneration and cell-autonomous excitotoxicity in Drosophila glutamatergic neurons. J Neurosci. 2018;38:7741–52. doi: 10.1523/JNEUROSCI.0908-18.2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Perkins EM, Burr K, Banerjee P, Mehta AR, Dando O, Selvaraj BT, et al. Altered network properties in C9ORF72 repeat expansion cortical neurons are due to synaptic dysfunction. Mol Neurodegener. 2021;16:13. doi: 10.1186/s13024-021-00433-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Chen-Plotkin AS, Martinez-Lage M, Sleiman PMA, Hu W, Greene R, Wood EMC, et al. Genetic and clinical features of progranulin-associated frontotemporal lobar degeneration. Arch Neurol. 2011;68:488–97. doi: 10.1001/archneurol.2011.53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Eriksen JL, Mackenzie IRA. Progranulin: normal function and role in neurodegeneration. J Neurochem. 2008;104:287–97. doi: 10.1111/j.1471-4159.2007.04968.x. [DOI] [PubMed] [Google Scholar]
- 41.Petkau TL, Neal SJ, Milnerwood A, Mew A, Hill AM, Orban P, et al. Synaptic dysfunction in progranulin-deficient mice. Neurobiol Dis. 2012;45:711–22. doi: 10.1016/j.nbd.2011.10.016. [DOI] [PubMed] [Google Scholar]
- 42.Longhena F, Zaltieri M, Grigoletto J, Faustini G, La Via L, Ghidoni R, et al. Depletion of progranulin reduces GluN2B-containing NMDA receptor density, tau phosphorylation, and dendritic arborization in mouse primary cortical neurons. J Pharm Exp Ther. 2017;363:164–75. doi: 10.1124/jpet.117.242164. [DOI] [PubMed] [Google Scholar]
- 43.Huang M, Modeste E, Dammer E, Merino P, Taylor G, Duong DM, et al. Network analysis of the progranulin-deficient mouse brain proteome reveals pathogenic mechanisms shared in human frontotemporal dementia caused by GRN mutations. Acta Neuropathol Commun. 2020;8:163. doi: 10.1186/s40478-020-01037-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Lui H, Zhang J, Makinson SR, Cahill MK, Kelley KW, Huang HY, et al. Progranulin deficiency promotes circuit-specific synaptic pruning by microglia via complement activation. Cell. 2016;165:921–35. doi: 10.1016/j.cell.2016.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Tapia L, Milnerwood A, Guo A, Mills F, Yoshida E, Vasuta C, et al. Progranulin deficiency decreases gross neural connectivity but enhances transmission at individual synapses. J Neurosci. 2011;31:11126–32. doi: 10.1523/JNEUROSCI.6244-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Foster NL, Wilhelmsen K, Sima AAF, Jones MZ, D’Amato CJ, Gilman S. Frontotemporal dementia and parkinsonism linked to chromosome 17: a consensus conference. Ann Neurol. 1997;41:706–15. doi: 10.1002/ana.410410606. [DOI] [PubMed] [Google Scholar]
- 47.Buée L, Delacourte A. Comparative biochemistry of tau in progressive supranuclear palsy, corticobasal degeneration, FTDP-17 and Pick’s disease. Brain Pathol. 1999;9:681–93. doi: 10.1111/j.1750-3639.1999.tb00550.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Dickson DW, Kouri N, Murray ME, Josephs KA. Neuropathology of frontotemporal lobar degeneration-tau (FTLD-tau) J Mol Neurosci. 2011;45:384–9. doi: 10.1007/s12031-011-9589-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Avila J, Lucas JJ, Pérez M, Hernández F. Role of tau protein in both physiological and pathological conditions. Physiol Rev. 2004;84:361–84. doi: 10.1152/physrev.00024.2003. [DOI] [PubMed] [Google Scholar]
- 50.Dixit R, Ross JL, Goldman YE, Holzbaur ELF. Differential regulation of dynein and kinesin motor proteins by tau. Science (80-) 2008;319:1086–9. doi: 10.1126/science.1152993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Fulga TA, Elson-Schwab I, Khurana V, Steinhilb ML, Spires TL, Hyman BT, et al. Abnormal bundling and accumulation of F-actin mediates tau-induced neuronal degeneration in vivo. Nat Cell Biol. 2007;9:139–48. doi: 10.1038/ncb1528. [DOI] [PubMed] [Google Scholar]
- 52.Yoshiyama Y, Higuchi M, Zhang B, Huang SM, Iwata N, Saido TCC, et al. Synapse loss and microglial activation precede tangles in a P301S tauopathy mouse model. Neuron. 2007;53:337–51. doi: 10.1016/j.neuron.2007.01.010. [DOI] [PubMed] [Google Scholar]
- 53.Hoover BR, Reed MN, Su J, Penrod RD, Kotilinek LA, Grant MK, et al. Tau mislocalization to dendritic spines mediates synaptic dysfunction independently of neurodegeneration. Neuron. 2010;68:1067–81. doi: 10.1016/j.neuron.2010.11.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Vogel JW, Iturria-Medina Y, Strandberg OT, Smith R, Levitis E, Evans AC, et al. Spread of pathological tau proteins through communicating neurons in human Alzheimer’s disease. Nat Commun. 2020;11:1–15.. doi: 10.1038/s41467-019-13993-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Mudher A, Colin M, Dujardin S, Medina M, Dewachter I, Alavi Naini SM, et al. What is the evidence that tau pathology spreads through prion-like propagation? Acta Neuropathol Commun. 2017;5:1–20.. doi: 10.1186/s40478-016-0408-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Kim E-J, Hwang J-HL, Gaus SE, Nana AL, Deng J, Brown JA, et al. Evidence of corticofugal tau spreading in patients with frontotemporal dementia. Acta Neuropathol. 2020;139:27. doi: 10.1007/s00401-019-02075-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Benussi A, Di Lorenzo F, Dell’Era V, Cosseddu M, Alberici A, Caratozzolo S, et al. Transcranial magnetic stimulation distinguishes Alzheimer disease from frontotemporal dementia. Neurology. 2017;89:665–72. doi: 10.1212/WNL.0000000000004232. [DOI] [PubMed] [Google Scholar]
- 58.Niciu MJ, Kelmendi B, Sanacora G. Overview of glutamatergic neurotransmission in the nervous system. Pharm Biochem Behav. 2012;100:656–64. doi: 10.1016/j.pbb.2011.08.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Benussi A, Alberici A, Buratti E, Ghidoni R, Gardoni F, Di Luca M, et al. Toward a glutamate hypothesis of frontotemporal dementia. Front Neurosci. 2019;13:1–9. doi: 10.3389/fnins.2019.00304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Ferrer I. Neurons and their dendrites in frontotemporal dementia. Dement Geriatr Cogn Disord. 1999;10:55–60. doi: 10.1159/000051214. [DOI] [PubMed] [Google Scholar]
- 61.Padovani A, Benussi A, Cantoni V, Dell’Era V, Cotelli MS, Caratozzolo S, et al. Diagnosis of mild cognitive impairment due to Alzheimer’s disease with transcranial magnetic stimulation. J Alzheimer’s Dis. 2018;65:221–30. doi: 10.3233/JAD-180293. [DOI] [PubMed] [Google Scholar]
- 62.Borroni B, Stanic J, Verpelli C, Mellone M, Bonomi E, Alberici A, et al. Anti-AMPA GluA3 antibodies in Frontotemporal dementia: a new molecular target. Sci Rep. 2017;7:1–10.. doi: 10.1038/s41598-017-06117-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Adamczyk A, Mejias R, Takamiya K, Yocum J, Krasnova IN, Calderon J, et al. GluA3-deficiency in mice is associated with increased social and aggressive behavior and elevated dopamine in striatum. Behav Brain Res. 2012;229:265–72. doi: 10.1016/j.bbr.2012.01.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Wu C, Sun D. GABA receptors in brain development, function, and injury. Metab Brain Dis. 2015;30:367–79. doi: 10.1007/s11011-014-9560-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Paredes RG, Ågmo A. GABA and behavior: the role of receptor subtypes. Neurosci Biobehav Rev. 1992;16:145–70. doi: 10.1016/S0149-7634(05)80177-0. [DOI] [PubMed] [Google Scholar]
- 66.Klein MO, Battagello DS, Cardoso AR, Hauser DN, Bittencourt JC, Correa RG. Dopamine: functions, signaling, and association with neurological diseases. Cell Mol Neurobiol. 2019;39:31–59. doi: 10.1007/s10571-018-0632-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Rinne JO, Laine M, Kaasinen V, Norvasuo-Heilä MK, Någren K, Helenius H. Striatal dopamine transporter and extrapyramidal symptoms in frontotemporal dementia. Neurology. 2002;58:1489–93. doi: 10.1212/WNL.58.10.1489. [DOI] [PubMed] [Google Scholar]
- 68.Siuda J, Fujioka S, Wszolek ZK. Parkinsonian syndrome in familial frontotemporal dementia. Park Relat Disord. 2014;20:957–64. doi: 10.1016/j.parkreldis.2014.06.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Park HK, Chung SJ. New perspective on parkinsonism in frontotemporal lobar degeneration. J Mov Disord. 2013;6:1–8. doi: 10.14802/jmd.13001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Broft A, Shingleton R, Kaufman J, Liu F, Kumar D, Slifstein M, et al. Striatal dopamine in bulimia nervosa: a PET imaging study. Int J Eat Disord. 2012;45:648–56. doi: 10.1002/eat.20984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Ahmed RM, Irish M, Kam J, Van Keizerswaard J, Bartley L, Samaras K, et al. Quantifying the eating abnormalities in frontotemporal dementia. JAMA Neurol. 2014;71:1540–6. doi: 10.1001/jamaneurol.2014.1931. [DOI] [PubMed] [Google Scholar]
- 72.Ciranna L. Serotonin as a modulator of glutamate- and GABA-mediated neurotransmission: implications in physiological functions and in pathology. Curr Neuropharmacol. 2006;4:101–14. doi: 10.2174/157015906776359540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Donovan MH, Tecott LH. Serotonin and the regulation of mammalian energy balance. Front Neurosci. 2013;7:36. doi: 10.3389/fnins.2013.00036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Maillet A, Krack P, Lhommée E, Météreau E, Klinger H, Favre E, et al. The prominent role of serotonergic degeneration in apathy, anxiety and depression in de novo Parkinson’s disease. Brain. 2016;139:2486–502. doi: 10.1093/brain/aww162. [DOI] [PubMed] [Google Scholar]
- 75.Bowen DM, Procter AW, Mann DMA, Snowden JS, Esiri MM, Neary D, et al. Imbalance of a serotonergic system in frontotemporal dementia: Implication for pharmacotherapy. Psychopharmacol (Berl) 2008;196:603–10. doi: 10.1007/s00213-007-0992-8. [DOI] [PubMed] [Google Scholar]
- 76.Vermeiren Y, Janssens J, Aerts T, Martin JJ, Sieben A, Van Dam D, et al. Brain serotonergic and noradrenergic deficiencies in behavioral variant frontotemporal dementia compared to early-onset Alzheimer’s disease. J Alzheimer’s Dis. 2016;53:1079–96. doi: 10.3233/JAD-160320. [DOI] [PubMed] [Google Scholar]
- 77.Yang Y, Schmitt HP. Frontotemporal dementia: evidence for impairment of ascending serotoninergic but not noradrenergic innervation. Immunocytochemical and quantitative study using a graph method. Acta Neuropathol. 2001;101:256–70. doi: 10.1007/s004010000293. [DOI] [PubMed] [Google Scholar]
- 78.Schwarz LA, Luo L. Organization of the locus coeruleus-norepinephrine system. Curr Biol. 2015;25:R1051–R1056.. doi: 10.1016/j.cub.2015.09.039. [DOI] [PubMed] [Google Scholar]
- 79.Xing B, Li YC, Gao WJ. Norepinephrine versus dopamine and their interaction in modulating synaptic function in the prefrontal cortex. Brain Res. 2016;1641:217–33. doi: 10.1016/j.brainres.2016.01.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Gannon M, Che P, Chen Y, Jiao K, Roberson ED, Wang Q. Noradrenergic dysfunction in Alzheimer’s disease. Front Neurosci. 2015;9:220. doi: 10.3389/fnins.2015.00220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Chandler DJ, Gao WJ, Waterhouse BD. Heterogeneous organization of the locus coeruleus projections to prefrontal and motor cortices. Proc Natl Acad Sci USA. 2014;111:6816–21. doi: 10.1073/pnas.1320827111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Picciotto MR. Acetylcholine as a neuromodulator. Neuron. 2013;76:116–29. doi: 10.1016/j.neuron.2012.08.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Teipel S, Raiser T, Riedl L, Riederer I, Schroeter ML, Bisenius S, et al. Atrophy and structural covariance of the cholinergic basal forebrain in primary progressive aphasia. Cortex. 2016;83:124–35. doi: 10.1016/j.cortex.2016.07.004. [DOI] [PubMed] [Google Scholar]
- 84.Nelissen N, Bormans G, Vandenberghe R, Dupont P, Vandenberghe R, Vandenberghe R. Cholinergic depletion and basal forebrain volume in primary progressive aphasia. NeuroImage Clin. 2017;13:271–9. doi: 10.1016/j.nicl.2016.11.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.López-Álvarez J, Sevilla-Llewellyn-Jones J, Agüera-Ortiz L. Anticholinergic drugs in geriatric psychopharmacology. Front Neurosci. 2019;13:1309. doi: 10.3389/fnins.2019.01309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Kertesz A, Morlog D, Light M, Blair M, Davidson W, Jesso S, et al. Galantamine in frontotemporal dementia and primary progressive aphasia. Dement Geriatr Cogn Disord. 2008;25:178–85. doi: 10.1159/000113034. [DOI] [PubMed] [Google Scholar]
- 87.Smith SM, Fox PT, Miller KL, Glahn DC, Fox PM, Mackay CE, et al. Correspondence of the brain’s functional architecture during activation and rest. Proc Natl Acad Sci USA. 2009;106:13040–5. doi: 10.1073/pnas.0905267106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Lee SE, Khazenzon AM, Trujillo AJ, Guo CC, Yokoyama JS, Sha SJ, et al. Altered network connectivity in frontotemporal dementia with C9orf72 hexanucleotide repeat expansion. Brain. 2014;137:3047–60. doi: 10.1093/brain/awu248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Dopper EGP, Rombouts SARB, Jiskoot LC, Heijer T Den, De Graaf JRA, De Koning I, et al. Structural and functional brain connectivity in presymptomatic familial frontotemporal dementia. Neurology. 2014;83:e19–26. doi: 10.1212/WNL.0000000000000583. [DOI] [PubMed] [Google Scholar]
- 90.Seeley WW, Crawford RK, Zhou J, Miller BL, Greicius MD. Neurodegenerative diseases target large-scale human brain networks. Neuron. 2009;62:42. doi: 10.1016/j.neuron.2009.03.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Filippi M, Agosta F, Scola E, Canu E, Magnani G, Marcone A, et al. Functional network connectivity in the behavioral variant of frontotemporal dementia. Cortex. 2013;49:2389–401. doi: 10.1016/j.cortex.2012.09.017. [DOI] [PubMed] [Google Scholar]
- 92.Farb NAS, Grady CL, Strother S, Tang-Wai DF, Masellis M, Black S, et al. Abnormal network connectivity in frontotemporal dementia: evidence for prefrontal isolation. Cortex. 2013;49:1856–73. doi: 10.1016/j.cortex.2012.09.008. [DOI] [PubMed] [Google Scholar]
- 93.Whitwell JL, Josephs KA, Avula R, Tosakulwong N, Weigand SD, Senjem ML, et al. Altered functional connectivity in asymptomatic MAPT subjects A comparison to bvFTD. Neurology. 2011;77:866–74. doi: 10.1212/WNL.0b013e31822c61f2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Zhou J, Greicius MD, Gennatas ED, Growdon ME, Jang JY, Rabinovici GD, et al. Divergent network connectivity changes in behavioural variant frontotemporal dementia and Alzheimer’s disease. Brain. 2010;133:1352–67. doi: 10.1093/brain/awq075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Sellami L, Bocchetta M, Masellis M, Cash DM, Dick KM, Van Swieten J, et al. Distinct neuroanatomical correlates of neuropsychiatric symptoms in the three main forms of genetic frontotemporal dementia in the GENFI Cohort. J Alzheimer’s Dis. 2018;65:147–63.. doi: 10.3233/JAD-180053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Premi E, Cauda F, Costa T, Diano M, Gazzina S, Gualeni V, et al. Looking for neuroimaging markers in frontotemporal lobar degeneration clinical trials: a multi-voxel pattern analysis study in granulin disease. J Alzheimer’s Dis. 2016;51:249–62. doi: 10.3233/JAD-150340. [DOI] [PubMed] [Google Scholar]
- 97.Lacomblez L, Bensimon G, Leigh PN, Guillet P, Meininger V. Dose-ranging study of riluzole in amyotrophic lateral sclerosis. Lancet. 1996;347:1425–31. doi: 10.1016/S0140-6736(96)91680-3. [DOI] [PubMed] [Google Scholar]
- 98.Bensimon G, Lacomblez L, Meininger V. A controlled trial of riluzole in amyotrophic lateral sclerosis. N. Engl J Med. 1994;330:585–91. doi: 10.1056/NEJM199403033300901. [DOI] [PubMed] [Google Scholar]
- 99.Casarotto PC, Girych M, Fred SM, Kovaleva V, Moliner R, Enkavi G, et al. Antidepressant drugs act by directly binding to TRKB neurotrophin receptors. Cell. 2021;184:1299–313.e19. doi: 10.1016/j.cell.2021.01.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Anneser JMH, Jox RJ, Borasio GD. Inappropriate sexual behaviour in a case of ALS and FTD: Successful treatment with sertraline. Amyotroph Lateral Scler. 2007;8:189–90. doi: 10.1080/17482960601073543. [DOI] [PubMed] [Google Scholar]
- 101.Hughes LE, Rittman T, Regenthal R, Robbins TW, Rowe JB. Improving response inhibition systems in frontotemporal dementia with citalopram. Brain. 2015;138:1961–75. doi: 10.1093/brain/awv133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Deakin JB, Rahman S, Nestor PJ, Hodges JR, Sahakian BJ. Paroxetine does not improve symptoms and impairs cognition in frontotemporal dementia: a double-blind randomized controlled trial. Psychopharmacol (Berl) 2004;172:400–8. doi: 10.1007/s00213-003-1686-5. [DOI] [PubMed] [Google Scholar]
- 103.Lebert F, Stekke W, Hasenbroekx C, Pasquier F. Frontotemporal dementia: A randomised, controlled trial with trazodone. Dement Geriatr Cogn Disord. 2004;17:355–9. doi: 10.1159/000077171. [DOI] [PubMed] [Google Scholar]
- 104.Chow TW, Mendez MF. Goals in symptomatic pharmacologic management of frontotemporal lobar degeneration. Am J Alzheimers Dis Other Demen. 2002;17:267–72. doi: 10.1177/153331750201700504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Huey ED, Section CN, Garcia C, Grant W, Clinical M, Wassermann EM, et al. Stimulant treatment of frontotemporal dementia in 8 patients. J Clin Psychiatry. 2008;69:1981–2. doi: 10.4088/JCP.v69n1219a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Moretti R, Torre P, Antonello RM, Cazzato G, Griggio S, Bava A. Olanzapine as a treatment of neuropsychiatric disorders of Alzheimer’s disease and other dementias: A 24-month follow-up of 68 patients. Am J Alzheimers Dis Other Demen. 2003;18:205–14. doi: 10.1177/153331750301800410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Steinberg M, Lyketsos CG. Atypical antipsychotic use in patients with dementia: managing safety concern. Am J Psychiatry. 2012;169:900–6. doi: 10.1176/appi.ajp.2012.12030342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Boeve BF, Boylan KB, Graff-Radford NR, Dejesus-Hernandez M, Knopman DS, Pedraza O, et al. Characterization of frontotemporal dementia and/or amyotrophic lateral sclerosis associated with the GGGGCC repeat expansion in C9ORF72. Brain. 2012;135:765–83. doi: 10.1093/brain/aws004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Kerrsens CJ, Pijnenburg YAL. Vulnerability to neuroleptic side effects in frontotemporal dementia. Eur J Neurol. 2008;15:111–2. doi: 10.1111/j.1468-1331.2007.02035.x. [DOI] [PubMed] [Google Scholar]
- 110.Bialer M. Why are antiepileptic drugs used for nonepileptic conditions? Epilepsia. 2012;53:26–33. doi: 10.1111/j.1528-1167.2012.03712.x. [DOI] [PubMed] [Google Scholar]
- 111.Dafinca R, Scaber J, Ababneh N, Lalic T, Weir G, Christian H, et al. C9orf72 hexanucleotide expansions are associated with altered endoplasmic reticulum calcium homeostasis and stress granule formation in induced pluripotent stem cell-derived neurons from patients with amyotrophic lateral sclerosis and frontotemporal demen. Stem Cells. 2016;34:2063–78. doi: 10.1002/stem.2388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Poetter CE, Stewart JT. Treatment of indiscriminate, inappropriate sexual behavior in frontotemporal dementia with carbamazepine. J Clin Psychopharmacol. 2012;32:137–8. doi: 10.1097/JCP.0b013e31823f91b9. [DOI] [PubMed] [Google Scholar]
- 113.Gupta S, Ivfesand PS, Frank BL, Lockwood KL, Keller PL. Topiramate in bipolar and schizoaffective disorders: Weight loss and efficacy. Prim Care Companion J Clin Psychiatry. 2000;2:96–100. doi: 10.4088/PCC.v02n0304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Minton GC, Miller AD, Bookstaver PB, Love BL. Topiramate: safety and efficacy of its use in the prevention and treatment of migraine. J Cent Nerv Syst Dis. 2011;3:JCNSD.S4365. doi: 10.4137/JCNSD.S4365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Limanaqi F, Biagioni F, Ryskalin L, Busceti CL, Fornai F. Molecular mechanisms linking ALS/FTD and psychiatric disorders, the potential effects of lithium. Front Cell Neurosci. 2019;13:1–10.. doi: 10.3389/fncel.2019.00450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Hampel H, Mesulam MM, Cuello AC, Farlow MR, Giacobini E, Grossberg GT, et al. The cholinergic system in the pathophysiology and treatment of Alzheimer’s disease. Brain. 2018;141:1917–33. doi: 10.1093/brain/awy132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Moretti R, Torre P, Antonello RM, Cattaruzza T, Cazzato G, Bava A. Rivastigmine in frontotemporal dementia: an open-label study. Drugs Aging. 2004;21:931–7. doi: 10.2165/00002512-200421140-00003. [DOI] [PubMed] [Google Scholar]
- 118.Mendez MF, Shapira JS, McMurtray A, Licht E. Preliminary findings? Behavioral worsening on donepezil in patients with frontotemporal dementia. Am J Geriatr Psychiatry. 2007;15:84–87. doi: 10.1097/01.JGP.0000231744.69631.33. [DOI] [PubMed] [Google Scholar]
- 119.Parsons CG, Stöffler A, Danysz W. Memantine: a NMDA receptor antagonist that improves memory by restoration of homeostasis in the glutamatergic system—too little activation is bad, too much is even worse. Neuropharmacology. 2007;53:699–723. doi: 10.1016/j.neuropharm.2007.07.013. [DOI] [PubMed] [Google Scholar]
- 120.Diehl‐Schmid J, Förstl H, Perneczky R, Pohl C, Kurz A. A 6‐month, open‐label study of memantine in patients with frontotemporal dementia. Int J Geriatr Psychiatry. 2008;23:754–9. doi: 10.1002/gps.1973. [DOI] [PubMed] [Google Scholar]
- 121.Boxer AL, Lipton AM, Womack K, Neuhaus J, Pavlic D, Gandhi A, et al. An open label study of memantine treatment in three subtypes of frontotemporal lobar degeneration. Alzheimer Disord Assoc Disord. 2010;23:211–7. doi: 10.1097/WAD.0b013e318197852f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Boxer AL, Knopman DS, Kaufer DI, Grossman M, Onyike C, Graf-Radford N, et al. Memantine in patients with frontotemporal lobar degeneration: a multicentre, randomised, double-blind, placebo-controlled trial. Lancet Neurol. 2013;12:149–56. doi: 10.1016/S1474-4422(12)70320-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Rahman S, Robbins TW, Hodges JR, Mehta MA, Nestor PJ, Clark L, et al. Methylphenidate (‘Ritalin’) can ameliorate abnormal risk-taking behavior in the frontal variant of frontotemporal dementia. Neuropsychopharmacology. 2006;31:651–8. doi: 10.1038/sj.npp.1300886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Moretti R, Torre P, Antonello RM, Cazzato G, Bava A. Effects of selegiline on fronto-temporal dementia: A neuropsychological evaluation. Int J Geriatr Psychiatry. 2002;17:391–2. doi: 10.1002/gps.602. [DOI] [PubMed] [Google Scholar]
- 125.Marken PA, Stuart, Munro J. Selecting a selective serotonin reuptake inhibitor: Clinically important distinguishing features. Prim Care Companion J Clin Psychiatry. 2000;2:205–10. doi: 10.4088/PCC.v02n0602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Su Z, Zhang Y, Gendron TF, Bauer PO, Chew J, Yang WY, et al. Discovery of a biomarker and lead small molecules to target r(GGGGCC)-associated defects in c9FTD/ALS. Neuron. 2014;83:1043–50. doi: 10.1016/j.neuron.2014.07.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Brown DG, Shorter J, Wobst HJ Emerging small-molecule therapeutic approaches for amyotrophic lateral sclerosis and frontotemporal dementia. Bioorganic Med Chem Lett. 2020;30:126942. [DOI] [PubMed]
- 128.Cappella M, Ciotti C, Cohen-tannoudji M, Biferi MG Gene therapy for ALS — A perspective. Int J Mol Sci. 2019;20:4388. [DOI] [PMC free article] [PubMed]
- 129.Giunta M, Solje E, Gardoni F, Borroni B, Benussi A. Experimental disease-modifying agents for frontotemporal lobar degeneration. J Exp Pharmacol. 2021;13:359–76. doi: 10.2147/JEP.S262352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Reba RC, Gibson R, Molchan S, Sunderland T, Coppola R, Jones DW, et al. The distribution of cerebral muscarinic acetylcholine receptors in vivo in patients with dementia: a controlled study with 123iqnb and single photon emission computed tomography. Arch Neurol. 1991;48:169–76. doi: 10.1001/archneur.1991.00530140061018. [DOI] [PubMed] [Google Scholar]
- 131.Procter AW, Qurne M, Francis PT. Neurochemical features of frontotemporal dementia. Dement Geriatr Cogn Disord. 1999;10:80–84. doi: 10.1159/000051219. [DOI] [PubMed] [Google Scholar]
- 132.Herrmann N, Black SE, Chow T, Cappell J, Tang-Wai DF, Lanctôt KL. Serotonergic function and treatment of behavioral and psychological symptoms of frontotemporal dementia. Am J Geriatr Psychiatry. 2012;20:789–97. doi: 10.1097/JGP.0b013e31823033f3. [DOI] [PubMed] [Google Scholar]
- 133.Wainger BJ, Kiskinis E, Mellin C, Wiskow O, Steve SW, Berry JD, et al. Intrinsic membrane hyperexcitability of ALS patient-derived motor neurons. Cell Rep. 2014;7:1–11. doi: 10.1016/j.celrep.2014.03.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Singam C, Walterfang M, Mocellin R, Evans A, Velakoulis D. Topiramate for abnormal eating behaviour in frontotemporal dementia. Behav Neurol. 2013;27:285–6. doi: 10.1155/2013/547853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Swanberg MM. Memantine for behavioral disturbances in frontotemporal dementia: a case series. Alzheimer Dis Assoc Disord. 2007;21:164–6. doi: 10.1097/WAD.0b013e318047df5d. [DOI] [PubMed] [Google Scholar]