Abstract
Background and Objectives
A quantitative evaluation of the PK of meropenem, a broad-spectrum β-lactam antibiotic, in plasma and interstitial space fluid (ISF) of subcutaneous adipose tissue of obese patients is lacking as of date. The objective of this study was the characterisation of meropenem population pharmacokinetics in plasma and ISF in obese and non-obese patients for identification of adequate dosing regimens via Monte-Carlo simulations.
Methods
We obtained plasma and microdialysate concentrations after administration of meropenem 1000 mg to 15 obese and 15 non-obese surgery patients from a prospective clinical trial. After characterizing plasma- and microdialysis-derived ISF pharmacokinetics via population pharmacokinetic analysis, we simulated thrice-daily (TID) meropenem short-term (0.5 h), prolonged (3.0 h), and continuous infusions. Adequacy of therapy was assessed by the probability of pharmacokinetic/pharmacodynamic (PK/PD) target attainment (PTA) analysis based on time unbound concentrations exceeded minimum inhibitory concentrations (MIC) on treatment day 1 (%fT > MIC) and the sum of PTA weighted by relative frequency of MIC values for infections by pathogens commonly treated with meropenem. To avoid interstitial tissue fluid concentrations below MIC for the entire dosing interval during continuous infusions, a more conservative PK/PD index was selected (%fT > 4 × MIC).
Results
Adjusted body weight (ABW) and calculated creatinine clearance (CLCRCG_ABW) of all patients (body mass index [BMI] = 20.5–81.5 kg/m2) explained a considerable proportion of the between-patient pharmacokinetic variability (15.1–31.0% relative reduction). The ISF:plasma ratio of %fT > MIC was relatively similar for MIC ≤ 2 mg/L but decreased for MIC = 8 mg/L over ABW = 60–120 kg (0.50–0.20). Steady-state concentrations were 2.68 times (95% confidence interval [CI] = 2.11–3.37) higher in plasma than in ISF, supporting PK/PD targets related to four times the MIC during continuous infusions to avoid suspected ISF concentrations constantly below the MIC. A 3000 mg/24 h continuous infusion was sufficient at MIC = 2 mg/L for patients with CLCRCG_ABW ≤ 100 mL/min and ABW < 90 kg, whereas 2000 mg TID prolonged infusions were adequate for those with CLCRCG_ABW ≤ 100 mL/min and ABW > 90 kg. For MIC = 2 mg/L and %fT> MIC = 95, PTA was adequate in patients over the entire investigated range of body mass and renal function using a 6000 mg continuous infusion. A prolonged infusion of meropenem 2000 mg TID was sufficient for MIC ≤ 8 mg/L and all investigated ABW and CLCRCG_ABW when employing the PK/PD target %fT > MIC = 40. Short-term infusions of 1000 mg TID were sufficient for CLCRCG_ABW ≤ 130 mL/min and distributions of MIC values for Escherichia coli, Citrobacter freundii, and Klebsiella pneumoniae but not for Pseudomonas aeruginosa.
Conclusions
This analysis indicated a need for higher doses (≥ 2000 mg) and prolonged infusions (≥ 3 h) for obese and non-obese patients at MIC ≥ 2 mg/L. Higher PTA was achieved with prolonged infusions in obese patients and with continuous infusions in non-obese patients.
Trial Registration
EudraCT: 2012-004383-22.
Supplementary Information
The online version contains supplementary material available at 10.1007/s40262-021-01070-6.
Key Points
| This study aimed to characterize the interstitial tissue fluid and plasma pharmacokinetics of meropenem in obese and non-obese patients followed by evaluation of commonly used dosing regimens via probability of target attainment analysis. |
| Adequacy of pharmacokinetic/pharmacodynamic targets: four times the minimum inhibitory concentration in probability of target attainment analysis for continuous infusions was corroborated in obese and non-obese patients based on lower meropenem interstitial tissue fluid versus plasma exposure. |
| Treatment optimization: higher probability of target attainment of meropenem was achieved with prolonged infusions in obese patients and with continuous infusions in non-obese patients. |
Introduction
Optimizing anti-infective dosing regimens is imperative for therapeutic success and to prevent the development of resistance, especially regarding integral components of the antibiotic armamentarium, such as meropenem. Meropenem is a carbapenem antibiotic with activity against a broad spectrum of Gram-positive and Gram-negative bacteria, including Pseudomonas aeruginosa and extended-spectrum β-lactamase-producing pathogens [1, 2]. Meropenem exhibits time-dependent bactericidal activity, and the pharmacokinetic/pharmacodynamic (PK/PD) parameter best predicting clinical and microbiological outcomes is the fraction of time that unbound (i.e., “free”) meropenem concentrations exceed the minimum inhibitory concentration (MIC) for a given pathogen during the dosing interval (%fT > MIC) [3].
MIC values cover non-species-related (when species information is not available in the clinical setting) and species-related susceptibility and resistance breakpoints of relevant pathogens defined by the European Committee on Antimicrobial Susceptibility Testing (EUCAST), e.g. Enterobacter spp., P. aeruginosa, Neisseria meningitidis [4]. A broad range of time-dependent PK/PD targets linking %fT > MIC to bactericidal activity and clinical cure has been discussed for β-lactams covering %fT > MIC = 40 for bactericidal effect (murine thigh-infection model) to %fT > MIC = 100 (severe bacterial infections in critical illness) [5, 6].
To date, two clinical studies have investigated the pharmacokinetic differences of meropenem in the plasma or serum of obese and non-obese patients [7, 8]. However, these studies lacked data on meropenem exposure at the site of infection, which is mostly outside of the systemic circulation. For example, whether stricter PK/PD targets related to multiples of MIC are needed to avoid concentrations continuously below MIC at the site of infection for continuous infusions is under debate, e.g. in the interstitial space fluid (ISF), where exposure has been reported to be lower than in plasma [9, 10]. Similarly, it is unclear whether the defined PK/PD targets apply to obese patients, who have higher rates of antibiotic therapy failure [11, 12], possibly due to reduced anti-infective exposure in ISF with large body mass [13, 14]. Knowledge of meropenem ISF exposure in obese individuals is urgently needed to answer these questions. Microdialysis is an established tool to measure unbound drug concentrations in ISF of tissues and organs [15].
Wittau et al. [16] integrated meropenem plasma and ISF concentrations obtained from microdialysis in a population pharmacokinetic model followed by probability of target attainment (PTA) analysis as recommended by the European Medicines Agency to support the selection of appropriate dosing regimens [15]. Yet, the low number of patients and lack of a control group compromised the PK/PD analysis and prevented the quantification of the effect of a body size descriptor on meropenem pharmacokinetics.
The current study aimed to investigate (1) whether reported PK/PD targets apply to obese patients by comparing ISF penetration over a wide range of body mass and (2) whether meropenem dosing regimens achieve effective concentrations in obese and non-obese patients and to identify patients at risk for not achieving effective concentrations. For this, data from a controlled clinical trial were analyzed in a population pharmacokinetic model to characterize plasma and ISF pharmacokinetics of meropenem after a single short-term intravenous infusion in obese and non-obese patients. Subsequently, we evaluated six clinically relevant dosing regimens of three different infusion lengths regarding the achievement of effective exposure using PTA analysis.
Methods
Study Population
Detailed information about study design, procedures, and data collection in this prospective, parallel-group, open-label, controlled single-center trial (EudraCT No. 2012-004383-22) have been described elsewhere [17]. Inclusion criteria were abdominal surgery, age ≥ 18 years, body mass index (BMI) = 18.5–30 kg/m2 for non-obese and BMI ≥ 35 kg/m2 for obese patients. Exclusion criteria comprised liver cirrhosis. Non-obese patients were age and sex matched to those in the obese patient group.
Meropenem Dosing and Pharmacokinetic Sampling
Patients received a standard (weight-independent) single intravenous infusion of meropenem 1000 mg after induction of anesthesia through an additional venous access over 30 min (60–30 min before incision).
Dense blood sampling (pre-dose and after 0.5, 1, 2, 3, 4, 5, 6, and 8 h) and collection of microdialysate samples in ISF of subcutaneous adipose tissue (pre-dose and 0–0.5, 0.5–1, 1–1.5, 1.5–2, 2–3, 3–4, 4–5, 5–6, 6–7, and 7–8 h) of both upper arms (one catheter per arm) were performed. Catheters were inserted into both upper arms (one catheter per arm) to allow quantification of catheter-related variability [18] and to allow precise estimation of pharmacokinetic parameter values associated with the ISF. To derive drug concentration in ISF from microdialysate concentrations, the retrodialysis calibration method was used [18–20]. Sampling, preparation, and storage are described by Simon et al. [17], and meropenem concentrations were quantified using validated high-performance liquid chromatography ultra-violet detection (lower limit of quantification = 0.1 mg/L in plasma and 0.02 mg/L in microdialysate) [21]. Based on in-process quality control samples (50, 15, and 0.5 mg/L in plasma or 15, 1.5, and 0.15 mg/L in 0.9% NaCl as the surrogate for microdialysate, respectively), the mean intra-/interassay imprecision and inaccuracy were < 4% coefficient of variation (CV). The autosampler stability (20–24 h/6°C) was 99.2 ± 3.6% (plasma) and 98.3 ± 4.1% (microdialysate/retrodialysate). Further details on the bioanalysis of meropenem are provided elsewhere [21].
Software
Nonlinear mixed-effects model development and simulations were performed in NONMEM v7.4.3 (Icon Development Solutions, Ellicott City, MD, USA, FOCE Interaction) accessed with PsN v4.8.1 through Pirana v2.9.6 (Certara, Princeton, NJ, USA). RStudio v1.2.1335 (Boston, MA, USA) was used for dataset preparation, model evaluation, and post-processing of results. Stepwise covariate modeling was performed in PsN v4.8.1.
Meropenem Population Pharmacokinetic Model Development
Meropenem standard (observations available in this clinical study) and alternative dosing regimens (Table S1 in the electronic supplementary material [ESM]) in both patient populations were evaluated via Monte Carlo simulations based on the developed population pharmacokinetic model. Two- and three-compartment (mammillary and serial) pharmacokinetic models with plasma data attributed to the central and ISF data to the central or peripheral compartments were evaluated using the integrated dialysate-based modeling approach [22, 23]. Elimination from the central compartment and intercompartmental flows were assessed using linear and nonlinear processes. Between-patient variability was implemented assuming a log-normal distribution of the individual parameters. Microdialysis technique-related variabilities between patients, within catheters, and between catheters were characterized assuming a logit-normal distribution to confine relative recovery values to 0–100%. Additive, proportional, combined additive and proportional, and log-normal models of residual unexplained variability were evaluated.
Statistical comparisons between nested models with additional covariates were made using the likelihood ratio test and based on the Akaike information criterion for non-nested models [24]. Model selection was based on statistical significance (p < 0.05) and the plausibility and precision of parameter estimates.
Impact of Body Size Descriptors and Renal Function on Meropenem Pharmacokinetics
Clinical and demographic characteristics were tested for inclusion as covariates. First, the relationship between pharmacokinetic parameters and body size descriptors was evaluated via allomeric scaling (fixed or estimated exponents) [25], and the impact of creatinine clearance (CLCRCG) on meropenem clearance was evaluated via linear and power relationships. Allometric scaling [26] was implemented based on body size descriptors (ideal [27], lean [28], total or adjusted body weight [ABW] [29], or BMI) to (1) ensure simplicity and therefore clinical applicability and (2) avoid type I errors by selecting numerous different combinations of covariates in allometric scaling. To test whether allometric principles held, allometric exponents of the selected body size descriptor were also estimated separately for volumes and flows and compared with the theory-based fixed exponents (1 for volumes and 0.75 for flows). The least biased body size descriptor in the calculation of CLCRCG [30] in obese patients was reported to be ABW, based on a retrospective study including 3678 patients with stable renal function [31]. CLCRCG based on other body size descriptors (ideal, lean, or total body weight) and predicted glomerular filtration rate via the Modification of Diet in Renal Disease (MDRD) equation [32] and Chronic Kidney Disease Epidemiology Collaboration (CKD-EPI) [33] equation (untransformed and “de-indexed”, i.e., de-normalized [34] via individual predicted body surface area [35]) were also evaluated.
ABW in male and female individuals was calculated according to Erstad [36].
Implementation of Remaining Covariate Relationships
After the implementation of allometric scaling, all other characteristics considered biologically plausible to affect the pharmacokinetics of meropenem were evaluated via stepwise covariate modeling (α = 0.05 for forward inclusion, α = 0.01 for backward deletion) [37].
Continuous covariates were normalized to the study median values, and linear, exponential, and power covariate pharmacokinetic parameter relationships were evaluated. Categorical covariates were incorporated in the model as index variables using the following general equation:
with θi as the individual model-predicted pharmacokinetic parameter for a patient with a covariate value covi (either 0 or 1), θpop as the population pharmacokinetic parameter, and θcov as the covariate effect parameter.
Remaining structural parameter correlations were estimated by implementation of covariance terms. Finally, we investigated whether the identified covariate relationships explained potential remaining differences in pharmacokinetic parameters between obese and non-obese patients by separate population-specific estimation of all pharmacokinetic parameters followed by likelihood ratio testing [24].
Population Pharmacokinetic Model Evaluation
Population pharmacokinetic models were evaluated with standard goodness-of-fit plots (e.g., observed vs. predicted meropenem concentrations and conditional weighted residuals vs. population prediction/time). The predictive model performance was assessed with visual predictive checks (n = 1000), and the uncertainty, bias, and stability of parameter estimates was evaluated using the nonparametric bootstrap (n = 1000) [38]. Statistically significant differences (p < 0.05) in individual parameter estimates between obese and non-obese patients were investigated using the Mann–Whitney–Wilcoxon test.
Target-Site Penetration in Obese and Non-Obese Patients
To demonstrate the impact of ABW and CLCRCG_ABW on meropenem exposure, simulations of meropenem plasma and ISF concentrations over 8 h following short-term, prolonged (both 1000 mg), and continuous (3000 mg/24 h) infusion were performed covering the study range of ABW (ABW = 60–120 kg) and CLCRCG_ABW (CLCRCG_ABW = 60–200 mL/min) covering the range of patient characteristics in the study cohort (Table 1).
Table 1.
Patient-specific and surgery-specific characteristics of obese and non-obese patients
| Characteristics | Population | ||
|---|---|---|---|
| Full (n = 30) | Obese (n = 15) | Non-obese (n = 15) | |
| Sex, female | 26 (86.7) | 13 (86.7) | 13 (86.7) |
| Age, years | 51.5 (30.0–65.0) | 52.0 (30.0–65.0) | 50.0 (31.0–64.0) |
| Total body weight [kg] | 101 (52.0–230) | 121 (96.0–230) | 65.0 (52.0–84.0) |
| BMI [kg/m2] | 32.6 (20.5–81.5) | 44.7 (38.1–81.5) | 23.6 (20.5–27.1) |
| ABW [kg] | 70.5 (51.4–128) | 81.6 (66.0–128) | 60.3 (51.4–72.8) |
| Serum creatinine concentration [µmol/L] | 63.0 (40.6–127) | 63.0 (40.6–103) | 66.4 (51.8–127) |
| CLCRCG_ABW [mL/min] | 87.9 (51.3–188) | 99.4 (51.3–188) | 76.0 (53.6–136) |
| Anesthesia duration [h] | 4.22 (2.38–8.63) | 4.08 (2.97–5.63) | 4.78 (2.38–8.63) |
| MAPa [mmHg] | 75.4 (48.3–130) | 76.7 (48.3–130) | 72.5 (53.3–105) |
| Intra-anesthetic | 72.7 (50.0–105) | 72.5 (50.0–102) | 75.0 (53.3–105) |
| Post-anesthetic | 85.0 (48.3–130) | 96.7 (48.3–130) | 81.2 (58.3–105) |
Data are presented as median (range) or count (%)
ABW adjusted body weight, BMI body mass index, CLCRCG_ABW creatinine clearance calculated via Cockcroft–Gault using ABW, MAP mean arterial pressure (summary statistics computed based on individual median of observed intra-anesthetic data and post-anesthetic data, respectively)
aTime-varying parameter
The extent of meropenem penetration into ISF in obese versus non-obese patients was evaluated by the commonly evaluated ratio of the area under the unbound drug concentration–time curve (AUC) between 0 and 8 h (fAUC0–8h) in ISF to plasma (i.e., [fAUC0–8h, ISF] : [fAUC0–8h, plasma]; penetration index). Given the negligible plasma protein binding (~ 2%) of meropenem, it was considered unbound in plasma [39].
Since the antibiotic effect of β-lactams such as meropenem has been demonstrated to be time dependent [5, 6], an AUC-related penetration index is irrelevant for the outcome of meropenem therapy, so we also evaluated the ratio of the effect-related %fT > MIC in ISF to plasma (i.e., [%fT > MIC, ISF] : [%fT > MIC, plasma]; effective penetration index). Both were evaluated for (1) ABW = 60–120 kg at healthy renal function (CLCRCG_ABW = 100 mL/min) and (2) CLCRCG_ABW = 60–140 mL/min at ABW = 60.0 kg. To investigate whether PK/PD targets related to 4 × MIC for continuous infusions are suitable to avoid ISF concentrations below MIC for the entire dosing interval (i.e., effective penetration index = 0) %fT > MIC was related to 4 × MIC in plasma and 1 × MIC in ISF.
Model-Based Evaluation of Dosing Regimens
To evaluate whether meropenem dosing regimens are sufficient to achieve effective concentrations in obese and non-obese patients when non-susceptible or resistant bacteria are encountered in the clinic, PTA analyses were performed for susceptibility and resistance breakpoints (EUCAST). To determine the PTA of various simulated dosing regimens, Monte-Carlo simulations (n = 1000 per covariate combination) of meropenem concentrations over time in plasma and ISF for the first day of treatment were performed using the developed population pharmacokinetic model and virtual patients without the effect of anesthesia. PTA for achieving a PK/PD target of %fT > MIC = 95 [6] and %fT> MIC = 40 [5] was calculated for MIC = 0.25 mg/L, 2 mg/L (EUCAST epidemiological cut-off value for P. aeruginosa and Enterobacteriaceae), and 8 mg/L (non-species-related resistance breakpoint). These PK/PD targets were selected to cover a broad range of PK/PD targets and to ensure comparability with other PK/PD evaluations of meropenem in (morbidly) obese patients [7, 8, 16]. The PK/PD target of %fT > MIC = 95 was selected instead of %fT > MIC = 100, since treatment at day 1 was evaluated during PTA analysis and meropenem concentrations in all matrices are zero before the first dose, preventing any concentration–time profile from attaining %fT > MIC = 100. For continuous infusions, stricter PK/PD targets were selected: %fT > MIC was related to 4 × MIC [10].
To determine whether meropenem dosing regimens are sufficient to achieve effective concentrations in obese and non-obese patients in routine clinical settings, we calculated the sum of PTA weighted by the relative frequency of MIC values (cumulative fraction of response [CFR] [40]) for specific pathogens. Infections by pathogens commonly treated with meropenem [41] were selected (E. coli, C. freundii, K. pneumoniae, P. aeruginosa).
A dosing regimen was considered adequate if the PTA or CFR was ≥ 90% [15]. We also assessed whether the meropenem toxicity threshold concentration was reached, for which a 50% risk of developing a neurotoxicity event has been reported [42]. The dosing regimens evaluated via simulations were thrice-daily (TID) intravenous short-term (30 min) or prolonged (3 h) infusions of 1000 and 2000 mg meropenem over 24 h, and continuous infusions of 3000 and 6000 mg meropenem starting immediately after a 1000-mg 30-min infusion loading dose (Table S1 in the ESM). PTA was assessed for different combinations of ABW (60–120 kg) and CLCRCG_ABW (60–200 mL/min).
Results
Database
In total, 30 patients (15 obese and 15 non-obese) scheduled for elective abdominal surgery were recruited according to study protocol [17]. The 15 obese (two class II and 13 class III) patients were scheduled for bariatric surgery, and the 15 non-obese patients were undergoing elective abdominal surgery, mainly tumor resection (11 patients underwent gynecological operations, and the remaining four operations involved the stomach, liver, kidneys, or appendix). The cohort was predominantly female (26/30 patients) and covered a wide BMI range (BMInon–obese = 20.5–27.1 kg/m2 and BMIobese = 38.1–81.5 kg/m2). As expected, heart rate and blood pressure were reduced during anesthesia versus the post-anesthetic period, as indicated by the derived mean arterial blood pressure (Table 1).
In total, 915/932 (observed/planned) meropenem concentrations were available, comprising plasma concentrations (n = 239/240), microdialysate concentrations collected via two catheters (to investigate microdialysis-related variability) in the ISF of subcutaneous adipose tissue over 8 h (n = 292/300 + 293/300), and retrodialysate concentrations (n = 46/46 + 45/46).
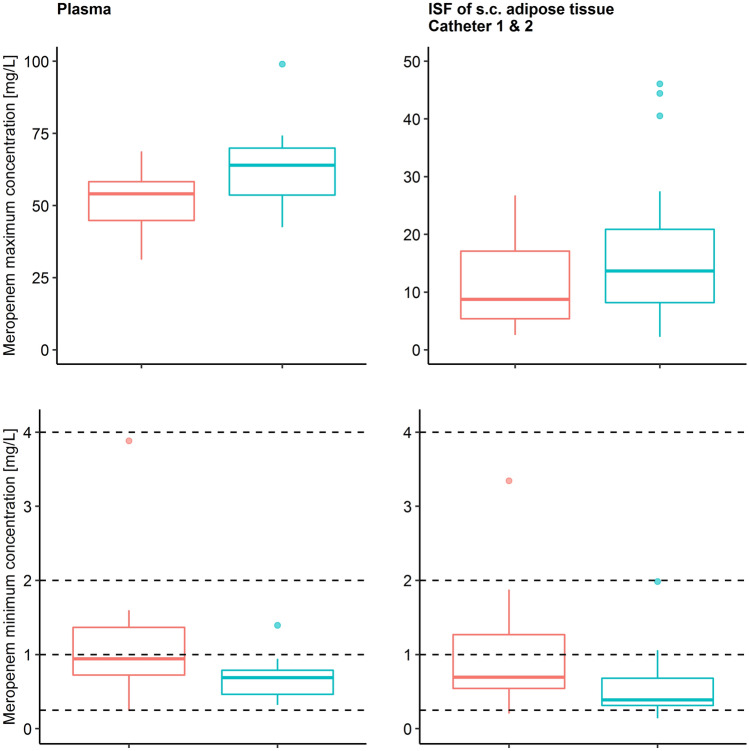
The geometric mean, selected to account for the heteroscedasticity of observed maximum meropenem concentrations (Cmax) in plasma and at target site were lower in obese than in non-obese patients (ΔCmax, plasma = − 19.6% and ΔCmax, target site = − 38.5 to − 40.9%; Fig. 1; Fig. S1 in the ESM), whereas observed minimum concentrations (Cmin) were higher in obese than in non-obese patients (ΔCmin, plasma = + 49.7% and ΔCmin, target site = + 38.6 to + 54.3%).
Fig. 1.
Observed maximum (top panel) and minimum (bottom panel) meropenem concentrations in plasma (total) and in the interstitial space fluid of subcutaneous adipose tissue obtained via microdialysis (unbound) using two catheters for obese (red, n = 15) and non-obese (green, n = 15) patients. Boxplots show the second to third quartile (box) and observations within (whiskers) and outside (dots) the interquartile range. Dashed horizontal lines indicate the minimum inhibitory concentrations. ISF interstitial space fluid, s.c. subcutaneous
Population Pharmacokinetic Model
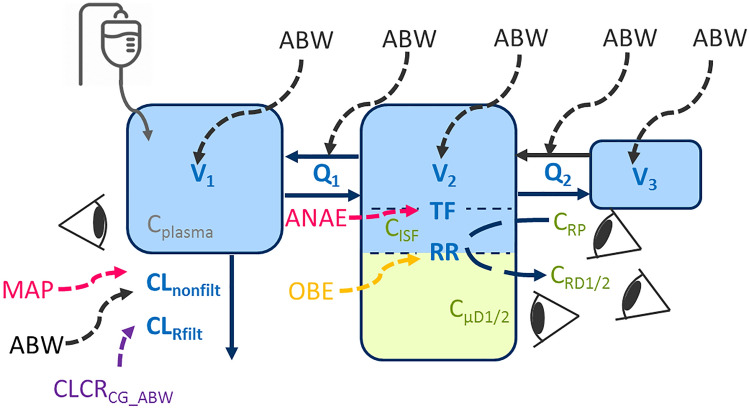
The developed pharmacokinetic model was a serial three-compartment model (Table 2, Fig. 2) with a significantly higher total meropenem volume of distribution (V1 + V2 + V3, median [range]) in obese (22.8 L [18.0–38.8]) than in non-obese patients (15.4 L [12.1–18.0]; p < 0.001) and similar clearance for obese (CL = 12.2 L/h [6.33–20.6]) and non-obese patients (CL = 10.5 L/h [5.38–14.6]; p = 0.250; Table 3). Individual volumes of distribution were higher in obese than in non-obese patients for V1, V2, and V3 (V1,obese = 10.1 L [6.09–17.5]; V1,non–obese = 6.67 L [3.87–11.5]; p = 0.001; V2,obese = 11.7 L [8.04–17.9]; V2,non–obese = 7.49 L [5.28–9.95]; p < 0.001; V3,obese = 3.06 L [2.28–4.41], V3,non–obese = 2.07 L [1.77–2.51]; p < 0.001). Concentrations in ISF were related to the first peripheral compartment, which was best described by a tissue factor, scaling predicted meropenem concentrations in this peripheral compartment to ISF concentrations (Fig. 2).
Table 2.
Parameter estimates (pharmacokinetic parameters for meropenem and microdialysis methodology-related parameters) including bootstrap results of the final model of meropenem in obese and non-obese patients
| Parameter | Final model | Bootstrapa |
|---|---|---|
| Estimate (RSEb, %) | Median (95% CI) | |
| Structural and covariate parameters | ||
| CLRfilt scaled via CLCRCG_ABWc, L/h | 5.48 (29.0) | 5.34 (1.01–8.91) |
| CLnonfilt scaled via ABWd, L/h | 4.99 (32.5) | 5.13 (1.91–9.91) |
| MAP_CLnonfilte,% | 0.618 (37.1) | 0.594 (0.141–2.01) |
| V1 scaled via ABWd [L] | 7.24 (10.7) | 7.24 (5.67–8.68) |
| Q1d scaled via ABWd [L/h] | 31.3 (10.2) | 31.4 (26.1–38.5) |
| V2d scaled via ABWd [L] | 9.39 (8.00) | 9.37 (7.96–11.1) |
| Q2d scaled via ABWd [L/h] | 3.06 (34.3) | 3.20 (0.73–5.56) |
| V3d scaled via ABWd [L] | 2.14 (19.2) | 2.14 (1.32–3.06) |
| TF, % | 37.2 (11.4) | 37.1 (29.7–47.4) |
| ANAE_TFf, % | 19.0 (20.4) | 19.1 (11.8–26.4) |
| RROBE, % | 31.0 (10.1) | 31.0 (25.8–37.6) |
| RRNOBE, % | 55.3 (9.90) | 55.7 (45.6–65.8) |
| Interindividual variability parameters, %CV | ||
| CL | 20.8 (13.4) | 19.9 (13.9–25.4) |
| V1 | 34.1 (18.9) | 32.9 (19.2–48.4) |
| Q1 | 43.1 (19.3) | 41.8 (22.0–57.7) |
| V2 | 32.2 (15.6) | 31.5 (19.3–40.5) |
| TF | 46.2 (15.0) | 44.9 (31.4–58.6) |
| Coefficients of correlation | ||
| V2–Q1 | 0.630 (41.1) | 0.624 (0.140–0.859) |
| Q1–TF | 0.281 (27.1) | 0.298 (0.0343–0.586) |
| TF–RR | − 0.764 (31.2) | − 0.797 (− 0.938 to − 0.477) |
| Microdialysis technique-related variability parameters | ||
| σ2Interindividual RR | 0.388 (38.1) | 0.355 (0.341–0.820) |
| σ2Intercatheter RR | 0.157 (49.8) | 0.151 (0.214–0.583) |
| σ2Intracatheter RR | 0.515 (44.9) | 0.519 (0.373–1.01) |
| Residual variability parameters | ||
| σadditive, plasma, mg/L | 0.0600 (28.2) | 0.0603 (0.0187–0.140) |
| σproportional, plasma, %CV | 8.43 (12.3) | 8.25 (6.24–10.4) |
| σproportional, microdialysis, %CV | 18.3 (9.70) | 17.9 (14.9–21.6) |
| σproportional, retrodialysis, %CV | 2.3 FIXg | 2.3 FIXg |
ABW adjusted body weight, ANAE_TF anesthesia effect on TF, CI confidence interval, CL clearance, CLCRCG_ABW creatinine clearance calculated via Cockcroft-Gault based on ABW, CLnonfilt clearance accounting for the remaining elimination scaling with ABW, CLRfilt renally filtered part of CL related to CLCRCG_ABW, CMT1/CMT2/CMT3 central/peripheral/deep peripheral compartment, CV coefficient of variation, MAP mean arterial pressure, OBE obesity, Q1/Q2 intercompartmental flow of meropenem from CMT1/CMT2 to CMT2/CMT3, RROBE/RRNOBE relative recovery for obese/non-obese patients, RSE relative standard error, TF tissue factor, V1/V2/V3 volume of distribution parameters for CMT1/CMT2/CMT3 of meropenem, σ residual unexplained variability, σ2 variance associated with retrodialysis
aConvergence rate of nonparametric bootstrap (n = 1000): 91.6%
bRSE of random effects are reported on approximate standard deviation scale
cScaled (linear relationship) with the ratio of individual CLCRCG_ABW to the median CLCRCG_ABW in overall population (87.9 mL/min)
dAllometrically scaled with ABW centered to median in overall population (70.5 kg) with exponent of 1 for volumes and 0.75 for flows
eChange of CLnonfilt per mmHg deviation of MAP from 75 mmHg (linear relationship)
fChange of TF after anesthesia
gFixed to interassay variability
Fig. 2.
Illustration of the final meropenem population pharmacokinetic model. Impact of patient (black: body size descriptors; orange: categorical difference between obese and non-obese patients; purple: kidney function) or therapy factors (pink: anesthesia-related factors) on pharmacokinetic parameters (blue); green: microdialysis-related observation types; eyes: observations. ABW adjusted body weight, ANAE anesthesia, CLCRCG_ABW creatinine clearance calculated via Cockcroft–Gault based on ABW, CLnonfilt clearance accounting for the remaining elimination scaling with ABW, CLRfilt renally filtered part of CL related to CLCRCG_ABW, CMT1/CMT2/CMT3 central/peripheral/deep peripheral compartment, CISF concentration in the interstitial space fluid of subcutaneous adipose tissue, Cplasma total plasma concentration, CRD1/2 retrodialysate concentration from catheter 1/2, CRP retroperfusate concentration, CµD1/2 microdialysate concentration from catheter 1/2, MAP mean arterial pressure, OBE obesity, Q1/Q2 intercompartmental flow of meropenem from CMT1/CMT2 to CMT2/CMT3, RR relative recovery, TF tissue factor, V1/V2/V3 volume of distribution parameters for CMT1/CMT2/CMT3 of meropenem
Table 3.
Individual meropenem parameter estimates and exposure predictions of the final population pharmacokinetic model in (morbidly) obese and non-obese patients
| Parameter | Obese population (n = 15) | Non-obese population (n = 15) | p-valuea |
|---|---|---|---|
| Parameter estimateb | |||
| Vtotal, L | 22.8 (18.0–38.8) | 15.4 (12.1–18.0) | < 0.001 |
| Vtotal, L/kgc | 0.179 (0.144–0.260) | 0.236 (0.159–0.315) | 0.00167 |
| Vtotal, L/kgd | 0.259 (0.217–0.395) | 0.262 (0.201–0.360) | 0.967 |
| CLtotal, L/h | 12.2 (6.33–20.6) | 10.5 (5.38–14.6) | 0.250 |
| Exposure predictione | |||
| Cmax, mg/L | |||
| Plasma | 48.6 (28.1–80.8) | 63.0 (38.8–99.0) | < 0.001 |
| Interstitial tissue fluid | 10.9 (3.40–32.5) | 14.6 (1.05–44.8) | < 0.001 |
| Cmin, mg/L | |||
| Plasma | 0.930 (0.0994–4.11) | 1.01 (0.0847–4.48) | 0.550 |
| Interstitial tissue fluid | 0.619 (0.08–3.24) | 0.641 (0.08–3.27) | 0.320 |
CI confidence interval, CLtotal total clearance, Cmax maximum concentration, Cmin minimum concentration, Vtotal total volume of distribution
aStudent t-test between obese and non-obese subpopulation
bEntries are median (range)
cNormalized to total body weight
dNormalized to adjusted body weight
eEntries are median (95% CI) based on 1000 Monte Carlo simulations
The evaluation of the relationship of individual parameter estimates (empirical Bayes estimates) before covariate implementation and potential covariates revealed an impact (p < 0.01) of different body size descriptors on volumes of distribution (total body weight, BMI, ABW, lean body weight; Fig. S2 in the ESM) and CL (total body weight, BMI, ABW; Fig. S3 in the ESM).
The effect of ABW on central and peripheral volumes (V1–3), CL, and intercompartmental flows (Q1–2) was implemented using theory-based allometric scaling with fixed exponents [25] (i.e., positive relationships of pharmacokinetic parameters with body size with the exponents 1 for V1–3 and 0.75 for CL and Q1–2, respectively) based on likelihood ratio testing (Table S2 in the ESM). When, instead, exponents were estimated separately for flows and volumes, the decrease in objective function value (OFV) was not significant (p = 0.104, ΔOFV = − 2.64) and the 90% confidence interval (CI) of estimates included the theory-based allometric exponents of 0.75 for flows (0.505; 90% CI = 0.135–0.876) and 1 for volumes (0.757; 90% CI = 0.408–1.10).
Additionally, positive relationships between CLCRCG (via total, lean, and adjusted body weight) and predicted glomerular filtration rate (via the MDRD and CKD-EPI equations de-indexed by individual body surface area; Fig. S3 in the ESM) on CL were evident. The very high concordance (Lin’s concordance correlation coefficient ≥ 0.962; Fig. S4 in the ESM) between CLCRCG_ABW and de-indexed predicted glomerular filtration rate via the MDRD and CKD-EPI equations resulted in similar model performance (Table S2 in the ESM). Since ABW was selected during allometric scaling and to enhance simplicity, CLCRCG_ABW was selected in the final model.
CL was successfully split into a renally filtered part related to CLCRCG_ABW (CLRfilt = 5.48 L/h; 29% relative standard error [RSE]) and CL accounting for remaining elimination pathways scaling with ABW (CLnonfilt = 4.99 L/h, 32.5% RSE).
Implementation of these two patient characteristics as covariates entirely explained differences in population parameter estimates between obese and non-obese patients.
Relative recovery estimates for catheters were variable and significantly lower for obese (31.0% [26.4–61.6]) than non-obese patients (55.4% [31.5–81.5]; p < 0.001). Additionally, the anesthesia status of the patient and mean arterial blood pressure impacted the tissue factor and CLnonfilt, respectively: post anesthesia, the tissue factor was 19.0% (11.4% RSE) larger than during anesthesia, and a 10% increase of mean arterial blood pressure increased CLnonfilt by 6.2% (37% RSE).
Inclusion of these covariates considerably decreased the between-patient and retrodialysis-related variability compared with the base model (15.1–31.0% relative reduction in CV). Microdialysis/retrodialysis data with repeated measurements from two catheters allowed quantification of microdialysis technique-related variabilities, which were large between patients (σ2 = 0.388) and within catheters (σ2 = 0.515) and comparably low between catheters (σ2 = 0.157).
The key model development steps are detailed in Table S2 in the ESM. Adequate model prediction for all three matrices was demonstrated by visual predictive checks (Figs. S5–6 in the ESM): The final pharmacokinetic model adequately captured both the central tendency and the variability of meropenem pharmacokinetics in all investigated matrices. Additionally, all meropenem pharmacokinetic- and microdialysis technique-related parameters were precisely estimated (RSE ≤ 49.8%, Table 2), and the results of model evaluation demonstrated appropriate model performance (Figs. S7–8 in the ESM). A small trend of underpredicting low meropenem plasma concentrations in obese patients and overpredicting low concentrations in non-obese patients remained (Fig. S7 in the ESM, top panel). This trend remained when allometric exponents were estimated instead of using theory-based allometric exponents (abolsute average fold error [AAFE]: AAFEobese = 0.996, AAFEnon–obese = 1.05 and slope of observed vs. predicted plasma concentrations: Slopeobese = 0.985; 95% CI = 0.938–1.03 and Slopenon–obese = 0.958; 95% CI = 0.910–1.01). No trends were found when inspecting random-effects variables for structural pharmacokinetic parameters versus the identified covariates (Figs. S9–10 in the ESM). Additionally, after implementation of the impact of body mass on pharmacokinetic parameters, no additional impact of obesity on any pharmacokinetic parameter remained in the final pharmacokinetic model (p > 0.05) as judged by likelihood ratio testing. Visual predictive checks stratified by obesity status demonstrated that, for low plasma concentrations, the trend in the median of observed values for obese and non-obese patients was also observable. However, for obese and non-obese patients, not only the median of observed values was within the 95% CI of the median of predicted concentrations (Fig. S6 in the ESM, top panel) [43]. This also applied to the 5th and 95th percentiles of meropenem plasma concentrations. This qualified the model application for the intended use of simulating with interindividual variability in both subpopulations.
Interstitial Space Fluid Penetration in Obese and Non-Obese Patients
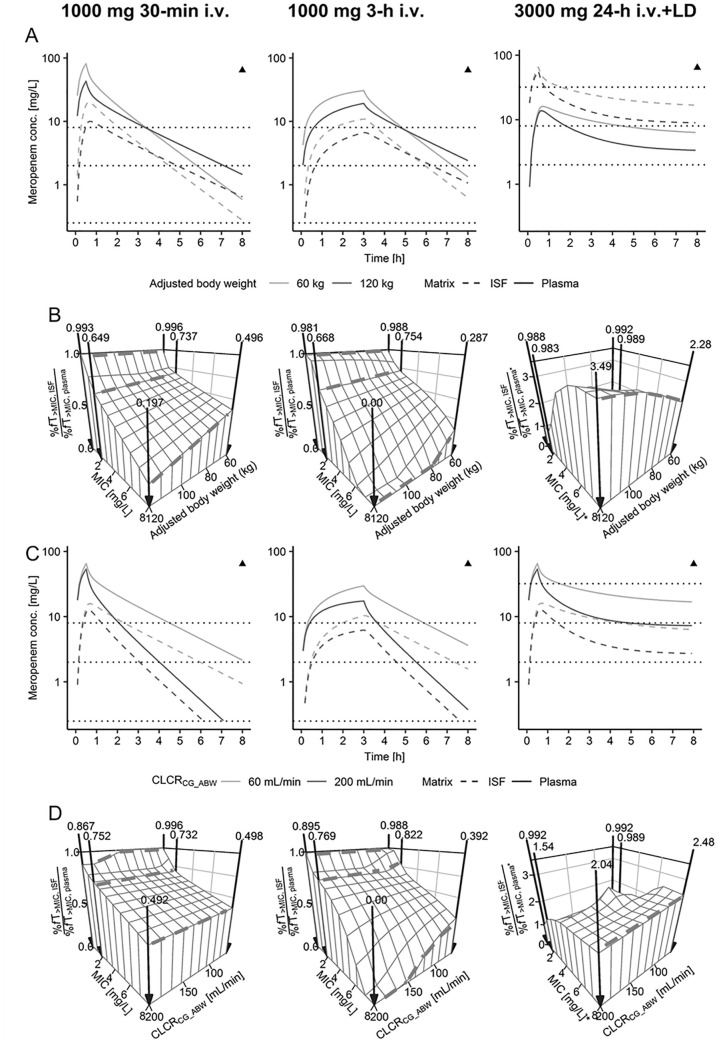
Simulated maximum concentrations in patients with ABW = 120 kg (obese) compared with ABW = 60 kg (non-obese) were lower in plasma and in ISF (34.9–47.8% decrease throughout all dosing regimens, Fig. 3A). In the final descending phase within one dosing interval (8 h), an inverse trend was observed for short-term and prolonged infusion dosing regimens (Fig. 3A, left and middle panels), with patients of ABW = 120 kg having higher minimum plasma and ISF concentrations (50.0–332% increase). For continuous infusions (Fig. 3A, right panel), this inverse effect was not evident: Simulated meropenem plasma and ISF concentrations in patients with ABW = 120 kg were continuously below simulated concentrations in patients with ABW = 60 kg. Throughout all simulated dosing regimens, patients with CLCRCG_ABW = 200 mL/min had lower minimum plasma and ISF concentrations than patients with CLCRCG_ABW = 60 mL/min (from 10.8% reduction to even no remaining concentration; Fig. 3C).
Fig. 3.
Simulations of meropenem concentration–time profiles (A, C) and the ISF:plasma ratio of %fT > MIC (B, D) for three different dosing regimens (panels) with varying adjusted body weight (A, B) and varying creatinine clearance calculated via Cockcroft–Gault based on adjusted body weight (CLCRCG_ABW, C, D). *For continuous infusions %fT > MIC, ISF is related to 1 × minimum inhibitory concentrations (MIC) and %fT > MIC, plasma is related to 4 × MIC. Dashed horizontal lines (A, B) or bold arrows (C, D): 1 × MIC of 0.25, 2, and 8 mg/L (short-term and prolonged infusions) and 4 × MIC of 2, 8, and 32 mg/L (continuous infusion; related to plasma). Triangles: Neurotoxicity thresholds for minimum plasma concentrations at steady-state reported by Imani et al. [42]. %fT > MIC time that unbound meropenem concentrations exceeded MIC, ISF interstitial space fluid of the subcutaneous adipose tissue, IV intravenous, LD 1000 mg 30-min IV loading dose
The model-predicted, commonly evaluated ISF to plasma ratio of fAUC0–8h (penetration index) was similar over the ABW range: 30.7% (95% CI = 25.0–37.8) for 120 kg vs. 30.2% (95% CI = 24.7–37.2) for 60 kg. Similarly, for simulated short-term and prolonged infusions (Fig. 3B; Fig. S11 in the ESM, left and middle panels), the ISF to plasma ratio of %fT > MIC (effective penetration index) was relatively similar over the investigated ABW range for MIC ≤ 2 mg/L (≤ 11.9% relative reduction). In contrast, the effective penetration index for MIC = 8 mg/L decreased from 60 to 120 kg ABW (≥60.3% relative reduction, Fig. 3B; Fig. S11 in the ESM, upper panels). Similar trends were observed for varying CLCRCG_ABW, yet relative differences were lower than for ABW (Fig. 3D; Fig. S11 in the ESM, lower panels).
For simulated continuous infusions (%fT > MIC related to 4 × MIC in plasma to avoid suspected ISF concentrations constantly below the MIC and 1 × MIC in ISF; Fig. 3B, right panel) ISF:plasma %fT > MIC ratios were ≥0.983 over the entire ABW and CLCRCG_ABW range (Fig. 3B, D; Fig. S11 in the ESM, right panels). With increasing MIC values, the ISF:plasma %fT > MIC ratio also increased since %fT > MIC in plasma (related to 4 × MIC) decreased faster than %fT > MIC in ISF (compare Fig. S11 in the ESM). Simulated unbound steady-state concentrations were 2.68 times (95% CI = 2.11–3.37) higher in plasma than in ISF over the whole ABW range (Fig. 3A, right panel).
Model-Based Evaluation of Dosing Regimens
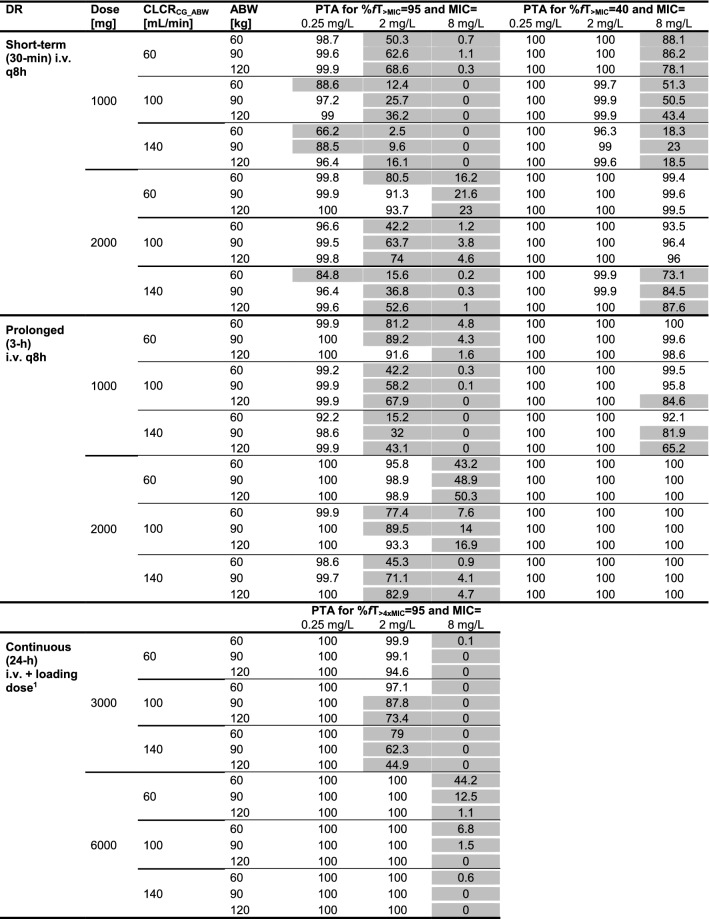
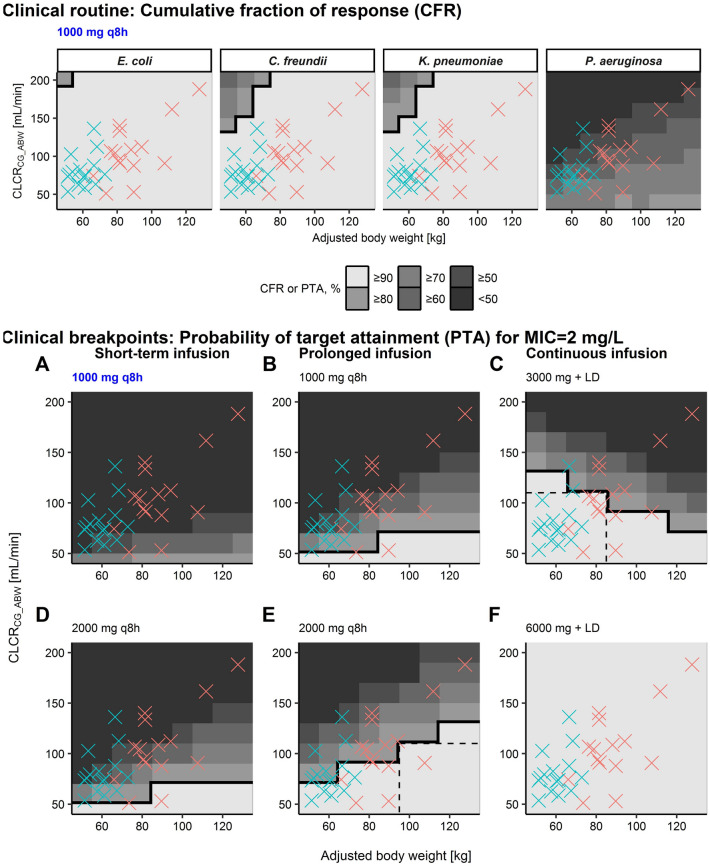
When evaluating susceptibility and resistance breakpoints, for short-term and prolonged infusions, PTA (%fT > MIC = 95) increased with increasing ABW because of the aforementioned inversion of concentration at the end of the dosing interval (Figs. S12–13 in the ESM). Consequentially, at ABW > 90 kg and CLCRCG_ABW ≤ 100 mL/min for %fT > MIC = 95 and MIC = 2 mg/L, PTA was adequate with 2000 mg TID prolonged infusions (Table 4, Fig. 4E). However, for the continuous infusion, higher body mass resulted in decreased PTA, leading to adequate PTA only for ABW < 90 kg (%fT> MIC = 95) with a 3000 mg continuous infusion for MIC = 2 mg/L (inadequate PTA for all patients at MIC = 8 mg/L) and patients with CLCRCG_ABW ≤ 100 mL/min (Table 4, Fig. 4C).
Table 4.
Meropenem probability of target attainment in plasma for (1) different dosing regimens, (2) adjusted body weights, (3) creatinine clearance values, and (4) PK/PD targets defined for %fT > MIC = 95 and %fT > MIC = 40 at three minimum inhibitory concentrations (0.25, 2 and 8 mg/L) each
Grey shading indicates that the probability of target attainment is < 90%
ABW adjusted body weight, CLCRCG_ABW serum creatinine clearance calculated using the Cockcroft–Gault equation based on ABW, DR dosing regimen, IV intravenous, MIC minimum inhibitory concentration, PTA probability of target attainment, q8h every 8 h, 95%fT > MIC/40%fT > MIC unbound meropenem plasma concentrations exceeding the MIC 95%/40% of the time over 24 h
a30-min 1000 mg IV loading dose
Fig. 4.
Plasma-based cumulative fraction of response (CFR, top panel) and probability of target attainment for MIC = 2 mg/L (bottom panels) versus ABW and CLCR after the standard meropenem dosing regimen (0.5–h i.v. infusion of 1000 mg, blue label) and alternative dosing regimens (black labels) based on MIC distributions* of four pathogens associated with severe skin and soft tissue infections. Bold black line: separates CFR or PTA ≥ 90% (adequate therapy) from CFR or PTA < 90%. Crosses denote observed combinations of ABW and CLCRCG_ABW of the 15 obese (red) and 15 non-obese (green) patients in this study. ABW adjusted body weight, CLCRCG_ABW creatinine clearance calculated via Cockcroft–Gault equation based on adjusted body weight, IV intravenous, LD 30-min intravenous loading dose of 1000 mg, MIC minimum inhibitory concentration, q8h every 8 h, 95%fT > MIC unbound meropenem plasma concentrations exceeding MIC 95% of the time over 24 h. *EUCAST.org, accessed 12 March 2021
Overall, PTA analysis showed lower PTA at higher CLCRCG_ABW (Table 4). In all patients for %fT > MIC = 95, a TID prolonged infusion of 1000 mg was sufficient to achieve adequate PTA for MIC = 0.25 mg/L, whereas only a continuous infusion of 6000 mg sufficed for MIC = 2 mg/L. None of the investigated dosing regimens was adequate for MIC = 8 mg/L. For the lower target, %fT > MIC = 40, the TID short-term infusion of 2000 mg and both prolonged infusion dosing regimens were adequate for MIC ≤ 2 mg/L, and a 2000 mg TID prolonged infusion was even sufficient for MIC = 8 mg/L. PTA results for additional covariate combinations are presented in Fig. S12–S14 in the ESM.
The CFR for the standard meropenem dosing regimen (short-term infusions of 1000 mg TID) was sufficient for CLCRCG_ABW ≤ 130 mL/min and commonly encountered MIC distributions for E. coli, C. freundii, and K. pneumoniae (Fig. 4, top panel). For P. aeruginosa, the CFR of the meropenem standard dosing regimen was inadequate for all virtual patients (Fig. 4, top panel).
In addition to efficacy, the reported neurotoxicity threshold [42] was not reached for the scenario with the highest meropenem exposure, i.e., the highest investigated daily dose (meropenem 6000 mg continuous infusion with 1000 mg loading dose) and the lowest investigated CLCRCG_ABW and ABW after 24 h (Fig. S15B in the ESM).
Discussion
In this controlled clinical trial, dosing regimens attaining target meropenem concentrations were identified for obese and non-obese patients. Employing a strict PK/PD target (%fT > MIC = 95), a higher PTA was achieved with short-term and prolonged infusions in obese patients (because of a higher volume of distribution and thus longer meropenem half-life) and with continuous infusions in non-obese patients. For patients with CLCRCG_ABW ≤ 100 mL/min, 2000 mg TID prolonged infusions were sufficient for patients with ABW > 90 kg, and lower doses of 3000 mg/24 h (continuous infusion) were sufficient for patients with ABW < 90 kg. For a less conservative PK/PD target (%fT > MIC = 40) and MIC ≤ 2 mg/L, adequate PTA was achieved for all patients irrespective of ABW via the standard dosing regimen (1000 mg short-term infusion TID).
PTA of the standard short-term infusion meropenem dosing regimen is inadequate throughout ABW = 60–120 kg for MIC ≥ 2 mg/L (EUCAST epidemiological cut-off value for P. aeruginosa and Enterobacteriaceae), whereas CFR (evaluation of empirical therapy) was adequate for E. coli, C. freundii, and K. pneumoniae (but not for P. aeruginosa). In contrast, for MIC ≤ 2 mg/L, we demonstrated that a continuous infusion of meropenem 6000 mg after a 1000 mg loading dose may be sufficient to avoid treatment adjustments according to body mass. The 2.68 times (95% CI = 2.11–3.37) higher steady-state meropenem concentrations in plasma versus ISF corroborated the use of PK/PD targets related to 4 × MIC to avoid sustained ISF concentrations below MIC during continuous infusion.
For the less conservative PK/PD target (%fT > MIC = 40), the same trends with ABW and renal function as for %fT > MIC = 95 were evident, but we demonstrated that a prolonged infusion of 2000 mg was sufficient for all ABW and CLCRCG_ABW values for MIC = 8 mg/L. These results indicate that the reported superiority of prolonged and continuous infusions (given infusion bags are prepared accordingly to account for the chemical instability of meropenem [44]) versus short-term infusion, regarding mortality and duration of therapy [45, 46] may well also apply to obese patients.
The relation between CLCRCG_ABW and meropenem CL was consistent with previous studies [8, 47–50], and the additional impact of body mass on meropenem clearance (CLnonfilt) was in line with meropenem elimination besides glomerular filtration such as tubular secretion [51]. Importantly, the final pharmacokinetic model did not include simple allometric scaling of total meropenem CL with ABW but rather a fraction of CL (i.e., by the nonfiltered elimination pathway, 47.7% of total CL) was scaled by ABW and the dominant renally filtered elimination pathway by CLCR (see Sect. 3.2). Discrimination between these two elimination pathways was possible because of the very large range of ABW (51.4–128 kg, scaling of CLnonfilt) and CLCRCG_ABW (51.3–188 mL/min, scaling of CLRfilt) in the leveraged clinical data set. However, the lack of a standardized prediction of glomerular filtration in obese patients has been identified as a major challenge in dosing of antibiotics [52]. Among all tested prediction methods, CLCRCG_ABW resulted in the lowest OFV and was hence selected as a covariate on meropenem CL (de-indexed predicted glomerular filtration rate via the MDRD or CKD-EPI equations performed equally well and might be used interchangeably given their frequent use in clinics). This was in line with results by Winter et al. [31], who identified the use of ABW-based CLCRCG as most accurate over the entire range of BMI classes. Future clinical trials should include individual measurements of glomerular filtration via standards such as 51Cr EDTA or measurement of meropenem concentrations in urine. This would allow a more accurate characterization of the distinct elimination processes of meropenem.
The larger CLCRCG_ABW in obese patients obtained in our analysis is supported by the reported initial state of glomerular hyperfiltration in obesity [53]. Second, a small but decisive influence of ABW on meropenem PTA was shown, corroborating the effect of body mass demonstrated in clinical studies for simulated continuous infusions in obese [7, 16] and non-obese patients [54–60]. Importantly, Chung et al. [8] identified no significant relationship between obesity status and any pharmacokinetic parameter in a clinical study in 40 critically ill non-obese and (morbidly) obese patients, although the estimates for differences were substantially in agreement with ours for some parameters. Whilst Chung et al. [8] selected total body weight as a covariate in the forward-selection step in their stepwise covariate modelling, the impact of this body size descriptor was removed in the more stringent backwards-deletion step. The impact of this influence depended on the selected dosing regimen: PTA was adequate for patients with healthy renal function (CLCRCG_ABW = 100 mL/min) and high ABW (> 90 kg) for %fT > MIC = 95 receiving 2000 mg TID prolonged infusions but inadequate when receiving a 3000 mg continuous infusion.
Analysis of ISF data further revealed that, for MIC > 2 mg/L, PTA analysis solely based on plasma concentration in obese patients was compromised by a lower %fT > MIC in ISF than in non-obese patients for whom PK/PD indices were developed and confirmed [5, 6]. Hence, we conclude that for MIC > 2 mg/L, PTA exclusively based on plasma concentration might not be informative for the treatment of obese patients when current PK/PD plasma targets developed in non-obese patients are applied. In future, more reliable PTA should be aimed for by using the developed pharmacokinetic model to define PK/PD targets adjusted to the population-specific ISF penetration.
In addition to the availability of ISF concentration data, which allowed inferences on meropenem distribution and its consequences on the interpretation of PTA, the strengths of this study were the high-quality, rich sampling data (915 samples) obtained prospectively under clinical trial conditions and the inclusion of a control group. Concentrations were measured in plasma and subcutaneous adipose tissue ISF in this study after a single dose, whereas sampling after multiple doses might have allowed evaluation of, for example, nonlinearity of elimination processes in obese patients, where pharmacokinetic data are sparse. The extent to which concentrations at this target site can be transferred to other target sites is still unclear, so results regarding ISF penetration should be applied to skin and soft tissue infections and not to other infection sites.
Notably, single-dose administration of meropenem as perioperative antibiotic prophylaxis was used as a standardized surrogate for critically ill patients. Importantly, key pharmacokinetic parameters such as CL and volumes of distribution can substantially differ between critically ill and non-critically ill patients [61]. Hence, the absence of serious secondary diseases limits the transferability of the results. In fact, population pharmacokinetic parameter estimates of the total volume of distribution of non-obese patients in this study (15.4 L) were lower than literature-reported values in non-obese critically ill patients (21.7–24.0 L [47, 62, 63]), whereas CL estimates were similar (10.5 vs. 8.40–13.0 L/h [47, 62, 63]), although results in critically ill patients with augmented renal clearance (e.g., in sepsis) are expected to differ. Similarly, body weight-normalized estimates of volume of distribution were lower in obese individuals in this study (0.179 L/kg) than literature-reported estimates (0.21–0.50 L/kg [8, 64]), whereas estimates of CL were relatively similar (12.2 vs. 10.0–18.0 L/h [8, 64]). Nevertheless, our analysis represents an important basis for future clinical PK/PD investigations of meropenem in obese and non-obese critically ill patients.
Conclusion
Employing a strict PK/PD target (%fT > MIC = 95), higher PTA was achieved in obese patients with TID prolonged infusions (3 h) and in non-obese patients with continuous infusions: This suggests dosing adaptations for MIC = 2 mg/L and patients with healthy renal function (CLCRCG_ABW = 100 mL/min) and ABW > 90 kg receiving a 3000 mg continuous infusion but ABW < 90 kg receiving TID 2000 mg prolonged infusions. Only a continuous infusion of meropenem 6000 mg following a loading dose resulted in adequate effective exposure over the entire investigated range of ABW and CLCRCG_ABW for MIC ≤ 2 mg/L, but this was still inadequate for MIC = 8 mg/L.
For a less conservative PK/PD target (%fT > MIC = 40), no dosing adjustment was necessary for MIC ≤ 2 mg/L, irrespective of ABW, and a prolonged infusion of meropenem 2000 mg TID was sufficient for MIC = 8 mg/L and all investigated ABW and CLCRCG_ABW.
Supplementary Information
Below is the link to the electronic supplementary material.
Acknowledgements
The authors gratefully acknowledge Dr Jens Borghardt and Dr Niklas Hartung for helpful discussions; the High-Performance Computing Service at Freie Universitaet Berlin (http://www.zedat.fu-berlin.de/Compute) for providing high-performance computing capacities enabling our modeling and simulation activities; the team at the Clinical Trial Center of the University of Leipzig for organizational support, study promotion, and on-site monitoring; Christiane Prettin for the excellent study management; and Frank Mehner, Nancy Neumann, Sophie Hochstädt, Melanie Simon, Sven Walther, and Jana Heyde for help in obtaining the study data.
Declarations
Preliminary results of parts of this analysis have been presented as abstracts at the annual meetings of the Population Approach Group in Europe (PAGE) 2019 and the European Congress of Clinical Microbiology and Infectious Diseases (ECCMID) 2020 and via oral presentations at the ECCMID 2021 and the International Society of Anti-infective Pharmacology meeting 2021.
Conflicts of Interest
CK and WH have received grants from an industry consortium (AbbVie Deutschland GmbH & Co. KG, AstraZeneca GmbH, Boehringer Ingelheim Pharma GmbH & Co. KG, Grünenthal GmbH, F. Hoffmann-La Roche Ltd, Merck KGaA, and Sanofi) for the PharMetrX program. CK has received grants from the Innovative Medicines Initiative-Joint Undertaking ("DDMoRe”), Diurnal Ltd., the Federal Ministry of Education and Research within the Joint Programming Initiative on Antimicrobial Resistance Initiative (JPIAMR), and the European Commission within the Horizon 2020 framework programme (“FAIR”), all outside the submitted work. HW has received grants from Pfizer (Investigator Initiated Trial Program, Berlin, Germany) and InfectoPharm (Heppenheim, Germany), both for the clinical microdialysis trial, lecture fees from InfectoPharm (Heppenheim, Germany) and MSD (Konstanz, Germany), and consultant honoraria from Dräger Medical (Lübeck, Germany). PS has received grants from Pfizer (Investigator Initiated Trial Program, Berlin, Germany) and InfectoPharm (Heppenheim, Germany), both for the clinical microdialysis trial, and lecture fees from InfectoPharm (Heppenheim, Germany). D. Busse, L. Schmitt, D. Petroff, C. Dorn, A. Dietrich, M. Zeitlinger, and R. Michelet have no conflicts of interest that are directly relevant to the content of this article.
Funding
Open Access funding enabled and organized by Projekt DEAL.
Ethics approval and consent to participate
Approval for the trial was granted by the Leipzig University Ethics committee (No.: 121-13-28012013). The study was also approved by the Federal Institute of Drug and Medical Devices (BfArM – No.: 4038808) and registered in EU clinical trials register (Eudr-CT-No. 2012-004383-22) and German Clinical trials Register (DRKS00004776). The study was designed following the principles of the Declaration of Helsinki. Written informed consent was obtained from every enrolled patient upon request by the local German law.
Consent to publish
Not applicable.
Availability of data and material
The datasets generated during and/or analysed during the current study are available from the corresponding author on reasonable request.
Code availability
The code generated during this analysis is available from the corresponding author on reasonable request.
Author contributions
DB, RM, and CK designed the data analysis. PS and DP designed and conducted the clinical study and CD performed the assays. DB, RM, and CK performed the data analysis and simulation. DB, PS, LS, DP, CD, WH, RM, HW, and CK discussed the results. DB, LS, RM, and CK drafted the manuscript. All authors commented on and approved the manuscript.
Footnotes
D. Busse, P. Simon have contributed equally.
References
- 1.Carmeli Y, Lidji SK, Shabtai E, Navon-Venezia S, Schwaber MJ. The effects of group 1 versus group 2 carbapenems on imipenem-resistant Pseudomonas aeruginosa: an ecological study. Diagn Microbiol Infect Dis. 2011;70:367–372. doi: 10.1016/j.diagmicrobio.2011.03.009. [DOI] [PubMed] [Google Scholar]
- 2.Davies TA, Marie Queenan A, Morrow BJ, Shang W, Amsler K, He W, et al. Longitudinal survey of carbapenem resistance and resistance mechanisms in Enterobacteriaceae and non-fermenters from the USA in 2007–09. J Antimicrob Chemother. 2011;66:2298–2307. doi: 10.1093/jac/dkr290. [DOI] [PubMed] [Google Scholar]
- 3.Lodise TP, Lomaestro BM, Drusano GL. Application of antimicrobial pharmacodynamic concepts into clinical practice: Focus on β-lactam antibiotics. Pharmacother J Hum Pharmacol Drug Ther. 2006;26:1320–1332. doi: 10.1592/phco.26.9.1320. [DOI] [PubMed] [Google Scholar]
- 4.European Committee on Antimicrobial Susceptibility Testing. Breakpoint tables for interpretation of MICs and zone diameters, Version 11.0, 2021. 2021. http://www.eucast.org/fileadmin/src/media/PDFs/EUCAST_files/Breakpoint_tables/v_8.0_Breakpoint_Tables.pdf. Accessed 9 June 2021.
- 5.Mattoes HM, Kuti JL, Drusano GL, Nicolau DP. Optimizing antimicrobial pharmacodynamics: dosage strategies for meropenem. Clin Ther. 2004;26:1187–1198. doi: 10.1016/S0149-2918(04)80001-8. [DOI] [PubMed] [Google Scholar]
- 6.McKinnon PS, Paladino JA, Schentag JJ. Evaluation of area under the inhibitory curve (AUIC) and time above the minimum inhibitory concentration (T>MIC) as predictors of outcome for cefepime and ceftazidime in serious bacterial infections. Int J Antimicrob Agents. 2008;31:345–351. doi: 10.1016/j.ijantimicag.2007.12.009. [DOI] [PubMed] [Google Scholar]
- 7.Alobaid AS, Wallis SC, Jarrett P, Starr T, Stuart J, Lassig-Smith M, et al. Effect of obesity on the population pharmacokinetics of meropenem in critically ill patients. Antimicrob Agents Chemother. 2016;60:4577–4584. doi: 10.1128/AAC.00531-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Chung EK, Cheatham SC, Fleming MR, Healy DP, Kays MB. Population Pharmacokinetics and pharmacodynamics of meropenem in nonobese, obese, and morbidly obese patients. J Clin Pharmacol. 2017;57:356–368. doi: 10.1002/jcph.812. [DOI] [PubMed] [Google Scholar]
- 9.Wong G, Brinkman A, Benefield RJ, Carlier M, De Waele JJ, El Helali N, et al. An international, multicentre survey of β-lactam antibiotic therapeutic drug monitoring practice in intensive care units. J Antimicrob Chemother. 2014;69:1416–1423. doi: 10.1093/jac/dkt523. [DOI] [PubMed] [Google Scholar]
- 10.Mouton JW, den Hollander JG. Killing of Pseudomonas aeruginosa during continuous and intermittent infusion of ceftazidime in an in vitro pharmacokinetic model. Antimicrob Agents Chemother. 1994;38:931 LP–936. doi: 10.1128/AAC.38.5.931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Longo C, Bartlett G, MacGibbon B, Mayo N, Rosenberg E, Nadeau L, et al. The effect of obesity on antibiotic treatment failure: a historical cohort study. Pharmacoepidemiol Drug Saf. 2013;22:970–976. doi: 10.1002/pds.3461. [DOI] [PubMed] [Google Scholar]
- 12.Winfield RD, Reese S, Bochicchio K, Mazuski JE, Bochicchio GV. Obesity and the risk for surgical site infection in abdominal surgery. Am Surg. 2016;82:331–336. doi: 10.1177/000313481608200418. [DOI] [PubMed] [Google Scholar]
- 13.Ehmann L, Simon P, Busse D, Petroff D, Dorn C, Huisinga W, et al. Risk of target non-attainment in obese compared to non-obese patients in calculated linezolid therapy. Clin Microbiol Infect. 2020;26:1222–1228. doi: 10.1016/j.cmi.2020.04.009. [DOI] [PubMed] [Google Scholar]
- 14.Simon P, Busse D, Petroff D, Dorn C, Ehmann L, Hochstädt S, et al. Linezolid concentrations in plasma and subcutaneous tissue are reduced in obese patients, resulting in a higher risk of underdosing in critically ill patients: a controlled clinical pharmacokinetic study. J Clin Med. 2020;9:1067. doi: 10.3390/jcm9041067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.EMA. Guideline on the use of pharmacokinetics and pharmacodynamics in the development of antibacterial medicinal products. Amsterdam, The Netherlands, 2017.
- 16.Wittau M, Scheele J, Kurlbaum M, Brockschmidt C, Wolf AM, Hemper E, et al. Population pharmacokinetics and target attainment of meropenem in plasma and tissue of morbidly obese patients after laparoscopic intraperitoneal surgery. Antimicrob Agents Chemother. 2015;59:6241–6247. doi: 10.1128/AAC.00259-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Simon P, Petroff D, Dorn C, Ehmann L, Kloft C, Prettin C, et al. Measurement of soft tissue drug concentrations in morbidly obese and non-obese patients—a prospective, parallel group, open-labeled, controlled, phase IV, single center clinical trial. Contemp Clin Trials Commun. 2019 doi: 10.1016/j.conctc.2019.100375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Busse D, Simon P, Michelet R, Ehmann L, Mehner F, Dorn C, et al. Quantification of microdialysis related variability in humans: clinical trial design recommendations. Eur J Pharm Sci. 2021 doi: 10.1016/j.ejps.2020.105607. [DOI] [PubMed] [Google Scholar]
- 19.Plock N, Kloft C. Microdialysis—theoretical background and recent implementation in applied life-sciences. Eur J Pharm Sci. 2005;25:1–24. doi: 10.1016/j.ejps.2005.01.017. [DOI] [PubMed] [Google Scholar]
- 20.Burau D, Petroff D, Simon P, Ehmann L, Weiser C, Dorn C, et al. Drug combinations and impact of experimental conditions on relative recovery in in vitro microdialysis investigations. Eur J Pharm Sci. 2019;127:252–260. doi: 10.1016/j.ejps.2018.10.030. [DOI] [PubMed] [Google Scholar]
- 21.Simon P, Petroff D, Busse D, Heyne J, Girrbach F, Dietrich A, et al. Meropenem plasma and interstitial soft tissue concentrations in obese and nonobese patients-a controlled clinical trial. Antibiot (Basel, Switzerland) 2020 doi: 10.3390/antibiotics9120931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Tunblad K, Hammarlund-Udenaes M, Jonsson EN. An integrated model for the analysis of pharmacokinetic data from microdialysis experiments. Pharm Res. 2004;21:1698–1707. doi: 10.1023/B:PHAM.0000041468.00587.c6. [DOI] [PubMed] [Google Scholar]
- 23.Busse D, Schaeftlein A, Solms A, Ilia L, Michelet R, Zeitlinger M, et al. Which analysis approach is adequate to leverage clinical microdialysis data? A quantitative comparison to investigate exposure and reponse exemplified by levofloxacin. Pharm Res. 2021 doi: 10.1007/s11095-021-02994-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Mould DR, Upton RN. Basic concepts in population modeling, simulation, and model-based drug development—part 2: introduction to pharmacokinetic modeling methods. CPT Pharmacometrics Syst Pharmacol. 2013 doi: 10.1038/psp.2013.14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Anderson BJ, Holford NHG. Mechanism-based concepts of size and maturity in pharmacokinetics. Annu Rev Pharmacol Toxicol. 2008;48:303–332. doi: 10.1146/annurev.pharmtox.48.113006.094708. [DOI] [PubMed] [Google Scholar]
- 26.Holford N, Heo Y-A, Anderson B. A pharmacokinetic standard for babies and adults. J Pharm Sci. 2013;102:2941–2952. doi: 10.1002/jps.23574. [DOI] [PubMed] [Google Scholar]
- 27.McCarron MM, Devine BJ. Clinical pharmacy: case studies: case number 25 gentamicin therapy. Drug Intell Clin Pharm. 1974;8:650–655. doi: 10.1177/106002807400801104. [DOI] [Google Scholar]
- 28.Janmahasatian S, Duffull SB, Ash S, Ward LC, Byrne NM, Green B. Quantification of lean bodyweight. Clin Pharmacokinet. 2005;44:1051–1065. doi: 10.2165/00003088-200544100-00004. [DOI] [PubMed] [Google Scholar]
- 29.Schwartz SN, Pazin GJ, Lyon JA, Ho M, Pasculle AW. A controlled investigation of the pharmacokinetics of gentamicin and tobramycin in obese subjects. J Infectous Dis. 1978;138:499–505. doi: 10.1093/infdis/138.4.499. [DOI] [PubMed] [Google Scholar]
- 30.Cockcroft DW, Gault MH. Prediction of creatinine clearance from serum creatinine. Nephron. 1976;16:31–41. doi: 10.1159/000180580. [DOI] [PubMed] [Google Scholar]
- 31.Winter MA, Guhr KN, Berg GM. Impact of various body weights and serum creatinine concentrations on the bias and accuracy of the cockcroft-gault equation. Pharmacotherapy. 2012;32:604–612. doi: 10.1002/j.1875-9114.2012.01098.x. [DOI] [PubMed] [Google Scholar]
- 32.Levey AS, Coresh J, Greene T, Stevens LA, Zhang Y(, Hendriksen S, et al. Using standardized serum creatinine values in the modification of diet in renal disease study equation for estimating glomerular filtration rate. Ann Intern Med. 2006;145:247–254. doi: 10.7326/0003-4819-145-4-200608150-00004. [DOI] [PubMed] [Google Scholar]
- 33.Levey AS, Stevens LA, Schmid CH, Zhang Y(, Castro AF, Feldman HI, et al. A new equation to estimate glomerular filtration rate. Ann Intern Med. 2009;150:604–612. doi: 10.7326/0003-4819-150-9-200905050-00006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Vlasschaert C, Thibodeau S, Parmar MS. De-indexed estimated glomerular filtration rates: a simple step towards improving accuracy of drug dosing of renally excreted medications in moderate to severe obesity. Nephrology. 2020;25:29–31. doi: 10.1111/nep.13621. [DOI] [PubMed] [Google Scholar]
- 35.Mosteller RD. Simplified calculation of body-surface area. N Engl J Med. 1987;317:1098. doi: 10.1056/NEJM198710223171717. [DOI] [PubMed] [Google Scholar]
- 36.Erstad BL. Dosing of medications in morbidly obese patients in the intensive care unit setting. Intensive Care Med. 2004;30:18–32. doi: 10.1007/s00134-003-2059-6. [DOI] [PubMed] [Google Scholar]
- 37.Wählby U, Jonsson EN, Karlsson MO. Comparison of stepwise covariate model building strategies in population pharmacokinetic-pharmacodynamic analysis. AAPS PharmSci. 2002;4:68–79. doi: 10.1208/ps040427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Bonate PLPL. Pharmacokinetic-pharmacodynamic modeling and simulation. 2. New York: Springer; 2011. [Google Scholar]
- 39.Schießer S, Hitzenbichler F, Kees MG, Kratzer A, Lubnow M, Salzberger B, et al. Measurement of free plasma concentrations of beta-lactam antibiotics: an applicability study in intensive care unit patients. Ther Drug Monit. 2021;43:264–270. 10.1097/FTD.0000000000000827. [DOI] [PubMed]
- 40.Mouton JW, Dudley MN, Cars O, Derendorf H, Drusano GL. Standardization of pharmacokinetic/pharmacodynamic (PK/PD) terminology for anti-infective drugs: an update. J Antimicrob Chemother. 2005;55:601–607. doi: 10.1093/jac/dki079. [DOI] [PubMed] [Google Scholar]
- 41.Fritzenwanker M, Imirzalioglu C, Herold S, Wagenlehner FM, Zimmer K-P, Chakraborty T. Treatment options for carbapenem- resistant gram-negative infections. Dtsch Arztebl Int. 2018;115:345–352. doi: 10.3238/arztebl.2018.0345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Imani S, Marriott D, Buscher H, Sandaradura I, Gentili S. Too much of a good thing: a retrospective study of β-lactam concentration–toxicity relationships. J Antimicrob Chemother. 2017;72:2891–2897. doi: 10.1093/jac/dkx209. [DOI] [PubMed] [Google Scholar]
- 43.Post TM, Freijer JI, Ploeger BA, Danhof M. Extensions to the Visual Predictive Check to facilitate model performance evaluation. J Pharmacokinet Pharmacodyn. 2008;35:185–202. doi: 10.1007/s10928-007-9081-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Kuti JL, Nightingale CH, Knauft RF, Nicolau DP. Pharmacokinetic properties and stability of continuous-infusion meropenem in adults with cystic fibrosis. Clin Ther. 2004;26:493–501. doi: 10.1016/S0149-2918(04)90051-3. [DOI] [PubMed] [Google Scholar]
- 45.Chytra I, Stepan M, Benes J, Pelnar P, Zidkova A, Bergerova T, et al. Clinical and microbiological efficacy of continuous versus intermittent application of meropenem in critically ill patients: a randomized open-label controlled trial. Crit Care. 2012 doi: 10.1186/cc11405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Roberts JA, Abdul-Aziz M-H, Davis JS, Dulhunty JM, Cotta MO, Myburgh J, et al. Continuous versus intermittent β-lactam infusion in severe sepsis. A meta-analysis of individual patient data from randomized trials. Am J Respir Crit Care Med. 2016;194:681–691. doi: 10.1164/rccm.201601-0024OC. [DOI] [PubMed] [Google Scholar]
- 47.Ehmann L, Zoller M, Minichmayr IK, Scharf C, Huisinga W, Zander J, et al. Development of a dosing algorithm for meropenem in critically ill patients based on a population pharmacokinetic/pharmacodynamic analysis. Int J Antimicrob Agents. 2019;43:309–317. doi: 10.1016/j.ijantimicag.2019.06.016. [DOI] [PubMed] [Google Scholar]
- 48.Minichmayr IK, Roberts JA, Frey OR, Roehr AC, Kloft C, Brinkmann A. Development of a dosing nomogram for continuous-infusion meropenem in critically ill patients based on a validated population pharmacokinetic model. J Antimicrob Chemother. 2018;73:1330–1339. doi: 10.1093/jac/dkx526. [DOI] [PubMed] [Google Scholar]
- 49.Ehmann L, Zoller M, Minichmayr IK, Scharf C, Maier B, Schmitt MV, et al. Role of renal function in risk assessment of target non-attainment after standard dosing of meropenem in critically ill patients: a prospective observational study. Crit Care. 2017;21:263. doi: 10.1186/s13054-017-1829-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Liebchen U, Klose M, Paal M, Vogeser M, Zoller M, Schroeder I, et al. Evaluation of the MeroRisk calculator, a user-friendly tool to predict the risk of meropenem target non-attainment in critically ill patients. Antibiot. 2021 doi: 10.3390/antibiotics10040468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Moon YSK, Chung KC, Gill MA. Pharmacokinetics of meropenem in animals, healthy volunteers, and patients. Clin Infect Dis. 1997;24:249–255. doi: 10.1093/clinids/24.Supplement_2.S249. [DOI] [PubMed] [Google Scholar]
- 52.Meng L, Mui E, Holubar MK, Deresinski SC. Comprehensive guidance for antibiotic dosing in obese adults. Pharmacother J Hum Pharmacol Drug Ther. 2017;37:1415–1431. doi: 10.1002/phar.2023. [DOI] [PubMed] [Google Scholar]
- 53.Stefansson VTN, Schei J, Jenssen TG, Melsom T, Eriksen BO. Central obesity associates with renal hyperfiltration in the non-diabetic general population: a cross-sectional study. BMC Nephrol. 2016 doi: 10.1186/s12882-016-0386-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Ulldemolins M, Soy D, Llaurado-Serra M, Vaquer S, Castro P, Rodríguez AH, et al. Meropenem population pharmacokinetics in critically ill patients with septic shock and continuous renal replacement therapy: influence of residual diuresis on dose requirements. Antimicrob Agents Chemother. 2015;59:5520–5528. doi: 10.1128/AAC.00712-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Hanberg P, Öbrink-Hansen K, Thorsted A, Bue M, Tøttrup M, Friberg LE, et al. Population pharmacokinetics of meropenem in plasma and subcutis from patients on extracorporeal membrane oxygenation treatment. Antimicrob Agents Chemother. 2018 doi: 10.1128/AAC.02390-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Du X, Li C, Kuti JL, Nightingale CH, Nicolau DP. Population pharmacokinetics and pharmacodynamics of meropenem in pediatric patients. J Clin Pharmacol. 2006;46:69–75. doi: 10.1177/0091270005283283. [DOI] [PubMed] [Google Scholar]
- 57.Lee D-G, Choi S-M, Shin W-S, Lah H-O, Yim D-S. Population pharmacokinetics of meropenem in febrile neutropenic patients in Korea. Int J Antimicrob Agents. 2006;28:333–339. doi: 10.1016/j.ijantimicag.2006.05.033. [DOI] [PubMed] [Google Scholar]
- 58.Gonçalves-Pereira J, Póvoa P. Antibiotics in critically ill patients: a systematic review of the pharmacokinetics of β-lactams. Crit Care. 2011;15:R206. doi: 10.1186/cc10441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Li C, Kuti JL, Nightingale CH, Nicolau DP. Population pharmacokinetic analysis and dosing regimen optimization of meropenem in adult patients. J Clin Pharmacol. 2006;46:1171–1178. doi: 10.1177/0091270006291035. [DOI] [PubMed] [Google Scholar]
- 60.Frippiat F, Musuamba FT, Seidel L, Albert A, Denooz R, Charlier C, et al. Modelled target attainment after meropenem infusion in patients with severe nosocomial pneumonia: the PROMESSE study. J Antimicrob Chemother. 2015;70:207–216. doi: 10.1093/jac/dku354. [DOI] [PubMed] [Google Scholar]
- 61.McKenzie C. Antibiotic dosing in critical illness. J Antimicrob Chemother. 2011;66:25–31. doi: 10.1093/jac/dkq516. [DOI] [PubMed] [Google Scholar]
- 62.Kitzes-Cohen R, Farin D, Piva G, De Myttenaere-Bursztein SA. Pharmacokinetics and pharmacodynamics of meropenem in critically ill patients. Int J Antimicrob Agents. 2002;19:105–110. doi: 10.1016/S0924-8579(01)00474-5. [DOI] [PubMed] [Google Scholar]
- 63.Binder L, Schwörer H, Hoppe S, Streit F, Neumann S, Beckmann A, et al. Pharmacokinetics of meropenem in critically ill patients with severe infections. Ther Drug Monit. 2013;35:63–70. doi: 10.1097/FTD.0b013e31827d496c. [DOI] [PubMed] [Google Scholar]
- 64.Hites M, Taccone FS, Wolff F, Cotton F, Beumier M, De Backer D, et al. Case-control study of drug monitoring of β-lactams in obese critically ill patients. Antimicrob Agents Chemother. 2013;57:708–715. doi: 10.1128/AAC.01083-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.