Abstract
Objectives:
Low-threshold buprenorphine treatment aims to reduce barriers to evidence-based opioid use disorder treatment. We aimed to describe the treatment philosophy, practices, and outcomes of a low-threshold syringe services program (SSP)-based buprenorphine program developed through an SSP-academic medical center partnership.
Methods:
We included all SSP participants who received one or more buprenorphine prescription from Feb 5, 2019- October 9, 2020. We collected data on patient characteristics, substance use, buprenorphine prescriptions, and urine drug tests (UDTs). We evaluated buprenorphine treatment retention using prescription data and buprenorphine adherence using UDTs. We used two retention definitions: 1) percentage of patients with buprenorphine prescriptions at 30, 90 and 180 days; and 2) total percentage of days “covered” with buprenorphine prescriptions through 180 days.
Results:
One-hundred and eighteen patients received one or more buprenorphine prescriptions. Patients were largely middle-aged (mean age 44, SD 11), male (68%), Hispanic (31%) or Non-Hispanic Black (32%), with heroin (90%) and crack/cocaine (62%) use, and injection drug use (59%). Retention was 62%, 43% and 31% at 30, 90 and 180 days, respectively. The median percentage of days covered with buprenorphine prescriptions through 180 days was 43% (IQR 8–92%). Of the 82 patients who completed two or more UDTs, the median percentage of buprenorphine-positive UDTs was 71% (IQR 40–100%).
Conclusions:
In an SSP-based low-threshold buprenorphine treatment program, approximately one-third of patients continued buprenorphine treatment for 180 days or more, and buprenorphine adherence was high. SSPs can be a pathway to buprenorphine treatment for patients at high risk for opioid-related harms.
Introduction
Buprenorphine is a safe, effective, and underutilized opioid use disorder (OUD) treatment.1 People with OUD who have the greatest risk for opioid-related harms, including those with polysubstance use and injection drug use,2–7 often have difficulty engaging in office-based buprenorphine treatment.8 Programs with inflexible policies or those that require abstinence from all illicit substances create a high threshold for patients to enter and continue treatment.9,10 More accessible treatment models are necessary.
Low-threshold buprenorphine treatment prioritizes treatment access through same-day treatment entry, flexibility regarding program rules (e.g., missed appointments), availability in unconventional locations, and a harm reduction approach, which recognizes patient-identified treatment goals that may not include abstinence.11 A small number of studies have demonstrated the feasibility of providing low-threshold buprenorphine treatment in syringe services program (SSPs), homeless health programs, and mobile health settings.12–18
SSPs are ideally positioned to provide low-threshold buprenorphine treatment as they are trusted by many people with OUD, including those who do not inject drugs.19 SSPs provide diverse health promotion services, such as distributing naloxone, performing HIV or HCV testing, and providing mental health counseling to a populations at high-risk for fatal opioid overdose,2 and hepatitis C virus (HCV)3 and HIV transmission.4–7 While many people with OUD experience stigma in traditional health care settings, SSPs aim to provide non-judgmental services.20 SSP participants have reported preferring onsite buprenorphine treatment at the SSP rather than being referred to outside programs.21 Thus, SSPs could engage people with OUD who are reluctant to seek treatment elsewhere.
Though low-threshold buprenorphine treatment stresses access and flexibility, best practices for participant engagement and monitoring buprenorphine adherence in low-threshold settings are uncertain. Here we describe an innovative SSP-based buprenorphine treatment program, highlight the program’s treatment philosophy, and present data on patient outcomes.
Methods
Program description
Development
The SSP-based buprenorphine treatment program resulted from many years of collaboration between an academic medical center and an urban SSP.19,21–25 The SSP offered syringe exchange, safer injection counseling, overdose prevention training and naloxone distribution, fentanyl test strip distribution, rapid hepatitis C virus (HCV), HIV and sexually transmitted infection testing, case management, and support groups. The partnership began with SSP-based research and expanded to include clinical services. In February 2019, partners opened a medical clinic at the SSP’s office space to provide HCV treatment, HIV prevention, and buprenorphine treatment services. Staffing includes academic medical center employees (four physicians providing care three days per week, a program manager, and a care coordinator) and SSP employees (two registered nurses, one clinical care coordinator, and a peer educator). SSP employees work regular business hours (9 AM-4 PM). All visits are on a walk-in basis.
Program philosophy
The buprenorphine program is guided by principles of low-threshold treatment.11 Patients do not need an appointment to see a physician, and if buprenorphine treatment is indicated, they receive a prescription on the same day as their first evaluation. Patients may resume treatment if they have a gap in services. Consistent with a harm reduction approach, patient-centered treatment prioritizes patients’ own specific recovery goals. Broadly, the program’s philosophy emphasizes that during any period that SSP participants are taking buprenorphine– however short or long— their risk of opioid overdose, contracting infectious diseases, or criminal justice involvement will be reduced.26 Therefore, the program stresses treatment access, engagement, and re-engagement after lapses in treatment. The clinic also offers HCV treatment, HIV pre- and post-exposure prophylaxis, and short-term management of urgent and primary care needs. Patients who need comprehensive primary care are linked to the academic medical center.
Buprenorphine initiation
Providers take a focused substance use, medical and mental health history during the initial visit. The only contraindication to initiating buprenorphine treatment are absence of opioid use disorder and inability to follow a buprenorphine treatment plan (including initiation procedures) due to intoxication, uncontrolled mental illness, or cognitive impairment. As per Food and Drug Administration guidance, the risks of concomitant alcohol and benzodiazepine use and misuse are weighed against the risk of untreated OUD.27 During the first visit, providers review program expectations and counsel patients on unobserved ”home” initiation procedures.28 Patients are typically prescribed enough buprenorphine to take up to 16 mg daily for one week.
Monitoring
Providers adhere to OUD treatment guidelines29 while maintaining patient-centeredness and a harm reduction approach. The first follow-up visit is one week after the initial visit. Follow-up visit frequency is agreed upon by patients and providers and is generally shorter at the beginning of treatment as patients are stabilizing, and then monthly for patients who are adherent to buprenorphine and progressing toward their recovery goals. Urine drug tests (UDTs) are conducted at every in-person visit to evaluate buprenorphine adherence and promote conversations about substance use. The decision to include urine drug testing was informed by focus groups with SSP participants, which highlighted their concerns that if patients sold medication that was prescribed from the SSP, it could compromise the SSP’s operations.19 Opioid-positive UDTs do not prompt cessation of treatment but may lead to changes in visit frequency depending on the patient’s goals and preferences. Patients who have numerous opioid-positive and buprenorphine-negative UDTs are asked to come weekly until UDTs are positive for buprenorphine. If UDTs are consistently negative for buprenorphine, treatment may be discontinued (see section on Discharge).
Multidisciplinary clinic meetings
Staff members hold regular meetings to discuss clinic issues and conduct case conferencing. All clinical staff attend these meetings and occasionally other SSP staff, such as case managers, join to discuss patients who are struggling to meet their recovery goals. All staff contribute to a plan for supporting the patient.
Patient support
Nursing and clinical coordinators play key roles in maintaining continuity with patients because providers are only at the clinic three days per week. If patients are unable to attend an in-person visit, they can request buprenorphine refills through a medication hotline staffed by nursing and clinical coordinators. Providers may conduct a brief televisit with the patient or provide a bridging prescription until the patient can attend an in-person visit. Nursing and clinical coordinators help patients with insurance problems (prior authorizations, activating insurance); link patients to other SSP services, such as support groups, case management and housing support; and make appointments at outside facilities for mental health and primary care.
Discharge
The primary expectation of patients is adherence to buprenorphine. Generally, the only reason patients are discharged from treatment is if they have a pattern of buprenorphine-negative UDTs while being prescribed buprenorphine, suggesting non-adherence. Providers have multiple discussions with patients when they suspect non-adherence and multidisciplinary case conferencing focuses on extending supports. Discharged patients are welcome to return for treatment in the future, if they are ready to take buprenorphine. Discharged patients can continue to receive other harm reduction and clinical services.
COVID-19 pandemic
At the beginning of the COVID-19 pandemic, the SSP closed completely for a period of three weeks then resumed providing syringe exchange and naloxone distribution through a window four hours per day. During this time, a medication refill (including buprenorphine) hotline was created and staffed by nursing and clinical coordinators. Initially, the clinic referred participants interested in initiating buprenorphine elsewhere due to concerns about lack of provider and staff capacity. After several months, the clinic began accepting new patients by telehealth visit. The SSP opened to participants in June 2020 and the clinic reopened completely in October 2020. From the start of the pandemic in March 2020 to October 2020, only three patients completed telehealth buprenorphine initiations.
Evaluation of OUD treatment outcomes
We conducted a retrospective study of all patients seen at the SSP clinic who received one or more buprenorphine prescriptions from Feb 5, 2019- October 9, 2020. We evaluated treatment retention at 30, 90, and 180 days after treatment initiation and compared sociodemographic and clinical characteristics between patients retained at 180 days and those not retained at 180 days. We also examined whether patterns of buprenorphine adherence were associated with the amount of time patients remained in treatment using survival analysis. The study received IRB approval from the Albert Einstein College of Medicine.
Data collection
Data were manually extracted from two sources: an electronic database of SSP intake forms and the electronic health record (EHR). SSP staff members use standardized intake forms to collect information on all new SSP participants. All prescription and UDT data were abstracted from the EHR. Two clinicians (AJ and BH) reviewed EHR charts to evaluate buprenorphine treatment outcomes. Reasons for treatment discontinuation were ascertained by reviewing three notes in the EHR: the note dated closest to the date of the last prescription, and one note immediately before and after.
Main outcomes
We evaluated patient retention, buprenorphine adherence, and illicit opioid use over time. We operationalized retention in two ways: 1) time in treatment during the first treatment episode (we report continuous days in treatment and percentage retained at 30, 90 and 180 days); and 2) total percentage of days “covered” with a buprenorphine prescription during the first 180 days of treatment, which could span multiple treatment episodes. We chose these definitions because the first allows for comparison to the buprenorphine treatment literature, while the second reflects our flexibility toward entering and exiting treatment. Buprenorphine adherence and use of illicit opioids were based on UDTs.
Continuous days in treatment during the first episode
We examined the time between the first day of the first prescription and the last day of the last prescription with no gaps greater than 30 days.13 Prescriptions written after a gap greater than 30 days were categorized as belonging to the next treatment episode. For survival analysis, treatment “failure” was defined as patient self-discharge, provider discharge due to non-adherence or other reason, or patients requiring a higher level of OUD treatment (transfer to an opioid treatment program (OTP) or an inpatient program). Patients were censored if they were still in treatment at the end of the study period (either at the SSP or at the academic medical center), were incarcerated, died, experienced a disruption in treatment due to medical illness, or moved out of state.
Percentage retained at 30, 90, and 180 days during the first treatment episode
We evaluated retention at 30, 90 and 180 days after treatment initiation as a dichotomous measure (retained vs. no longer in treatment). Retention was defined as having greater than or equal to 30, 90 and 180 continuous days in treatment, as defined above.
Buprenorphine Coverage
We defined coverage as the percentage of days with an active buprenorphine prescription during the first 180 days after starting treatment. Covered days did not need to be continuous and could span all treatment episodes.
Baseline substance use
Baseline substance use was defined as either the presence of the substance on the first UDT completed at the clinic or self-reported current substance use from the standardized intake form. Alcohol was not evaluated with UDTs, so data is only based on data from the intake form. Methadone use was based only on UDTs.
Adherence to buprenorphine and illicit opioid use
Adherence to buprenorphine was defined as presence of buprenorphine on UDTs. Norbuprenorphine testing was inconsistent and therefore was not used to evaluate adherence. Illicit opioid use was defined as the presence of opiates, methadone, or oxycodone. Adherence to buprenorphine and illicit opioid use were evaluated for all patients with two or more UDTs completed.
Patterns of UDTs
We categorized patients by whether the majority (greater than 50%) of their urine samples were: 1) buprenorphine-positive/other opioid-negative (high adherence); 2) buprenorphine-positive/other opioid-positive (high adherence, persistent opioid use); 3) buprenorphine-negative/opioid-positive or negative (low adherence); or 4) mixed– a combination of buprenorphine positive/opioid negative, buprenorphine-positive/opioid-positive and buprenorphine negative UDTs without any majority (intermittent adherence). We chose to categorize patients this way because we hypothesized that patients who were generally adherent to buprenorphine would have longer time in treatment than patients who were usually non-adherent to buprenorphine.
Covariates
Data on housing and baseline substance use were derived from intake forms. Housing was categorized as 1) homeless (street or shelter), 2) permanent housing (own or rent home or apartment), 3) staying with family or friends; and 4) residential drug treatment, group home or transitional housing. Data on patient age, sex, race and ethnicity were abstracted from the EHR.
Statistical analysis
Patient sociodemographic characteristics are reported as frequencies. We report the number and percentage of patients with one or more treatment episode and categorized patients by number of prescriptions received. For time-in treatment and percentage of patients retained, we restricted our analysis to the first treatment episode because the majority of patients had a single episode. We generated survival curves and report the median time in treatment. We report the median percentage of opioid-positive and buprenorphine-positive UDTs among patients with two or more UDTs, number and percentage of patients with buprenorphine-positive and opioid-negative UDTs over time, and the number and percentage of patients with different UDT patterns. We generated survival curves by UDT pattern and compared time in treatment between the groups using the log rank test. Additionally, we report the percentage of patients retained at 30, 90, and 180 days after initiating buprenorphine. We evaluated associations between patient characteristics and 180-day retention using t-tests, chi-squared tests, and Fisher’s exact tests where appropriate.
Results
Patient characteristics and baseline substance use
From Feb 5, 2019- October 9, 2020, 118 patients received one or more buprenorphine prescriptions at the SSP clinic. Sociodemographic characteristics and substance use data for these 118 patients are presented in Table 1. Patients were largely middle aged (mean age 44, SD 11), male (68%), Hispanic (31%) or Non-Hispanic Black (32%), homeless (46%), with current heroin (90%) and crack/cocaine (62%) use and current injection drug use (59%).
Table 1.
Characteristics of patients who received one or more buprenorphine prescriptions from Feb 5, 2019- October 9, 2020 (N=118)
| Total (N=118) | Retained at 180 days1 | Not retained at 180 days1 | p-value2 | |
|---|---|---|---|---|
|
| ||||
| N (%) | 36 (31) | 81 (69) | ||
|
| ||||
| Age Mean (SD) | 44 (11) | 46 (9) | 43 (12) | 0.31 |
|
| ||||
| Male | 81 (68) | 25 (69) | 55 (68) | 0.89 |
|
| ||||
| Race/ethnicity | 0.20 | |||
| Hispanic | 37 (31) | 15 (42) | 22 (27) | |
| Non-Hispanic Black | 38 (32) | 12 (33) | 26 (32) | |
| Non-Hispanic White | 26 (21) | 7 (19) | 18 (22) | |
| Non-Hispanic Other | 17 (14) | 2 (6) | 15 (19) | |
|
| ||||
| Housing 3 | ||||
|
| ||||
| Homeless (street or shelter) | 50 (46) | 16 (46) | 33 (45) | 0.08 |
|
| ||||
| Permanent housing | 24 (22) | 9 (26) | 15 (21) | |
|
| ||||
| With family or friends | 19 (17) | 2 (6) | 17 (23) | |
|
| ||||
| Residential drug treatment, group home, transitional housing | 16 (15) | 8 (23) | 8 (11) | |
|
| ||||
| Current substances used 4 | ||||
| Heroin | 106 (90) | 31 (86) | 74 (91) | 0.28 |
| Crack/Cocaine | 73 (62) | 23 (64) | 50 (62) | 0.82 |
| Cannabis | 57 (48) | 17 (47) | 40 (49) | 0.83 |
| Benzodiazepines5 | 39 (33) | 15 (42) | 24 (30) | 0.22 |
| Alcohol5 | 33 (28) | 13 (36) | 20 (25) | 0.22 |
| Other opiates/analgesics5 | 23 (20) | 7 (19) | 16 (20) | 0.95 |
| Methadone6 | 16 (14) | 4 (11) | 12 (16) | 0.49 |
| Amphetamines | 11 (9) | 3 (8) | 8 (10) | 1.00 |
|
| ||||
| Injection drug use | ||||
|
| ||||
| Current5 | 69 (59) | 15 (42) | 53 (66) | 0.01 |
|
| ||||
| Ever | 83 (70) | 22 (61) | 60 (74) | 0.16 |
|
| ||||
N=117 patients included in analyses of 180-day retention. One patient excluded due to insufficient follow-up time.
p-values are for t-tests, Pearson chi-squared or Fisher’s exact tests, where appropriate
N=109 due to missing data
Defined as either the presence of the substance on the first urine drug test completed at the clinic or self-reported current substance use from the standardized intake form.
N=117 due to missing data
N=111 due to missing data
Number of treatment episodes and prescriptions received
Seventy-eight percent of patients had a single treatment episode. Twenty percent had two episodes, and two percent had three episodes. Twenty-six percent of patients received only one buprenorphine prescription, 24% received two to four, and 50% received five or more prescriptions.
Treatment retention and buprenorphine coverage
Of the 118 patients who received one or more buprenorphine prescriptions, one patient was excluded from the analysis because they initiated buprenorphine fewer than 30 days before the end of the observation period. The other 117 patients were all observed for 180 or more days so were included in analyses of 30, 90 and 180 day retention. Sixty-two percent were retained for 30 or more days, 43% for 90 or more days, and 31% for 180 or more days. The median percentage of days covered with a buprenorphine prescription during the first 180 days was 43% (IQR 8–92%).
Association between 180-day retention and patient characteristics
Associations between 180-day retention and patient characteristics are presented in Table 1. Compared with patients who were not retained at 180 days, those that were retained had lower baseline injection drug use (42% vs. 66%, p=0.01).
Time in treatment for the first treatment episode
The Kaplan-Meier survival curve is presented in Figure 1. Median time in treatment was 64 days (IQR 8–253). Of the 118 patients who received one or more buprenorphine prescriptions, 22% were censored (14% still in treatment at the end of the study period, three percent were incarcerated, three percent moved out of state, one percent experienced a disruption in treatment due to medical illness, and one percent died), and 78% experienced treatment “failure” (64% self-discharged, six percent were discharged due to non-adherence or other reason, five percent transferred to an OTP, and three percent transferred to an inpatient program).
Figure 1.
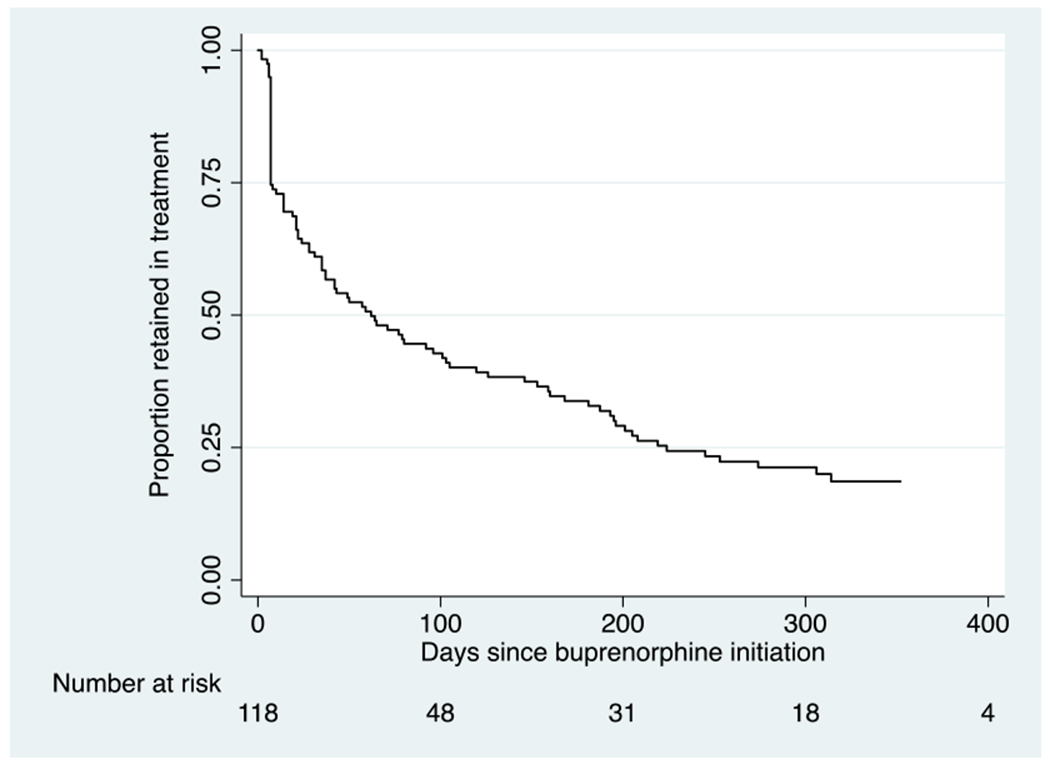
Time in treatment during the first treatment episode for all patients receiving one or more buprenorphine prescriptions (N=118)
Adherence to buprenorphine and illicit opioid use
One-hundred and eleven patients completed one or more UDTs. The median number of UDTs collected per patient was 5 (IQR 1–9). Eighty-two patients completed two or more UDTs. Of these 82 patients, the median percentage of buprenorphine-positive UDTs was 71% (IQR 40–100%) and opioid-positive UDTs was 50% (IQR 11–100%). The percentage of buprenorphine-positive and opioid-negative UDTs over time is presented in Figure 2.
FIGURE 2.
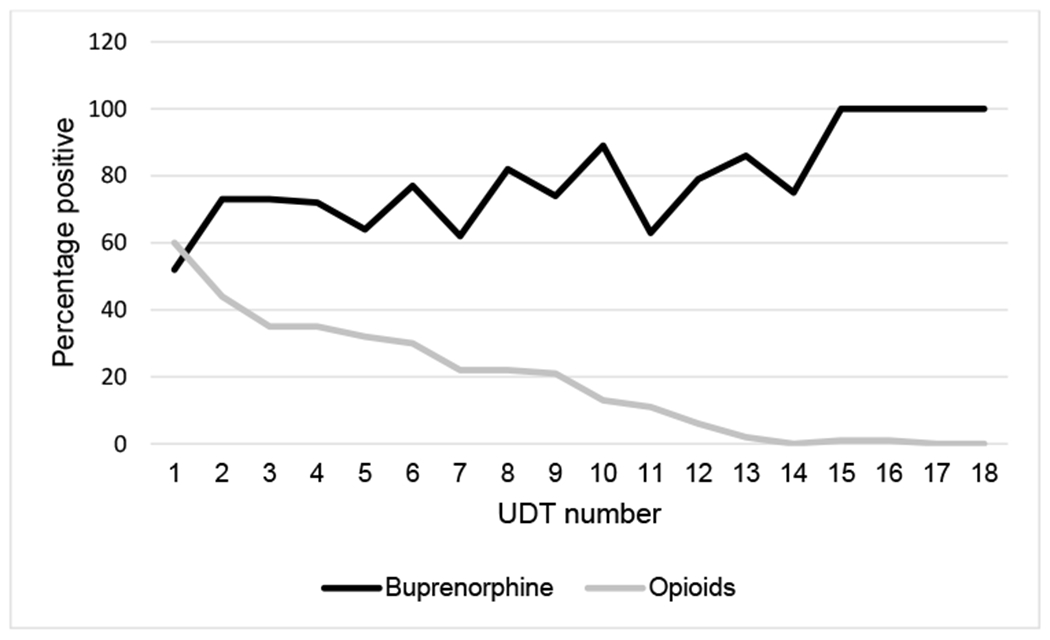
Percentage of buprenorphine and opioid positive UDTs over time among patients completing 2 or more UDTs (N ¼ 82). UDTs indicates urine drug tests.
Patterns of UDTs
Of the 82 patients who completed two or more UDTs, 34% had a majority of buprenorphine-positive/opioid-negative UDTs, 15% had a majority of buprenorphine-positive/opioid-positive UDTs, 27% had a majority of buprenorphine-negative (opioid-positive or opioid-negative) UDTs, and 24% had a mixed pattern without any majority.
Time in treatment by UDT pattern
The Kaplan-Meier survival curve by UDT pattern is presented in Figure 3. There were statistically significant differences in time in treatment by UDT pattern (Log-rank p<0.05). In some categories fewer than 75% of patients experienced treatment “failure”, in which case the upper limit of the IQR is denoted by an asterisk. Median days in treatment was 306 (IQR 103-*) for patients with majority buprenorphine-positive/opioid-negative UDTs, 168 (IQR 42–245) for buprenorphine-positive/opioid-positive, 195 days (IQR 43-*) for patients with a mixed pattern, and 49 days (IQR 22–71) for patients with majority buprenorphine-negative UDTs. Of the 22 patients with majority buprenorphine-negative UDTs (opioid positive and opioid negative), 13 self-discharged, five were discharged by a provider, three transferred to an OTP, and one was incarcerated.
Figure 3.
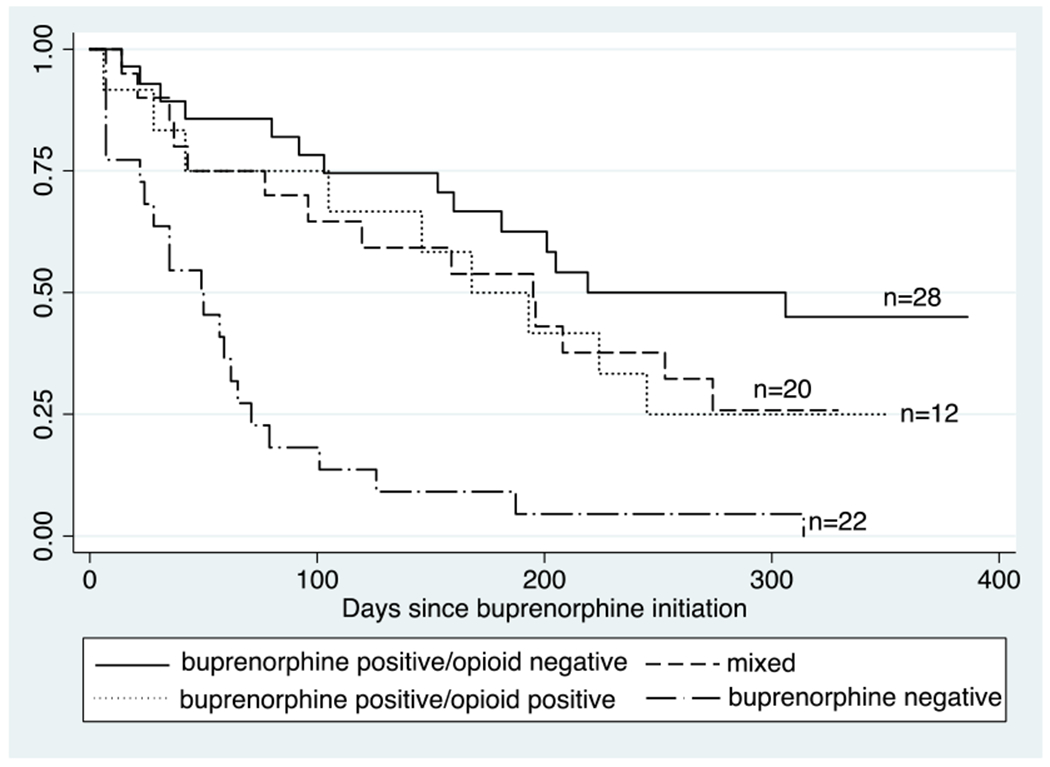
Time in treatment by UDT pattern among patients completing two or more UDTs (N=82)
Log rank p<0.05
Discussion
An academic medical center and an SSP collaborated to engage patients at high risk for opioid-related harms in buprenorphine treatment. Using UDT data, we identified four patterns of buprenorphine adherence and illicit opioid use. Even in this low threshold setting, many patients were adherent to buprenorphine and abstinent from illicit opioids. Importantly, treatment retention was only low among patients who struggled with buprenorphine adherence, and most of these patients stopped treatment on their own. These data suggest that when buprenorphine treatment is easily accessible, patients “self-select” to continue or stop treatment, likely based on whether or not it is helping them. Allowing patients to then re-engage in OUD treatment when they are ready in the future is important for continued engagement.
Our study highlights the benefits and challenges associated with low-threshold treatment. Among retained patients, buprenorphine adherence was high and illicit opioid use low, as has been reported in other low-threshold settings.13,14 Despite homelessness and polysubstance use, 31% of patients were retained at 180 days. This is comparable to other low barrier programs described in the literature (28% at a mobile clinic in Philadelphia,17 27% at a street medicine based-program in San Francisco,12 31% in an SSP in NYC),15 but lower than retention reported from traditional office-based buprenorphine treatment programs (typically, 40–70%).30 These differences in retention likely stem from selection bias – whereas patients accessing SSPs have more risk factors for treatment discontinuation, such as polysubstance and injection drug use, than patients accessing traditional office-based programs. It is therefore reassuring that this low threshold model provides access to a “hard to reach” population, and a plurality of patients do well with treatment. That said, there is still room for improving treatment retention in low-threshold settings.
Improving medication adherence and retention in low-threshold settings will likely require multi-pronged approaches. More robust patient navigation services could potentially improve retention. When buprenorphine treatment was integrated into HIV treatment settings, dedicated buprenorphine coordinators sustained programs by providing counseling, case management services and facilitating communication between patients and providers.31 In other settings, peer recovery coaches have increased patient engagement in buprenorphine treatment by providing motivational support, assistance with psychosocial treatment barriers, system navigation and behavior change and harm reduction counseling.32 Onsite mental health treatment for individuals with co-occurring anxiety, depression, and PTSD may also improve outcomes.33 Ongoing research should examine interventions to improve retention in low-threshold settings.
Another important component of low-threshold treatment is allowing for breaks in treatment. One-fifth of patients in this study had two or more care episodes, and in another SSP-based program one-third of patients had multiple care episodes.13 Our analysis based on UDT patterns suggest that half of participants (low and intermittent adherence groups) had periods where they were not taking prescribed buprenorphine. Not all patients wish to engage continuously with buprenorphine treatment. Because opioid overdose mortality is greatly reduced during periods when people with OUD receive buprenorphine,34 patients who want to re-engage in care should be able to do so easily and without shame.
This study has limitations. Our outcomes were limited by data collected during routine clinical care. Buprenorphine-positive UDTs are an estimate, not a true measure of adherence. We did not examine patient experiences with receiving care in this setting. Future studies could evaluate patient experiences with receiving low-threshold services at SSPs and examine other outcomes such as quality of life, mental and physical health, housing and employment to evaluate treatment outcomes from a more holistic and patient-centered perspective.
Conclusions
Onsite buprenorphine treatment engages a population at high risk for opioid-related harms. Nearly one-third of patients were continuously retained in treatment for 180 days. Non-adherent patients tended to self-discharge. These data indicate that a significant number of patients benefit from buprenorphine treatment in low-threshold settings. Buprenorphine treatment should be expanded in SSPs and other low-threshold settings such as homeless shelters and mobile health sites.. Efforts should be made to support patient engagement in these settings to maximize the time patients receive lifesaving OUD treatment.
Acknowledgements
Aaron D. Fox is supported by R01 DA044878 (NIH/NIDA). Andrea Jakubowski is funded by K12 HS026396–03 (AHRQ). Brianna L. Norton is supported by K23 DA039060 (NIH/NIDA). This research is supported by NIAID P30AI124414.
Sources of support:
Aaron D. Fox is supported by R01 DA044878 (NIH/NIDA)
Andrea Jakubowski is funded by K12 HS026396–03 (AHRQ)
Brianna L. Norton is supported by K23 DA039060 (NIH/NIDA)
This research is supported by NIAID P30AI124414
References
- 1.Huhn AS, Dunn KE. Why aren’t physicians prescribing more buprenorphine? J Subst Abuse Treat. 2017;78:1–7. doi: 10.1016/j.jsat.2017.04.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Piper TM, Rudenstine S, Stancliff S, et al. Overdose prevention for injection drug users: lessons learned from naloxone training and distribution programs in New York City. Harm Reduct J. 2007;4:3. doi: 10.1186/1477-7517-4-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Zibbell JE, Asher AK, Patel RC, et al. Increases in Acute Hepatitis C Virus Infection Related to a Growing Opioid Epidemic and Associated Injection Drug Use, United States, 2004 to 2014. Am J Public Health. 2018;108(2):175–181. doi: 10.2105/AJPH.2017.304132 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Burnett JC, Broz D, Spiller MW, Wejnert C, Paz-Bailey G. HIV infection and HIV-associated behaviors among persons who inject drugs - 20 cities, United States, 2015. Morb Mortal Wkly Rep. 2018;67(1):23–28. doi: 10.15585/mmwr.mm6701a5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Golden MR, Lechtenberg R, Glick SN, et al. Outbreak of human immunodeficiency virus infection among heterosexual persons who are living homeless and inject drugs — Seattle, Washington, 2018. Morb Mortal Wkly Rep. 2019;68(15):344–349. doi: 10.15585/mmwr.mm6815a2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Peters PJ, Pontones P, Hoover KW, et al. HIV infection linked to injection use of oxymorphone in Indiana, 2014–2015. N Engl J Med. 2016;375(3):229–239. doi: 10.1056/NEJMoa1515195 [DOI] [PubMed] [Google Scholar]
- 7.Evans ME, Labuda SM, Hogan V, et al. HIV infection investigation in a rural area — West Virginia, 2017. Morb Mortal Wkly Rep. 2018;67(8):257–258. doi: 10.15585/mmwr.mm6708a6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Simon CB, Tsui JI, Merrill JO, Adwell A, Tamru E, Klein JW. Linking patients with buprenorphine treatment in primary care: Predictors of engagement. Drug Alcohol Depend. 2017;181(October):58–62. doi: 10.1016/j.drugalcdep.2017.09.017 [DOI] [PubMed] [Google Scholar]
- 9.Mclean K, Kavanaugh PR. “They’re making it so hard for people to get help:” Motivations for non-prescribed buprenorphine use in a time of treatment expansion. 2019. doi: 10.1016/j.drugpo.2019.06.019 [DOI] [PubMed] [Google Scholar]
- 10.Gryczynski J, Mitchell SG, Jaffe JH, O’Grady KE, Olsen YK, Schwartz RP. Leaving buprenorphine treatment: Patients’ reasons for cessation of care. J Subst Abuse Treat. 2014;46(3):356–361. doi: 10.1016/j.jsat.2013.10.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Jakubowski A, Fox A. Defining Low-threshold Buprenorphine Treatment. J Addict Med. September 2019:1. doi: 10.1097/ADM.0000000000000555 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Carter J, Zevin B, Lum PJ. Low barrier buprenorphine treatment for persons experiencing homelessness and injecting heroin in San Francisco. Addict Sci Clin Pract. 2019;14(1):20. doi: 10.1186/s13722-019-0149-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hood JE, Banta-Green CJ, Duchin JS, et al. Engaging an unstably housed population with low-barrier buprenorphine treatment at a syringe services program: Lessons learned from Seattle, Washington. Subst Abus. August 2019:1–9. doi: 10.1080/08897077.2019.1635557 [DOI] [PubMed] [Google Scholar]
- 14.Bachhuber MA, Thompson C, Prybylowski A, Benitez J, Mazzella S, Barclay D. Description and outcomes of a buprenorphine maintenance treatment program integrated within Prevention Point Philadelphia, an urban syringe exchange program. Subst Abus. 2018;39(2):167–172. doi: 10.1080/08897077.2018.1443541 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Stancliff S, Joseph H, Fong C, Furst T, Comer SD, Roux P. Opioid maintenance treatment as a harm reduction tool for opioid-dependent individuals in New York City: the need to expand access to buprenorphine/naloxone in marginalized populations. J Addict Dis. 2012;31(3):278–287. doi: 10.1080/10550887.2012.694603 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hill K, Nussdorf L, Mount JD, et al. Initiation of Low-threshold Buprenorphine in Nontreatment Seeking Patients With Opioid Use Disorder Engaged in Hepatitis C Treatment. 2021;00(00):1–8. doi: 10.1097/ADM.0000000000000807 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.O’Gurek DT, Jatres J, Gibbs J, Latham I, Udegbe B, Reeves K. Expanding buprenorphine treatment to people experiencing homelessness through a mobile, multidisciplinary program in an urban, underserved setting. J Subst Abuse Treat. 2021;127(August 2020):108342. doi: 10.1016/j.jsat.2021.108342 [DOI] [PubMed] [Google Scholar]
- 18.Krawczyk N, Buresh M, Gordon MS, Blue TR, Fingerhood MI, Agus D. Expanding low-threshold buprenorphine to justice-involved individuals through mobile treatment: Addressing a critical care gap. J Subst Abuse Treat. 2019;103:1–8. doi: 10.1016/j.jsat.2019.05.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Frost T, Deutsch S, Brown S, et al. “ We ‘ ll be able to take care of ourselves ” – A qualitative study of client attitudes toward implementing buprenorphine treatment at syringe services programs. Subst Abus. 2021;0(0):1–13. doi: 10.1080/08897077.2021.1901173 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Van Boekel LC, Brouwers EPM, Van Weeghel J, Garretsen HFL. Stigma among health professionals towards patients with substance use disorders and its consequences for healthcare delivery: Systematic review. Drug Alcohol Depend. 2013;131(1–3):23–35. doi: 10.1016/j.drugalcdep.2013.02.018 [DOI] [PubMed] [Google Scholar]
- 21.Fox AD, Chamberlain A, Sohler NL, Frost T, Cunningham CO. Illicit buprenorphine use, interest in and access to buprenorphine treatment among syringe exchange participants. J Subst Abuse Treat. 2015;48(1):112–116. doi: 10.1016/j.jsat.2014.07.015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Fox AD, Shah PA, Sohler NL, Lopez CM, Starrels JL, Cunningham CO. I heard about it from a friend: Assessing interest in buprenorphine treatment. Subst Abus. 2014;35(1):74–79. doi: 10.1080/08897077.2013.804484 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Fox AD, Chamberlain A, Frost T, Cunningham CO. Harm reduction agencies as a potential site for buprenorphine treatment. Subst Abus. 2015;36(2):155–160. doi: 10.1080/08897077.2015.1011820 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Fox AD, Sohler NL, Frost T, Lopez C, Cunningham CO. Development and evaluation of a community-based buprenorphine treatment intervention. Harm Reduct J. 2017;14(1):23. doi: 10.1186/s12954-017-0149-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Sohler NL, Weiss L, Egan JE, et al. Consumer attitudes about opioid addiction treatment: A focus group study in New York City. J Opioid Manag. 2013;9(2):111–119. doi: 10.5055/jom.2013.0152 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Santo T, Clark B, Hickman M, et al. Association of Opioid Agonist Treatment With All-Cause Mortality and Specific Causes of Death Among People With Opioid Dependence. JAMA Psychiatry. 2021:1–14. doi: 10.1001/jamapsychiatry.2021.0976 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Center for Drug Evaluation and Research. Drug Safety and Availability - FDA Drug Safety Communication: FDA urges caution about withholding opioid addiction medications from patients taking benzodiazepines or CNS depressants: careful medication management can reduce risks. FDA.gov. 2017;2:1–6. [Google Scholar]
- 28.Cunningham CO, Giovanniello A, Li X, Kunins HV., Roose RJ, Sohler NL. A comparison of buprenorphine induction strategies: Patient-centered home-based inductions versus standard-of-care office-based inductions. J Subst Abuse Treat. 2011;40(4):349–356. doi: 10.1016/j.jsat.2010.12.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.New York State Department of Health AIDS Institute Clinical Guidelines Program. Substance Use Disorder Guideline Committee: Treatment of Opioid Use Disorder; 2021. https://cdn.hivguidelines.org/wp-content/uploads/20210129154949/NYSDOH-AI-Treatment-of-Opioid-Use-Disorder_1-29-2021_HG.pdf. Accessed June 8, 2021.
- 30.Timko C, Schultz NR, Cucciare MA, et al. Retention in medication-assisted treatment for opiate dependence: A systematic review. J Addict Dis. 2016;35(1):22–35. doi: 10.1080/10550887.2016.1100960.Retention [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Weiss L, Netherland J, Egan JE, et al. Integration of buprenorphine/naloxone treatment into HIV clinical care: Lessons from the BHIVES collaborative. J Acquir Immune Defic Syndr. 2011;56(SUPPL. 1):68–75. doi: 10.1097/QAI.0b013e31820a8226 [DOI] [PubMed] [Google Scholar]
- 32.Magidson JF, Regan S, Powell E, et al. Peer recovery coaches in general medical settings: Changes in utilization, treatment engagement, and opioid use. J Subst Abuse Treat. 2021;122(June 2020):108248. doi: 10.1016/j.jsat.2020.108248 [DOI] [PubMed] [Google Scholar]
- 33.Carroll KM, Weiss RD. The Role of Behavioral Interventions in Buprenorphine Maintenance Treatment: A Review. Am J Psychiatry. 2017;174(8):738–747. doi: 10.1176/appi.ajp.2016.16070792 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Larochelle MR, Bernson D, Land T, et al. Medication for Opioid Use Disorder After Nonfatal Opioid Overdose and Association With Mortality: Ann Intern Med. 2018;169(3):137–145. doi: 10.1007/s13679-019-00335-3.Metformin [DOI] [PMC free article] [PubMed] [Google Scholar]


