Abstract
Background
Individuals with a higher lifelong cardiorespiratory fitness show better vascular health with aging. Studies on fitness-related effects on endothelial function either analyzed samples with a narrow age-range or incompletely assessed endothelial responsiveness. This study aims to assess the impact of cardiorespiratory fitness on the association of brachial-arterial flow-mediated vasodilation (FMD) and low flow-mediated vasoconstriction (L-FMC) with age in healthy adults and patients with cardiovascular diseases.
Methods
FMD, L-FMC and O2peak were prospectively measured in a population-based sample including 360 healthy adults and 99 patients with cardiovascular disease of European descend. Non-linear models were applied to assess O2peak-associated variations in age-related differences of endothelial function independent of classical cardiovascular risk factors.
Results
FMD was negatively associated with age in healthy adults (adjusted R2 = 0.27, partial R2 = 0.07, p < 0.001) and in cardiovascular patients (adjusted R2 = 0.29, partial R2 = 0.05, p = 002). L-FMC showed no association with age. In models predicting the change of FMD with higher age, O2peak accounted for 2.8% of variation in FMD (χ2(5) = 5.37, p = 0.372, s = 1.43). Thereby, O2peak-stratified changes of FMD started to fan out at around 30 years of age in women and 50 years of age in men, with 7–12% lower values at old age with O2peak ≤3rd percentile compared to O2peak ≥97th percentile) in both, the healthy sample and in cardiovascular patients.
Conclusion
The statistical effect of cardiorespiratory fitness on the association of FMD with age independent of classical cardiovascular risk factors was small in both, healthy aging adults as well as patients with cardiovascular diseases. Its clinical significance should be assessed further.
Keywords: aging, cardiovascular disease, primary prevention, secondary prevention, ultrasound, peripheral vascular disease, atherosclerosis
Introduction
Cardiorespiratory fitness is a strong predictor of cardiovascular morbidity and mortality (1, 2). However, less than 50% of the protective effects of regular exercise on the cardiovascular system can be explained by traditional risk factors (3–5). The remaining risk factor gap of more than 50% may, at least partly, be explained by direct effects of exercise-induced hemodynamic changes on functional and structural properties of the vascular walls (6, 7).
This has been demonstrated with brachial arterial flow-mediated vasodilation (FMD), which reflects nitric oxide dependent vasodilator responsiveness of the endothelium toward acute changes in shear stress (8–10). Studies demonstrated that FMD can be improved by exercise, especially when endothelial function is already impaired (11, 12). This seems to be associated to higher peak oxygen consumption (O2peak), the gold-standard biomarker of cardiorespiratory fitness (13). However, previous works on samples including individuals of a specific age infrequently report an association between O2peak and FMD (14). Especially in young healthy individuals, FMD appears independent of O2peak (15). Instead, two studies in confined samples of young and older adults found favorable cross-sectional associations of O2peak with low flow-mediated constriction (L-FMC), which reflects the endothelial vasoconstrictor responsiveness toward altered shear stress (16, 17). Therefore, FMD and L-FMC have been supposed as complementary biomarkers of endothelial function (18, 19).
Recently, age-related differences of FMD and L-FMC were demonstrated in a healthy European population sample (20). Some evidence indicates that a lifelong high O2peak might effectively support preservation of good endothelial function into old age (13, 15). It was speculated that O2peak might modify the association of FMD with age, more so in the elderly compared to young adults (15). Finally, a recent meta-analysis concluded that existing evidence is inconclusive about whether exercise, irrespective of its impact on O2peak, is effective to improve FMD in previously sedentary older adults (21). Accordingly, adaptive capacities of the vascular endothelium toward exercise might vary across the life span and depend on the amount of high-intensity exercise. However, direct effects of O2peak on the association of endothelial function with age, independent of classical cardiovascular risk factors, have only been inconsistently studied so far, and only endothelial vasodilator function (FMD), but not vasoconstrictor function (L-FMC), has been analyzed.
In addition, no studies involving patients with cardiovascular diseases exist, that include both, FMD and L-FMC. However, FMD and L-FMC are impaired with apparent cardiovascular diseases, such as heart failure (22), coronary artery disease (18) and arterial hypertension (23). In these patients, increased O2peak was associated with improved FMD (24, 25) and L-FMC (26), leading to lower rates of morbidity and mortality (27).
A better understanding of the interactions between cardiorespiratory fitness, aging and endothelial function is necessary to improve precision of exercise-based prescriptions in promotion of healthy aging. Therefore, this study analyzed the impact of cardiorespiratory fitness on the interrelation between endothelial function and age in healthy adults and cardiovascular patients. Specifically, it investigated (I) whether age is independently associated with FMD and L-FMC throughout adult lifespan and (II) whether O2peak moderates age-related differences of endothelial function. Based on existing evidence (15, 28), the assumption is that age is associated with FMD and L-FMC in a non-linear relationship and that age-related differences of vascular biomarkers depend on the level of O2peak.
Materials and Methods
Study Design and Participant Characteristics
This prospective study was approved by the Ethics Committee of Northwestern and Central Switzerland (EKNZ 2017–01451) and conducted between 2018 and 2020 according to the Declaration of Helsinki. Written informed consent was obtained from all study participants before study participation. It was part of the COmPLETE-project (29). A detailed description of the study design is presented elsewhere (29). In summary, the project aimed to analyze age-related differences in physical functioning and to assess the role of physical fitness in this context. For the current study, targeting the interplay between cardiorespiratory fitness and vascular aging, 564 healthy adults between 20 and 91 years of age were recruited based on a randomized and blinded invitation procedure involving rural and urban areas. 144 patients with cardiovascular diseases aged 26 to 91 years stemmed from the vicinity of the cantons Basel-City and Basel-Country. Details about the recruitment procedure can be found elsewhere (29). After post-procedural data cleaning, full datasets of 360 healthy participants and 99 patients with cardiovascular diseases were used for statistical analyses (Figure 1). Empiric and anthropometric data were collected during a medical interview and examination (29).
FIGURE 1.
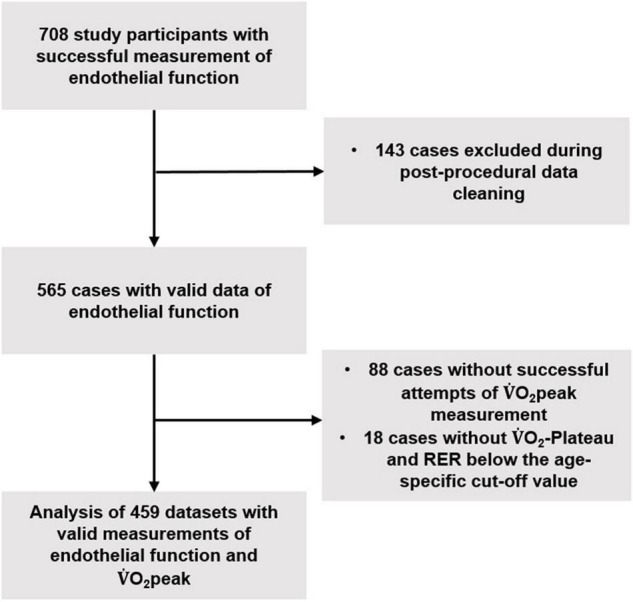
Flow-chart of data acquisition and cleaning. O2peak = peak oxygen consumption, RER = respiratory exchange quotient.
Measurement of Endothelial Function
FMD and L-FMC were measured semi-automatically with an ECG-guided high-resolution B-mode ultrasound system (UNEX-EF-38G, UNEX Corp., Nagoya, Japan) following a standardized procedure according to up-to-date methodological recommendations (30). Excellent reliability and precision of this method have been demonstrated before (31). Details of measurement procedure, post-procedural data cleaning and automatic calculation of FMD and L-FMC have been described elsewhere (20). Importantly, lower L-FMC values indicate better vasoconstrictor responsiveness, whereas higher FMD values indicate better vasodilator responsiveness.
Measurement of Cardiorespiratory Fitness
Cardiopulmonary exercise testing was conducted on an electromagnetically braked cycle ergometer (Ergoselect-200; Ergoline, Bitz, Germany). Gas exchange and ventilatory variables were continuously analyzed (breath-by-breath) using a computer-based system (MetaMax-3B; Cortex Biophysik GmbH, Leipzig, Germany). Before and during tests, participants were encouraged to reach their individual level of maximal exertion (i.e., volitional exhaustion, dyspnea, or fatigue). O2peak was defined as the highest 30-second average of O2 at any point during the test. Participants without a O2-plateau who did not fulfill secondary O2peak criteria were excluded from analysis. A complete description of testing procedures, including ramp protocols based on predicted performance can be found elsewhere (32).
Statistical Analyses
Data analysis was performed using “R” version 3.6.1. Means and standard deviations were calculated for general description of the dataset and the level of significance was set at p = 0.05 for all tests. All tests were two-sided. Independent samples t-test was used for sex-specific differences between healthy adults and patients with cardiovascular diseases. Linear regression models were used to predict associations of age with FMD and L-FMC. Sample selection accounted for age-related variations of disease burden at different ages, and models were additionally adjusted for sex, mean arterial pressure, heart rate and pre-cuff-inflation diameter, which are major effectors of FMD and L-FMC. All continuous variables were modeled using restricted cubic splines with four knots placed at appropriate quantiles. To evaluate the evidence of a moderating effect of O2peak on the relationship between age and FMD, a likelihood ratio test was used comparing a model with an interaction between O2peak and age to a model without such an interaction. Model fits were inspected using diagnostic residual plots. The model for FMD exhibited heteroscedastic residuals. Therefore, heteroscedasticity-robust p-values and 95%–confidence intervals are presented for this model. To visualize the relationship of FMD with age for different levels of cardiorespiratory fitness, model-predicted change of FMD was plotted for different centiles of O2peak (3rd, 15th, 50th, 85th, and 97th) (33) while other variables were fixed at their sex-specific mean. Partial R2 was presented to quantify the relative importance of the included variables. To aid interpretation, p-values were converted to surprisal values (s-values) when appropriate using the formula s = −log2(p) (34). The s-value provides an intuitive interpretation of the information conveyed by a certain p-value against the tested hypothesis. For a specific s-value, p conveys roughly the same evidence against the tested null hypothesis as seeing all heads in s independent tosses of a fair coin.
Results
Sample Characteristics
Data of 360 healthy non-smoking adults without cardiovascular disease (48.1% women) and 99 patients with cardiovascular diseases (28.3% women) were analyzed (Figure 1 and Table 1). Physical activity of the subjects was above a population specific average [details provided elsewhere (33)]. Healthy participants had a very low average 10-year risk of cardiovascular disease of 8.2 ± 8.6% (35) and their age- and sex-specific O2peak was similar (36) or higher (37, 38) than in other large cohorts of healthy adults. Cardiovascular diseases among the patients were arterial hypertension (N = 42), coronary artery disease (N = 41), aortic/mitral valve regurgitation (N = 5), atrial fibrillation (N = 4), dilative cardiomyopathy (N = 4), aortic/mitral valve stenosis (N = 2) and pulmonary hypertension (N = 1). 95 participants received antihypertensive medication including β-blockers in 47 cases. Lipid-lowering and anti-diabetic medication was taken by 52 and 8 patients, respectively. 4 patients were active smokers, 17 were ex-smokers. Left ventricular ejection fraction was mildly reduced (40 – 60%) in 11 patients and severely reduced (<40%) in 18 patients. According to NYHA dyspnea classification, 68, 19, 12 and 0 patients were class I, II, III and IV, respectively. Average FMD was lower in patients with cardiovascular diseases than in healthy adults (men: 4.6 ± 3.2% vs. 6.6 ± 4.0%, women: 4.3 ± 3.5% vs. 7.8 ± 4.3%, p < 0.001), whereas there was only little evidence for a difference in L-FMC between groups (men: −0.6 ± 2.8 vs. −0.3 ± 2.0, p = 0.30, women: −0.7 ± 3.1 vs. −1.2 ± 3.0, p = 0.38). Further measurement data of endothelial function (i.e., shear rate, blood flow, vascular wall thickness) are presented in the Supplementary Table 1.
TABLE 1.
Sample characteristics.
| Without cardiovascular disease (N = 360) |
With cardiovascular disease (N = 99) |
|||
| Male (N = 187) |
Female (N = 173) |
Male (N = 71) |
Female (N = 28) |
|
| Age [years] | 49.0 ± 18.3 | 51.2 ± 19.0 | 68.5 ± 12.5* | 72.0 ± 13.4* |
| O2peak [ml/min/kg] | 40.5 ± 9.5 | 32.4 ± 8.0 | 24.9 ± 8.5* | 19.4 ± 5.9* |
| BMI [kg/m2] | 24.0 ± 2.4 | 22.6 ± 2.5 | 27.3 ± 3.5* | 25.5 ± 3.3* |
| MAP [mmHg] | 94.9 ± 8.2 | 91.1 ± 9.5 | 97.3 ± 10.8 | 97.7 ± 10.7* |
| HbA1c [%] | 5.2 ± 0.3 | 5.2 ± 0.4 | 5.8 ± 0.6* | 5.6 ± 0.4* |
| Triglycerides [mg/dL] | 131.3 ± 71.5 | 99.8 ± 48.9 | 125.8 ± 73.1 | 140.5 ± 88.8* |
| LDL-cholesterol [mg/dL] | 118.2 ± 25.9 | 123.6 ± 29.2 | 101.4 ± 31.4* | 123.6 ± 31.1 |
| hs-CrP [mg/L] | 1.8 ± 4.1 | 1.9 ± 3.7 | 3.0 ± 3.8* | 2.5 ± 2.0 |
| Creatinine [mg/L] | 0.9 ± 0.1 | 1.3 ± 0.8 | 1.1 ± 0.4* | 0.8 ± 0.2 |
| NT-pro-BNP [pg/mL] | 77.9 ± 75.1 | 128.5 ± 96.1 | 468.1 ± 667.3* | 564.8 ± 829.5* |
| FMD [%] | 6.6 ± 4.0 | 7.8 ± 4.3 | 4.6 ± 3.2* | 4.3 ± 3.5* |
| L-FMC [%] | −0.6 ± 2.8 | −0.7 ± 3.1 | −0.3 ± 2.0 | −1.2 ± 3.0 |
| Pre-cuff-infl. diam. [mm] | 4.2 ± 0.5 | 3.4 ± 0.4 | 4.4 ± 0.6 | 3.7 ± 0.6* |
| Resting heart rate [bpm] | 57.1 ± 9.2 | 58.7 ± 8.6 | 58.4 ± 8.9 | 61.5 ± 9.8 |
O2peak = peak oxygen consumption; BMI = Body mass index; MAP = Mean arterial pressure; HbA1c = glycated hemoglobin; LDL-cholesterol = Low-density lipoprotein-cholesterol; hs-CrP = high-sensitivity C-reactive protein; NT-pro-BNP = n-terminal-pro-brain natriuretic peptide; FMD = flow-mediated vasodilation; L-FMC = low flow-mediated vasoconstriction; Pre-cuff-infl. diam. = pre-cuff-inflation diameter; *significantly different from “without cardiovascular disease” (independent student’s t-test, significance level p = 0.05).
Association of Endothelial Function With Age
FMD showed a significant negative association with age in the healthy sample without burden of chronic diseases (adjusted R2 = 0.27, partial R2 = 0.07, p < 0.001; Supplementary Table 2) and in patients with cardiovascular diseases (adjusted R2 = 0.29, partial R2 = 0.05, p = 002). L-FMC showed no significant association with age in both samples (Supplementary Table 3).
Moderating Effects of Cardiorespiratory Fitness on the Decline of Flow-Mediated Vasodilation With Aging
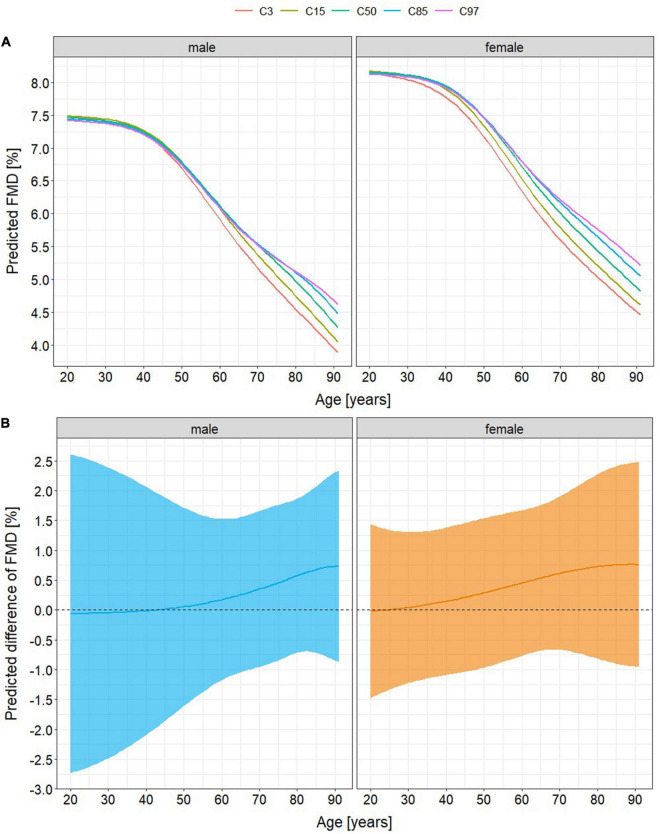
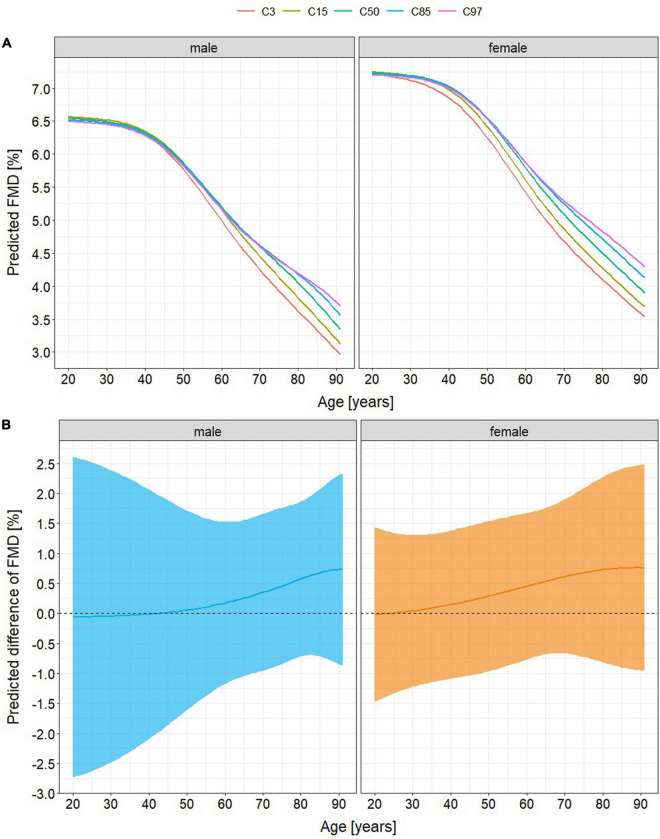
There was little evidence that the relationship of FMD with age was moderated by O2peak (χ2(5) = 5.37, p = 0.372, s = 1.43). Therefore, models containing no interaction between O2peak and age were presented. The model explained 30.3% of the variance of FMD with O2peak accounting for 2.8%. It predicted a 41% decline of FMD in very high-fit healthy men (O2peak ≥97th percentile) from 20 to 90 years of age compared to 48% in very low-fit healthy men (O2peak ≤3rd percentile) and of 36% in very high-fit healthy women compared to 45% in very low-fit healthy women (Figures 2A,B). In patients with cardiovascular diseases, predicted age-related differences of FMD were 43% in very high-fit men compared to 55% in very low-fit men and 41% in very high-fit women compared to 52% in very low-fit women (Figures 3A,B).
FIGURE 2.
Age-related differences of flow-mediated vasodilation (FMD) in healthy adults (A) stratified by percentile of peak oxygen consumption (O2peak) and (B) predicted difference between FMD in the lowest-fit (O2peak ≤3rd percentile) and FMD in the highest fit (O2peak ≥97th percentile) individuals. There are no differences associated with cardiorespiratory fitness until the age of 30 (women) and 50 years (men). Afterward, models predict a lower FMD at old age of 7% in very low-fit men (maximum age-related difference of 41% if O2peak ≥97th percentile versus 48% if O2peak ≤3rd percentile) and of 9% in very low-fit women (36% versus 45%). Absolute mean differences of FMD between O2peak percentiles ≤3rd versus ≥97th deviate from 0 in tendency at middle and old age (maximum 0.75%, 95% confidence intervals –0.8 – 2.3% (men) and –1.0 – 2.5% (women)). C3, 15, 50, 85, and 97 = O2peak percentiles (3rd, 15th, 50th, 85th, and 97th).
FIGURE 3.
Age-related differences of flow-mediated vasodilation (FMD) in patients with cardiovascular diseases (A) stratified by percentile of peak oxygen consumption (O2peak) and (B) predicted difference between FMD in the lowest-fit (O2peak ≤3rd percentile) and FMD in the highest fit (O2peak ≥97th percentile) individuals. There are no differences associated with cardiorespiratory fitness until the age of 30 (women) and 50 years (men). Afterward, models predict a lower FMD alt old age of 12% in very low-fit men (maximum age-related difference of 43% if O2peak ≥97th percentile versus 55% if O2peak ≤3rd percentile) and of 11% in very low-fit women (41% versus 52%). Absolute mean differences of FMD between O2peak percentiles ≤3rd versus ≥97th deviate from 0 in tendency at middle and old age (maximum 0.75%, 95% confidence intervals –0.8 – 2.3% (men) and –1.0 – 2.5% (women)). C3, 15, 50, 85, and 97 = O2peak percentiles (3rd, 15th, 50th, 85th, and 97th).
Discussion
Independent of classical cardiovascular risk factors FMD, but not L-FMC, is negatively associated with higher age in both, healthy aging adults and patients with cardiovascular diseases. Additionally, the current study provides some novel insights into the effects of cardiorespiratory fitness on the age-related changes of endothelial function. First, O2peak may account only for a small proportion of the age-related change of FMD. Thereby, a constantly low O2peak might lead to a 7 (men) – 9% (women) lower FMD at old age in healthy individuals and to a 11 (women) – 12% (men) lower FMD at old age in patients with cardiovascular diseases. Second, this accelerated decline of FMD may become apparent around the age of 30 years in women, thus 20 years earlier than in men.
These study results demonstrate a negative association of FMD with age, but provide little statistical evidence for an influence of O2peak on this association. This is in accordance with the results of Montero et al. (14) who reported an age-independent association of O2peak with FMD in only 46% of all cross-sectional studies. Furthermore, they also reported that FMD in healthy young adults did not differ in low-fit individuals from those being high-fit, which might be due to generally high adaptive capacities in the vascular walls of the young (15). Assuming that these capacities decline with aging, it seems a logical consequence that the theoretical models in this study suggest relatively lower FMD of 7 – 12% in the lowest-fit old-aged individuals compared to those with the highest O2peak (maximum difference of total FMD values was 0.75%). Favorable effects of a high O2peak on endothelial function at any age were previously demonstrated (13) emphasizing its importance for a healthy vascular aging independent from other cardiovascular risk factors.
Three potential explanations are proposed for why the statistically small effect of O2peak on the negative association of FMD with age might carry a clinical relevance. First, a high cardiorespiratory fitness might exert favorable effects on vascular function that are unrelated to vasodilator function. Therefore, L-FMC was included in the analyses, which was suggested as a complementary biomarker to FMD for the thorough clinical interpretation of endothelial function (18, 19). However, only very little evidence was found for an association of age with L-FMC in this study’s sample, probably due to the large variation of L-FMC values despite strict adherence to current measurement guidelines (30). Furthermore, a previous study comparing L-FMC of the radial with the brachial artery confirmed vasoconstriction upon cuff-occlusion in the radial but not in the brachial artery (39). The authors concluded, that endothelial dysfunction would manifest as attenuated L-FMC in the radial artery but not in the brachial artery. Accordingly, lower vasoconstrictor responsiveness with higher age might rather occur in the smaller resistance arteries instead of the larger ones.
Second, structural remodeling of the vascular wall in terms of thinner thickness with higher O2peak was also discussed as an explanation for a partial non-detectability of favorable vascular effects by measurement of FMD and L-FMC (40). However, there was no evidence for an association between brachial arterial wall thickness and O2peak in this study’s analyses (Supplementary Table 4) leaving it open for discussion, whether favorable structural adaptations in the vascular walls could mitigate improved FMD and L-FMC (41).
A third explanation can be derived from the assumption, that not maximal shear stress, but rather repeated bouts of increased shear stress induce favorable improvements of endothelial function by exercise (42). Accordingly, light- or moderate intensity exercise that do not induce changes of O2peak, might still induce favorable vascular effects (43). At this point, longitudinal studies collecting detailed information about the cumulative load of exercise and physical activity at different intensities are lacking. Furthermore, interventional studies including high-, moderate and low-intensity activities are needed as well, to clarify how different modes of exercise effectuate long-term endothelial function independent of O2peak.
Clinical Considerations
Although statistically non-significant, the predicted 7 – 9% reduction of FMD with higher age in association with a constantly low O2peak in the healthy sample should not be ignored for their potential clinical relevance on an individual level. Notably, a 1% higher FMD may lead to a 13% reduction of cardiovascular morbidity (44, 45) and, thus, might reduce the risk of cardiovascular morbidity considerably, regardless of sex, age or other cardiovascular risk factors (44, 45).
In the older patients with cardiovascular diseases, FMD was 11 (women) – 12% (men) lower in the lowest O2peak percentile compared to the highest, which equals an absolute difference of 0.8%. This is similar to previously observed effects of exercise training in heart failure patients increasing FMD by 1.08% (11), which, again seems clearly relevant, in terms of cardiovascular morbidity (44, 45). This is supported by further observations. For example, one study on Japanese individuals with coronary artery disease observed 50% lower rates of cardiovascular events with every 3.1% total improvement of FMD (46). Furthermore, a reduced FMD was previously associated with higher rates of in-stent restenosis (47) and occurrence of postoperative cardiovascular sequelae (48) in patients with coronary artery disease.
Differences in FMD stratified by O2peak percentile became visible at the age of 30 years in women, thus 20 years earlier than in men. This seems counter-intuitive, as previous evidence suggests a 10 year earlier decline of endothelial function in men than in women indicating their higher risk for early cardiovascular morbidity and mortality (49). However, this study’s observations support the assumption, that O2peak-improving exercise during young adulthood might exert higher favorable effects on vasodilator responsiveness in women, than in men (13).
Methodological Considerations
The quality of phenotyping with direct state-of-the art measurement of cardiorespiratory fitness and endothelial function as well as the large and well-phenotyped samples representing the healthy population and a population with high cardiovascular disease burden are major strengths of this study.
Importantly, results from the regression analyses are visualized in Figures 2, 3 but not based on visual inspection of these graphs. Furthermore, they are not descriptive and do not provide clinical cut-offs for FMD with high or low O2peak. For this, longitudinal studies with multiple measures are needed. Due to their cross-sectional nature, the models do not allow for the conclusion, that people with a high O2peak throughout their adult life will show considerably better FMD than those with low cardiorespiratory fitness, especially as the process of aging does not follow a linear course, thus ultimately requesting longitudinal studies to be assessed. Yet, a person will most likely have a better individual FMD, if a high O2peak is maintained into old age. However, the current results give valuable insights into the relationship between vascular aging and cardiorespiratory fitness, supporting previous claims that direct effects of exercise on vascular function and structure might constitute a relevant part of the beneficial effects of exercise in humans independent of classical cardiovascular risk factors (6, 50).
Conclusion and Perspectives
Flow-mediated vasodilation is negatively associated with age in a healthy population as well as in patients with cardiovascular diseases, whereas L-FMC is not. O2peak explains only a small proportion of the age-related change of FMD independent of traditional cardiovascular risk factors. This small statistical effect might account for up to 7–12% lower values at old age in low-fit healthy adults and patients with cardiovascular diseases compared to those with higher lifelong O2peak. Especially in the patients, this might be clinically relevant in terms of increased morbidity and mortality, emphasizing the need to aim for a lifelong preservation of cardiorespiratory fitness and endothelial function. As a consequence, clinicians should promote the implementation of regular intensive exercise at any age in both, healthy adults as well as patients with cardiovascular diseases. Scientists should conduct long-term observational studies as well as training interventions to analyze O2peak-independent benefits of cumulative exercise loads and of different exercise intensities on endothelial function.
Data Availability Statement
The original contributions presented in the study are included in the article/Supplementary Material, further inquiries can be directed to the corresponding authors.
Ethics Statement
The studies involving human participants were reviewed and approved by Ethikkommission Nordwestschweiz, Switzerland. The patients/participants provided their written informed consent to participate in this study.
Author Contributions
KK wrote the manuscript, conducted data analysis and participated in the conception, and conduction of the study. JW participated in the conception and conduction of the study and extensively engaged in preparation, and revision of the manuscript drafts. DI participated in the conception of the study, data analysis, and revision of the manuscript drafts. RK, GN, CK, and JC participated in the conduction of the study and extensively engaged in preparation, and revision of the manuscript drafts. TH and AS-T participated in the conception of the study, supervised the conduction and data analysis and extensively engaged in preparation, and revision of the manuscript drafts. All authors contributed to the article and approved the submitted version.
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s Note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Acknowledgments
We thank Mirijam Frei, Jennifer Eymann, Marina Häberli, Rahel Vögeli, and Barbara Wegmann who participated in data acquisition. We also thank Achim Schwarz (ALF Distribution GmbH, Aachen, Germany) for the technical support with the UNEX EF 38G device (UNEX Corp., Nagoya, Japan) and all participants who contributed with their engagement during data acquisition to the success of this project.
Abbreviations
- FMD
Flow-mediated dilation
- L-FMC
Low flow-mediated constriction
- O2peak
Peak oxygen consumption.
Funding
This research was funded by the Swiss National Science Foundation (SNSF), a competitive governmental funding body (grant no. 182815).
Supplementary Material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fcvm.2022.870847/full#supplementary-material
References
- 1.Israel A, Kivity S, Sidi Y, Segev S, Berkovitch A, Klempfner R, et al. Use of exercise capacity to improve SCORE risk prediction model in asymptomatic adults. Eur Heart J. (2016) 37:2300–6. 10.1093/eurheartj/ehw053 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ahmed HM, Al-Mallah MH, McEvoy JW, Nasir K, Blumenthal RS, Jones SR, et al. Maximal exercise testing variables and 10-year survival: fitness risk score derivation from the FIT Project. Mayo Clin Proc. (2015) 90:346–55. 10.1016/j.mayocp.2014.12.013 [DOI] [PubMed] [Google Scholar]
- 3.Hamer M, Ingle L, Carroll S, Stamatakis E. Physical activity and cardiovascular mortality risk: possible protective mechanisms? Med Sci Sports Exerc. (2012) 44:84–8. 10.1249/MSS.0b013e3182251077 [DOI] [PubMed] [Google Scholar]
- 4.Mora S, Cook N, Buring JE, Ridker PM, Lee IM. Physical activity and reduced risk of cardiovascular events: potential mediating mechanisms. Circulation. (2007) 116:2110–8. 10.1161/CIRCULATIONAHA.107.729939 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Taylor RS, Unal B, Critchley JA, Capewell S. Mortality reductions in patients receiving exercise-based cardiac rehabilitation: how much can be attributed to cardiovascular risk factor improvements? Eur J Cardiovasc Prev Rehabil. (2006) 13:369–74. 10.1097/01.hjr.0000199492.00967.11 [DOI] [PubMed] [Google Scholar]
- 6.Green DJ, O’Driscoll G, Joyner MJ, Cable NT. Exercise and cardiovascular risk reduction: time to update the rationale for exercise? J Appl Physiol. (1985) 105:766–8. 10.1152/japplphysiol.01028.2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Green DJ, Hopman MT, Padilla J, Laughlin MH, Thijssen DH. Vascular adaptation to exercise in humans: role of hemodynamic stimuli. Physiol Rev. (2017) 97:495–528. 10.1152/physrev.00014.2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ashor AW, Lara J, Siervo M, Celis-Morales C, Oggioni C, Jakovljevic DG, et al. Exercise modalities and endothelial function: a systematic review and dose-response meta-analysis of randomized controlled trials. Sports Med. (2015) 45:279–96. 10.1007/s40279-014-0272-9 [DOI] [PubMed] [Google Scholar]
- 9.Dawson EA, Green DJ, Cable NT, Thijssen DH. Effects of acute exercise on flow-mediated dilatation in healthy humans. J Appl Physiol. (1985) 115:1589–98. 10.1152/japplphysiol.00450.2013 [DOI] [PubMed] [Google Scholar]
- 10.Green DJ, Bilsborough W, Naylor LH, Reed C, Wright J, O’Driscoll G, et al. Comparison of forearm blood flow responses to incremental handgrip and cycle ergometer exercise: relative contribution of nitric oxide. J Physiol. (2005) 562(Pt 2):617–28. 10.1113/jphysiol.2004.075929 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Pearson MJ, Smart NA. Effect of exercise training on endothelial function in heart failure patients: a systematic review meta-analysis. Int J Cardiol. (2017) 231:234–43. 10.1016/j.ijcard.2016.12.145 [DOI] [PubMed] [Google Scholar]
- 12.Early KS, Stewart A, Johannsen N, Lavie CJ, Thomas JR, Welsch M. The effects of exercise training on brachial artery flow-mediated dilation: a meta-analysis. J Cardiopulm Rehabil Prev. (2017) 37:77–89. 10.1097/HCR.0000000000000206 [DOI] [PubMed] [Google Scholar]
- 13.Black MA, Cable NT, Thijssen DH, Green DJ. Impact of age, sex, and exercise on brachial artery flow-mediated dilatation. Am J Physiol Heart Circ Physiol. (2009) 297:H1109–16. 10.1152/ajpheart.00226.2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Montero D. The association of cardiorespiratory fitness with endothelial or smooth muscle vasodilator function. Eur J Prev Cardiol. (2015) 22:1200–11. 10.1177/2047487314553780 [DOI] [PubMed] [Google Scholar]
- 15.Montero D, Padilla J, Diaz-Canestro C, Muris DM, Pyke KE, Obert P, et al. Flow-mediated dilation in athletes: influence of aging. Med Sci Sports Exerc. (2014) 46:2148–58. 10.1249/MSS.0000000000000341 [DOI] [PubMed] [Google Scholar]
- 16.Bell PL, Kelley ET, McCoy SM, Credeur DP. Influence of aerobic fitness on vasoreactivity in young men. Eur J Appl Physiol. (2017) 117:2075–83. 10.1007/s00421-017-3698-6 [DOI] [PubMed] [Google Scholar]
- 17.O’Brien MW, Mekary S, Robinson SA, Johns JA, Kimmerly DS. The relationship between aerobic fitness and low-flow-mediated constriction in older adults. Eur J Appl Physiol. (2019) 119:351–9. 10.1007/s00421-018-4044-3 [DOI] [PubMed] [Google Scholar]
- 18.Gori T, Muxel S, Damaske A, Radmacher MC, Fasola F, Schaefer S, et al. Endothelial function assessment: flow-mediated dilation and constriction provide different and complementary information on the presence of coronary artery disease. Eur Heart J. (2012) 33:363–71. 10.1093/eurheartj/ehr361 [DOI] [PubMed] [Google Scholar]
- 19.Humphreys RE, Green DJ, Cable NT, Thijssen DH, Dawson EA. Low-flow mediated constriction: the yin to FMD’s yang? Expert Rev Cardiovasc Ther. (2014) 12:557–64. 10.1586/14779072.2014.909728 [DOI] [PubMed] [Google Scholar]
- 20.Konigstein K, Wagner J, Frei M, Knaier R, Klenk C, Carrard J, et al. Endothelial function of healthy adults from 20 to 91 years of age: prediction of cardiovascular risk by vasoactive range. J Hypertens. (2021) 39:1361–9. 10.1097/HJH.0000000000002798 [DOI] [PubMed] [Google Scholar]
- 21.Campbell A, Grace F, Ritchie L, Beaumont A, Sculthorpe N. Long-term aerobic exercise improves vascular function into old age: a systematic review, meta-analysis and meta regression of observational and interventional studies. Front Physiol. (2019) 10:31. 10.3389/fphys.2019.00031 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Drexler H, Hornig B. Importance of endothelial function in chronic heart failure. J Cardiovasc Pharmacol. (1996) 27(Suppl. 2):S9–12. 10.1097/00005344-199600002-00003 [DOI] [PubMed] [Google Scholar]
- 23.Gokce N, Holbrook M, Duffy SJ, Demissie S, Cupples LA, Biegelsen E, et al. Effects of race and hypertension on flow-mediated and nitroglycerin-mediated dilation of the brachial artery. Hypertension. (2001) 38:1349–54. 10.1161/hy1201.096575 [DOI] [PubMed] [Google Scholar]
- 24.Belardinelli R, Capestro F, Misiani A, Scipione P, Georgiou D. Moderate exercise training improves functional capacity, quality of life, and endothelium-dependent vasodilation in chronic heart failure patients with implantable cardioverter defibrillators and cardiac resynchronization therapy. Eur J Cardiovasc Prev Rehabil. (2006) 13:818–25. 10.1097/01.hjr.0000230104.93771.7d [DOI] [PubMed] [Google Scholar]
- 25.Hornig B, Maier V, Drexler H. Physical training improves endothelial function in patients with chronic heart failure. Circulation. (1996) 93:210–4. 10.1161/01.cir.93.2.210 [DOI] [PubMed] [Google Scholar]
- 26.Dawson EA, Alkarmi A, Thijssen DH, Rathore S, Marsman DE, Cable NT, et al. Low-flow mediated constriction is endothelium-dependent: effects of exercise training after radial artery catheterization. Circ Cardiovasc Interv. (2012) 5:713–9. 10.1161/CIRCINTERVENTIONS.112.971556 [DOI] [PubMed] [Google Scholar]
- 27.Tarro Genta F, Eleuteri E, Temporelli PL, Comazzi F, Tidu M, Bouslenko Z, et al. Flow-mediated dilation normalization predicts outcome in chronic heart failure patients. J Card Fail. (2013) 19:260–7. 10.1016/j.cardfail.2013.01.014 [DOI] [PubMed] [Google Scholar]
- 28.Celermajer DS, Sorensen KE, Spiegelhalter DJ, Georgakopoulos D, Robinson J, Deanfield JE. Aging is associated with endothelial dysfunction in healthy men years before the age-related decline in women. J Am Coll Cardiol. (1994) 24:471–6. 10.1016/0735-1097(94)90305-0 [DOI] [PubMed] [Google Scholar]
- 29.Wagner J, Knaier R, Infanger D, Arbeev K, Briel M, Dieterle T, et al. Functional aging in health and heart failure: the COmPLETE Study. BMC Cardiovasc Disord. (2019) 19:180. 10.1186/s12872-019-1164-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Thijssen DHJ, Bruno RM, van Mil A, Holder SM, Faita F, Greyling A, et al. Expert consensus and evidence-based recommendations for the assessment of flow-mediated dilation in humans. Eur Heart J. (2019) 40:2534–47. 10.1093/eurheartj/ehz350 [DOI] [PubMed] [Google Scholar]
- 31.Tomiyama H, Kohro T, Higashi Y, Takase B, Suzuki T, Ishizu T, et al. Reliability of measurement of endothelial function across multiple institutions and establishment of reference values in Japanese. Atherosclerosis. (2015) 242:433–42. 10.1016/j.atherosclerosis.2015.08.001 [DOI] [PubMed] [Google Scholar]
- 32.Wagner J, Niemeyer M, Infanger D, Hinrichs T, Streese L, Hanssen H, et al. New data-based cutoffs for maximal exercise criteria across the lifespan. Med Sci Sports Exerc. (2020) 52:1915–23. 10.1249/MSS.0000000000002344 [DOI] [PubMed] [Google Scholar]
- 33.Wagner J, Knaier R, Infanger D, Konigstein K, Klenk C, Carrard J, et al. Novel CPET reference values in healthy adults: associations with physical activity. Med Sci Sports Exerc. (2020) 53:26–37. 10.1249/MSS.0000000000002454 [DOI] [PubMed] [Google Scholar]
- 34.Greenland S. Valid P-values behave exactly as they should: some misleading criticisms of P-values and their resolution with S-values. Am Statistician. (2019) 73:106–14. 10.1080/00031305.2018.1529625 [DOI] [Google Scholar]
- 35.Wilson PW, D’Agostino RB, Levy D, Belanger AM, Silbershatz H, Kannel WB. Prediction of coronary heart disease using risk factor categories. Circulation. (1998) 97:1837–47. 10.1161/01.cir.97.18.1837 [DOI] [PubMed] [Google Scholar]
- 36.Mylius CF, Krijnen WP, van der Schans CP, Takken T. Peak oxygen uptake reference values for cycle ergometry for the healthy Dutch population: data from the LowLands fitness registry. ERJ Open Res. (2019) 5:00056–2018. 10.1183/23120541.00056-2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Rapp D, Scharhag J, Wagenpfeil S, Scholl J. Reference values for peak oxygen uptake: cross-sectional analysis of cycle ergometry-based cardiopulmonary exercise tests of 10 090 adult German volunteers from the prevention first registry. BMJ Open. (2018) 8:e018697. 10.1136/bmjopen-2017-018697 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Kaminsky LA, Imboden MT, Arena R, Myers J. Reference standards for cardiorespiratory fitness measured with cardiopulmonary exercise testing using cycle ergometry: data from the fitness registry and the importance of exercise national database (FRIEND) registry. Mayo Clin Proc. (2017) 92:228–33. 10.1016/j.mayocp.2016.10.003 [DOI] [PubMed] [Google Scholar]
- 39.Weissgerber TL, Davies GA, Tschakovsky ME. Low flow-mediated constriction occurs in the radial but not the brachial artery in healthy pregnant and nonpregnant women. J Appl Physiol (1985) 108:1097–105. 10.1152/japplphysiol.00815.2009 [DOI] [PubMed] [Google Scholar]
- 40.Thijssen DH, Cable NT, Green DJ. Impact of exercise training on arterial wall thickness in humans. Clin Sci (Lond). (2012) 122:311–22. 10.1042/CS20110469 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Laughlin MH. Endothelium-mediated control of coronary vascular tone after chronic exercise training. Med Sci Sports Exerc. (1995) 27:1135–44. [PubMed] [Google Scholar]
- 42.Pyke KE, Tschakovsky ME. Peak vs. total reactive hyperemia: which determines the magnitude of flow-mediated dilation? J Appl Physiol (1985) 102:1510–9. 10.1152/japplphysiol.01024.2006 [DOI] [PubMed] [Google Scholar]
- 43.Currie KD, McKelvie RS, Macdonald MJ. Flow-mediated dilation is acutely improved after high-intensity interval exercise. Med Sci Sports Exerc. (2012) 44:2057–64. 10.1249/MSS.0b013e318260ff92 [DOI] [PubMed] [Google Scholar]
- 44.Inaba Y, Chen JA, Bergmann SR. Prediction of future cardiovascular outcomes by flow-mediated vasodilatation of brachial artery: a meta-analysis. Int J Cardiovasc Imaging. (2010) 26:631–40. 10.1007/s10554-010-9616-1 [DOI] [PubMed] [Google Scholar]
- 45.Ras RT, Streppel MT, Draijer R, Zock PL. Flow-mediated dilation and cardiovascular risk prediction: a systematic review with meta-analysis. Int J Cardiol. (2013) 168:344–51. 10.1016/j.ijcard.2012.09.047 [DOI] [PubMed] [Google Scholar]
- 46.Sugamata W, Nakamura T, Uematsu M, Kitta Y, Fujioka D, Saito Y, et al. Combined assessment of flow-mediated dilation of the brachial artery and brachial-ankle pulse wave velocity improves the prediction of future coronary events in patients with chronic coronary artery disease. J Cardiol. (2014) 64:179–84. 10.1016/j.jjcc.2014.01.004 [DOI] [PubMed] [Google Scholar]
- 47.Patti G, Pasceri V, Melfi R, Goffredo C, Chello M, D’Ambrosio A, et al. Impaired flow-mediated dilation and risk of restenosis in patients undergoing coronary stent implantation. Circulation. (2005) 111:70–5. 10.1161/01.CIR.0000151308.06673.D2 [DOI] [PubMed] [Google Scholar]
- 48.Gokce N, Keaney JF, Jr., Hunter LM, Watkins MT, Menzoian JO, Vita JA. Risk stratification for postoperative cardiovascular events via noninvasive assessment of endothelial function: a prospective study. Circulation. (2002) 105:1567–72. 10.1161/01.cir.0000012543.55874.47 [DOI] [PubMed] [Google Scholar]
- 49.Skaug EA, Aspenes ST, Oldervoll L, Morkedal B, Vatten L, Wisloff U, et al. Age and gender differences of endothelial function in 4739 healthy adults: the HUNT3 Fitness Study. Eur J Prev Cardiol. (2013) 20:531–40. 10.1177/2047487312444234 [DOI] [PubMed] [Google Scholar]
- 50.Green DJ, Eijsvogels T, Bouts YM, Maiorana AJ, Naylor LH, Scholten RR, et al. Exercise training and artery function in humans: nonresponse and its relationship to cardiovascular risk factors. J Appl Physiol. (1985) 117:345–52. 10.1152/japplphysiol.00354.2014 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The original contributions presented in the study are included in the article/Supplementary Material, further inquiries can be directed to the corresponding authors.