Abstract
Whereas the link between psychosocial stress and health complications has long been established, the influence of psychosocial stress on brain activity is not yet completely understood. Electroencephalography (EEG) has been regularly employed to investigate the neural aspects of the psychosocial stress response, but these results have not yet been unified. Therefore, in this article, we systematically review the current EEG literature in which spectral analyses were employed to investigate the neural psychosocial stress response and interpret the results with regard to the three stress phases (anticipatory, reactive, and recovery) in which the response can be divided. Our results show that three EEG measures, alpha power, beta power and frontal alpha asymmetry (FAA), are commonly utilized and that alpha power consistently decreases, beta power shows a tendency to increase, and FAA varies inconsistently. We furthermore found that whereas changes in alpha power are independent of the stress phase, and changes in beta power show a relative stress phase independent trend, other EEG measures such as delta power, theta power, relative gamma and theta-alpha power ratio show less stress phase independent changes. Meta-analyses conducted on alpha power, beta power and FAA further revealed a significant effect size (hedge's g = 0.6; p = 0.001) for alpha power, but an insignificant effect size for beta power (hedge's g = −0.31; p = 0.29) and FAA (hedge's g = 0.01, p = 0.93). From our results, it can be concluded that psychosocial stress results in significant changes in some spectral EEG indices, but that more research is needed to further uncover the precise (temporal) mechanisms underlying these neural responses.
Keywords: Psychosocial stress, Social exclusion, Ostracism, Mental stress, Electroencephalography, EEG, Spectral analysis
1. Introduction
Throughout recent years, the incidence of mental health problems has risen worldwide (Liu et al., 2020). The recent outbreak of COVID-19 further exacerbated the major challenges regarding mental health due to its large impact on both society (economic uncertainty) and on a personal level (loss of close friends/family and social isolation) (Salari et al., 2020). An important factor in the current problems regarding mental health is stress, the latter being an important catalyzer of mental disorders such as depression and anxiety disorders (Daviu et al., 2019; Mazure, 1998). Stress can be defined as the perception of personal or environmental stimuli as more taxing than the direct mitigating capability of the body, which results in an acute stress response in the body (Folkman and Lazarus, 1984). A key player in the stress response is nevertheless the brain, that alters various effector systems mainly through the hypothalamic-pituitary-adrenal (HPA) and sympathetic-adreno-medullar (SAM) axes, resulting in physiological (such as increased heart rate and blood pressure) as well as psychological changes (Dickerson and Kemeny, 2004; Godoy et al., 2018; Kudielka and Kirschbaum, 2005; McEwen and Seeman, 1999).
One specific stressor subtype, the psychosocial stressor, seems to have a prominent role in the stress-disease link, making it an important subject for extensive research (Backé et al., 2012; Greenwood et al., 1996; Kemeny and Schedlowski, 2007; MD et al., 2002; Siegrist, 2008). In the current paper, based on a review of the literature, we define psychosocial stressors as “threatening or stressful stimuli arising from social interactions mainly due to their novel, unpredictable or uncontrollable characteristics or the presence of social-evaluative threats”. Psychosocial stress can then be defined as the result of a cognitive appraisal or interpretation of a psychosocial stressor that taxes or exceeds the coping capabilities of an individual. The crucial role of psychosocial stressors as contributors to stress-imposed (mental) health complications has multiple reasons (Epel et al., 2018; Kogler et al., 2015). From an evolutionary point of view, humans are a social species, making social interactions and the need to belong innate properties of every individual (Baumeister and Leary, 1995). Psychosocial stressors may disrupt these core human needs and are therefore highly impactful on the general well-being of a person. Furthermore, psychosocial stressors are not limited to a specific part of an individual's life, as everyday life consists of an abundance of social interactions. This makes chronic exposure to psychosocial stressors more probable compared to other types of stressors such as physical stressors (e.g. receiving electrical shocks) or cognitive stressors (e.g. reaction time tasks), further explaining its dominant presence in stress-related diseases (Dupre et al., 2015, Melchior et al., 2007, Phelan, 1991, Tennant, 2001).
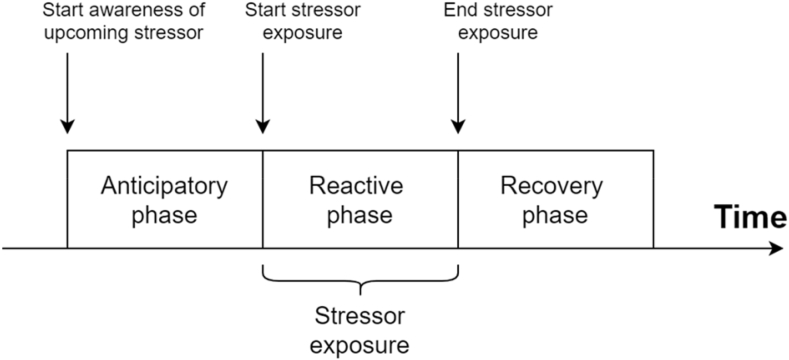
It is noticeable that various articles investigating the response to psychosocial stressors do not investigate the stress response in its entirety, but rather focus on specific phases of it. This subdivision of the acute stress response in discrete phases is logical as various distinct neurological, psychological, and physiological processes are active throughout the stress response. When identifying different phases of an acute stress response as a function of its occurrence in time in relationship to the present stressor, three phases become apparent: the anticipatory, the reactive, and the recovery phase (see Fig. 1). The existence of distinct phases in the acute stress response has long been established, and the allostatic load theory has defined how repeated exposure to stressors can lead to maladaptive trajectories of these phases such as the lack of adaptation (abnormal reactive phase) or a prolonged response to stressors (abnormal recovery phase) (Juster et al., 2010; McEwen and Seeman, 1999). The first phase, the anticipatory phase, can be defined as the moment a person is aware of the upcoming stressor, but is not yet directly exposed to it. This phase is defined by a high uncertainty about the near future and the possible presence of a social evaluative threat, therefore evoking a stress response (Engert et al., 2013). The second phase, the reactive phase, is defined as the time when an individual is directly exposed to the stressor. It could be argued that the anticipatory and reactive phase of the stress response can be seen as one, since uncertainty and social evaluative threat are present in both phases, but this distinction is valid since in the reactive phase, participants are actively engaged with the actual stressor whereas during the anticipatory phase they might not be. The third phase that can be defined is the recovery phase and starts directly after the ending of the stressor exposure. The difference between this phase and the anticipatory and reactive phase is larger, since the uncertain, uncontrollable, and socially evaluative threatening stimuli are no longer present. The recovery phase therefore consists mainly of the possible reversal of psychological and physiological alterations caused by the stressor. Depending on the physiological measure, the duration of these restorations can take from 30 min to 1 h (i.e., cortisol, Goodman et al., 2017) to up to 2 h (i.e., functional magnetic resonance imaging, Vaisvaser et al., 2013; van Oort et al., 2017). Although all three phases may contain unique alterations in physiological and psychological mechanisms, these alterations are all induced by the same underlying mechanism, namely, the evoked stress response due to the presence of a psychosocial stressor.
Fig. 1.
Visualization of the three phases of the stress response with respect to time.
The pivotal role of the brain as the orchestrating organ of the different phases of the psychosocial stress response, makes the brain a principal focus of (psychosocial) stress-related studies (McEwen, 2007; McEwen and Gianaros, 2011). A central investigative tool in this research endeavor has been the use of neuroimaging, with the most commonly used neuroimaging methods in stress research being functional magnetic resonance imaging (fMRI) and electroencephalography (EEG). fMRI has, due to its high spatial resolution, mostly been employed to identify the involved brain regions in the psychosocial stress response. The main findings from these efforts are the involvement of three major brain networks (the default mode, salience and central executive network; for reviews see Kogler et al., 2015; van Oort et al., 2017) as well as a bilateral cluster comprising the insula, claustrum, and inferior frontal gyrus (for a review, see Berretz et al., 2021). EEG has, due to its high temporal resolution, been employed to investigate the more time-sensitive aspects of the neural response to psychosocial stress. EEG studies investigating the neural psychosocial stress response either focus on event-related potentials (ERPs) or analyze changes in the prominent oscillations present in the EEG signal through spectral analysis methods. ERP analyses focus on short-term (mostly within 1 s), task-evoked variations in neural activity in response to sensory stimuli and investigate known event-related potentials such as the N2 and P3 (Cohen, 2014; Kawamoto et al., 2013; van der Veen et al., 2016). The main drawback of ERP analysis, if applied to the investigation of the various phases of the psychosocial stress response, is its dependency on clearly defined stimuli, thus limiting its application potential for uncovering the whole neural response to stressful stimuli. This limitation is not shared by spectral analysis methods, since these methods can be applied to either the stimulus-defined time windows to investigate task-evoked neural activity, but also longer, continuous EEG recordings for the investigation of task-induced variations in neural activity. Therefore, spectral analyses that investigate EEG signal properties in both the time and frequency domain (Cohen, 2014) are more useful to fully investigate the different phases of stress response. Various spectral analyses have been applied to investigate psychosocial stress-related variations in the delta (0.5–4 Hz), theta (4–7 Hz), alpha (8–13 Hz), beta (15–30 Hz) and gamma (>30 Hz) frequency bands (although the exact ranges can vary across articles). The most common spectral analysis technique is spectral band power, which calculates the average power in a specific frequency band and is reflective of the neuronal activity in this frequency range (Cohen, 2014). Commonly studied frequency bands are the theta band, linked to working memory functionality, sensory and motor processing, cognitive interference, and emotional memory consolidation during sleep (Karakaş, 2020; Klimesch, 1999; Nigbur et al., 2011; Nishida et al., 2009); the alpha band, believed to be inversely correlated with cortical activity and reflecting coordination mechanisms of brain networks (Allen et al., 2004; Jensen and Mazaheri, 2010; Mathewson et al., 2011) and the beta band, associated with attention and sustaining the current cognitive or sensorimotor state (Engel and Fries, 2010; Wróbel, 2000). Another spectral analysis technique commonly selected is frontal alpha asymmetry (FAA), generally obtained by subtracting the natural log transformed alpha power value of a left frontal electrode (mostly F3 or F7) from that of a right frontal electrode (mostly F4 or F8), is indicative of the relative difference in alpha power between the frontal parts of the left and right hemisphere. It is suggested that a relative higher left hemispherical activity indicates a tendency for approach-oriented behavior and that relative higher right hemispherical activity signifies more withdrawal-oriented behavior (Smith et al., 2017). FAA is ubiquitous in psychological and psychiatric EEG research and has been linked to a wide variety of psychological constructs and psychiatric disorders (Smith et al., 2017). Most commonly studied are the relationships between FAA and motivational/emotional variables or psychiatric disorders such as depression and posttraumatic stress disorder (Allen et al., 2004; Meyer et al., 2015; Smith et al., 2017; van der Vinne et al., 2017). Research suggests that FAA changes on a group level are variable and are highly dependent on a variety of factors such as personality traits, age and gender (Coan et al., 2006; Miller et al., 2002; Stewart et al., 2010; van der Vinne et al., 2017). Moreover, a recent review shows that stress influences hemispheric laterality in both animal and human brains, where stress seems to mostly induce higher activity in the right hemisphere (Ocklenburg et al., 2016). A proposed explanation of the varying results by Ocklenburg et al. (2016) might then be that acute stressors generally induce higher right hemispherical activity, and that depending on the hemispheric dominance of the cognitive functions performed by the brain during the presence of a stressor, FAA might increase or decrease. An article by Berretz et al. (2020) further suggests that stress is highly influential in both the development of psychiatric disorders as well as the alteration of hemispheric laterality, further showing the relevance of FAA as an EEG index for psychosocial stress. Aside from FAA, other power-derived measures are utilized such as relative gamma and the theta/alpha ratio (Minguillon et al., 2016; Subhani et al., 2013). More complex spectral analysis methods, such as functional connectivity (FC), which investigates the temporal relationships between distinct neurophysiological events (Friston, 1994), are also explored, but to a lesser extent. Only three functional connectivity measures, phase-amplitude coupling, amplitude-amplitude correlation, and coherence have been reported (Poppelaars et al., 2018, 2021; Subhani et al., 2016a, 2016b).
Although the current body of literature using spectral EEG measures to identify the neural processes related to psychosocial stress is substantial, to our knowledge a systematic review and meta-analysis is currently lacking, making it difficult to have a concise overview of what has been undertaken and uncovered. Interestingly, two reviews have been recently published which review EEG results regarding the more general concepts of mental/psychological stress. Katmah et al. (2021) reviewed articles focusing on the detection of mental stress through means of machine learning algorithms. Although some overlap exists between this review and the current article regarding included articles, due the more technical focus on the machine learning algorithms used to detect mental stress, Katmah et al. (2021) employed a broader definition of mental stress, did not differentiate between the different phases of the stress response when interpreting the results and did not conduct meta-analyses. In another review, Giannakakis et al. (2019) examined the different physiological measurement possibilities for stress detection. However, similarly to Katmah et al. (2021), a more general definition of psychological stress is employed and no differentiation between stress phases nor meta-analyses are present. For the reader interested in mental stress detection through means of EEG or the possibilities regarding physiological stress measurement options, we refer to the aforementioned articles.
In this paper, we focus on the three (anticipation, reactive and recovery) phases of the stress response induced by acute (short-term) laboratory (conducted in a controlled environment) psychosocial stressors in healthy, unmedicated adults measured using EEG and interpreted with spectral analysis methods. The main research questions investigated in this systematic review are: 1) Which spectral EEG measures have been employed in the investigation of the psychosocial stress response; 2) Whether the identified spectral EEG measures are stress phase-sensitive or phase-independent. In the subsequent meta-analyses, performed for alpha power, beta power, and FAA, the main research question is if changes in these EEG measures induced by acute laboratory stressors result in a significant effect size, regardless of the stressor phase in which they have been investigated.
2. Material and methods
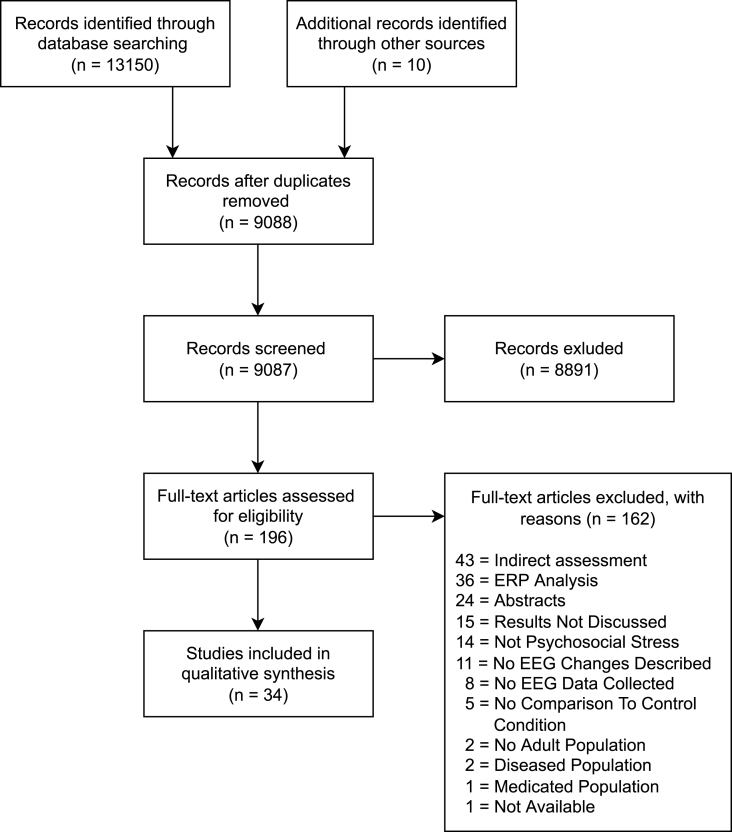
The guidelines of ‘The Preferred Reporting Items for Systematic Reviews and Meta-Analyses statement (PRISMA, Moher et al., 2009) were followed and a protocol was designed and registered in the PROSPERO database (registration number: CRD42020177226, registration date: April 7th, 2020). During the first screening of the obtained papers, a small adjustment to the protocol, the inclusion of the additional search term “criticism”, was made (PROSPERO registration data: July 9th, 2020). The PRISMA flow diagram is shown in Fig. 2.
Fig. 2.
The preferred reporting items for systematic review and meta-analyses (PRISMA) flow diagram.
2.1. Search strategy
Three databases, The National Library of Medicine (MEDLINE-PubMed), Web of Science and Embase, were searched to find articles whose content matched with the defined research questions. The keywords used for this search strategy are closely aligned with those of Kogler et al. (2015), with the following basic structure: (“Electroencephalography” OR “Electroencephalogram” OR “EEG”) AND (“Stress” OR “Social Exclusion” OR “Social Rejection” OR “Ostracism” OR “Social Pain” OR “Criticism”). The choice of using “Stress” and its derivatives as the main term rather than “psychological” or “psychosocial stress” was made due to the lack of consistent typology in the literature (Epel et al., 2018). The full search strategy can be found in the PROSPERO registration.
2.2. Study selection
All obtained articles were firstly reviewed based on the title/abstract and later based on the full text independently by two reviewers (G.V. and S.D.S.). Inclusion/exclusion disagreements were resolved by reaching a consensus between both reviewers. Only studies that reported data of unmedicated healthy adults (18 years and older with no history of neurological or psychiatric disorders) that were exposed to an acute laboratory psychosocial stressor (as defined in the introduction) and underwent an EEG recording within 24 h after the stressor exposure were included. Papers which investigated event-related potentials were included only if a spectral analysis was performed on the stimulus-defined time windows. Other results regarding latencies or amplitudes of ERP components discussed in the included articles are not discussed in the current review article. Studies that employed an acute psychosocial stressor, but used other neuroimaging methods (eg. fMRI, fNIRS) combined with EEG were included when the results of the EEG data were reported separately. Studies that investigated specific populations (eg. psychiatric patients, specific metabolic interactions (eg. pharmacological interventions) or specific interventions (eg. meditation), but also included a control group satisfying the previously defined criteria were also included if the data of the control group was reported separately. The authors of studies that did not report data of the EEG results or control groups separately were contacted and these studies were included when additional data was provided. Review articles, meta-analyses, conference proceedings, editorials, letters, case reports, or non-peer-reviewed articles were excluded. Conference abstracts were also excluded, but the authors were contacted and asked if a publication had been completed from the work of the abstract. No limitation on the publication date was imposed. All articles until April 8th, 2021, were included.
2.3. Quality assessment and risk of bias analysis
Quality assessment and risk of bias analysis of the included papers was done using an adapted version of the Standard Quality Assessment Criteria for evaluating primary research papers from a variety of fields (Kmet et al., 2004). This assessment tool consists of 14 questions that investigate the various aspects of an article regarding comprehensibility, reproducibility and validity. For each question, three answers are possible: yes (= 2) if the article reports all necessary information, partial (= 1) if some information is reported, but not all, and no (= 0) if the information is not present. A final score is obtained by summing all separate scores and dividing the final score by the maximum amount of points possible. A list of the used questions as well as the reasoning for the scoring of each question can be found in the supplementary materials (Risk of Bias Analysis – Explanation Of Scoring). The results of the risk of bias assessment are discussed in Section 3.2.
2.4. Data extraction
Data from the included articles were extracted by one reviewer (G.V.) and were checked by a second reviewer (S.D.S.). The following variables were extracted: population demographics (sample size, mean age + standard deviation, men/women distribution, inclusion and exclusion criteria, recruiting method), experimental protocol (study type, stressor type, control condition, cover story, timing considerations), EEG recording specifics (amount of EEG channels, equipment brand, electrode placement position, impedance values, presence of electrooculography (EOG) electrodes, sampling rate, online reference), EEG analysis specifics (downsample frequency, low pass frequency, high pass frequency, utility frequency removal procedure, interpolation procedure, offline reference, artifact removal procedures, epoch length, EEG analysis method), additional physiological data collection (cortisol, heart rate, heart rate variability (HRV), electrodermal skin activity (EDA), blood pressure, respiratory rate (RR), state questionnaires and whether or not these measures changed significantly), EEG-related results (test value (T-test or F-test), p-value, effect size and a short summary) and (if present) a priori sample size or power calculations. This information can be found in the supplementary materials (Systematic Review - Extracted Information).
2.5. Data analysis
Three EEG measures, frontal alpha asymmetry (FAA), alpha power, and beta power, were selected for meta-analyses as these measures were utilized in more than five articles (FAA: 13 articles, alpha power: 12 articles, beta power: 8 articles; Tufanaru et al., 2015). Although theta power was also utilized in a sufficient number of articles (i.e., 6 articles), no meta-analysis was conducted for this EEG measure due to an important difference between the articles. Three articles calculated theta power from stimulus-locked epochs and investigated stimulus-evoked neural activity whereas the other three articles used continuous EEG data which was not stimulus-locked. Although it is likely that some relation exists between the mechanisms underlying evoked and induced neuronal activity, it remains difficult to accurately compare the obtained power values from each response type (David et al., 2006). When no relevant values (means + standard deviations) were given in the articles, the authors were contacted and if no response was obtained, values were extracted from the figures using GRABIT, a MATLAB toolbox designed for extracting data points from figures (Jiro, 2021). Both the data extraction method for each paper in the meta-analyses and the obtained values for the meta-analyses can be found in the supplementary materials (Meta-Analysis – Data Extraction Procedure; Meta-Analysis - Numbers). Data in several articles were pooled to obtain a single value for the EEG measure using (1), (2). In both formulas, ni denotes the number of participants in study i.
| (1) |
| (2) |
Since different articles report results from different phases of the stress response (anticipatory, reactive, recovery), values for the meta-analyses are defined as the difference between the mean value from a phase of the study in which no psychosocial stressor is present (baseline: recording at the start of the experiment; control condition: a participant performs a task without the additional presence of a psychosocial stressor) and the mean value from a phase of the study where a psychosocial stressor is present (anticipatory phase: a participant is aware of the upcoming task; reactive phase: a participant is directly exposed to a psychosocial stressor) or where the effects of a psychosocial stressor are still present (recovery phase: right after a participant has been exposed to a psychosocial stressor). Differences between two phases are interpreted as changes in the EEG measure due to the presence of a psychosocial stressor. An overview of the phase-specific comparisons which have been used in the meta-analyses is shown in Fig. 3. For two articles, multiple phase comparisons were possible (see Fig. 3). Only one comparison from each study was included to not introduce possible biases in the meta-analysis that might have been present in the study. For the article by Betti and colleagues (2017), we chose to include the baseline-recovery comparison since both phases report data from resting-state EEG, which is not the case with the baseline-reactive comparison. For the study by Wang et al. (2015), we chose to include the baseline-anticipatory comparison, since both phases report data from resting-state EEG, which is not the case with the baseline-reactive comparison. The decision to include the baseline-anticipatory instead of the baseline-recovery comparison was made because the baseline phase is closer to the anticipatory phase compared to the recovery phase in time. One article by Izhar et al. (2019) contained theta power values and thus was eligible for the meta-analysis but was not included since the reactive phase was compared to a baseline recording. Since participants were engaged with a task in the reactive phase, differences between this phase and the resting-state baseline recording to which the obtained EEG data was compared, could not be uniquely attributed to the presence of a psychosocial stressor.Whereas the comparison of two phases within an article makes it possible to investigate the influence of psychosocial stress in the different phases of the stress response, a considerable amount of variability is still present between the articles that are included in the meta-analyses. Therefore, it is likely that the reported results are not only representative of the underlying effect of psychosocial stress but are also influenced by other factors as well, resulting in possible differences regarding reported results and effect sizes. For these reasons, a random effects model is chosen to perform the meta-analyses, since random effects models in meta-analyses do not assume statistical homogeneity or a common effect size between included studies (Tufanaru et al., 2015). To assess possible publication bias, funnel plots are generated, and the Egger's test is conducted to assess funnel plot asymmetry (Egger et al., 1997; Sterne et al., 2011). The generation of the funnel plots and subsequent Egger's tests are conducted in R (version 4.1.1). The complete analysis can be found in the supplementary materials (Meta-Analysis – Publication Bias Analysis) and the R files can be found on GitHub.1 No subgroup analyses are conducted regarding the specific stressor phases due to the limited number of included articles. To correct for small samples, which tend to overestimate effect sizes, hedges' g is used for effect size calculation. The meta-analyses were conducted using Review Manager (RevMan), version 5.4.1 (Review Manager (Revman), 2012). The MATLAB code used for data pooling as well as the final RevMan file containing the meta-analytic results can be found on GitHub.1
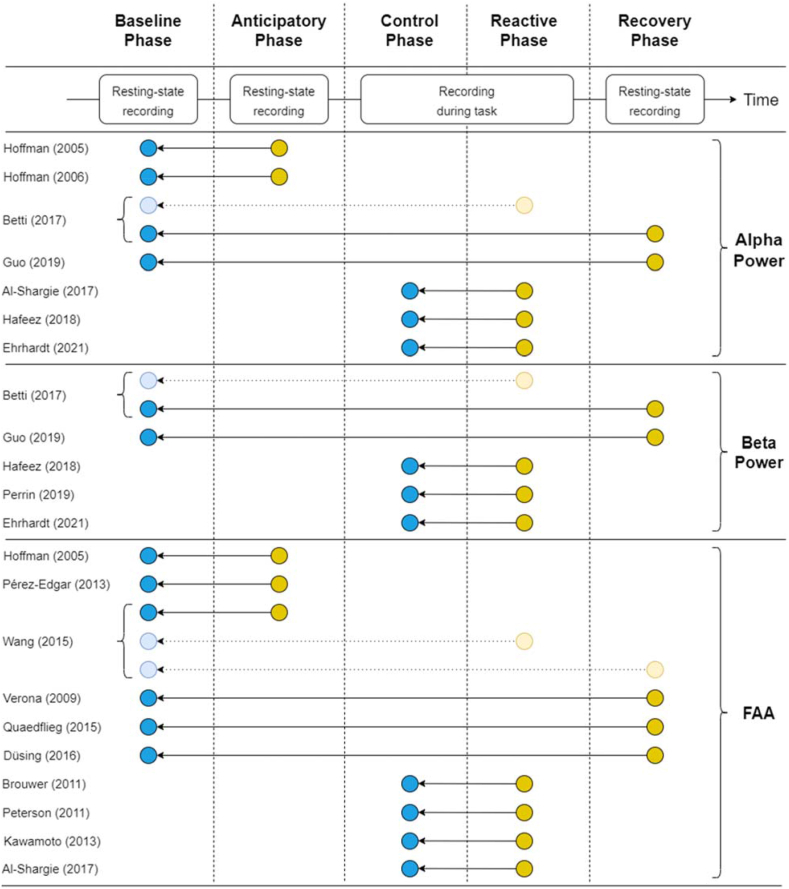
Fig. 3.
Figure showing which data from each article is used in the meta-analyses. Top part of the figure: identification of the different phases which can be present in each article. Middle part of the figure: identification of the type of EEG data which is collected during the corresponsing phase and the occurrence of the different phases with respect to time. Resting-state recording: indicates that during this phase, resting-state EEG data is collected. Recording during task: indicates that during this phase, EEG data is recorded while a participant is actively engaged with a task. Lower part of the figure: shows which data from each article is used in the meta-analysis. A blue circle denotes data used in the meta-analysis from a phase in which no psychosocial stressor is present. These phases are either the baseline phase or the control phase. A yellow circle denotes data used in the meta-analysis from a phase in which a psychosocial stressor is present or a phase where the effects of a psychosocial stressor are still present. These phases are either the anticipatory phase, the reactive phase or the recovery phase. An arrow indicates the comparison made between the data from the phase in which a psychosocial stressor is present and the phase without a psychosocial stressor. A light blue/yelow circle indicates that the corresponding article contains data from the indicated phases, but that this data is not used in the meta-analysis. The articles are defined on the left of the figure, and are grouped based on the EEG measure on which a meta-analysis is conducted (indicated at the right of the figure). (For interpretation of the references to colour in this figure legend, the reader is referred to the Web version of this article.)
3. Results
3.1. Search results
After deduplication of the articles, a total of 9088 publications were pinpointed as potentially relevant (3427 from PubMed, 3571 from Web of Science, 6152 from Embase and 10 from reference lists of publications). After abstract review, 8891 publications were excluded, leaving 196 articles for full text review. After a full text review, 162 publications were excluded, leaving 34 articles suitable for this systematic review. From these 34 articles, 7 were identified as reporting from the same population (Subhani et al., 2013, Subhani et al., 2016a, Subhani et al., 2016b): and (Al-Shargie et al., 2016; 2017a; 2017b, 2018). Therefore, the 34 articles selected for this systematic review report on 29 distinct participant populations (n = 1213, age range = 18–50 years, the characteristics of the studies are presented in Table 1).
Table 1.
Demographics, investigated neural activity, employed stressor paradigm, employed phase comparison, employed EEG measure, and employed frequency ranges of the included articles. Legend: Ppts = Participants; M = Mean; STD = Standard Deviation; Activity = type of neural activity which is investigated; Induced = task-induced neural activity; Evoked = task-evoked neural activity; Paradigm = employed psychosocial stressor; MIST = Montreal Imaging Stress Task (Dedovic et al., 2005); MAST = Maastricht Acute Stress Test (Smeets et al., 2012); SET = paradigms employing social-evaluative threat as stressor; TSST = Trier Social Stress Test (Kirschbaum et al., 1993); Cyberball = Cyberball paradigm (Williams et al., 2000); SJP = Social Judgment Paradigm (van der Molen et al., 2017); SPT = Social Performance Task (Harrewijn et al., 2016); Anticipation = Anticipatory phase; Reactive = Reactive Phase; Recovery = Recovery phase; Recoverycontrol = a recovery phase from a non-psychosocial stressed situation; Control = Control Condition; Baseline = Baseline Recording; Measure = The EEG measure used in the article; Apow = Alpha Power; Bpow = Beta Power; Tpow = Theta Power; Dpow = Delta Power; Spow = Sigma Power; FAA = Frontal Alpha Asymmetry; RG = Relative Gamma; SR = Slowing Ratio; AAC = Alpha Attenuation Coefficient; dPAC = Debiased Phase-Amplitude Coupling; AAC = Amplitude-Amplitude Correlation; PR = Theta-Alpha Power Ratio. (↓) = Significant decrease; (↑) = Significant increase; (−) = insignificant change; FR = Frequency ranges used in the article.
| Author | Year | Ppts | Pptsmale | Age (M ± STD) | Activity | Paradigm | Stress Phase | No-Stress Phase | Measure | FR (Hz) |
|---|---|---|---|---|---|---|---|---|---|---|
| Al-Shargie | 2016 | 22 | 22 | 26 ± 4 | Induced | MIST | Reactive | Control | Apow (↓) | Alpha: 8–12.5 Beta: 12.5–30 |
| Bpow (−) | ||||||||||
| Al-Shargie | 2017b | 25 | 25 | 22 ± 3 | Induced | MIST | Reactive | Control | Apow (↓) | Alpha: 8 - 13 |
| Al-Shargie | 2017a | 22 | 22 | 22 ± 2 | Induced | MIST | Reactive | Control | Apow (↓) | Alpha: 8 - 16 |
| Al-Shargie | 2018 | 18 | 18 | – | Induced | MIST | Reactive | Control | Apow (↓) | Alpha: 8 - 16 |
| FAA (↓) | ||||||||||
| Betti | 2017 | 12 (from 15) | 8 | 40.8 ± 9.5 | Induced | MAST | Reactive | Baseline | Apow (↑) | Alpha1: 8–9 |
| Bpow (−) | Alpha2: 10–12 | |||||||||
| Recovery | Baseline | Apow (↓) | Beta1: 13–17 | |||||||
| Bpow (−) | Beta2: 18 - 30 | |||||||||
| Brouwer | 2011 | 9 | 6 | – | Induced | SET | Reactive | Control | FAA (−) | Alpha: 8 - 13 |
| Crost | 2008 | 89 | 89 | 24.2 | Induced | SET | Reactive | Control | FAA (−) | Alpha: 8–10.25 |
| Düsing | 2016 | 49 | 17 | 22.48 ± 3.33 | Induced | TSST | Recovery | Baseline | FAA (↑) | Alpha: 8 - 13 |
| Ehrhardt | 2021 | 34 (from 38) | 19 | 25.76 ± 6.03 | Induced | SET | Reactive | Control | Apow (−) | Alpha: 8–13 Beta: 13 - 30 |
| Bpow (−) | ||||||||||
| Guo | 2020 | 150 | 75 | 23.75 ± 1.01 | Induced | TSST | Recovery | Baseline | Apow (↓) | Alphalow: 8–10 Alphahigh: 10–12 Betalow: 12–20 Betahigh: 20 - 30 |
| Bpow (↑) | ||||||||||
| Hafeez | 2018 | 14 | 11 | – | Induced | MIST | Reactive | Control | Tpow (↓) | Theta: 4–8 Alpha: 8–16 Beta: 16 - 31 |
| Apow (↓) | ||||||||||
| Bpow (↑) | ||||||||||
| Hofmann | 2005 | 27 (from 40) | 27 | 19 ± 1.29 | Induced | TSST | Anticipation | Baseline | Apow (↓) | Alpha: 8–13 |
| FAA (−) | ||||||||||
| Hofmann | 2006 | 32 | 0 | 18.53 ± 0.67 | Induced | TSST | Anticipation | Baseline | Apow (↓) | Alpha: 8 - 13 |
| Izhar | 2019 | 4 (Stable) 4 (Neurotic) | 8 | 19.5 ± 0.76 | Induced | TSST | Reactive | Baseline | Bpow (↑) | Beta: 13 - 30 |
| Minguillon | 2016 | 6 | – | 26.3 ± 6.4 | Induced | MIST | Reactive Recovery |
Control Control |
Tpow (↓) | Theta: 4–7 Alpha: 8–13 Beta: 14–24 Gamma: 25 - 45 |
| Apow (↓) | ||||||||||
| FAA (↓) | ||||||||||
| Bpow (↑) | ||||||||||
| RG (↑) | ||||||||||
| Minguillon | 2017 | 6 (from 12) | – | 25.3 ± 4.8 | Induced | MIST | Reactive | Recovery | RG (↑) | Theta: 4–7 |
| Alpha: 8–13 | ||||||||||
| Gamma: 25 - 45 | ||||||||||
| Kawamoto | 2013 | 19 | 8 | 18.3 | Induced | Cyberball | Reactive | Control | FAA (−) | Alpha: 8 - 13 |
| Kortdink | 2018 | 65 (from 78) | 0 | 19.69 ± 1.45 | Evoked | SJP | Reactive | Control | Tpow (↑) | Theta: 4 - 8 |
| Papousek | 2019 | 62 (from 67) | 12 | 24 ± 4 | Induced | TSST | Anticipation | Baseline | FAA (−) | Alpha: 8 - 12 |
| Recovery | ||||||||||
| Perrin | 2019 | 24 | 24 | 26.5 ± 4 | Induced | TSST | Recovery | RecoveryControl | Dpow (↓) | Delta: 0.5–4.5 Theta: 4.5–8 Alpha: 8–12 Sigma: 13–15 Beta: 15 - 32 |
| Tpow (↑) | ||||||||||
| SR (↓) | ||||||||||
| AAC (↓) | ||||||||||
| Spow (−) | ||||||||||
| Bpow (↑) | ||||||||||
| Peterson | 2011 | 40 | 20 | – | Induced | Cyberball | Reactive | Control | FAA (−) | Alpha: 10.25–12.5 |
| Pérez-Edgar | 2013 | 45 | 19 | 21.1 ± 5.34 | Induced | TSST | Anticipation | Baseline | FAA (−) | Alpha: 8 - 13 |
| Poppelaars | 2018 | 20 (HSA) | 52 | 19.7 ± 1.5 | Induced | SPT | Anticipation | Baseline | dPAC (−) | Delta: 1–4 |
| 32 (LSA) | 20 ± 1.6 | Recovery | AAC (−) | Beta: 14 - 30 | ||||||
| Poppelaars | 2021 | 64 (from 85) | 34 | 22.4 ± 2.6 (M) | Induced | SPT | Anticipation | Baseline | dPAC (−) | Delta: 1–4 |
| 22.9 ± 2.8 (F) | Recovery | AAC (−) | Beta: 14 - 30 | |||||||
| Quaedflieg | 2015 | 70 | 30 | 20.83 ± 2.67 | Induced | MAST | Recovery | Baseline | FAA (−) | Alpha: 8 - 13 |
| Subhani | 2013 | 10 | 10 | – | Induced | MIST | Reactive | Control | PR (↑) | Theta: 4–8 |
| Recovery | Baseline | Alpha: 8 - 12 | ||||||||
| Subhani a | 2016 | 22 | – | – | Induced | MIST | Reactive | Control | Coherence (↑,↓) | Alpha: 8–12 |
| Beta: 13 - 30 | ||||||||||
| Subhani b | 2016 | 22 | – | 22 ± 1.5 | Induced | MIST | Recovery | Baseline | Coherence (↑,↓) | Alpha: 8–12 |
| Beta: 13 - 25 | ||||||||||
| van der Veen | 2016 | 194 | 48 | 20.9 ± 2.3 | Evoked | SJP | Reactive | Control | Tpow (↑) | Delta: 2 - 3 |
| Dpow (↓) | Theta: 5–7 | |||||||||
| Vaquero-Blasco | 2020 | 17 (from 20) | – | 24.2 ± 4.03 | Induced | MIST | Reactive | Control | RG (↑) | Theta: 4–8 Alpha: 8–13 Gamma: 25 - 45 |
| Recovery | Control | RG (↓) | ||||||||
| Vaquero-Blasco | 2021 | 19 (from 23) | 8 | 22.65 ± 5.48 | Induced | MIST | Reactive | Control | RG (↑) | Theta: 4–8 Alpha: 8–13 Gamma: 25 - 45 |
| Recovery | Reactive | RG (↓) | ||||||||
| Verona | 2009 | 43 (from 135) | – | 24.6 ± 6.46 | Induced | SET | Recovery | Baseline | FAA (−) | Alpha: 8 - 13 |
| Wang | 2015 | 25 (from 80) | 13 | 23.12 ± 2.15 | Induced | TSST | Anticipation | Baseline | FAA (↓) | Alpha: 8 - 12 |
| Reactive | FAA (↓) | |||||||||
| Recovery | FAA (−) | |||||||||
| Yao | 2020 | 50 (HPS) 50 (LPS) |
23 29 |
20.4 ± 0.29 20.16 ± 0.32 |
Evoked | Cyberball | Reactive | Control | Dpow (↑) | Alpha: 7–13 |
| Tpow (↑) | Theta: 4–8 | |||||||||
| Apow (↓) | Delta: 1 - 4 |
= Subhani, A. R., Malik, A. S., Kamil, N., & Saad, M. N. M.
Subhani, A. R., Malik, A. S., Kamil, N., Naufal, M., & Saad, M. N. M.
3.2. Quality assessment and analysis of bias
The results of the quality assessment and analysis of bias are shown in Table 2. The average score from the risk of bias analysis is 74.47% (standard deviation = 9.95; minimum = 57%; maximum = 93%), showing a high variability in the final scores. Overall, all articles precisely describe their research questions and hypotheses (question one) and that almost all articles employ an appropriate study design (question two). Large differences however are found in the description of the demographic information of the sample population (question three), the explanation of the blinding of the participants (question seven), the estimate of variance (question 11) and the control of confounding factors (question 12). It is notable that most articles scored poorly on question nine, indicating that small sample sizes were common across the studies. One article did not include any information, 32 out of the 34 included articles scored “partial” (= 1, see above) and one article included all information on question 10. The extended risk of bias analysis with answers to the subquestions eight and ten can be found in the supplementary materials (Risk Of Bias Analysis - Extended Calculation). It should be noted that the research questions of this systematic review do not always align with the research questions posed in the articles. This can lead to lower scores for multiple articles regarding questions such as the reporting of statistical analysis (question ten c) or the estimation of variance (question 11).
Table 2.
Risk of Bias analysis. To obtain the percentage score, the total score was divided by 28 (= 14*2) and multiplied by 100.
| Author | Q1 | Q2 | Q3 | Q4 | Q5 | Q6 | Q7 | Q8 | Q9 | Q10 | Q11 | Q12 | Q13 | Q14 | Total | Percentage (%) |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Al-Shargie et al. (2016) | 2 | 2 | 0 | 2 | 2 | 2 | 1 | 2 | 0 | 1 | 0 | 2 | 1 | 1 | 18 | 64 |
| Al-Shargie (2017a, 2017b) | 2 | 2 | 0 | 2 | 2 | 2 | 1 | 2 | 0 | 1 | 1 | 2 | 2 | 2 | 21 | 75 |
| Al-Shargie (2017a, 2017b) | 2 | 2 | 0 | 2 | 2 | 2 | 1 | 1 | 0 | 1 | 0 | 1 | 2 | 2 | 18 | 64 |
| Al-Shargie (2017a, 2017b) | 2 | 2 | 0 | 1 | 2 | 2 | 1 | 2 | 0 | 1 | 2 | 1 | 1 | 1 | 18 | 64 |
| Betti et al. (2018) | 2 | 2 | 2 | 2 | 2 | 2 | 1 | 1 | 0 | 0 | 2 | 1 | 2 | 1 | 20 | 71 |
| Brouwer et al. (2011) | 2 | 1 | 1 | 1 | 2 | 2 | 2 | 2 | 0 | 1 | 1 | 1 | 2 | 1 | 19 | 68 |
| Crost et al. (2008) | 2 | 1 | 1 | 1 | 1 | 2 | 1 | 2 | 2 | 1 | 1 | 1 | 2 | 2 | 20 | 71 |
| Düsing et al. (2016) | 2 | 2 | 2 | 2 | 2 | 2 | 1 | 2 | 1 | 2 | 2 | 1 | 2 | 2 | 25 | 89 |
| Ehrhardt et al. (2021) | 2 | 2 | 0 | 2 | 2 | 2 | 1 | 2 | 1 | 1 | 2 | 1 | 2 | 2 | 22 | 79 |
| Guo et al. (2019) | 2 | 2 | 1 | 2 | 2 | 2 | 1 | 1 | 2 | 1 | 2 | 1 | 1 | 2 | 22 | 79 |
| Hafeez et al. (2018) | 2 | 2 | 1 | 1 | 2 | 2 | 1 | 2 | 0 | 1 | 1 | 1 | 2 | 1 | 19 | 68 |
| Hofmann et al. (2005) | 2 | 2 | 2 | 2 | 2 | 2 | 2 | 2 | 0 | 1 | 1 | 1 | 2 | 2 | 23 | 82 |
| Hofmann (2006) | 2 | 2 | 2 | 2 | 2 | 2 | 1 | 1 | 0 | 1 | 1 | 1 | 2 | 2 | 21 | 75 |
| Izhar et al. (2019) | 2 | 2 | 1 | 2 | 2 | 2 | 1 | 2 | 0 | 1 | 2 | 1 | 2 | 2 | 22 | 79 |
| Kawamoto et al. (2013) | 2 | 2 | 0 | 1 | 2 | 2 | 0 | 2 | 0 | 1 | 1 | 1 | 2 | 2 | 18 | 64 |
| Kortink et al. (2018) | 2 | 2 | 1 | 2 | 2 | 2 | 2 | 2 | 1 | 1 | 0 | 1 | 2 | 2 | 22 | 79 |
| Minguillon et al. (2016) | 2 | 2 | 0 | 1 | 2 | 2 | 1 | 1 | 0 | 1 | 0 | 1 | 2 | 2 | 17 | 61 |
| Minguillon et al. (2017) | 2 | 2 | 1 | 1 | 2 | 2 | 1 | 1 | 0 | 1 | 0 | 1 | 2 | 2 | 18 | 64 |
| Papousek et al. (2019) | 2 | 2 | 1 | 2 | 2 | 2 | 2 | 2 | 2 | 1 | 0 | 2 | 2 | 2 | 24 | 86 |
| Pérez-Edgar et al. (2013) | 2 | 2 | 2 | 2 | 2 | 2 | 1 | 1 | 1 | 1 | 2 | 1 | 2 | 2 | 23 | 82 |
| Perrin et al. (2019) | 2 | 1 | 1 | 2 | 2 | 2 | 2 | 1 | 0 | 1 | 2 | 1 | 1 | 2 | 20 | 71 |
| Peterson et al. (2011) | 2 | 2 | 2 | 1 | 2 | 2 | 2 | 1 | 0 | 1 | 2 | 1 | 2 | 2 | 22 | 79 |
| Poppelaars et al. (2018) | 2 | 2 | 2 | 2 | 2 | 2 | 2 | 2 | 2 | 1 | 2 | 1 | 2 | 2 | 26 | 93 |
| Poppelaars et al. (2021) | 2 | 2 | 1 | 2 | 2 | 2 | 1 | 2 | 2 | 1 | 2 | 1 | 2 | 2 | 24 | 86 |
| Quaedflieg et al. (2015) | 2 | 2 | 1 | 2 | 2 | 2 | 2 | 2 | 2 | 1 | 2 | 1 | 2 | 2 | 25 | 89 |
| Subhani et al. (2013) | 2 | 2 | 0 | 1 | 2 | 2 | 1 | 2 | 0 | 1 | 2 | 1 | 1 | 2 | 19 | 68 |
| Subhani et al. (2016)a | 2 | 2 | 0 | 0 | 2 | 2 | 1 | 2 | 0 | 1 | 0 | 1 | 2 | 1 | 16 | 57 |
| Subhani et al. (2016)b | 2 | 2 | 1 | 1 | 2 | 2 | 1 | 2 | 0 | 1 | 0 | 1 | 1 | 1 | 17 | 61 |
| van der Veen et al. (2016) | 2 | 2 | 1 | 2 | 2 | 2 | 1 | 2 | 2 | 1 | 1 | 1 | 2 | 2 | 23 | 82 |
| Vaquero-Blasco et al. (2020) | 2 | 2 | 0 | 1 | 2 | 2 | 1 | 2 | 0 | 1 | 0 | 1 | 2 | 2 | 18 | 64 |
| Vaquero-Blasco et al. (2021) | 2 | 2 | 1 | 2 | 2 | 2 | 1 | 1 | 0 | 1 | 0 | 1 | 1 | 2 | 18 | 64 |
| Verona et al. (2009) | 2 | 2 | 1 | 2 | 2 | 2 | 2 | 2 | 1 | 1 | 2 | 1 | 2 | 2 | 24 | 86 |
| Wang et al. (2015) | 2 | 2 | 2 | 2 | 2 | 2 | 1 | 2 | 2 | 1 | 2 | 1 | 2 | 2 | 25 | 89 |
| Yao et al. (2020) | 2 | 2 | 0 | 2 | 2 | 2 | 2 | 2 | 2 | 1 | 0 | 1 | 2 | 2 | 22 | 79 |
= Subhani, A. R., Malik, A. S., Kamil, N., & Saad, M. N. M.
Subhani, A. R., Malik, A. S., Kamil, N., Naufal, M., & Saad, M. N. M.
3.3. Systematic review
The results of the articles included in this systematic review will be discussed with a focus on which stress phase was investigated and how the measured EEG variable changed due to the presence of an acute psychosocial stressor. All results are discussed by making a comparison between a phase where a psychosocial stressor is present (anticipatory, reactive or recovery phase) and a phase without the presence of a psychosocial stressor (a baseline recording at the start of the study or a control condition where participants perform the same task as during the reactive phase, but without the presence of a psychosocial stressor). The anticipatory and recovery phase are compared to a baseline condition if possible since EEG data from both stress response phases is resting-state data, which is also the case for a baseline recording. The reactive phase is compared to a control condition and not a baseline recording, if possible, since differences between a baseline recording and EEG data recorded during the reactive phase can not only be attributed to the psychosocial stressor, but also to the task which is performed during the reactive phase. An overview of the stress response phases which are investigated (Stress Phase column) and the phases without psychosocial stressors to which they are compared (No-Stress Phase column), as well as the type of neural activity which is investigated (Activity column), employed psychosocial stressor (Paradigm column), investigated EEG measure and reported findings (Measure column) and specific frequency ranges (FR column) can be found in Table 1. Three types of results have been identified in the included articles: spectral power features, spectral power derived features, and functional connectivity features. Spectral power features are calculated by obtaining the power spectral density, usually through a Fourier transformation, and selecting a frequency band of interest to calculate the average power within it (Cohen, 2014). Spectral power derived features use spectral power measures for their calculation but combine multiple power values from distinct spatial locations or different frequency ranges. Functional connectivity features investigate temporal dependencies between spatially distinct neurophysiological events and give insight into the communicational mechanics of the brain (Friston, 1994). Results are grouped by the type of EEG feature, namely power, power-derived and functional connectivity features. The employed psychosocial stress paradigms are not discussed, unless necessary to understand the results, but can be found in Table 1. When discussing results, “significant” is defined as statistically significant (p < 0.05) after multiple comparison correction (if applied in the article itself).
3.3.1. Power features
3.3.1.1. Delta power
In three articles, delta power was employed as a measure to investigate psychosocial stress. van der Veen and colleagues (2016) investigated the reactive phase by employing the social judgment paradigm (SJP), where participants need to guess whether other participants would like or dislike them after seeing a picture of them and afterwards receive the corresponding, computer-generated, feedback. This paradigm leads to four possible conditions: expected or unexpected acceptance/rejection, and the delta power has been calculated from the corresponding ERP segments (Somerville et al., 2006). They found a significantly decreased delta power when participants were rejected (expected or unexpected) or unexpectedly accepted compared to the expected acceptance condition. This effect was the only significant effect and was found at electrode FCz. Yao et al. (2020) also employed the social judgment paradigm and found that delta power was increased when participants experienced feedback in a social context (reactive phase) compared to a nonsocial context (control condition). Perrin et al. (2019) looked at the delta frequency band (electrodes Fz, C3, C4, Cz, Pz, Oz) throughout the day after a public speaking task in the morning (recovery phase). They found that the delta power was significantly lower after this speaking task compared to a control condition (i.e., no speaking task in the morning).
3.3.1.2. Theta power
In six articles, results regarding theta power are reported. In three articles, task-evoked neural activity in the reactive phase was investigated during the SJP (see section 3.3.1. for clarity) (Somerville et al., 2006). In two articles, significantly increased theta power during the unexpected rejection condition compared to the other conditions was reported (Kortink et al., 2018; van der Veen et al., 2016), whereas in the third article an overall increase in theta power during social feedback was reported (reactive phase) compared to a control condition (Yao et al., 2020). Minguillon et al. (2016) investigated the reactive and recovery phase and found that theta power was decreased during the reactive phase when compared to both a control condition or the recovery phase. This decrease in theta power during the reactive phase compared to a control condition was also found by Hafeez et al. (2018). Perrin et al. (2019) investigated the recovery phase and found that theta power was significantly higher when compared to the recovery phase of a control condition.
3.3.1.3. Alpha power
In twelve articles, alpha power changes from (mainly) the frontal electrodes are reported (Al-Shargie et al., 2016, 2017a, 2017b, 2018; Betti et al., 2018; Ehrhardt et al., 2021; Guo et al., 2019; Hafeez et al., 2018; Hofmann, 2006; Hofmann et al., 2005; Minguillon et al., 2016; Yao et al., 2020). In eight articles, the reactive phase, compared to a control condition, was investigated and seven times a significant decrease in alpha power during the reactive phase (Al-Shargie et al., 2016, 2017a, 2017b, 2018; Hafeez et al., 2018; Minguillon et al., 2016; Yao et al., 2020) was found. In one article however, a nonsignificant drop in alpha power between the reactive phase (when participants perform the paced auditory serial addition task (PASAT, Gronwall and Sampson, 1974) with time constraint and social feedback) and a control condition (PASAT with only a time constraint) was found (Ehrhardt et al., 2021). In one article the reactive phase was compared to a baseline recording instead of a control condition and an increase in alpha power during the reactive phase was found, which is contrary to all other articles in which alpha power was employed (Betti et al., 2018). The anticipatory phase, compared to a baseline recording, was investigated in two articles and a significant reduction in alpha power during the anticipatory phase was found in both articles (Hofmann, 2006; Hofmann et al., 2005). Finally, in two articles results from the recovery phase, compared to a baseline recording, were reported and here a significant decrease in alpha power during this phase of the psychosocial stress response was found (Betti et al., 2018; Guo et al., 2019).
3.3.1.4. Sigma power
One article investigated the influence of psychosocial stress on sleep quality and investigated if sigma power (13–15 Hz) was affected during the recovery phase (Perrin et al., 2019). Sigma power, linked with sleep quality of sleeping individuals (Spiegelhalder et al., 2012), was not significantly affected by psychosocial stress in the recovery phase.
3.3.1.5. Beta power
Beta power variations due to psychosocial stress were investigated in eight articles, which report results from the reactive and recovery phase (Al-Shargie et al., 2016; Betti et al., 2018; Ehrhardt et al., 2021; Guo et al., 2019; Hafeez et al., 2018; Izhar et al., 2019; Minguillon et al., 2016; Perrin et al., 2019). In five articles, the reactive phase was compared to a control condition (Al-Shargie et al., 2016; Ehrhardt et al., 2021; Hafeez et al., 2018; Minguillon et al., 2016; Perrin et al., 2019). Hafeez et al. (2018) as well as Perrin et al. (2019) reported significantly higher beta power during the reactive phase. Al-Shargie et al. (2016) also reported higher beta power during the reactive phase, but this difference is not significant. Minguillon et al. (2016) reported a significantly higher beta power during the reactive phase compared to the recovery phase for both prefrontal (Fp1, Fp2) and frontal (Fz, F3, F4, F7, F8) electrodes. When compared to a control condition, the beta power is significantly higher during the reactive phase, but only at the frontal electrodes. Ehrhardt et al. (2021) reported, contrary to other articles investigating the reactive phase - control condition difference, a decrease in beta power during the reactive phase, although this difference is nonsignificant. In two articles, the reactive phase was also investigated, but was compared to a baseline recording instead of a control condition (Betti et al., 2018; Izhar et al., 2019). Izhar et al. (2019) reported a significant increase in beta power during the reactive phase, whereas Betti et al. (2018) reported a reduction in beta power, although this was not significant. Finally, in two articles results from the recovery phase, compared to a baseline recording were described (Betti et al., 2018; Guo et al., 2019). Guo et al. (2019) reported a significant increase in beta power during the recovery phase, whereas Betti et al. (2018) reported an insignificant increase.
3.3.2. Power-derived features
3.3.2.1. Slowing ratio
In one paper, the slowing ratio (an EEG measure reflecting cortical arousal during sleep, defined as the ratio of the power in the slower (delta, theta, 0.5–8Hz) frequency ranges by the power of the faster (alpha, sigma, beta, 12–32Hz) ranges (D'Rozario et al., 2013)) measured during the recovery phase was significantly lower after the psychosocial stress exposure compared to a control condition (Perrin et al., 2019).
3.3.2.2. Theta-alpha power ratio
Subhani et al. (2013) used theta-alpha power ratio (an index reflecting the internal and external load on an individual, defined as the ratio of the theta power value at electrode Fz by the alpha power value at electrode Pz (Holm et al., 2009)) in both the reactive and recovery phase. In the reactive phase, they found that the theta-alpha power ratio was significantly higher during the stress condition compared to the control condition. This difference did not persist in the recovery phase.
3.3.2.3. Relative gamma
In four papers, relative gamma (RG, an index initially identified in research regarding meditation and defined as the ratio of gamma band power by the average power of the combined theta and alpha band (Lutz et al., 2004; Steinhubl et al., 2015)) was used. Minguillon and colleagues (2016, 2017) investigated RG during the reactive and recovery phase (2016) and during the recovery phase (2017) at the prefrontal (Fp1, Fp2), frontal (Fz, F3, F4, F7, F8), central (Cz, C3, C4) and parietal (Pz, T5, T6) electrodes. During the reactive phase, RG was significantly higher during the stress condition compared to the control condition for all electrode locations (Minguillon et al., 2016). During the recovery phase, relative gamma decreased significantly compared to the reactive phase, again for all electrode positions (Minguillon et al., 2016, 2017). Vaquero-Blasco and colleagues (2020, 2021) reported alterations in RG during the reactive and recovery phase. RG decreased from the reactive to the recovery phase, although it is not clear whether this was significant or not due to the specific research question of the studies and corresponding absence of relevant p-values.
3.3.2.4. Frontal alpha asymmetry
Frontal Alpha Asymmetry (FAA) is computed by obtaining the natural log transformed power in the alpha frequency band from two frontal EEG electrodes that are opposite symmetric compared to the midline (mostly F3/F4 or F7/F8) and subtracting the power value of the left electrode from the right (see formula 3). FAA reflects approximately the relative difference between the alpha power of the frontal part of the left and right hemisphere, and a positive FAA value indicates a relative greater alpha power of the right frontal hemisphere. Changes in FAA are believed to denote emotional or motivational responses of an individual whereby an increase in FAA (indicating an increase of relative right hemispheric activity) likely indicates more withdrawal-related states (Smith et al., 2017).
| (3) |
In thirteen articles, FAA was used to investigate the various phases of the psychosocial stress response (Al-Shargie et al., 2018; Brouwer et al., 2011; Crost et al., 2008; Düsing et al., 2016; Hofmann et al., 2005; Kawamoto et al., 2013; Minguillon et al., 2016; Papousek et al., 2019; Pérez-Edgar et al., 2013; Peterson et al., 2011; Quaedflieg et al., 2015; Verona et al., 2009; Wang et al., 2015). In four articles, the anticipatory phase, compared to a baseline recording, was investigated (Hofmann et al., 2005; Papousek et al., 2019; Pérez-Edgar et al., 2013; Wang et al., 2015). In three articles a decrease in FAA was reported, one significant (Wang et al., 2015), and two insignificant (Hofmann et al., 2005; Pérez-Edgar et al., 2013). Papousek et al. (2019) however, reported an increase in the laterality coefficient (which can be understood as a normalized version of FAA) but these results were also insignificant. In seven articles the reactive phase was investigated. In six articles, it was compared to a control condition (Al-Shargie et al., 2018; Brouwer et al., 2011; Crost et al., 2008; Kawamoto et al., 2013; Minguillon et al., 2016; Peterson et al., 2011), whereas in one article it was compared to a baseline recording (Wang et al., 2015). In four articles, a decrease in FAA was reported during the reactive phase compared to either a control condition or a baseline recording, three times significant (Al-Shargie et al., 2018; Minguillon et al., 2016; Wang et al., 2015), and one time insignificant (Kawamoto et al., 2013). Twice an increase in FAA during the reactive phase was reported, but neither were significant (Brouwer et al., 2011; Peterson et al., 2011). In one article the participant population was divided by anxiety and defensiveness scores, so an overall result of FAA changes is not present (Crost et al., 2008). Finally, in four articles the recovery phase, compared to a baseline recording was investigated (Düsing et al., 2016; Quaedflieg et al., 2015; Verona et al., 2009; Wang et al., 2015). In three articles, small increases in FAA were reported, but aside from the results of electrode pair F3/F4 from Düsing et al. (2016), no results were significant (Düsing et al., 2016; Quaedflieg et al., 2015; Verona et al., 2009). In one article, no change at all between baseline and recovery was found (Wang et al., 2015).
Aside from frontal alpha asymmetry, 3 articles also reported alpha asymmetry results, but from the parietal regions (Crost et al., 2008; Hofmann et al., 2005; Pérez-Edgar et al., 2013). Hofmann et al. (2005) as well as Pérez-Edgar et al. (2013) investigated the anticipatory phase, compared to a baseline recording, and neither found a significant result. Crost et al. (2008) investigated the reactive phase, compared to a control condition, and found no significant results.
3.3.2.5. Alpha attenuation coefficient
In one paper, the alpha attenuation coefficient (AAC, an index of sleepiness and defined as the division of the mean alpha power during an eyes-closed resting state recording by the mean alpha power during an eyes-open resting state recording (Stampi et al., 1995)) was used and a significant lower AAC during the recovery phase when participants were exposed to a psychosocial stressor compared to the recovery after a control condition was found (Perrin et al., 2019).
3.3.3. Functional connectivity features
3.3.3.1. Coherence
In two articles, reporting results of the same experiment and population, coherence (a FC measure that infers the similarity between the power spectra of two time series and can roughly be understood as the frequency equivalent of cross-correlation (Cohen, 2014)) was used to investigate psychosocial stress-related brain activity alterations. Subhani et al. (2016a) reported the results of the reactive phase of the stress response compared to a control condition and found increased coherence within the left and right frontal central electrode clusters as well as decreased coherence between both clusters in the delta band, increased coherence mainly between the right fronto-central electrodes in the theta band, increased coherence mainly between the prefrontal, frontal and central electrodes in the alpha band and increased coherence between the frontal, central and temporal/parietal electrodes in the beta band. Subhani et al. (2016b) compared the stress recovery phase to a baseline recording collected before the start of the experiment and found decreased coherence mainly between the right frontal and central electrodes in the delta band, decreased coherence mainly between the occipital, temporal, parietal and right frontal electrodes as well as increased coherence between the prefrontal and left central electrodes in the theta band, some decreased coherence between occipital electrodes in the alpha band and increased coherence between the Pz electrode and frontal and prefrontal electrode in the beta band. It should be noted that the coherence between all electrodes (19 in total) and within multiple frequency bands had been calculated and that the results were not corrected for multiple comparisons, so these results should be interpreted with caution.
3.3.3.2. Phase-amplitude coupling and amplitude-amplitude correlation”
Poppelaars and colleagues (2018, 2021) used both phase-amplitude coupling (a FC measure investigating the relationship between the phase of an EEG signal in a low frequency band with the amplitude of an EEG signal in a high frequency band (Tort et al., 2010)) and amplitude-amplitude correlation (a FC measure investigating the relationship between the amplitudes of EEG signals in different frequency ranges (Knyazev, 2011)) between the delta and beta band (Poppelaars et al., 2018, 2021). They investigated the anticipatory and recovery phase but found no significant effects due to the psychosocial stressor using either the phase-amplitude coupling or the amplitude-amplitude correlation.
3.4. Meta-analytic results
3.4.1. Alpha power
From the 12 articles in which results regarding alpha power changes were reported, five articles were omitted from further meta-analysis. In four articles, results from the same population were reported (Al-Shargie et al., 2016; 2017a; 2017b, 2018), so the article reporting results from the largest population was included in the meta-analysis (Al-Shargie et al., 2017b), whereas the other three were discarded (Al-Shargie et al., 2016; 2017a, 2018). Two articles were omitted as it was not possible to extract the results (Minguillon et al., 2016; Yao et al., 2020). Therefore, seven articles are included in the meta-analysis (Al-Shargie et al., 2017a, 2017b; Betti et al., 2018; Ehrhardt et al., 2021; Guo et al., 2019; Hafeez et al., 2018; Hofmann, 2006; Hofmann et al., 2005). As mentioned in section 2.5., the article from Betti et al. (2018) investigated alpha power in both the reactive and recovery phase. Since the EEG data from both phases is compared with a baseline, resting-state recording at the start of the experiment, results from the recovery phase are chosen for the meta-analysis, since resting-state data is also recorded in this stage, which is not the case in the reactive phase (see Fig. 3). Interestingly, the result of the reactive phase from Betti et al. (2018) reports a small to moderate negative effect size (hedge's g = −0.23) and is the only negative effect size (which can be understood as an increase in alpha power) of all articles included in the alpha power meta-analysis.
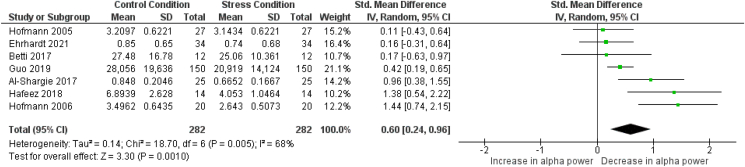
Fig. 4 shows the details regarding the alpha power meta-analysis. Overall, a significant effect was found (SMD = 0.6; [0.24, 0.96]; Z = 3.30; p = 0.001), showing that alpha power significantly decreases due to psychosocial stress, regardless of the stress phase which was investigated. Heterogeneity tests show that high heterogeneity is present in the meta-analysis (I2 = 68%; p = 0.005). Due to the limited amount of included studies, no further subgroup analysis can be performed to further investigate the reasons for this heterogeneity, but the low number of articles as well as the low number of participants in multiple studies might be the reason. Analyses suggest that no publication bias is present (p = 0.336, see supplementary material Meta-Analysis – Publication Bias Analysis).
Fig. 4.
Forest plot illustrating the standardized mean differences (SMD), individual effect sizes, overall effect size and heterogeneity statistics for the meta-analysis examining changes in alpha power from a control (non-stressed) condition to a stress condition. Hofmann et al., 2005 and Hofmann (2006) report a baseline - anticipatory phase comparison; Al-Shargie 2017a, 2017b, Hafeez et al., 2018 and Ehrhardt et al., 2021 report a control condition - reactive phase comparison; Betti et al., 2018 and Guo et al., 2019 report a baseline - recovery phase comparison.
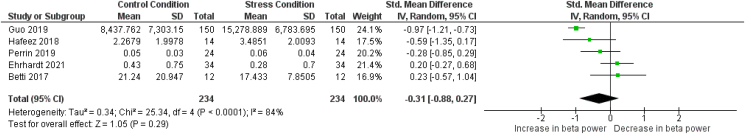
3.4.2. Beta power
From the eight articles in which beta power results are described, five articles were included in the meta-analysis (Betti et al., 2018; Ehrhardt et al., 2021; Guo et al., 2019; Hafeez et al., 2018; Perrin et al., 2019). Three articles were omitted, two due to the absence of data or figures (Al-Shargie et al., 2016; Minguillon et al., 2016) and one due to the fact that the reactive phase (EEG data during a task) was investigated, whereas only a baseline, resting-state EEG recording was present for comparison (Izhar et al., 2019). Similarly to the alpha power meta-analysis, Betti et al. (2018) report results from both the reactive and recovery phase. To eliminate bias in the meta-analysis, only one result was chosen to be included and, similarly to the alpha power meta-analysis, we chose to include the result reporting changes in the recovery phase (see section 2.5. and Fig. 3). The effect size reported by Betti et al. (2018) for the reactive phase compared to the baseline recording is small (hedge's g = 0.14), showing a small decrease in overall beta power during the reactive phase compared to the baseline measurement.
Fig. 5 shows the details regarding the beta power meta-analysis. Overall, no significant effect was found (SMD = −0.31; [−0.88 0.27]; Z = 1.05; p = 0.29), meaning that beta power did not change significantly from a non stressed condition to a psychosocially stressed condition. Due to the limited number of articles included in the meta-analysis, no further subgroup analysis can be performed. Heterogeneity tests show that high heterogeneity is present (I2 = 80%; p = 0.0001). Similarly to the alpha power meta-analysis, the low amount of results combined with the small sample size is likely the reason for this heterogeneity. The Egger's test indicated that no publication bias is present (p = 0.13, see supplementary material Meta-Analysis – Publication Bias Analysis). However, due to the low number of included studies, this result should be interpreted with caution since the Egger's test might lack sufficient power to correctly identify publication bias (Sterne et al., 2011).
Fig. 5.
Forest plot illustrating the standardized mean differences (SMD), individual effect sizes, overall effect size and heterogeneity statistics for the meta-analysis examining changes in beta power from a control (non-stressed) condition to a stress condition. Hafeez et al., 2018, Perrin et al., 2019 and Ehrhardt et al., 2021 report a control condition - reactive phase comparison; Betti et al., 2018 and Guo et al., 2019 report a baseline - recovery phase comparison.
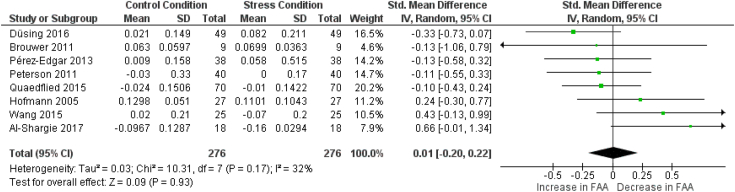
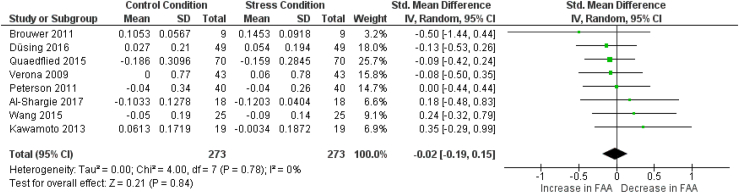
3.4.3. Frontal alpha asymmetry
From the thirteen articles in which FAA results are reported, 10 articles are eligible for concurrent meta-analyses. Three articles were omitted from the analysis due to the absence of data or figures (Crost et al., 2008; Minguillon et al., 2016; Papousek et al., 2019). Two meta-analyses have been run for FAA, since FAA can be calculated for multiple electrode pairs and results from two electrode pairs, the F3/F4 and F7/F8 pairs, were commonly reported. Therefore, a meta-analysis has been conducted for each of these electrode pairs. In each meta-analysis eight articles are included. In six articles, FAA results were reported for both electrode pairs (Al-Shargie et al., 2018; Brouwer et al., 2011; Düsing et al., 2016; Peterson et al., 2011; Quaedflieg et al., 2015; Wang et al., 2015). In two articles, the results for the F3/F4 electrode pair alone were reported (Hofmann et al., 2005; Pérez-Edgar et al., 2013), whereas results for only the F7/F8 electrode pair were reported in two other articles (Kawamoto et al., 2013; Verona et al., 2009). Wang et al. (2015) reported results from multiple phases (anticipatory, reactive, and recovery), so to avoid bias in the meta-analyses, only one result is included. We chose to include the results from the anticipatory phase as this phase lies closest to the baseline recording in time, therefore making it the least likely to be affected by unknown influences introduced throughout time (see section 2.5. and Fig. 3).
3.4.3.1. F3/F4 electrode pair
Fig. 6 shows the details regarding the FAA meta-analysis for electrode pair F3–F4. Overall, no significant effect was found (SMD = −0.01; [−0.20 0.22]; Z = 0.09; p = 0.93), showing that over the various phases of the psychosocial stress response, FAA when calculated using frontal electrodes F3 and F4 does not change significantly. No subgroup analysis was performed as not enough articles are present in the meta-analysis. Heterogeneity tests indicate low heterogeneity (I2: 32%, p = 0.17), indicating that the articles in this meta-analysis likely report the same effect. No publication bias was detected by the Egger's test (p = 0.15, see supplementary material Meta-Analysis – Publication Bias Analysis).
Fig. 6.
Forest plot illustrating the standardized mean differences (SMD), individual effect sizes, overall effect size and heterogeneity statistics for the meta-analysis examining changes in frontal alpha asymmetry (electrode pair F3–F4) from a control (non-stressed) condition to a stress condition. Hofmann et al., 2005, Pérez-Edgar et al., 2013 and Wang et al., 2015 report a baseline - anticipatory phase comparison; Quaedflieg et al., 2015 and Düsing et al., 2016 report a baseline - recovery phase comparison; Brouwer et al., 2011, Peterson et al., 2011 and Al-Shargie 2017a, 2017b report a control condition - reactive phase comparison.
3.4.3.2. F7/F8 electrode pair
Fig. 7 shows the details regarding the FAA meta-analysis for electrode pair F7–F8. Overall, no significant effect was found (SMD = −0.02; [−0.19 0.15]. Z = 0.21; p = 0.84), showing that FAA, calculated using electrodes F7 and F8, does not change consistently throughout the psychosocial stress response. Due to the low number of included studies, no subgroup analysis investigating the variations in FAA between the various phases were conducted. Heterogeneity tests showed no heterogeneity in this meta-analysis (I2 = 0%; p = 0.78). No publication bias was detected by the Egger's test (p = 0.54, see supplementary material Meta-Analysis – Publication Bias Analysis).
Fig. 7.
Forest plot illustrating the standardized mean differences (SMD), individual effect sizes, overall effect size and heterogeneity statistics for the meta-analysis examining changes in frontal alpha asymmetry (electrode pair F7–F8) from a control (non-stressed) condition to a stress condition. Wang et al., 2015 report a baseline - anticipatory phase comparison; Quaedflieg et al., 2015, Verona et al., 2009 and Düsing et al., 2016 report a baseline - recovery phase comparison; Al-Shargie 2017a, 2017b, Brouwer et al., 2011, Kawamoto et al., 2013 and Peterson et al., 2011 report a control condition - reactive phase comparison.
4. Discussion
In this systematic review and subsequent meta-analyses, we investigated how brain activity of healthy adults, measured by means of EEG spectral analyses, changes due the presence of an acute, laboratory-controlled psychosocial stressor and how these induced changes might vary throughout the three phases (anticipatory, reactive and recovery) of the psychosocial stress response. Results from the systematic review show that a large variety (13 in total) of EEG measures have been employed. Stress phase dependence seems present for some EEG measures, whereas other measures seem more phase independent, show inconsistencies within or between stress phases or are not affected by psychosocial stress. Alpha power shows a relative congruent trend of decreasing under the influence of psychosocial stress, regardless of the stress phase, as ten of the twelve articles investigating alpha power report a significant decrease. This trend is also found in the subsequent meta-analysis of alpha power, since a significant effect size is found (SMD = 0.6, p = 0.001) when evaluating changes in alpha power over all stress phases. Beta power shows a tendency to increase due to psychosocial stress as five out of the eight articles investigating beta power report a significant increase, whereas one article reports an insignificant increase. Two articles report decreases in beta power, although insignificant. However, when investigating changes of beta power over all stress phases through a meta-analysis, no significant effect was found (SMD = −0.31, p = 0.29). Other measures such as delta power, theta power, relative gamma and theta-alpha power ratio seem to indicate more stress phase dependent changes whereas FAA and coherency do not show consistency between or within stress phases, and the phase-amplitude coupling and amplitude-amplitude correlation do not seem affected by psychosocial stressors. The assessment of stress phase dependence or independence is difficult to assess given that certain stress phases are either overrepresented (reactive phase in alpha power), not investigated (anticipatory phase in beta power), or too little articles have used the specific EEG measure to assess phase dependence systematically. The results will be further discussed by the type of measure (power, power-derived and functional connectivity) which has been investigated. When employing the term “significant” during the discussion, statistical significance (p < 0.05) after multiple comparison correction (if applied in the articles) is implied.
Delta power increased during the reactive phase and decreased in the recovery phase (Perrin et al., 2019; van der Veen et al., 2016; Yao et al., 2020). The observed short-term increase in delta power during exposure to a stressor might thus reflect the high salience and motivational relevance of the applied psychosocial stressors within ERP segments (Knyazev, 2012). The observed decrease in delta power after exposure to a psychosocial stressor (i.e., during the recovery phase (Perrin et al., 2019)) might reflect the heightened vigilance of participants. Although the fact that both an increase and decrease in delta power might reflect similar reactions sounds contradictory at first, it should be noted that the articles likely investigate fundamentally different brain mechanisms since van der Veen et al. (2016) as well as Yao et al. (2020) calculate delta power from ERP segments (less than a second long and evoked by a specific stimulus) and therefore investigate stimulus-evoked neural activity whereas Perrin et al. (2019) use longer segments without any stimuli, therefore investigating induced rather than evoked neural activity.
Theta power increased during the reactive phase when analyzing ERP segments from the social judgment paradigm (Somerville et al., 2006). This increase has been found for all ERP segments which contained a psychosocial stressor (Yao et al., 2020), which could, similarly to the increase in delta power, reflect the heightened salience of the stimuli (Knyazev, 2012). Heightened theta power was however also found only in the unexpected rejection condition of the social judgment paradigm (Kortink et al., 2018; van der Veen et al., 2016), which might reflect the involvement of theta oscillations in mechanisms related to both social rejection as well as prediction errors (Hajihosseini and Holroyd, 2013; van der Molen et al., 2017). Contrary to the results from studies investigating evoked neural activity in ERP segments, studies investigating induced neural activity through theta power throughout the reactive phase of the psychosocial stress response report a decrease in theta power compared to a control condition (Hafeez et al., 2018; Minguillon et al., 2016). Research investigating theta power (specifically frontal midline theta) and their relationship with non-psychosocial stressors also reported decreases in theta power, and suggested that difficulties in keeping attention under a stressful condition might be the cause of this decrease (Gärtner et al., 2014, 2015). This discrepancy of evoked and induced theta power variations within the reactive phase shows that theta power is reflective of multiple processes within the brain and shows that spectral analysis within ERP segments likely reveals fundamentally different aspects of brain functioning compared to spectral analyses over longer time periods. Whereas the difference between evoked and induced neural activity might explain the contradictory findings, differences between the articles such as participant population and employed psychosocial stressor also likely influence the results, so conclusions regarding contradictory findings should be interpreted with caution.
Alpha power shows a relative congruent trend of decreasing due to psychosocial stress throughout all stress phases. From the twelve articles reporting alpha power results from the various phases of the stress response, ten found significant decreases in alpha power (Al-Shargie et al., 2016, 2017a, 2017b, 2018; Guo et al., 2019; Hafeez et al., 2018; Hofmann, 2006; Hofmann et al., 2005; Minguillon et al., 2016; Yao et al., 2020). This congruency is confirmed by the meta-analysis which shows that the alpha power does, regardless of the stress phase, decrease significantly. The found effect size equals 0.6 (p = 0.001), indicating a moderate to large effect size and showing that frontal alpha power is influenced by psychosocial stress. A common assumption regarding alpha power, backed by a significant body of research, is that alpha power is inversely proportional to cortical activity as it likely reflects an inhibitory control system of the brain (Allen et al., 2004; Jensen and Mazaheri, 2010; Mathewson et al., 2011). The reduction of alpha power can therefore be explained by the fact that the psychosocial stress response leads to a state of higher arousal and vigilance as well as the activation of the HPA axis (Campbell and Ehlert, 2012; Kudielka et al., 2004), which leads to higher cortical activity so the individual can correctly cope and adapt to the applied stressor. The results and the accompanying meta-analysis provide convincing evidence to conclude that frontal alpha power is significantly impacted (i.e., reduced) by psychosocial stress, although some caution is warranted. In multiple studies (adaptations of) the Montreal Imaging Stress Task (MIST) have been used to induce psychosocial stress (Al-Shargie et al., 2017a, 2017b, 2017b, 2018; Hafeez et al., 2018; Minguillon et al., 2016). The main difference between the control and stress condition in this paradigm is the presence of a psychosocial stressor (presented by a false comparison to peers as well as the threat from the researchers that if participants do not perform adequately their data is not useable), but time pressure is also added. Ehrhardt et al. (2021) untangled this co-occurrence by letting participants perform a task (the PASAT, Gronwall and Sampson, 1974) with time pressure and with pressure combined with a psychosocial stressor. A drop in alpha power was found in the time pressure + psychosocial stressor condition compared to the time pressure condition, although this drop was not significant. This might indicate that time pressure could be the main driver of alpha power reduction instead of psychosocial stress. Nevertheless, the current meta-analysis only contained two articles employing the MIST (Al-Shargie et al., 2017b; Hafeez et al., 2018), and if these results are removed the effect is still significant (SMD = 0.42; [−0.06 0.79]. Z = 2.25; p = 0.02; see supplementary materials for the additional analysis (Meta-Analysis – Additional Calculation)), robustly showing that frontal alpha power is reduced by psychosocial stress.
Sigma power, investigated by a single article (Perrin et al., 2019), did not change in the recovery phase of the psychosocial stress response. This frequency band (13–15 Hz), can be interpreted as a low beta frequency band and is mostly investigated during sleep (D'Rozario et al., 2013). Since sigma power was calculated when participants were awake, the absence of a difference in the recovery phase might be explained by the fact that neural processes in this frequency band are more involved when individuals are sleeping.
Beta power shows a tendency to increase due to psychosocial stress in the reactive and recovery phase. Of the eight articles reporting beta power changes, six report an increase during both phases of the stress response and five articles report a significant increase (Al-Shargie et al., 2016; Guo et al., 2019; Hafeez et al., 2018; Izhar et al., 2019; Minguillon et al., 2016; Perrin et al., 2019). However, both Betti et al. (2018) and Ehrhardt et al. (2021) report a decrease in beta power in response to a psychosocial stressor, although insignificantly. The corresponding meta-analysis shows that overall, no significant effect regarding beta power is found in the reactive and recovery phase (SMD = −0,34; p = 0,19). The lack of a significant effect found in the meta-analysis should however be interpreted with caution. Betti et al. (2018) split the beta band in low (13–17 Hz) and high (18–30 Hz) and found that low beta power did increase significantly. Due to the rules applied for the pooling of the data (see supplementary materials for more information (Meta-Analysis – Data Extraction Procedure)), both beta bands were combined, resulting in a decrease in beta power due to the larger range and therefore higher weight of the high beta band. Ehrahrdt and colleagues (2021) show, similarly to their results regarding alpha power, that when the psychosocial stressor is split in a time pressure and a social component, no significant difference is found. When both results, however, are compared to a baseline recording, a significant increase is reported, again hinting at the fact that beta power increases due to psychosocial stress. Finally, it should be noted that the three articles excluded from the meta-analysis (due to the absence of data or figures or due to a comparison between a resting-state recording and an EEG recording during task engagement) all reported an increase in beta power and thus, although the reasons for exclusion are correct, reaffirm the overall tendency of beta power to increase due to psychosocial stress. The trend of increasing beta power due to psychosocial stress conforms to previous research regarding beta power and stress (Lewis et al., 2007; Tran et al., 2007). Activity in the beta band is assumed to be indicative of cognitive processing (Engel and Fries, 2010; Miller, 2007; von Stein and Sarnthein, 2000), so higher beta power throughout the stress response could be understood as more ongoing cognitive processing. This is likely explained by the fact that neuronal circuits are recruited under the influence of stress, so the individual is capable of coping and adapting to the demands of psychosocial stressors (De Kloet et al., 2005; Liston et al., 2009; McEwen, 2007). However, the absence of articles investigating beta power in the anticipatory phase and the overall insignificant effect size found in the meta-analysis indicate that more research is needed to validate the identified trend of increases in beta power and to answer the possible stress phase dependence of beta power.
Aside from power measures, another frequently used EEG measure type was power-derived measures. These measures investigate power changes similarly to power measures but combine multiple power values from spatial distinct locations (e.g., FAA, alpha-theta power ratio) or from different frequency ranges (e.g., RG). The most employed power-derived EEG measure is FAA. FAA is an extensively researched measure in psychology and psychiatry research and has been linked to a multitude of psychological constructs and psychiatric disorders (Smith et al., 2017). The recent article by Ocklenburg et al. (2016) has shown the importance of hemispheric laterality, for which FAA can be seen as index, as well as how both chronic and acute stressors can influence this laterality. Building upon the foundation of this article, Berretz et al. (2020) have further expended these principles to neurodevelopmental and psychiatric disorders and show how hemispheric laterality is altered in these clinical populations and how stress can influence this laterality, further showing the importance of FAA as a possible index of psychosocial stress. Thirteen included articles employed FAA and investigated it with respect to psychosocial stress (Al-Shargie et al., 2018; Brouwer et al., 2011; Crost et al., 2008; Düsing et al., 2016; Hofmann et al., 2005; Kawamoto et al., 2013; Minguillon et al., 2016; Papousek et al., 2019; Pérez-Edgar et al., 2013; Peterson et al., 2011; Quaedflieg et al., 2015; Verona et al., 2009; Wang et al., 2015). The meta-analyses for FAA show that, across the three phases, FAA does not change consistently due to psychosocial stress regardless of the electrode pair (for electrode pair F3/F4: SMD = −0,01; p = 0.93; for F7/F8: SMD = −0,02; p = 0.84). These results are in line with another meta-analysis regarding FAA as a biomarker for major depressive disorder, as no overall effect was also found (van der Vinne et al., 2017). This lack of overall effect on a group level might be explained by the fact that the relative difference between alpha power between the left and right hemisphere is highly dependent on individual characteristics. FAA is considered as an electrophysiological correlate of approach motivation, which could further explain the high interindividual variability (Smith et al., 2017). The individual variation in FAA is acknowledged by multiple included articles, as their research questions are often aimed at exploring which characteristics are influential for FAA changes. Factors investigated were anger and control (Peterson et al., 2011), defensiveness and anxiety (Crost et al., 2008), action orientation (Düsing et al., 2016), social anxiety (Hofmann et al., 2005), trait positive affect (Papousek et al., 2019), individual peak alpha frequency (Quaedflieg et al., 2015), time period within a stress phase (Kawamoto et al., 2013) and the change in activation (Pérez-Edgar et al., 2013). It is noticeable that the subdivisions investigated often result in significant differences in FAA, whereas overall no significant effect is found for the investigation of psychosocial stress. Interestingly, only three articles investigated the influence of gender (Pérez-Edgar et al., 2013; Quaedflieg et al., 2015; Verona et al., 2009) and no effect of gender was found in these articles. Another reason for the variable results might be the differences between the cognitive processes which are active when FAA is assessed. The right-shift model, defined by Ocklenburg et al. (2016), proposes that stress often results in an increased activation of mostly the right hemisphere. Changes in FAA are therefore not only dependable on the presence of a psychosocial stressor, but also on the cognitive functions which are active at the same time. If these functions are more left-lateralized, FAA would decrease due to the increased right hemispheric activity induced by a psychosocial stressor. When the active cognitive functions are, however, more right-lateralized, FAA would increase (Ocklenburg et al., 2016). The differences between the stress phases within a single study as well as the differences between studies regarding employed psychosocial stressor likely results in various cognitive functions that are active at different times throughout the stress response, possibly explaining the absence of a uniform change in FAA due to psychosocial stress. Asymmetric brain activity can be assessed from multiple locations. Aside from frontal alpha asymmetry, parietal alpha asymmetry has also been investigated by three articles (Crost et al., 2008; Hofmann et al., 2005; Pérez-Edgar et al., 2013). However, no significant change has been found in this measure. A possible reason for this might be that FAA assesses activity from mainly the (pre)frontal cortex, which is known to contain brain regions involved in higher order cognitive functioning. Given the importance of brain activity reflecting cognitive functioning by the right-shift model of Ocklenburg et al. (2016), it might be possible that the absence of significant changes in parietal alpha asymmetry can be explained by the fact that the parietal regions of the brain are less involved in cognitive processes and asymmetric activity alterations are therefore not found due to the presence of a psychosocial stressor. While no significant changes were reported for parietal alpha asymmetry, it should be noted that parietal alpha asymmetry changes have been observed in patients with major depressive disorder, a stress-related disorder (Jaworska et al., 2012). An absence of significant changes could therefore also be due to methodological aspects such as the employed psychosocial stressor or investigated stressor phase.
Aside from the investigation of hemispheric laterality, another recurring principle of brain activity, the differing functional role of low and high frequency oscillations, is employed in several EEG indices. Low frequency (delta and theta) EEG oscillations seem more prominent during brain states requiring little vigilance or attention, such as drowsiness or sleep (Santamaria and Chiappa, 1987; Susmáková, 2005). Contrary to this, high frequency EEG (alpha, beta, and gamma) oscillations seem to be more commonly associated with brain states requiring attention and alertness. Alpha oscillations are believed to reflect top-down inhibitory control processes (Jensen and Mazaheri, 2010; Klimesch et al., 2007), beta oscillations have been linked to both emotional and cognitive tasks (Ray and Cole, 1985), and gamma oscillations are also linked to cognitive as well as learning processes (Fitzgibbon et al., 2004; Miltner et al., 1999). When comparing activity from the low frequency ranges to the high frequency ranges, indices reflecting these differences can be constructed. One such index is the slowing ratio, initially defined as an index of cortical arousal in the context of sleepiness of individuals, which is obtained by dividing the power of the lower frequency ranges by the power of the higher frequency ranges (D'Rozario et al., 2013). Perrin et al. (2019) report a decrease in the slowing ratio during the recovery phase (compared to the recovery of a non-psychosocial stressor). This decrease reflects a relative increase of activity in the higher frequency ranges compared to the lower frequency ranges, which possibly shows this heightened state of cortical activity and alertness after psychosocial stress exposure. Another such index is theta-alpha power ratio, which compares theta power (measured at Fz) to alpha power (measured at Pz). The theta-alpha power ratio is reported as indicative of the mental load put on an individual whereby an increase in the ratio corresponds to an increase in mental load (Holm et al., 2009). Under this assumption, an increase in the theta-alpha power ratio during the reactive phase under stressful conditions compared to a control condition, as reported by Subhani et al. (2013), and the disappearance of this difference during the recovery phase, is logical as stress increases the mental load experienced by people, which disappears when the stressor itself is no longer present. The disappearance of the difference during the recovery phase also hints at stress phase dependence, but since only a single article reports results from this measure, no concrete conclusions can be formed. A third index investigating the low-high frequency range differences is relative gamma, which was initially identified in studies investigating effect of meditation on neural activity (Lutz. Et al, 2004; Steinhubl et al., 2015). Here, gamma power is compared to the combined power in the theta and alpha frequency bands. Results from the reactive phase report higher relative gamma values compared to a control condition and a decrease during the recovery phase compared to the reactive phase (Minguillon et al., 2016, 2017; Vaquero-Blasco et al., 2020, 2021). These results seem mostly driven by changes in the theta and alpha band rather than the gamma band, and high variability exists between participants, showing that psychosocial stress exposure leads to different neurological adjustments between people. Finally, the alpha attenuation coefficient, reflective of sleepiness and drowsiness in participants (Stampi et al., 1995), was decreased during the recovery phase of a psychosocial stressor. This might show that even after the stressor exposure, the brain is still in a state of heightened vigilance, thus possibly showing the long-lasting effects of psychosocial stress on an individual, even when only acute psychosocial stressors are present.
The use of functional connectivity (FC) measures to investigate how the brain adapts to psychosocial stress is less represented in the current literature compared to EEG power (derived) measures, as only four articles have employed such measures (Poppelaars et al., 2018, 2021; Subhani et al., 2016a, Subhani et al., 2016b). Poppelaars and colleagues (2018, 2021) employed both phase-amplitude coherence and amplitude-amplitude correlation, FC measures that investigate the interplay between low and high frequency bands. Neither article found significant effects related to the induction of psychosocial stress. The lack of significant effects could have multiple reasons. One possible reason is that the choice of FC measure might be suboptimal for the discovery of brain function alterations related to psychosocial stress. A multitude of FC measures exist, and currently no consensus has yet been reached regarding which FC measure captures the underlying communication between regions best (Bastos and Schoffelen, 2016). Another reason could be that the induction of psychosocial stress was insufficient, but this is not likely as cortisol, considered the gold standard for HPA activation detection, did significantly increase during the paradigm. Subhani et al. (2016a,b) employed coherence and found a multitude of changes due to psychosocial stress. These results should however be interpreted with caution since no multiple comparison correction was employed. Furthermore, the used FC measure (magnitude-squared coherence) overestimates the connections between closely spaced electrodes due to volume conduction, further complicating the interpretation of the results (Nunez et al., 1997).
Overall, the results reviewed in this systematic review and subsequent meta-analyses generally align with the current understanding of the mechanisms of stress. A vast amount of studies investigating psychosocial stress show that, similar to other types of stress, psychosocial stress is taxing on the coping abilities of an individual, therefore requiring additional resources from an individual to correctly cope with the perceived challenges (Berretz et al., 2021; Folkman and Lazarus, 1984; Kogler et al., 2015; McEwen et al., 2015; van Oort et al., 2017). fMRI research regarding psychosocial stress has shown that during the psychosocial stress response, specific brain regions (the insula, claustrum, and inferior frontal gyrus, for a review and meta-analysis, see (Berretz et al., 2021) are more active, likely showing the additional employed resources by the brain. Our results show that, similarly to the results from fMRI studies, increased cortical activity is present during the various phases of the psychosocial stress response. This increased brain activity is mainly found by the fact that alpha power, mainly from the (pre)frontal lobes, decreases consistently throughout the whole stress response, thus indicating the increased activity of the frontal brain regions. Beta power, associated with cognitive processing, shows a trend of increasing throughout the reactive and recovery phase of the stress response, further implicating the increase in brain activity due to psychosocial stress. More complex power and FC measures however are less consistent regarding the psychosocial stress response. This could be because they reflect more specific brain functions and are therefore more subject-specific. The absence of a uniform effect of psychosocial stress on FAA (both in the systematic review as well as in the meta-analyses) could be indicative of this subject-specific brain activity induced by psychosocial stress, as well as the possible different cognitive functions that are active due to the specific circumstances in which psychosocial stress was induced.
Although a multitude of results are already present in the current literature, acquiring a general conclusion regarding psychosocial stress and EEG measured brain activity remains difficult due to methodological and technical reasons. The most notable methodological concern is the large difference in sample sizes (ranging from 6 to 150). The main concern here lies with the articles that use small samples, as this increases the probability of making a type II error. A likely reason for this large variability is the absence of a priori power calculations in all articles, a common occurrence in EEG research (Clayson et al., 2019). This variety however also has implications regarding the meta-analyses conducted in this article, as the overall small number of included articles in the meta-analyses combined with the large variability in sample size limits the certainty of the obtained conclusions. It should be noted however that the variability between the included articles of the meta-analyses has been limited as much as possible through the usage of a random effects model and the strict rules regarding phase comparisons which were allowed. Other methodological aspects that further complicate the integration of results are the high variety of employed EEG measures, psychosocial stressor paradigms, and research questions. Variability introduced through the different stressor paradigms is partly reduced since, aside from the articles by Crost et al. (2008) and Verona et al. (2009), all articles employed a within-subjects design, therefore avoiding possible differences between the compared groups when comparing a stress phase to its non-stressed counterpart. It is still possible however that changes in the EEG measures are partially due to differences between the obtained baseline recordings or control conditions. Differences due to the employed stress paradigm are most likely present in the reactive phase, since during this phase participants are engaged with a task and task-specific neural activity is likely present in the EEG signal. The high diversity of research questions also explains part of the low scores regarding the risk of bias analysis since this discrepancy leads to the absence of information necessary for our research questions.
From a technical point of view, some aspects should also be considered when interpreting the results. Variations in the selected electrodes, preprocessing and analysis steps as well as the definitions of the used frequency bands between the articles could introduce small differences in the reported findings (Gutmann et al., 2018; Robbins et al., 2020). Another technical aspect that should be considered is the influence of volume conduction. Volume conduction, understood as the propagation of electrical fields through biological tissue, results in the recording of electrical activity from a single source within the brain by multiple electrodes across the scalp. For the results of FAA, and specifically FAA calculated from the F3/F4 electrode pair (as these electrodes lie closely together), volume conduction probably has an influence. It is likely that both electrodes recorded alpha activity from similar sources, further complicating the results. When evaluating the results of other EEG measures that employ location-specific electrodes for their calculation, such as coherence and theta-alpha power ratio, the influence of volume conduction should also be considered. Future research should therefore consider additional preprocessing or analysis steps, such as a surface laplacian, to reduce the influence of volume conduction (Kayser and Tenke, 2015). Finally, it should be mentioned that up until now source imaging, referring to the mathematical operations that allow one to calculate the activity of various regions in the brain from surface recordings (Michel and Brunet, 2019), has not yet been employed with regard to psychosocial stress. This technique could be considered in future research to further investigate how psychosocial stress affects the brain.
5. Conclusion
In conclusion, the current literature regarding psychosocial stress and spectral EEG analyses shows that psychosocial stress results in measurable changes in several EEG indices, which exhibit both stressor response phase specific and aspecific tendencies. Alpha power consistently decreases due to acute, laboratory psychosocial stressors, regardless of the stressor phase. Beta power shows a tendency of increasing during the reactive and recovery phase, but this tendency should be interpreted with caution since no significant effect was found in the subsequent meta-analysis. The results from other EEG measures, such as delta power, theta power, relative gamma and theta-alpha power ratio, imply that these measures show stress phase dependence. Other measures, such as FAA and coherence show variable results regarding their stress phase dependency. Although significant work has already been performed which shows the importance of stress response phase when investigating EEG measures, the high variability in both methodological as well as technical aspects complicates the integration of the results towards an overall conclusion regarding psychosocial stressors and EEG-measured brain activity. Therefore, future work is needed to clear up how the stressor phases, stress paradigms and analysis steps have an influence on neural activity and their corresponding spectral EEG measures.
Author contributions
G.V., S. D.S and M.A.V. designed the study. G.V. and S.D.S. searched the literature and conducted the analyses. G.V. wrote the first draft of the article. All authors were involved in the interpretation of the results, the revision of the article and gave approval of the current version of the publication.
Disclosures
Ir. G. V. was supported by a grant from the Bijzonder Onderzoeksfonds (BOF) (BOFSTA2017002501) from the university of Ghent. S.D.S. is funded by an FWO-Flanders PhD fellowship (Grant Number: 11J7521N). All authors report no other financial interests of potential conflicts of interest related to this publication.
Declaration of competing interest
Ir. G. V. was supported by a grant from the Bijzonder Onderzoeksfonds (BOF) (Grant Number: BOFSTA2017002501) from the university of Ghent. S.D.S was supported by a grant from the Fonds Wetenschappelijk Onderzoek Vlaanderen (FWO-Flanders) (Grant Number: 11J7521N). All authors report no other financial interests of potential conflicts of interest related to this publication.
Acknowledgments
The authors certify that this publication is not considered for publication elsewhere and has not been published previously. The manuscript was approved by all authors and all authors contributed significantly.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.ynstr.2022.100452.
Appendix A. Supplementary data
The following are the Supplementary data to this article:
Data availability
The data extracted from the articles is available as supplementary materials. All other data is also available as supplementary materials.
References
- Al-Shargie F., Kiguchi M., Badruddin N., Dass S.C., Hani A.F.M., Tang T.B. Mental stress assessment using simultaneous measurement of EEG and fNIRS. Biomed. Opt Express. 2016;7(10):3882–3898. doi: 10.1364/BOE.7.003882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Al-Shargie F., Tang T.B., Kiguchi M. Stress assessment based on decision fusion of EEG and fNIRS signals. IEEE Access. 2017 doi: 10.1109/ACCESS.2017.2754325. [DOI] [Google Scholar]
- Al-Shargie F., Tang T.B., Kiguchi M. Assessment of mental stress effects on prefrontal cortical activities using canonical correlation analysis: an fNIRS-EEG study. Biomed. Opt Express. 2017;8(5):2583–2598. doi: 10.1364/BOE.8.002583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Al-Shargie F., Tang T.B., Badruddin N., Kiguchi M. Towards multilevel mental stress assessment using SVM with ECOC: an EEG approach. Med. Biol. Eng. Comput. 2018;56(1):125–136. doi: 10.1007/s11517-017-1733-8. [DOI] [PubMed] [Google Scholar]
- Allen J.J.B., Coan J.A., Nazarian M. Issues and assumptions on the road from raw signals to metrics of frontal EEG asymmetry in emotion. Biol. Psychol. 2004;67(1):183–218. doi: 10.1016/j.biopsycho.2004.03.007. [DOI] [PubMed] [Google Scholar]
- Backé E.-M., Seidler A., Latza U., Rossnagel K., Schumann B. The role of psychosocial stress at work for the development of cardiovascular diseases: a systematic review. Int. Arch. Occup. Environ. Health. 2012;85(1):67–79. doi: 10.1007/s00420-011-0643-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bastos A.M., Schoffelen J.-M. A tutorial review of functional connectivity analysis methods and their interpretational pitfalls. Front. Syst. Neurosci. 2016 doi: 10.3389/fnsys.2015.00175. 0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baumeister R.F., Leary M.R. The need to belong: desire for interpersonal attachments as a fundamental human motivation. Psychol. Bull. 1995;117(3):497–529. doi: 10.1037/0033-2909.117.3.497. [DOI] [PubMed] [Google Scholar]
- Berretz G., Wolf O.T., Güntürkün O., Ocklenburg S. Atypical lateralization in neurodevelopmental and psychiatric disorders: what is the role of stress? Cortex. 2020;125:215–232. doi: 10.1016/j.cortex.2019.12.019. [DOI] [PubMed] [Google Scholar]
- Berretz G., Packheiser J., Kumsta R., Wolf O.T., Ocklenburg S. The brain under stress-A systematic review and activation likelihood estimation meta-analysis of changes in BOLD signal associated with acute stress exposure. Neurosci. Biobehav. Rev. 2021;124:89–99. doi: 10.1016/j.neubiorev.2021.01.001. [DOI] [PubMed] [Google Scholar]
- Betti S., Lova R.M., Rovini E., Acerbi G., Santarelli L., Cabiati M., Ry S.D., Cavallo F. Evaluation of an integrated system of wearable physiological sensors for stress monitoring in working environments by using biological markers. IEEE (Inst. Electr. Electron. Eng.) Trans. Biomed. Eng. 2018;65(8):1748–1758. doi: 10.1109/TBME.2017.2764507. [DOI] [PubMed] [Google Scholar]
- Brouwer A.-M., Neerincx M.A., Kallen V., van der Leer L., Brinke M.T. EEG alpha asymmetry, heart rate variability and cortisol in response to virtual reality induced stress. J. CyberTherapy Rehab. 2011;4(1):27. [Google Scholar]
- Campbell J., Ehlert U. Acute psychosocial stress: does the emotional stress response correspond with physiological responses? Psychoneuroendocrinology. 2012;37(8):1111–1134. doi: 10.1016/j.psyneuen.2011.12.010. [DOI] [PubMed] [Google Scholar]
- Clayson P.E., Carbine K.A., Baldwin S.A., Larson M.J. Methodological reporting behavior, sample sizes, and statistical power in studies of event-related potentials: barriers to reproducibility and replicability. Psychophysiology. 2019;56(11) doi: 10.1111/psyp.13437. [DOI] [PubMed] [Google Scholar]
- Coan J.A., Allen J.J.B., McKnight P.E. A capability model of individual differences in frontal EEG asymmetry. Biol. Psychol. 2006;72(2):198–207. doi: 10.1016/j.biopsycho.2005.10.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen M.X. MIT press; 2014. Analyzing Neural Time Series Data: Theory and Practice. [Google Scholar]
- Crost N.W., Pauls C.A., Wacker J. Defensiveness and anxiety predict frontal EEG asymmetry only in specific situational contexts. Biol. Psychol. 2008;78(1):43–52. doi: 10.1016/j.biopsycho.2007.12.008. [DOI] [PubMed] [Google Scholar]
- David O., Kilner J.M., Friston K.J. Mechanisms of evoked and induced responses in MEG/EEG. Neuroimage. 2006;31(4):1580–1591. doi: 10.1016/j.neuroimage.2006.02.034. [DOI] [PubMed] [Google Scholar]
- Daviu N., Bruchas M.R., Moghaddam B., Sandi C., Beyeler A. Neurobiological links between stress and anxiety. Neurobiol. Stress. 2019;11 doi: 10.1016/j.ynstr.2019.100191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Kloet E.R., Joëls M., Holsboer F. Stress and the brain: from adaptation to disease. Nat. Rev. Neurosci. 2005;6(6):463–475. doi: 10.1038/nrn1683. [DOI] [PubMed] [Google Scholar]
- Dedovic K., Renwick R., Mahani N.K., Engert V., Lupien S.J., Pruessner J.C. The Montreal Imaging Stress Task: using functional imaging to investigate the effects of perceiving and processing psychosocial stress in the human brain. J. Psychiatry Neurosci. 2005;30(5):319. [PMC free article] [PubMed] [Google Scholar]
- Dickerson S.S., Kemeny M.E. Acute stressors and cortisol responses: a theoretical integration and synthesis of laboratory research. Psychol. Bull. 2004;130(3):355–391. doi: 10.1037/0033-2909.130.3.355. [DOI] [PubMed] [Google Scholar]
- Dupre M.E., Linda G.K., Liu G., Peterson E.D. Association between divorce and risks for acute myocardial infarction. Circ.: Cardiovasc. Qual. Outcome. 2015;8(3):244–251. doi: 10.1161/CIRCOUTCOMES.114.001291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Düsing R., Tops M., Radtke E.L., Kuhl J., Quirin M. Relative frontal brain asymmetry and cortisol release after social stress: the role of action orientation. Biol. Psychol. 2016;115:86–93. doi: 10.1016/j.biopsycho.2016.01.012. [DOI] [PubMed] [Google Scholar]
- D'Rozario A.L., Kim J.W., Wong K.K., Bartlett D.J., Marshall N.S., Dijk D.J., et al. A new EEG biomarker of neurobehavioural impairment and sleepiness in sleep apnea patients and controls during extended wakefulness. Clin. Neurophysiol. 2013;124(8):1605–1614. doi: 10.1016/j.clinph.2013.02.022. [DOI] [PubMed] [Google Scholar]
- Egger M., Smith G.D., Schneider M., Minder C. Bias in meta-analysis detected by a simple, graphical test. BMJ. 1997;315(7109):629–634. doi: 10.1136/bmj.315.7109.629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ehrhardt N.M., Fietz J., Kopf-Beck J., Kappelmann N., Brem A.-K. Separating EEG correlates of stress: cognitive effort, time pressure, and social-evaluative threat. Eur. J. Neurosci. 2021 doi: 10.1111/ejn.15211. [DOI] [PubMed] [Google Scholar]
- Engel A.K., Fries P. Beta-band oscillations—signalling the status quo? Curr. Opin. Neurobiol. 2010;20(2):156–165. doi: 10.1016/j.conb.2010.02.015. [DOI] [PubMed] [Google Scholar]
- Engert V., Efanov S.I., Duchesne A., Vogel S., Corbo V., Pruessner J.C. Differentiating anticipatory from reactive cortisol responses to psychosocial stress. Psychoneuroendocrinology. 2013;38(8):1328–1337. doi: 10.1016/j.psyneuen.2012.11.018. [DOI] [PubMed] [Google Scholar]
- Epel E.S., Crosswell A.D., Mayer S.E., Prather A.A., Slavich G.M., Puterman E., Mendes W.B. More than a feeling: a unified view of stress measurement for population science. Front. Neuroendocrinol. 2018;49:146–169. doi: 10.1016/j.yfrne.2018.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fitzgibbon S.P., Pope K.J., Mackenzie L., Clark C.R., Willoughby J.O. Cognitive tasks augment gamma EEG power. Clin. Neurophysiol. 2004;115(8):1802–1809. doi: 10.1016/j.clinph.2004.03.009. [DOI] [PubMed] [Google Scholar]
- Folkman S., Lazarus R.S. Springer Publishing Company; 1984. Stress, Appraisal, and Coping. [Google Scholar]
- Friston K.J. Functional and effective connectivity in neuroimaging: a synthesis. Hum. Brain Mapp. 1994;2(1–2):56–78. doi: 10.1002/hbm.460020107. [DOI] [Google Scholar]
- Gärtner M., Rohde-Liebenau L., Grimm S., Bajbouj M. Working memory-related frontal theta activity is decreased under acute stress. Psychoneuroendocrinology. 2014;43:105–113. doi: 10.1016/j.psyneuen.2014.02.009. [DOI] [PubMed] [Google Scholar]
- Gärtner M., Grimm S., Bajbouj M. Frontal midline theta oscillations during mental arithmetic: effects of stress. Front. Behav. Neurosci. 2015 doi: 10.3389/fnbeh.2015.00096. 0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giannakakis G., Grigoriadis D., Giannakaki K., Simantiraki O., Roniotis A., Tsiknakis M. Review on psychological stress detection using biosignals. IEEE Trans. Affect. Comput. 2019 [Google Scholar]
- Godoy L.D., Rossignoli M.T., Delfino-Pereira P., Garcia-Cairasco N., de Lima Umeoka E.H. A comprehensive overview on stress neurobiology: basic concepts and clinical implications. Front. Behav. Neurosci. 2018;12 doi: 10.3389/fnbeh.2018.00127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goodman W.K., Janson J., Wolf J.M. Meta-analytical assessment of the effects of protocol variations on cortisol responses to the Trier Social Stress Test. Psychoneuroendocrinology. 2017;80:26–35. doi: 10.1016/j.psyneuen.2017.02.030. [DOI] [PubMed] [Google Scholar]
- Greenwood D.C., Muir K.R., Packham C.J., Madeley R.J. Coronary heart disease: a review of the role of psychosocial stress and social support. J. Publ. Health. 1996;18(2):221–231. doi: 10.1093/oxfordjournals.pubmed.a024483. [DOI] [PubMed] [Google Scholar]
- Gronwall D.M., Sampson H. Auckland University Press; Auckland, New Zealand: 1974. The Psychological Effects of Concussion. [Google Scholar]
- Guo L.-N., Zhao R.-L., Ren A.-H., Niu L.-X., Zhang Y.-L. Stress recovery of campus street trees as visual stimuli on graduate students in autumn. Int. J. Environ. Res. Publ. Health. 2019;17(1) doi: 10.3390/ijerph17010148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gutmann B., Hülsdünker T., Mierau J., Strüder H.K., Mierau A. Exercise-induced changes in EEG alpha power depend on frequency band definition mode. Neurosci. Lett. 2018;662:271–275. doi: 10.1016/j.neulet.2017.10.033. [DOI] [PubMed] [Google Scholar]
- Hafeez M.A., Shakil S., Jangsher S. 2018 14th International Conference On Emerging Technologies (ICET) 2018. Stress effects on exam performance using EEG; pp. 1–4. [DOI] [Google Scholar]
- Hajihosseini A., Holroyd C.B. Frontal midline theta and N200 amplitude reflect complementary information about expectancy and outcome evaluation. Psychophysiology. 2013;50(6):550–562. doi: 10.1111/psyp.12040. [DOI] [PubMed] [Google Scholar]
- Harrewijn A., Van der Molen M.J.W., Westenberg P.M. Putative EEG measures of social anxiety: comparing frontal alpha asymmetry and delta–beta cross-frequency correlation. Cognit. Affect Behav. Neurosci. 2016;16(6):1086–1098. doi: 10.3758/s13415-016-0455-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hofmann S.G. The emotional consequences of social pragmatism: the psychophysiological correlates of self-monitoring. Biol. Psychol. 2006;73(2):169–174. doi: 10.1016/j.biopsycho.2006.03.001. [DOI] [PubMed] [Google Scholar]
- Hofmann S.G., Moscovitch D.A., Litz B.T., Kim H.-J., Davis L.L., Pizzagalli D.A. The worried mind: autonomic and prefrontal activation during worrying. Emotion (Washington, D.C.) 2005;5(4):464–475. doi: 10.1037/1528-3542.5.4.464. [DOI] [PubMed] [Google Scholar]
- Holm A., Lukander K., Korpela J., Sallinen M., Müller K.M. Estimating brain load from the EEG. TheScientificWorldJOURNAL. 2009;9:639–651. doi: 10.1100/tsw.2009.83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Izhar L.I., Roslan N.S., Feng Y.X., Faye I., Ho E.T.W., Rahman M.A. Classification of neuroticism using psychophysiological signals during speaking task based on two different baseline measurements. Int. J. Integrat. Eng. 2019;11(3) [Google Scholar]
- Jaworska N., Blier P., Fusee W., Knott V. Alpha power, alpha asymmetry and anterior cingulate cortex activity in depressed males and females. J. Psychiatr. Res. 2012;46(11):1483–1491. doi: 10.1016/j.jpsychires.2012.08.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jensen O., Mazaheri A. Shaping functional architecture by oscillatory alpha activity: gating by inhibition. Front. Hum. Neurosci. 2010;4 doi: 10.3389/fnhum.2010.00186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiro . 2021. GRABIT. [Google Scholar]
- Juster R.P., McEwen B.S., Lupien S.J. Allostatic load biomarkers of chronic stress and impact on health and cognition. Neurosci. Biobehav. Rev. 2010;35(1):2–16. doi: 10.1016/j.neubiorev.2009.10.002. [DOI] [PubMed] [Google Scholar]
- Karakaş S. A review of theta oscillation and its functional correlates. Int. J. Psychophysiol.: Off. J. Int. Organiz. Psychophysiol. 2020;157:82–99. doi: 10.1016/j.ijpsycho.2020.04.008. [DOI] [PubMed] [Google Scholar]
- Katmah R., Al-Shargie F., Tariq U., Babiloni F., Al-Mughairbi F., Al-Nashash H. A review on mental stress assessment methods using EEG signals. Sensors. 2021;21(15):5043. doi: 10.3390/s21155043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kawamoto T., Nittono H., Ura M. Cognitive, affective, and motivational changes during ostracism: an ERP, EMG, and EEG study using a computerized Cyberball task. Neurosci. J. 2013 doi: 10.1155/2013/304674. 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kayser J., Tenke C.E. Issues and considerations for using the scalp surface Laplacian in EEG/ERP research: a tutorial review. Int. J. Psychophysiol. 2015;97(3):189–209. doi: 10.1016/j.ijpsycho.2015.04.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kemeny M.E., Schedlowski M. Understanding the interaction between psychosocial stress and immune-related diseases: a stepwise progression. Brain Behav. Immun. 2007;21(8):1009–1018. doi: 10.1016/j.bbi.2007.07.010. [DOI] [PubMed] [Google Scholar]
- Kirschbaum C., Pirke K.M., Hellhammer D.H. The ‘Trier Social Stress Test’–a tool for investigating psychobiological stress responses in a laboratory setting. Neuropsychobiology. 1993;28(1–2):76–81. doi: 10.1159/000119004. [DOI] [PubMed] [Google Scholar]
- Klimesch W. EEG alpha and theta oscillations reflect cognitive and memory performance: a review and analysis. Brain Res. Rev. 1999;29(2):169–195. doi: 10.1016/S0165-0173(98)00056-3. [DOI] [PubMed] [Google Scholar]
- Klimesch W., Sauseng P., Hanslmayr S. EEG alpha oscillations: the inhibition–timing hypothesis. Brain Res. Rev. 2007;53(1):63–88. doi: 10.1016/j.brainresrev.2006.06.003. [DOI] [PubMed] [Google Scholar]
- Kmet L.M., Cook L.S., Lee R.C. 2004. Standard Quality Assessment Criteria for Evaluating Primary Research Papers from a Variety of Fields. [Google Scholar]
- Knyazev G.G. Cross-frequency coupling of brain oscillations: an impact of state anxiety. Int. J. Psychophysiol.: Off. J. Int. Organiz. Psychophysiol. 2011;80(3):236–245. doi: 10.1016/j.ijpsycho.2011.03.013. [DOI] [PubMed] [Google Scholar]
- Knyazev G.G. EEG delta oscillations as a correlate of basic homeostatic and motivational processes. Neurosci. Biobehav. Rev. 2012;36(1):677–695. doi: 10.1016/j.neubiorev.2011.10.002. [DOI] [PubMed] [Google Scholar]
- Kogler L., Müller V.I., Chang A., Eickhoff S.B., Fox P.T., Gur R.C., Derntl B. Psychosocial versus physiological stress—meta-analyses on deactivations and activations of the neural correlates of stress reactions. NeuroImage. 2015;119:235–251. doi: 10.1016/j.neuroimage.2015.06.059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kortink E.D., Weeda W.D., Crowley M.J., Gunther Moor B., van der Molen M.J.W. Community structure analysis of rejection sensitive personality profiles: a common neural response to social evaluative threat? Cognit. Affect Behav. Neurosci. 2018;18(3):581–595. doi: 10.3758/s13415-018-0589-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kudielka B.M., Kirschbaum C. Sex differences in HPA axis responses to stress: a review. Biol. Psychol. 2005;69(1):113–132. doi: 10.1016/j.biopsycho.2004.11.009. [DOI] [PubMed] [Google Scholar]
- Kudielka B.M., Schommer N.C., Hellhammer D.H., Kirschbaum C. Acute HPA axis responses, heart rate, and mood changes to psychosocial stress (TSST) in humans at different times of day. Psychoneuroendocrinology. 2004;29(8):983–992. doi: 10.1016/j.psyneuen.2003.08.009. [DOI] [PubMed] [Google Scholar]
- Lewis R.S., Weekes N.Y., Wang T.H. The effect of a naturalistic stressor on frontal EEG asymmetry, stress, and health. Biol. Psychol. 2007;75(3):239–247. doi: 10.1016/j.biopsycho.2007.03.004. [DOI] [PubMed] [Google Scholar]
- Liston C., McEwen B.S., Casey B.J. Psychosocial stress reversibly disrupts prefrontal processing and attentional control. Proc. Natl. Acad. Sci. Unit. States Am. 2009;106(3):912–917. doi: 10.1073/pnas.0807041106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu Q., He H., Yang J., Feng X., Zhao F., Lyu J. Changes in the global burden of depression from 1990 to 2017: findings from the global burden of disease study. J. Psychiatr. Res. 2020;126:134–140. doi: 10.1016/j.jpsychires.2019.08.002. [DOI] [PubMed] [Google Scholar]
- Lutz A., Greischar L.L., Rawlings N.B., Ricard M., Davidson R.J. Long-term meditators self-induce high-amplitude gamma synchrony during mental practice. Proc. Natl. Acad. Sci. 2004;101(46):16369–16373. doi: 10.1073/pnas.0407401101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mathewson K.E., Lleras A., Beck D.M., Fabiani M., Ro T., Gratton G. Pulsed out of awareness: EEG alpha oscillations represent a pulsed-inhibition of ongoing cortical processing. Front. Psychol. 2011;2 doi: 10.3389/fpsyg.2011.00099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mazure C.M. Life stressors as risk factors in depression. Clin. Psychol. Sci. Pract. 1998;5(3):291–313. doi: 10.1111/j.1468-2850.1998.tb00151.x. [DOI] [Google Scholar]
- McEwen B.S. Physiology and neurobiology of stress and adaptation: central role of the brain. Physiol. Rev. 2007;87(3):873–904. doi: 10.1152/physrev.00041.2006. [DOI] [PubMed] [Google Scholar]
- McEwen B.S., Gianaros P.J. Stress- and allostasis-induced brain plasticity. Annu. Rev. Med. 2011;62(1):431–445. doi: 10.1146/annurev-med-052209-100430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McEwen B.S., Seeman T. Protective and damaging effects of mediators of stress: elaborating and testing the concepts of allostasis and allostatic load. Ann. N. Y. Acad. Sci. 1999;896(1):30–47. doi: 10.1111/j.1749-6632.1999.tb08103.x. [DOI] [PubMed] [Google Scholar]
- McEwen B.S., Bowles N.P., Gray J.D., Hill M.N., Hunter R.G., Karatsoreos I.N., Nasca C. Mechanisms of stress in the brain. Nat. Neurosci. 2015;18(10):1353–1363. doi: 10.1038/nn.4086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Md C.N.B.M., PhD J.D., Mph C.K.N., PhD K.G.W., PhD J.W.S., Md R.H.S. Psychosocial stress and cardiovascular disease: pathophysiological links. Behav. Med. 2002;27(4):141–147. doi: 10.1080/08964280209596039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Melchior M., Caspi A., Milne B.J., Danese A., Richie P., Moffitt T.E. Work stress precipitates depression and anxiety in young, working women and men. Psychol. Med. 2007;37:1119–1129. doi: 10.1017/S0033291707000414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meyer T., Smeets T., Giesbrecht T., Quaedflieg C.W.E.M., Smulders F.T.Y., Meijer E.H., Merckelbach H.L.G.J. The role of frontal EEG asymmetry in post-traumatic stress disorder. Biol. Psychol. 2015;108:62–77. doi: 10.1016/j.biopsycho.2015.03.018. [DOI] [PubMed] [Google Scholar]
- Michel C.M., Brunet D. EEG source imaging: a practical review of the analysis steps. Front. Neurol. 2019;10:325. doi: 10.3389/fneur.2019.00325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller R. Theory of the normal waking EEG: from single neurones to waveforms in the alpha, beta and gamma frequency ranges. Int. J. Psychophysiol. 2007;64(1):18–23. doi: 10.1016/j.ijpsycho.2006.07.009. [DOI] [PubMed] [Google Scholar]
- Miller A., Fox N.A., Cohn J.F., Forbes E.E., Sherrill J.T., Kovacs M. Regional patterns of brain activity in adults with a history of childhood-onset depression: gender differences and clinical variability. Am. J. Psychiatr. 2002;159(6):934–940. doi: 10.1176/appi.ajp.159.6.934. [DOI] [PubMed] [Google Scholar]
- Miltner W.H., Braun C., Arnold M., Witte H., Taub E. Coherence of gamma-band EEG activity as a basis for associative learning. Nature. 1999;397(6718):434–436. doi: 10.1038/17126. [DOI] [PubMed] [Google Scholar]
- Minguillon J., Lopez-Gordo M.A., Pelayo F. Stress assessment by prefrontal relative gamma. Front. Comput. Neurosci. 2016;10 doi: 10.3389/fncom.2016.00101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Minguillon J., Lopez-Gordo M.A., Renedo-Criado D.A., Sanchez-Carrion M.J., Pelayo F. Blue lighting accelerates post-stress relaxation: results of a preliminary study. PLoS One. 2017;12(10) doi: 10.1371/journal.pone.0186399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moher D., Liberati A., Tetzlaff J., Altman D.G. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. Ann. Intern. Med. 2009;151(4):264–269. doi: 10.7326/0003-4819-151-4-200908180-00135. [DOI] [PubMed] [Google Scholar]
- Nigbur R., Ivanova G., Stürmer B. Theta power as a marker for cognitive interference. Clin. Neurophysiol. 2011;122(11):2185–2194. doi: 10.1016/j.clinph.2011.03.030. [DOI] [PubMed] [Google Scholar]
- Nishida M., Pearsall J., Buckner R.L., Walker M.P. REM sleep, prefrontal theta, and the consolidation of human emotional memory. Cerebr. Cortex. 2009;19(5):1158–1166. doi: 10.1093/cercor/bhn155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nunez P.L., Srinivasan R., Westdorp A.F., Wijesinghe R.S., Tucker D.M., Silberstein R.B., Cadusch P.J. EEG coherency: I: statistics, reference electrode, volume conduction, Laplacians, cortical imaging, and interpretation at multiple scales. Electroencephalogr. Clin. Neurophysiol. 1997;103(5):499–515. doi: 10.1016/S0013-4694(97)00066-7. [DOI] [PubMed] [Google Scholar]
- Ocklenburg S., Korte S.M., Peterburs J., Wolf O.T., Güntürkün O. Stress and laterality–The comparative perspective. Physiol. Behav. 2016;164:321–329. doi: 10.1016/j.physbeh.2016.06.020. [DOI] [PubMed] [Google Scholar]
- Papousek I., Wimmer S., Lackner H.K., Schulter G., Perchtold C.M., Paechter M. Trait positive affect and students' prefrontal EEG alpha asymmetry responses during a simulated exam situation. Biol. Psychol. 2019;148 doi: 10.1016/j.biopsycho.2019.107762. [DOI] [PubMed] [Google Scholar]
- Pérez-Edgar K., Kujawa A., Nelson S.K., Cole C., Zapp D.J. The relation between electroencephalogram asymmetry and attention biases to threat at baseline and under stress. Brain Cognit. 2013;82(3):337–343. doi: 10.1016/j.bandc.2013.05.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perrin S.L., Jay S.M., Vincent G.E., Sprajcer M., Lack L., Ferguson S.A., Vakulin A. Waking qEEG to assess psychophysiological stress and alertness during simulated on-call conditions. Int. J. Psychophysiol. 2019;141:93–100. doi: 10.1016/j.ijpsycho.2019.04.001. [DOI] [PubMed] [Google Scholar]
- Peterson C.K., Gravens L.C., Harmon-Jones E. Asymmetric frontal cortical activity and negative affective responses to ostracism. Soc. Cognit. Affect Neurosci. 2011;6(3):277–285. doi: 10.1093/scan/nsq027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Phelan J., et al. Work stress, family stress and depression in professional and managerial employees. Psychol. Med. 1991;4:999–1012. doi: 10.1017/S0033291700029998. [DOI] [PubMed] [Google Scholar]
- Poppelaars E.S., Harrewijn A., Westenberg P.M., van der Molen M.J.W. Frontal delta-beta cross-frequency coupling in high and low social anxiety: an index of stress regulation? Cognit. Affect Behav. Neurosci. 2018;18(4):764–777. doi: 10.3758/s13415-018-0603-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poppelaars E.S., Klackl J., Pletzer B., Jonas E. Delta-beta cross-frequency coupling as an index of stress regulation during social-evaluative threat. Biol. Psychol. 2021;160 doi: 10.1016/j.biopsycho.2021.108043. [DOI] [PubMed] [Google Scholar]
- Quaedflieg C.W.E.M., Meyer T., Smulders F.T.Y., Smeets T. The functional role of individual-alpha based frontal asymmetry in stress responding. Biol. Psychol. 2015;104:75–81. doi: 10.1016/j.biopsycho.2014.11.014. [DOI] [PubMed] [Google Scholar]
- Ray W.J., Cole H.W. EEG alpha activity reflects attentional demands, and beta activity reflects emotional and cognitive processes. Science. 1985;228(4700):750–752. doi: 10.1126/science.3992243. [DOI] [PubMed] [Google Scholar]
- Robbins K.A., Touryan J., Mullen T., Kothe C., Bigdely-Shamlo N. How sensitive are EEG results to preprocessing methods: a benchmarking study. IEEE Trans. Neural Syst. Rehabil. Eng. 2020;28(5):1081–1090. doi: 10.1109/TNSRE.2020.2980223. [DOI] [PubMed] [Google Scholar]
- Salari N., Hosseinian-Far A., Jalali R., Vaisi-Raygani A., Rasoulpoor S., Mohammadi M., Rasoulpoor S., Khaledi-Paveh B. Prevalence of stress, anxiety, depression among the general population during the COVID-19 pandemic: a systematic review and meta-analysis. Glob. Health. 2020;16(1):57. doi: 10.1186/s12992-020-00589-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Santamaria J., Chiappa K.H. The EEG of drowsiness in normal adults. J. Clin. Neurophysiol. 1987;4(4):327–382. doi: 10.1097/00004691-198710000-00002. [DOI] [PubMed] [Google Scholar]
- Siegrist J. Chronic psychosocial stress at work and risk of depression: evidence from prospective studies. Eur. Arch. Psychiatr. Clin. Neurosci. 2008;258(5):115. doi: 10.1007/s00406-008-5024-0. [DOI] [PubMed] [Google Scholar]
- Smeets T., Cornelisse S., Quaedflieg C.W., Meyer T., Jelicic M., Merckelbach H. Introducing the Maastricht Acute Stress Test (MAST): a quick and non-invasive approach to elicit robust autonomic and glucocorticoid stress responses. Psychoneuroendocrinology. 2012;37(12):1998–2008. doi: 10.1016/j.psyneuen.2012.04.012. [DOI] [PubMed] [Google Scholar]
- Smith E.E., Reznik S.J., Stewart J.L., Allen J.J.B. Assessing and conceptualizing frontal EEG asymmetry: an updated primer on recording, processing, analyzing, and interpreting frontal alpha asymmetry. Int. J. Psychophysiol. 2017;111:98–114. doi: 10.1016/j.ijpsycho.2016.11.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Somerville L.H., Heatherton T.F., Kelley W.M. Anterior cingulate cortex responds differentially to expectancy violation and social rejection. Nat. Neurosci. 2006;9(8):1007–1008. doi: 10.1038/nn1728. [DOI] [PubMed] [Google Scholar]
- Spiegelhalder K., Regen W., Feige B., Holz J., Piosczyk H., Baglioni C., et al. Increased EEG sigma and beta power during NREM sleep in primary insomnia. Biol. Psychol. 2012;91(3):329–333. doi: 10.1016/j.biopsycho.2012.08.009. [DOI] [PubMed] [Google Scholar]
- Stampi C., Stone P., Michimori A. A new quantitative method for assessing sleepiness: the Alpha Attenuation Test. Work. Stress. 1995;9(2–3):368–376. [Google Scholar]
- Steinhubl S.R., Wineinger N.E., Patel S., Boeldt D.L., Mackellar G., Porter V., et al. Cardiovascular and nervous system changes during meditation. Front. Hum. Neurosci. 2015;9:145. doi: 10.3389/fnhum.2015.00145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sterne J.A., Sutton A.J., Ioannidis J.P., Terrin N., Jones D.R., Lau J., et al. Recommendations for examining and interpreting funnel plot asymmetry in meta-analyses of randomised controlled trials. BMJ. 2011;343 doi: 10.1136/bmj.d4002. [DOI] [PubMed] [Google Scholar]
- Stewart J.L., Bismark A.W., Towers D.N., Coan J.A., Allen J.J. Resting frontal EEG asymmetry as an endophenotype for depression risk: sex-specific patterns of frontal brain asymmetry. J. Abnorm. Psychol. 2010;119(3):502. doi: 10.1037/a0019196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Subhani A.R., Xia L., Malik A.S., Othman Z. 2013 35th Annual International Conference of the IEEE Engineering in Medicine and Biology Society (EMBC) 2013. Quantification of physiological disparities and task performance in stress and control conditions; pp. 2060–2063. [DOI] [PubMed] [Google Scholar]
- Subhani A.R., Malik A.S., Kamil N., Naufal M., Saad M.N.M. 2016 6th International Conference On Intelligent And Advanced Systems (ICIAS) 2016. Using resting state coherence to distinguish between low and high stress groups; pp. 1–4. [DOI] [Google Scholar]
- Subhani A.R., Malik A.S., Kamil N., Saad M.N.M. 2016 IEEE EMBS Conference on Biomedical Engineering and Sciences (IECBES) 2016. Difference in brain dynamics during arithmetic task performed in stress and control conditions; pp. 695–698. [DOI] [Google Scholar]
- Susmáková K. Human sleep and sleep EEG. 2005. https://www.semanticscholar.org/paper/Human-Sleep-and-Sleep-EEG-Susm%C3%A1kov%C3%A1/702b1942f24f73208edbf7fdaf3d881ffe7e033a
- Tennant C. Work-related stress and depressive disorders. J. Psychosom. Res. 2001;5:697–704. doi: 10.1016/S0022-3999(01)00255-0. [DOI] [PubMed] [Google Scholar]
- Tort A.B.L., Komorowski R., Eichenbaum H., Kopell N. Measuring phase-amplitude coupling between neuronal oscillations of different frequencies. J. Neurophysiol. 2010;104(2):1195–1210. doi: 10.1152/jn.00106.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tran Y., Thuraisingham R.A., Wijesuriya N., Nguyen H.T., Craig A. 2007 3rd International IEEE/EMBS Conference on Neural Engineering. 2007. Detecting neural changes during stress and fatigue effectively: a comparison of spectral analysis and sample entropy; pp. 350–353. [DOI] [Google Scholar]
- Tufanaru C., Munn Z., Stephenson M., Aromataris E. Fixed or random effects meta-analysis? Common methodological issues in systematic reviews of effectiveness. Int. J. Evid. Base. Healthc. 2015;13(3):196–207. doi: 10.1097/XEB.0000000000000065. [DOI] [PubMed] [Google Scholar]
- Vaisvaser S., Lin T., Admon R., Podlipsky I., Greenman Y., Stern N., Fruchter E., Wald I., Pine D.S., Tarrasch R., Bar-Haim Y., Hendler T. Neural traces of stress: cortisol related sustained enhancement of amygdala-hippocampal functional connectivity. Front. Hum. Neurosci. 2013;7 doi: 10.3389/fnhum.2013.00313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van der Molen M.J.W., Dekkers L.M.S., Westenberg P.M., van der Veen F.M., van der Molen M.W. Why don't you like me? Midfrontal theta power in response to unexpected peer rejection feedback. NeuroImage. 2017;146:474–483. doi: 10.1016/j.neuroimage.2016.08.045. [DOI] [PubMed] [Google Scholar]
- van der Veen F.M., van der Molen M.J.W., van der Molen M.W., Franken I.H.A. Thumbs up or thumbs down? Effects of neuroticism and depressive symptoms on psychophysiological responses to social evaluation in healthy students. Cognit. Affect Behav. Neurosci. 2016;16(5):836–847. doi: 10.3758/s13415-016-0435-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van der Vinne N., Vollebregt M.A., van Putten M.J.A.M., Arns M. Frontal alpha asymmetry as a diagnostic marker in depression: fact or fiction? A meta-analysis. NeuroImage: Clinical. 2017;16:79–87. doi: 10.1016/j.nicl.2017.07.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Oort J., Tendolkar I., Hermans E.J., Mulders P.C., Beckmann C.F., Schene A.H., Fernández G., van Eijndhoven P.F. How the brain connects in response to acute stress: a review at the human brain systems level. Neurosci. Biobehav. Rev. 2017;83:281–297. doi: 10.1016/j.neubiorev.2017.10.015. [DOI] [PubMed] [Google Scholar]
- Vaquero-Blasco M.A., Perez-Valero E., Lopez-Gordo M.A., Morillas C. Virtual reality as a portable Alternative to chromotherapy rooms for stress relief: a preliminary study. Sensors. 2020;20(21):6211. doi: 10.3390/s20216211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vaquero-Blasco M.A., Perez-Valero E., Morillas C., Lopez-Gordo M.A. Virtual reality customized 360-degree experiences for stress relief. Sensors. 2021;21(6):2219. doi: 10.3390/s21062219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verona E., Sadeh N., Curtin J.J. Stress-induced asymmetric frontal brain activity and aggression risk. J. Abnorm. Psychol. 2009;118(1):131–145. doi: 10.1037/a0014376. [DOI] [PubMed] [Google Scholar]
- von Stein A., Sarnthein J. Different frequencies for different scales of cortical integration: from local gamma to long range alpha/theta synchronization. Int. J. Psychophysiol. 2000;38(3):301–313. doi: 10.1016/S0167-8760(00)00172-0. [DOI] [PubMed] [Google Scholar]
- Wang F., Wang C., Yin Q., Wang K., Li D., Mao M., Zhu C., Huang Y. Reappraisal writing relieves social anxiety and may be accompanied by changes in frontal alpha asymmetry. Front. Psychol. 2015;6 doi: 10.3389/fpsyg.2015.01604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams K.D., Cheung C.K., Choi W. Cyberostracism: effects of being ignored over the Internet. J. Pers. Soc. Psychol. 2000;79(5):748. doi: 10.1037//0022-3514.79.5.748. [DOI] [PubMed] [Google Scholar]
- Wróbel A. Beta activity: a carrier for visual attention. Acta Neurobiol. Exp. 2000;60(2):247–260. doi: 10.55782/ane-2000-1344. [DOI] [PubMed] [Google Scholar]
- Yao M., Lei Y., Li P., Ye Q., Liu Y., Li X., Peng W. Shared sensitivity to physical Pain and social evaluation. J. Pain. 2020;21(5):677–688. doi: 10.1016/j.jpain.2019.10.007. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The data extracted from the articles is available as supplementary materials. All other data is also available as supplementary materials.