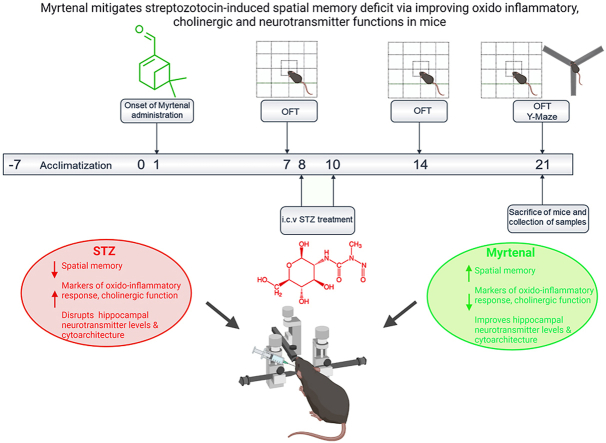
Abstract
The occurrence of chronic neurodegenerative disorders is on the rise, but with no effective treatment due to the paucity of information on the pathological mechanism underlying these disorders. Thus, this study investigated the role of oral administration of myrtenal in mitigating memory deficits and neuro-biochemical alterations in streptozotocin-demented mice model. Mice (n = 35) were randomly allocated into five cohorts consisting of 7 mice each; Group I: Control mice received vehicle alone; Group II: streptozotocin; Group III: streptozotocin + 100 mg/kg myrtenal; Group IV: streptozotocin +200 mg/kg myrtenal; and Group V: streptozotocin + donepezil 0.5 mg/kg. Data from this study demonstrated that the administration of streptozotocin (STZ) impaired spatial memory and induced alterations in markers of oxido-inflammatory response, cholinergic function, cytoarchitecture, and neurotransmitter levels in mice hippocampus. Notably, administration of myrtenal enhanced spatial memory performance in STZ-demented mice by improving the activities of endogenous antioxidant enzymes to protect the brain from oxido-inflammatory stress. Treatment with myrtenal also restored cholinergic function and stabilized the homeostasis of neurotransmitters in STZ-demented mice. The authors infer that fruits rich in myrtenal may be beneficial for treating patients living with dementia associated with Alzheimer's disease.
Keywords: Myrtenal, Memory, Antioxidant, Streptozotocin, Dementia, Mice
Graphical abstract
Highlights
-
•
Data from the present study demonstrates that the administration of streptozotocin impairs spatial memory in mice and induces alterations in markers of oxido-inflammatory response, cholinergic function, histoarchitecture, and neurotransmitter levels in the hippocampus.
-
•
The administration of myrtenal enhances spatial memory performance in streptozotocin-demented mice by improving the activities of endogenous antioxidant enzymes to protect the brain from oxido-inflammatory stress.
-
•
Treatment with myrtenal restores cholinergic function and stabilizes the homeostasis of neurotransmitters in streptozotocin-demented mice.
1. Introduction
A substantial number of contemporary studies have reported a rise in the global occurrence of dementia. Among the different types of dementia identified, dementia of Alzheimer's type (DAT) is considered the most severe (Stephan et al., 2010), with an incident of approximately 6% in women and 5% in men above the age 60 years (Kawai et al., 2018). Furthermore, a survey carried out by Ageing Demographics and Memory Study (ADAMS) estimated that about 4.5 million Americans live with dementia, a number likely to rise to about 16 million by 2050 (Langa et al., 2005; Plassman et al., 2011).
Pathologically, the hallmarks of Alzheimer's disease include a deposition of neurofibrillary tangles, accumulation of amyloid-beta peptides, and hyperphosphorylated tau protein associated with microtubules (Bloom, 2014; Serrano-Pozo et al., 2011). These classic features bear strong correlations with neuronal and synaptic loss – which ultimately manifests as cognitive impairment in Alzheimer's patients – and inform contemporary treatments that aim to improve the activity of healthy neurons without inhibiting the progressive degeneration of neuronal cells (DeTure and Dickson, 2019). There is no definite cure to reverse the neuropathological hallmarks of the disease process partly because the specific aetiological factor of dementia of Alzheimer's type (DAT) is yet to be identified, but also, the signalling pathways are complex (Serrano-Pozo et al., 2011). Recent studies have implicated increased oxidative stress and lipid peroxidation in the development of DAT (Fracassi et al., 2021; Kouhestani et al., 2018), factors that also reduce brain glucose utilization, neuroinflammation and impair cholinergic neurotransmission (Buccellato et al., 2021; Peña-Bautista et al., 2021; Tönnies and Trushina, 2017). In line with these theories, we believe that targeting oxidative stress and enhancing the antioxidant status of the brain is a vital strategy in minimising the pathophysiology and improving contemporary understanding of dementia of Alzheimer's type.
However, research into antioxidant agents that can potentially minimise the pathophysiology of memory deficits and neuro-biochemical alterations in Alzheimer's disease are scanty. Notably, the antioxidant properties of myrtenal (6,6-Dimethyl-2-norpinene-2-carboxaldehyde) remain under-investigated. Myrtenal is an organic compound commonly derived from many plant-essential oils, including rice (Cho et al., 2014), astartea (Lowe et al., 2005), Lavandula spp (Smigielski et al., 2009), and Cuminum cyminum (Moraghebi, 2013) making it easily accessible. This bicyclic monoterpenoid compound (Fig. 1) is an isoprenoid lipid molecule possessing several physiological functions, including antioxidant (Tancheva et al., 2020), anti-inflammatory (Babu et al., 2012), anti-apoptotic and anti-tumour activities (Venkatachalam et al., 2014), alongside the potential to regulate enzymatic activities in the mitochondrion and lysosomes (Begum et al., 2012).
Fig. 1.
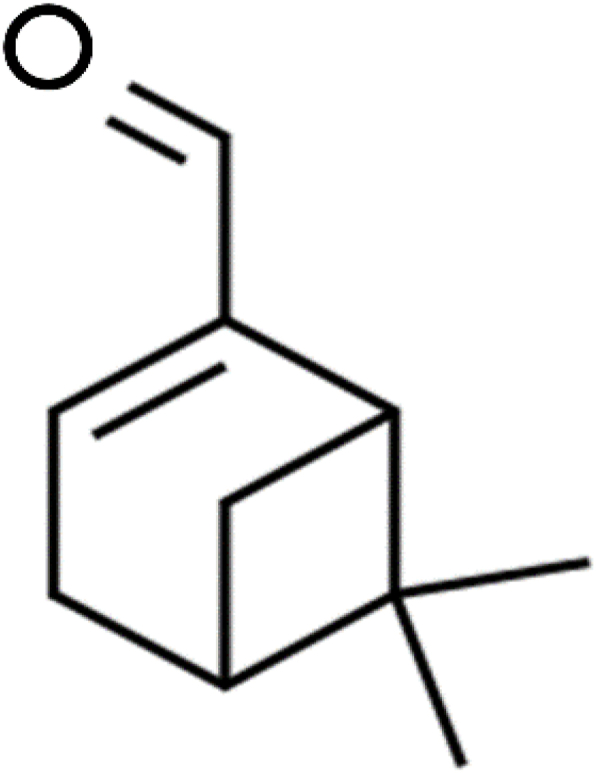
Chemical structure and IUPAC name of myrtenal.
Although, myrtenal has been suggested to possess neuro-protective effects in experimental rat models of Parkinson's disease (Tancheva et al., 2020), there is no information on the memory-enhancing potential of myrtenal and its therapeutic benefit in improving DAT induced by STZ treatment. Consequently, the current study aimed to create mice models of dementia using streptozotocin and investigate the potential of mitigating the pathophysiology of memory deficits and neuro-biochemical changes using myrtenal.
2. Materials and methods
2.1. Animals
This study was approved by the Animal Welfare and Central Research Committee of the Faculty of Veterinary Medicine, University of Maiduguri (AWCRC/2017/141), and all animals were treated following the National Institute of Health Guide for Care and Use of Laboratory Animals (National Research Council Committee for the Update of the Guide for the and Use of Laboratory, 2011). Adult male albino mice (n = 35; 25–35 g) were used for this study. The mice were housed in plastic cages at room temperature (22 ± 2 °C) within the animal facility and were exposed to 12 h light/dark cycle with access to feed and water ad libitum. The mice were examined for health and pathogen-free status throughout the study period and stress was minimized during experimental procedures by ensuring minimal handling. All the animals were allowed to acclimatize to the laboratory condition for five days prior to behavioural experiments.
2.2. Drug dosage and administration
Donepezil (DP) powder was dissolved in 0.9% saline and administered at a dose of 0.5 mg/kg i.e. (0.9% w/v) (Kumar and Singh, 2017). Streptozotocin (Sigma Aldrich Co. Ltd., St. Louis) was dissolved in freshly prepared artificial cerebrospinal fluid (aCSF) as vehicle and administered at a dose of 3 mg/kg, 10 μL on each side i.c.v (Ramezani et al., 2016; Kouhestani et al., 2018). Artificial cerebrospinal fluid was simultaneously administered at a dose of 10 μL on each side i.c.v to the control group on the 1st and 3rd day of Streptozotocin administration. Myrtenal (Sigma Aldrich Co. Ltd., St. Louis) was dissolved in corn oil and administered orally at doses of 100 mg/kg and 200 mg/kg while 10 ml/kg of corn oil was administered simultaneously to the control group. The dosage of myrtenal was selected based on previous LD50 report (Ishida et al., 1989). All drugs were freshly prepared before use.
2.3. Induction of experimental dementia by streptozotocin
Dementia was induced by administering two divided doses of streptozotocin by intracerebroventricular injection on the first and third day (Kouhestani et al., 2018). In brief, under anaesthesia, the animals were bilaterally cannulated using a stereotaxic equipment (Stoelting, Chicago, IL, USA) according to the following ventricular coordinate: anterior-posterior = −0.8; medio-lateral = ± 1.5; and dorso-ventral = −3.4 (Paxinos and Watson, 2007). Mice in control group were administered intracerebroventricular injection of artificial cerebrospinal fluid only.
2.4. Experimental design
The mice were randomly divided into five groups consisting of seven animals each, and were administered with the dose regimen presented in Table 1:
Table 1.
An outline of the experimental groups and the agents administered.
| Groups (n = 7 each) | Agents administered |
|---|---|
| Group I (control) | Control mice received vehicle alone |
| Group II (negative control) | Streptozotocin (3 mg/kg) |
| Group III | Streptozotocin (3 mg/kg) + myrtenal (100 mg/kg) |
| Group IV | Streptozotocin (3 mg/kg) + myrtenal (200 mg/kg) |
| Group V | Streptozotocin (3 mg/kg) + donepezil 0.5 mg/kg |
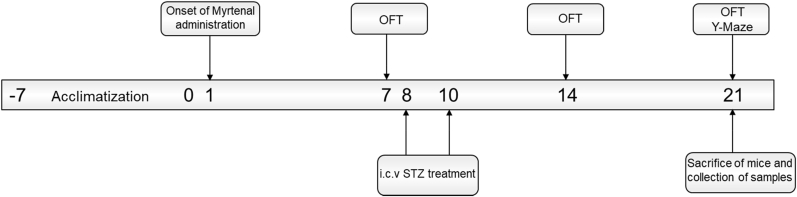
Pre-treatment with myrtenal was carried out for 7 days prior the first administration of streptozotocin and continued for 14 days thereafter. Donepezil and myrtenal were administered 30 min prior the administration of streptozotocin (Fig. 2).
Fig. 2.
Diagrammatic illustration of the experimental design in the current study. The numbers on the central bar indicate the days when behavioural or treatment interventions were conducted.
2.5. Assessment of memory performance
Memory performance was assessed using the Y-maze Test as previously described by Casadesus et al. (2007). The test was applied to determine spontaneous alterations in the behaviour of the mice as an indication of short-term memory (Casadesus et al., 2007). The test apparatus comprised of 3 identical arms (120°, 41 × 5 × 15 cm) marked as ‘A’, ‘B’, and ‘C’, and involved consistently positioning each mouse in the ‘home’ arm labelled as A. Thereafter, the mouse was left to explore the maze unrestricted for 5 min. Alternation performance was recorded as consecutive entries into all three arms (ABC, CAB or BCA). The performance chamber was wiped using 75% ethanol after completing each assessment session to avoid odour cues. The percentage alternation was evaluated as total complete alternations/total entries – 2) x 100.
2.6. Open field test
The open-field device was composed of a wooden box (50 × 50 × 45 cm) lined with a clear Plexiglas on the inner surface (base). The floor of the box was delineated into 25 squares of equal sizes. As previously described by Zhu et al. (2001), locomotor activity and anxiety were assessed using horizontal locomotion and the frequency of rearing (Zhu et al., 2001). Evaluating the locomotor activity involved placing each mouse in the central point of the box and allowing it to walk freely for 3 min. This period was also used to habituate the mouse with the environment. Thereafter, the number of squares crossed with all the paws by each mouse was recorded to assess locomotion while rearing was determined by measuring the vertical counts (frequency at which the mice stood on its hind-limbs; with the forepaws lifted freely or leaned against the wall of the apparatus). These parameters were evaluated during the next 2 min. A 75% v/v ethanol solution was used to clean the cage following each test session to avoid residual odours. All mice were tested on days 1, 7, 14, and 21 of the treatment.
2.7. Preparation of brain tissue homogenate for biochemical analysis
At the end of the behavioural experiments, all the mice were anesthetized using 200 mg/kg, pentobarbital sodium (i.p) and then euthanised by cervical dislocation. Next, brain tissues were removed and dissected to isolate the hippocampus, which was weighed and further processed following the method of Shen et al. (2011). The supernatants were separated and used to estimate biochemical parameters including activities of hippocampal antioxidant enzymes (superoxide dismutase (SOD), glutathione peroxidase (GPx), catalase (CAT)), acetylcholinesterase activity (AChE), lipid peroxidation (malondialdehyde; MDA concentration), nitric oxide (NO) concentrations, and in addition, inflammatory marker (TNF-α) and neurotransmitters (namely dopamine (DA), serotonin (5-HT), glutamate, norepinephrine (NE) and gamma-aminobutyric acid (GABA) were also assessed following standard laboratory procedures (Shen et al., 2011).
3. Brain oxidative stress markers
3.1. Determination of brain lipid peroxidation
The concentration of malondialdehyde (MDA) as a product of lipid peroxidation, was assayed spectrophotometrically in hippocampal homogenates as previously described by Ohkawa et al. (1979). The change in absorbance was read spectrophotometrically at 532 nm. The levels of MDA in hippocampal samples were estimated using a molar extinction coefficient of 1.56 × 105 M−1 cm−1, and expressed as μmoles of MDA/tissue weight in grams (Ohkawa et al., 1979).
3.2. Estimation of brain nitric oxide (NO) concentration
The concentration of NO was assayed calorimetrically using the Griess reagent as described by Green et al. (1982). The principle of the Griess reaction is based on the estimation of the formation of nitrite as a reddish-pink coloration detected in the sample following treatment with the Griess reagent (Green et al., 1982). Thereafter, the absorbance was read at 548 nm.
3.3. Estimation of brain proinflammatory cytokine levels
Tumour Necrosis Factor-alpha (TNF-α) was used as a marker to assess the extent of inflammation. The concentration of TNF-α in hippocampal tissues was estimated with the aid of a mice TNF-α ELISA kit of MyBioSource (USA) catalogue No. MBS2507393) and read spectrophotometrically at 540 nm using a microplate reader.
3.4. Western blot
For protein extraction, hippocampal tissues were homogenized in an ice-cold extraction buffer and then the sample homogenates centrifuged at 10,000 rpm for 5 min at 4 °C. The respective protein concentrations were then measured using a Protein Assay Kit (Sigma–Aldrich) according to the manufacturer's guidelines.
3.5. Assessment of brain acetylcholinesterase (AChE) activity
The activity of acetylcholinesterase was estimated in hippocampal tissues following the method described by Ellman (1961), the colour observed was read spectrophotometrically at 412 nm, and the activity of AChE was measured in μM per SH group (μMSH) based on a standard curve (Ellman, 1959).
3.6. Estimation of brain antioxidant capacity
Hippocampal tissue concentrations of antioxidants markers, glutathione peroxidase (GPx), superoxide dismutase (SOD), and catalase (CAT) were determined using ELISA kits of MyBioSource (USA) catalogue No. MBS265966, MBS036924, and MBS726781, respectively according to the manufacturer's instructions.
3.7. Histopathological investigation
The preparation of haematoxylin and eosin (H & E) stained sections of the collected hippocampal samples were carried out as described by Suvarna and Bancroft (Suvarna et al., 2018). The sections were investigated and photographed by a blind pathologist for the pathological findings.
3.8. Estimation of brain neurotransmitter content
Determination of the levels of serotonin, glutamate, dopamine, noradrenaline, and gamma-aminobutyric acid (GABA) in hippocampal tissues were measured as previously described by Pagel et al. (2000), using high-performance liquid chromatography (HPLC) (Pagel et al., 2000). The supernatant was centrifuged at 10, 000×g for 10 min at 4 °C and divided into two portions. The first portion was used to detect the levels of noradrenaline, serotonin, and dopamine, while the second portion was used to estimate GABA and glutamate. The levels of the different neurotransmitters were determined by comparing the sample retention time with that of the standard and expressed as ng/g (for noradrenaline, dopamine, and serotonin) and ug/g (for GABA and glutamate). Concentration change was determined using ANOVA, followed by Tukey's posthoc test is indicated by asterisks ∗ p < 0.05, ∗∗p < 0.01, ∗∗∗p < 0.001, compared to the streptozotocin group.
3.9. Data analysis
To estimate the significant difference among the different experimental groups in the present study, the data collected from all experiments were expressed as mean ± standard error of the mean (±SEM) and subjected to a repeated-measures analysis of variance (ANOVA), followed by Tukey's post-hoc test. GraphPad Prism, version 7.0 (GraphPad Software, USA) was used to analyse the collected data. P values of <0.05 were considered statistically significant. Photomicrographs were obtained using a microscope equipped with a camera (Olympus BX43; Olympus, Centre Valley, PA). Images were minimally adjusted to enhance contrast and brightness only.
4. Results
4.1. Myrtenal improves streptozotocin-induced impairment in memory and open-field performance
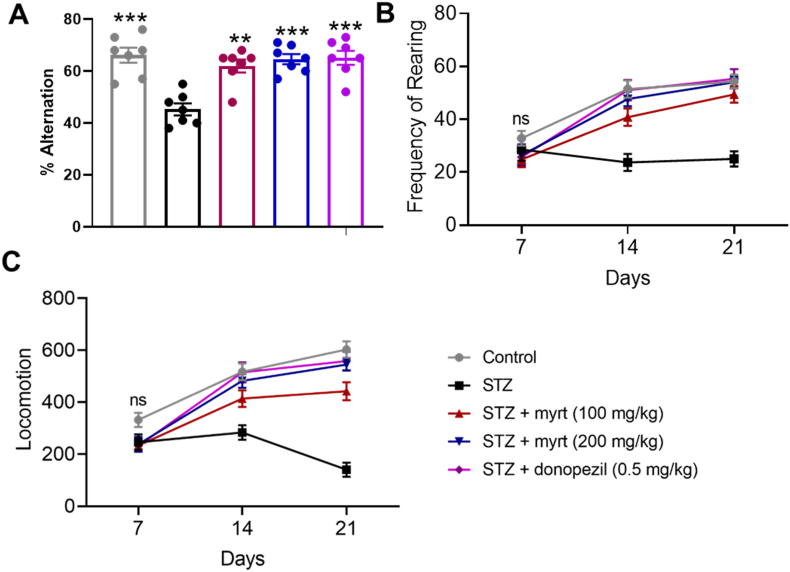
Streptozotocin induced impairment in the working memory of mice subjected to the Y maze test as indicated by a significant decrease (p < 0.01) in the alternation percentage of all experimental groups when compared to the control group (Fig. 3. However, treatment with myrtenal (100 and 200 mg/kg, p.o.) significantly (p < 0.01 and p < 0.001, respectively) attenuated streptozotocin-induced memory impairment as depicted by the improved percentage alternation recorded in the treatment groups compared to the streptozotocin group (Group II) which had the lowest memory performance (Fig. 3A).
Fig. 3.
Effect of myrtenal on memory and open-field performance in Streptozotocin (STZ)-induced demented mice (Mean ± SEM, n = 7). Behavioural assessment of mice showing effect of myrtenal on A) memory performance, B) locomotion, and C) frequency of rearing. Memory performance was assessed using the Y-maze Test. Alternation performance was recorded as consecutive entries into all three arms. The percentage alternation was evaluated as total complete alternations/total entries – 2) x 100. Open-field performance was used to assess horizontal locomotor activity and vertical frequency of rearing (vertical counts). Assessing locomotor activity involved placing each mouse in the central point of the box and allowing it to walk freely for 3 min and familiarize with the environment. Thereafter, and for the next 2 min, the number of squares crossed with all the paws by each mouse was recorded to assess locomotion while rearing was determined by measuring the vertical counts. The significance of the alteration in performance of mice determined using ANOVA, followed by Tukey's multiple comparison test is indicated by asterisks ∗ P < 0.05, ∗∗P < 0.01, ∗∗∗P < 0.001, compared to the STZ group.
The effect of myrtenal on voluntary locomotion of mice in the open field as noted by the number of lines traversed following streptozotocin treatment is as displayed in Fig. 3B. Streptozotocin significantly decreased spontaneous motor activity compared to the control group. However, treatment with myrtenal (100 and 200 mg/kg, p.o.) significantly (p < 0.01 and p < 0.001, respectively) reversed the effect compared to the streptozotocin group, which had the least locomotor activity (Fig. 3B).
The effect of myrtenal on rearing of mice as depicted by the number of vertical counts following streptozotocin treatment is as shown in Fig. 3C. Streptozotocin-treated mice exhibited a significant decrease in rearing compared to the control group. However, treatment with myrtenal (100 and 200 mg/kg, p.o.) significantly (p < 0.05 and p < 0.01 respectively) increased the number of vertical counts compared to the streptozotocin-treated animals, which had the least vertical counts (Fig. 3C).
4.2. Myrtenal mitigates streptozotocin-induced oxidative stress
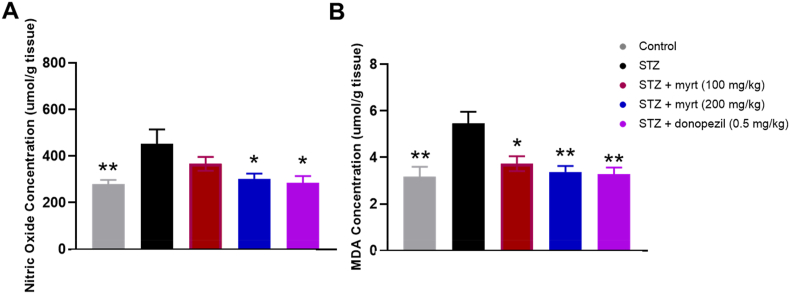
The effect of administering myrtenal on elevated nitric oxide concentration in hippocampal tissues of mice exposed to streptozotocin is shown in Fig. 4a. The result showed that administering 200 mg/kg of myrtenal in streptozotocin-demented mice significantly decreased (p < 0.05) the elevated hippocampal nitric oxide levels in mice brains compared to streptozotocin alone. There was no significant difference between the group treated with streptozotocin + myrtenal (200 mg/kg) and streptozotocin + donepezil (0.5 mg/kg).
Fig. 4.
Effect of myrtenal on brain oxidative stress markers in Streptozotocin (STZ)-induced demented mice (Mean ± SEM, n = 7). Following the completion of behavioural experiments, all the mice were anesthetized and sacrificed. Thereafter, brain tissues were instantly removed and dissected to isolate about 1 mg of hippocampal tissue which was homogenized in 10 mL of ice-cold phosphate-buffered solution (pH 7.0), centrifuged at 4 °C and 3000g for 5min. The supernatant was retrieved and used to determine NO and MDA concentrations. The concentration of MDA was assayed spectrophotometrically and the change in absorbance was read at 532 nm. The levels of MDA in hippocampal samples were estimated using a molar extinction coefficient of 1.56 × 105 M−1 cm−1 and expressed as μmoles of MDA/tissue weight in grams. The concentration of NO was assayed calorimetrically using the Griess reaction and thereafter, the absorbance was read at 548 nm. The assayed nitrite levels in each sample were extrapolated from the standard curve of sodium nitrite (0–100 μM). The significance of the change in concentration determined using ANOVA, followed by Tukey's post-hoc test is indicated by asterisks ∗ p < 0.05, ∗∗p < 0.01, compared to the Streptozotocin group.
The effect of administering myrtenal in combination with streptozotocin on MDA levels (umol/g tissue) in the hippocampal tissues is shown in Fig. 4b. The results showed that administration of either 100 or 200 mg/kg of myrtenal in streptozotocin-demented mice significantly decreased (p < 0.05 and p < 0.01, respectively) MDA levels in mice brains compared to streptozotocin alone. However, there was no significant difference between the group treated with streptozotocin + myrtenal (200 mg/kg) and streptozotocin + donopezil (0.5 mg/kg). Similarly, the reference drug, donepezil also decreased MDA levels in mice brains, as there was no significant difference between both groups (Fig. 4b). In contrast, streptozotocin alone significantly elevated MDA levels (p < 0.01) in hippocampal tissues compared to the control (Fig. 4b).
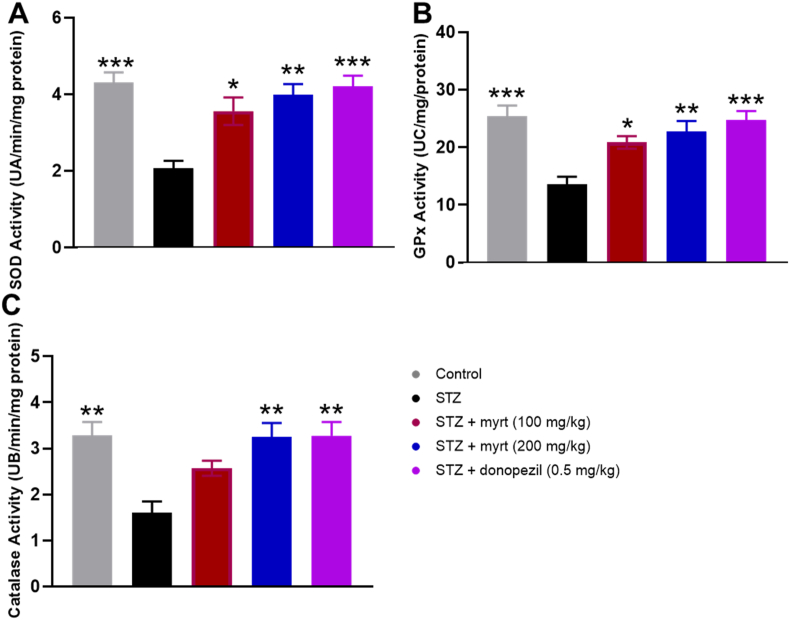
4.3. Myrtenal enhances activities of endogenous antioxidant enzymes
The effect of administering myrtenal on the activities of endogenous antioxidants is demonstrated in Fig. 5. Streptozotocin induced a significant decrease in the activities of hippocampal superoxide dismutase (SOD), glutathione peroxidase (GPx), and catalase (CAT). Conversely, in a grade dependent manner, treatment with 100 and 200 mg/kg of myrtenal enhanced the activity of hippocampal SOD (3.557 ± 0.33; 3.986 ± 0.41 UA/min/mg protein), GPx (20.86 ± 2.31; 22.71 ± 0.61 UC/mg protein), and CAT (2.57 ± 0.31; 3.21 ± 0.51 UB/min/mg protein), in streptozotocin-demented mice. Similarly, the reference drug, donepezil (0.5 mg/kg) enhanced the activities of SOD, GPx, and CAT in hippocampal tissues (3.98 ± 0.41 UA/min/mg protein, 24.71 ± 2.32 UC/mg protein; 3.24 ± 0.39 UB/min/mg protein, respectively) and was not significantly different from the streptozotocin +200 mg/kg myrtenal group (p > 0.05). The lowest antioxidant activities were observed in the streptozotocin-treated group (2.071 ± 0.41 UA/min/mg protein, 13.57 ± 2.31 UC/mg protein, 1.600 ± 0.39 UB/min/mg protein; Fig. 5).
Fig. 5.
Effect of myrtenal on brain antioxidant enzymes activity in STZ-induced demented mice (Mean ± SEM, n = 7). Activities of SOD, GPx, and CAT were estimated using hippocampal tissues and significance of the change in concentration was determined using ANOVA, followed by Tukey's posthoc test is indicated by asterisks ∗ p < 0.05, ∗∗p < 0.01, ∗∗∗p < 0.001, compared to the STZ group.
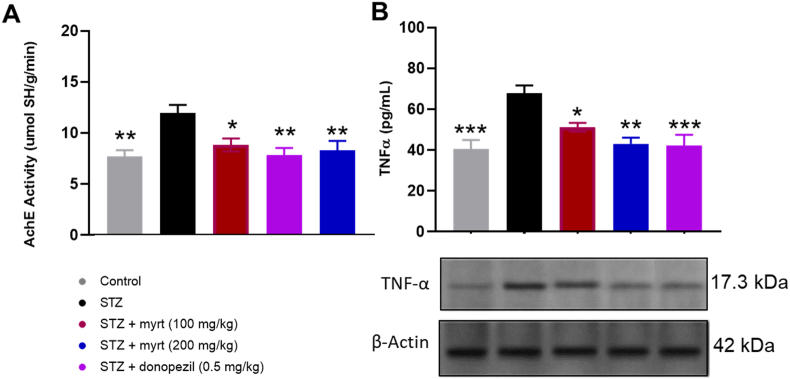
4.4. Myrtenal enhances hippocampal AchE activity in streptozotocin-induced demented mice
Streptozotocin induced a significant increase (p < 0.01) in the activity of AChE compared to control mice. Conversely, treatment with 100 and 200 mg/kg of myrtenal significantly inhibited AChE activity in mice brains compared to streptozotocin alone (8.84 ± 0.97 μmol SH/g/min; p < 0.05 and 8.33 ± 0.81 μmol SH/g/min; p < 0.01, respectively). Similarly, the reference drug, donepezil (0.5 mg/kg) inhibited AChE activity in hippocampal tissues (7.83 ± 0.91 μmol SH/g/min) and was not significant different (p > 0.05) from the streptozotocin +200 mg/kg myrtenal group (Fig. 6a).
Fig. 6.
Effect of myrtenal on brain acetylcholinesterase activity and TNF-α in STZ-induced demented mice (Mean ± SEM, n = 7/group). Activities of acetylcholinesterase and TNF-α was estimated in hippocampal tissues and the significance of the change in concentration determined using ANOVA, followed by Tukey's posthoc test is indicated by asterisks ∗ p < 0.05, ∗∗p < 0.01, ∗∗∗p < 0.001, compared to the STZ group.
4.5. Myrtenal improves TNF-α levels streptozotocin-induced demented mice
Streptozotocin induced a significant increase (p < 0.01) in the levels of TNF-α (67.9 ± 5.51 pg/mL) compared to the control. Conversely, treatment with 100 and 200 mg/kg of myrtenal significantly reduced TNF-α activity in mice brains compared to streptozotocin alone (51.14 ± 0.97 pg/mL; p < 0.05 and 43.0 ± 5.81 pg/mL; p < 0.01 respectively). Similarly, the reference drug, donepezil (0.5 mg/kg) reduced TNF-α activity in hippocampal tissues (42.29 ± 4.01 pg/mL) and was not significantly different from the streptozotocin +200 mg/kg myrtenal group (p > 0.05). The lowest TNF-α level was observed in the control group (40.43 ± 6.07 pg/mL). Also, the results of western blot showed a reduction in the levels of TNF-α in the groups treated with myrtenal (100 and 200 mg/kg; Fig. 6b) (see Fig. 7).
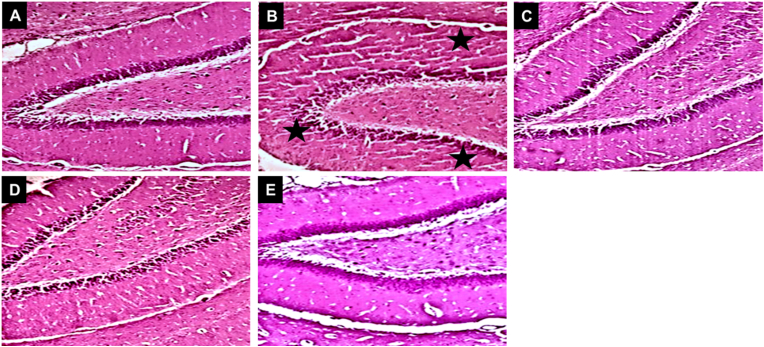
Fig. 7.
Photomicrograph showing the mitigating effect of myrtenal on the hippocampus of STZ-demented mice stained with H & E. Stars indicate neuronal degeneration and loss of neuronal cells in STZ treated mice (X20).
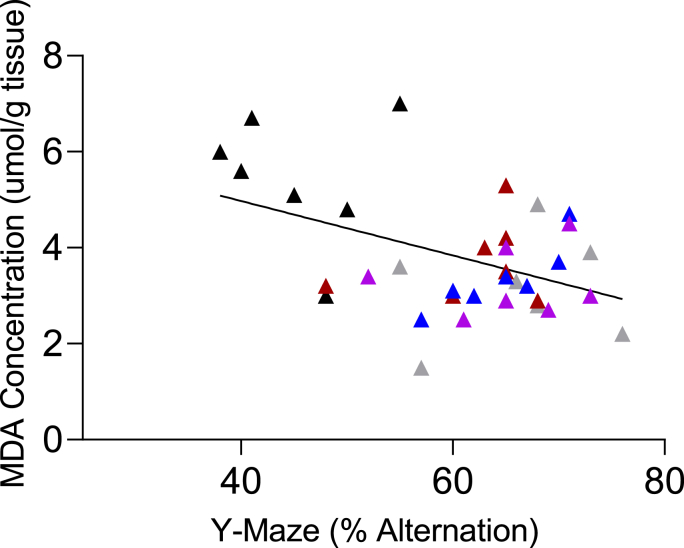
4.6. Relationship between MDA concentration and memory performance following treatment with myrtenal
A significant correlation between the concentration of MDA and memory performance was observed. Pearson's correlation coefficient of r = −0.569 and p = 0.006 was observed (Fig. 8).
Fig. 8.
Correlation of MDA concentration and memory performance in STZ-demented mice (Mean ± SEM, n = 7/group). r = Pearson's correlation coefficient, p < 0.05 was considered significant.
4.7. Myrtenal modulates neurotransmitter levels in streptozotocin-induced demented mice
The administration of streptozotocin intracerebroventricularly significantly disrupted the neurotransmitter levels including serotonin (ng/g), glutamate (ug/g), GABA (ug/g) and dopamine (ng/g). The levels of Noradrenaline (ng/g) were however not significantly different among the different groups. The administration of myrtenal (200 mg/kg), significantly restored the levels of neurotransmitters to normal. The lowest neurotransmitter levels were observed in the STZ treated group while the highest levels were observed in the donepezil treated group (Table 2).
Table 2.
Effect of myrtenal on neurotransmitter levels in streptozotocin (STZ)-induced demented mice (Mean ± SEM, n = 7/group).
| Neurotransmitters |
|||||
|---|---|---|---|---|---|
| Dopamine (ng/g) | Noradrenaline (ng/g) | Serotonin (ng/g) | Glutamate (ug/g) | GABA (ug/g) | |
| Control | 746.8 ± 54.4∗∗ | 854.7 ± 78.6 | 551.8 ± 51.2∗ | 1667.2 ± 119.4∗∗ | 2705.1 ± 123.5∗∗ |
| STZ (3 mg/kg) | 581.9 ± 33.1 | 815.6 ± 66.1 | 438.5 ± 69.2 | 1201.8 ± 171.2 | 2397.4 ± 111.4 |
| STZ (3 mg/kg) + myrtenal (100 mg/kg) |
698.2 ± 43.9∗ | 824.2 ± 52.3 | 502.2 ± 53.8 | 1389.4 ± 210.6 | 2574.2 ± 223.5 |
| STZ (3 mg/kg) + myrtenal (200 mg/kg) |
730.9 ± 23.8∗∗ | 830.9 ± 46.1 | 565.8 ± 39.6∗∗ | 1458.5 ± 217.9∗∗ | 2658.8 ± 312∗∗ |
| STZ (3 mg/kg) + donepezil (0.5 mg/kg) |
745.1 ± 53.5∗∗ | 853.5 ± 67.5 | 568.4 ± 57.3∗∗ | 1591.4 ± 198.2∗∗∗ | 2692.8 ± 340.2∗∗ |
4.8. Myrtenal modulates neurotransmitter levels in streptozotocin-induced demented mice
The administration of streptozotocin intracerebroventricularly significantly disrupted the neurotransmitter levels including serotonin (ng/g), glutamate (ug/g), GABA (ug/g) and dopamine (ng/g). The levels of Noradrenaline (ng/g) were however not significantly different among the different groups. The administration of myrtenal (200 mg/kg), significantly restored the levels of neurotransmitters to normal. The lowest neurotransmitter levels were observed in the STZ treated group while the highest levels were observed in the donepezil treated group (Table 2).
5. Discussion
In the current study, we aimed to better understand the therapeutic effects of myrtenal in dementia, particularly of Alzheimer's type. We leveraged the ability of streptozotocin to produce an experimental model of dementia and demonstrated the capacity of myrtenal to alleviate the neurodegenerative deficits simulated in experimental models of dementia of Alzheimer's type (DAT). A notable effect of myrtenal was an improvement in memory deficits, which can be ascribed to the antihyperglycemic properties of myrtenal, enhancing the levels and utilization of glucose in the central nervous system (Dragomanova et al., 2018). This effect corroborates the findings of Dragomanova et al. (2018) who demonstrated significantly improved cognitive functions following the administration of myrtenal to rat models of anxiety and memory loss created by repeated scopolamine injections. The evidence suggests that myrtenal plays a crucial role as a therapeutic agent that mitigates the neurodegenerative processes resulting in memory loss in dementia. However, full proofing the hypothesis requires experimental trials with alternate cholinergic agents capable of simulating dementia.
We used streptozotocin - a naturally occurring chemical derived from Streptomyces achromogenes - to induce dementia in the current study. Although streptozotocin is a glucosamine-nitrosourea compound primarily categorised as an antibiotic (Zhang et al., 2020), it induces an insulin-resistant neuronal state associated with progressive cholinergic deficits, hyperglycaemia, oxidative stress, neuroinflammation, biochemical alterations, neurodegeneration, and memory impairment in humans and animals (Qi et al., 2021; Zhang et al., 2020). These manifestations match the classical signs of DAT in humans, thus justifying its use in creating an experimental model of DAT. Moreover, as a negative control in the current study, the behaviour of animals injected with streptozotocin and evaluated for percentage alternation, rearing and locomotion progressively deteriorated (Fig. 3), indicating the recruitment of the same signalling pathways as those of DAT. It is worthy of note that streptozotocin-associated alterations suggest an influence over multiple cell-signalling and metabolic pathways in the brain as suggested by the stack, but consistently significant changes were observed in both behavioural and biochemical parameters. For instance, nitric oxide and MDA concentrations - crucial brain oxidative markers - reflected a spike in streptozotocin injected animals in the current study (Fig. 4). These findings, together with those of severe hyperglycaemia and an imbalance in glutamate and dopamine neurotransmitters levels (Alvarez et al., 2009; Biessels et al., 1998; Stranahan et al., 2008) disrupted by injecting streptozotocin are evidence of complex signalling. However, preliminary deductions can be made by empirically deploying a range of behavioural paradigms.
Using the Y-Maze test, the current study showed that treatment with streptozotocin induced spatial memory deficit and decreased the total percentage alternation (Fig. 3). We believe that this finding stems from reduced neuronal glucose uptake, consequently hampering actual firing capacity and creating a neurotransmitter blockade to minimise metabolism within the hippocampus. To further deepen understanding, we also used open field tests to demonstrate anxiogenesis – as indicated by deficits in exploration observed in the group of mice administered STZ. The mechanism underlying the anxiogenic potential of STZ involves elevated activity in the hypothalamic-pituitary-adrenal axis and changes in serotonergic and dopaminergic neurotransmitters in the hypothalamus (Kumar and Singh, 2017; Qi et al., 2021). On the other hand, the administration of myrtenal significantly reduced the frequency of rearing, indicating a potential anxiolytic effect, suggestive of a modulatory influence of myrtenal on glutamate and GABA-ergic neurotransmission processes (Li et al., 2016). Interestingly, previous studies have reported that the antioxidant potential of myrtenal on cognitive functions correlates significantly with increased locomotion (Dragomanova et al., 2018), as observed in the current study. Similarly, increased locomotion is associated with improved levels of corticosterone and corticotrophin-releasing hormone (Babu et al., 2012). It is therefore plausible that myrtenal may modulate neurobehavioral patterns in rodents by reducing the secretion of corticotropin-releasing hormone and modulating oxidative processes.
The dynamics of oxidants and antioxidants in neural anabolic and catabolic processes are well documented. However, investigations into agents that disrupt these processes remain ongoing. Here, we observed that treatment with streptozotocin induced a significant reduction in the activities of the endogenous antioxidants and impaired the function of the antioxidant defence machinery (Fig. 5). This finding suggests that myrtenal possesses biologically active molecules capable of donating electrons to free radicals to neutralize them and consequently inhibit cellular damage via their free radical scavenging property. Notably, the administration of myrtenal restored the activities of endogenous antioxidant enzymes in the hippocampus to normal levels (Fig. 5). Besides, treatment with myrtenal mitigated oxidative stress by stabilising the levels of lipoperoxidation (MDA) and nitric oxide in the hippocampus. Perhaps the decrease in NO concentration by myrtenal is due to its potential to modulate the expression of inducible nitric oxide synthase (iNOS) and downregulate its levels in the hippocampus (Dragomanova et al., 2018; González-Burgos and Gómez-Serranillos, 2012). Thus, we infer that myrtenal possesses properties that could be beneficial for the management of disorders associated with the central nervous system, particularly neurodegenerative diseases. However, to explore this potential further, the reactions deployed by myrtenal to interfere with neurotransmission processes must be explored further.
Notably, AChE primarily metabolises acetylcholine, and hence, inhibitors of AChE are potent therapeutic agents for the mitigation of cognition deficits (Kumar and Singh, 2017; Qi et al., 2021). Data from the current study revealed an inhibition in the cholinergic neurotransmission functions with increased AChE activity in mice brains following streptozotocin injection. Conversely, treatment with myrtenal inhibited the AChE activity in the hippocampal tissues, thus, enhancing cholinergic neurotransmission function. The alteration in AChE activity may be due to the underlying hyperglycaemia in the brain, associated with streptozotocin administration (Kouhestani et al., 2018). The ability of myrtenal to restore the activity of AChE may be ascribed to its potential to modulate insulin signalling, stabilise glucose metabolism and improve memory deficits induced by streptozotocin (Babu et al., 2012; Dragomanova et al., 2018; Venkatachalam et al., 2014).
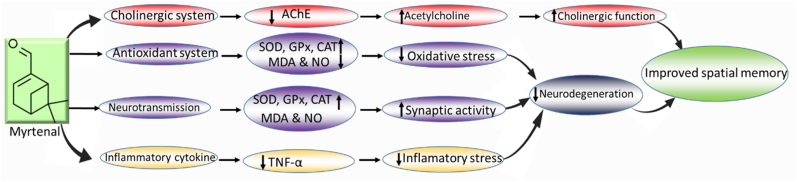
Relatedly, dopamine also plays a crucial role in learning and memory (Edelmann and Lessmann, 2018; Small, 2017). Specifically, D1 and D2 dopamine receptors play crucial roles in activating nuclear factors, including CREM and CREB (Dudman et al., 2003). Learning and memory consolidation in the Y-maze behavioural paradigm requires an intact neurotransmitter system to activate CREB protein which is critical for memory retrieval and consolidation (Edelmann and Lessmann, 2018). Also, specific inflammatory markers, including TNF-α, have been reported to enhance insulin resistance and further impair the activation of CREB (Verdile et al., 2015). In the current study, the administration of myrtenal significantly restored the levels of neurotransmitters, consequently enhancing spatial memory in the treated animals. This finding indicates that myrtenal enhanced the neurotransmitter patency of local neuronal circuits and enabled the activation of CREB protein. Additionally, the increased levels of TNF-α (a pro-inflammatory cytokine) in the brains of mice treated with streptozotocin indicated an increased level of inflammation (González-Burgos and Gómez-Serranillos, 2012). However, the administration of myrtenal mitigated oxido-inflammatory stress by decreasing lipid peroxidation, nitric oxide levels, and concentrations of TNF-α via upregulation of the activities of endogenous antioxidants in the mice brain. This action protects the brain from oxidative damage and improves spatial memory performance (Kouhestani et al., 2018). The histopathological result from this study further substantiates the potential of myrtenal to improve the structural integrity of neuronal cells in the hippocampus, possibly by modulating the cholinergic system, improving antioxidant activities, and ameliorating inflammation in streptozotocin-treated mice brains (Tancheva et al., 2020; Xourgia et al., 2019) (Fig. 9).
Fig. 9.
Schematic representation of the possible underlying mechanism of memory enhancement by myrtenal.
6. Conclusion
Data from the present study demonstrates that the administration of streptozotocin impairs spatial memory in mice and induces alterations in markers of oxido-inflammatory response, cholinergic function, histoarchitecture, and neurotransmitter levels in the hippocampus. However, the administration of myrtenal enhances spatial memory performance in streptozotocin-demented mice by improving the activities of endogenous antioxidant enzymes to protect the brain from oxido-inflammatory stress. Also, we show that treatment with myrtenal restores cholinergic function and stabilizes the homeostasis of neurotransmitters in streptozotocin-demented mice. Based on the latitude of neuro-biochemical factors considered in the current study, the authors infer that myrtenal plays a crucial role in modulating memory signalling. Therefore, it is conceivable that the consumption of fruits rich in myrtenal may be beneficial for the treatment of patients living with dementia associated with Alzheimer's disease.
Funding
No external fund was received for this work.
CRediT authorship contribution statement
Isaac Oluwatobi Akefe: Conducted experiments, Data collection, statistical analysis, data interpretation, figures, initial draft. Victoria Aderonke Adegoke: Conducted experiments, Data collection, statistical analysis, data interpretation, figures, initial draft. Ibrahim Yusuf Lamidi: Conducted experiments, data interpretation, revised manuscript. Matthew Phillip Ameh: Conducted experiments, data interpretation, revised manuscript. Enokela Shaibu Idoga: Conducted experiments, data interpretation, revised manuscript. Simon Azubuike Ubah: Conducted experiments, data interpretation, revised manuscript. Itopa Etudaye Ajayi: Administered the project, Interpretation, final revision .
Declaration of competing interest
The authors declare that they have no conflict of interest.
References
- Alvarez E.O., Beauquis J., Revsin Y., Banzan A.M., Roig P., De Nicola A.F., Saravia F. Cognitive dysfunction and hippocampal changes in experimental type 1 diabetes. Behav. Brain Res. 2009;198(1):224–230. doi: 10.1016/j.bbr.2008.11.001. [DOI] [PubMed] [Google Scholar]
- Babu L.H., Perumal S., Balasubramanian M.P. Myrtenal, a natural monoterpene, down-regulates TNF-α expression and suppresses carcinogen-induced hepatocellular carcinoma in rats. Mol. Cell. Biochem. 2012;369(1–2):183–193. doi: 10.1007/s11010-012-1381-0. [DOI] [PubMed] [Google Scholar]
- Begum S., Ali M., Gul H., Ahmad W., Alam S., Khan M., et al. In vitro enzyme inhibition activities of Myrtus communis L. Afr. J. Pharm. Pharmacol. 2012;6:1083–1087. [Google Scholar]
- Biessels G.-J., Kamal A., Urban I.J., Spruijt B.M., Erkelens D.W., Gispen W.H. Water maze learning and hippocampal synaptic plasticity in streptozotocin-diabetic rats: effects of insulin treatment. Brain Res. 1998;800(1):125–135. doi: 10.1016/s0006-8993(98)00510-1. [DOI] [PubMed] [Google Scholar]
- Bloom G.S. Amyloid-β and tau: the trigger and bullet in Alzheimer disease pathogenesis. JAMA Neurol. 2014;71(4):505–508. doi: 10.1001/jamaneurol.2013.5847. [DOI] [PubMed] [Google Scholar]
- Buccellato F.R., D'Anca M., Fenoglio C., Scarpini E., Galimberti D. Role of oxidative damage in Alzheimer's disease and neurodegeneration: from pathogenic mechanisms to biomarker discovery. Antioxidants. 2021;10(9):1353. doi: 10.3390/antiox10091353. https://pubmed.ncbi.nlm.nih.gov/34572985 Retrieved from. doi:10.3390/antiox10091353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Casadesus G., Milliken E.L., Webber K.M., Bowen R.L., Lei Z., Rao C.V., et al. Increases in luteinizing hormone are associated with declines in cognitive performance. Mol. Cell. Endocrinol. 2007;269(1–2):107–111. doi: 10.1016/j.mce.2006.06.013. http://europepmc.org/abstract/MED/17376589 Retrieved from. doi.org/10.1016/j.mce.2006.06.013. [DOI] [PubMed] [Google Scholar]
- Cho S., Nuijten E., Shewfelt R.L., Kays S.J. Aroma chemistry of African Oryza glaberrima and Oryza sativa rice and their interspecific hybrids. J. Sci. Food Agric. 2014;94(4):727–735. doi: 10.1002/jsfa.6329. [DOI] [PubMed] [Google Scholar]
- DeTure M.A., Dickson D.W. The neuropathological diagnosis of Alzheimer's disease. Mol. Neurodegener. 2019;14(1) doi: 10.1186/s13024-019-0333-5. https://pubmed.ncbi.nlm.nih.gov/31375134 32-32. Retrieved from. doi:10.1186/s13024-019-0333-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dragomanova S., Tancheva L., Georgieva M. A review: Biological activity of myrtenal and some myrtenal-containing medicinal plant essential oils. 2018. 2018;5(2):22–33. doi: 10.14748/ssp.v5i2.5614. [DOI] [Google Scholar]
- Dudman J.T., Eaton M.E., Rajadhyaksha A., Macías W., Taher M., Barczak A., et al. Dopamine D1 receptors mediate CREB phosphorylation via phosphorylation of the NMDA receptor at Ser897–NR1. J. Neurochem. 2003;87(4):922–934. doi: 10.1046/j.1471-4159.2003.02067.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edelmann E., Lessmann V. Dopaminergic innervation and modulation of hippocampal networks. Cell Tissue Res. 2018;373(3):711–727. doi: 10.1007/s00441-018-2800-7. [DOI] [PubMed] [Google Scholar]
- Ellman G.L. Tissue sulfhydryl groups. Arch. Biochem. Biophys. 1959;82(1):70–77. doi: 10.1016/0003-9861(59)90090-6. Retrieved from, doi:10.1016/0003-9861(59)90090-6. [DOI] [PubMed] [Google Scholar]
- Fracassi A., Marcatti M., Zolochevska O., Tabor N., Woltjer R., Moreno S., Taglialatela G. Oxidative damage and antioxidant response in frontal cortex of demented and nondemented individuals with Alzheimer's neuropathology. J. Neurosci. 2021;41(3):538–554. doi: 10.1523/jneurosci.0295-20.2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- González-Burgos E., Gómez-Serranillos M.P. Terpene compounds in nature: a review of their potential antioxidant activity. Curr. Med. Chem. 2012;19(31):5319–5341. doi: 10.2174/092986712803833335. [DOI] [PubMed] [Google Scholar]
- Green L.C., Wagner D.A., Glogowski J., Skipper P.L., Wishnok J.S., Tannenbaum S.R. Analysis of nitrate, nitrite, and [15N]nitrate in biological fluids. Anal. Biochem. 1982;126(1):131–138. doi: 10.1016/0003-2697(82)90118-x. [DOI] [PubMed] [Google Scholar]
- Ishida T., Toyota M., Asakawa Y. Terpenoid biotransformation in mammals. V. Metabolism of (+)-citronellal, (+-)-7-hydroxycitronellal, citral, (-)-perillaldehyde, (-)-myrtenal, cuminaldehyde, thujone, and (+-)-carvone in rabbits. Xenobiotica. 1989;19(8):843–855. doi: 10.3109/00498258909043145. [DOI] [PubMed] [Google Scholar]
- Kawai T., Oda K., Funao N., Nishimura A., Matsumoto Y., Mizokami Y., et al. Vonoprazan prevents low-dose aspirin-associated ulcer recurrence: randomised phase 3 study. Gut. 2018;67(6):1033–1041. doi: 10.1136/gutjnl-2017-314852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kouhestani S., Jafari A., Babaei P. Kaempferol attenuates cognitive deficit via regulating oxidative stress and neuroinflammation in an ovariectomized rat model of sporadic dementia. Neural. Regen. Res. 2018;13(10):1827–1832. doi: 10.4103/1673-5374.238714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumar A., Singh N. Inhibitor of Phosphodiestearse-4 improves memory deficits, oxidative stress, neuroinflammation and neuropathological alterations in mouse models of dementia of Alzheimer's Type. Biomed. Pharmacother. 2017;88:698–707. doi: 10.1016/j.biopha.2017.01.059. [DOI] [PubMed] [Google Scholar]
- Langa K.M., Plassman B.L., Wallace R.B., Herzog A.R., Heeringa S.G., Ofstedal M.B., et al. The aging, Demographics, and memory study: study design and methods. Neuroepidemiology. 2005;25(4):181–191. doi: 10.1159/000087448. [DOI] [PubMed] [Google Scholar]
- Li Y., Sun H., Chen Z., Xu H., Bu G., Zheng H. Implications of GABAergic neurotransmission in Alzheimer's disease. Front. Aging Neurosci. 2016;8(31) doi: 10.3389/fnagi.2016.00031. https://www.frontiersin.org/article/10.3389/fnagi.2016.00031 Retrieved from. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lowe R.F., Russell M.F., Southwell I.A., Day J. Astartea, a new source of (+)-(1S,5R)-Myrtenal. J. Essent. Oil Res. 2005;17(6):683–685. doi: 10.1080/10412905.2005.9699032. Retrieved from. [DOI] [Google Scholar]
- Moraghebi F. 2013. Introduction of Mirtenal as an Indicator Component in Essential Oil of Cuminum Cyminum Isfahan Variety. [Google Scholar]
- Ohkawa H., Ohishi N., Yagi K. Assay for lipid peroxides in animal tissues by thiobarbituric acid reaction. Anal. Biochem. 1979;95(2):351–358. doi: 10.1016/0003-2697(79)90738-3. [DOI] [PubMed] [Google Scholar]
- Pagel P., Blome J., Wolf H.U. High-performance liquid chromatographic separation and measurement of various biogenic compounds possibly involved in the pathomechanism of Parkinson's disease. J. Chromatogr. B Biomed. Sci. Appl. 2000;746(2):297–304. doi: 10.1016/s0378-4347(00)00348-0. [DOI] [PubMed] [Google Scholar]
- Paxinos G., Watson C. Edition t. Elsevier Press; New York: 2007. The Rat Brain in Stereotaxic Co-ordinates. [Google Scholar]
- Peña-Bautista C., Álvarez-Sánchez L., Ferrer I., López-Nogueroles M., Cañada-Martínez A.J., Oger C., et al. Lipid peroxidation assessment in preclinical Alzheimer disease diagnosis. Antioxidants. 2021;10(7):1043. doi: 10.3390/antiox10071043. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC8300760/ Retrieved from. doi:10.3390/antiox10071043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Plassman B.L., Langa K.M., McCammon R.J., Fisher G.G., Potter G.G., Burke J.R.…Wallace R.B. Incidence of dementia and cognitive impairment, not dementia in the United States. Ann. Neurol. 2011;70(3):418–426. doi: 10.1002/ana.22362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qi C.-C., Chen X.-X., Gao X.-R., Xu J.-X., Liu S., Ge J.-F. Impaired learning and memory ability induced by a bilaterally hippocampal injection of streptozotocin in mice: involved with the Adaptive changes of synaptic plasticity. Front. Aging Neurosci. 2021;13(87) doi: 10.3389/fnagi.2021.633495. https://www.frontiersin.org/article/10.3389/fnagi.2021.633495 Retrieved from. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramezani M., Darbandi N., Khodagholi F., Hashemi A. Myricetin protects hippocampal CA3 pyramidal neurons and improves learning and memory impairments in rats with Alzheimer's disease. Neural. Regen. Res. 2016;11(12):1976–1980. doi: 10.4103/1673-5374.197141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Serrano-Pozo A., Frosch M.P., Masliah E., Hyman B.T. Neuropathological alterations in Alzheimer disease. Cold Spring Harbor Perspect. Med. 2011;1(1) doi: 10.1101/cshperspect.a006189. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC3234452/ a006189-a006189. Retrieved from. doi:10.1101/cshperspect.a006189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shen K., Ji L.L., Chen Y., Yu Q., Wang Z.T. Influence of glutathione levels and activity of glutathione-related enzymes in the brains of tumor-bearing mice. Biosci. Trends. 2011;5 1:30–37. doi: 10.5582/bst.2011.v5.1.30. [DOI] [PubMed] [Google Scholar]
- Small D.M. Dopamine adaptations as a common pathway for neurocognitive impairment in diabetes and obesity: a neuropsychological perspective. Front. Neurosci. 2017;11:134. doi: 10.3389/fnins.2017.00134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smigielski K., Raj A., Krosowiak K., Gruska R. Chemical composition of the essential oil of Lavandula angustifolia cultivated in Poland. J. Essent. Oil Bear. Plants. 2009;12(3):338–347. doi: 10.1080/0972060X.2009.10643729. Retrieved from. [DOI] [Google Scholar]
- Stephan B.C., Wells J.C., Brayne C., Albanese E., Siervo M. Increased fructose intake as a risk factor for dementia. J. Gerontol. A Biol. Sci. Med. Sci. 2010;65(8):809–814. doi: 10.1093/gerona/glq079. [DOI] [PubMed] [Google Scholar]
- Stranahan A.M., Lee K., Mattson M.P. Central mechanisms of HPA axis regulation by voluntary exercise. NeuroMolecular Med. 2008;10(2):118–127. doi: 10.1007/s12017-008-8027-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suvarna K.S., Layton C., Bancroft J.D. Elsevier Health Sciences; 2018. Bancroft's Theory and Practice of Histological Techniques E-Book. [Google Scholar]
- Tancheva L.P., Lazarova M.I., Alexandrova A.V., Dragomanova S.T., Nicoletti F., Tzvetanova E.R., et al. Neuroprotective mechanisms of three natural antioxidants on a rat model of Parkinson's disease: a comparative study. Antioxidants. 2020;9(1):49. doi: 10.3390/antiox9010049. https://www.mdpi.com/2076-3921/9/1/49 Retrieved from. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tönnies E., Trushina E. Oxidative stress, synaptic dysfunction, and Alzheimer's disease. J. Alzheim. Dis. : JAD. 2017;57(4):1105–1121. doi: 10.3233/JAD-161088. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC5409043/ Retrieved from. doi:10.3233/JAD-161088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- National Research Council Committee for the Update of the Guide for the, C., & Use of Laboratory, A . Guide for the Care and Use of Laboratory Animals. National Academies Press (US). Copyright © 2011, National Academy of Sciences; Washington (DC): 2011. The National Academies Collection: reports funded by National Institutes of Health. [Google Scholar]
- Venkatachalam S., Boobathi L., Balasubramanian M.P. Salubrious Therapeutic efficacy of Myrtenal on Colon Carcinoma induced by 1, 2-Dimethylhydrazine studied in experimental albino rats. Res. J. Pharmacol. Pharmacodyn. 2014;6(3):146–152. [Google Scholar]
- Verdile G., Keane K.N., Cruzat V.F., Medic S., Sabale M., Rowles J., et al. Mediators of inflammation, 2015; 2015. Inflammation and Oxidative Stress: the Molecular Connectivity between Insulin Resistance, Obesity, and Alzheimer's Disease. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xourgia E., Papazafiropoulou A., Melidonis A. Antidiabetic treatment on memory and spatial learning: from the pancreas to the neuron. World J. Diabetes. 2019;10(3):169–180. doi: 10.4239/wjd.v10.i3.169. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC6422855/ Retrieved from. doi:10.4239/wjd.v10.i3.169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang J., Yakovlieva L., de Haan B.J., de Vos P., Minnaard A.J., Witte M.D., Walvoort M.T.C. Selective modification of streptozotocin at the C3 position to improve its bioactivity as antibiotic and reduce its cytotoxicity towards insulin-producing β cells. Antibiotics. 2020;9(4):182. doi: 10.3390/antibiotics9040182. https://www.mdpi.com/2079-6382/9/4/182 Retrieved from. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu H., Rockhold R.W., Baker R.C., Kramer R.E., Ho I.K. Effects of single or repeated dermal exposure to methyl parathion on behavior and blood cholinesterase activity in rats. J. Biomed. Sci. 2001;8(6):467–474. doi: 10.1007/bf02256609. [DOI] [PubMed] [Google Scholar]