Abstract
Akabane virus (AKAV), belonging to the genus Orthobunyavirus and family Peribunyaviridae, causes reproductive and congenital abnormalities in ruminants. Its envelope glycoprotein Gc is a neutralizing antigen, on which at least five distinct antigenic regions have been identified. We attempted to identify the domains using truncated recombinant AKAV Gc proteins expressed in Escherichia coli and monoclonal antibodies (mAbs) with AKAV-neutralizing activity. Dot blot analysis revealed that amino acid positions 1–97 and 189–397 (Gc1–97 and Gc189–397) in the truncated recombinant proteins reacted with the mAbs. Additionally, AKAV was neutralized by sera from mice immunized with these recombinant proteins. The results suggested that the two domains contain neutralizing epitopes and could be potential subunit vaccines against AKAV.
Keywords: Akabane virus, envelope glycoprotein Gc, neutralizing antibody, neutralizing domain, subunit vaccine
Akabane virus (AKAV), a member of the Simbu serogroup, genus Orthobunyavirus, and family Peribunyaviridae, is primarily transmitted by species of biting midges in the genus Culicoides. It is widely distributed throughout Australia, Southeast Asia, East Asia, the Middle East, and Africa [16]. Prototypical AKAV, the JaGAr39 strain, was first isolated from Aedes vexans and Culex tritaeniorhynchus mosquitoes in central Japan in 1959 [10]; however, its pathogenesis was unknown at the time. OBE-1, an AKAV strain isolated from a naturally infected bovine fetus, was identified during a major outbreak in Japan between 1972 and 1974 [6] and is currently used in vaccination. Although pregnant cows, ewes, and goats infected with AKAV do not present any clinical signs by experimental infection, in utero infections result in abortion, premature birth, stillbirth, and congenital deformities such as arthrogryposis-hydranencephaly syndrome. AKAV has caused heavy economic losses in the Japanese livestock industry. However, due to the widespread use of a live vaccine based on an attenuated OBE-1 strain, only sporadic cases have been reported [5].
Members of the genus Orthobunyavirus are enveloped, and their genomes consist of three segments of single-stranded negative-sense RNA: L (large), M (medium), and S (small) [13]. Genetic studies have shown that the L RNA segment encodes an RNA-dependent RNA polymerase, whereas the S RNA segment encodes a nucleoprotein (N) and a nonstructural protein (NSs). The M RNA segment encodes two envelope glycoproteins (Gn and Gc) and a nonstructural protein (NSm). Gc is responsible for viral neutralization and attachment to mammalian cells, whereas Gn may help attachment to insect cells [7]. The NSm protein is involved in virion assembly [14].
Glycoprotein Gc, the AKAV-neutralizing antigen, has been reported to contain at least five antigenic regions, indicated by a competitive binding method using neutralizing monoclonal antibodies (mAbs); however, the specific regions containing these epitopes are unknown [1, 18]. This study aimed to identify the neutralizing domain using dot blot analysis with truncated recombinant proteins expressed in Escherichia coli and neutralizing mAbs. Furthermore, we examined whether mice immunized with the identified domain produced neutralizing antibodies.
For transcription, the purified recombinant proteins (100 µg/ml) were blotted onto a nitrocellulose membrane (pore size, 0.45 µm; GE Healthcare, Chicago, IL, USA) using the Mini-PROTEAN II Multiscreen Apparatus (Bio-Rad Laboratories, Hercules, CA, USA), and a recombinant trigger factor (TF) protein as the negative control. The membrane was immersed in phosphate-buffered saline (PBS) containing 5% skim milk for blocking. Subsequently, the membrane was rotated 90° and replaced on the blotting apparatus described above. The supernatant from hybridoma cultures was used as the source of mAbs [1]. Each mAb reacted to a different antigenic region of the AKAV Gc protein as follows: mAbs 1B3 and 4F9 reacted to antigenic region A; mAb 1D3 reacted to antigenic region B; mAb 3A5 reacted to antigenic region C; mAbs 4A10, 4D4, 5G4, 5E5, and 2F1 reacted to antigenic region D; and mAb 9G10 reacted to antigenic region E. All mAbs, except mAb 2F1, exhibited neutralizing activity. The membrane was immersed in mAb (from hybridoma culture supernatant), diluted 10-fold with PBS containing 5% skim milk, allowed to stand for 1 hr, and then washed three times with PBS. Furthermore, the membrane was incubated with horseradish peroxidase-conjugated goat antibody against mouse IgG (Invitrogen Corp., Carlsbad, CA, USA) diluted 1,000-fold with PBS containing 5% skim milk at room temperature for 1 hr, followed by washing with PBS three times. Color was developed using 3,3′-diaminobenzidine tetrahydrochloride (Fujifilm Wako Pure Chemical Corp., Osaka, Japan) as a substrate.
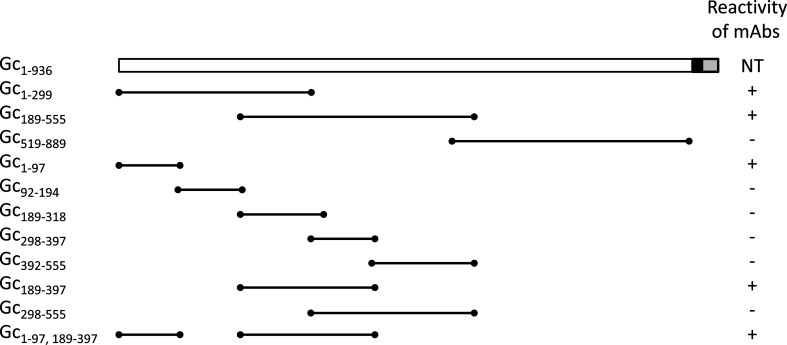
Eleven truncated recombinant AKAV Gc proteins were expressed using the E. coli expression system to identify the epitope for viral neutralization (Fig. 1). The expression of histidine (His)- and TF-tagged fusion proteins was performed using the pCold-TF system (Takara Bio Inc., Kusatsu, Japan). pRF42/M containing the sequence of the M segment from the OBE-1 strain was used as a template [8]. DNA fragments were polymerase chain reaction (PCR)-amplified using KOD FX polymerase (TOYOBO Co., Ltd., Osaka, Japan) and the primers listed in Supplementary Table 1. The PCR products were purified using the Wizard SV gel and PCR clean-up system (Promega, Madison, WI, USA). The purified DNA fragments were digested with restriction enzymes and cloned into a pCold-TF expression vector that had been predigested with the same enzymes (Supplementary Table 1). Gc1–299, Gc298–397, Gc298–555, and Gc519–889 were cloned using the internal restriction enzyme sites (HindIII) within the amplified fragments. Two PCR fragments were fused using overlap extension PCR to express a fusion protein. The constructs were transformed into E. coli JM109 cells (TOYOBO), and positive clones were confirmed by sequencing (BigDye Terminator v3.1 cycle sequencing kit and ABI PRISM 3130XL, Thermo Fisher Scientific Inc., Waltham, MA, USA). Recombinant protein expression was performed according to the manufacturer’s protocol for the pCold-TF system. His-tagged recombinant proteins were purified using Ni-NTA agarose (Qiagen Inc., Valencia, CA, USA) according to the manufacturer’s protocol. The fractions containing recombinant protein were pooled, dialyzed with PBS at 4°C, and subsequently used for dot blot analysis and immunization. The purified protein concentration was determined using a Pierce BCA Protein Assay Kit (Pierce Biotechnology, Rockford, IL, USA). Except for the transmembrane region, the Gc protein was divided into three regions: the N-terminal, middle, and C-terminal regions. Dot blot analysis of the three recombinant proteins and mAbs showed that mAbs reacted to those with the N-terminal region (Gc1–299) and middle region (Gc189–555), but not the C-terminal region (Gc519–889) (Fig. 2). mAb 1D3, which recognizes the antigenic region B of AKAV Gc, reacted with the N-terminal region of AKAV Gc. mAbs 4A10, 4D4, 5G4, and 2F1 recognized antigenic region D, whereas 9G10 recognized antigenic region E; all five mAbs reacted with the middle region of AKAV Gc. mAbs that recognized antigenic regions A and C did not react with any of the recombinant proteins expressed by E. coli. Next, we divided the N-terminal and middle regions into five regions (Gc1–97, Gc92–194, Gc189–318, Gc298–397, and Gc392–555). mAb 1D3 reacted with Gc1–97 recombinant protein. mAbs 4A10, 4D4, 5G4, 2F1, and 9G10 did not react with the five truncated recombinant proteins. These results suggested that Gc1–97 contains an active domain in the Gc1–299 region; however, it was not possible to identify the active domain in the Gc189–555 region. We generated two recombinant proteins (Gc189–397 and Gc298–555) from the middle region. mAbs 4A10, 4D4, 5G4, and 9G10 reacted with the Gc189–397 recombinant protein, whereas mAb 2F1 did not react with either recombinant protein. Furthermore, we generated a fusion protein (Gc1–97, 189–397) consisting of two recombinant proteins (Gc1–97 and Gc189–397). Five mAbs, 1D3, 4A10, 4D4, 5G4, and 9G10, reacted with the fusion protein.
Fig. 1.
Schematic representation of recombinant proteins. Black and gray boxes indicate transmembrane regions and cytoplasmic domain, respectively. Numbers indicate positions of amino acids in glycoprotein Gc. The reactivity to mAb is shown on the right: + (positive), −(negative), NT (not tested). mAb: monoclonal antibodies
Fig. 2.
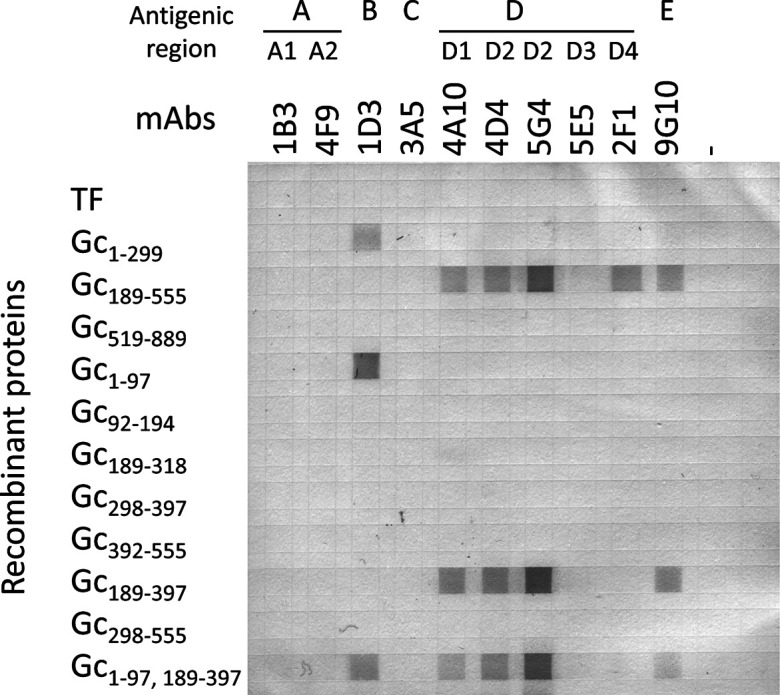
Dot blot analysis. Recombinant proteins are dotted on a nitrocellulose membrane. The membrane was reacted to mAbs. Antigenic region of mAbs [1] are indicated above the clone number of mAbs. mAb: monoclonal antibodies. TF: trigger factor.
The animal experiments in this study were approved by the Animal Ethics Committee of the National Institute of Animal Health, Tsukuba, Ibaraki, Japan (12-097). Four groups of three 5-week-old female BALB/c mice (Japan SLC, Inc., Hamamatsu, Japan) were immunized with each recombinant protein to examine the immunogenicity of the recombinant proteins. Each mouse was injected subcutaneously with 100 μl (100 μg) of each recombinant protein sample emulsified in Freund’s complete adjuvant (Difco Laboratories Inc., Franklin Lakes, NJ, USA). Three mice were injected with recombinant TF protein containing the adjuvant as a control. At two and four weeks, the mice were boosted at the same injection sites with the same dose of the same protein sample emulsified in Freund’s incomplete adjuvant (Difco Laboratories Inc.). One week after final boosting, blood was collected from each mouse, and the sera were assessed for an antibody response against TF protein using ELISA; anti-TF antibodies were detected. ELISA plate wells (MaxiSorp Nunc-Immuno plate; Thermo Fisher Scientific, Roskilde, Denmark) were coated with 10 µg/ml recombinant TF protein. After incubation and washing with PBS containing 0.1% Tween 20, the wells were blocked with 5% bovine serum albumin in PBS (pH 7.4; blocking buffer). The plates were washed, and 100 μl serum, diluted 1:100 with a dilution buffer (blocking buffer containing 0.1% Tween 20), was applied to each well. After incubation and washing, the bound antibodies were detected using horseradish peroxidase-conjugated secondary immunoglobulin G antibody and visualized with 3,3′,5,5′- tramethylbenzidine substrate (SeraCare Life Sciences, Inc., Milford, MA, USA). The mice were immunized with two recombinant proteins (Gc1–97 and Gc189–397) and one fusion protein (Gc1–97, 189–397); the mice immunized with TF were the negative control. Antibodies against TF were confirmed by ELISA, and neutralization tests against the AKAV OBE-1 strain [2] were subsequently performed using sera from the immunized mice. Serial two-fold dilutions of murine serum were mixed with an equal volume of maintenance medium containing 200 TCID50/0.05 ml AKAV OBE-1 strain and incubated at 37°C for 1 hr. The virus–serum mixtures were inoculated in 0.1 ml volumes of HmLu-1 cells in a 96-well plate [9], incubated at 37°C for five days, and then examined for cytopathic effects (CPEs). All samples were tested in quadruplicate, and the antibody titer was expressed as the reciprocal of the highest serum dilution showing complete CPE inhibition. Neutralization tests showed that the neutralizing antibody titers of murine sera immunized with the recombinant proteins, including the N-terminal and middle regions, were 42.7-fold and 74.7-fold, respectively (Fig. 3). Sera from mice immunized with the fusion protein showed a 42.7-fold increase in neutralizing activity. In contrast, the neutralizing antibody titer of the serum from mice immunized with the TF recombinant protein (control) was four-fold.
Fig. 3.
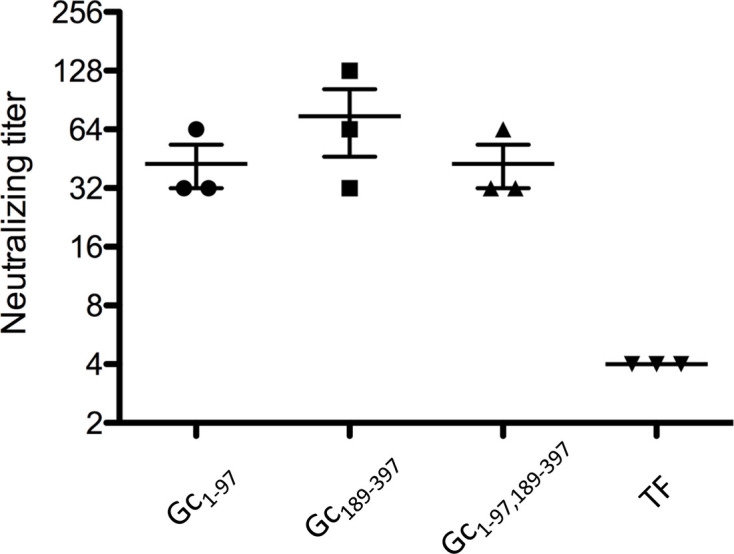
Neutralizing tests. Dots represents the titer of individual serum. Center bars indicate means; upper and lower bars indicate standard error of the mean. TF: trigger factor.
Roman-Sosa et al. [12] reported that the 234 amino acid residues of the N-terminal of Schmallenberg virus (SBV) Gc (Gc amino) contained a neutralizing epitope in SBV, which, like AKAV, belongs to the Simbu serogroup. Similarly, in AKAV, the neutralizing mAb 1D3 reacted with the recombinant protein of the N-terminal region (Gc1–299). Further analysis suggested a neutralizing epitope at positions 1–97. Although it has been reported that cross-immunity cannot be acquired between SBV and AKAV [3, 17], the presence of a neutralizing epitope in the N-terminal region of AKAV and SBV suggests that it may be an important site involved in viral neutralization common to the Simbu serogroup.
Furthermore, neutralizing mAbs recognizing different antigenic regions reacted with the recombinant protein in the middle (189–555) region, suggesting the existence of a different neutralizing epitope. Different mAbs reacted with recombinant proteins in the N-terminal (1–299) or middle (189–555) regions, suggesting that the region common to both recombinant proteins (189–299) lacked an antigenic site. The regions Gc189–318, Gc298–397, and Gc392–555 showed no reactivity with mAbs (Fig. 2). Thus, these three fragments were restructured to obtain two fragments, Gc189–397 and Gc298–555, for further examination. It was observed that the mAbs reacted with Gc189–397; although the common part of this region does not contain an epitope, it might be necessary for antigen recognition. These results suggested that there were at least two neutralizing epitopes in AKAV. As the mAb has been reported to react with SBV Δamino [12], it is not specific to AKAV and may be common to the Simbu serogroup.
We produced recombinant proteins using the E. coli expression system; however, Roman-Sosa et al. utilized a mammalian expression system [12]. Furthermore, mice vaccinated with SBV Gc amino recombinant proteins expressed by E. coli developed no detectable immune response and were not protected against viral challenge [17]. In this study, mAbs recognized the antigenic regions A and C of AKAV Gc, but showed no reactivity with any recombinant proteins expressed by E. coli, and mAb 5E5, one of the mAbs that recognized antigenic region D, did not show reactivity with them either (Fig. 2). However, the mAbs might recognize posttranslational modifications and conformation because the two domains we identified contained N-linked glycosylation sites (positions 23 and 220). However, dot blot analysis indicated that the two recombinant proteins, Gc1–97 and Gc189–397, reacted with mAbs. Thus, our results suggested that immunization with recombinant proteins expressed by E. coli induces neutralizing antibodies in a mouse model. To express the recombinant proteins, we utilized the pCold-TF system [11, 15]. In this system, E. coli expressed recombinant proteins fused to the TF chaperoning protein during culture at low temperatures. Culturing at low temperatures and fusion with a TF may have affected the stability or folding of the recombinant proteins [4, 11]. As the recombinant protein became shorter, the reactivity between mAb 2F1 and the recombinant protein disappeared (Fig. 2), suggesting that the epitope of mAb 2F1 was involved in the conformation of the Gc protein.
In this study, two recombinant proteins containing the N-terminal (1–97) and middle (189–397) regions of AKAV glycoprotein Gc reacted with neutralizing mAbs, and neutralizing antibodies were induced in sera from mice immunized with these recombinant proteins and their fused protein products. The results suggested that the glycoprotein Gc domains contain AKAV-neutralizing epitopes. These recombinant proteins could have potential applications as vaccine antigens. Further research is needed to determine whether the immunity of these recombinant proteins establishes protection against infection in ruminants, such as cattle.
CONFLICT OF INTEREST
The authors declare that they have no conflicts of interest.
Supplementary Material
Acknowledgments
ACKNOWLEDGMENTS. This study was supported by a research project for Innovative Technologies for Vaccine Development of the Ministry of Agriculture, Forestry, and Fisheries of Japan (Project number 11105375). We thank Katsuyoshi Matsuura of the National Institute of Animal Health for preparing hybridoma supernatants.
REFERENCES
- 1.Akashi H., Inaba Y.1997. Antigenic diversity of Akabane virus detected by monoclonal antibodies. Virus Res. 47: 187–196. doi: 10.1016/S0168-1702(96)01415-3 [DOI] [PubMed] [Google Scholar]
- 2.Akashi H., Kaku Y., Kong X., Pang H.1997. Antigenic and genetic comparisons of Japanese and Australian Simbu serogroup viruses: evidence for the recovery of natural virus reassortants. Virus Res. 50: 205–213. doi: 10.1016/S0168-1702(97)00071-3 [DOI] [PubMed] [Google Scholar]
- 3.Hechinger S., Wernike K., Beer M.2013. Evaluating the protective efficacy of a trivalent vaccine containing Akabane virus, Aino virus and Chuzan virus against Schmallenberg virus infection. Vet. Res. (Faisalabad) 44: 114. doi: 10.1186/1297-9716-44-114 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hoffmann A., Bukau B., Kramer G.2010. Structure and function of the molecular chaperone Trigger Factor. Biochim. Biophys. Acta 1803: 650–661. doi: 10.1016/j.bbamcr.2010.01.017 [DOI] [PubMed] [Google Scholar]
- 5.Inaba Y., Matsumoto M.1990. Akabane virus. pp. 467−480. In: Virus Infections of Ruminants (Dinter, Z. and Morein, B. eds.), Elsevier Science Publishers, Amsterdam. [Google Scholar]
- 6.Kurogi H., Inaba Y., Takahashi E., Sato K., Omori T., Miura Y., Goto Y., Fujiwara Y., Hatano Y., Kodama K., Fukuyama S., Sasaki N., Matumoto M.1976. Epizootic congenital arthrogryposis-hydranencephaly syndrome in cattle: isolation of Akabane virus from affected fetuses. Arch. Virol. 51: 67–74. doi: 10.1007/BF01317835 [DOI] [PubMed] [Google Scholar]
- 7.Ludwig G. V., Israel B. A., Christensen B. M., Yuill T. M., Schultz K. T.1991. Role of La Crosse virus glycoproteins in attachment of virus to host cells. Virology 181: 564–571. doi: 10.1016/0042-6822(91)90889-J [DOI] [PubMed] [Google Scholar]
- 8.Ogawa Y., Sugiura K., Kato K., Tohya Y., Akashi H.2007. Rescue of Akabane virus (family Bunyaviridae) entirely from cloned cDNAs by using RNA polymerase I. J. Gen. Virol. 88: 3385–3390. doi: 10.1099/vir.0.83173-0 [DOI] [PubMed] [Google Scholar]
- 9.Okumura H.1968. Spontaneous malignant transformation of hamster lung cells in tissue culture. pp. 292−298. In: Cancer Cells in Culture (Katsuta, H. ed.), University of Tokyo Press, Tokyo. [Google Scholar]
- 10.Oya A., Okuno T., Ogata T., Kobayashii, Matsuyama T.1961. Akabane, a new arbor virus isolated in Japan. Jpn. J. Med. Sci. Biol. 14: 101–108. doi: 10.7883/yoken1952.14.101 [DOI] [PubMed] [Google Scholar]
- 11.Qing G., Ma L. C., Khorchid A., Swapna G. V., Mal T. K., Takayama M. M., Xia B., Phadtare S., Ke H., Acton T., Montelione G. T., Ikura M., Inouye M.2004. Cold-shock induced high-yield protein production in Escherichia coli. Nat. Biotechnol. 22: 877–882. doi: 10.1038/nbt984 [DOI] [PubMed] [Google Scholar]
- 12.Roman-Sosa G., Brocchi E., Schirrmeier H., Wernike K., Schelp C., Beer M.2016. Analysis of the humoral immune response against the envelope glycoprotein Gc of Schmallenberg virus reveals a domain located at the amino terminus targeted by mAbs with neutralizing activity. J. Gen. Virol. 97: 571–580. doi: 10.1099/jgv.0.000377 [DOI] [PubMed] [Google Scholar]
- 13.Schmaljohn C. S., Hooper J. W.2001. Bunyaviridae: the viruses and their replication. pp. 1581−1602. In: Fields Virology, 4th ed. (Knipe, D. M. and Howley, P. M. eds.), Lippincott Williams and Wilkins, Philadelphia. [Google Scholar]
- 14.Shi X., Kohl A., Léonard V. H., Li P., McLees A., Elliott R. M.2006. Requirement of the N-terminal region of orthobunyavirus nonstructural protein NSm for virus assembly and morphogenesis. J. Virol. 80: 8089–8099. doi: 10.1128/JVI.00579-06 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Stoller G., Rücknagel K. P., Nierhaus K. H., Schmid F. X., Fischer G., Rahfeld J. U.1995. A ribosome-associated peptidyl-prolyl cis/trans isomerase identified as the trigger factor. EMBO J. 14: 4939–4948. doi: 10.1002/j.1460-2075.1995.tb00177.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Uema A., Ogawa Y., Akashi H.2016. Akabane virus. pp. 521−532. In: Molecular Detection of Animal Viral Pathogens (Liu, D. ed.), CRC press, Boca Raton. [Google Scholar]
- 17.Wernike K., Aebischer A., Roman-Sosa G., Beer M.2017. The N-terminal domain of Schmallenberg virus envelope protein Gc is highly immunogenic and can provide protection from infection. Sci. Rep. 7: 42500. doi: 10.1038/srep42500 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Yoshida K., Tsuda T.1998. Rapid detection of antigenic diversity of Akabane virus isolates by dot immunobinding assay using neutralizing monoclonal antibodies. Clin. Diagn. Lab. Immunol. 5: 192–198. doi: 10.1128/CDLI.5.2.192-198.1998 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.