Abstract
High-intensity exercise and competition are associated with depressed immune function. Young horses, which participate in high-intensity exercise and competitions, are at increased risk for the development of infectious disease due to depression of immune function. The effects of branched-chain amino acid (BCAA) supplementation on the immune status of young racing horses were evaluated, determining whether BCAA might help to avoid or reduce immune suppression during exercise and competitions. Twenty horses (10 male and 10 female) were treated with BCAA supplementation; another twenty untreated horses (10 male and 10 female) constituted control group. Peripheral blood was collected from each animal and evaluated for lymphocyte subsets, phagocytosis analysis of monocytes and granulocytes, lymphocyte proliferative response, and expression of cytokine-encoding messenger ribonucleic acids (mRNAs). The numbers of CD4+, CD8+, and major histocompatibility complex (MHC) class II+ cells in females of the treated group were significantly higher than those in females of the control group. The lymphocyte proliferative response in female of the treated group also was significantly higher than that in females of the control group. In addition, expression of mRNAs encoding interleukin-1β (IL-1β) and interferon-γ (IFN-γ) in females of the treated group was significantly higher than that in females of the control group. There were no significant differences between males of the treated and control groups. The results of this study indicated the positive effects of BCAA supplementation in counteracting immunosuppression in young female racing horses during and following high-intensity exercise.
Keywords: branched-chain amino acid, immune status, young racing horse
Repetitive cycles and long periods of exercise create stress conditions and can cause immunosuppression [27, 48]. The immune systems of man [34], cattle [8], and rat [5] may be deeply affected by high-intensity exercise or treadmill operating, which can serve as a physical stressor.
In horses, racing and high-intensity exercise contribute to many metabolic and hormonal alterations, with resulting impacts on the immune system. The severity of changes varies according to the intensity, duration, and repetition of exercise, and also are affected by breed and gender differences [11, 25, 33, 44]. Based on alterations of immunological functions, exercise may potentiate or attenuate the symptoms of infectious disease [17, 24].
Moderate exercise reduces the suppression of the immune system compared to the effects seen with high-intensity exercise [36, 39, 40]. Several studies on horses have demonstrated the marked influence of high-intensity exercise on the immune system [30, 54]. Young racing horses attending competitive tournaments are especially at risk from infectious disease [43]. For instance, epidemiological research has shown that the occurrence of upper respiratory tract infection is elevated following high-intensity exercise [34, 54].
To avoid immunosuppression during exercise, the effects of some supplements on high-intensity exercise-induced changes in the immune system were evaluated [55]. For racing horses that run different distances, amino acid supplements such as glutamine, arginine, and sulfur-containing amino acids often have been used [2, 9, 10, 35, 38]. However, previous studies have been shown about limited effects of glutamine [15, 19, 29] and arginine [1, 21, 53] supplements which did not recommend for long time treatment during exercise training. Although some researchers have evaluated the influence of branched-chain amino acids (BCAA) on the immune system [9, 10], many aspects of BCAA and their effects on immune function, especially during the training regime, have received little or no attention. Therefore, this study sought to evaluate the effect of BCAA supplementation, during exercise, on alterations of immune status in young racing horses; this work also evaluated possible gender differences in immune status.
MATERIALS AND METHODS
Animals, diets, and experimental design
Forty young (approximately 1-year-old) clinically healthy Thoroughbred racing horses (20 male, 20 female) from the Miyazaki Yearling Training Farm of the Japan Racing Association (JRA) were used for this research. The 20 male horses were divided into two groups (n=10 each) that were oral administered BCAA at 15 g/day (treated group) or untreated (control group); the 20 female horses also were divided into two groups (n=10 each) that were administered BCAA at 15 g/day (treated group) or untreated (control group). The dose of 15 g/day for the treated horses was adjusted according to the previous research on horses [47]. The treatment was applied in 2017 and 2019, each year for five months, during an interval from December to April. The feeding time was around 6 a.m., before starting exercise. The BCAA ratio for L-valine, L-leucine, and L-isoleucine was 1:2:1, respectively, and was provided by ASKA Animal Health Co. (Tokyo, Japan). All horses in this study were housed in the same training center and trained under the same conditions. The morning exercise was performed between 9:00 to 11:00 a.m., and the exercise protocol was designed with different distances and speed levels (Table 1). The experimental procedures were approved by the Animal Care and Use Committee at University of Miyazaki (2019-001).
Table 1. Exercise protocol during the period of study.
| Month | Walking (m) | Trot (m) | Gallop (m) | Total (m) | Walking machine | Note | |
|---|---|---|---|---|---|---|---|
| October | Middle | 1,600 | 2,000 | 1,000 | 4,600 | ||
| Late | 1,600 | 2,000 | 1,600 | 5,200 | |||
| November | Early | 1,600 | 2,000 | 1,600 | 5,200 | ||
| Middle | 1,600 | 2,000 | 2,000 | 5,600 | |||
| Late | 1,600 | 2,000 | 2,400 | 6,000 | |||
| December | Early | 2,000 | 2,000 | 2,800 | 6,800 | 3,000 m for 30 min (6 km/hr) | Speed up to 600 m/min |
| Late | 2,000 | 2,000 | 2,800 | 6,800 | |||
| January | Early | 2,000 | 1,200 | 2,000 | 5,200 | Speed up to 667 m/min | |
| Late | 2,000 | 1,200 | 2,400 | 5,600 | |||
| February | Early | 2,000 | 1,200 | 2,000 | 5,200 | Speed up to 800 m/min | |
| Late | 2,000 | 1,200 | 2,400 | 5,600 | |||
| March | Early | 2,000 | 1,200 | 2,000 | 5,200 | Speed up to 857 m/min | |
| Late | 2,000 | 1,200 | 2,400 | 5,600 | |||
| April | Early | 2,000 | 1,200 | 2,000 | 5,200 | Speed up to 900 m/min | |
| Late | 2,000 | 1,200 | 2,400 | 5,600 | |||
Sample collection and isolation of peripheral blood mononuclear cells (PBMCs)
Blood samples were obtained by jugular vein puncture and transferred into sterile EDTA-containing tubes; samples were analyzed immediately after transfer. These samplings were performed at the end of each month between 8:00 and 9:00 a.m., before morning exercise. The PBMCs were purified by density gradient centrifugation with Ficoll-Paque PLUS (GE Healthcare UK, Little Chalfont, Buckinghamshire, UK). Red blood cells were removed using NH4Cl lysis buffer according to our previously described article [3]; the remaining cells then were washed with phosphate-buffered saline (PBS) (Fujifilm Wako Pure Chemical Co., Tokyo, Japan).
Lymphocyte subset analyses
PBMCs were suspended in PBS supplemented with 0.5% bovine serum albumin (Nacalai Tesque, Inc., Kyoto, Japan) and 0.05% sodium azide (Fujifilm Wako Pure Chemical Co.) (BSA-PBS). Viable cells (at densities ranging from 1 × 105 to 1 × 106 cells) were specified and counted by trypan blue exclusion test and incubated with fluorescently labeled monoclonal antibodies (mAbs) at 4°C for 1 hr. The stained cells then were washed three times with BSA-PBS and resuspended in BSA-PBS containing propidium iodide (1 µg/ml, Sigma-Aldrich, St. Louis, MO, USA). The relative immunofluorescence intensities were determined by flow cytometry with a FACS CantoTM II system (Becton Dickinson, Franklin Lakes, NJ, USA). Anti-CD4 (200-fold dilution, HB61A, Monoclonal Antibody Center at Washington State University, Pullman, WA, USA), anti-CD8 (200-fold dilution, HT14A, Monoclonal Antibody Center at Washington State University), and anti-MHC class II (200-fold dilution, TH14B, Monoclonal Antibody Center at Washington State University) were used. For fluorescent labeling of mAbs, the FITC labeling kit-NH2 (Dojindo Laboratories, Kumamoto, Japan), HiLyteTM Fluor 555 (F555) labeling kit-NH2 (Dojindo Laboratories), and HiLyteTM Fluor 647 (F647) labeling kit-NH2 (Dojindo Laboratories) were used according to the manufacturer’s instructions. Absolute white blood cell (WBC) numbers were counted using a pocH-100iV Diff (Sysmex, Kobe, Japan). The percentage of lymphocytes in WBCs was measured by Giemsa staining of blood smears. The numbers of cells in each subset were calculated via the following formula: cell densities (cells/µl)=relative population from FACS × percentage of lymphocytes in WBC from smears × WBC numbers.
Phagocytosis assay
Blood sample was centrifuged at 500 g for 10 min at room temperature; the buffy coat then was collected. Red blood cells were removed using NH4Cl lysis buffer; the remaining cells then were washed with PBS. The cells (4.0 × 106 cells/ml) were suspended in RPMI 1640 medium (Fujifilm Wako Pure Chemical Co.) supplemented with 10% fetal calf serum (Sigma-Aldrich) and antibiotics (Fujifilm Wako Pure Chemical Co.). One microliter of a 2.5% FITC-labeled latex bead suspension (1-µm diameter, L1030, Sigma-Aldrich) was added and the mixture was incubated at 37°C for 1 hr in a 5% CO2 humidified atmosphere. Following the incubation, cell-free beads were removed with ice-cold 1 mM EDTA-PBS, and the cells were stained with F647-labeled anti-MHC class II (200-fold dilution, TH14B) and F555 labeled anti-granulocyte (100-fold dilution, HACT39A, Monoclonal Antibody Center at Washington State University) mAbs and then analyzed by flow cytometry with a FACS CantoTM II system. Monocytes and granulocytes were gated according to their relative size (forward scatter) and complexity (side scatter). The phagocytic index was calculated as the percentage of FITC+ TH14B+ cells relative to all TH14B+ cells, or the percentage of FITC+ HACT39A+ cells relative to all HACT39A+ cells.
Immunoproliferation
PBMCs (2 × 105 cells/well) in RPMI1640 medium were plated in triplicate in 96-well flat-bottom plates and stimulated with concanavalin A (Con A, Sigma-Aldrich) at a final concentration of 3.13 μg/ml. Cells were incubated at 37°C for 72 hr in a 5% CO2 humidified atmosphere. Four hr before the end of the culture, 20 μl of 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyl tetrazolium bromide (MTT, 5 mg/ml, Fujifilm Wako Pure Chemical Co.) were added to each well. After 4 hr, the formazan crystals were collected by centrifugation, and then 100 μl of dimethyl sulfoxide were added to each well to dissolve the crystals. The optical density (OD) was measured with a microplate reader (Benchmark Plus, measurement wavelength 570 nm, reference wavelength 610 nm, Bio-Rad, Hercules, CA, USA). Proliferation of lymphocyte cultures were calculated as the stimulation index (SI)=OD of the experimental group divided by OD of the control group.
Analysis of PBMC expression of mRNAs encoding IFN-γ, IL-1β, IL-4, IL-6, IL-8, IL-12, and tumor necrosis factor-α (TNF-α) using real-time PCR
Total RNA was extracted from PBMCs using the RNeasy Mini Kit (Qiagen, Valencia, CA, USA) according to the manufacturer’s protocol. Real-time RT-PCR was performed using a one-step TB Green PrimeScript PLUS RT-PCR Kit (Takara Bio, Tokyo, Japan) according to the manufacturer’s protocol. Real-time PCR primer pairs were designed using Oligo7 software (Molecular Biology Insights, Colorado Springs, CO, USA) and are described in Table 2. Real-time PCR conditions consisted of reverse transcription at 42°C for 5 min and initial PCR activation at 95°C for 10 sec, followed by 40 cycles at 95°C for 5 sec, 57°C for 30 sec and 70°C for 30 sec; the results were used to generate a dissociation curve. The real-time RT-PCR assay was performed using the QuantStudio™ Real-Time PCR system (Applied Biosystems, Carlsbad, CA, USA). The mRNA expression level of each target gene was normalized against that of the gene encoding the housekeeping protein glyceraldehyde phosphate dehydrogenase (GAPDH) in the respective sample. GAPDH expression levels in the sample did not differ significantly among the samples, confirming that GAPDH could be used as the reference gene for real-time PCR. For quantification of target mRNA, a comparative Ct method (2-ΔΔCt method/ Livak method) using GAPDH as an internal control was used. Data were analyzed using QuantStudio™ software (Thermo Fisher Scientific) [31].
Table 2. Primer sequences used for real-time PCR.
| Gene | Primer | Sequences | Accession number | Length(base pairs) |
|---|---|---|---|---|
| GAPDH | F | CTCCTTCTGCTGATGCCCCAA | NM_001163856 | 155 |
| R | GAGTCCCTCCACGATGCCAAA | |||
| IL-1 β | F | ACAAGACTTTCAAACATGCCAT | NM_001317261 | 180 |
| R | TGGCTGCATCACATACATAACCT | |||
| IL-4 | F | CTTACTAGCATGTACCAGCAAC | AF305617 | 143 |
| R | CAGCAAAGGCATCCGCTAC | |||
| IL-6 | F | CACATTAAGTACATCCTCGGCAA | NM_001082496 | 179 |
| R | ATTTTCATCAGGCAGGTCTC | |||
| IL-8 | F | GCATCAAGACGCACTCCAA | AY184956 | 160 |
| R | ACAATAATCTGCACCCACT | |||
| IL-12p40 | F | GCCATTCTCACCTGCTGCTTC | Y11129 | 141 |
| R | GCCACCAGCATGTGAAACGTC | |||
| IFN- γ | F | AAAATAATTCAGAGCCAAATCG | D28520 | 172 |
| R | CCGGAATCTGAATCAGCTTT | |||
| TNF- α | F | AATGCCTTCCAGTCAATCAACCC | NM_001081819 | 172 |
| R | TGTCTGTCAGCTTCACGCCATT |
Statistical analysis
Statistical analysis was performed among groups using a two-tailed one-way analysis of variance (ANOVA); differences were determined using a post hoc Tukey’s honestly significant difference (HSD) test. Pearson’s regression analysis also was used to clarify the relationship between exercise intensity and T cell number. Results are expressed as mean ± standard deviation (SD). P-values <0.05 were considered as statistically significant. Statistical analysis was performed using program R version 3.6.3.
RESULTS
Lymphocyte subset analysis and correlation
In the female treated group, a significant increase was recorded for the number of CD4+ cells (in February P=0.008 and April P=0.0001) and of CD8+ cells (in April P=0.021) than those in the female control group, as shown in Figs. 1 and 2. Additionally, the numbers of MHC class II+ cells in the female treated group in February P=0.002 and April P=0.0001 were significantly higher than those in the female control group, as shown in Fig. 3. Furthermore, according to the correlation data between the exercise distance and the numbers of CD4+ and CD8+ cells, the female treated group exhibited a weak negative correlation (y= −0.79 x + 8987.4, R= −0.05), while the negative correlation in the female control group was stronger (y= −0.14 x + 7405.9, R= −0.31) than that in the treated group.
Fig. 1.
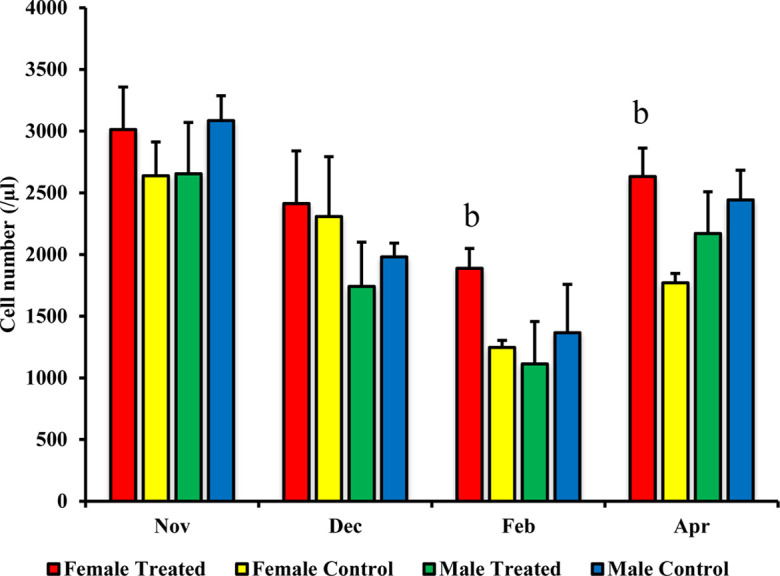
Effect of branched-chain amino acid (BCAA) on male and female CD4+ cell population. The treated group was fed by BCAA supplementation daily and the untreated group was considered a control. The data are presented as mean ± SD. Letters (b: P<0.01 vs. control) indicate significant differences.
Fig. 2.
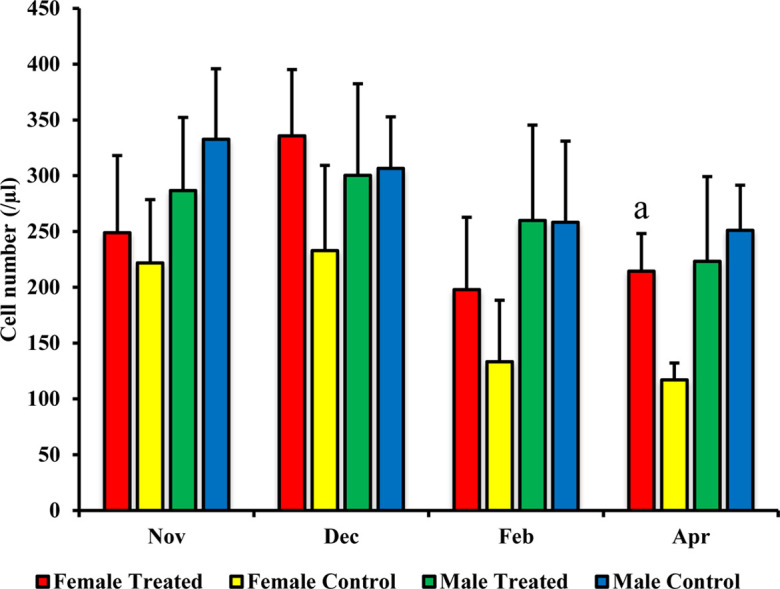
Effect of branched-chain amino acid (BCAA) on male and female CD8+ cell populations. The treated group was fed by BCAA supplementation daily and the untreated group was considered a control. The data are presented as mean ± SD. Letters (a: P<0.05 vs. control) indicate significant differences.
Fig. 3.
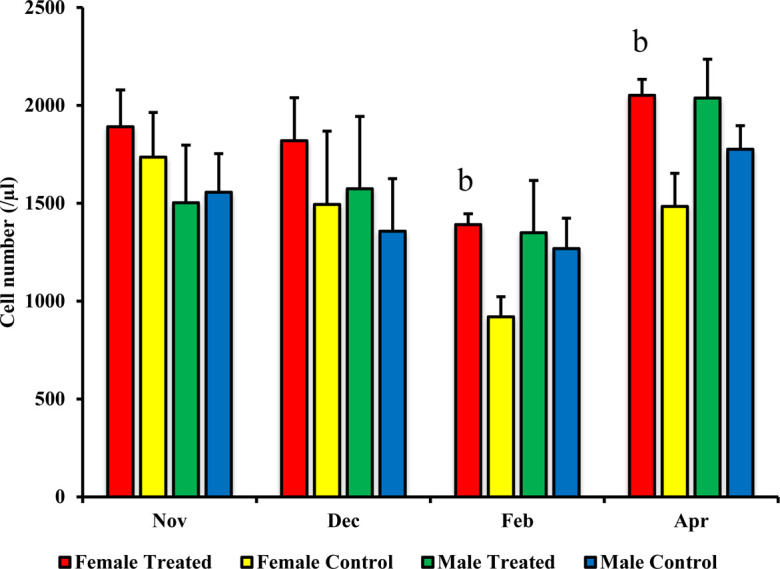
Effect of branched-chain amino acid (BCAA) on male and female major histocompatibility complex (MHC) class II+ cell populations. The treated group was fed by BCAA supplementation daily and the untreated group was considered a control. The data are presented as mean ± SD. Letters (b: P<0.01 vs. control) indicate significant differences.
However, between the male treated and control groups, no significant change was observed in subset analysis for CD4+ cells, CD8+ cells, and MHC class II+ cells (Figs. 1, 23). There was weak and little negative correlation between the exercise distance and the numbers of CD4+ and CD8+ cells in both males treated (y= −0.18 x + 7,490.6, R= −0.08) and control (y= −0.17 x + 7,485.1, R= −0.06) groups. On the other hand, no significant difference was observed for the counts of WBCs, lymphocytes, and monocytes when comparing between treated and control groups for the males or for the females (data not shown).
Lymphocyte proliferation and phagocytosis
The lymphocyte proliferative response to Con A in the female treated group was significantly higher than that in the control group in both December P=0.047 and April P=0.0001 (Fig. 4). On the other hand, there were no significant changes for the lymphocyte proliferative response to Con A between the male treated group and male control group (Fig. 4). The phagocytic indexes for granulocytes and MHC class II+ monocytes did not differ significantly between male, and female treated and control groups (Figs. 5 and 6).
Fig. 4.
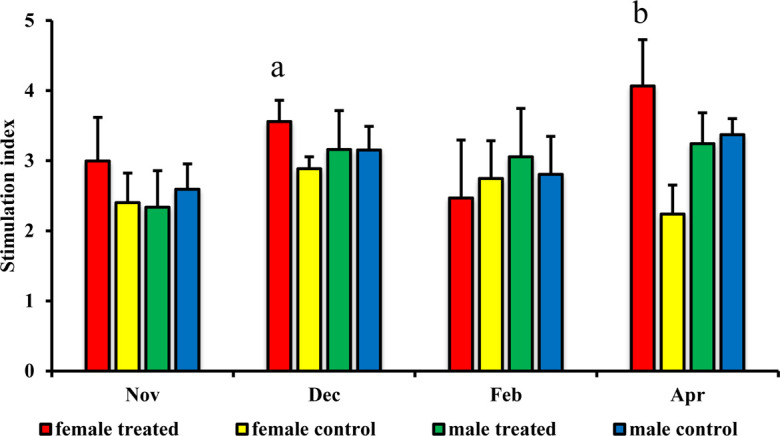
Effect of branched-chain amino acid (BCAA) on male and female lymphocyte proliferative response to concanavalin A (Con A). The stimulation index was evaluated for the treated group, fed by BCAA supplementation daily, and for the untreated group, which was considered a control. The data are presented as mean ± SD. Letters (a: P<0.05 vs. control, b: P<0.01 vs. control) indicate significant differences.
Fig. 5.
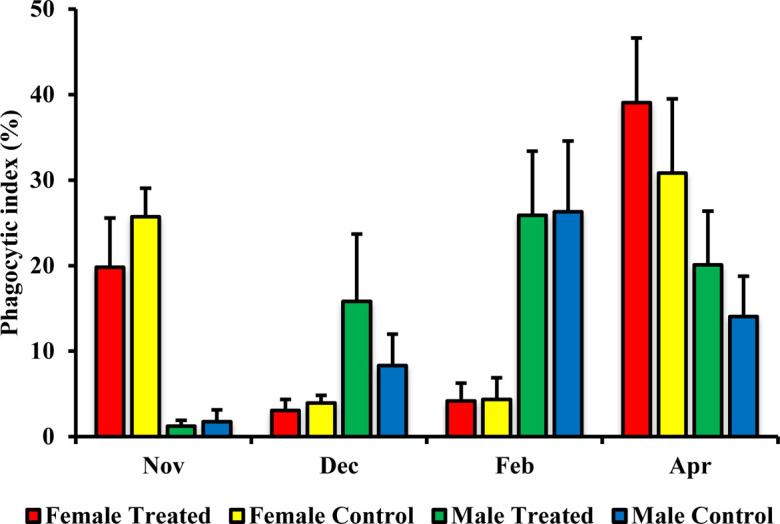
Effect of branched-chain amino acid (BCAA) on male and female phagocytic index for granulocytes. The treated group was fed by BCAA supplementation daily and the untreated group was considered a control. The data are presented as mean ± SD.
Fig. 6.
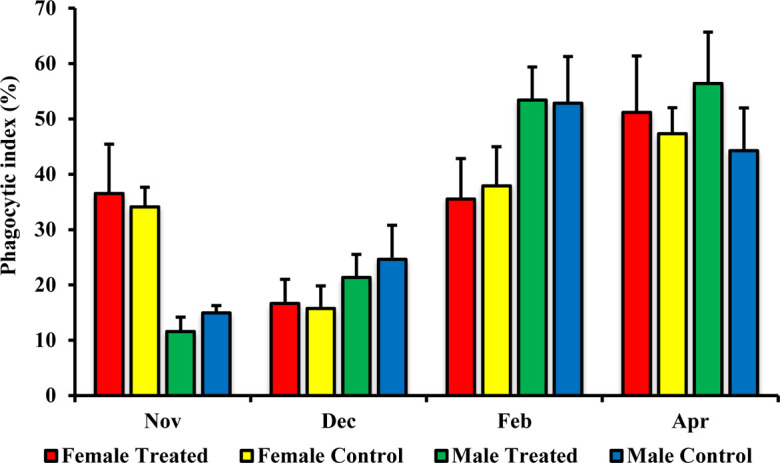
Effect of branched-chain amino acid (BCAA) on male and female phagocytic index for monocytes. The treated group was fed by BCAA supplementation daily and the untreated group was considered a control. The data are presented as mean ± SD.
Cytokine-encoding mRNA expression
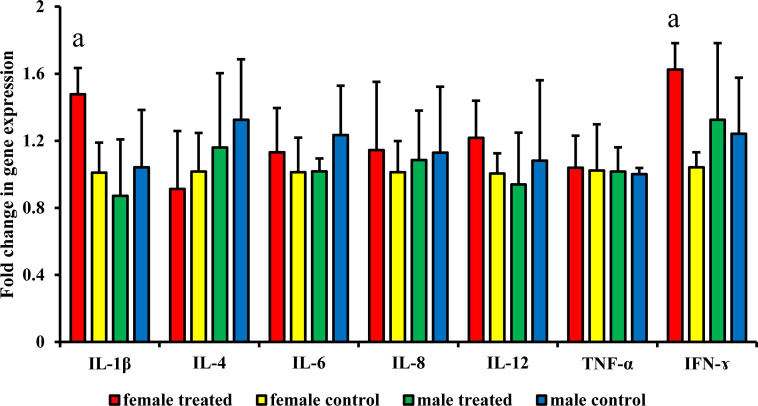
The expression of cytokine-encoding mRNAs was analyzed for the male and female treated and control groups in April. Expression of the IFN-γ- and IL-1β-encoding mRNAs in the female treated group was significantly higher than that in the control group (P=0.021 and P=0.041, respectively). However, there were no statistically significant differences in the levels of other cytokine-encoding transcripts between treated and control groups (Fig. 7).
Fig. 7.
Effect of branched-chain amino acid (BCAA) on expression (in April for male and female) of mRNAs encoding interferon-γ (IFN-γ), interleukin-1β (IL-1β), IL-4, IL-6, IL-8, IL-12, and tumor necrosis factor-α (TNF-α). The treated group was fed by BCAA supplementation daily and the untreated group was considered a control. Expression levels were measured by real-time PCR. The data are presented as mean ± SD. Letters (a: P<0.05 vs. control) indicate significant differences.
DISCUSSION
Prolonged heavy exertion is widely recognized as a reproducible stressor, which may influence and suppress the immune system [38] and enhance susceptibility to infection [35]. Thus, susceptibility to infection and disease are increased during high-intensity exercise and competitions [16].
To develop their performance, athletes competing in events must attend numerous strenuous preparatory sessions. Moderate-intensity exercise is an effective way to decrease susceptibility to infectious disease [20], and according to animal welfare, training sessions for racing horses start with moderate-intensity exercise and gradually continue to stronger prolonged or exhaustive physical exercises [14]. Thus, it is necessary to protect and evaluate the health condition of the horses during such training. In the present study, we evaluated the immunity of racing horses during session training and assessed the effect of BCAA supplements on the immune system.
Exercise has been shown to decrease different subsets of lymphocytes [52], such as CD8+ T cells, which are important for defense against viral infection [13, 28]. According to correlations data for T cell population and exercise intensity, T lymphocyte population was decreased during high intensity exercise. However, exercise associated decline in T lymphocyte population for female control group was stronger than that in female treated group. Also, our results indicated that BCAA supplementation significantly increases the peripheral blood lymphocyte subset populations of CD4+ cells, CD8+ cells and MHC class II+ cells in female treated horses compared to female control horses. Therefore, the high-intensity exercise-associated decline of lymphocyte subpopulations in females appeared to be counteracted by BCAA treatment. In previous work in rat, BCAA supplementation was shown to provide significant increases in the numbers of CD4+ cells, CD8+ cells, and natural killer cells in the liver compared to a control group [51]. In the present study, increases in lymphocyte subset populations by BCAA supplementation in the female treated group suggested immune enhancement resulting from inclusion of BCAA in the feed.
In this study, the response of lymphocyte proliferation to Con A was used to evaluate the effects of BCAA on immune status. It has been reported that the lymphocyte proliferative response in horses decreases at 12 to 16 hr post-race [33]. The response of lymphocyte proliferation to Con A in the female treated group was significantly higher than that in the control group. Therefore, BCAA supplementation appeared to be effective in preventing the decline of lymphocyte proliferation following high-intensity exercise. Thus, both in vivo and in vitro examinations, reveal that BCAA supplementation can increase lymphocyte proliferation responses [6, 14].
Phagocytic capacity is normal in moderate exercise but is suppressed and altered in the case of high-intensity exercise [4, 22, 37, 46]. In the present work, the phagocytosis of granulocytes and monocytes did not differ significantly between the treated and control groups. In horses subjected to routine exercise, phagocytic activity is suppressed after exercise but is re-stimulated upon training on the next day, preventing decreases in phagocytotic function in the peripheral blood [12]. Study in rats has shown that BCAA supplementation could not improve the diminished phagocytic function of macrophages during four weeks of high-intensity exercise [56]. In addition, study in mouse, BCAA supplementation did not ameliorate the phagocytic function of peritoneal macrophages against S. typhimurium [42]. According to our data and previous related research [12] we speculated that during routine training the phagocytosis activity in peripheral blood would not decrease for long time, and recover to the normal condition.
Cytokines have an important role in the regulation of both natural and acquired immune responses. Previous studies evaluated the effect of exercise on cytokine production [23, 49]. In the present work, BCAA supplementation in female treated horses significantly stimulated the systemic levels of transcripts encoding IFN-γ and IL-1β (compared to those in the female control group); these cytokines are known to be secreted by Th1 cells [41] and innate immune cells such as macrophage [32], respectively. In previous work, oral administration of BCAA in humans enhanced the ex vivo production of IFN-γ by PBMCs isolated from patients with advanced cirrhosis [26]. Other work has shown that BCAA supplementation increases IL-1β production by lymphocytes isolated from human athletes and cultivated for 48 hr in the presence of lipopolysaccharide or phytohemagglutinin [7]. These results indicated that BCAA supplementation prevent the decline of IFN-γ and IL-1β cytokine production otherwise seen after long-distance running. We speculate that BCAA supplementation is important for the regulation of Th1 cells and innate immune cell responses after high-intensity exercise, which may help to decrease the risk of infectious disease.
In the present study, BCAA supplementation mostly was effective on the immune status of female horses in the last months of the training plan. In the last months of the training plan the intensity of exercise would have been sufficiently high to suppress immunity and enhance susceptibility to infection. We infer that this dose of BCAA in the female treated group was effective in reducing immunosuppression that otherwise would be seen following high-intensity exercise.
On the other hand, the data indicated no significant changes between the male treated and male control groups in the subset analysis for T cells and MHC class II+ cells, the lymphoproliferative response to Con A, or the expression of cytokine-encoding mRNAs. The gender-specific differences may be attributable to different reasons, as described below.
1- The effective dose of BCAA supplement to enhance immune status may be different in males and females. As suggested previously by Tarnopolsky [50], amino acid requirements in male athletes likely would exceed those in females. In the present case, a sufficient dose of BCAA is necessary to make positive changes in the immune system. Treatment with a sufficient dose of BCAA may be key to supporting immune cell function [10].
2- The changes in the immune system due to exercise in males and females are different [18]. In our study, the intensity of the exercise protocol for males may not have been sufficient to create exercise-induced stress and immune suppression in males. If so, the effect of BCAA on male immune status would not have been evaluated properly. As reported previously, distinct gender-specific high-intensity protocols must be used for males and females, since physiological differences between males and females may affect training performance [45].
In conclusion, our results suggest that the dose of BCAA used in this research may be effective in promoting immune function in female horses. Increases in the number of T cell subsets and MHC class II+ cells in peripheral blood may enhance the lymphocyte proliferative response by T cells and stimulate the Th1 cell response. BCAA supplementation may prevent or reduce immunosuppression after high-intensity exercise, especially in female horses; this treatment has the potential to decrease or avoid the incidence of infectious disease after exercise.
CONFLICT OF INTEREST
The authors declare no conflicts of interest.
Acknowledgments
The authors thank ASKA Animal Health Co., Ltd., for providing the BCAA supplement used in this study.
REFERENCES
- 1.Álvares T. S., Conte C. A., Paschoalin V. M. F., Silva J. T., Meirelles C. M., Bhambhani Y. N., Gomes P. S. C.2012. Acute l-arginine supplementation increases muscle blood volume but not strength performance. Appl. Physiol. Nutr. Metab. 37: 115–126. doi: 10.1139/h11-144 [DOI] [PubMed] [Google Scholar]
- 2.Assenza A., Bergero D., Tarantola M., Piccione G., Caola G.2004. Blood serum branched chain amino acids and tryptophan modifications in horses competing in long-distance rides of different length. J. Anim. Physiol. Anim. Nutr. (Berl.) 88: 172–177. doi: 10.1111/j.1439-0396.2004.00493.x [DOI] [PubMed] [Google Scholar]
- 3.Azizi A. F. N., Miyazaki R., Yumito T., Ohashi Y., Uno S., Miyajima U., Kumamoto M., Uchiyama S., Yasuda M.2018. Effect of maternal supplementation with seaweed powder on immune status of liver and lymphoid organs of piglets. J. Vet. Med. Sci. 80: 8–12. doi: 10.1292/jvms.17-0537 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Baj Z., Kantorski J., Majewska E., Zeman K., Pokoca L., Fornalczyk E., Tchórzewski H., Sulowska Z., Lewicki R.1994. Immunological status of competitive cyclists before and after the training season. Int. J. Sports Med. 15: 319–324. doi: 10.1055/s-2007-1021067 [DOI] [PubMed] [Google Scholar]
- 5.Baldwin D. R., Wilcox Z. C., Zheng G.1997. The effects of voluntary exercise and immobilization on humoral immunity and endocrine responses in rats. Physiol. Behav. 61: 447–453. doi: 10.1016/S0031-9384(96)00459-3 [DOI] [PubMed] [Google Scholar]
- 6.Bassit R. A., Sawada L. A., Bacurau R. F. P., Navarro F., Martins E., Jr, Santos R. V. T., Caperuto E. C., Rogeri P., Costa Rosa L. F. B. P.2002. Branched-chain amino acid supplementation and the immune response of long-distance athletes. Nutrition 18: 376–379. doi: 10.1016/S0899-9007(02)00753-0 [DOI] [PubMed] [Google Scholar]
- 7.Bassit R. A., Sawada L. A., Bacurau R. F. P., Navarro F., Costa Rosa L. F. B. P.2000. The effect of BCAA supplementation upon the immune response of triathletes. Med. Sci. Sports Exerc. 32: 1214–1219. doi: 10.1097/00005768-200007000-00005 [DOI] [PubMed] [Google Scholar]
- 8.Blecha F., Minocha H. C.1983. Suppressed lymphocyte blastogenic responses and enhanced in vitro growth of infectious bovine rhinotracheitis virus in stressed feeder calves. Am. J. Vet. Res. 44: 2145–2148. [PubMed] [Google Scholar]
- 9.Calder P. C., Yaqoob P.2003. Amino acids and immune function. pp. 305–320. In: Metabolic and Therapeutic Aspects of Amino Acids in Clinical Nutrition, 2nd ed., CRC Press, Boca Raton. [Google Scholar]
- 10.Calder P. C.2006. Branched-chain amino acids and immunity. J. Nutr. 136Suppl: 288S–293S. doi: 10.1093/jn/136.1.288S [DOI] [PubMed] [Google Scholar]
- 11.Cywińska A., Wyszyńska Z., Górecka R., Szarska E., Witkowski L., Dziekan P., Winnicka A., Schollenberger A.2010. The effect of the 162 km endurance ride on equine peripheral blood neutrophil and lymphocyte functions. Pol. J. Vet. Sci. 13: 279–285. [PubMed] [Google Scholar]
- 12.Cywinska A., Szarska E., Degorski A., Guzera M., Gorecka R., Strzelec K., Kowalik S., Schollenberger A., Winnicka A.2013. Blood phagocyte activity after race training sessions in Thoroughbred and Arabian horses. Res. Vet. Sci. 95: 459–464. doi: 10.1016/j.rvsc.2013.04.020 [DOI] [PubMed] [Google Scholar]
- 13.Doherty P. C., Hou S., Tripp R. A.1994. CD8+ T-cell memory to viruses. Curr. Opin. Immunol. 6: 545–552. doi: 10.1016/0952-7915(94)90139-2 [DOI] [PubMed] [Google Scholar]
- 14.Evans D. L.2007. Welfare of the racehorse during exercise training and racing. pp. 181–201. In: The Welfare of Horses, Springer Netherlands, Dordrecht. [Google Scholar]
- 15.Fister I., Fister I., Fister D.2019. Sports nutrition. pp. 247–277. In: Adaptation, Learning, and Optimization, Springer, Singapore. [Google Scholar]
- 16.Fitzgerald L.1991. Overtraining increases the susceptibility to infection. Int. J. Sports Med. 12Suppl 1: S5–S8. doi: 10.1055/s-2007-1024742 [DOI] [PubMed] [Google Scholar]
- 17.Folsom R. W., Littlefield-Chabaud M. A., French D. D., Pourciau S. S., Mistric L., Horohov D. W.2001. Exercise alters the immune response to equine influenza virus and increases susceptibility to infection. Equine Vet. J. 33: 664–669. doi: 10.2746/042516401776249417 [DOI] [PubMed] [Google Scholar]
- 18.Gillum T. L., Kuennen M. R., Schneider S., Moseley P.2011. A review of sex differences in immune function after aerobic exercise. Exerc. Immunol. Rev. 17: 104–121. [PubMed] [Google Scholar]
- 19.Gleeson M.2008. Dosing and efficacy of glutamine supplementation in human exercise and sport training. J. Nutr. 138: 2045S–2049S. doi: 10.1093/jn/138.10.2045S [DOI] [PubMed] [Google Scholar]
- 20.Gleeson M.2007. Immune function in sport and exercise. J Appl Physiol (1985) 103: 693–699. doi: 10.1152/japplphysiol.00008.2007 [DOI] [PubMed] [Google Scholar]
- 21.Greer B. K., Jones B. T.2011. Acute arginine supplementation fails to improve muscle endurance or affect blood pressure responses to resistance training. J. Strength Cond. Res. 25: 1789–1794. doi: 10.1519/JSC.0b013e3181e07569 [DOI] [PubMed] [Google Scholar]
- 22.Hack V., Strobel G., Weiss M., Weicker H.1994. PMN cell counts and phagocytic activity of highly trained athletes depend on training period. J Appl Physiol (1985) 77: 1731–1735. doi: 10.1152/jappl.1994.77.4.1731 [DOI] [PubMed] [Google Scholar]
- 23.Henson D. A., Nieman D. C., Blodgett A. D., Butterworth D. E., Utter A., Davis J. M., Sonnenfeld G., Morton D. S., Fagoaga O. R., Nehlsen-Cannarella S. L.1999. Influence of exercise mode and carbohydrate on the immune response to prolonged exercise. Int. J. Sport Nutr. 9: 213–228. doi: 10.1123/ijsn.9.2.213 [DOI] [PubMed] [Google Scholar]
- 24.Hines M. T., Schott H. C., 2nd, Bayly W. M., Leroux A. J.1996. Exercise and immunity: a review with emphasis on the horse. J. Vet. Intern. Med. 10: 280–289. doi: 10.1111/j.1939-1676.1996.tb02063.x [DOI] [PubMed] [Google Scholar]
- 25.Hosoya M., Inoue A., Kimura N., Arai T.2004. Enzyme activities in some types of peripheral leukocytes of thoroughbred race horses before and after the races. Res. Vet. Sci. 77: 101–104. doi: 10.1016/j.rvsc.2004.04.001 [DOI] [PubMed] [Google Scholar]
- 26.Kakazu E., Ueno Y., Kondo Y., Fukushima K., Shiina M., Inoue J., Tamai K., Ninomiya M., Shimosegawa T.2009. Branched chain amino acids enhance the maturation and function of myeloid dendritic cells ex vivo in patients with advanced cirrhosis. Hepatology 50: 1936–1945. doi: 10.1002/hep.23248 [DOI] [PubMed] [Google Scholar]
- 27.Kohut M. L., Boehm G. W., Moynihan J. A.2001. Prolonged exercise suppresses antigen-specific cytokine response to upper respiratory infection. J. Appl. Physiol. (1985) 90: 678–684. doi: 10.1152/jappl.2001.90.2.678 [DOI] [PubMed] [Google Scholar]
- 28.Kos F. J., Engleman E. G.1996. Role of natural killer cells in the generation of influenza virus-specific cytotoxic T cells. Cell. Immunol. 173: 1–6. doi: 10.1006/cimm.1996.0245 [DOI] [PubMed] [Google Scholar]
- 29.Krzywkowski K., Petersen E. W., Ostrowski K., Kristensen J. H., Boza J., Pedersen B. K.2001. Effect of glutamine supplementation on exercise-induced changes in lymphocyte function. Am. J. Physiol. Cell Physiol. 281: C1259–C1265. doi: 10.1152/ajpcell.2001.281.4.C1259 [DOI] [PubMed] [Google Scholar]
- 30.Kurcz E. V., Lawrence L. M., Kelley K. W., Miller P. A.1988. The effect of intense exercise on the cell-mediated immune response of horses. J. Equine Vet. Sci. 8: 237–239. doi: 10.1016/S0737-0806(88)80015-7 [DOI] [Google Scholar]
- 31.Livak K. J., Schmittgen T. D.2001. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Δ Δ C(T)) Method. Methods 25: 402–408. doi: 10.1006/meth.2001.1262 [DOI] [PubMed] [Google Scholar]
- 32.Lopez-Castejon G., Brough D.2011. Understanding the mechanism of IL-1β secretion. Cytokine Growth Factor Rev. 22: 189–195. doi: 10.1016/j.cytogfr.2011.10.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Nesse L. L., Johansen G. I., Blom A. K.2002. Effects of racing on lymphocyte proliferation in horses. Am. J. Vet. Res. 63: 528–530. doi: 10.2460/ajvr.2002.63.528 [DOI] [PubMed] [Google Scholar]
- 34.Nieman D. C.1994. Exercise, infection, and immunity. Int. J. Sports Med. 15Suppl 3: S131–S141. doi: 10.1055/s-2007-1021128 [DOI] [PubMed] [Google Scholar]
- 35.Nieman D. C., Johanssen L. M., Lee J. W., Arabatzis K.1990. Infectious episodes in runners before and after the Los Angeles Marathon. J. Sports Med. Phys. Fitness 30: 316–328. [PubMed] [Google Scholar]
- 36.Nieman D. C., Nehlsen-Cannarella S. L., Fagoaga O. R., Henson D. A., Shannon M., Davis J. M., Austin M. D., Hisey C. L., Holbeck J. C., Hjertman J. M., Bolton M. R., Schilling B. K.1999. Immune response to two hours of rowing in elite female rowers. Int. J. Sports Med. 20: 476–481. doi: 10.1055/s-1999-8827 [DOI] [PubMed] [Google Scholar]
- 37.Nieman D. C., Nehlsen-Cannarella S. L., Fagoaga O. R., Henson D. A., Shannon M., Hjertman J. M. E., Schmitt R. L., Bolton M. R., Austin M. D., Schilling B. K., Thorpe R.2000. Immune function in female elite rowers and non-athletes. Br. J. Sports Med. 34: 181–187. doi: 10.1136/bjsm.34.3.181 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Nieman D. C.1997. Immune response to heavy exertion. J. Appl. Physiol. (1985) 82: 1385–1394. doi: 10.1152/jappl.1997.82.5.1385 [DOI] [PubMed] [Google Scholar]
- 39.Nieman D. C., Kernodle M. W., Henson D. A., Sonnenfeld G., Morton D. S.2000. The acute response of the immune system to tennis drills in adolescent athletes. Res. Q. Exerc. Sport 71: 403–408. doi: 10.1080/02701367.2000.10608923 [DOI] [PubMed] [Google Scholar]
- 40.Nieman D. C., Nehlsen-Cannarella S. L.1994. The immune response to exercise. Semin. Hematol. 31: 166–179. [PubMed] [Google Scholar]
- 41.O’Garra A.1998. Cytokines induce the development of functionally heterogeneous T helper cell subsets. Immunity 8: 275–283. doi: 10.1016/S1074-7613(00)80533-6 [DOI] [PubMed] [Google Scholar]
- 42.Petro T. M., Bhattacharjee J. K.1981. Effect of dietary essential amino acid limitations upon the susceptibility to Salmonella typhimurium and the effect upon humoral and cellular immune responses in mice. Infect. Immun. 32: 251–259. doi: 10.1128/iai.32.1.251-259.1981 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Powell D. G., Watkins K. L., Li P. H., Shortridge K. F.1995. Outbreak of equine influenza among horses in Hong Kong during 1992. Vet. Rec. 136: 531–536. doi: 10.1136/vr.136.21.531 [DOI] [PubMed] [Google Scholar]
- 44.Raidal S. L., Love D. N., Bailey G. D., Rose R. J.2000. Effect of single bouts of moderate and high intensity exercise and training on equine peripheral blood neutrophil function. Res. Vet. Sci. 68: 141–146. doi: 10.1053/rvsc.1999.0349 [DOI] [PubMed] [Google Scholar]
- 45.Schmitz B., Niehues H., Thorwesten L., Klose A., Krüger M., Brand S. M.2020. Sex differences in high-intensity interval training–are HIIT protocols interchangeable between females and males? Front. Physiol. 11: 38. doi: 10.3389/fphys.2020.00038 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Smith J. A., Telford R. D., Mason I. B., Weidemann M. J.1990. Exercise, training and neutrophil microbicidal activity. Int. J. Sports Med. 11: 179–187. doi: 10.1055/s-2007-1024788 [DOI] [PubMed] [Google Scholar]
- 47.Stefanon B., Bettini C., Guggia P.2000. Administration of branched-chain amino acids to standardbred horses in training. J. Equine Vet. Sci. 20: 115–119. doi: 10.1016/S0737-0806(00)80469-4 [DOI] [Google Scholar]
- 48.Sugiura H., Nishida H., Inaba R., Mirbod S. M., Iwata H.2000. Immunomodulation by 8-week voluntary exercise in mice. Acta Physiol. Scand. 168: 413–420. doi: 10.1046/j.1365-201x.2000.00674.x [DOI] [PubMed] [Google Scholar]
- 49.Suzuki K., Yamada M., Kurakake S., Okamura N., Yamaya K., Liu Q., Kudoh S., Kowatari K., Nakaji S., Sugawara K.2000. Circulating cytokines and hormones with immunosuppressive but neutrophil-priming potentials rise after endurance exercise in humans. Eur. J. Appl. Physiol. 81: 281–287. doi: 10.1007/s004210050044 [DOI] [PubMed] [Google Scholar]
- 50.Tarnopolsky M. A.2003. Females and males: Should nutritional recommendations be gender specific? Schweizerische Zeitschrift fur Sport. und Sport. 51: 39–46. [Google Scholar]
- 51.Tsukishiro T., Shimizu Y., Higuchi K., Watanabe A.2000. Effect of branched-chain amino acids on the composition and cytolytic activity of liver-associated lymphocytes in rats. J. Gastroenterol. Hepatol. 15: 849–859. doi: 10.1046/j.1440-1746.2000.02220.x [DOI] [PubMed] [Google Scholar]
- 52.Tvede N., Kappel M., Klarlund K., Duhn S., Halkjaer-Kristensen J., Kjaer M., Galbo H., Pedersen B. K.1994. Evidence that the effect of bicycle exercise on blood mononuclear cell proliferative responses and subsets is mediated by epinephrine. Int. J. Sports Med. 15: 100–104. doi: 10.1055/s-2007-1021028 [DOI] [PubMed] [Google Scholar]
- 53.Wax B., Kavazis A. N., Webb H. E., Brown S. P.2012. Acute L-arginine alpha ketoglutarate supplementation fails to improve muscular performance in resistance trained and untrained men. J. Int. Soc. Sports Nutr. 9: 17. doi: 10.1186/1550-2783-9-17 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Wong C. W., Smith S. E., Thong Y. H., Opdebeeck J. P., Thornton J. R.1992. Effects of exercise stress on various immune functions in horses. Am. J. Vet. Res. 53: 1414–1417. [PubMed] [Google Scholar]
- 55.Woods J. A.2000. Cancer, nutrition, and exercise immunology. pp. 155–174. In: Nutrition and Exercise Immunology, CRC Press, Boca Raton. [Google Scholar]
- 56.Xiao W., Chen P., Liu X., Zhao L.2015. The impaired function of macrophages induced by strenuous exercise could not be ameliorated by BCAA supplementation. Nutrients 7: 8645–8656. doi: 10.3390/nu7105425 [DOI] [PMC free article] [PubMed] [Google Scholar]