Abstract
The enzyme-linked immunosorbent assay (ELISA) was applied to detect antibodies against Brucella abortus in serum samples from four seal species at nine coastal locations of Hokkaido, Japan. These antibodies were detected in 27% (32/118) of Western Pacific harbor seals (Phoca vitulina stejnegeri) at Cape Erimo. The antibodies were observed in spotted seals (P. largha) in one out of six at Nemuro, in two out of three at Rebun Island, in one out of two at Bakkai, and in examined one at Soya. They were also found in respective examined one ribbon seal (Histriophoca fasciata) and one ringed seal (Pusa hispida) at Akkeshi. Harbor seals that tested positive were mostly yearlings (35%, 20/57) and juveniles (45%, 10/22), while only one pup (1/13) and one subadult (1/5) tested positive with low titers of the antibody; no antibodies were observed in adults (n=21). These results suggest that Brucella mainly infected harbor seals from the environment while weaning, and the bacteria were cleared during the early life stage of the seals. In spotted seals, however, antibodies were also detected in adults, suggesting that spotted seals could become infected with Brucella even as adults. It is also possible that a different, more persistent strain of Brucella may have infected the spotted seals.
Keywords: antibody, Brucella, pinniped, seal
Brucella are gram-negative intracellular pathogenic bacteria that infect many mammalian species and cause reproductive disorders including abortion [2]. Marine Brucella were first isolated from seals and dolphins in 1994 [4, 26]. Several epidemiological studies have shown that marine Brucella infect various marine mammal species in waters throughout the world [5, 9, 10, 21, 24]. Based on their preferred hosts and molecular biological features, the isolates from cetaceans and pinnipeds are referred to as B. ceti and B. pinnipedialis, respectively [6, 27, 28]. Although several pathological changes including reproductive disorders and abortions have been reported in B. ceti, no lesions have been found in B. pinnipedialis-infected seals [5, 10, 21]. However, some cases of placentitis have been reported in two eared seal species: California sea lions (Zalophus californianus) and Northern fur seals (Callorhinus ursinus) inhabiting the eastern Pacific Ocean [3, 8].
The coasts of Hokkaido, the northernmost main island of Japan, accommodate several pinniped species. Western Pacific harbor seals (Phoca vitulina stejnegeri), a subspecies of the harbor seal (P. vitulina), have several haul-out sites along the eastern coast of Hokkaido, which is the southern limit of their habitat. Cape Erimo is their southernmost haul-out site. The seals deliver their pups on rocks from April to May, and nurse them for approximately four weeks [11]. Human activities caused the pinniped population to decrease until the 1970s, and with legal protection since then, the population has been recovering [16]. The spotted seal (P. largha), ribbon seal (Histriophoca fasciata) and ringed seal (Pusa hispida) are ice seal species distributed mainly in the Sea of Okhostk and the Bering Sea. They appear with ice floes in winter along the northeastern coast of Hokkaido, close to the southernmost limit of their habitat, and give birth and nurse their pups on the ice [12,13,14]. Spotted seals have recently been expanding their distribution not only to the eastern coast but also the western coast of Hokkaido [15, 20].
Although information on B. pinnipedialis infections in worldwide waters has been growing, it is still very limited regarding the western North Pacific. In 2017, we performed the first serologic study at Cape Erimo and the Shiretoko Peninsula in Hokkaido [1]. Anti-Brucella antibodies were found in all pinniped species examined: Western Pacific harbor seals, spotted seals, ribbon seals, and Steller’s sea lions (Eumetopias jubatus jubatus). We continued serologic surveys at these locations and added several sampling locations, including the western coast of Hokkaido. In this study, we report on the epidemiological results and discuss the age profiles — both life-stage-dependent and -independent — of the seals where antibodies were present.
MATERIALS AND METHODS
Samples
The seal samples and locations where they were collected are summarized in Table 1 and Fig. 1. Blood samples were obtained from living seals which were captured in scientific capture-release surveys by Tokyo University of Agriculture and in population management by the Japanese Ministry of the Environment. When we found living seals which were incidentally caught by fishery nets, the blood samples were also collected. Bloods were taken from the vein of flippers, or of dura mater in euthanized animals. Serum samples were collected by centrifugation of the bloods and stored at −20°C until use. Permissions for the sampling were given from the Japanese Ministry of the Environment and the Hokkaido Government (Supplementary Table 1). All studies were conducted in accordance with the guideline of the Animal Ethics Committee of Tokyo University of Agriculture.
Table 1. Biological information on seals providing sera samples in this study.
| Location a) | Species | Sampling Date | Number of seals | Seal biological feature | ||
|---|---|---|---|---|---|---|
| Year | Period | Sex (M/F) b) | Life stage (P/Y/J/S/A) c) | |||
| Cape Erimo (A) | Harbor seal (Phoca vitulina stejnegeri) | 2013 | Aug 31−Oct 23 | 3 | 1/2 | 0/3/0/0/0 |
| 2014 | Aug 22−Nov 4 | 5 | 0/5 | 0/2/3/0/0 | ||
| 2015 | May 25−Oct 14 | 21 | 10/11 | 1/16/3/0/1 | ||
| 2016 | May 24−Nov 1 | 34 | 18/16 | 1/26/3/1/3 | ||
| 2017 | May 23−Oct 19 | 55 | 16/39 | 11/10/13/4/17 | ||
| Total | 118 | 45/73 | 13/57/22/5/21 | |||
| Spotted seal (Phoca largha) | 2017 | Sep 14 | 1 | 0/1 | 0/0/0/1/0 | |
| Nemuro (B) | Spotted seal (Phoca largha) | 2016 | Dec 5 | 1 | 0/1 | 0/1/0/0/0 |
| 2017 | Dec 1–8 | 5 | 1/4 | 0/0/0/1/4 | ||
| Total | 6 | 1/5 | 0/1/0/1/4 | |||
| Akkeshi (C) | Harbor seal (Phoca vitulina stejnegeri) | 2013 | Apr 22 | 1 | 0/1 | 0/0/0/0/1 |
| 2016 | Jun 8 | 1 | 1/0 | 1/0/0/0/0 | ||
| Total | 2 | 1/1 | 1/0/0/0/1 | |||
| Ribbon seal (Histriophoca fasciata) | 2014 | May 1 | 1 | 0/1 | 0/0/1/0/0 | |
| Ringed seal (Phoca hispida) | 2014 | Sep 2 | 1 | 0/1 | 0/1/0/0/0 | |
| Rebun Island (D) | Spotted seal (Phoca largha) | 2013 | Dec 18–25 | 3 | 0/3 | 0/0/1/0/2 |
| Bakkai (E) | Spotted seal (Phoca largha) | 2013 | Dec 24–26 | 2 | 0/2 | 0/1/0/0/1 |
| Soya (F) | Spotted seal (Phoca largha) | 2017 | Oct 20 | 1 | 1/0 | 0/0/1/0/0 |
| Teuri Island (G) | Spotted seal (Phoca largha) | 2016 | May 20 | 1 | 0/1 | 0/0/1/0/0 |
| Rausu (H) | Spotted seal (Phoca largha) | 2018 | May 17 | 1 | 0/1 | 0/1/0/0/0 |
| Hidaka (I) | Ribbon seal (Histriophoca fasciata) | 2014 | May 13 | 1 | 1/0 | 1/0/0/0/0 |
a) Capital letters in parentheses refer to the sampling locations in Fig. 1. b) M/F: Male/Female. c) P/Y/J/S/A: Pup/Yearling/Juvenile/Subadult/Adult.
Fig. 1.
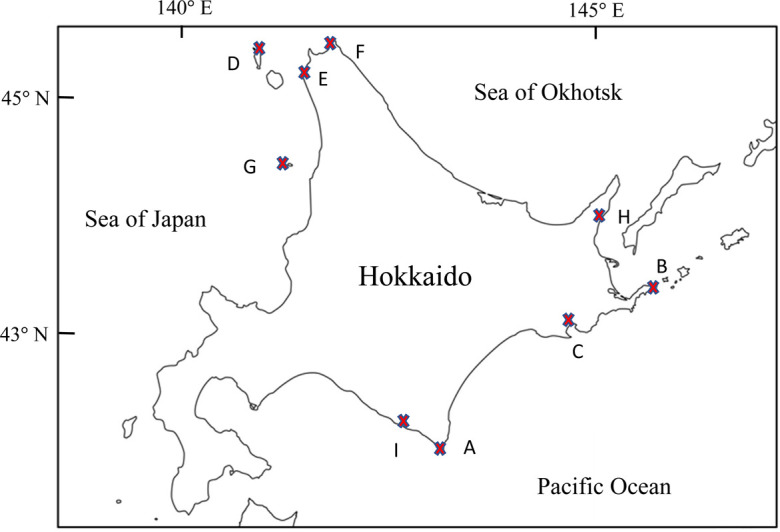
Sampling sites in this study. A: Cape Erimo; B: Nemuro; C: Akkeshi; D: Rebun Island; E: Bakkai; F: Soya; G: Teuri Island; H: Rausu; I: Hidaka.
The overall health conditions of the seals were observed, and their body sizes were measured. Seal ages were estimated from body length based on data from previous studies (Kobayashi unpublished data). Ages of some harbor seals were also determined by counting growth layers in the cementum of the canine teeth. Their life stages were classified based on these estimations. Individuals receiving milk–less than one month old−were categorized as pup. Those less than one year old, 1 or 2 years old, 3 or 4 years old, and more than 5 years old, were respectively categorized as yearling, juvenile, subadult, and adult.
Enzyme-linked immunosorbent assay
Anti-Brucella serum antibodies were detected in an enzyme-linked immunosorbent assay (ELISA) according to the previously described protocol with slight modification [1]. Commercially available inactivated B. abortus strain 125 (Kaketsuken Co., Kumamoto, Japan) was absorbed to the inner surface of each well (protein weight 7.6 μg/well) of a 96-well microtiter plate using a solid phasing buffer (0.1 M carbonate-bicarbonate buffer). After blocking with Block Ace (1 g/100 ml; Yukijirushi Co., Tokyo, Japan), the sera diluted at 1:100 were used as the primary antibody. After washing four times with PBS, horseradish peroxidase-conjugated Protein A/G (Thermo Fisher Scientific Inc., Waltham, MA, USA) diluted at 1:5,000 was used for detection. Color development was performed by adding a substrate solution (Kirkegaard & Perry Laboratories, Inc., Gaithersburg, MD, USA), and stopped with a stop solution (Kirkegaard & Perry Laboratories, Inc.). Absorbance at 405 nm was measured with a microplate reader (BIO-RAD Co., Hercules, CA, USA) and expressed as optical density (OD). The arithmetic mean of triplicate absorbance was then calculated. Because the absorbances of the serum samples from captive seals that were negative were in the range of 0.056–0.093 OD (n=16), absorbances lower than 0.1 were regarded as negative, and the absorbances of serum samples that were shown to be positive by Western blot analysis [1] and were higher than 0.2 OD, were regarded as positive; absorbances of 0.1–0.2 OD were regarded as intermediate.
RESULTS
The absorbances in ELISA and the positive rates are shown in Table 2. Anti-Brucella antibodies were detected in 32 serum samples (27%) from harbor seals at Cape Erimo. The antibodies were frequently detected in yearlings (35%; 95% Confidence Interval [CI]: 23–49%) and juveniles (45%; 95% CI: 24–68%) (Table 3). By contrast, there was only one positive individual among pups (8%; 95% CI: 0.2–36%) and one among subadults (20%; 95% CI: 0.5–72%); no positive antibodies were detected in adults (Table 3).
Table 2. ELISA absorbances of the serum samples and positive rates.
| Location a) | Species | Number of seals | Positive rate b) | |||
|---|---|---|---|---|---|---|
| Total | OD<0.1 | 0.1≤OD<0.2 | 0.2≤OD | |||
| Cape Erimo (A) | Harbor seal | 118 | 72 | 14 | 32 | 32/118 (27%) |
| Spotted seal | 1 | 0 | 1 | 0 | ⁃ | |
| Nemuro (B) | Spotted seal | 6 | 4 | 1 | 1 | 1/6 |
| Akkeshi (C) | Harbor seal | 2 | 1 | 1 | 0 | ⁃ |
| Ribbon seal | 1 | 0 | 0 | 1 | 1/1 | |
| Ringed seal | 1 | 0 | 0 | 1 | 1/1 | |
| Rebun Island (D) | Spotted seal | 3 | 1 | 0 | 2 | 2/3 |
| Bakkai (E) | Spotted seal | 2 | 0 | 1 | 1 | 1/2 |
| Soya (F) | Spotted seal | 1 | 0 | 0 | 1 | 1/1 |
| Teuri Island (G) | Spotted seal | 1 | 0 | 1 | 0 | ⁃ |
| Rausu (H) | Spotted seal | 1 | 1 | 0 | 0 | ⁃ |
| Hidaka (I) | Ribbon seal | 1 | 1 | 0 | 0 | ⁃ |
a) Capital letters in parentheses refer to the sampling locations in Fig. 1. b) Number positive/number tested. Absorbances higher than 0.2 OD (optical density) were regarded as positive.
Table 3. Relationships between sex and life stage regarding the presence of the anti-Brucella antibody in pinnipeds.
| Location a) | Species | Number of positives | Breakdown of positives based on sex or life stage | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Sex | Life stage | ||||||||
| Male | Female | Pup | Yearling | Juvenile | Subadult | Adult | |||
| Cape Erimo (A) | Harbor seal | 32 | 13 (29%, n=45) b) | 19 (26%, n=73) | 1 (8%, n=13) | 20 (35%, n=57) | 10 (45%, n=22) | 1 (20%, n=5) | 0 |
| Spotted seal | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | |
| Nemuro (B) | Spotted seal | 1 | 0 | 1 (n=5) | 0 | 1 (n=1) | 0 | 0 | 0 |
| Akkeshi (C) | Harbor seal | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Ribbon seal | 1 | 0 | 1 (n=1) | 0 | 0 | 1 (n=1) | 0 | 0 | |
| Ringed seal | 1 | 0 | 1 (n=1) | 0 | 1 (n=1) | 0 | 0 | 0 | |
| Rebun Island (D) | Spotted seal | 2 | 0 | 2 (n=3) | 0 | 0 | 1 (n=1) | 0 | 1 (n=2) |
| Bakkai (E) | Spotted seal | 1 | 0 | 1 (n=2) | 0 | 0 | 0 | 0 | 1 (n=1) |
| Soya (F) | Spotted seal | 1 | 1 (n=1) | 0 | 0 | 0 | 1 (n=1) | 0 | 0 |
| Teuri Island (G) | Spotted seal | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Rausu (H) | Spotted seal | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Hidaka (I) | Ribbon seal | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
a) Capital letters in parentheses refer to the sampling locations in Fig. 1. b) Percentages in harbor seals at Cape Erimo indicate the positive ratios to the total number of animals in the sex or life stage.
The relationships between absorbances in ELISA and the life stages are shown in Fig. 2. One pup had positive serum with relatively low absorbance (OD=0.255). That of one subadult, estimated to be 3 years old, was positive but close to the threshold (OD=0.204). On the other hand, higher absorbances were observed in positive sera from pups and juveniles. No relationship was found between Brucella antibody positivity and sex (males: 29%, 95% CI: 16–44%; females: 26%, 95% CI: 17–38%) (Table 3).
Fig. 2.
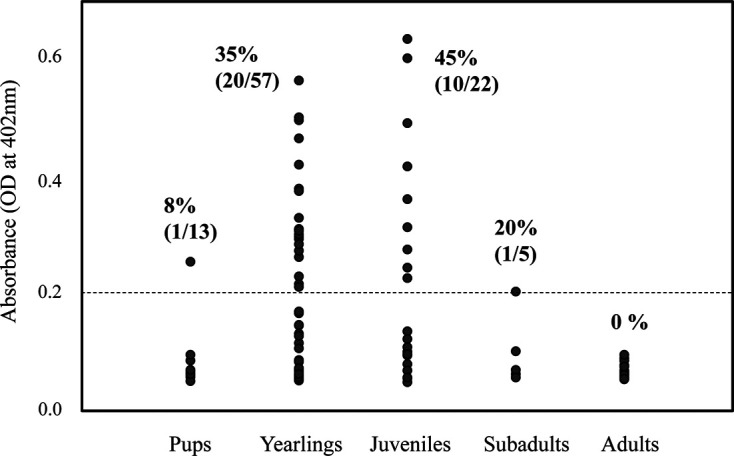
Antibody titers and the life stages of harbor seals. Absorbance (optical density (OD) at 405 nm) in ELISA is indicated along the y-axis. Black closed circles indicate the arithmetic mean of the values for each seal sample, and those above the dotted line (0.2 OD) are regarded as positive.
Anti-Brucella antibodies were also detected in spotted seals at Nemuro (1/6), Rebun Island (2/3), Bakkai (1/2), Soya (1/1), and in one ribbon seal (n=1) and one ringed seal (n=1) at Akkeshi (Tables 2, 3). Among the spotted seals, one adult was positive at Bakkai and another at Rebun Island.
DISCUSSION
The present serologic study, together with a previous work [1], showed that Brucella are prevalent in various pinniped populations of multiple species inhabiting the coasts of Hokkaido, Japan. Life-stage-dependent antibody prevalence was found in harbor seals at Cape Erimo. A similar antibody pattern was reported in our previous study of harbor seals at this location, though information on the yearling stage is lacking [1]. As samples from seals in a variety of life stages are included in the present study, life stage dependency of the antibody profile is more clearly illustrated (Table 3, Fig. 2). A similar antibody pattern was reported in hooded seals (Cystophora cristata) in the North Atlantic population [22], Eastern Pacific harbor seals (P. vitulina richardii) in Washington state, USA [17], as well as harbor seals, spotted seals, ribbon seals, and ringed seals in Alaska [23].
The life-stage-dependent antibody profile suggests that the seals are exposed to the Brucella during the post-weaning period and that the bacteria are cleared from the infected seals within their early life stage. These observations may provide some important points concerning the pathogenicity and transmission of B. pinnipedialis. One point is that maternal transmission via the placenta and milk is not likely to occur. The Brucella may come from the environment during the post-weaning period via still unidentified routes that are avoided or rarely selected by adults. Large-scale studies on stomach contents showed only slight differences among life stages (Kobayashi unpublished data). Though we could not identify the source of Brucella, the infection possibly occurs via prey that juveniles prefer or other factors yet to be identified.
Lungworm, Parafilaroides sp., may be involved in the process [7, 25]. The B. pinnipedialis-infected lungworms may directly invade the seals, or be indirectly taken via lungworm-infected marine animals.
Another important point is that the Brucella may remain in the seals for only a short period. Larsen et al. [18, 19] have shown through in vitro experiments that B. pinnipedialis can enter macrophages and epithelial cells in seals but fails to replicate in those cells.
In the present study, however, two adult spotted seals were shown to be positive. This result is consistent with our previous study that a high prevalence of anti-Brucella antibodies was found in adult spotted seals (54%, n=24) at Rausu, on the Shiretoko Peninsula. The study also showed positive test results in adult ribbon seals (15%, n=13) and sea lions (20%, n=12) [1]. This discrepancy suggests that these seals can come into contact with sources of bacteria not only in their early life stage but also as adults, resulting in repeated infections. It is also possible that a different strain of Brucella whose infection is more persistent may invade these seal populations. The Western blot analysis in our previous study suggested the presence of two different Brucella strains in seals inhabiting the coast of Hokkaido. Band patterns formed by the sera in samples from spotted seals, ribbon seals, and sea lions were different from those formed by the sera from harbor seals [1]. Isolation and characterization of the Brucella from these seals would explain the different antibody response patterns. In addition to ongoing serologic surveys, comprehensive studies focusing on molecular biology and pathology are also important in gaining an understanding of the characteristics of marine Brucella and its impact on marine ecosystems in the western North Pacific Ocean.
Supplementary Material
CONFLICTS OF INTEREST
All authors have no conflicts of interest to disclose.
Acknowledgments
The authors extend their thanks to many students of Tokyo University of Agriculture and members of the Erimo Seal Club for their support in collecting samples. We also thank Dr. Etsuko Katsumata of the Kamogawa Sea World Aquarium for providing the control serum. This study was partially supported by funding from the Ecological Monitoring Survey of Harbor Seals in the Erimo Area (2014–2017) by the Japanese Ministry of the Environment.
REFERENCES
- 1.Abe E., Ohishi K., Ishinazaka T., Fujii K., Maruyama T.2017. Serologic evidence of Brucella infection in pinnipeds along the coast of Hokkaido, the northernmost main island of Japan. Microbiol. Immunol. 61: 114–122. doi: 10.1111/1348-0421.12474 [DOI] [PubMed] [Google Scholar]
- 2.Corbel M. J., Banai M. B.2005. Genus Brucella. pp. 370–386. In: Bergey’s Manual of Systematic Bacteriology, Vol. 2 (Brenner, D. J., Kreig, N. R. and Staley, J. T. eds.), Springer Press, New York. [Google Scholar]
- 3.Duncan C. G., Tiller R., Mathis D., Stoddard R., Kersh G. J., Dickerson B., Gelatt T.2014. Brucella placentitis and seroprevalence in northern fur seals (Callorhinus ursinus) of the Pribilof Islands, Alaska. J. Vet. Diagn. Invest. 26: 507–512. doi: 10.1177/1040638714532647 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ewalt D. R., Payeur J. B., Martin B. M., Cummins D. R., Miller W. G.1994. Characteristics of a Brucella species from a bottlenose dolphin (Tursiops truncatus). J. Vet. Diagn. Invest. 6: 448–452. doi: 10.1177/104063879400600408 [DOI] [PubMed] [Google Scholar]
- 5.Foster G., MacMillan A. P., Godfroid J., Howie F., Ross H. M., Cloeckaert A., Reid R. J., Brew S., Patterson I. A.2002. A review of Brucella sp. infection of sea mammals with particular emphasis on isolates from Scotland. Vet. Microbiol. 90: 563–580. doi: 10.1016/S0378-1135(02)00236-5 [DOI] [PubMed] [Google Scholar]
- 6.Foster G., Osterman B. S., Godfroid J., Jacques I., Cloeckaert A.2007. Brucella ceti sp. nov. and Brucella pinnipedialis sp. nov. for Brucella strains with cetaceans and seals as their preferred hosts. Int. J. Syst. Evol. Microbiol. 57: 2688–2693. doi: 10.1099/ijs.0.65269-0 [DOI] [PubMed] [Google Scholar]
- 7.Garner M. M., Lambourn D. M., Jeffries S. J., Hall P. B., Rhyan J. C., Ewalt D. R., Polzin L. M., Cheville N. F.1997. Evidence of Brucella infection in Parafilaroides lungworms in a Pacific harbor seal (Phoca vitulina richardsi). J. Vet. Diagn. Invest. 9: 298–303. doi: 10.1177/104063879700900311 [DOI] [PubMed] [Google Scholar]
- 8.Goldstein T., Zabka T. S., Delong R. L., Wheeler E. A., Ylitalo G., Bargu S., Silver M., Leighfield T., Van Dolah F., Langlois G., Sidor I., Dunn J. L., Gulland F. M.2009. The role of domoic acid in abortion and premature parturition of California sea lions (Zalophus californianus) on San Miguel Island, California. J. Wildl. Dis. 45: 91–108. doi: 10.7589/0090-3558-45.1.91 [DOI] [PubMed] [Google Scholar]
- 9.Guzmán-Verri C., González-Barrientos R., Hernández-Mora G., Morales J. A., Baquero-Calvo E., Chaves-Olarte E., Moreno E.2012. Brucella ceti and brucellosis in cetaceans. Front. Cell. Infect. Microbiol. 2: 3. doi: 10.3389/fcimb.2012.00003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hernández-Mora G., Palacios-Alfaro J. D., González-Barrientos R.2013. Wildlife reservoirs of brucellosis: Brucella in aquatic environments. Rev. Sci. Tech. 32: 89–103. doi: 10.20506/rst.32.1.2194 [DOI] [PubMed] [Google Scholar]
- 11.Kobayashi M.2009. Phoca vitulina Linnaeus, 1758. pp. 272–273. In: The Wild Mammals of Japan (Ohdachi, S. D., Ishibashi, Y., Iwasa, M. A. and Saitoh, T. eds.), Shoukadoh, Kyoto. [Google Scholar]
- 12.Kobayashi M.2009. Phoca largha Pallas, 1851. pp. 274–277. In: The Wild Mammals of Japan (Ohdachi, S. D., Ishibashi, Y., Iwasa, M. A. and Saitoh, T. eds.), Shoukadoh, Kyoto. [Google Scholar]
- 13.Kobayashi M.2009. Pusa hispida (Schreber, 1775). pp. 278–279. In: The Wild Mammals of Japan (Ohdachi, S. D., Ishibashi, Y., Iwasa, M. A. and Saitoh, T. eds.), Shoukadoh, Kyoto. [Google Scholar]
- 14.Kobayashi M.2009. Histriophoca fasciata (Zimmermann, 1783). pp. 280–281. In: The Wild Mammals of Japan (Ohdachi, S. D., Ishibashi, Y., Iwasa, M. A. and Saitoh, T. eds.), Shoukadoh, Kyoto. [Google Scholar]
- 15.Kobayashi M.2011. Fisheries damage by wintering seals around Hokkaido. pp. 239–245. In: Proceedings of the Japan-Russia Cooperation Symposium on the Conservation of the Ecosystem in Okhotsk (Ministry of Foreign Affairs and Ministry of the Environment of Japan eds.), Office of Japan-Russia Cooperation Symposium on the Conservation of the Ecosystem in Okhotsk, Tokyo (in Japanese).
- 16.Kobayashi Y., Kariya T., Chishima J., Fujii K., Wada K., Baba S., Itoo T., Nakaoka T., Kawashima M., Saito S., Aoki N., Hayama S., Osa Y., Osada H., Niizuma A., Suzuki M., Uekane Y., Hayashi K., Kobayashi M., Ohtaishi N., Sakurai Y.2014. Population trends of the Kuril harbour seal Phoca vitulina stejnegeri from 1974 to 2010 in southeastern Hokkaido, Japan. Endanger. Species Res. 24: 61–72. doi: 10.3354/esr00553 [DOI] [Google Scholar]
- 17.Lambourn D. M., Garner M., Ewalt D., Raverty S., Sidor I., Jeffries S. J., Rhyan J., Gaydos J. K.2013. Brucella pinnipedialis infections in Pacific harbor seals (Phoca vitulina richardsi) from Washington State, USA. J. Wildl. Dis. 49: 802–815. doi: 10.7589/2012-05-137 [DOI] [PubMed] [Google Scholar]
- 18.Larsen A. K., Nymo I. H., Boysen P., Tryland M., Godfroid J.2013. Entry and elimination of marine mammal Brucella spp. by hooded seal (Cystophora cristata) alveolar macrophages in vitro. PLoS One 8: e70186. doi: 10.1371/journal.pone.0070186 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Larsen A. K., Godfroid J., Nymo I. H.2016. Brucella pinnipedialis in hooded seal (Cystophora cristata) primary epithelial cells. Acta Vet. Scand. 58: 9. doi: 10.1186/s13028-016-0188-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Mizuno A. W., Suzuki M., Ohtaishi N.2001. Distribution of the spotted seal Phoca largha along the coast of Hokkaido. Mam. Study 26: 109–118. doi: 10.3106/mammalstudy.26.109 [DOI] [Google Scholar]
- 21.Nymo I. H., Tryland M., Godfroid J.2011. A review of Brucella infection in marine mammals, with special emphasis on Brucella pinnipedialis in the hooded seal (Cystophora cristata). Vet. Res. 42: 93. doi: 10.1186/1297-9716-42-93 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Nymo I. H., Tryland M., Frie A. K., Haug T., Foster G., Rødven R., Godfroid J.2013. Age-dependent prevalence of anti-Brucella antibodies in hooded seals Cystophora cristata. Dis. Aquat. Organ. 106: 187–196. doi: 10.3354/dao02659 [DOI] [PubMed] [Google Scholar]
- 23.Nymo I. H., Rødven R., Beckmen K., Larsen A. K., Tryland M., Quakenbush L., Godfroid J.2018. Brucella antibodies in Alaskan true seals and eared seals−two different stories. Front. Vet. Sci. 5: 8. doi: 10.3389/fvets.2018.00008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ohishi K., Fujise Y., Maruyama T.2008. Brucella spp. in the western North Pacific and Antarctic cetaceans: a review. J. Cet. Res. Manag. 10: 67–72. [Google Scholar]
- 25.Rhyan J., Garner M., Spraker T., Lambourn D., Cheville N.2018. Brucella pinnipedialis in lungworms Parafilaroides sp. and Pacific harbor seals Phoca vitulina richardsi: proposed pathogenesis. Dis. Aquat. Organ. 131: 87–94. doi: 10.3354/dao03291 [DOI] [PubMed] [Google Scholar]
- 26.Ross H. M., Foster G., Reid R. J., Jahans K. L., MacMillan A. P.1994. Brucella species infection in sea-mammals. Vet. Rec. 134: 359. doi: 10.1136/vr.134.14.359-b [DOI] [PubMed] [Google Scholar]
- 27.Whatmore A. M., Koylass M. S., Muchowski J., Edwards-Smallbone J., Gopaul K. K., Perrett L. L.2016. Extended multilocus sequence analysis to describe the global population structure of the genus Brucella: phylogeography and relationship to biovars. Front. Microbiol. 7: 2049. doi: 10.3389/fmicb.2016.02049 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Whatmore A. M., Dawson C., Muchowski J., Perrett L. L., Stubberfield E., Koylass M., Foster G., Davison N. J., Quance C., Sidor I. F., Field C. L., St Leger J.2017. Characterisation of North American Brucella isolates from marine mammals. PLoS One 12: e0184758. doi: 10.1371/journal.pone.0184758 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


