Abstract
Introduction
Andexanet alfa (andexanet) is an approved antidote used to reverse the bleeding effects of Direct Oral Anticoagulant (Direct-Xa agents) agents because it reverses anti-Xa activity. Unfractionated heparin (UFH) and low molecular weight heparins (LMWHs) exhibit anti-Xa activity. The purpose is to investigate the neutralization of UFH and LMWH by andexanet in activated clotting time (ACT), thrombelastography (TEG), and anti-Xa due to the protamine sulfate shortage.
Methods
UFH and LMWH were studied with andexanet, PS, or saline as potential reversal agents/controls at varying concentrations in ACT, TEG, and anti-Xa and compared to each other.
Results
Andexanet partially neutralized both drugs several TEG parameters at high andexanet concentrations, but it was not as effective as protamine sulfate in any of the assays used. Most TEG parameters were correlated with andexanet concentration. In ACT, significant neutralization was demonstrated at many andexanet concentrations for UFH, but not LMWH. UFH was completely neutralized by PS in ACT, while LMWH was partially neutralized by PS in ACT. Andexanet alfa was a less effective neutralization agent than the protamine sulfate as it only partially neutralized UFH in ACT and was ineffective at neutralizing LMWH when tested at the same concentration as PS (10 ug/mL).
Conclusion
Andexanet partially neutralized UFH and LMWH with variability between assays, necessitating investigation into assay-dependent differences.
Keywords: neutralization, andexanet alfa, UFH, LMWH, thrombelastography, activated clotting time
Introduction
Andexanet alfa is a clinically used antidote for the neutralization of the bleeding effects of direct oral anticoagulants (DOACs), specifically, direct anti-Xa agents such as rivaroxaban and apixaban. 1 DOACs have become an alternative to vitamin K antagonists and are useful because their effects are not impacted by variable intake of dietary vitamin K. 2 Because DOACs are not reversed by administration of a common dietary components, an antidote had to be developed to reverse anticoagulation in emergency situations.
Andexanet alfa is a molecularly modified factor Xa decoy protein with high specificity for factor Xa inhibitors 3 that competitively binds to factor Xa inhibitors without exhibiting any catalytic activity. 4 Unfractionated heparin (UFH) and low molecular weight heparins (LMWHs) exhibit both anti-Xa and anti-IIa activities, however LMWHs exhibit a higher relative anti-Xa to anti-IIa effect when compared to unfractionated heparins. 5 The anticoagulant effects of UFH and LMWH are reversed by a polycationic polypeptide, protamine sulfate, although protamine sulfate neutralization of low molecular weight heparin is less effective.6,7 The mechanism of protamine reversal is through electrostatic interactions directly with heparin molecules due to the differing charges on each molecule. 8 While protamine sulfate is an effective neutralization agent, there is evidence of harmful side effects associated with protamine sulfate neutralization such as thrombocytopenia, systemic hypotension, leukopenia, pulmonary artery hypertension, 9 and allergic reaction. 10 There is an ongoing shortage of protamine sulfate, 11 and unfractionated heparin, low molecular weight heparins, and protamine sulfate are all on the World Health Organization's Model List of Essential Medicines. 12 Due to the shortage and these side effects, a search for an alternative neutralization agent is warranted.
Andexanet alfa was studied because of its reversal of anti-Xa DOACs, providing a potential antidote to UFH and LMWH. It is hypothesized that andexanet alfa would be an effective neutralizing agent if it has an effect on inhibited Factor Xa (FXa) directly. A case report from 2019 noted that a patient was unresponsive to UFH anticoagulation during surgery following an andexanet alfa reversal.13,14 Previous research has also shown that recombinant factor Xa may be effective at reversing LMWH 15 and UFH. 16 Furthermore, it appears that andexanet alfa binds directly with UFH and LMWH and interestingly, binds to heparin-activated antithrombin. 17 Although there is evidence of binding between andexanet alfa and anticoagulants, that observation is insufficient to determine if andexanet is an effective reversal agent. The purpose of this study was to investigate the neutralization of the anticoagulant effects of UFH and enoxaparin by andexanet alfa in whole blood assays such as activated clotting time (ACT) and thrombelastography (TEG) and in the Anti-Xa plasma assay. Activated clotting time is a clinically used assessment for the management of anticoagulation during interventional procedures and tracks anticoagulation by determining the length of time for whole blood to clot at various points during surgery and comparing it to a baseline sample taken before a patient is anticoagulated. TEG is a more sensitive assay, which measures several parameters of clotting, and gives a more precise overview of the anti-coagulation effects. Anti-Xa is an industry standard method for determining the potency of anticoagulants and the subsequent reversals. It was hypothesized that there would be partial or complete neutralization of unfractionated heparins and enoxaparin, and enoxaparin would be more thoroughly neutralized due to its higher relative anti-Xa effect.
Materials and Methods
Materials and Instrumentation
Unfractionated Heparin of porcine origin was obtained from Medefil Laboratories (Glendale Heights, IL). The heparin was provided in lyophilized powder form and was dissolved in saline solution to obtain a stock solution of 1 mg/mL. Enoxaparin, a low molecular weight heparin, was purchased from Sanofi-Aventis (Bridgewater, NJ, USA). A working solution of 1 mg/mL was made by diluting the content of the syringe containing a 100 mg/mL solution. Andexanet alfa was obtained from North Shore University Hospital Systems (Glenbrook, IL, USA) in solution containing 10 mg/mL and subsequently diluted to 1 mg/mL. Haemonetics TEG 5000 instrument (Haemonetics, Boston, MA) was used for the TEG measurements. Hemochron clot analyzer (Werfen, Barcelona, Spain) was used for measuring activated clotting time (ACT). Automated clotting laboratory (ACL) Elite fast kinetics coagulation analyzer (Beckman-Coulter, Hialeah, FL) was used to measure Anti-Xa percent inhibition. All reagents used were of analytical grade quality and obtained from commercial vendors. Whole blood collected from volunteers for these studies was obtained under an approved Institutional review board (IRB) and all of the donors were deidentified.
The neutralization profiles of UFH and enoxaparin by andexanet alfa were studied at various concentrations. The final concentration (FC) of UFH used for activated clotting time (ACT) was 10 ug/mL, and for enoxaparin was 25 ug/mL. These concentrations were selected as they represent the ideal final concentrations of heparin or LMWH in percutaneous coronary interventions. 18 Andexanet alfa was used at the following final concentrations: 200, 100, 50, 25, 12.5, and 6.25 ug/mL to neutralize UFH and LMWH. In order to compare andexanet alfa with PS, both were tested in at 10 ug/mL in ACT. For the thrombelastography (TEG) analysis the concentrations of all drugs were reduced by a factor of ten.
Methods for TEG
In order to prepare the six experimental conditions (UFH + Saline, LMWH + Saline, UFH + andexanet alfa, LMWH + andexanet alfa, UFH + protamine sulfate, LMWH + protamine sulfate) for TEG, the solutions indicated in the materials section were made. Once all the solutions were made, the TEG 5000 instrument was calibrated, followed by adding all the sample information into the computer with proper labeling. Next, the plastic TEG cups and pins were inserted into the instrument. For each individual sample, 20 ul of 0.2 M CaCl2 was pipetted into TEG cup, followed by 36 µl of UFH or LMWH (depending on condition), followed by 36 ul of saline, andexanet alfa, or protamine sulfate into the TEG cup (depending on condition). Once all the cups were filled with the proper conditions, 268 ul of citrated blood was pipetted into TEG cup, followed by gently mixing by pipetting up and down. After, the carrier was lifted into the run position and the computer was started. This was repeated until all samples were run.
Methods for ACT
In order to prepare the syringes for ACT, starting concentrations mentioned in the materials section above were prepared. Next, 200 ul of UFH or LMWH were added to each syringe, followed by 200 ul of saline, andexanet alfa, or protamine sulfate to create 6 experimental conditions (UFH + Saline, LMWH + Saline, UFH + andexanet alfa, LMWH + andexanet alfa, UFH + protamine sulfate, LMWH + protamine sulfate). Once all of the syringes were prepared, blood was then drawn to the 2 mL mark in each syringe to achieve the desired final concentration. After blood was drawn into the syringe, it was inverted to mix the contents, and then it was put into the celite ACT tubes and shaken briefly, but vigorously, to activate the blood. Then the ACT tube was then put into Hemochron ACT machine and analyzed. Once finished, the data were recorded and charted into a spreadsheet. This process was repeated until all runs of the experiment were complete.
Methods for Anti-Xa
Samples were prepared by placing 250 ul of plasma into the ACL test cups, which were then placed in an ACL test-cup-carousel. Normal human pool plasma and saline samples were used as controls. Bovine factor Xa (1.0 mg/mL) (Enzyme Research Laboratories, South Bend, IN) was reconstituted with 4 mL (factor Xa FC: 6 ug/mL) of Xa/IIa buffer (50 mM Tris, 175 mM NaCL, 7.5 mM Na2 EDTA, 800 mL distilled H2O, pH = 8.4, 25 °C) prior to use. The substrate, Spectrozyme FXa (5 umole/vial) (Sekisui Diagnostics, Stamford, CT, USA) was reconstituted by adding 2 mL of distilled water. The reagents were inserted into the ACL Elite machine. A clean ACL rotor was placed in the instrument. Once the reagents and samples were in their proper places, the ACL keypad was used to select the program for the anti-Xa assay. An aliquot of 10 ul of plasma was incubated for 1 minute at 37 °C, followed by the addition of 100 ul of bovine factor Xa. After five minutes incubation at 37 °C, 75 ul of Spectrozyme FXa was added and the optical density change at 405 nm was measured for 30 seconds.
Data Analysis
The results were analyzed using R open-source statistical software. Separate analyzes were done for UFH and enoxaparin. Results within each type of anticoagulant were grouped by andexanet alfa concentration. In order to analyze the ACT and TEG results, Paired T-Tests and Pearson Correlations were run for each parameter. A significance level of 0.05 was used to evaluate difference.
Results
TEG
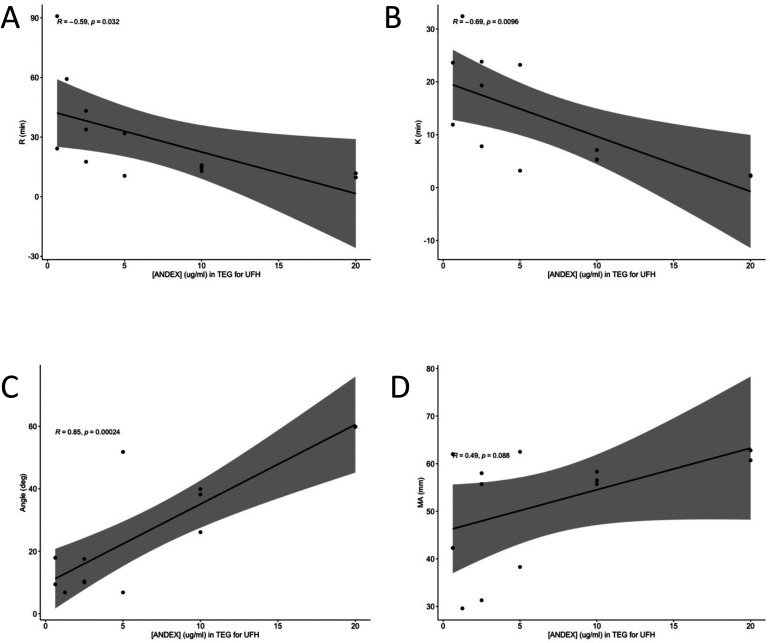
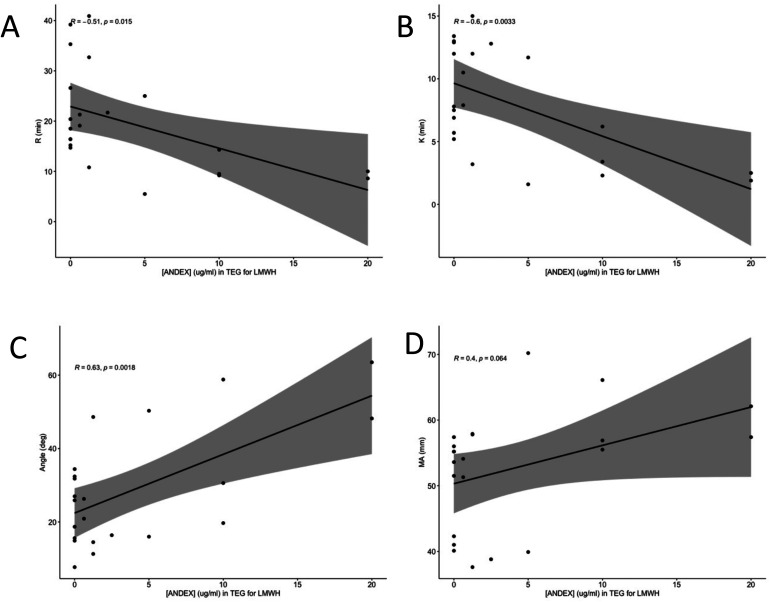
In order to determine if andexanet alfa is an effective neutralization agent for UFH and LMWH, andexanet alfa was supplemented in at various final concentrations in order to establish a concentration-dependent relationship. Correlations were run between andexanet alfa concentration and each of the four measured TEG parameters (R, K, angle, and MA). Andexanet alfa had significant negative correlations with R time, K time, and angle (P < .05) (Figure 1A to C) but not with MA (Figure 1D). The same series of correlations were run for LMWH with significant results for R, K, and angle again (Figure 2A to C), but not for MA (Figure 2D).
Figure 1.
Pearson Correlation of TEG parameters in UFH at varying andexanet alfa concentrations. The x-axis is andexanet alfa concentration in ug/mL and the y-axis is the TEG parameter in its proper units. Figure 1A is for R time, 1B is for K time, 1C is for angle, and 1D is MA.
Figure 2.
Pearson Correlation of TEG parameters in LMWH at varying andexanet alfa concentrations. The x-axis is andexanet alfa concentration in ug/mL and the y-axis is the TEG parameter in its proper units. Figure 2A is for R time, 2B is for K time, 2C is for angle, and 2D is MA.
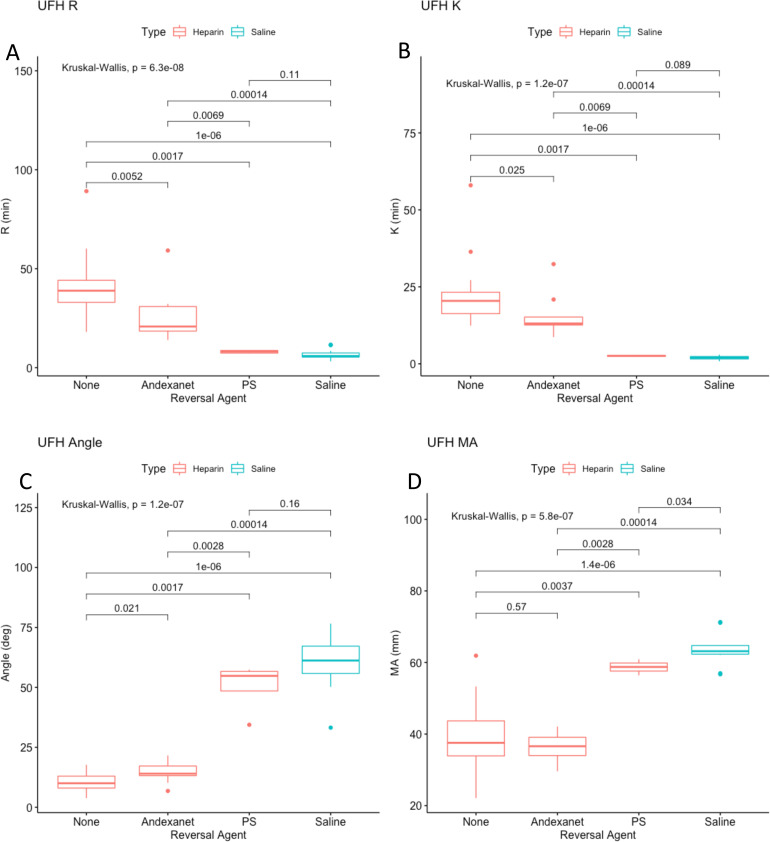
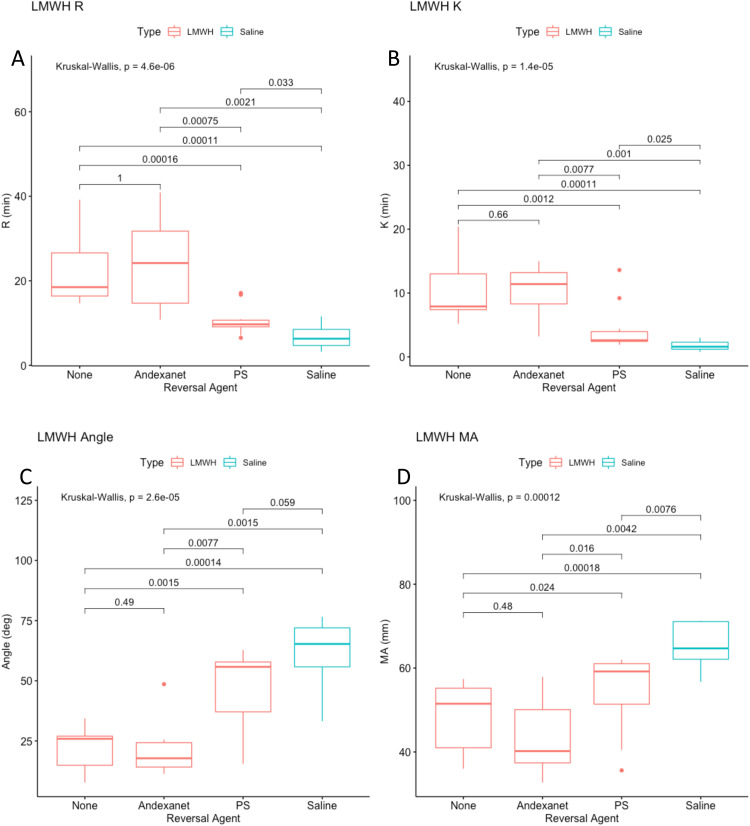
PS and andexanet alfa were tested at a final concentration of 1 ug/mL for in order to determine which is the superior neutralization agent in TEG. All the parameters showed greater reversal of anticoagulation for both UFH and LMWH when neutralized with PS as compared to andexanet alfa (Figure 3A to D & Figure 4A to D). Andexanet partially neutralized all factors in UFH except for MA which had no significant neutralization. In LMWH, no significant neutralization was observed for any parameter when supplemented with andexanet. PS completely neutralized all clotting parameters in UFH except MA (no significant neutralization), and partially neutralized all parameters in LMWH except for angle, which was completely neutralized.
Figure 3.
(A-D). TEG results for UFH (FC 1 ug/mL) combined with various reversal agents (1 ug/mL). The x-axis indicates that the type of reversal agent. The color indicates whether UFH (red) or saline (blue) is used as the anticoagulant. The y-axis is the TEG parameter. Figure 3A is R time, 3B is K time, 3C is angle, 3D is MA.
Figure 4.
(A-D). TEG results for LMWH (FC 2.5 ug/mL) combined with various reversal agents (1 ug/mL). The x-axis indicates that the type of reversal agent. The color indicates whether LMWH (red) or saline (blue) is used as the anticoagulant. The y-axis is the TEG parameter. Figure 4A is R time, 4B is K time, 4C is angle, 4D is MA.
ACT
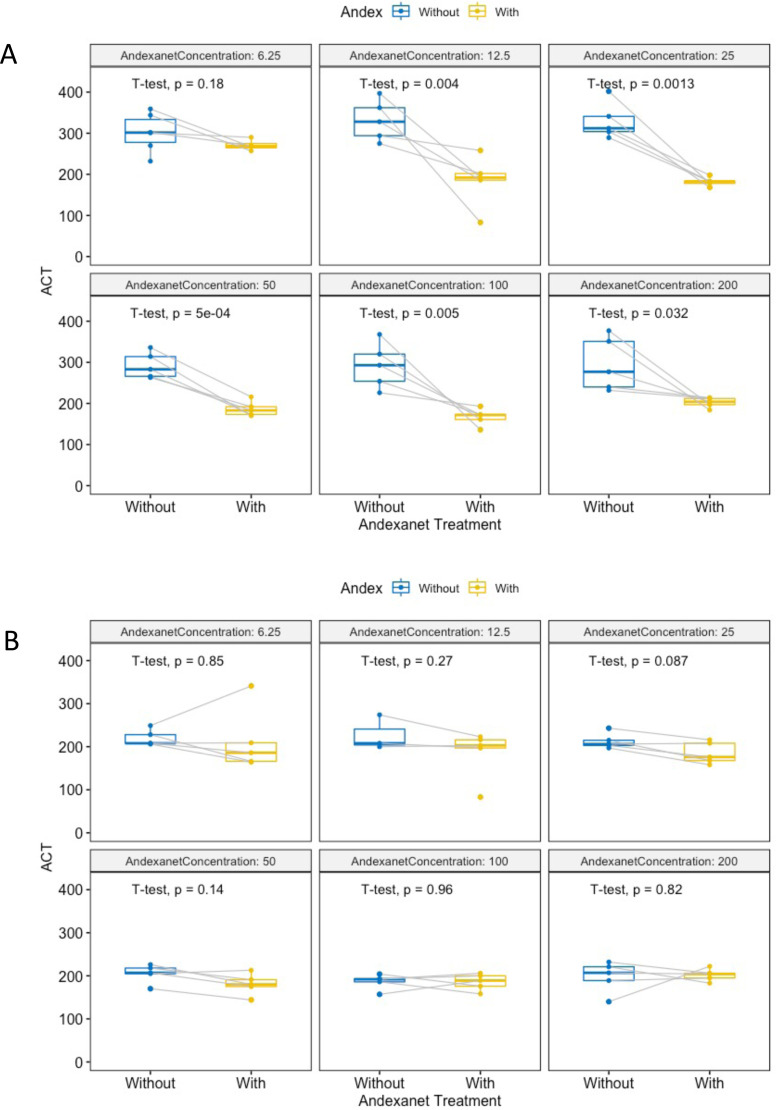
In order to determine if andexanet alfa is an effective neutralizing agent in ACT, the samples were paired and then analyzed with T-Tests such that the before and after results were tested in the same blood that was drawn at the sample time. For UFH, there was a significant difference in ACT between UFH and UFH treated with andexanet alfa, indicating that there is neutralization occurring at all tested concentrations (Figure 5A). There was not a significant neutralization for any of the concentrations tested in LMWH (Figure 5B).
Figure 5.
Paired box and whisker plots of ACT before and after andexanet alfa treatment. The t-tests were run for the samples at each tested concentration. The andexanet alfa concentrations are in ug/mL, the ACT is in seconds. The lines connecting two dots indicated that the samples are paired. The color blue indicates that it is a sample before andexanet alfa treatment and the color yellow indicates that the sample is after andexanet alfa treatment. Figure 3a is for UFH and Figure 3b is for LMWH.
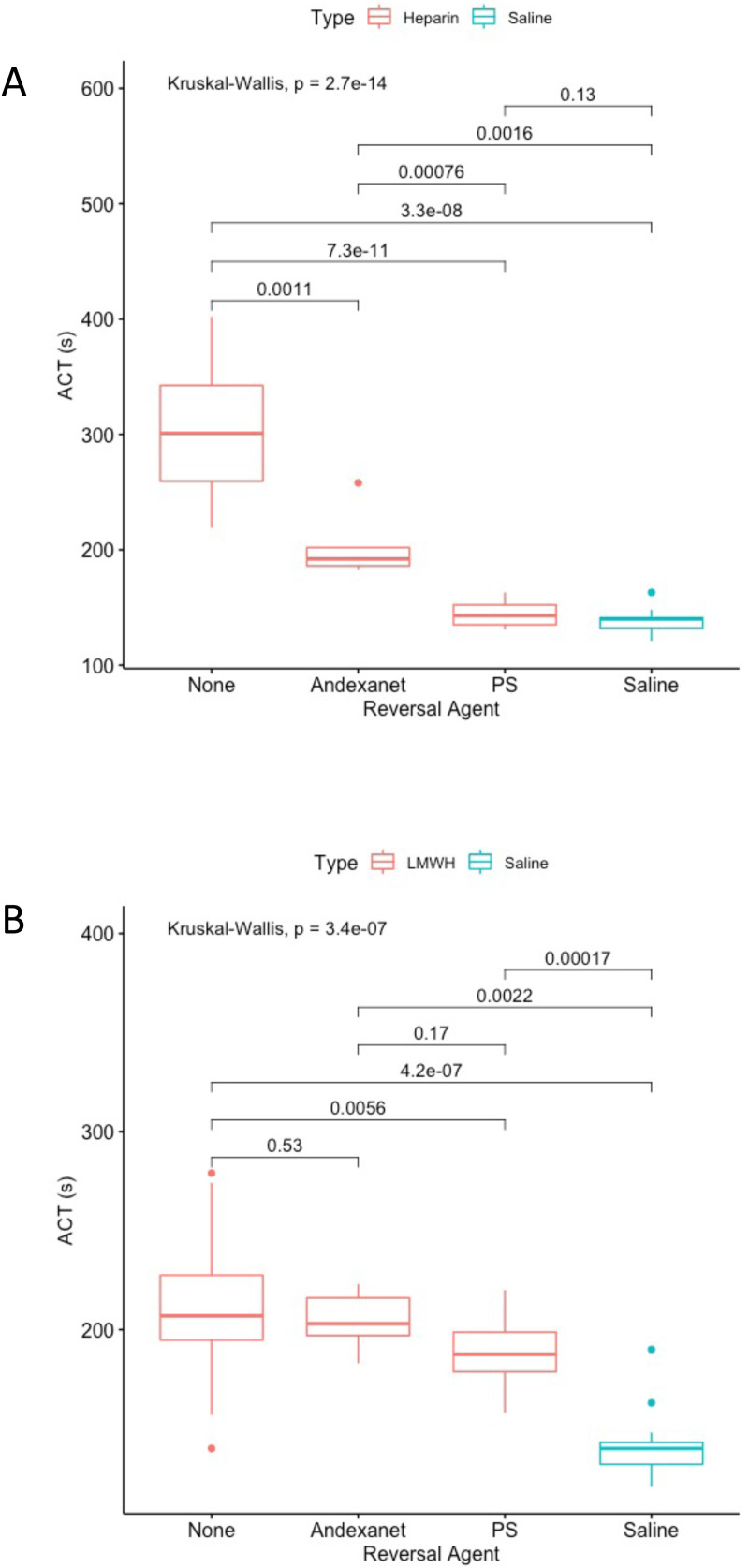
In order to compare PS directly with andexanet, PS and andexanet were run at a final concentration of 10 ug/mL in both UFH (FC 10 ug/mL) and LMWH (FC 25 ug/mL) and compared to one another, the positive control (anticoagulant only) and the negative control (saline instead of anticoagulant).
For ACT, all groups were compared using T tests in order to determine if there were significant differences between the various groups (Figure 6A). P values of the pairwise comparisons are listed above the brackets. In UFH, both andexanet and PS had significant neutralizations when compared to the UFH without any neutralization agent. The ACT of UFH with andexanet was significantly higher than the ACT of UFH with PS and significantly lower than the ACT of UFH with saline, suggesting that andexanet neutralization is partial and less effective than PS. The PS condition was not significantly different than saline condition (no anticoagulant), demonstrating that PS completely neutralized UFH.
Figure 6.
A: ACT results of UFH (FC 10 ug/mL) combined with various reversal agents (10 ug/mL). The x-axis indicates the type of reversal agent. The color indicates whether UFH (red) or saline (blue) is used as the anticoagulant. The y-axis is the activated clotting time in seconds. 6 B: ACT results of LMWH (FC 25 ug/mL) combined with various reversal agents (10 ug/mL). The x-axis indicates the type of reversal agent. The color indicates whether LMWH (red) or saline (blue) is used as the anticoagulant. The y-axis is the activated clotting time in seconds.
In LMWH, there was not a significant difference between the LMWH and LMWH with andexanet (Figure 6B), indicating that there was no reversal of anticoagulation in this assay by andexanet alfa. LMWH with PS was significantly lower than LMWH, but was still significantly higher than the saline (no anticoagulant) condition, suggesting partial reversal with the use of PS.
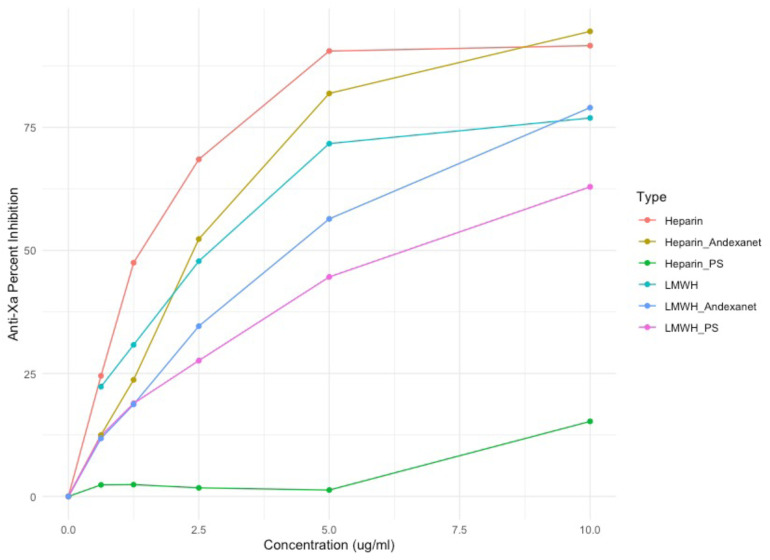
Anti-Xa Percent Inhibition
Protamine sulfate neutralized unfractionated heparin completely, while andexanet alfa only neutralized LMWH partially (Figure 7). This would indicate that andexanet alfa has a lower utility as a neutralization agent for unfractionated heparin than protamine sulfate and thus would not be a replacement for protamine sulfate when protamine sulfate is available. In LMWH, the andexanet alfa did not neutralize anticoagulant effects at all at the concentrations tested below and therefore offers no utility based on the current testing, while protamine sulfate does neutralize LMWH partially. In both instances, protamine sulfate remains the superior neutralization agent for reversal of anticoagulation in heparin and its derivatives. In an instance where protamine sulfate may not be available, andexanet alfa could provide a possible alternative for UFH only.
Figure 7.
Anti-Xa percent inhibition of anticoagulants with and without andexanet and protamine sulfate. The x-axis is the concentration in ug/mL. The y-axis is the anti-Xa percent inhibition calculated from the absorbance measured by ACL elite.
Discussion
The results demonstrated incomplete neutralization of unfractionated heparin and incomplete (or ineffective neutralization) of enoxaparin. UFH was more thoroughly neutralized than LMWH in both ACT and TEG assays in this study which ran contrary to our predictions. One possible explanation for this is that there may be some effect that andexanet alfa is having in a place other than the anti-Xa portion of the heparin's (and associated derivatives) effects. Further investigation into the mechanism of andexanet alfa is necessary to understand why the neutralization effect is not as powerful with LMWH as it is with UFH. In addition, there was no concentration-dependent neutralization of heparin after andexanet alfa was supplemented at concentrations greater than 12.5 ug/mL as measured by the ACT and no apparent neutralization for LMWH. The concentration-dependent relationship up to 12.5 ug/mL of andexanet alfa must be explored further, as it is not testable statistically post-hoc with the data collected and therefore remains an observation that warrants further investigation rather than a result of the experiment. A way to determine if there is a concentration dependent effect would be to vary the concentration of anticoagulant used and see if increasing the anticoagulant decreases the neutralization effects. Andexanet alfa was able to partially neutralize the effect of unfractionated heparin, and as noted, the partial neutralization could not be furthered by adding more andexanet alfa, which suggests that andexanet alfa's mechanism is limited in its targets for neutralizing anticoagulation.
The TEG results demonstrate a statistically significant correlation in three parameters for both UFH and LMWH. R time is the amount of time it takes for blood to being clotting, and increased andexanet alfa concentration was significantly negatively correlated with R time for both UFH and LMWH. K time is the amount of time it takes for blood to reach a standardized clot size once it has started clotting, and again a significant negative correlation was seen in this parameter for both UFH and LMWH. Angle is used as a measure of the rate of clot formation, and generally, decreased angle denotes anticoagulation. A positive correlation was noted for both UFH and LMWH supplemented samples. These results suggest that andexanet alfa reverses anticoagulation in a concentration-dependent manner. However, MA, which is a measure of clot strength was not significantly correlated with andexanet alfa concentration in either UFH or LMWH. This would indicate that there is not a concentration-dependent effect on restoring clot strength using andexanet alfa. The paired T-tests (Table 1) indicated that andexanet alfa only significantly reversed UFH-induced anticoagulation at higher concentrations (10 or 20 ug/mL) for most parameters. For LMWH, the only significant neutralization was in R time at only the highest andexanet concentration. These suggest that andexanet alfa is more effective at reversing anticoagulation induced by UFH than LMWH when measured by TEG. One important note is that the power of the TEG study is low, and therefore significant neutralization may be there, but not captured due to small sample sizes.
Table 1.
All Significant Results from Paired T-Tests in TEG.
| TEG Paired T-Test | Concentration | Before | After | P-value | |
|---|---|---|---|---|---|
| Significant Results | |||||
| UFH | R | 10 | 36.4 | 14.6 | .006 |
| UFH | K | 20 | 14.7 | 2.2 | .001 |
| UFH | K | 10 | 17.9 | 5.9 | .02 |
| UFH | Angle | 20 | 59.9 | 13.9 | .0001 |
| UFH | Angle | 10 | 34.7 | 11 | .016 |
| LMWH | R | 20 | 15.8 | 9.8 | .021 |
The TEG results were supported by the ACT results for UFH. Neutralization of the prolongation of ACT by all concentrations of andexanet was observed, supporting the TEG correlation. However, in TEG correlation results, andexanet alfa had concentration-dependent reversal LMWH's anticoagulant effects in three out of four parameters, but the TEG paired T-tests and ACT results do not demonstrate a significant neutralization. There was also a discrepancy in the ACT and TEG results as the TEG results suggest dose dependent effects at the concentrations tested but the ACT results do not. Further investigation must be done to determine why there are assay dependent differences and if there are, which assay is a better model for representing the interaction in vivo. It is certainly possible that the TEG reader, due to its increased precision, is documenting statistically significant results at lower power than the ACT, which would require many more samples to truly recognize. In regards to the dose-dependent relationship discrepancy, it may also be due to the lower concentrations tested in the TEG system relative to the ACT system. Even if andexanet alfa is found to be an effective partial neutralizing agent for UFH and LMWH in a statistical sense, whether the reversal is clinically useful must be explored.
The anti-Xa results show that andexanet alfa does decrease the anti-Xa effect in both UFH and LMWH but does not reverse anti-Xa effects as well in either anticoagulant as PS does. As seen in Figure 5, PS reverses almost all the anti-Xa activity in UFH, while being relatively less effective at reversing anti-Xa activity in LMWH. The reversal of anti-Xa in both circumstances suggests that the neutralization of anticoagulation by andexanet alfa is likely at least in part through the Xa mechanism. The incomplete anti-Xa reversal for both UFH and LMWH may also explain why andexanet alfa is not completely effective in other assays. Incomplete reversal of anti-Xa effects by andexanet alfa suggests that PS is the preferable neutralization unless it cannot be used. Although there is reversal of anti-Xa effects in LMWH by andexanet alfa, LMWH does not seem to have diminished anticoagulation in the whole blood assays that were tested. The difference between these three assays must be investigated further. Perhaps the reversal of anti-Xa is not strong enough in LMWH to significantly reduce the anticoagulant effect. It is also possible that because the anti-Xa effect is relatively stronger in LMWH, it may in fact take a higher ratio of andexanet alfa to LMWH to reverse the anti-Xa effect. The anti-Xa percent inhibition relates the effects seen in both ACT and TEG, roughly approximating the results seen in those assays and can serve as the common link that can partially explain the similarities in results seen in those assays.
The anti-Xa assay in unmodified form has been demonstrated to be insufficient for measuring the reversal of anti-Xa effects of DOACs by andexanet alfa. 19 Although UFH and LMWH are structurally distinct from DOACs and thus the concerns presented for DOACs may not apply, the interaction between andexanet alfa and UFH/LMWH is not fully understood. Without a complete understanding of this interaction, it may not be possible to fully validate the anti-Xa assay for this experiment in its entirety at this time. From the results, there is consistency across ACT, TEG, and anti-Xa that may provide evidence to the validity of the anti-Xa results and serves as a starting point for determining ways to measure this interaction empirically.
Differences in the ability of protamine sulfate and andexanet alfa to reverse anticoagulation may be explained by differences in the mechanism of each drug. Primarily, the effect of andexanet alfa is thought to be through the direct binding to heparin activated antithrombin (although there is some binding to the UFH and LMWH) 17 and protamine reverses the anticoagulation through direct binding to the UFH and LMWH. Examining the differences in mechanism may illuminate how to get more complete neutralization from andexanet alfa or which other drug to combine with andexanet alfa to yield complete neutralization.
Dabigatran is another DOAC which mediates its anticoagulant effects through interaction with thrombin. 20 The reversal of dabigatran is accomplished using Idarucizumab, which binds to dabigatran and dabigatran bound to thrombin, 21 making it most likely an ineffective agent for reversal of UFH and LMWH. However, I hypothesize that a hypothetical modified factor IIa in conjunction with andexanet alfa may be able fully neutralize unfractionated heparin if the Factor IIa (FIIa) decoy can bind with high enough affinity to AT activated by heparin, thus causing a disruption of that interaction without having any catalytic activity on its own.
It is exceedingly important to consider the cost of using pharmaceuticals such as andexanet alfa, which costs an estimated $29,700 (low dose)-$59,400 (high dose) per reversal as of 2019. 22 The cost effectiveness of andexanet alfa used on label is also low at current prices 23 and there is some evidence of increased thrombogenesis in andexanet alfa reversals. 24 Identifying new uses for drugs that are already developed is an important area of study. New pharmaceutical drugs cost an estimated average of between $870 million 25 and $2.8 billion dollars 26 to bring to market. Niche pharmaceuticals tend to be exceedingly expensive, with the high cost of development and small use rate justifying expenses.
It is also important to consider the case reports that mentioned patients who were resistant to unfractionated heparin anticoagulation after andexanet reversal, as resistance to anticoagulation may be dangerous in emergencies. A risk-benefit analysis would be necessary to determine if this potential alternative has an acceptable safety profile.
Our results differed from the previous study conducted on LMWH, 15 in that we did not find a significant reversal of LMWH anticoagulation. One difference is in the methodology and administration of andexanet alfa, where our study was run in vitro, and the other study was performed in vivo in rats. Furthermore, because the dosing was determined by body weight of the rat and it was run in vivo, the concentrations we tested at are not necessarily comparable to those tested previously.
The search for a perfect reversal agent to heparin should be continuously pursued for the betterment of overall human health, especially given some of the risks associated with protamine sulfate use. Due to the widespread use of protamine sulfate, a small improvement in effectiveness or safety can have large effects on public health. Having drugs with redundant and multiple functions and clinical uses also increases the options available to patients and in some instances may be lifesaving, as in the case of allergies.
Conclusions
Andexanet alfa partially reverses the anticoagulant effects of UFH in vitro, but does not seem to reverse the effects of LMWH in vitro based on the assays that we employed. In the unfractionated heparin group, reversal was not furthered by adding more andexanet alfa after 12.5 ug/mL in TEG, suggesting that andexanet alone cannot fully neutralize the anticoagulant effects of UFH on its own. Further work needs to be done in order to determine why there are inconsistencies between similar studies and this study. The partial neutralization of UFH is promising as a starting point to develop alternative neutralization agents to protamine sulfate and decrease reliability on a single source and to potentially mitigate some of the suggested dangers associated with protamine sulfate usage.
Limitations to This Study
Given that the heparin and andexanet alfa were supplemented ex vivo to whole blood, the study results may not reflect all potential interactions between heparins, andexanet, and endogenous molecules and may not fully reflect local and systemic effects in the body. Furthermore, only one porcine heparin and one porcine low molecular weight heparin were used in this study, so generalization to the whole drug class must be investigated further as there are some differences between manufacturers. In addition, ACT results across systems are not necessarily comparable, 27 and therefore this study would need to be repeated using other instrumentation in order to compare anticoagulation and neutralization done in other ACT systems. The volunteers who donated blood are presumed healthy and thus may not be representative of pathological conditions.
Acknowledgements
The authors are grateful to Northshore Hospital pharmacy for providing the andexanet. A special thanks to the staff of the Hemostasis and Thrombosis laboratories in completing this study. We are also thankful to Dr Wojcik, Chairperson of the Department of Pathology and laboratory medicine, Dr Meharvan Singh, Vice Provost of Research, Loyola University Chicago and Dr Lowell Steen, Chief of cardiology in the Department of medicine for their support for this study. A special thanks to Ms. Erin Healy-Erickson for her assistance in finalizing this manuscript.
Footnotes
Author Contributions: The main manusript was prepared by JL. Statistical support and proofreading, implementation and design of this study were equally shared by all authors.
Declaration of Conflicting Interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) received no financial support for the research, authorship, and/or publication of this article.
ORCID iDs: Joseph Lewis https://orcid.org/0000-0001-6118-3259
Debra Hoppensteadt https://orcid.org/0000-0001-9342-4213
Fakiha Siddiqui https://orcid.org/0000-0002-2219-7049
Jawed Fareed https://orcid.org/0000-0003-3465-2499
References
- 1.Siegal DM, Curnutte JT, Connolly SJ, et al. Andexanet alfa for the reversal of factor Xa inhibitor activity. N Engl J Med. 2015;373(25):2413‐2424. [DOI] [PubMed] [Google Scholar]
- 2.Chen A, Stecker E, Warden BA. Direct oral anticoagulant use: a practical guide to common clinical challenges. J Am Heart Assoc. 2020;9(13):e017559 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kaatz S, Bhansali H, Gibbs J, Lavender R, Mahan CE, Paje DG. Reversing factor Xa inhibitors - clinical utility of andexanet alfa. J Blood Med. 2017;8(8):141‐149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Lu G, DeGuzman FR, Hollenbach SJ, et al. A specific antidote for reversal of anticoagulation by direct and indirect inhibitors of coagulation factor Xa. Nat Med. 2013;19(4):446‐451. [DOI] [PubMed] [Google Scholar]
- 5.Gray E, Mulloy B, Barrowcliffel TW. Heparin and low-molecular-weight heparin. Thromb Haemostasis. 2008;99(5):807‐818. [DOI] [PubMed] [Google Scholar]
- 6.Kouta A, Jeske W, Cera L, et al. Protamine sulfate neutralization profile of various dosages of bovine, ovine and porcine UFHs and their depolymerized derivatives in non-human primates. Clin Appl Thromb Hemost. 2021;27(1):10760296211005544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Thachil J. Protamine-The journey from DNA to heparin neutralization to gene therapy. Semin Thromb Hemost. 2021;48(2):240-243. [DOI] [PubMed] [Google Scholar]
- 8.Carr JA, Silverman N. The heparin-protamine interaction. A review. J Cardiovasc Surg (Torino). 1999;40(5):659‐666. [PubMed] [Google Scholar]
- 9.Lindblad B, Wakefield TW, Whitehouse WM, Jr., Stanley JC. The effect of protamine sulfate on platelet function. Scand J Thorac Cardiovasc Surg. 1988;22(1):55‐59. [DOI] [PubMed] [Google Scholar]
- 10.Nybo M, Madsen JS. Serious anaphylactic reactions due to protamine sulfate: a systematic literature review. Basic Clin Pharmacol Toxicol. 2008;103(2):192‐196. [DOI] [PubMed] [Google Scholar]
- 11.United States Food and Drug Administration Drug Shortages. Current and Resolved Drug Shortages and Discontinuations Reported to FDA. fda.gov: U.S. Department of Health and Human Services; 2021. [Google Scholar]
- 12.World Health Organization Model List of Essential Medicines – 22nd List. In: Organization WH ed. Geneva; 2021.
- 13.Eche IM, Elsamadisi P, Wex N, et al. Intraoperative unfractionated heparin unresponsiveness during endovascular repair of a ruptured abdominal aortic aneurysm following administration of Andexanet Alfa for the reversal of rivaroxaban. Pharmacotherapy. 2019;39(8):861‐865. [DOI] [PubMed] [Google Scholar]
- 14.Watson CJ, Zettervall SL, Hall MM, Ganetsky M. Difficult intraoperative heparinization following andexanet alfa administration. Clin Pract Cases Emerg Med. 2019;3(4):390‐394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lu GM, DeGuzman FR, Hollenbach SJ, et al. Reversal of low molecular weight heparin and fondaparinux by a recombinant antidote (r-antidote, PRT064445). Circulation. 2010;122(21):446-51. [Google Scholar]
- 16.Lu GM, Lin J, Curnutte JT, Conley PB. Reversal of heparin-induced anticoagulation by andexanet alfa, a universal antidote for factor Xa inhibitors. Blood. 2015;126(23):2329. [Google Scholar]
- 17.Kalathottukaren MT, Creagh AL, Abbina S, et al. Comparison of reversal activity and mechanism of action of UHRA, andexanet, and PER977 on heparin and oral FXa inhibitors. Blood Adv. 2018;2(16):2104‐2114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Levine GN, Bates ER, Blankenship JC, et al. 2011 ACCF/AHA/SCAI guideline for percutaneous coronary intervention: executive summary: a report of the American college of cardiology foundation/American heart association task force on practice guidelines and the society for cardiovascular angiography and interventions. Circulation. 2011;124(23):2574‐2609. [DOI] [PubMed] [Google Scholar]
- 19.Bourdin M, Perrotin D, Mathieu O, et al. Measuring residual anti-Xa activity of direct factor Xa inhibitors after reversal with andexanet alfa. Int J Lab Hematol. 2021;43(4):795‐801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hankey GJ, Eikelboom JW. Dabigatran etexilate a new oral thrombin inhibitor. Circulation. 2011;123(13):1436‐1450. [DOI] [PubMed] [Google Scholar]
- 21.Eikelboom JW, Quinlan DJ, van Ryn J, Weitz JI. Idarucizumab: the antidote for reversal of dabigatran. Circulation. 2015;132(25):2412‐2422. [DOI] [PubMed] [Google Scholar]
- 22.Carpenter E, Singh D, Dietrich E, Gums J. Andexanet alfa for reversal of factor Xa inhibitor-associated anticoagulation. Ther Adv Drug Saf. 2019;10(1):2042098619888133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Micieli A, Demchuk AM, Wijeysundera HC. Economic evaluation of andexanet versus prothrombin Complex concentrate for reversal of factor Xa-associated intracranial hemorrhage. Stroke. 2021;52(4):1390‐1397. [DOI] [PubMed] [Google Scholar]
- 24.Siddiqui F, Tafur A, Ramacciotti LS, et al. Reversal of factor Xa inhibitors by andexanet alfa may increase thrombogenesis compared to pretreatment values. Clinical and Applied Thrombosis-Hemostasis. 2019;25(1):1076029619863493. doi: 10.1177/1076029619863493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Paul SM, Mytelka DS, Dunwiddie CT, et al. How to improve R&D productivity: the pharmaceutical industry's Grand challenge. Nat Rev Drug Discovery. 2010;9(3):203‐214. [DOI] [PubMed] [Google Scholar]
- 26.Wouters OJ, McKee M, Luyten J. Estimated research and development investment needed to bring a new medicine to market, 2009–2018. Jama-Journal of the American Medical Association. 2020;323(9):844‐853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Nilsson S, Appelblad M, Svenmarker S. Can we rely on the activated clotting time to measure heparin anticoagulation? A clinical evaluation of two ACT monitors. J Extra Corpor Technol. 2020;52(3):212‐217. [DOI] [PMC free article] [PubMed] [Google Scholar]