Abstract
Male-specific bacteriophage (MSB) densities were determined in animal and human fecal wastes to assess their potential impact on aquatic environments. Fecal samples (1,031) from cattle, chickens, dairy cows, dogs, ducks, geese, goats, hogs, horses, seagulls, sheep, and humans as well as 64 sewerage samples were examined for MSB. All animal species were found to harbor MSB, although the great majority excreted these viruses at very low levels. The results from this study demonstrate that in areas affected by both human and animal wastes, wastewater treatment plants are the principal contributors of MSB to fresh, estuarine, and marine waters.
Diseases resulting from the consumption of molluscan shellfish have been reported in the United States for many decades (16, 17). In the last two decades the number of shellfish-associated illnesses due to enteric viral pathogens, such as the Norwalk and Norwalk-like viruses, has increased (16, 18). Few municipalities are able to consistently produce sewage effluents of good virological quality; thus, shellfish-growing areas continue to be affected by human viral pathogens (8, 9), with adverse effects on human health.
Total and fecal coliform groups colonize the intestinal tracts of warm-blooded animals (7), including humans, and have been employed as indicators of sanitation for many years. These two bacterial groups are the only microbial indicators accepted by the National Shellfish Sanitation Program and the Interstate Shellfish Sanitation Conference to classify the harvest acceptability of estuarine and marine shellfish-growing waters (23). Many researchers and public health officials have challenged the reliability of coliform bacteria as an indicator of the presence of enteric viral pathogens (10, 16, 21) for several reasons. Enteric viruses appear to be more resistant to inactivation by certain environmental factors (e.g., sunlight and salinity) (1, 12, 19) and to wastewater treatment, especially chlorine disinfection (6, 14), than bacterial indicators. Molluscan shellfish generally concentrate and retain viruses (including male-specific bacteriophage [MSB]) at higher rates than total and fecal coliform bacteria (2, 3). As a consequence, enteric viruses are a distinct public health hazard that is not reliably indexed by the total or fecal coliform groups.
In recent years, an MSB group has been suggested as an auxiliary indicator of fecal coliforms for indexing enteric viral pollution (9, 13, 21). The MSB group was proposed for several reasons. (i) It is more resistant to chlorination and inactivation by environmental factors than are coliforms (10, 22); (ii) the method used to enumerate MSB is direct, precise, rapid, facile, and cost-effective (5, 15); (iii) MSB occurs in high densities in both treated and untreated sewage effluents (5, 6, 11); and (iv) it is unlikely to multiply in the estuarine environment (24).
The application of MSB as an indicator of viral contamination from human sources is contingent, at least in part, on its ecology, particularly its occurrence in feral and domestic animals (11). Human enteric viral disease is considered to be predominantly associated with the ingestion of human-derived wastes because of the host-specific nature of these enteric pathogens. Although related viral strains can cause diarrhea in animal species, data from neither areas of endemicity nor outbreak show zoonotic transmission as having an important role in human disease (4, 20). To further explore the use of MSB as an indicator of enteric viral contamination, the contribution of these bacteriophages in both human and animal waste was examined.
Escherichia coli HS(pFamp)RR was used as the host for bacteriophage enumeration because this strain is relatively resistant to most somatic bacteriophages, and thus more than 95% of the bacteriophages infecting it are male specific (5). Fecal samples (n = 1,081) were collected from domestic animals (cattle, chickens, dairy cows, dogs, goats, hogs, horses, and sheep) and waterfowl (ducks, geese, and seagulls). Fresh fecal samples were collected from at least five distinct geographical locations, to include animals representing a variety of habitats and dietary conditions. The majority of samples (51%) were collected from animals at farms in Rhode Island. The remainder of the samples were retrieved from freshwater ponds, the shores of Narragansett Bay, and area landfills. In most cases, fecal wastes from these animals reach the aquatic environment as a result of runoff from the land during rainfall. For each fecal specimen, at least 1 g of freshly voided feces was collected and placed in a sterile 100-ml specimen container (Falcon, Lincoln Park, N.J.) by using a sterile hardwood tongue depressor. Containers were capped and stored on ice until arrival at the laboratory. There, 1 g of each fecal sample was transferred into a sterile 50-ml conical screw-cap centrifuge tube (Falcon) and diluted 1:25 (wt/vol) with tryptone broth. These fecal slurries could be stored for up to 2 weeks at −80°C. By the direct-plating method, sample volumes were placed directly in the top agar tube at 48°C, to which 0.3 ml of a log-phase culture of host strain HS(pFamp)RR and a supplemental volume of tryptone broth were added, for a total volume of 10 ml. The contents of the tubes were mixed, poured onto prewarmed bottom agar plates, and distributed evenly over the surface (5). The remainder of the fecal slurry (21 ml) was utilized for the enrichment procedure, in which 0.5 ml of a log-phase culture of HS(pFamp)RR was added, followed by incubation at 35°C for 18 to 24 h. The slurry was then spot tested for the presence of MSB.
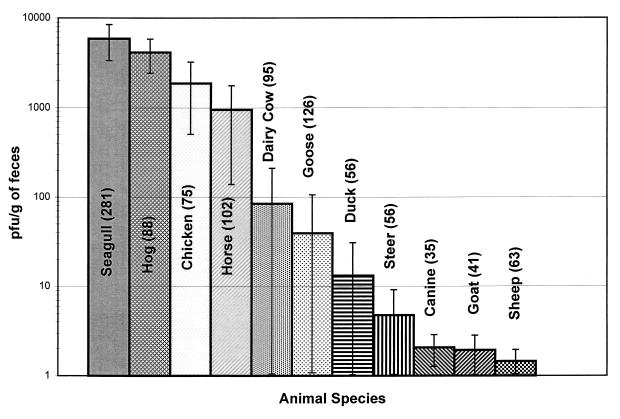
The results from both the direct-plating procedures and enrichment demonstrate the great variability in the occurrence of MSB among animal species (Fig. 1). Sheep, goats, and canines had the lowest frequencies of occurrence. Of the 35 canine fecal specimens examined, none were positive by the direct-plating procedure, whereas more than 25% of fecal samples examined from seagulls, hogs, chickens, and horses were positive for MSB by direct analysis.
FIG. 1.
Mean density and standard deviation of male-specific bacteriophage from animal feces.
Most of the 11 animal species examined in this study shed relatively low numbers of MSB (Fig. 1). Only four species (chickens, hogs, horses, and seagulls) were found to excrete a wide range of MSB; in these species, densities of >104 PFU/g were observed. More than 75% of the horses shed relatively low numbers of MSB (<10 PFU/g of feces), although a small percentage (8.8%) shed MSB at levels >103 PFU/g. More than 53% of the chickens shed low numbers of MSB (<10 PFU/g feces). For both horses and chickens, an inverse relationship was found between the levels of MSB in feces and the corresponding percentage of contributing animals. That is, the greater the number of MSB detected in feces was, the lower the number of animals found to harbor these increasing densities was. This trend was not evident for hogs. Instead, about 15% of the hog population shed MSB in log10 ranges between <100 and >104 PFU/g.
Septic tank samples, obtained from single-family dwellings in southern Rhode Island, were collected from a septage depth of about 0.5 m. The samples were placed in sterile screw-cap centrifuge tubes (Falcon) and held on ice until they were examined. All samples were assayed within 24 h of collection. MSB was not detected by the direct-plating procedure in any of the 13 human fecal samples examined (Table 1) but was found only following enrichment. These results are consistent with the findings of other investigators (10). The wastewater from 22 residential sewage lift stations was examined by the direct-plating method. These lift stations, which pumped residential wastewater into a main sewerage system as they filled, each serviced a single-family home. Only two of these stations (9%) were positive for MSB by direct analysis, with densities of 0.1 × 104 to 2.5 × 104 PFU/100 ml. The wastewater from 17 single-family residential septic tanks was examined by both direct analysis and the enrichment technique. Fifty-eight percent of the samples were positive for MSB by direct enumeration. Densities ranged from <0.1 × 106 to 1.0 × 106 PFU/100 ml. Of 11 wastewater samples collected from main sewer lines, all were positive for MSB by the direct-plating method. Densities ranged from 4.3 × 103 to 8.7 × 105 PFU/100 ml. Similarly, high levels of MSB were found in all sewage treatment plant influents examined from locations around the coastal United States. The locations were as follows: Maine, Rhode Island, Virginia, South Carolina, Florida, Alabama, Louisiana, California (n = 4), Oregon, and Washington (n = 2).
TABLE 1.
Densities of MSB in human-associated wastewaters
| Waste type | No. of samples | MSB densitya
|
Mean MSBb | ||
|---|---|---|---|---|---|
| By qualitative enrichment method (%) | By quantitative direct-plating method (%) | Range | |||
| Human feces | 13 | 1 (8) | 0.0 (0) | <1.0–6.25 | <1 |
| Residential lift station | 22 | —c | 2 (9) | <50.0–2.5 × 104 | 1.3 × 103 |
| Sewage plant effluent | 14 | — | 11 (79) | <100–2.1 × 105 | 3.0 × 104 |
| Septage | 17 | 11 (65) | 10 (59) | <10–1.0 × 106 | 1.0 × 105 |
| In-line sewage | 11 | — | 11 (100) | 4.5 × 103–8.7 × 105 | 2.3 × 105 |
| Sewage plant influent | 14 | — | 14 (100) | 8.5 × 104–3.4 × 106 | 5.2 × 105 |
PFU per gram of feces or per 100 ml of sewage. Range was determined by the direct-plating procedure.
Arithmetic mean MSB values were determined from quantitative direct-plating results.
—, not determined.
The daily magnitudes of the environmental contributions from animal sources and wastewater treatment plants are compared in Table 2. The potential contributions of MSB to the environment from the average animal within each species were calculated as the product of the mean fecal density of MSB determined for each species and the per capita fecal output in a 24-h period. Daily per capita fecal outputs were estimated by us or cited from other sources (7). Though relatively low in MSB density, the daily fecal wastes from a dairy cow are a more significant source of MSB than the daily wastes from a chicken or bay-associated seagull because of the cow’s comparatively large fecal output per day. Horses and hogs excrete the highest number of MSB, 1.9 × 107 and 1.1 × 107 PFU/animal/day, respectively. The magnitude of MSB from human sources was calculated by multiplying the mean density of MSB for effluents from 14 wastewater treatment plants located around the coastal United States (3 × 104 per 100 ml) by 1 million gallons/day (1 MGD). Calculations demonstrate that MSB inputs from animal sources are insignificant compared with those from wastewater effluents. To equal the MSB levels discharged by a wastewater treatment plant averaging 1 MGD (1012 PFU/day) would require the daily fecal contribution of more than 60,000 horses, 100,000 hogs, 180,000 landfill-associated seagulls, or 550,000 dairy cows. A plant serving a relatively small community of about 10,000 would discharge this volume of wastewater. It is apparent from the number of inputs that animals are not a significant source of MSB compared to wastewater treatment plants servicing even relatively small communities which discharge treated wastes into the aquatic environment. We found that MSB recovered from waters or shellfish, excluding those from the most-rural coastal areas, can be presumed to be of anthropogenic origin. As such, MSB may be a reliable indicator of enteric viral pathogens in environmental waters and molluscan shellfish.
TABLE 2.
Daily per capita MSB loadings for different animal species and for a hypothetical 1-MGD wastewater treatment plant, calculated from mean MSB densities determined for respective animal feces and wastewaters
| Waste source or type | Mean MSB density (PFU/g)a | Excrement generated (g/day)b | Estimated MSB released into the environment (PFU/day) |
|---|---|---|---|
| Source | |||
| Canine | 2.1 | 412.5 | 8.6 × 102 |
| Sheep | 1.5 | 1,130.0 | 1.7 × 103 |
| Goat | 1.9 | 1,200.0 | 2.3 × 103 |
| Duck | 13.1 | 336.0 | 4.4 × 103 |
| Goose | 39.2 | 500.0 | 2.0 × 104 |
| Steer | 4.7 | 23,600.0 | 1.1 × 105 |
| Chicken | 1,867.0 | 182.3 | 3.4 × 105 |
| Bay seagull | 888.2 | 500.0 | 4.4 × 105 |
| Dairy cow | 84.3 | 23,600.0 | 2.0 × 105 |
| Landfill seagull | 11,848.8 | 500.0 | 5.9 × 106 |
| Hog | 4,136.5 | 2,700.0 | 1.1 × 107 |
| Horse | 950.2 | 20,000.0 | 1.9 × 107 |
| Type | |||
| Septage (n = 17) | 1,000.0 | 1.5 × 106 | 1.5 × 109 |
| Sewage effluent (n = 14) | 300.0 | 3.8 × 109 | 1.1 × 1012 |
| Sewage influent (n = 14) | 5,200.0 | 3.8 × 109 | 2.0 × 1013 |
For types of waste, mean MSB density is expressed as PFU/milliliter.
For types of waste, excrement generated is expressed as milliliters/day.
While no single water quality indicator can reliably assess both the bacterial and viral contamination of aquatic environments in all circumstances, the results of this study strongly support the use of MSB as the indicator of choice for assessing the potential presence of human enteric viruses in estuarine and marine environments impacted by wastewater sources.
REFERENCES
- 1.Borrego J J, Arrabal F, deVicente A, Gomez L F, Romero P. Study of microbial inactivation in the marine environment. J Water Pollut Control Fed. 1983;55:297–302. [Google Scholar]
- 2.Burkhardt W, III, Rippey S R, Watkins W D. Depuration rates of northern quahogs, Mercenaria mercenaria (Linnaeus, 1758), and eastern oysters, Crassostrea virginica (Gmelin, 1791), in ozone- and ultraviolet light-disinfected seawater systems. J Shellfish Res. 1992;11:105–109. [Google Scholar]
- 3.Burkhardt W, III, Watkins W D, Rippey S R. Seasonal effects on accumulation of microbial indicator organisms by Mercenaria mercenaria. Appl Environ Microbiol. 1992;58:826–831. doi: 10.1128/aem.58.3.826-831.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Centers for Disease Control and Prevention. Viral agents of gastroenteritis. Morbid Mortal Weekly Rep. 1990;39:1–24. [Google Scholar]
- 5.DeBartolomeis J, Cabelli V J. Evaluation of an Escherichia coli host strain for enumeration of F male-specific bacteriophages. Appl Environ Microbiol. 1991;57:1301–1305. doi: 10.1128/aem.57.5.1301-1305.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Donnison A M, Ross C M. Somatic and F-specific coliphages in New Zealand waste treatment lagoons. Water Res. 1995;29:1105–1110. [Google Scholar]
- 7.Geldreich R H, Bordner R H, Huff C B, Clark H F, Kabler P W. Type distribution of coliform bacteria in the feces of warm-blooded animals. J Water Pollut Control Fed. 1962;34:295–301. [Google Scholar]
- 8.Goyal S M. Indicators of viruses. In: Berg G, editor. Viral pollution of the environment. Boca Raton, Fla: CRC Press, Inc.; 1983. pp. 1–32. [Google Scholar]
- 9.Grabow W O K, Coubrough P, Nupen E M, Bateman B W. Evaluation of coliphages as indicators of the virological quality of sewage-polluted water. Water Res. 1984;10:7–14. [Google Scholar]
- 10.Havelaar A R, van Olphen M, Drost Y C. F-specific RNA bacteriophage are adequate model organisms for enteric viruses in fresh water. Appl Environ Microbiol. 1993;59:2956–2962. doi: 10.1128/aem.59.9.2956-2962.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Havelaar A R, Pot-Hogeboom W M, Furuse K, Pot R, Hormann M P. F-specific RNA bacteriophages and sensitive host strains in faeces and wastewater of human and animal origin. J Appl Bacteriol. 1990;69:30–37. doi: 10.1111/j.1365-2672.1990.tb02908.x. [DOI] [PubMed] [Google Scholar]
- 12.Kapuscinski R B, Mitchell R. Processes controlling virus inactivation in coastal waters. Water Res. 1977;14:363–371. [Google Scholar]
- 13.Kator H, Rhodes M. Evaluation of male-specific coliphages as candidate indicators of fecal contamination in point and nonpoint source impacted shellfish growing areas. Report on National Oceanic and Atmospheric Administration, award no. NA17FQ0546. Gloucester Point, Va: College of William and Mary Virginia Institute of Marine Science; 1993. [Google Scholar]
- 14.Keswick B H, Satterwhite T K, Johnson P C, DuPont H L, Secor S I, Bitsura J A, Gary G W, Hoff J C. Inactivation of Norwalk virus in drinking water by chlorine. Appl Environ Microbiol. 1985;50:261–264. doi: 10.1128/aem.50.2.261-264.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kott Y, Rozi N, Sperber S, Betzer N. Bacteriophages as viral pollution indicators. Water Res. 1974;8:165–171. [Google Scholar]
- 16.Richards G P. Outbreaks of shellfish-associated enteric virus illness in the United States: requisite for development of viral guidelines. J Food Prot. 1985;48:815–823. doi: 10.4315/0362-028X-48.9.815. [DOI] [PubMed] [Google Scholar]
- 17.Rippey S R. Infectious diseases associated with molluscan shellfish consumption. Clin Microbiol Rev. 1994;7:419–425. doi: 10.1128/cmr.7.4.419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Rippey S R, Calci K R, Burkhardt III W, Watkins W D. Factors responsible for an outbreak of shellfish-associated gastroenteritis. Food and Drug Administration Science Forum on Regulatory Sciences. Washington, D.C: Food and Drug Administration; 1994. [Google Scholar]
- 19.Roszak D B, Colwell R R. Survival strategies of bacteria in the natural environment. Microbiol Rev. 1987;51:365–379. doi: 10.1128/mr.51.3.365-379.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Stelma G N, McCabe L. Nonpoint pollution from animal sources and shellfish sanitation. J Food Prot. 1992;55:649–656. doi: 10.4315/0362-028X-55.8.649. [DOI] [PubMed] [Google Scholar]
- 21.Stetler R E. Coliphages as indicators of enteroviruses. Appl Environ Microbiol. 1984;48:668–670. doi: 10.1128/aem.48.3.668-670.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Straub T M, Gerba C P, Zhou X, Price R, Yahya M T. Synergistic inactivation of Escherichia coli and MS-2 coliphage by chloramine and cupric chloride. Water Res. 1995;29:811–818. [Google Scholar]
- 23.U.S. Food and Drug Administration. National shellfish sanitation program manual of operations. I. Sanitation of shellfish growing areas. Washington, D.C: Office of Seafood, Food and Drug Administration; 1995. [Google Scholar]
- 24.Woody M A, Cliver D O. Effects of temperature and host cell growth phase on replication of F-specific RNA coliphage Qβ. Appl Environ Microbiol. 1995;61:1520–1526. doi: 10.1128/aem.61.4.1520-1526.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]