Summary
Background
Vietnam has one of the greatest disease burdens from chronic viral hepatitis. Comprehensive prevalence data are essential to support its elimination as a public health threat.
Methods
We searched Medline and Embase from 1990 to 2021 for seroprevalence data relating to Hepatitis B (HBV), C (HCV) and D (HDV) in Vietnam. We estimated pooled prevalence with a DerSimonian-Laird random-effects model and stratified study populations into i) low-risk ii) high-risk exposure and iii) liver disease. We further estimated prevalence by decade and region and rates of HIV-coinfection.
Findings
We analysed 72 studies, including 120 HBV, 114 HCV and 23 HDV study populations. Pooled HBV prevalence was low in blood donors (1.86% [1.82-1.90]) but high in antenatal populations (10.8% [10.1-11.6]) and adults in the general population (10.5% [10.0-11.0]). It was similar or modestly increased in groups at highest risk of exposure, suggesting the epidemic is largely driven by chronic infections acquired in childhood. HCV pooled prevalence in the general population was lower than historical estimates: 0.26% (0.09-0.51) have active infection defined by detectable antigen or HCV RNA. In contrast, there is an extremely high prevalence of active HCV infection in people who inject drugs (PWID) (57.8% [56.5-59.1]), which has persisted through the decades despite harm-reduction interventions. HDV appears mainly confined to high-risk groups.
Interpretation
Blood safety has improved, but renewed focus on HBV vaccination at birth and targeted HCV screening and treatment of PWID are urgently required to meet elimination targets. Large cross-sectional studies are needed to better characterize HDV prevalence, but mass screening may not be warranted.
Funding
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Keywords: Hepatitis B/epidemiology, Hepatitis C/epidemiology, Delta virus, HIV, Vietnam, Prevalence, Risk factors, PWID
Research in context.
Evidence before this study
Despite Vietnam suffering one of the highest burdens of viral hepatitis in the world, prevalence estimates for Hepatitis B virus (HBV), HCV and HDV vary widely. We searched PubMed and Embase for systematic reviews published between January 1st 1990 and 31st December 2021 using ‘Hepatitis’ and ‘Vietnam’ and ‘prevalence’. We found two relevant publications: a 2019 meta-analysis of 16 studies relating to HBV and HCV infections in dialysis patients, and a 2017 systematic review of HCV control efforts. The latter identified basic epidemiological and public health data as a significant gap in the literature and concluded there is an urgent need for an up-to-date assessment of hepatitis disease burden in Vietnam.
Added value of this study
We systematically reviewed all studies relating to prevalence of HBV, HCV and HDV in Vietnam since 1990. By stratifying cohorts as either low- or high-risk we were able to characterise the epidemics with a view to informing elimination policy. By separating HCV antibody studies from HCV antigen/RNA studies we were able to gage true disease burden and treatment need. We found a low pooled prevalence of HBV and HCV in blood donors, but a high pooled prevalence of HBV in other low risk populations, which remains around 9.5% in studies the last 10 years. High risk exposures in adulthood have marginal impact on rates of HBV infection, reinforcing the view that chronic HBV infection in Vietnam is largely driven by vertical transmission. We found HCV and HDV prevalence is lower in the general population than previous estimates, with these infections increasingly concentrated in specific high-risk groups, particularly people who inject drugs.
Implications of all the available evidence
HBV is by far the biggest contributor to hepatitis-related morbidity and mortality in Vietnam and elimination efforts must focus on screening and treatment of pregnant women and improved provision of active and passive immunisation at birth to prevent vertical transmission. The number of individuals requiring treatment for HCV is likely smaller than previous estimates, but improved screening and treatment of PWID is urgently required to meet elimination targets. More data is required to characterise the HDV epidemic, which may be an under-recognised cause of acute hepatitis in Vietnam.
Alt-text: Unlabelled box
Introduction
Vietnam, population 97.3 million, is one of twenty countries reported to shoulder 75% of the world's burden of viral hepatitis.1 Globally, around 96% of viral hepatitis deaths are attributable to Hepatitis B virus (HBV) and Hepatitis C virus (HCV)1 but the prevalence of these infections in Vietnam is poorly characterised. Reliable prevalence data is now considered a central policy indicator by which to measure a country's progress towards elimination.1,2 In a recent analysis of policy scores and rankings of 66 countries with the highest burden of viral hepatitis, Vietnam was singled out as scoring poorly.2 indicating urgent action is required to focus elimination efforts.
Morbidity from viral hepatitis in Vietnam is largely driven by Hepatitis B virus (HBV), with most chronic infections acquired through mother-to-child transmission3 and horizontal transmission in early childhood.4 Vietnam's Ministry of Health (MOH) approximates the prevalence of chronic HBV infection to range from 8-25%.5 The World Health Organisation (WHO) Vietnam Office estimates there were 7,697,525 chronic infections in 2017 (8.1% prevalence),6 and the most recent Global Burden of Disease (GBD) modelling from 2019 estimates a prevalence of 6.6% (95% C.I. 6.30 - 6.92)7 based on data from eight studies.8 The Vietnam Viral Hepatitis Alliance concedes that since these models are based on a small number of studies, the true burden of HBV in Vietnam remains uncertain.5
Prevalence estimates for HCV are similarly variable. WHO estimate that approximately one million Vietnamese (∼1%) have chronic active infection,5 while the most recent GBD modelling suggests this figure may be over 60% higher (1.66% [95% C.I 1.35 – 2.0]).7 A major reason for this discrepancy is that a high proportion of HCV infections are believed to result from unsafe health-care associated activities9, 10, 11 and community services,12, 13, 14 making it difficult to accurately assess the size of the population at risk. Prevalence estimates from both low- and high-risk populations and data relating to co-infection with HIV are needed to better characterize the epidemic.
Hepatitis D virus (HDV) also makes an important contribution to Vietnam's hepatitis burden. Worldwide HDV prevalence is estimated to be around 4.5% (95% CI 3.6-5.7) among all HBsAg-positive individuals and around 16.4% (14.6-18.6)15 in those attending hepatology clinics. Prevalence in Asia is highly variable and does not parallel rates of HBV infection.15 HDV is not currently screened for in Vietnam and is not included in national treatment guidelines. However, improved HDV therapeutics are forthcoming,16 so HDV prevalence estimates are urgently required.
Finally, in the last thirty years Vietnam has undergone unprecedented change.17 Economic and political reforms under ‘Doi Moi’, launched in 1986, established Vietnam as the fastest-growing economy in the world.18 It is estimated that between 2002 and 2018, 45 million people were lifted out of poverty,17 bringing remarkable improvements in public health. Notable progress has been made in access to HBV vaccination, blood donor screening and, more recently, government subsidisation of HBV and HCV therapy, altering the shape and scope of the hepatitis epidemic and shifting public health priorities. This is the first systematic review of its kind in Vietnam, and assimilates all published data on HBV, HCV and HDV seroprevalence since 1990, in one of the highest burdened countries in the world. We evaluate HBV, HCV and HDV seroprevalence chronologically, geographically and by individual's exposure risk, with the aim of informing future health policy.
Methods
This systematic review was performed in accordance with PRISMA guidance and a full checklist is provided in the supplements section. It was registered with Centre for Reviews and Dissemination (CRD) in September 2020 (PROSPERO CRD42020202567). The study conforms to its original protocol, with subsequent addition of chronological and HIV co-infection analyses.
We searched Medline, Embase and Global Health - Ovid® (Wolters Kluwer) from 1st January 1990 to 31st December 2021 for all reports that contained data for HBV, HDV and HCV seroprevalence in Vietnam. Using a free text search strategy, we entered the search terms [‘Hepatitis B’ OR ‘Hepatitis C’ OR ‘Hepatitis D’] AND [‘Vietnam’] AND [‘Prevalence’] (full details in appendix). We included both prospective and retrospective studies with manuscripts published in English, French or Vietnamese language. This included published surveys from screening programmes, antenatal clinics, blood donations, sexual health and HIV clinics, needle and syringe programmes for people who inject drugs (PWID), commercial sex worker initiatives, and inpatient, outpatient and community serosurveys.
For HBV we included studies reporting HBsAg, the diagnostic marker of infection. For HCV and HDV, we separated studies reporting antibody (a marker of past exposure, but not necessarily active infection), from those reporting antigen or RNA (markers of active infection) for separate analyses.
Non-peer reviewed conference abstracts, and studies not stating sample size or HBV, HCV or HDV seroprevalence were excluded, as were studies involving Vietnamese patient populations from outside of Vietnam (e.g. USA) or studies exclusively published in Vietnamese without evidence of peer review.
BF (first author) performed the literature search, screened all abstracts, and extracted data from prevalence studies. HVTK (third author) independently reviewed the abstract search, reviewed all manuscripts written in Vietnamese, checked 49 excluded studies, and assisted with data entry. Discrepancies regarding study eligibility were resolved through discussion between investigators (HVTK, BF) and the senior author (GC).
Study title, authors, study type & design and seroprevalence data for extraction and synthesis was documented on a spreadsheet with predetermined dropdown lists where applicable. We recorded year of publication, year(s) of data collection, region of Vietnam, study population, study type, exposure risk, sample size, HBsAg and/or HCV antibody seroprevalence, HCV antigen and/or PCR prevalence, HDV antibody and HDV RNA prevalence, and prevalence of HIV co-infection. We also recorded HBV and HCV co-infection prevalence in HIV infected populations.
Study populations were classified by risk. ‘High risk’ populations were subcategorized into patients with known liver disease (acute hepatitis, chronic hepatitis, hepatocellular carcinoma (HCC)), and patients with high-risk exposure to blood borne viruses. The latter included i) people who inject drugs (PWID), ii) commercial sex workers (CSW) iii) men who have sex with men (MSM) attending sexual health services, iv) dialysis patients v) individuals who have had multiple transfusions or major surgery and vi) (for HBV only) children of HBsAg positive mothers.
‘Low risk’ was defined as absence of any of the above risk factors. To limit sampling bias we further subdivided low-risk groups into blood donors and non-blood donors in recognition that blood donors are risk-assessed prior to testing and may not representative of the general population. Non-blood donors were further split into i) antenatal patients, ii) adults in the general population (including community studies, outpatient studies and occupational surveys), iii) children in the general population and iv) inpatients with non-hepatic illness. Methodological quality of all selected studies was assessed using the Joanna Briggs Institute (JBI) critical appraisal checklist for prevalence data.19
Meta-analysis
For most populations our systematic review and study quality assessment met pre-determined conditions for sub-group meta-analysis – namely that study populations, years of data, locations, diagnostic tests, and sampling strategies were adequately described and comparable. However, the qualitative heterogeneity between the blood donor studies and other low-risk populations studies was so great that it did not make sense to combine all low-risk populations (including blood donors) to derive a single summary estimate.
Statistical Analysis
We determined point estimates and 95% CIs for the proportion of people with HBsAg, HCV antibody, HCV core antigen or PCR, HDV antibody and HDV RNA where available. In light of substantial between-study heterogeneity, data from each study were pooled with a DerSimonian-Laird random-effects model,20 which estimates between-study variance, allowing that the true effect size may vary between studies. Different populations from the same study were combined provided they were from same decade, population and geographical region. From this we estimated the overall prevalence of HBsAg, HCV antibody and HCV antigen in low-risk groups (blood donors and other low risk populations), those at high risk of exposure and those with liver disease. We further determined prevalence of each virus by population subgroup.
For the chronological analysis, we assessed pooled prevalence by decade (1990-2000; 2001-2010; 2011-2020). In high-risk populations we used a different approach for each virus. For HBV the difference in risk between various high-risk exposures is small, and it is recommended that all high-risk individuals are vaccinated. Therefore, we estimated pooled prevalence by decade in all high-risk exposures combined. For HCV there is no vaccine, and PWID are at highest risk of infection by far. Therefore, we restricted chronological and regional analysis of HCV to PWID populations, in which between study heterogeneity was minimal.
We utilised the t2 statistic to assess between-study heterogeneity for the estimates of pooled prevalence by population and by decade. The variance of raw proportions was stabilised with a Freeman-Tukey type arcsine square root transformation.21 There are several methods available for pooling proportions; the Freeman-Tukey method works well with both fixed-effects and random-effects meta-analysis.22 Statistical analysis was performed on R version 4.1.23
Role of the funding source
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Results
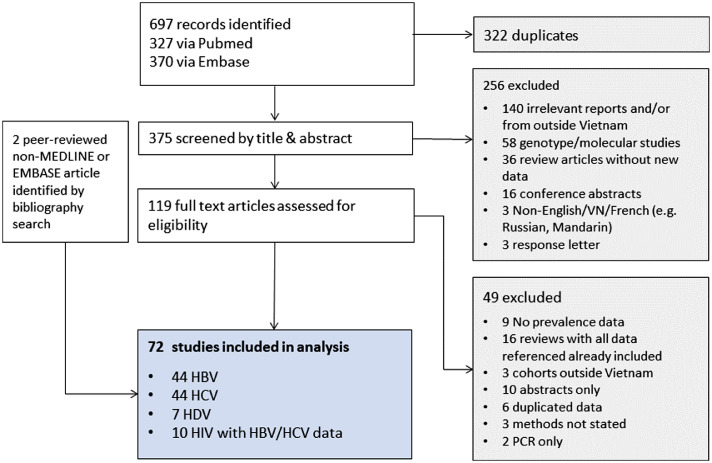
We analysed 72 studies in total, representing 22 different locations in Vietnam (Figure 1). This included 501,543 individuals tested for HBsAg in 120 cohorts, 448,765 individuals tested for HCV (antibody or antigen/RNA) in 114 HCV cohorts, and 7055 individuals tested for HCV or HBV co-infection in 13 HIV cohorts. Most studies included populations from the three largest cities in Vietnam, HCMC (29), Hanoi (24) and Hai Phong (16), while rural representation was low.
Figure 1.
Study selection.
Study quality was generally good, but only 8 studies met all nine critical appraisal checklist criteria for prevalence data19 (Table 1; supplementary table 6). The most frequently identified deficiency was in sampling, with 82% (59/72) studies relying on non-random consecutive, or response-driven sampling or including an entire single centre population. Sampling was most rigorous in the low-risk general population, with 6/14 studies apparently truly cross sectional in nature.
Table 1.
Summary of 72 included studies.
| Study | Year(s) of data | Region(s) | Study population(s) | Population categories | Prevalence data | JB score* | Potential bias |
|---|---|---|---|---|---|---|---|
| Barcus et al 2002 | 1992-96 | HCMC | Inpatients with severe malaria | Low risk | HBsAg | 7 | Non-random consecutive sampling, non- representative sample (severe malaria) |
| Binh et al 2018 | 2013-15 | Hanoi | HBV positive outpatients | HDV | HDV RNA | 7 | Non-random consecutive sampling, non-representative sample (85% male) |
| Boettiger et al 2015 | 1998-13 | Hanoi | HIV outpatients | HIV population | HBsAg, HCV Ab | 8 | Non-random consecutive sampling |
| Buchy et al 2004 | 2002 | Nha Trang | Inpatients with hepatitis | High risk (liver disease) | HBsAg, HCV Ab | 7 | Non-random consecutive sampling, underpowered |
| Bùi et al 2014 | 2005-11 | Hanoi | HIV outpatients | HIV population | HBsAg, HCV Ab | 8 | Non-random; entire centre's population |
| Chau et al 2002 | 1991-96 | HCMC | Inpatient PWID with malaria, inpatient non-PWID with malaria | High risk (exposure), low risk | HBsAg, HCV Ab | 8 | Non-random consecutive sampling |
| Clatts et al 2009 | 2005-6 | Hanoi | PWID | High risk (exposure) | HCV Ab, HIV coinfection | 7 | Non-random sampling; non-representative sample (male IVDU only) |
| Clatts et al 2015 | 2010-11 | Hanoi, HCMC, Nha Trang | MSM sex workers | High risk (exposure) | HCV Ab | 8 | Non-random; non-representative (sex workers) |
| Colby et al 2016 | 2014 | HCMC | MSM sex workers | High risk (exposure) | HCV Ab, HIV coinfection | 8 | Non-random sampling; non-representative (sex workers) |
| Cordier et al 1993 | 1989-92 | Hanoi | Inpatients, patients with HCC | Low risk, high risk (liver disease) | HBsAg, HCV Ab | 8 | Non-random, non-representative, male HCC only |
| Corwin et al 1996 | 1993-95 | Hanoi | Adults in general population, inpatients with hepatitis | Low risk, high risk (liver disease) | HBsAg, HCV Ab | 8 | Non-random consecutive sampling |
| Dang et al 2020 | 2019 | Hanoi | MSM with HIV and non-MSM with HIV | HIV population | HBsAg, HCV Ab | 8 | Non-random consecutive sampling |
| Do et al 2015 | 2012 | Binh Thuan | Adults in general population | Low risk | HBsAg, HCV Ab, HCV Ag | 9 | |
| Dunford et al 2012 (HBV) | 2008-9 | Hanoi, Haiphong, Danang, Khanh Hoa, Can Tho | Military, antenatal, adults in general population, blood donors, PWID, dialysis, CSW, multiple transfusions, surgical | Low risk, high risk (exposure), HDV | HBsAg, HDV Ab | 8 | Non-random consecutive sampling |
| Dunford et al 2012 (HCV) | 2008-9 | Hanoi, Haiphong, Danang, Khanh Hoa, Can Tho | Military, antenatal, adults in general population, blood donors, PWID, dialysis, CSW, multiple transfusions, surgical | Low risk, high risk (exposure) | HCV Ag/RNA | 8 | Non-random consecutive sampling |
| Duong et al 2009 | 2006 | Thai Nguyen | Adults in general population | Low risk | HBsAg | 8 | Cross sectional but non-representative rural sample |
| Duong et al 2015 i | 2012-2013 | HCMC | Dialysis | High risk (exposure) | HCV Ag/RNA | 8 | Non-random consecutive sampling |
| Duong et al 2015 ii | 2012-2013 | HCMC | Dialysis | High risk (exposure) | HBsAg, HCV Ag | 8 | Non-random consecutive sampling |
| Duong et al 2016 | 2012-2014 | HCMC | Dialysis | High risk (exposure) | HBsAg, HCV Ag | 8 | Non-random; entire centre population |
| Duong et al 2018 | 2014 | Hai Phong | PWID | High risk (exposure) | HCV Ab | 8 | Non-random consecutive sampling |
| Duong et al 2019 | 2012-2014 | HCMC | Dialysis | High risk (exposure) | HCV Ab, ACV Ag, HCV RNA | 8 | Non-random; entire centre population |
| Follezou et al 1999 | 1996 | HCMC | PWID | High risk (exposure) | HBsAg, HCV Ab | 6 | Non-representative sample (very high rates HIV) |
| Goto et al 2005 | 2003 | Nghe An | Antenatal | Low risk | HBsAg | 8 | Non-random consecutive sampling |
| Hall et al 2015 | 2010-11 | Hanoi, Hai phong, Da Nang, Khanh Hoa, Can Tho | HBV positive PWID | High risk (exposure) | HDV Ab, HDV RNA | 7 | Non-random consecutive sampling, lacks baseline characteristics |
| Hipgrave et al 2003 | 1990-99 | Thanh Hoa | Children in general population, adults in general population | Low risk | HBsAg | 9 | |
| Hoang et al 2015 | 2009 | Hai Phong, HCMC | PWID | High risk (exposure) | HBsAg, HCV Ab | 8 | Non-random consecutive sampling |
| Ishizaki et al 2017 | 2007-2012 | Hai Phong | Blood donors, antenatal, PWID, CSW | Low risk, high risk (exposure) | HBsAg, HCV Ag, HIV coinfection | 8 | Non-random consecutive sampling |
| Kakumu et al 1998 | 1994-96 | HCMC, Da Lat | Chronic hepatitis; adults in general population | Low risk, high risk (liver disease) | HBsAg, HCV Ab | 8 | Non-random consecutive sampling of hepatitis patients. Details of sampling strategy for general population lacking |
| Katelaris et al 1995 | 1993 | Dong Nai | children in general population | Low risk | HBsAg, HCV Ab | 8 | Under powered for HCV prevalence |
| Kha To et al 2020 | 2017-19 | HCMC | blood donors | Low risk | HBsAg, HCV Ab | 8 | Non-random consecutive sampling |
| Lan et al 2008 | 2006 | Hanoi | Adults in general population | Low risk | HBsAg | 8 | Non-representative sample (married women age 18-49) |
| Lien et al 1997 | 1994 | HCMC | Adults general population, CSW, PWID, HIV patients | Low risk, high risk (exposure) HIV population | HCV Ab | 8 | Non-cross sectional sampling |
| Linh-Vi et al 2019 | 2013 | Hanoi, Hai Phong, HCMC | CSW | High risk (exposure) | HBsAg, HCV Ab, HCV Ag, HIV coinfection | 9 | |
| Minh et al 2021 | 2018-20 | Hue | Adults in general population | Low risk | HBsAg | 7 | Non-random, non-representative sample (males from infertile couples) |
| Miyakawa et al 2021 | 2009-12 | Khan Hoa | antenatal, children in general population | Low risk | HBsAg | 7 | Non-random sample, high drop out >30% |
| Mohan et al 2017 | 2012-15 | Hanoi, Pho Yen, Thai Nguyen | HIV outpatients | HIV population | HBsAg, HCV Ab | 8 | Non-random retrospective chart review |
| Molès et al 2020 | 2014 | Hai Phong | PWID | High risk (exposure) | HCV Ab | 8 | Non-random response-driven sampling |
| Nadol et al 2015 | 2009-10 | Hanoi, Hai Phong, Quang Ninh, Nghe An, Yen Bai. Da Nang, Dong Nai, HCMC. Can Tho, An Giang | PWID | High risk (exposure) | HBsAg, HCV Ag, HIV coinfection | 8 | Non-random response-driven sampling |
| Nadol et al 2016 | 2009-10 | Hanoi, Hai Phong, HCMC, Can Tho | MSM | High risk (exposure) | HBsAg, HCV Ag, HIV coinfection | 8 | Non-random response-driven sampling |
| Nakata et al 1994 | 1993 | Hanoi, HCMC | inpatients, multiple transfusions, PWID. Dialysis, CSW, prisoners | Low risk, high risk (exposure) | HBsAg, HCV Ab | 8 | Non-random consecutive and retrospective sampling |
| Nerurkar et al 1999 | 1997-98 | Hanoi | PWID | High risk (exposure) | HCV Ab, HIV coinfection | 7 | Non-random sampling, diagnostics were combo of sera or filter paperblotted whole blood |
| Nghiem et al 2021 | 2018-19 | Hanoi | HBV positive outpatients | HDV | HDV RNA | 8 | Non-random, consecutive sampling |
| Ngo et al 2009 | 2007 | HCMC | General inpatients | Low risk | HBsAg, HCV Ab | 6 | Non-random, consecutive sampling, non-representative sample (inpatients and outpatients), minimal baseline characteristics |
| Nguyen et al 1997 | 1995 | HCMC | Inpatients with Dengue | Low risk | HBsAg, HCV Ab | 6 | Non-random consecutive sampling, non-representative sample (patients with severe Dengue), under-powered for HCV |
| Nguyen et al 2006 | 2002 | Thai Binh | Adults general population | Low risk | HBsAg | 9 | |
| Nguyen et al 2007 | 2002 | Thai Binh | Adults general population | Low risk | HCV Ab | 9 | |
| Nguyen et al 2011 | 2007 | Hai Phong | Adults in general population, antenatal, blood donors | Low risk | HBsAg | 8 | Non-random consecutive sampling |
| Nguyen et al 2014 | 2011 | Vietnam (national) | Children in general population | Low risk | HBsAg | 9 | |
| Nguyen et al 2017 | 2015 | Da Nang | HBV positive outpatients | HDV | HDV RNA | 7 | Non-random consecutive sampling, non- representative |
| Nguyen et al 2021 | 2017 | Thai Nguyen | MSM in sexual health clinics, PWID | High risk (exposure) | HCV Ab | 7 | Non-random sampling, Oraquick diagnostics |
| Nguyen-Dinh et al 2018 | 2010-16 | HCMC | Patients with HCC | High risk (liver) | HBsAg, HCV Ab | 8 | Non-random retrospective sample |
| Pham et al 2020 | 2017-18 | Hai Phong | Antenatal, children of HBV infected mothers | Low risk, high risk (exposure) | HBsAg | 9 | |
| Pham et al 2020 ii | 2018 | Central Highlands | Adults in general population | Low risk | HBsAg | 9 | |
| Quan et al 2009 | 2003 | Bac Ninh | PWID | High risk (exposure) | HBsAg, HCV Ab, HIV coinfection | 8 | Non-random snowball sampling using peer recruiters |
| Quesada et al 2015 | 1994-05 | HCMC | Adults in general population | Low risk | HCV Ab | 8 | Non-representative sample (females only) |
| Rangarajan et al 2016 | 2013-14 | HCMC | HIV outpatients | HIV population | HBsAg, HCV Ab | 8 | Non-random consecutive sampling |
| Riondel et al 2020 | 2016-17 | Hai Phong | PWID | High risk (exposure) | HCV Ab | 8 | Non-random response-driven sampling |
| Sinh et al 2012 | 1992-09 | HCMC | Dialysis | High risk (exposure) | HCV Ab | 7 | Non-random consecutive sample, lacking baseline characteristics |
| Son et al 2014 | 2006-09 | Hai Phong | HIV inpatients | HIV population | HBsAg, HCV Ab | 6 | Non-random consecutive sampling, non-representative (inpatients with penicilliosis), under powered for HBV/HCV prevalence |
| Song et al 1994 | 1992 | HCMC, Hanoi | blood donors | Low risk | HBsAg, HCV Ab | 8 | Non-random sampling |
| Sy et al 2013 | 2000-09 | Hanoi | HBV positive outpatients | HDV | HDV RNA | 7 | Non-random consecutive sampling, non-representative (HCV and HIV positive patients excluded) |
| Tanimoto et al 2010 | 2007 | Hai Phong | PWID | High risk (exposure) | HCV Ab, HCV PCR | 8 | Non-random response driven sampling |
| Tanuma et al 2017 | 2007-13 | Hanoi | HIV outpatients | HIV population | HBsAg, HCV Ab | 8 | Non-random consecutive sampling |
| Terakawa et al 2011 | 2009-10 | HCMC | Adults in general population | Low risk | HBsAg, HCV Ab | 7 | Non-random sampling, non-representative (healthy workers at major companies) |
| Thanh et al 2020 | 2016-17 | HCMC | Hepatitis outpatients | High risk (liver) | HBsAg | 7 | Non-random consecutive sample, non-representative (HCV-infected outpatients) |
| Tran et al 2003 | 1998-02 | HCMC | Adults in general population, patients with liver disease | Low risk | HCV RNA, HDV Ab | 7 | Non-random sampling, non-representative (healthy outpatients) |
| Truong et al 2016 | 2014 | Hai Phong | HIV outpatients | HIV population | HBsAg | 8 | Non-random consecutive sample |
| Trung et al 2010 | 2006-08 | HCMC | Inpatients with dengue and non-dengue acute infections | Low risk | HBsAg | 8 | Non-random consecutive sampling |
| Van Be et al 1992 | 1989-91 | HCMC | Blood donors, adults in general population, inpatients, prsioners, CSW, PWID | Low risk, high risk (exposure) | HBsAg | 6 | Non-random sampling, unclearly defined study populations, no basseline characteristics |
| Van Quang et al 2019 | 2010-17 | Hanoi | Patients with HCC | High risk (liver) | HBsAg | 8 | Non-random, restrospective sample |
| Viet et al 2012 | 2007 | Quang Tri | Blood donors | Low risk | HBsAg, HCV Ab | 7 | Non-random sampling; non-representative (potential blood donors, HBV-vaccinated individuals excluded) |
| Zhang et al 2015 | 2005-07 | Thai Nguyen | PWID | High risk (exposure) | HCV Ab, HIV coinfection | 8 | Non-random sampling |
HBsAg = Hepatitis B surface antigen; HCV Ab = Hepatitis C antibody; HCV Ag = Hepatitis C antigen; HDV Ab = Hepatitis D antibody; RNA = ribonucleic acid.
Blood donors
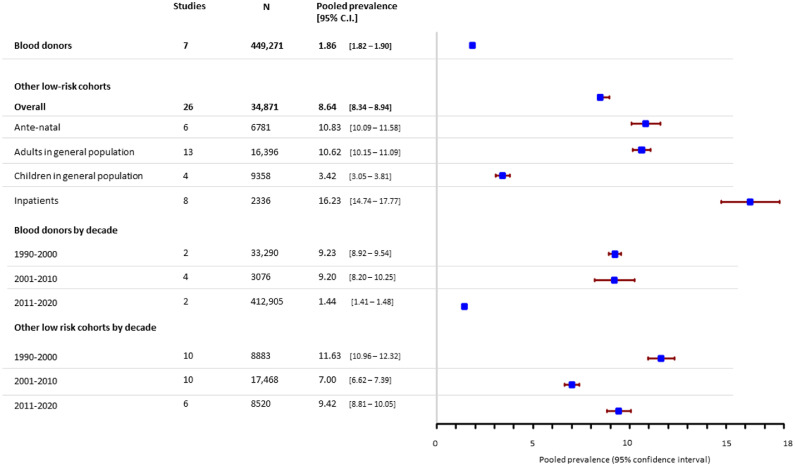
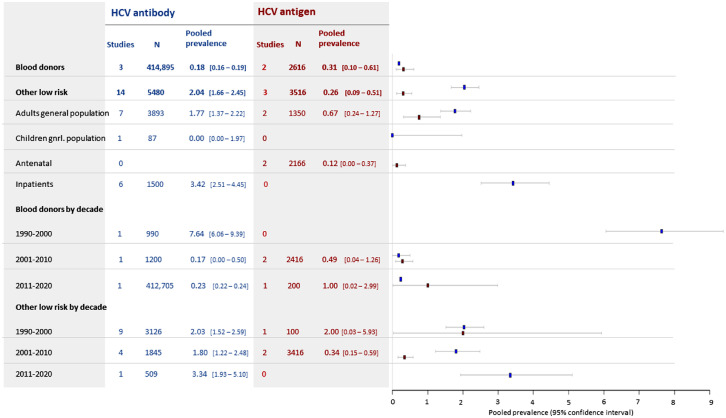
Blood donor screening studies contributed the largest study populations, such that 90% of all included individuals tested for HBsAg and 93% tested for HCV were blood donors. Overall infection rates in this population were lower than is reported in the general population (see next section), with an HBsAg point prevalence of 1.86% (95% C.I. 1.82 - 1.90) (Figure 2), an HCV antibody prevalence of 0.18% (0.16 - 0.19) and HCV antigen prevalence of 0.31% (0.10 – 0.61) (Figure 3). Pooled HBsAg prevalence in blood donor cohorts from prior to 2011 was around 9%, with apparent improvement in pre-screening in the last decade, when it fell to 1.44% (1.41 – 1.48). HCV prevalence in blood donors was extremely high when it was first discovered in the 1990s (7.6% [6.1 – 9.4]) but is around 0.2% overall in studies since 2001.
Figure 2.
Estimated pooled seroprevalence of HBsAg in low-risk populations.
Figure 3.
Estimated pooled seroprevalence of HCV antibody (blue) and HCV antigen/PCR (red) in low-risk populations.
Low risk (non-blood donors)
Overall prevalence of HBsAg in non-donor low-risk groups was 8.6% (8.3 – 8.9). It was lowest in children in the general population (3.4% (3.1 – 3.8)) but was 10.8% [10.1-11.6] in antenatal women and 10.6% [10.2-11.1] in adults from the general population. HBsAg prevalence was high in inpatients presenting with non-hepatic illness (16.2% (14.7 – 17.8)), which included patients admitted with Dengue24 and Malaria.25,26 Pooled prevalence of HBsAg in low-risk non-donors fluctuated from 11.6% (11.0 – 12.3) in studies from 1990 to 2000, 7.0% (6.6 – 7.4)) in 2001-2010 and 9.4% (8.8 – 10.1) in studies since 2011.
For HCV, pooled antibody prevalence in non-blood donor low-risk populations was 2.0% (1.7 – 2.5) and HCV antigen prevalence was 0.26% (0.09 – 0.51). We found no significant change in prevalence of HCV antibody in non-blood donor low-risk populations by decade. There was insufficient data to assess whether prevalence of HCV antigen has changed (Figure 3).
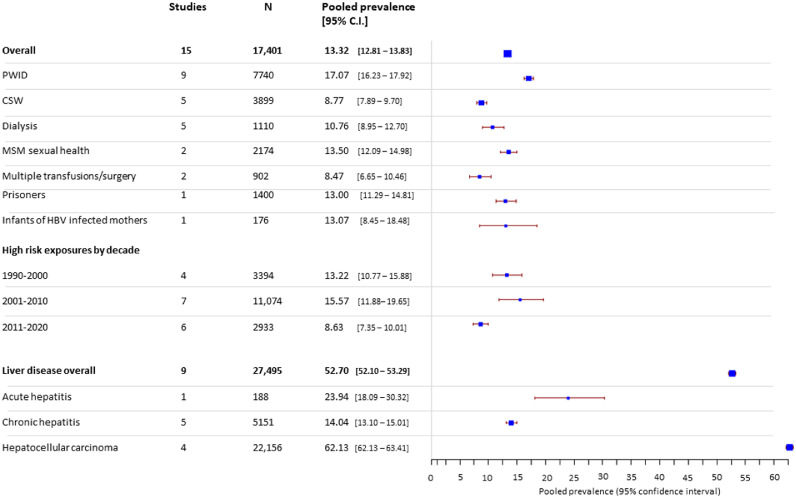
High-risk
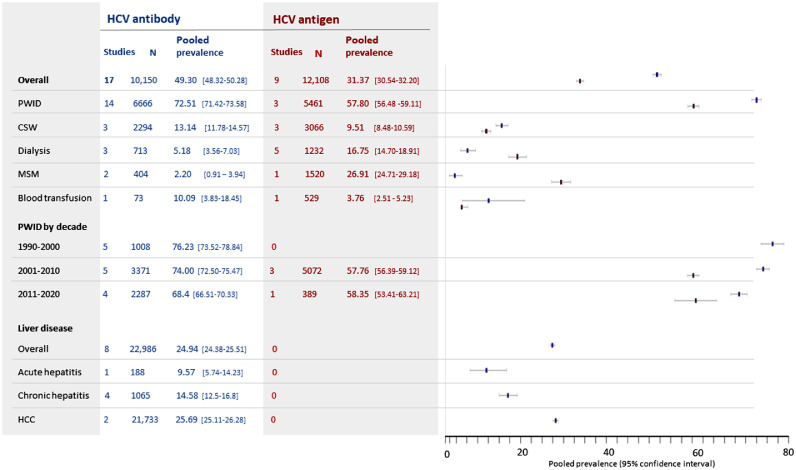
In high-risk groups, overall pooled prevalence of HBsAg was 13.3% (12.8 – 13.8) (Figure 4). Prevalence of HBsAg in individuals undergoing haemodialysis, blood transfusion or surgery was similar or lower than observed in non-blood donor low-risk groups (8-10%). In contrast, rates of HCV infection were significantly elevated in these populations, with 16.8% (14.7-18.9) of dialysis patients showing evidence of active HCV infection (Figure 5).
Figure 4.
Estimated pooled seroprevalence of HBV in high-risk populations.
Figure 5.
Estimated pooled prevalence of HCV antibody (blue) and HCV antigen/PCR (red) in high-risk groups.
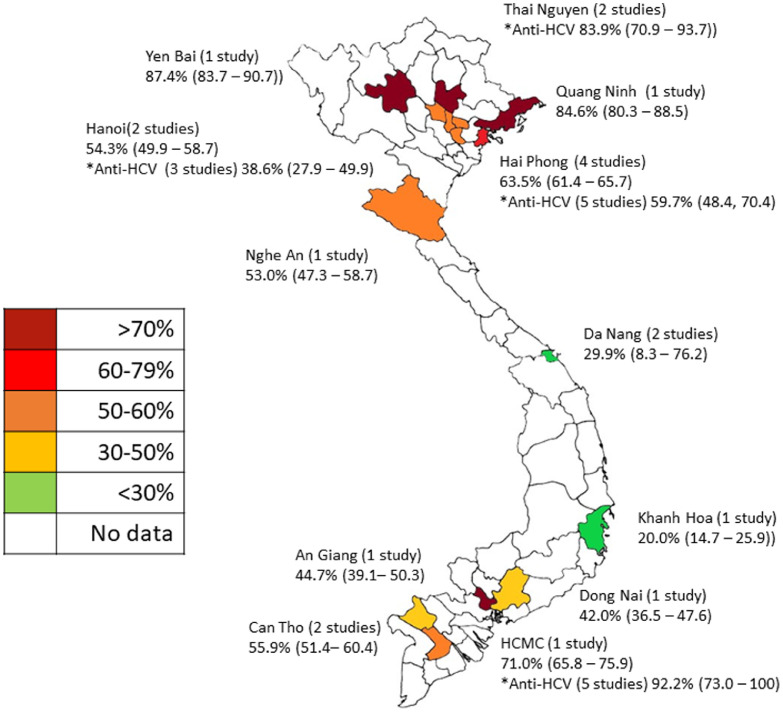
Overall, for HCV in high-risk groups, pooled prevalence was 49.3% [48.3 – 50.3]) for HCV antibody and 31.4% (30.6 – 32.2) for HCV antigen (Figure 5). These figures were heavily influenced by very high prevalence of HCV in PWID, with evidence of past HCV exposure in 72.5% (71.4 – 73.6) of PWID tested, and active infection in 57.8% (56.5 – 59.1) (Figure 5). Extremely high rates of active HCV infection were reported in in the northern provinces of Yen Bai (87.4% [83.7 – 90.7])) and Quang Ninh (84.6% [80.3 – 88.5]))27 (Figure 6) and the southern metropolis of Ho Chi Minh City (92.2% [73.0 –100] HCV antibody, five studies26,28, 29, 30, 31 and 71.0% (65.8 – 75.9) HCV antigen, one study27) (Figure 6).
Figure 6.
HCV antigen prevalence (and antibody where available) in PWID by region.
Prevalence pooled for locations with more than one study.
Only six studies described both HCV antibody and antigen/RNA prevalence in the same individuals (Table S6). In one PWID cohort32 79.3% (74.4 – 83.6) of individuals testing positive for HCV antibody had evidence of current infection. This proportion was lower among individuals with liver disease (60.9% [48.3 – 72.4])33 and in sex workers (58.5% [52.4 – 64.4],34 and in adults the general population (50.0% [26.0 – 74.0]35 and 44.4% [13.70 - 78.8],33 which likely reflects less frequent exposure. A fifth study in individuals undergoing haemodialysis36 found HCV antigen prevalence exceeded antibody prevalence (12.9% [8.6 – 18.4] vs 5.5% [2.8 - 9.6]). This surprising finding may reflect a high number of acute HCV infections associated with the dialysis unit concerned, or defective antibody generation in the context of frequent exposure to HCV from haemodialysis. However, numbers were small.
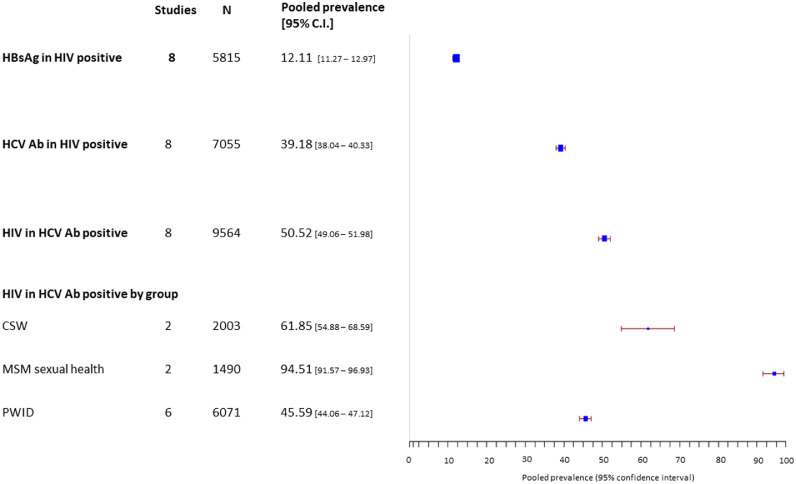
In HIV positive cohorts, 12.1% (11.3 – 13.0) were HBsAg positive and 39.2% (38.0 – 40.3) were HCV antibody positive (Figure 7). Although baseline characteristics were available, it was not possible to ascertain specific risk factors in coinfected individuals, such as past injecting drug use or high-risk sexual activity. Only one study compared HCV co-infection in HIV positive MSM vs HIV positive heterosexual men attending the same HIV service.37 Injecting drug use was more prevalent in HIV positive heterosexuals than in MSM (46.8% vs 2.4%). Consequently, HIV-HCV co-infection was higher in heterosexual males (55%) than MSM (4.9%).
Figure 7.
Estimated pooled prevalence of i) HBsAg and ii) HCV antibody in HIV positive populations and iii) HIV co-infection in HCV-antibody positive populations.
Among 4676 HCV-infected individuals in 27 high-risk cohorts screened for HIV, 50.52% (49.1 – 51.2) were HIV co-infected. HIV co-infection was extremely prevalent in HCV antibody positive MSM, with 94.5% (91.6 – 97.0) testing positive. It was less prevalent in PWID (45.6% (44.1 – 47.1), reflecting different routes of exposure: HCV is more likely to be accompanied by HIV when sexually acquired.
Among over 20,000 individuals with Hepatocellular carcinoma (HCC), a very high prevalence of both HBsAg (62.8% (62.1 – 63.4)) (Figure 4) and HCV antibody (25.7% (25.1 – 26.3)) (Figure 5) was observed, highlighting the devastating consequences of these infections.
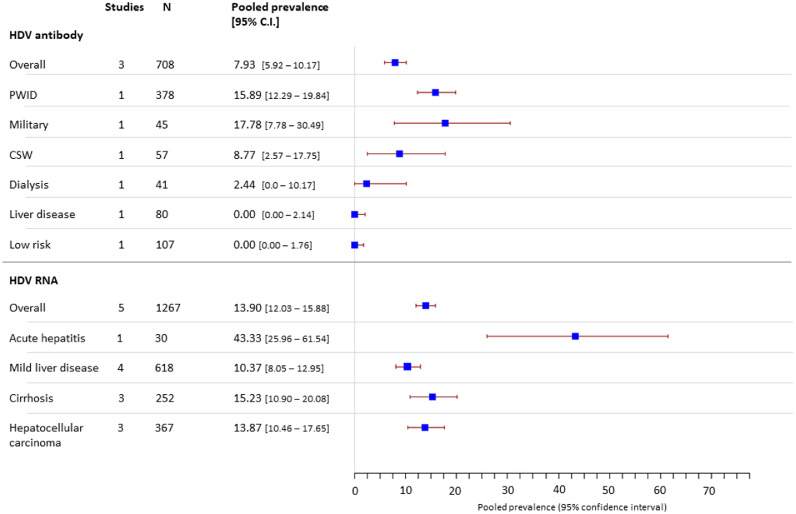
Hepatitis D
We included 1975 individuals tested for HDV antibody or HDV RNA in 23 HDV cohorts. Of 708 HBsAg positive individuals tested for HDV antibodies in 12 cohorts, 7.9% (5.9 – 10.2) were positive (Figure 8). Highest rates of HDV infection were seen in PWID. One study of 45 HBsAg positive military recruits found 17% (8 - 32) were HDV antibody positive38 but no cases of HDV were detected in other larger low risk cohorts. We found highest prevalence of HDV RNA in individuals presenting with acute hepatitis (43.3% [26.0 – 61.5]), suggesting HDV may be an under-recognised cause of this presentation in Vietnam. Only 4 studies included genotype data in 115 individuals. Of these 74% had genotype 1 infection and 26% had genotype 2.
Figure 8.
Estimated pooled prevalence of HDV antibody and HDV RNA in HBsAg positive cohorts.
Discussion
This study is the most comprehensive review of HBV, HCV and HDV seroprevalence in Vietnam and adds important granularity to our understanding of the hepatitis epidemic.
We found that pre-screening of blood donors in Vietnam has improved significantly in the last 30 years, with very low rates of both HBV and HCV infection detected in blood donors compared with the general population. This improvement may be attributable to the prohibition of paid and family/replacement blood donation since 2013,39 with a successful switch to voluntary unpaid blood donation, supported by an annual “All People's Voluntary Blood Donation Day”.40 In addition, in the last decade rapid HBsAg testing has become mandatory for all new blood donors prior to blood donation.39
In contrast, the pooled prevalence of HBV in other low-risk populations was high, exceeding 10% in all non-donor adult cohorts. A high HBsAg prevalence in inpatients with non-hepatic illness may reflect the increased all-cause morbidity associated with chronic liver disease. HBV prevalence in groups at high-risk of exposure was similar, or only modestly elevated compared to low-risk populations. This may be explained by high rates of vaccination in high-risk groups and a reduced risk (<10%) of chronic infection when exposed to HBV in adulthood.41
The lower HBsAg prevalence in children (3.4% (3.1 – 3.8)) is somewhat reassuring. HBsAg positivity in children was >15% in two studies from the 1990s42,43 compared to 2.7% (2.2 - 3.3) in a national study from 201144 and 1.9% (1.2 - 2.7) in a study from Central Vietnam with data from 2009-2012.45 This change is a direct consequence of vaccination, which has been included in Vietnam's national vaccine program since 1997 and was expanded to a cost-free 4-dose schedule for all new-borns in 2004, including birth dose vaccination within 24h of delivery. Scale up of this vaccine series has had a profound impact on horizontal transmission in early childhood,44 which will become apparent in future surveys of the adult population. However, this has made vertical transmission proportionally more dominant.46
Despite a concerted effort in the last decade to improve delivery of birth dose vaccine, coverage is not yet perfect, being below the WHO target of 90%. A 2019 study found that only 63% of children in Vietnam received birth dose vaccine, with lowest uptake seen in poor, rural communities and among ethnic minorities.47 Recent data from Haiphong showed that 13.1% (8.5-18.5) of children of HBV-infected mothers were HBsAg positive.3 Given the high rates of chronic infection resulting from HBV acquired in infancy (>90%),41 more needs to be done to reduce the perinatal transmission driving Vietnam's HBV epidemic.
Prophylactic antiviral treatment for HBsAg positive expectant mothers in the final trimester of pregnancy has been recommended by Vietnam's MOH since 2014, and nucleos(t)ide analogue drugs for this purpose are now covered by health insurance. However, many pregnant women lack this basic cover, and antenatal care in rural settings is frequently inadequate, with one study indicating only one fifth of rural women receive sufficient core antenatal services according to national recommendations.48 Hepatitis B immunoglobulin (HBIG) is also recommended at birth for all children born of HBsAg-positive mothers. However, HBIG is a blood product that requires infection screening and a cold chain, making delivery to resource-poor regions problematic. Where available, HBIG costs around US$100/dose, which must be fully paid by the parents and access is very limited. Even when these preventative measures are implemented appropriately, 8–30% vertical transmission still occur in infants born to HBeAg-positive women,49,50 indicating research into additional interventions is warranted.
In contrast to HBV, our findings indicate that HCV infection is probably less common in the general population than previous estimates suggest. One possible reason for this is that studies prior to 2012 were generally limited to measuring HCV antibody - a marker of past HCV exposure but not active infection. Estimates for the number of active infections were inferred from population studies measuring both antibody and antigen, in which high-risk individuals from high-income settings are over-represented.51 Given 15-45% of acute HCV infections spontaneously resolve without treatment,52 the proportion of antibody positive individuals with active infection will be lower in low-risk groups compared to those with repeated exposure, such as PWID. Our data illustrate this point, with very low prevalence of active HCV infections in general population cohorts, and a high prevalence of active infections in PWID, MSM and dialysis patients. The risk of over-estimating prevalence of active HCV infection may be greater in low- and middle-income countries, in which a greater proportion of antibody positive individuals are low-risk and repeat exposure risk is hard to quantify. This has important implications for health policy, which is currently insufficiently loaded towards those at highest risk.
We identified an extremely high prevalence of HCV in PWID, with antibody positivity (72.5% [71.4 – 73.6]) 39% higher than global estimates from a 2017 meta-analysis (52.3% [42·4–62·1]).53 PWID represent a key target population for ending the HCV epidemic and are estimated to contribute to nearly 40% of disability-adjusted life-years (DALYs) due to HCV worldwide.54 In Vietnam preventative interventions have been implemented since 2008, including opioid substitution therapy, universal antiretroviral treatment (ART) for HIV-infected PWID, and financing of community-based organisations to deliver harm reduction and distribute free syringes.55 In 2015 the MOH expanded methadone treatment to at least 30 provinces to provide treatment for more than 80,000 drug users.56 While these initiatives have been effective in reducing the incidence of HIV, incidence of HCV seroconversions in PWID remains very high57 and we found no significant reduction in HCV antigen prevalence in the last decade.
Accumulating evidence shows that PWID can achieve high cure rates with DAA drugs, comparable with other populations,58 reducing the risk of onward transmission of HCV in the process. In 2019 the government began subsidizing 50% HCV treatment costs for those with health insurance. However, many PWID lack coverage and treatment remains expensive - a 12 week course of sofosbuvir and daclatasvir currently costing US$ 1347.59 In 2021 the Global Fund committed to providing free DAA therapy to 16,000 HIV/hepatitis C co-infected patients at HIV treatment facilities across Vietnam.60 This represents a positive step, but given less than 50% of HCV antibody positive PWID in our study are co-infected with HIV, most won't be eligible for free treatment through this scheme. This highlights important limitations in current global funding. Provision of free HCV screening and treatment for PWID would have a major impact on reducing the scale of the HCV epidemic in Vietnam.61
Our finding of a high prevalence of active HCV infection among patients on dialysis is concerning. Given that chronic HCV infection is associated with a range of renal pathologies including type 1 membranoproliferative glomerulonephritis, focal segmental glomerulosclerosis, and interstitial nephritis, some of these patients may have end-stage renal failure because of HCV acquired some years previously. However, given the high rates of HCV antigen in this population it is likely many individuals are acquiring HCV from dialysis, highlighting a need for improved infection control and an HCV vaccine.
HDV infection in Vietnam remains poorly characterised. We found just seven studies assessing HDV prevalence and most cohorts were small. The largest HDV antibody cohort surveyed just 97 HBV infected individuals across five provinces, and the largest RNA cohort included just 250 individuals. Across all studies, only 115 HDV infections were genotyped, with genotype 1 appearing dominant.
Our finding that 7.9% (5.9 – 10.7) of individuals with HBV may have HDV co-infection is highly skewed by the nature of the cohorts surveyed, with >50% individuals tested for HDV antibody coming from PWID cohorts. Globally HDV prevalence is frequently over-estimated, as surveys tend to be performed in patients with known HBV infection and liver disease, representing the more severe end of the HBV disease spectrum.15 Despite a high prevalence of HBV in Vietnam, from the limited data available, it appears HDV is not endemic and seems to be concentrated in high-risk groups at risk of repeated exposure to blood borne viruses. This is in keeping with the HDV distribution in Japan, Hong Kong and parts of Europe and quite different to the high community prevalence reported in other parts of Asia such as Pakistan62 and Mongolia.15
HDV is not routinely screened for in Vietnam, partly because of its perceived rarity, but also because drugs licensed to treat HDV (PEGylated IFN alfa-2a, PEGylated IFN alfa-2b and bulevirtide) are not available. Our findings would not support mass screening. However, given the high prevalence in individuals with acute hepatitis, diagnostics should be available and HDV should be included in future treatment guidelines. Given the high prevalence of HBV in Vietnam, the most effective measure to minimise the impact on HDV will be vaccinating high-risk HBV-susceptible individuals against HBV and improving birth dose vaccine coverage.
The major strength of our study is in its breakdown of population groups. The significant difference in HBV and HCV prevalence between blood donors and other low-risk adult populations, highlights the bias inherent in including pre-screened blood donors in prevalence estimates. By estimating pooled prevalence of both HCV antibody and antigen we also highlight the groups most in need of screening and treatment.
Our study has some important limitations. Over 70% of study populations analysed came from just three large cities (figure S1): Ho Chi Minh City, Hanoi and Hai Phong. While these are the most populous cities, this urban focus limits generalisability to the entire country, particularly rural areas where there may be increased risk of community transmission of HCV and lower rates of HBV vaccination. In addition, our search was limited to peer-reviewed articles retrieved from three databases and published in one of three languages; an expanded search including grey literature and non-peer reviewed Vietnamese manuscripts, such as government reports, might contribute additional useful data. However, a lack of methodological detail in the grey literature, such as the sampling strategy or diagnostic tests used, would introduce bias that is harder to quantify.
The quality of studies included was generally good, with Joanna Briggs Institute Systematic Review Index scores of 6-9 (see table S5). However, most were not randomized cross-sectional surveys, and only 11% (8/72) met all nine quality criteria. Low-risk ‘general population’ groups are at highest risk of selection bias, but we mitigated this to some extent by separating adults from children and inpatient studies from community surveys. In addition, MSM studies were universally high-risk populations attending sexual health clinics or engaged in commercial sex work, such that pooled prevalence should not be extrapolated to the wider MSM community. Finally, the DerSimonian–Laird random effects model has been criticized for producing confidence bounds that are too narrow when the number of studies is small or when there are substantive differences among study estimates.63 This is because it fails to capture uncertainty in estimations of between-study and within-study variance when few studies are available for comparison. This may be especially relevant to our smallest subgroups, such as pooled prevalence of HCV in PWID by region and HDV by population.
Larger randomised cross-sectional surveys using high-quality HBV, HCV and HDV PCR as well as serological markers in both urban and rural settings will provide more robust prevalence estimates to inform future hepatitis strategy in Vietnam.
Contributors
BF and GC designed the study. BF drafted the manuscript. BF and DDH conducted the analyses. BF and HVTK verified the data. JD, RG and PMK provided study oversight. All authors have reviewed and approved the final manuscript.
Declaration of interests
No conflicts declared
Acknowledgments
Acknowledgements
GC is supported in part by the NIHR Biomedical Research Centre of Imperial College NHS Trust and an NIHR Professorship.
Data availability
All raw data from 72 analysed studies is provided in Microsoft Excel format in the supplements.
Editor note
The Lancet Group takes a neutral position with respect to territorial claims in published maps and institutional affiliations.
Footnotes
Supplementary material associated with this article can be found in the online version at doi:10.1016/j.lanwpc.2022.100468.
Appendix. Supplementary materials
References
- 1.Cooke GS, Andrieux-Meyer I, Applegate TL, et al. Accelerating the elimination of viral hepatitis: a Lancet Gastroenterology & Hepatology Commission. Lancet Gastroenterol Hepatol. 2019;4(2):135–184. doi: 10.1016/S2468-1253(18)30270-X. [DOI] [PubMed] [Google Scholar]
- 2.Palayew A, Razavi H, Hutchinson SJ, Cooke GS, Lazarus J V. Do the most heavily burdened countries have the right policies to eliminate viral hepatitis B and C? Lancet Gastroenterol Hepatol. 2020;5(10):948–953. doi: 10.1016/S2468-1253(20)30011-X. [DOI] [PubMed] [Google Scholar]
- 3.Khue PM, Thuy Linh NT, Vinh VH, Dung LV. Nguyen Van B. Hepatitis B Infection and Mother-to-Child Transmission in Haiphong, Vietnam: A Cohort Study with Implications for Interventions. Biomed Res Int. 2020;2020 doi: 10.1155/2020/4747965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Huy Do S. Epidemiology of Hepatitis B and C Virus Infections and Liver Cancer in Vietnam. Euroasian J hepato-gastroenterology. 2015;5(1):49–51. doi: 10.5005/jp-journals-10018-1130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Dao D, Mize G, Pham LT, et al. EASL International Liver Fundation: The Vietnam Viral Hepatitis Alliance (V-VHA) dedicated to viral hepatitis initiatives in Vietnam. In: Vol 41.; 2019:22-25.
- 6.Nguyen VTT, Quang TD, Anh NT, et al. P1-011: Estimates and projection of disease burden and economic analysis for hepatitis B in Viet Nam. J Viral Hepat. 2018;25(June):38. doi: 10.1111/jvh.07_12923. [DOI] [Google Scholar]
- 7.Coalition for Global Hepatitis Elimination (A Programme of the Taskforce for Gloabl Health). Data dashboards - Viet Nam HBV & HCV. https://www.globalhep.org/country-progress/viet-nam. Published 2019.
- 8.Global Health Data Exchange. Global Burden of Disease Study 2019 Data Input Sources Tool: Causes of death and disability in Vietnam. http://ghdx.healthdata.org/gbd-2019/data-input-sources?components=3&locations=20. Published 2019.
- 9.Hauri AM, Armstrong GL, H YJF. The global burden of disease attributable to contaminated injections given in health care settings. Int J STD AIDS. 2004;15(1):7–16. doi: 10.1258/095646204322637182. [DOI] [PubMed] [Google Scholar]
- 10.Dunford L, Carr MJ, Dean J, et al. Hepatitis C virus in Vietnam: high prevalence of infection in dialysis and multi-transfused patients involving diverse and novel virus variants. PLoS One. 2012;7(8):e41266. doi: 10.1371/journal.pone.0041266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Averbukh LD, Wu GY. Highlights for Dental Care as a Hepatitis C Risk Factor: A Review of Literature. J Clin Transl Hepatol. 2019;7(4):346–351. doi: 10.14218/JCTH.2019.00023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Jafari S, Copes R, Baharlou S, Etminan M, Buxton J. Tattooing and the risk of transmission of hepatitis C: a systematic review and meta-analysis. Int J Infect Dis. 2010;14(11):e928–e940. doi: 10.1016/j.ijid.2010.03.019. [DOI] [PubMed] [Google Scholar]
- 13.Yang S, Wang D, Zhang Y, et al. Transmission of Hepatitis B and C Virus Infection Through Body Piercing: A Systematic Review and Meta-Analysis. Medicine (Baltimore) 2015;94(47):e1893. doi: 10.1097/MD.0000000000001893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.El-Ghitany EM, Abdel Wahab MM, Abd El-Wahab EW, Hassouna S, Farghaly AG. A comprehensive hepatitis C virus risk factors meta-analysis (1989–2013); Do they differ in Egypt? Liver Int. 2015;35(2):489–501. doi: 10.1111/liv.12617. [DOI] [PubMed] [Google Scholar]
- 15.Stockdale AJ, Kreuels B, Henrion MYR, et al. The global prevalence of hepatitis D virus infection: Systematic review and meta-analysis. J Hepatol. 2020;73(3):523–532. doi: 10.1016/j.jhep.2020.04.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Loureiro D, Castelnau C, Tout I, et al. New therapies for hepatitis delta virus infection. Liver Int. 2021;41(S1):30–37. doi: 10.1111/liv.14838. [DOI] [PubMed] [Google Scholar]
- 17.World Bank. World Bank in Vietnam: Overview.; 2021. https://www.worldbank.org/en/country/vietnam/overview#1.
- 18.Pricewaterhouse Coopers The long view: How will the global economic order change by 2050? Pwc. 2017:1–72. [Google Scholar]
- 19.JBI. Critical Appraisal Checklist for Systematic Reviews and Research Syntheses.; 2017. http://joannabriggs.org/research/critical-appraisal-tools.htmlwww.joannabriggs.org%0Awww.joannabriggs.org.
- 20.DerSimonian R, Laird N. Meta-analysis in clinical trials. Control Clin Trials. 1986;7(3):177–188. doi: 10.1016/0197-2456(86)90046-2. [DOI] [PubMed] [Google Scholar]
- 21.Freeman MF, Tukey JW. Transformations related to the angular and the square root. Ann Math Stat. 1950:607–611. [Google Scholar]
- 22.Newcombe RG. Interval estimation for the difference between independent proportions: comparison of eleven methods. Stat Med. 1998;17(8):873–890. doi: 10.1002/(SICI)1097-0258(19980430)17:8<873::AID-SIM779>3.0.CO;2-I. [DOI] [PubMed] [Google Scholar]
- 23.R Core Team. R: A language and environment for statistical computing. R Foundation for Statistical Computing. 2021. https://www.r-project.org/.
- 24.Trung DT, Thao LTT, Hien TT, et al. Liver involvement associated with dengue infection in adults in Vietnam. Am J Trop Med Hyg. 2010;83(4):774–780. doi: 10.4269/ajtmh.2010.10-0090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Barcus MJ, Hien TT, White NJ, et al. Short report: hepatitis b infection and severe Plasmodium falciparum malaria in Vietnamese adults. Am J Trop Med Hyg. 2002;66(2):140–142. doi: 10.4269/ajtmh.2002.66.140. [DOI] [PubMed] [Google Scholar]
- 26.Chau TTH, Mai NTH, Phu NH, et al. Malaria in Injection Drug Abusers in Vietnam. Clin Infect Dis. 2002;34(10):1317–1322. doi: 10.1086/340053. [DOI] [PubMed] [Google Scholar]
- 27.Nadol P, O'connor S, Duong H, et al. Findings from integrated behavioral and biologic survey among males who inject drugs (MWID) - Vietnam, 2009-2010: evidence of the need for an integrated response to HIV, hepatitis B virus, and hepatitis C virus. PLoS One. 2015;10(2) doi: 10.1371/journal.pone.0118304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Hoang TV, Ha TTT, Hoang TM, et al. Impact of a methadone maintenance therapy pilot in Vietnam and its role in a scaled-up response. Harm Reduct J. 2015;12:39. doi: 10.1186/s12954-015-0075-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Follézou JY, Lan NY, Lien TX, et al. Clinical and biological characteristics of human immunodeficiency virus-infected and uninfected intravascular drug users in Ho Chi Minh City, Vietnam. Am J Trop Med Hyg Am J Trop Med Hyg Am J Trop Med Hyg. 1999;61(3):420–424. doi: 10.4269/ajtmh.1999.61.420. [DOI] [PubMed] [Google Scholar]
- 30.Nakata S, Song P, Duc D, et al. Hepatitis C and B virus infections in populations at low or high risk in Ho Chi Minh and Hanoi, Vietnam. J Gastroenterol Hepatol. 1994;9(4):416–419. doi: 10.1111/j.1440-1746.1994.tb01265.x. [DOI] [PubMed] [Google Scholar]
- 31.Lien TTX, Trimoulet P, Yen VT, Lepers JP, Fleury HJA. Epidémiologie de l'infection par le virus de l'hépatite C au Vietnam. Médecine Mal Infect. 1997;27(11):893–897. doi: 10.1016/S0399-077X(97)80245-4. [DOI] [Google Scholar]
- 32.Tanimoto T, Nguyen HC, Ishizaki A, et al. Multiple routes of hepatitis C virus transmission among injection drug users in Hai Phong, Northern Vietnam. J Med Virol. 2010;82(8):1355–1363. doi: 10.1002/jmv.21787. [DOI] [PubMed] [Google Scholar]
- 33.Kakumu S, Sato K, Morishita T, et al. Prevalence of hepatitis B, hepatitis C, and GB virus C/hepatitis G virus infections in liver disease patients and inhabitants in Ho Chi Minh, Vietnam. J Med Virol. 1998;54(4):243–248. doi: 10.1002/(SICI)1096-9071(199804)54::4<243:AID-JMV2>3.0.CO;2-4. [DOI] [PubMed] [Google Scholar]
- 34.Le L-VN, O'Connor S, Tran TH, et al. High hepatitis C virus infection among female sex workers in Viet Nam: strong correlation with HIV and injection drug use. West Pacific Surveill response J WPSAR. 2019;10(3):9–18. doi: 10.5365/wpsar.2019.10.1.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Do SH, Yamada H, Fujimoto M, et al. High prevalences of hepatitis B and C virus infections among adults living in Binh Thuan province, Vietnam. Hepatol Res. 2015;45(3):259–268. doi: 10.1111/hepr.12350. [DOI] [PubMed] [Google Scholar]
- 36.Duong MC, McLaws ML. Screening haemodialysis patients for hepatitis C in Vietnam: The inconsistency between common hepatitis C virus serological and virological tests. J Viral Hepat. 2019;26(1):25–29. doi: 10.1111/jvh.12994. [DOI] [PubMed] [Google Scholar]
- 37.Dang LVP, Nguyen QH, Ishizaki A, et al. Prevalence of Opportunistic Infections and Associated Factors in HIV-Infected Men Who Have Sex With Men on Antiretroviral Therapy in Bach Mai Hospital, Hanoi, Vietnam: A Case-Control Study. Am J Mens Health. 2020;14(3) doi: 10.1177/1557988320926743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Dunford L, Carr MJ, Dean J, et al. A multicentre molecular analysis of hepatitis B and blood-borne virus coinfections in Viet Nam. PLoS One. 2012;7(6):e39027. doi: 10.1371/journal.pone.0039027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Vietnam MOH.Thông Tư 26/2013/TT-BYT Circular No. 26/2013/TT-BYT, Providing Guidance on the Blood Transfusion. Hanoi; 2013. https://vanbanphapluat.co/circular-no-26-2013-tt-byt-guidance-on-the-blood-transfusion.
- 40.Vietnam Plus. Voluntary blood donation a popular movement in Vietnam.https://en.vietnamplus.vn/voluntary-blood-donation-a-popular-movement-in-vietnam/224689.vnp. Published April 12, 2022.
- 41.Hyams KC. Risks of Chronicity Following Acute Hepatitis B Virus Infection: A Review. Clin Infect Dis. 1995;20(4):992–1000. doi: 10.1093/clinids/20.4.992. [DOI] [PubMed] [Google Scholar]
- 42.Katelaris PH, Robertson G, Bradbury R, Tippett G, Hoa DQ, Ngu MC. Seroprevalence of hepatitis viruses in children in rural Viet Nam. Trans R Soc Trop Med Hyg. 1995;89(5):487. doi: 10.1016/0035-9203(95)90077-2. [DOI] [PubMed] [Google Scholar]
- 43.Hipgrave DB, Nguyen T V, Vu MH, et al. Hepatitis B infection in rural Vietnam and the implications for a national program of infant immunization. Am J Trop Med Hyg. 2003;69(3):288–294. [PubMed] [Google Scholar]
- 44.Nguyen TH, Vu MH, Nguyen VC, et al. A reduction in chronic hepatitis B virus infection prevalence among children in Vietnam demonstrates the importance of vaccination. Vaccine. 2014;32(2):217–222. doi: 10.1016/j.vaccine.2013.11.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Miyakawa M, Yoshida L-M, Nguyen H-AT, et al. Hepatitis B virus infection among pregnant mothers and children after the introduction of the universal vaccination program in Central Vietnam. Sci Rep. 2021;11(1):8676. doi: 10.1038/s41598-021-87860-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.de Villiers MJ, Nayagam S, Hallett TB. The impact of the timely birth dose vaccine on the global elimination of hepatitis B. Nat Commun. 2021;12(1):6223. doi: 10.1038/s41467-021-26475-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Nguyen Si Anh H, Vo H-L, Hoang Bao L, Tran Minh H, Tran Thi Thu H, Kien VD. Hepatitis B Birth Dose Vaccination among Vietnamese Children: Implications for the Expanded Program on Immunization. Liu Y, editor. Hepatitis B Birth Dose Vaccination among Vietnamese Children: Implications for the Expanded Program on ImmunizationBiomed Res Int. 2019;2019 doi: 10.1155/2019/3453105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Tran TK, Gottvall K, Nguyen HD, Ascher H, Petzold M. Factors associated with antenatal care adequacy in rural and urban contexts-results from two health and demographic surveillance sites in Vietnam. BMC Health Serv Res. 2012;12(1):40. doi: 10.1186/1472-6963-12-40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Zou H, Chen Y, Duan Z, Zhang H, Pan C. Virologic factors associated with failure to passive–active immunoprophylaxis in infants born to HBsAg-positive mothers. J Viral Hepat. 2012;19(2):e18–e25. doi: 10.1111/j.1365-2893.2011.01492.x. [DOI] [PubMed] [Google Scholar]
- 50.Wen W-H, Chang M-H, Zhao L-L, et al. Mother-to-infant transmission of hepatitis B virus infection: Significance of maternal viral load and strategies for intervention. J Hepatol. 2013;59(1):24–30. doi: 10.1016/j.jhep.2013.02.015. [DOI] [PubMed] [Google Scholar]
- 51.Gower E, Estes C, Blach S, Razavi-Shearer K, Razavi H. Global epidemiology and genotype distribution of the hepatitis C virus infection. J Hepatol. 2014;61(1):S45–S57. doi: 10.1016/j.jhep.2014.07.027. [DOI] [PubMed] [Google Scholar]
- 52.WHO. Hepatitis C Fact Sheet.; 2021. https://www.who.int/news-room/fact-sheets/detail/hepatitis-c. Accessed 8 December 2021.
- 53.Degenhardt L, Peacock A, Colledge S, et al. Global prevalence of injecting drug use and sociodemographic characteristics and prevalence of HIV, HBV, and HCV in people who inject drugs: a multistage systematic review. Lancet Glob Heal. 2017;5(12):e1192–e1207. doi: 10.1016/S2214-109X(17)30375-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.(WHO) WHO. Global health sector strategy on viral hepatitis 2016–2021. Towards ending viral hepatitis; 2016.
- 55.Rapoud D, Quillet C, Pham Minh K, et al. Towards HCV elimination among people who inject drugs in Hai Phong, Vietnam: study protocol for an effectiveness-implementation trial evaluating an integrated model of HCV care (DRIVE-C: DRug use & Infections in ViEtnam–hepatitis C) BMJ Open. 2020;10(11) doi: 10.1136/bmjopen-2020-039234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Nguyen TTM, Nguyen LT, Pham MD, Vu HH, Mulvey KP. Methadone Maintenance Therapy in Vietnam: An Overview and Scaling-Up Plan. Adv Prev Med. 2012;2012 doi: 10.1155/2012/732484. Bruce RD. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Molès J-P, Vallo R, Khue PM, et al. HIV control programs reduce HIV incidence but not HCV incidence among people who inject drugs in HaiPhong, Vietnam. Sci Rep. 2020;10(1):6999. doi: 10.1038/s41598-020-63990-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Grebely J, Dalgard O, Conway B, et al. Sofosbuvir and velpatasvir for hepatitis C virus infection in people with recent injection drug use (SIMPLIFY): an open-label, single-arm, phase 4, multicentre trial. Lancet Gastroenterol Hepatol. 2018;3(3):153–161. doi: 10.1016/S2468-1253(17)30404-1. [DOI] [PubMed] [Google Scholar]
- 59.Clinton Health Access Initiative. Hepatitis C Market Report.; 2020.
- 60.Vietnam Ministry of Health. Increased Access to Treatment for HIV/HCV Co-Infected Patients in Vietnam.; 2021. https://vaac.gov.vn/tang-cuong-tiep-can-dieu-tri-viem-gan-c-doi-voi-nguoi-benh-dong-nhiem-hiv-viem-gan-c-tai-viet-nam.html.
- 61.Blake A, Smith JE. Modeling Hepatitis C Elimination Among People Who Inject Drugs in New Hampshire. JAMA Netw Open. 2021;4(8) doi: 10.1001/jamanetworkopen.2021.19092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Abbas Z, Jafri W, Raza S. Hepatitis D: Scenario in the Asia-Pacific region. World J Gastroenterol. 2010;16(5):554–562. doi: 10.3748/wjg.v16.i5.554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Cornell JE, Mulrow CD, Localio R, et al. Random-Effects Meta-analysis of Inconsistent Effects: A Time for Change. Ann Intern Med. 2014;160(4):267–270. doi: 10.7326/M13-2886. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
All raw data from 72 analysed studies is provided in Microsoft Excel format in the supplements.