Abstract
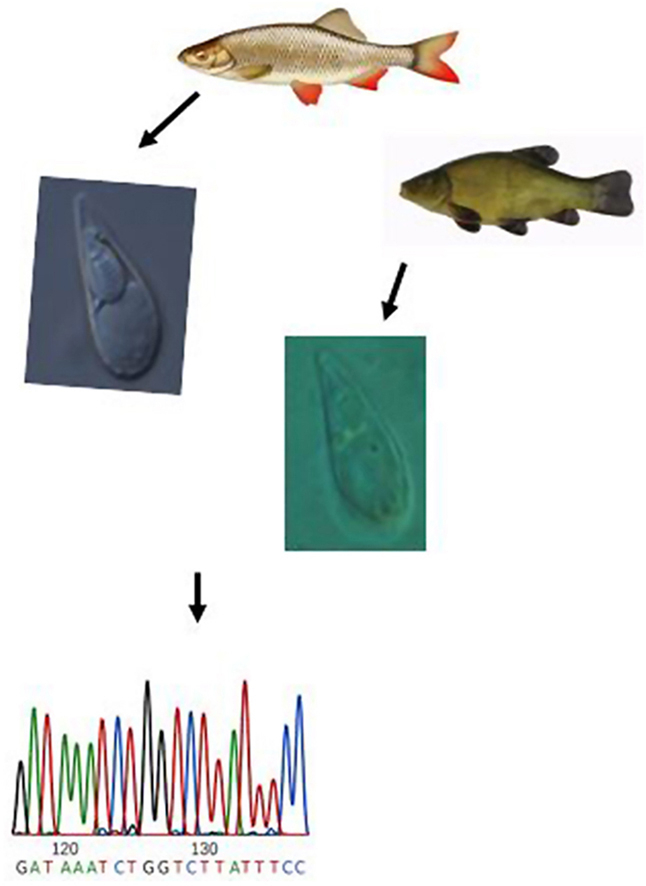
During surveys on myxosporean parasites of Lake Balaton and River Danube fishes, two Thelohanellus spp. were found on tench (Tinca tinca) and on common nase (Chondrostoma nasus). They were identified as Thelohanellus pyriformis and Thelohanellus cf. fuhrmanni, respectively. Myxospores of T. pyriformis from tench were collected from arteria branchialis afferens of gill filaments. The mature myxospores of this species were pyriform in shape and 19 ± 0.6l (18–19.5) long, 8.2 ± 0.54 (7.5–9) wide, 7.3 ± 0.25 (7–7.5) thick containing polar tubules with 9–10 turns. The plasmodia of Thelohanellus cf. fuhrmanni were collected from under the skin of snout of the common nase. The myxospores were pyriform, 16.3 ± 0.39l (15.5–16.5) long, 6.5 ± 0.55 (6.3–7) wide, 6.3 ± 0.53 (5.8–7) thick containing polar tubules with 6 turns. Small subunit ribosomal DNA sequences of both Thelohanellus species differed from other known myxozoans. The myxospores morphology, histopathology and ssrDNA sequences supported a diagnosis of T. pyriformis from tench and T. cf. fuhrmanni from common nase.
Keywords: Thelohanellus, Myxozoan, Tench, Common nase, ssrDNA, Histology
Graphical abstract
Highlights
-
•
Two Thelohanellus spp. were redescribed from cyprinid fishes.
-
•
Thelohanellus pyriformis was recorded and described from the type host tench (Tinca tinca).
-
•
Thelohanellus cf. fuhrmanni was found in an atypical host, common nase (Chondrostoma nasus).
-
•
The morphology of both Thelohanellus species was supported by histology and ssrDNA sequence data.
1. Introduction
Members of the genus Thelohanellus Kudo, 1933 are among the most common myxosporeans usually infecting freshwater fishes. Zhang et al. (2013) in their synopsis reported on 108 nominal species, but this number has been increased recently with several newly described species. Most of the described Thelohanellus species infect cyprinid fishes but majority of species are originated from India and China (Basu et al., 2006; Zhang et al., 2013; Chen and Ma 1998). After a general revision, most probably the large number of these species will prove to be synonyms. Similar problems concern the host range and tissue specificity of members of this genus. Often several fish species are recorded as hosts for a given Thelohanellus sp. (Kudo, 1919; Shulman, 1966; Donec and Shulman, 1984), while others argue that Thelohanellus spp. are rather host specific parasites infecting mostly a single species (Akhmerov, 1955, 1960; Molnár, 1982; Shin et al., 2014). Moreover, recent studies prove that most species can be characterized by strict tissue and organ specificity (Akhmerov, 1955; Molnár and Eszterbauer, 2015; Liu et al., 2016; Zhang et al., 2017). The synopsis on Thelohanellus by Zhang et al. (2013) focused to designate only the host and organ from where the Thelohanellus species were first described. The Thelohanellus fauna of the common carp and the goldfish has been thoroughly studied, however little is known on species infecting other cyprinids. Donec and Shulman (1984) recorded Thelohanellus spp. in more than a dozen cyprinids fishes but they classified them to some already known species such as T. pyriformis (Thélohan, 1892), T. fuhrmanni (Auerbach, 1909), or T. oculileucisci (Trojan, 1909). Type host of T. pyriformis is the tench while the type host of T. fuhrmanni is the roach (Rutilus rutilus L.). Little is known on the Thelohanellus infection of the nase. Only a single report was presented up to this time on its occurrence on this fish by Molnár (1979). More intensive studies were conducted by Dyková and Lom (1987) who studied the histology and the morphology of a case of T. pyriformis infection in tench by electron microscope. Data are scarce on the ssrDNA sequences of the majority of Thelohanellus species, which may answer the validity of these morphologically similar species.
In the present study, we document Thelohanellus pyriformis in the type host, tench, and report a Thelohanellus. cf. fuhrmanni infection found in nase. Morphological details and histopathological analysis of these species are supported by ssrDNA sequences.
2. Materials and methods
During a long-term fish health survey of natural water in Lake Balaton and River Danube, Hungary, fishes were regularly examined for myxosporean infections. Large sized fish specimens were obtained from fishing companies and sport anglers, and others were selected from the trap of Lake Balaton Drainage System. Small fishes were collected by a 15 m long seine net. All caught fishes were carried to the institute alive in oxygenated water, kept in aquarium in aerated water, and dissected within 3–5 days after collection. Tench (Tinca tinca) is a widespread but rare fish in both Lake Balaton and River Danube, only seven specimens (8–31 cm in length) were examined in 2019. Common nase (Chondrostoma nasus) is one of the most common fish in River Danube. In the case of common nase, parasitological dissections were conducted on 6 specimens of 3–4 year old and 12 specimens of two year old fish in 2019. Formerly, Thelohanellus infection was documented in 2004 in a 34 cm long nase collected from the Danube close to city Győr.
Fish were sedated prior to examination with clove oil and were given a cervical cut. Firstly, skin and fins were examined for myxosporean parasites. Subsequently, after hemibranch of the gills, and inner organs of fishes were carefully dissected, all organs were examined under a dissecting microscope for myxosporeans. The intestines were opened and pieces of muscle were compressed between two glass plates. In the case of a single cyst, small portion of myxospores was absorbed by a syringe for morphological and molecular studies and the cyst and the surrounding tissues were saved for histology. Myxospores from the cysts were studied native, and then some of the myxospores were placed in glycerine-jelly under a coverslip on a slide and preserved as a reference preparation. Myxospores were also collected into Eppendorf tubes in 95% ethanol for subsequent molecular studies. Tissue samples from infected organs had been fixed in Bouin's solution, embedded in paraffin wax, sectioned at 4–5 μm and stained with hematoxylin and eosin. The vitality of the spores was checked by 0.4% solution of urea; spores of a given plasmodium were regarded as mature when at least 90% of them extruded polar filaments in that solution. Unfixed spores were studied using differential interference contrast optics on an Olympus BH2 microscope. Fresh spores were photographed with an Olympus DP20 digital camera or recorded on videotapes; digitized images were obtained and measurements were taken from fresh spores and digitized photos. All measurements are given in micrometers (μm). In description, we use the term “polar tubule” instead of “polar filament” as Ben-David et al. (2016) suggested it.
3. Genomic DNA isolation and amplification
Preserved isolated plasmodia or myxospores in 95% ethanol were centrifuged at 8000×g for 10 min then ethanol was removed by pipetting and evaporation. Genomic DNA was extracted from the pellet using the Genaid Tissue Genomic DNA Mini kit (Geneaid Tawai City, Taiwan), following the manufacturer's recommended protocol for animal tissue. The ssrDNA was amplified by semi-nested PCR: first round with universal primers ERIB1 and ERIB10 (Barta et al., 1997). This was followed by two semi-nested PCR reactions to generate over-lapping sequences, ERIB1 with CR1R (Székely et al., 2015) and ERIB10 with CR1F (Székely et al., 2015) were paired. Reaction mixtures and thermal conditions followed the protocol previously published by Goswami et al. (2021). The primers used for PCR amplification and sequencing are listed in Table 1. PCR products were analysed in 1% agarose gel and the specific bands were excised from the gel.
Table 1.
Polymerase chain reaction primers used for the amplification and sequencing of the ssrDNA gene.
| Primers | Sequences | References |
|---|---|---|
| ERIB1 | ACCTGGTTGATCCTGCA | Barta et al. (1997) |
| ERB10 | CTTCCGCAGGTTCACCTACGG | Barta et al. (1997) |
| CR1R | CTAGGACGGTATCTGATCGTCTTCG | Székely et al. (2015) |
| CR1F | CGAAGACGATCAGATACCGTCCTAG | Székely et al. (2015) |
| MYXGEN4F | GTGCCTTGAATAAATCAGAG | Diamant et al. (2004) |
| ACT1FR | TTGGGTAATTTGCGCGCCTGCTGCC | Hallett and Diamant (2001) |
| ACT3R | ATT GTT CGT TCC ATG | Rocha et al. (2014) |
PCR products cut off from the gel were purified with the Gel/PCR DNA-Fragments Extraction Kit (Geneaid Tawai City, Taiwan) and directly sequenced with sequencing primers (Table 1) in both directions using the BigDye Terminator v3.1 Cycle Sequencing Kit (Life Technologies, Waltham, MA USA) with an ABI PRISM 3100 Genetic Analyser (Life Technologies, Waltham, MA USA), using the amplification and inner primers.
4. Phylogenetic analysis
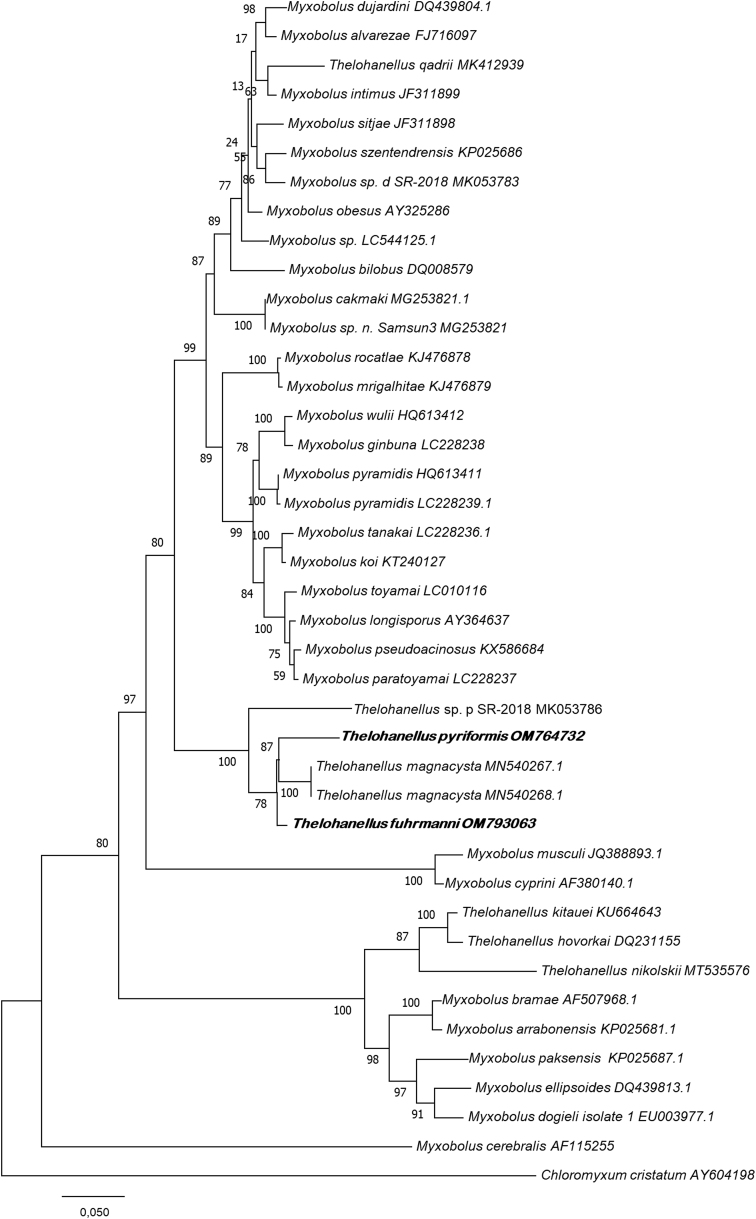
Assembly of the sequences was conducted in MEGA X (Kumar et al., 2018) and were corrected by manual adjustments. Assembled ssrDNA sequences were verified as myxozoan by GenBank BLAST search and related myxozoan sequences were added to the alignment. The selected sequences were restricted to only those of at least 1000 base pairs in length and 89% similarity. Gblocks 0.91b (Castresana, 2000) was used to remove ambiguous regions and highly variable sections. The sequences were aligned by using Clustal W (Thompson et al., 1994) implemented in the MEGA X. Pairwise distances were computed by using the p-distance model matrix in MEGA X. Evolutionary model for Maximum Likelihood analysis was determined by model testing of the dataset for the nucleotide substitution model of judging by the Akaike information criterion (AIC), which suggested the GTR + G + I. 1000 bootstrap replicates were performed to assess node support. Chloromyxum cristatum (AY604198) was chosen as an out-group in the final alignment.
5. Results
5.1. Infection in tench (Tinca tinca)
Thelohanellus infection in tench was found in June 2019 in a fish of 29 cm in length caught in Lake Balaton at Balatonszemes (46°48′36.4″N 17°45′55.9″E). When inspecting hemibranchia under dissecting microscope, small dark nodules were found in the arteria afferens, which proved to be plasmodia filled with spores of a Thelohanellus sp. When studying the gill filament under a compound microscope round or oval plasmodia were located in the lumen of the arteria afferens (Fig. 1). Round cysts measured 25 × 25 and elongate ones 25 × 160. They contained from 500 to 3000 spores. Each of the eight arches was infected with 2–8 plasmodia. Based on the shape and size of the spores and the specific location, this species was identified as T. pyriformis (Thélohan, 1892).
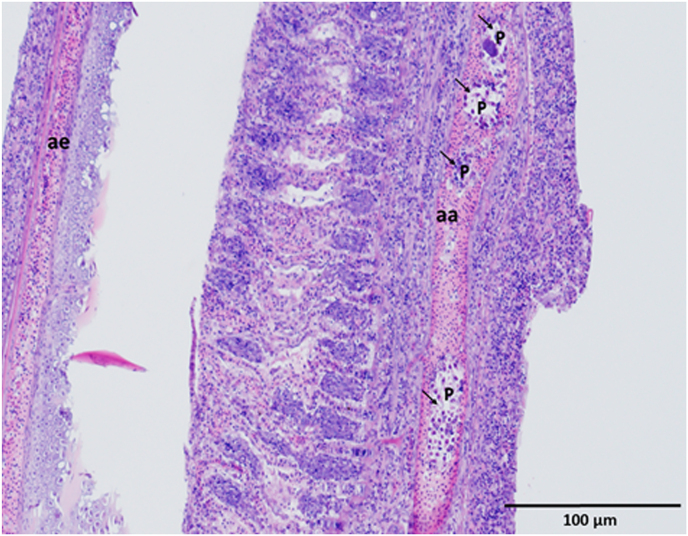
Fig. 1.
Plasmodia (arrows) of Thelohanellus pyriformis in the lumen of arteria branchialis afferens (aa). Arteria branchialis efferens (ae) is uninfected. Histological section. H. and E. staining.
5.2. Description of spores
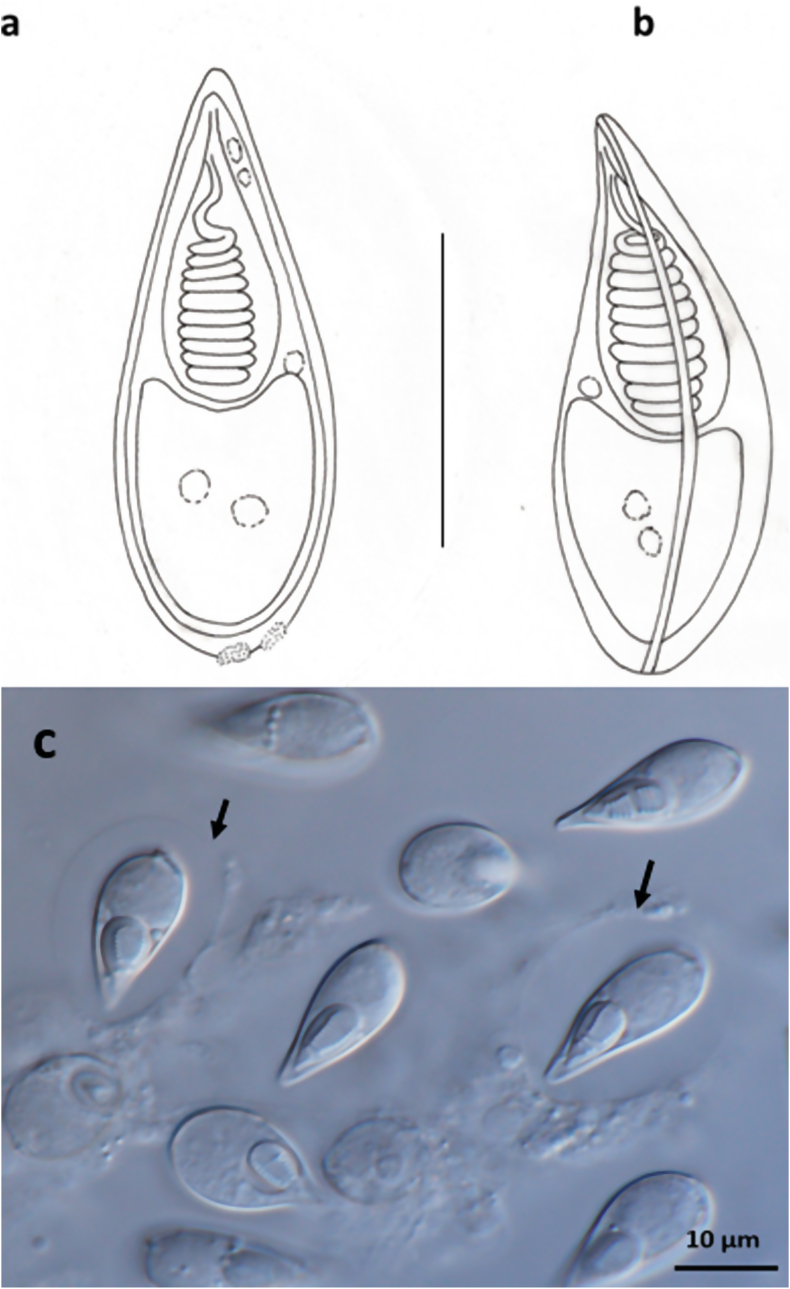
Mature spores (Fig. 2a and b and 2c) were pyriform both in frontal and sutural view, slightly tapering toward the anterior end and round at posterior end, 19.0 ± 0.6 l (18.0–19.5) long (n = 50), 8.2 ± 0.54 (7.5–9.0) wide (n = 50) and 7.3 ± 0.25 (7.0–7.5) thick (n = 25). In some spores the anterior end slightly bent. Spore wall formed by two shell valves of equal-size separated by sutural ridge, and light microscopically showing an about 0.55 in thickness. The wall at the posterior pole of the spore thickened showing some nodules on its surface, regarded as sutural notches by Dyková and Lom (1987. Single pyriform polar capsule presents close to apex of spore, 8.4 ± 055 (7.5–9.0) (n = 50) long, 4.6 ± 0.3 (4.0–5.0) (n = 50) wide. Polar tubules closely coiled with 9, or 10 turns, arranged perpendicular to the longitudinal axis of capsule. At one side of the polar capsule close to the blunt end a bright nucleus of the capsulogenic cell, about 1 in diameter is present. Sporoplasm located at the posterior pole of spore, contains two round nuclei. No iodinophilous vacuole had been seen. At the anterior end of the spore besides the polar capsule two bright round globules (probably the nuclei of the valvogenic cells), about 1 in diameter are located. Some of the spores are surrounded by on average 15.8 × 22.7 mucous envelope (Fig. 2c arrows).
Fig. 2.
Spores of Thelohanellus pyriformis a) Schematic drawing of a spore in frontal view, b) Schematic drawing of a spore in sutural view. c) Microscopic photos of spores.
5.2.1. Taxonomic summary
Host: Tench, Tinca tinca L.
Locality: Lake Balaton, Balatonszemes, Hungary (46°48′36.4″N 17°45′55.9″E).
Site of tissue development: Arteria branchialis afferens.
Material: Photo-types and histological preparations were deposited in the parasitological collection of the Zoological Department, Hungarian Natural History Museum, Budapest, Coll. No. HNHM-PAR-72082. The ssrDNA sequence (1973 bp, long) of T. pyriformis was deposited in the GenBank under accession numbers OM764632.
Prevalence: 1 specimen from 7 fish.
Molecular data: The phylogenetic analysis revealed the closest relative of this species to T. cf. fuhrmanni (present study data) with 96.4% nucleotide sequence identity. 95.1% similarity was found with T. magnacysta (MN540268) and 91.6% similarity was observed to a Thelohanellus species-2018 (MK053786) from intestinal epithelium of the Cobitis paludica in Portugal.
5.3. Infection in common nase (Chondrostoma nasus)
During our long-termed examinations on myxosporean infections of the common nase, Thelohanellus infection was detected only in two cases. Firstly, in 2004 in a 34 cm long nase collected in the Danube close to the city Győr (46°48′36.4″N 17°45′55.9″E). Secondly, in 2019 when Thelohanellus infection was found in one of the six nase of 31–33 cm long caught from the Danube at Nagymaros (47° 47′ 16.9368″ N 18° 57′ 14.9256″ E). In both cases, a single large, flat, 4 × 4 mm size cyst was found on the snout of the specimens. The cysts were visible to the naked eye. Inside the cysts, 5–6 global shape plasmodia were located filled by about 10.000 myxospores.
5.4. Description of spores
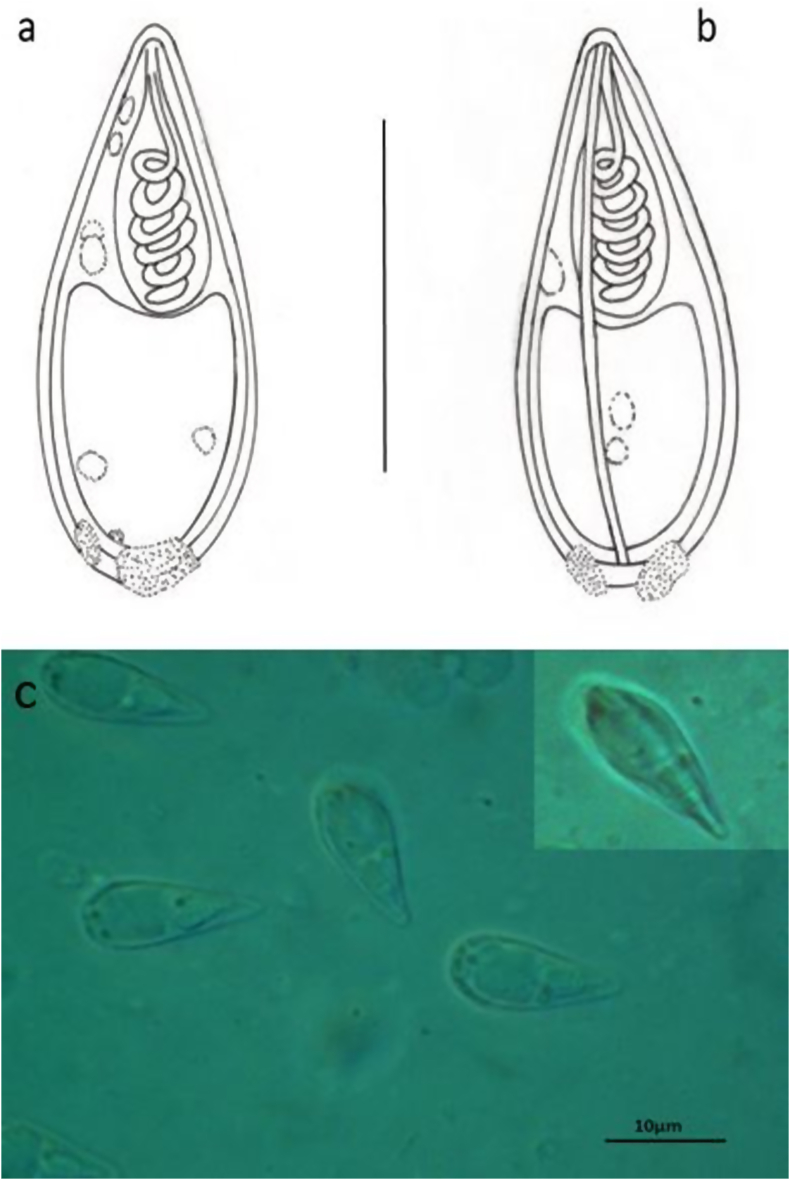
Mature myxospores were pyriform both in frontal and sutural view, slightly tapering toward the conical anterior end and rounded at the posterior end 16.3 ± 0.39 (15.5–16.5) long (n = 50), 6.5 ± 0.55 (6.3–7.0) wide (n = 50) and 6.3 ± 0.53 (5.8–7.0) thick (n = 25) (Fig. 3a, b, c, d). Myxospore wall formed by two shell valves of equal-size separated by a thick and fairly well marked sutural ridge. Light microscopically in sutural plane, the spore wall was about 0.5–0.6 in thickness but at the posterior end the myxospore thickened and bearing some nodules on its surface. Single flask-shaped polar capsule presents close to apex of spore, 6.5 ± 0.8 (5.5–7.0) (n = 50) long, 3.0 ± 0.1 (3.0–3.2) (n = 20) thick. Polar tubule coiled with 6 turns arranged obliquely to the longitudinal axis of capsule. At one side close to the blunt end of the polar capsule a bright nucleus of the capsulogenic cell, about 1 in diameter is present. Sporoplasm located close to posterior pole of myxospore, containing two round nuclei. At the anterior end of the myxospore besides the thickened polar capsule one or two bright round globules (probably the nuclei of the valvogenic cells) are located. Sutural ridge thickened and fairly well marked. Most of the myxospores surrounded by a pale, oval shape on average 13.5 × 9.0 mucous envelope (Fig. 3 c, d arrows) attaching to the wall of the myxospore at the anterior pole. The spores of the species resembled to T. fuhrmanni described by Auerbach (1909).
Fig. 3.
Spores of Thelohanellus cf. fuhrmanni. a) Schematic drawing of a spore in frontal view, b) Schematic drawing of a spore in sutural view, c) Microscopic photos of spores in frontal view. A spore in sutural view (inset).
5.5. Histology
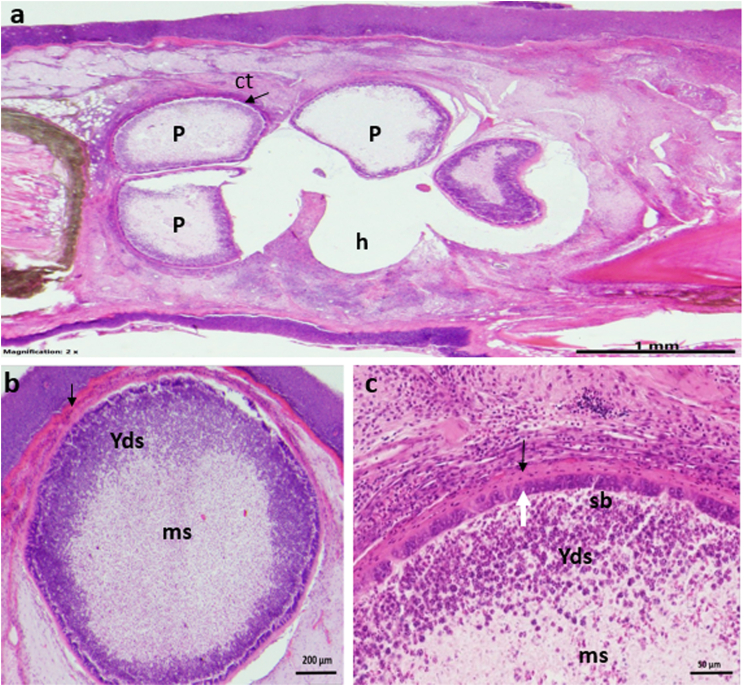
Plasmodia of this species located inside the loose connective tissue of the snout. Five to six round shape plasmodia with a diameter of 1–1.3 mm developed in close vicinity within an about 4.3–4.5 mm long and 3–3.2 mm wide cyst formed by the host (Fig. 4a). The thickness of the cyst was not measured. Plasmodia were surrounded by a relatively thin dense connective tissue wall of the host (Fig. 4). This layer wrapped plasmodia from outside and separated plasmodia from each other also in the interplasmodial space. Plasmodia were separated from the host capsule by their own very thin eosinophil staining ectoplasm (Fig. 4b and c). Dark staining early developmental stages located in a single layer at the periphery (Fig. 4c). Centrally, it was followed by a layer of sporoblasts and forming myxospores (Fig. 4c), while the centre of the plasmodium was filled by young and matured myxospores.
Fig. 4.
a) Plasmodia (p) of Thelohanellus cf. fuhrmanni enclosed in a cyst inside the loose connective tissue (ct) in the snout of the common nase. Empty holes (h) show the place of plasmodia fell out during histological processing or damaged by syringe at sampling spores b) Enlarged picture of T. cf. fuhrmanni plasmodia. Plasmodia are surrounded by a thin, dens connective tissue of the host (arrows) c) A part of T. cf. fuhrmanni plasmodium. Inside the connective tissue capsule (arrow) and the thin ectoplasm a dark staining layer of young developmental stages (white arrow) is located, followed by a zone of sporoblast (sb) and young spores (Yds) toward to the center. The center of the plasmodium is filled with matured spores (ms).
Remarks: Nodules at the thickened wall at the posterior end found also at T. pyriformis are more emphasized in this species.
5.5.1. Taxonomic summary
Host: Common nase, Chondrostoma nasus L.
Locality: River Danube, Nagymaros, Hungary (47° 47′ 16.9368″ N 18° 57′ 14.9256″ E).
Site of tissue development: Plasmodium under the skin of the snout.
Material: Photo-types were deposited in the parasitological collection of the Zoological Department, Hungarian Natural History Museum, Budapest, Coll. No. HNHM-PAR-72083. The ssrDNA sequence (1547 bp, long) of was deposited in the GenBank under accession number OM793063.
Prevalence: 2 specimens of 6 nases of 3–4 years old.
Molecular data: This species showed 97.6% similarity with Thelohanellus magnacysta (MN540267.1) described from the somatic muscles of Cyprinella venusta Girard by Ksepka et al. (2020), and 96.4% similarity was found to Thelohanellus pyriformis sequenced by us in this study from the gill of Tinca tinca. 93.4% similarity was observed with a Thelohanellus sp. p. SR-2018 (MK053786), obtained from the intestinal epithelium of Cobitis paludica (Fig. 5). This clade of Thelohanellus species was branched together with several Myxobolus spp. from cyprinid fishes apart from other notable Thelohanellus species, like T. nikolskii and T. hovorkai.
Fig. 5.
Phylogenetic tree generated by maximum likelihood analysis of ssrDNA sequences of Thelohanellus pyriformis and Thelohanellus fuhrmanni and other closely related myxozoan species identified by BLAST; GenBank accession numbers. Numbers at nodes indicate the bootstrap confidence values (ML). Chloromyxum cristatum was used as an outgroup.
6. Discussion
The genus Thelohanellus Kudo, 1933 is one of the richest myxosporean genus in species. Since then Zhang et al. (2013) in their synopsis reported on 108 Thelohanellus species, at least 14 more Thelohanellus spp. have been described from China, India, Brazil, Angola, Egypt and the USA. Most of them are plasmodia-forming parasites infecting various tissues of freshwater fishes, and some are pathogenic, causing economical losses in aquaculture (Zhang et al., 2013). Identification of several Thelohanellus spp. is difficult because descriptions of the old species are often relatively poor, lacking several important characteristics; moreover, many species were originally described as Myxobolus sp. with one polar capsule (Kudo, 1933).
Recently, publications from Asia (Yokoyama et al., 2012; Zhang et al., 2013; Singh and Kaur, 2012) described many new species or re-described old ones, but there is still a lot of questions regarding their host-, tissue-, and organ specificity. In our present study, T. pyriformis in the gills of tench was identified based on the correspondence of morphometrical characteristics of the spores, the location of the plasmodia and the identity of the type host. Morphometrically differing myxospores are classified to certain species described long ago despite their evolutionary distinct hosts, like T. pyriformis (Thelohan, 1892), T. oculileucisci (Trojan, 1909) or T. fuhrmanni (Auerbach, 1909). Many of the host fishes are probably infected by currently undescribed Thelohanellus species which are assumed to be one of those above-mentioned species. Akhmerov (1955, 1960) realized first the large morphological diversity and species richness of Thelohanellus spp. in common carp and in other cyprinids, though Shulman (1966) furtherly regarded most of his species’ synonyms. Therefore, the redescriptions of T. pyriformis and T. fuhrmanni are necessary. The myxospores of T. pyriformis were found in the same species as in the original description and spores corresponded to the original data. However, the myxospores of T. cf. fuhrmanni were found in common nase (Chondrostoma nasus) altering from the type host (Rutilus rutilus) in many respects but belonging to the same subfamily Leuciscinae. Regarding T. pyriformis, it was originally reported from the gills, kidneys, and spleen of tench. Later, however several new hosts were reported, like Molnár (1979) described this parasite from the gills and skin of T. tinca and C. nasus from Hungary, however the myxospores found in the skin probably corresponded to T. fuhrmanni. Donec and Shulman (1984) reported T. pyriformis from several additional fish hosts and from various organs. Dyková and Lom (1987) described plasmodia of T. pyriformis from the vessels of gills of the type host tench and concluded that the infection could lead to hypertrophy of endothelial cells in gills. Large plasmodia of T. cf. fuhrmanni found together in a cyst caused well observable tumors in the nostrils of the nase. Similar nodules with pathogenic characters were found in the skin of Cyprinus carpio infected by T. kitauei (Egusa and Nakajima, 1981; Zhai et al., 2016; Liu et al., 2019) and in Cyprinella venusta infected by T. magnacysta (Ksepka et al., 2020).
Molnár (1994) suggested that host species and infection site are important factors for myxosporean identification. Host specificity of myxosporeans was mostly studied on Myxobolus spp. Several Myxobolus species are known to have a strict host specificity causing infection of a single host or some closely related species (Molnár et al., 2011; Cech et al., 2012). In our assumption, host specificity of Thelohanellus spp. might be similar to Myxobolus spp. Tench as a single member of Tincinae, and nase as a member of Leuciscinae are relatively distinct inside the family Cyprinidae, therefore it is unlikely that T. pyriformis could infect other cyprinids than tench. Recent publications (Shin et al., 2014; Lewisch et al., 2015; Liu et al., 2014, 2019) using molecular data suggest the relatively strict host specificity on Thelohanellus spp. of the common carp and goldfish (Carassius auratus L.). The common carp and its closest relative, the goldfish e.g., have no documented Thelohanellus species infecting the both fish species (Molnár and Kovács-Gayer, 1982; Zhang et al., 2013, 2018; Shin et al., 2014). Therefore, the problem of the occurrence of T. cf. fuhrmanni in common nase requires more caution. The species from nase identified as T. cf. fuhrmanni has similar looking but somewhat different sized spores compared to the original description. Interestingly, the site of infection in the roach documented by Auerbach (1909) as “connective tissue under the mucous membrane of the mouth” fairly corresponds to our data, therefore we designated this species as T. cf. fuhrmanni until further evidence emerges. Both common nase and roach belong to the Leuciscinae subfamily of Cyprinidae but they differ greatly regarding their body structure and natural freshwater habitats. Phylogenetic studies have demonstrated that the origin and radiation of myxozoans reflect the evolution of their hosts (Carriero et al., 2013; Kodádková et al., 2015; Holzer et al., 2018; Patra et al., 2018). Thelohanellus cf. fuhrmanni showed the close similarity with T. magnacysta (Ksepka et al., 2020) described from the muscles of Cyprinella venusta Girard, 1856 and 96.4% similarity was found to Thelohanellus pyriformis (present study) from the gill of T. tinca. However, there are no previously published sequences of Thelohanellus species from tench or common nase, therefore further evolutionary comparisons cannot be made beside the fact that these two newly sequenced species in this study showed a close relationship to each other. The genus Thelohanellus is polyphyletic, as its members are always scattered around within the Myxobolus cluster which is consistent to the previous studies (Liu et al., 2014; Székely et al., 2009). Both the species of Thelohanellus also showed closer similarity with Myxobolus intimus Zaika, 1965 isolated from the capillary network of gill lamellae of Aspius aspius L., as well with many Myxobolus species from cyprinid fishes just like the other Thelohanellus species. Molecular data clearly indicate a close genetic relation existing among genera Myxobolus, Thelohanellus, and Henneguya (Zhang et al., 2019). The difference is in the number of polar capsules between Thelohanellus species (one polar capsule) and Myxobolus species (two polar capsules), however one of the two polar capsules in some Myxobolus species is too small to be recognized (Yokoyama and Ogawa, 2015; Griffin and Goodwin, 2011). Measurements given by different authors vary highly (Table 2, Table 3.), therefore there is a possibility that they identified both species incorrectly, and parasites regarded as T. pyriformis or T. fuhrmanni designated by them represent different but closely related species. Further molecular data of new isolates might answer these uncertain species designations.
Table 2.
Comparison of the spore measurements of Thelohanellus pyriformis (Thélohan, 1892) Kudo (1933) given by different authors from cyprinids.
| present paper | by Donec and Shulman (1984) | by Kudo (1919) | by Agapova (1966) | by Lom et Dyková and Lom, 1987 | by Lom and Dyková , 1992 | |
|---|---|---|---|---|---|---|
| Hosts | Tinca tinca | Cyprinids | Tinca tinca | Cyprinids | Cyprinids | Tinca tinca |
| Spore length | 19.0 ± 0.6l (18.0–19.5) | 14.0–22.0 | 18.0–20.0 | 16.0–18.0 | 16.0–23.0 | 19.5 (18.0–20.6) |
| Spore width | 8.2 ± 0.54 (7.5–9) | 7.0–10.0 | 8.0 | 7.0–8.0 | 6.0–8.0 | 9.4 (9.1–9.6) |
| Spore thickness | 7.3 ± 0.25 (7.0–7.5) | 6.5–6.7 | 6.0 | – | – | – |
| length of polar capsule | 8.4 ± 055 (7.5–9.0) | 6.0–10.5 | – | 5.0–7.5 | 8.2 | 9.0 (7.6–9.6) |
| Width of polar capsule | 4.6 ± 0.3 (4.0–5.0) | 4.5 | – | 3.0.5.0 | 4.2 | 4.3 (3.8–4.8) |
| Number of polar filament coil | 9 | – | 9 | – | 6 | 9 (7–11) |
Table 3.
Comparison of the spore measurements of Thelohanellus fuhrmanni (Auerbach, 1909) Kudo (1933) given by different authors.
| present paper | by Donec and Shulman (1984) | by Kudo (1919) | Petrushewski and Bikhovskaya-Pavlovskaya, 1935.cit. by Shulman (1966) | |
|---|---|---|---|---|
| Hosts | Chondrostoma nasus | Cyprinids | Rutilus rutilus | Nemachilus barbatulus |
| Spore length | 16.3 ± 0.39 (15.5–16.5) | 14.0–20.0 | 18.0–20.0 | 10.8–12.4 |
| Spore width | 6.5 ± 0.55 (6.3–7.0) | 5.0–8.0 | 8.0 | – |
| Spore thickness | 6.3 ± 0.53 (5.8–7.0) | – | 6.0 | – |
| length of polar capsule | 6.5 ± 08 (5.5–7.0) | 6.0–10.0 | 9.0–10.0 | – |
| Width of polar capsule | 3.0 ± 0.1 (3.0–3.2) | – | – | – |
| No. of polar filament coil | 6 - | – | – |
Unfortunately, our data relay on two cases, although we agree with Zhai et al. (2016) that measurements of spores should come from several different plasmodia to increase the reliability of the morphological data. Intraspecific morphometric variation of myxosporean species commonly occurs, therefore we are reluctant to describe Thelohanellus sp. found in nase as a new species. However, the great differences in morphology and size given for the two species (Table 2, Table 3), and the large number of genetically far distinct hosts suggest that identification of the two Thelohanellus species by different authors was not correct and by more thorough examinations several morphologically similar but host specific species can be separated.
Acknowledgements
The study was supported by the 2017-2.3.7-TET-IN-2017-00003 Indo-Hungarian Inter-Governmental Science and Technology Cooperation Program. We also thank for the support of the 243/1/2021/HF - Balatoni halfajok halegészségügyi monitoringja (Survey of health status of fishes of Lake Balaton) program and Ms. Györgyi Pataki for histological slides and Mr. Ádám Varga for helping in photo technics.
References
- Agapova A.I. Nauka; KazSSR: 1966. Parasites of Fish from Water Systems of Kazakhstan; p. 342. (In Russian) [Google Scholar]
- Akhmerov A.Kh. Paths of species formation in Myxosporidia of the genus Thelohanellus Kudo from the Amur carp. Dokl. Akad. Nauk SSSR. 1955;105(1) 129–1,131 (In Russian) [Google Scholar]
- Akhmerov A.Kh. Myxosporidia of fishes from the Amur river basin. Rybnoe Khozyaistvo Vnutrennykh Vodoemov Latvijskoi SSR. 1960;5:240–307. (In Russian) [Google Scholar]
- Auerbach M. Bemerkungen über Myxosporidien. Zool. Anz. 1909;34:65–82. [Google Scholar]
- Barta J.R., Martin D.S., Liberator P.A., Dashkevica M., Anderson J.W., Feighner S.D., Elbrecht A., Perkins-Barrow A., Jenkins M.C., Danforth H.D., Ruff M.D., Profous-Juchelka H. Phylogenetic relationships among eight Eimeria species infecting domestic fowl inferred using complete small subunit ribosomal DNA sequences. J. Parasitol. 1997;83:262–271. [PubMed] [Google Scholar]
- Basu S., Modak B.K., Haldar D.P. Synopsis of the Indian species of the genus Thelohanellus Kudo, 1933 along with the description of Thelohanellus disporomorphus sp. n. J. Parasitol. Appl. Anim. Biol. 2006;15:81–94. [Google Scholar]
- Ben-David J., Atkinson S.D., Pollak Y., Yossifon G., Shavit U., Bartholomew J.L., Lotan T. Myxozoan polar tubules display structural and functional variation. Parasites Vectors. 2016;9:549. doi: 10.1186/s13071-016-1819-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carriero M.M., Adriano E.A., Silva M.R.M., Ceccarelli P.S., Maia A.A.M. Molecular phylogeny of the Myxobolus and Henneguya genera with several new South American species. PLoS One. 2013;8 doi: 10.1371/journal.pone.0073713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Castresana J. Selection of conserved blocks from multiple alignments for their use in phylogenetic analysis. Mol. Biol. Evol. 2000;17:540–552. doi: 10.1093/oxfordjournals.molbev.a026334. [DOI] [PubMed] [Google Scholar]
- Cech G., Molnár K., Székely Cs. Molecular genetic studies on morphologically indistinguishable Myxobolus spp. infecting cyprinid fishes, with the description of three new species, M. alvarezae sp. nov., M. sitjae sp. nov. and M. eirasianus sp. nov. Acta Parasitol. 2012;57:354. doi: 10.2478/s11686-012-0045-2. ISSN 1230-2821. [DOI] [PubMed] [Google Scholar]
- Chen C., Ma C. Science Press; Beijing: 1998. Fauna Sinica. Myxozoa, Myxosporea; p. 993. (In Chinese) [Google Scholar]
- Diamant A., Whipps C.M., Kent M.L. A new species of Sphaeromyxa (Myxosporea: Sphaeromyxina: Sphaeromyxidae) in devil firefish. Pterois miles (Scorpaenidae), from the northern Red Sea: morphology, ultrastructure, and phylogeny. J. Parasitol. 2004;90:1434–1442. doi: 10.1645/GE-336R. [DOI] [PubMed] [Google Scholar]
- Donec Z.S., Shulman S.S. In: Bauer O.N., editor. vol. 1. Nauka; Leningrad: 1984. Knidosporidii (Cnidosporidia) pp. 88–251. (Key to the Parasites of Freshwater Fishes of the USSR). (in Russian) [Google Scholar]
- Dyková I., Lom J. Host cell hypertrophy induced by contact with trophozoites of Thelohanellus pyriformis (Myxozoa: Myxosporea) Arch. Protistenkd. 1987;133:285–293. [Google Scholar]
- Egusa S., Nakajima K. A new Myxozoa Thelohanellus kitauei, the cause of intestinal giant cystic disease of carp. Fish Pathol. 1981;15 213-2. [Google Scholar]
- Goswami U., Molnár K., Cech G., Eiras J.C., Bandyopadhyay P.K., Ghosh S., Czeglédi I., Székely C. Evidence of the American Myxobolus dechtiari was introduced along with its host in Europe: molecular and histological data. Int. J. Parasitol.: Parasites Wildl. 2021;15:51–57. doi: 10.1016/j.ijppaw.2021.04.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Griffin M.J., Goodwin A.E. Thelohanellus toyamai (Syn. Myxobolus toyamai) infecting the gills of Koi Cyprinus carpio in the Eastern United States. J. Parasitol. 2011;97:493–502. doi: 10.1645/GE-2674.1. [DOI] [PubMed] [Google Scholar]
- Hallett S.L., Diamant A. Ultrastructure and small-subunit ribosomal DNA sequence of Henneguya lesteri n. sp. (Myxosporea), a parasite of sand whiting (Sillaginidae) from the coast of Queensland, Australia. Dis. Aquat. Org. 2001;46:197–212. doi: 10.3354/dao046197. [DOI] [PubMed] [Google Scholar]
- Holzer A.S., Bartošová-Sojková P., Born-Torrijos A., Lövy A., Hartigan A., Fiala I. The joint evolution of the Myxozoa and their alternate hosts: a cnidarian recipe for success and vast biodiversity. Mol. Ecol. 2018:1651–1666. doi: 10.1111/mec.14558. [DOI] [PubMed] [Google Scholar]
- Kodádková A., Bartošová-Sojková P., Holzer A.S., Fiala I. Bipteria vetusta n. sp. an old parasite in an old host: tracing the origin of myxosporean parasitism in vertebrates. Int. J. Parasitol. 2015;45:269–276. doi: 10.1016/j.ijpara.2014.12.004. [DOI] [PubMed] [Google Scholar]
- Ksepka S.P., Whelan N., Whipps C.M., Bullard S.A. A new species of Thelohanellus Kudo, 1933 (Myxozoa: Bivalvulida) infecting skeletal muscles of muscle of Blacktail Shiner, Cyprinella venusta Girard, 1856 (Cypriniformes: Cyprinidae) in the Chattahoochee river basin. Georgia. J. Parasitol. 2020;106:350–359. doi: 10.1645/19-162. [DOI] [PubMed] [Google Scholar]
- Kudo R.R. Studies on Myxosporidia; a synopsis of genera and species of Myxosporidia. Ill Biol. Monogr. 1919;5:1–265. [Google Scholar]
- Kudo R.R. A taxonomic consideration of Myxosporidia. Trans. Am. Microsc. Soc. 1933;52:195–216. [Google Scholar]
- Kumar S., Stecher G., Li M., Knyaz C., Tamura K. Mega X: molecular evolutionary genetics analysis across computing platforms. Mol. Biol. Evol. 2018;35:1547–1549. doi: 10.1093/molbev/msy096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lewisch E., Soliman H., Schmidt P., El-Matbouli M. Morphological and molecular characterization of Thelohanellus hoffmanni sp. nov. (Myxozoa) infecting goldfish Carassius auratus auratus. Dis. Aquat. Org. 2015;29(1):37–46. doi: 10.3354/dao02870. 115. [DOI] [PubMed] [Google Scholar]
- Liu T., Wei W.Y., Wang K.Y., Yang Q., Wang E.L. Pathological and immunological analyses of Thelohanellus kitauei (Myxozoa:Myxosporea) infection in the scattered mirror carp, Cyprinus carpio. Sci. Rep. 2019;9(1):20014. doi: 10.1038/s41598-019-56752-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu Y., Jia L., Huang M.J., Gu Z.M. Thelohanellus testudineus n. sp. (Myxosporea: Bivalvulida) infecting the skin of allogynogenetic gibel carp Carassius auratus gibelio (Bloch) in China. J. Fish. Dis. 2014;37:535–542. doi: 10.1111/jfd.12141. [DOI] [PubMed] [Google Scholar]
- Liu Y., Zhai Y., Gu Z. Morphological and molecular characterization of Thelohanellus macrovacuolaris n. sp. (Myxosporea: Bivalvulida) infecting the palate in the mouth of common carp Cyprinus carpio L. in China. Parasitol. Int. 2016;65:303–307. doi: 10.1016/j.parint.2016.02.013. [DOI] [PubMed] [Google Scholar]
- Lom J., Dyková I. vol. 26. Elsevier; Amsterdam: 1992. Protozoan parasites of fishes; p. 315. (Developments in Aquaculture and Fisheries Science). [Google Scholar]
- Molnár K. Protozoan parasites of fish species indigenous in Hungary. Parasitol. Hung. 1979;12:5–8. [Google Scholar]
- Molnár K. Biology and histopathology of Thelohanellus nikolskii Achmerov, 1955 (Myxosporea, Myxozoa), a protozoan parasite of the common carp (Cyprinus carpio) Z. Parasitenkd. 1982;68:269–277. doi: 10.1007/BF00927405. [DOI] [PubMed] [Google Scholar]
- Molnár K. Comments on the host, organ and tissue specificity of fish myxosporeans and on the types of their intrapiscine development. Parasitol. Hung. 1994;27:5–20. [Google Scholar]
- Molnár K., Cech G., Székely Cs. Histological and molecular studies of species of Myxobolus Bütschli, 1882 (Myxozoa: Myxosporea) in the gills of Abramis, Blicca and Vimba spp. (Cyprinidae), with the redescription of M. macrocapsularis Reuss, 1906 and M. bliccae Donec & Tozyyakova, 1984. Syst. Parasitol. 2011;79:109–121. doi: 10.1007/s11230-011-9292-0. [DOI] [PubMed] [Google Scholar]
- Molnár K., Eszterbauer E. In: Myxozoan Evolution, Ecology and Development. Okamura B., Gruhl A., Bartolomew J.L., editors. Springer; Cham Heildelberg New York Dordrecht London: 2015. Specificity of infection sites in vertebrate hosts; pp. 2195–3013. [Google Scholar]
- Molnár K., Kovács-Gayer E. The occurrence of two Far-East origin Thelohanellus (Myxosporidia) species in common carp populations of the Hungarian pond farms. Parasitol. Hung. 1982;14:51–55. [Google Scholar]
- Patra S., Bartošová-Sojková P., Pecková H., Fiala I., Eszterbauer E., Holzer A.S. vol. 11. 2018. p. 347. (Biodiversity and Host-Parasite Cophylogeny of Sphaerospora (Sensu Stricto) (Cnidaria: Myxozoa) Parasite Vectors). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rocha G.C., Velasco M., Alves A., Matos E., Al-Quraishy S., Azevedo C. Morphology and phylogeny of Thelohanellus marginatus n. sp. (Myxozoa: Myxosporea), a parasite infecting the gills of the fish Hypophthalmus marginatus (Teleostei: Pimelodidae) in the Amazon river. J. Eukaryot. Microbiol. 2014 doi: 10.1111/jeu.12136. [DOI] [PubMed] [Google Scholar]
- Shin S.P., Nguen V.G., Jeong J.M., Jun J.W., Kim J.H., Han J.E., Baeck G.W., Park S.C. The phylogenetic study on Thelohanellus species (Myxosporea) in relation to host specificity and infection site tropism. Mol. Phylogenet. Evol. 2014;72:31–34. doi: 10.1016/j.ympev.2014.01.002. [DOI] [PubMed] [Google Scholar]
- Singh R., Kaur H. Two new and two already known species of genus Thelohanellus Kudo, 1933 (Myxozoa: Myxosporea: Bivalvulida) infecting Indian major carp fishes in Punjab wetlands (India) J. Parasit. Dis. 2012 doi: 10.1007/s12639-012-0190-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shulman S.S. Nauka; Moscow: 1966. Myxosporidia of the Fauna of the USSR. (in Russian) [Google Scholar]
- Székely C., Molnár K., Cech G. vol. 91. 2015. Description of Myxobolus balatonicus n. sp. (Myxozoa: Myxobolidae) from the common carp Cyprinus carpio L; pp. 71–79. (Lake Balaton. Syst. Parasitol.). [DOI] [PubMed] [Google Scholar]
- Székely C., Shaharom-Harrison F., Cech G., Mohamed K., Molnár K. Myxozoan pathogens of Malaysian fishes cultured in ponds and net-cages. Dis. Aquat. Org. 2009;83:49–57. doi: 10.3354/dao01990. [DOI] [PubMed] [Google Scholar]
- Thompson J.D., Higgins D.G., Gibson T.J. CLUSTAL W: improving the sensitivity of progressive multiple sequence alignment through sequence weighting, position-specific gap penalties and weight matrix choice. Nucleic Acids Res. 1994;22:4673–4680. doi: 10.1093/nar/22.22.4673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yokoyama H., Grabner D., Shirakashi S. In: Transmission Biology of the Myxozoa, Health and Environment in Aquaculture. Edmir Carvalho Dr., editor. 2012. [Google Scholar]
- Yokoyama H., Ogawa K. The resurrection of Myxobolus toyamai with a validation of a stunted polar capsule based on morphological evidence. Parasitol. Int. 2015;64:43–47. doi: 10.1016/j.parint.2015.01.008. [DOI] [PubMed] [Google Scholar]
- Zhai Y.H., Whipps C.M., Gu Z.M., Guo Q.X., Wu Z.Z., Wang H.M., Liu Y. Intraspecific morphometric variation in myxosporeans. Folia Parasitol. 2016;63 doi: 10.14411/fp.2016.011. [DOI] [PubMed] [Google Scholar]
- Zhang B., Zhai Y., Liu Y., Gu Z. Myxobolus pseudowulii sp. n. (Myxozoa: Myxosporea), a new skin parasite of yellow catfish Tachysurus fulvidraco (Richardson) and redescription of Myxobolus voremkhai (Akhmerov, 1960) Folia Parasitol. 2017;64 doi: 10.14411/fp.2017.030. [DOI] [PubMed] [Google Scholar]
- Zhang B., Zhai Y., Gu Z., Liu Y. 2018. Morphological, Histological and Molecular Characterization of Myxobolus Kingchowensis and Thelohanellus cf. Sinensis Infecting Gibel Carp Carassius auratus Gibelio (Bloch, 1782) p. 2. [DOI] [PubMed] [Google Scholar]
- Zhang J.Y., Gu Z.M., Kalavati C., Eiras J.C., Liu Y., Guo Q.Y., Molnár K. Synopsis of the species of Thelohanellus Kudo, 1933 (Myxozoa: Myxosporea: Bivalvulida. Syst. Parasitol. 2013;86:235–256. doi: 10.1007/s11230-013-9449-0. [DOI] [PubMed] [Google Scholar]
- Zhang X.P., Liu Y., Whipps C.M., Guo Q.X., Gu Z.M. Multiple evolutionary routes of the single polar capsule in Thelohanellus species (Myxozoa; Myxobolidae) Int. J. Parasitol. Parasites Wildl. 2019;8:56–62. doi: 10.1016/j.ijppaw.2018.11.006. [DOI] [PMC free article] [PubMed] [Google Scholar]