Abstract
Background
There have been few studies to verify factors associated with a false-negative interferon-gamma release assay (IGRA) in patients with tuberculous pleurisy. We investigated the clinical relevance of false-negative results of the blood QuantiFERON-TB Gold In-Tube (QFT-GIT) assay and its risk factors in patients diagnosed with pleural tuberculosis (TB).
Methods
Medical records of 650 pleural TB patients in a tertiary hospital between January 2009 and December 2020 were reviewed retrospectively. Patients who underwent the blood QFT-GIT assay and pleural fluid analysis before starting anti-TB medication were included.
Results
Of 199 patients with pleural TB who were performed QFT-GIT assay, 36 (18.1%) were false-negative results. These patients tended to be older than those with a positive result (P=0.060). The QFT-GIT-false-negative group of had significantly more comorbidities such as end-stage renal disease (ESRD), haematological cancer or pneumoconiosis than the QFT-GIT-positive group. Hypoproteinaemia and pH >6 in pleural fluid were associated with a false-negative QFT-GIT. Of the 199 patients, 163 (81.9%) were cured or completed anti-TB treatment; 13 patients (6.5%) died. The QFT-GIT-negative patients had significantly worse outcomes including mortality [unfavourable outcome: 33.3% (12/36 patients) in QFT-GIT-negative groups vs. 14.7% (24/163 patients) in QFT-GIT-positive groups, P<0.017; overall mortality: 16.7% (6/36 patients) vs. 4.3% (7/163 patients), respectively, P<0.015].
Conclusions
In pleural TB, a false-negative QFT-GIT result was 18.1% in a country of intermediate TB incidence. This discordant result in GFT-GIT was associated with ESRD, pneumoconiosis, hypoproteinaemia and a poor outcome. Clinicians should keep in mind the possibility of false-negativity in the blood IGRA test, especially in specific situations and its impact on TB outcome in managing patients with pleural TB.
Keywords: Interferon-gamma release assay (IGRA), pleural tuberculosis (TB), false negativity
Introduction
Tuberculosis (TB) pleurisy is the most common extra-pulmonary TB in South Korea, and the second most common in the world (1,2). The incidence ranges from 5% in TB non-endemic areas to 30% in TB endemic areas (3). However, diagnosing the disease is challenging in practice because of its obscure clinical manifestations, low bacillary burden in the involved lesion and relatively invasive procedure to obtain samples. Using pleural fluid specimens, diagnostic yields were reported as 17% in Mycobacterium tuberculosis (M. tuberculosis) culture and 14% in GeneXpert (4). While recent advances of diagnostic tools such as GeneXpert TB/RIF, or liquid culture media, the BACTEC-MGIT system, can enhance the microbiological yield of M. tuberculosis, the diagnostic performance of the new techniques remains unsatisfactory.
Interferon-gamma release assay (IGRA) is a widely and rapidly adopted method to detect latent TB infection. The assay determines the immune response to M. tuberculosis by measuring interferon-gamma (IFN-γ) or IFN-γ-releasing T lymphocytes under stimulation with TB specific antigens in peripheral blood. Although the World Health Organization has endorsed IGRA as a diagnostic test of latent TB infection, not active TB (5), some studies have shown the clinical usefulness of the IGRA in diagnosing active TB (6-8), especially in situations in which it has been difficult to obtain microbiological results for M. tuberculosis. Regarding TB pleurisy, research on the diagnostic performance of IGRA is conflicting (9-11), possibly because of variable results due to different sampling times or false-negative findings in the test in patients with active TB. When there is a clinical suspicion of TB pleurisy but with borderline results in analysis of pleural effusion, such as mild elevated adenosine deaminase (ADA) and no microbiological evidence, a positive IGRA result in the blood could help clinician support a diagnosis of TB pleurisy. On the other hand, a false-negativity of IGRA in presuming TB pleurisy could cause clinical confusion and delay the appropriate anti-TB treatment. However, few studies have verified the factors associated with false-negative results of IGRA in the blood undertaken in patients with TB pleurisy. In the current study, we aimed to investigate the clinical relevance of false-negative results of the QuantiFERON-TB Gold In-Tube (QFT-GIT; Cellestis Ltd., Carnegie, Australia) assay in the peripheral blood, and its associated risk factors in patients diagnosed with pleural TB. We present the following article in accordance with the STROBE reporting checklist (available at https://jtd.amegroups.com/article/view/10.21037/jtd-21-1723/rc).
Methods
Study design and population
This observational retrospective study reviewed medical charts of 650 patients diagnosed with pleural TB by ICD 10 code A165 between January 2009 and December 2020 at a tertiary, referral hospital (1,369 beds) in South Korea. The patients who underwent QFT-GIT in the peripheral blood and analysis of pleural fluid simultaneously before starting anti-TB medication were included. Patients aged <18 years were excluded.
Diagnostic definition
All patients were diagnosed based on clinical, histopathological, radiological and microbiological data. Pleural TB patients were categorized as confirmed or probable pleural TB. Confirmed pleural TB was defined when there was pleural effusion with microbiological, histopathological or genetic evidence for M. tuberculosis infection such as TB PCR or M. tuberculosis culture positivity in pleural fluid, or chronic granulomatous inflammation with or without caseous necrosis in the pleural tissue. Probable pleural TB was defined as lymphocyte dominant exudate in the pleural fluid based on Light’s criteria, with ADA >40 IU/L, and clinical improvement after anti-TB medication. Treatment outcome was classified into two groups, favourable vs. unfavourable outcome, referring to our previous study (12). Favourable outcome was either treatment cure or completion, and unfavourable outcome included treatment failure, discontinuation of anti-TB medication, follow-up loss or death.
QFT-GIT test
IGRA was performed using the QFT-GIT tool before anti-TB treatment according to the manufacturer’s instructions. The IGRA test was performed using the peripheral blood. The QFT-GIT result was defined as positive, indeterminate or negative. The tests were defined as positive if the IFN-γ level of Nil was ≤8.0 IU/mL, and that of TB antigen minus Nil was ≥0.35 IU/mL and 25% of the Nil value. The negative result was if the IFN-γ level of Nil was ≤8.0 IU/mL, that of mitogen minus Nil was ≥0.5 IU/mL, and that of TB antigen minus Nil was <0.35 IU/mL or 25% of the Nil value. An indeterminate result was defined when the IFN-γ level of Nil was ≤8.0 IU/mL, that of TB antigen minus Nil was <0.35 IU/mL or <25% of Nil value, and mitogen minus Nil was <0.5 IU/mL or if the INF-γ level of Nil was >8.0 IU/mL. In the current study, negative and indeterminate results of QFT-GIT were classified as a negative subgroup from a clinical point of view. QFT-Gold Plus, a new generation of QFT assay was used in three subjects in our study.
Data collection
Baseline characteristics were collected including patient’s age, sex, body mass index (BMI), smoking history and comorbidities. We assessed laboratory findings including the analysis of pleural fluid pH, white blood cell (WBC) with differentials, protein, glucose, albumin, lactate dehydrogenase (LDH) and ADA as well as microbiological or histological evidence for M. tuberculosis in respiratory specimens and radiological features at the initial diagnosis of pleural TB. Treatment outcome after anti-TB medication was evaluated. Sputum, bronchial washing fluid or pleural fluid were used for microbiological tests for M. tuberculosis such as acid-fast bacillus (AFB) stain or culture, TB PCR. AFB culture was performed with both tests such as Ogawa solid media and mycobacteria growth indicator tube (MGIT) liquid media. TB PCR test was done with the AdvanSure TB/non-tuberculous mycobacterium PCR (LG Life Sciences, Seoul, South Korea) or the Xpert MTB/resistance to rifampicin assay (Cepheid, Sunnyvale, CA, USA).
Statistical analysis
Categorical variables are reported as number and percentage, and continuous variables are expressed as mean ± standard deviation (SD) in normally distributed or as median [interquartile range (IQR)] if not normally distributed. Student’s t-test or the Mann-Whitney U test was used for analysis of continuous variables, and the χ2 test or Fisher’s exact test was used for categorical variables. Multivariate analysis using multiple logistic regression was performed for statistically significant predictors in the univariate analysis to determine the risk factors associated with negative QFT-GIT results. All statistical analyses were performed using both the R 4.0.2 version (R foundation, Vienna, Austria) and SPSS 21.0 (SPSS Inc., Chicago, IL, USA) and P<0.05 was considered statistically significant.
Ethical approval
The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). This study was conducted in accordance with the relevant legislation and approved by the Ethics Committee of Seoul St. Mary’s Hospital (KC21RISI0062). The need for informed consent from the subjects was waived by the Committee.
Results
Baseline characteristics of study participants
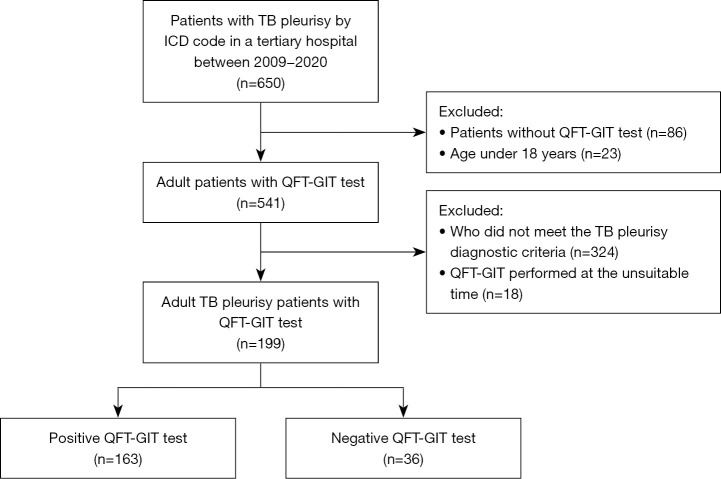
The flow sheet of the subjects in this study is shown in Figure 1. Out of 650 patients with TB pleurisy, 199 patients (126 men and 73 women) were included in the final study. Baseline characteristics of the patients are shown in Table 1. The median age of the patients was 55.0 (IQR, 33.5–73.0) years and males accounted for 63.3%. Median BMI was 21.7 kg/m2 and 7.8% of the participants had a BMI <18 kg/m2. Sixty-six patients (33.2%) had concomitant diseases, the most common of which were haematological malignancy and diabetes. Twenty patients (10.1%) had a previous history of pulmonary TB. On microbiological tests, only 2 patients (1.0%) were positive for AFB smears in the pleural fluid. Nineteen patients (11.1%) and 61 patients (31.1%) were positive for pleural PCR and culture, respectively. Of the total subjects, 34.7% had microbiological evidence of TB on pleural fluid analysis. The pleural effusion was located on the patient’s right side (54.3%), on the left side (28.9%) or on both sides (16.8%). 119 patients were classified to confirmed TB pleurisy and 80 patients were to probable TB pleurisy. Among participants, 37.2% had concomitant pulmonary TB and 13.6% had disseminated TB. Data are shown in Table 2.
Figure 1.
The patient selection process. TB, tuberculosis; QFT-GIT, QuantiFERON-TB Gold In-Tube.
Table 1. Comparison of baseline characteristics of the study population.
| Variable | Total (n=199) | QFT-GIT-negative group (n=36) | QFT-GIT-positive group (n=163) | P value |
|---|---|---|---|---|
| Age | 55.0 (33.5–73.0) | 65.5 (46.0–76.5) | 52.0 (32.0–72.0) | 0.060 |
| Sex, male | 126 (63.3%) | 20 (55.6%) | 106 (65.0%) | 0.381 |
| BMI, kg/m2 | 21.7 (19.4–23.5) | 21.1 (19.6–22.9) | 21.8 (19.4–23.8) | 0.445 |
| BMI <18, kg/m2 | 14 (7.0%) | 2 (5.6%) | 12 (7.4%) | 1.000 |
| Comorbidities | ||||
| Diabetes | 23 (11.6%) | 4 (11.1%) | 19 (11.7%) | 1.000 |
| ESRD | 15 (7.5%) | 6 (16.7%) | 9 (5.5%) | 0.034 |
| Autoimmune diseases† | 14 (7.0%) | 4 (11.1%) | 10 (6.1%) | 0.287 |
| Malignancy‡ | 7 (3.5%) | 1 (2.8%) | 6 (3.7%) | 1.000 |
| Liver cirrhosis | 5 (2.5%) | 2 (5.6%) | 3 (1.8%) | 0.223 |
| Haematologic malignancy§ | 24 (12.1%) | 8 (22.2%) | 16 (9.8%) | 0.049 |
| HIV infection | 1 (0.5%) | 1 (2.8%) | 0 (0.0%) | 0.181 |
| Pneumoconiosis | 8 (4.0%) | 4 (11.1%) | 4 (2.5%) | 0.037 |
| Smoking status | 0.071 | |||
| Never smoker | 137 (68.8%) | 27 (75.0%) | 110 (67.5%) | |
| Current smoker | 29 (14.6%) | 1 (2.8%) | 28 (17.2%) | |
| Former smoker | 33 (16.6%) | 8 (22.2%) | 25 (15.3%) | |
| Past TB history | 20 (10.1%) | 4 (11.1%) | 16 (9.8%) | 0.764 |
Categorical variables are presented as n (%). Continuous variables are presented as median (IQR). †, systemic lupus erythematosus, rheumatoid arthritis, ankylosing spondylitis, dermatomyositis, polymyositis, systemic sclerosis and ulcerative colitis were included in autoimmune diseases; ‡, bladder cancer, hepatocellular carcinoma, gastric cancer, ovary cancer and lung cancer were included in malignancy; §, lymphoma, acute myeloid leukaemia, acute lymphoblastic leukaemia, MDS, HLH, ITP and multiple myeloma were included in haematological malignancy. QFT-GIT, QuantiFERON-TB Gold In-Tube; BMI, body mass index; ESRD, end-stage renal disease; HIV, human immunodeficiency virus; TB, tuberculosis; IQR, interquartile range; MDS, myelodysplastic syndrome; HLH, hemophagocytic lymphohistiocytosis; ITP, immune thrombocytopenia.
Table 2. Comparison between QFT-GIT-negative and –positive patients with pleural TB.
| Variable | Total (n=199) | QFT-GIT-negative group (n=36) | QFT-GIT-positive group (n=163) | P value |
|---|---|---|---|---|
| Pleural AFB stain | 2 (1.0%) | 0 (0.0%) | 2 (1.2%) | 1.000 |
| Pleural TB PCR | 19 (9.5%) | 4 (11.1%) | 15 (9.2%) | 1.000 |
| Pleural M. tuberculosis culture | 61 (30.7%) | 7 (19.4%) | 54 (33.1%) | 0.172 |
| Pleural microbiological evidence for M. tuberculosis | 69 (34.7%) | 9 (25.0%) | 60 (36.8%) | 0.248 |
| Sputum AFB stain | 25 (12.6%) | 5 (13.9%) | 20 (12.3%) | 0.778 |
| Sputum TB PCR | 40 (20.1%) | 8 (22.2%) | 32 (19.6%) | 0.730 |
| Sputum M. tuberculosis culture | 84 (42.2%) | 12 (33.3%) | 72 (44.2%) | 0.413 |
| Sputum microbiological evidence for M. tuberculosis | 88 (44.2%) | 14 (38.9%) | 74 (45.4%) | 0.599 |
| Category of pleural TB | 0.446 | |||
| Confirmed TB | 119 (59.8%) | 19 (52.8%) | 100 (61.3%) | |
| Probable TB | 80 (40.2%) | 17 (47.2%) | 63 (38.7%) | |
| Site of pleural effusion | 0.846 | |||
| Right | 107 (53.8%) | 18 (50.0%) | 89 (54.6%) | |
| Left | 57 (28.6%) | 10 (27.8%) | 47 (28.8%) | |
| Both | 33 (16.6%) | 7 (19.4%) | 26 (16.0%) | |
| Group by infected site of TB | ||||
| Only pleural TB | 98 (49.2%) | 20 (55.6%) | 78 (47.9%) | 0.514 |
| Pleural and pulmonary TB | 74 (37.2%) | 14 (38.9%) | 60 (36.8%) | 0.966 |
| Disseminated TB | 27 (13.6%) | 2 (5.6%) | 25 (15.3%) | 0.178 |
Categorical variables are presented as n (%). QFT-GIT, QuantiFERON-TB Gold In-Tube; TB, tuberculosis; AFB, acid-fast bacillus; PCR, polymerase chain reaction; M. tuberculosis, Mycobacterium tuberculosis.
Comparisons between QFT-GIT-negative and -positive groups in patients with pleural TB
A total of 36 patients were false-negative for QFT-GIT assay, that is, a false-negative rate of 18.1%. The median age of patients with negative QFT-GIT assay was older than that of positive patients (65.5 vs. 52.0 years, P=0.060). The QFT-GIT-false-negative groups had significantly more comorbidities with end-stage renal disease (ESRD), haematological cancer and pneumoconiosis than the QFT-GIT-positive groups (Table 1). For microbiologic evidence for TB and clinical features, the two groups showed no differences in the positive results such as TB PCR or M. tuberculosis culture and lung or other organ involvement (Table 2).
We analysed blood tests and pleural fluid results between two groups (Table 3). QFT-GIT-negative groups showed remarkably low levels of protein and lymphocyte percentage in blood. In the pleural fluid, there were significant differences between the two groups in pH, protein and albumin. There was also a significantly lower proportion of patients with ADA >70 IU/L in the QFT-GIT-negative group than in the QFT-GIT-positive group. Quantitative levels of QFT-GIT test (TB antigen-Nil) between the confirmed pleural TB and probable pleural TB group were compared; the levels in the confirmed pleural TB were significantly higher than it in the probable pleural TB [confirmed vs. probable; 2.77 (0.95–8.72) vs. 1.83 (0.40–5.63), P=0.022] (Figure 2).
Table 3. Comparison of serum and pleural fluid study results according to QFT-GIT results in patients with pleural TB.
| Variable | Total (n=199) | QFT-GIT-negative group (n=36) | QFT-GIT-positive group (n=163) | P value |
|---|---|---|---|---|
| Serum | ||||
| Glucose (mg/dL) | 109.5 (96.0–125.0) | 112.0 (97.0–142.5) | 109.0 (96.0–125.0) | 0.365 |
| Protein (g/dL) | 6.7±0.8 | 6.3±0.9 | 6.8±0.7 | 0.008 |
| Hypoproteinaemia† | 36 (18.1%) | 15 (41.7%) | 21 (12.9%) | <0.001 |
| Albumin (g/dL) | 3.4±0.5 | 3.3±0.6 | 3.4±0.5 | 0.070 |
| LDH (U/L) | 416.0 (369.5–508.0) | 431.5 (386.0–537.0) | 414.0 (362.5–502.5) | 0.261 |
| CRP (mg/dL) | 5.3 (1.9–9.8) | 5.8 (1.3–11.5) | 5.2 (2.0–9.6) | 0.855 |
| WBC (cells/µL) | 6,540 (5,150–8,160) | 7,325 (5,340–9,095) | 6,480 (5,115–8,035) | 0.107 |
| Lymphocyte (%) | 17.7 (12.4–23.6) | 13.6 (9.6–24.0) | 18.0 (13.8–23.5) | 0.049 |
| Lymphocyte count | 1,121.1 (775.8–1,499.5) | 956.8 (589.8–1,507.9) | 1,123.2 (810.8–1,499.5) | 0.273 |
| Level in QFT-GIT (IU/mL) | 2.4 (0.6–7.5) | 0.0 (0.0–0.1) | 3.1 (1.6–8.7) | <0.001 |
| Pleural fluid | ||||
| pH >7.6 | 26 (13.1%) | 9 (25.0%) | 17 (10.4%) | 0.024 |
| WBC (cells/µL) | 1,760.0 (785.0–2,735.0) | 1,100.0 (520.0–3,760.0) | 1,847.5 (872.0–2,580.0) | 0.386 |
| Neutrophil (%) | 9.0 (1.0–23.0) | 7.0 (2.0–18.5) | 9.0 (1.0–24.0) | 0.860 |
| Lymphocyte (%) | 72.0 (52.0–88.0) | 74.0 (52.0–89.5) | 72.0 (52.0–87.0) | 0.850 |
| Lymphocyte count | 1,080.1 (498.0–1,788.5) | 786.6 (296.4–1,524.0) | 1,199.8 (522.0–1,800.0) | 0.184 |
| Protein (g/dL) | 4.8 (4.0–5.3) | 4.2 (3.9–4.8) | 4.9 (4.2–5.4) | 0.012 |
| Glucose (mg/dL) | 86.5 (66.0–104.0) | 88.0 (71.0–108.5) | 86.0 (65.0–102.0) | 0.546 |
| LDH (U/L) | 878.5 (519.5–1,323.5) | 727.0 (410.5–1,189.0) | 883.0 (546.0–1,324.0) | 0.171 |
| Albumin (g/dL) | 2.7 (2.3–3.0) | 2.5 (2.0–2.6) | 2.7 (2.3–3.0) | 0.007 |
| ADA (IU/L) | 102.64±38.86 | 99.39±52.31 | 103.35±35.44 | 0.589 |
| ADA >70 (IU/L) | 154 (77.4%) | 22 (61.1%) | 132 (81.0%) | 0.023 |
†, hypoproteinaemia was defined as serum protein <6.0 g/dL. Categorical variables are presented as n (%). Continuous variables are presented as mean ± SD or median (IQR). QFT-GIT, QuantiFERON-TB Gold In-Tube; TB, tuberculosis; LDH, lactate dehydrogenase; CRP, C-reactive protein; WBC, white blood cell; ADA, adenosine deaminase; SD, standard deviation; IQR, interquartile range.
Figure 2.
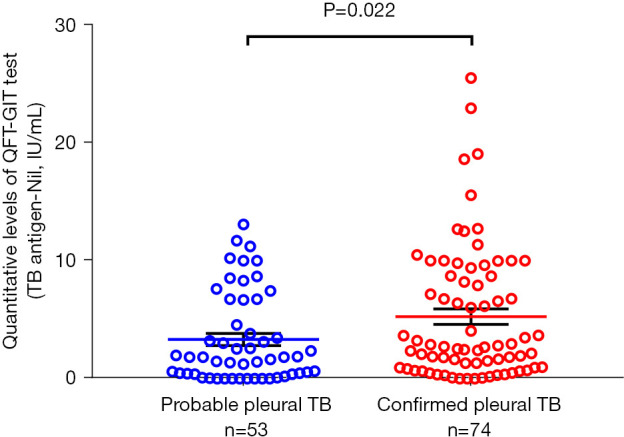
Difference of quantitative levels of QFT-GIT test according to the categorization of pleural TB, the vertical axis represents the TB antigen-Nil value. QFT-GIT, QuantiFERON-TB Gold In-Tube; TB, tuberculosis.
Treatment results in patients with pleural TB are shown in Table 4. Of 199 patients, 163 (81.9%) were cured or completed anti-TB treatment; 13 patients (6.5%) died. Comparing treatment results according to QFT-GIT results, QFT-GIT-negative groups had significantly more unfavourable outcomes such as mortality, stop to anti-TB treatment or follow-up loss [33.3% (12/36 patients) in QFT-GIT-negative groups vs. 14.7% (24/163 patients) in QFT-GIT-positive groups, P<0.017]. Notably, overall mortality and TB-unrelated death were higher in the QFT-GIT-negative group than in the QFT-GIT-positive group [16.7% (6/36 patients) vs. 4.3% (7/163 patients) in overall mortality, P<0.015; 13.9% vs. 2.5% in TB unrelated death, P<0.011, respectively].
Table 4. Comparison of treatment outcome according to QFT-GIT results in patients with pleural TB.
| Variable | Total (n=199) | QFT-GIT-negative group (n=36) | QFT-GIT-positive group (n=163) | P value |
|---|---|---|---|---|
| Favorable outcome | 163 (81.9%) | 24 (66.7%) | 139 (85.3%) | 0.017 |
| Unfavorable outcome | 36 (18.1%) | 12 (33.3%) | 24 (14.7%) | 0.017 |
| Discontinuation of anti-TB medication | 6 (3.0%) | 3 (8.3%) | 3 (1.8%) | 0.074 |
| Follow-up loss | 17 (8.5%) | 3 (8.3%) | 14 (8.6%) | 1.000 |
| Death | 13 (6.5%) | 6 (16.7%) | 7 (4.3%) | 0.015 |
| TB-related death | 4 (2.0%) | 1 (2.8%) | 3 (1.8%) | 0.553 |
| Not TB-related death | 9 (4.5%) | 5 (13.9%) | 4 (2.5%) | 0.011 |
QFT-GIT, QuantiFERON-TB Gold In-Tube; TB, tuberculosis.
Risk factors for false-negative QFT-GIT results in patients with pleural TB
In univariate analysis for risk factors associated with false-negative peripheral blood QFT-GIT results in pleural TB patients, false-negative results were associated with ESRD, haematological malignancy, pneumoconiosis, overall death or death due to other causes more than TB, discontinuation of anti-TB medication, serum and pleural protein, pleural fluid albumin, pleural pH >7.6 and patients with ADA <70 IU/L. The results are summarized in Table 5. In multivariate analysis, the odds ratios (ORs) were 8.96 [95% confidence interval (CI): 1.48–49.56; P=0.012] for pneumoconiosis, 3.99 (95% CI: 1.01–14.70; P=0.039) for ESRD, 5.25 (95% CI: 1.75–15.68; P=0.003) for pleural fluid pH >7.6, and 7.12 (95% CI: 2.82–18.78; P<0.001) for hypoproteinaemia <6.0 g/dL.
Table 5. Factors associated with the false-negative QFT-GIT assay results in patients with pleural TB.
| Factor | Univariate analysis | Multivariate analysis | |||||
|---|---|---|---|---|---|---|---|
| Crude OR | 95% CI | P value | Adjusted OR | 95% CI | P value | ||
| Age | 1.02 | 1.00–1.03 | 0.061 | ||||
| ESRD | 3.42 | 1.08–10.22 | 0.029 | 3.99 | 1.01–14.70 | 0.039 | |
| Haematological malignancy | 2.62 | 0.98–6.59 | 0.044 | ||||
| Pneumoconiosis | 4.97 | 1.12–22.02 | 0.029 | 8.96 | 1.48–49.56 | 0.012 | |
| Discontinuation of anti-TB medication | 2.55 | 0.15–0.69 | 0.027 | ||||
| Death | 4.46 | 1.35–14.35 | 0.011 | ||||
| Not TB-related death | 6.41 | 1.61–27.2 | 0.008 | ||||
| Serum lymphocyte (%) | 1.01 | 0.98–1.06 | 0.446 | ||||
| Serum protein | 2.17 | 1.35–3.62 | 0.002 | ||||
| Serum albumin | 1.88 | 0.95–3.77 | 0.072 | ||||
| Hypoproteinaemia† | 4.83 | 2.15–10.86 | <0.001 | 7.12 | 2.82–18.78 | <0.001 | |
| Pleural fluid pH‡ | 2.44 | 0.44–13.42 | 0.303 | ||||
| Pleural fluid protein | 1.22 | 0.88–1.69 | 0.216 | ||||
| Pleural fluid albumin | 2.33 | 1.16–4.72 | 0.018 | ||||
| Pleural ADA <70 | 2.69 | 1.20–5.92 | 0.015 | ||||
| Pleural fluid pH >7.6 | 3.03 | 1.18–7.45 | 0.017 | 5.25 | 1.75–15.68 | 0.003 | |
†, hypoproteinaemia was defined as serum protein <6.0 g/dL; ‡, per 1 unit decrease. QFT-GIT, QuantiFERON-TB Gold In-Tube; TB, tuberculosis; OR, odds ratio; CI, confidence interval; ESRD, end-stage renal disease; ADA, adenosine deaminase.
Discussion
In the current study, we observed that the false-negative rate of the QFT-GIT assay in 199 patients with pleural TB was 18.1% in the intermediate TB incidence country of South Korea. Patients with specific underlying diseases such as pneumoconiosis or ESRD, and with low protein in blood and pleural fluid had a higher rate of negative results in the QFT-GIT test. Furthermore, the false-negative groups in the assay showed poor clinical outcome including higher discontinuation with anti-TB treatment and mortality.
Diagnosis of pleural TB is a challenging task for physicians. Finding or proving microbiological evidence of M. tuberculosis in pleural effusion is difficult. Although, the diagnostic performance of ADA level in pleural fluid, the most widely used parameter for diagnosing pleural TB, is very high (sensitivity 0.92 and specificity 0.90), and the false-positive and -negative rates of ADA are 8% and 10%, respectively (13). On the contrary, IGRA does not confirm a diagnosis of pleural TB but it could be a useful test that supports the diagnosis of pleural TB in some situations. In a recent meta-analysis of IGRA performance for diagnosis of pleural TB, the sensitivity and specificity of the blood IGRA were 0.77 and 0.78, respectively, and for the pleural fluid IGRA, they were 0.72 and 0.78, respectively (9). The lower performance of IGRA for diagnosing TB pleurisy can reduce the reliability of the test results and limit its clinical use in the diagnosis of pleural TB. Therefore, to clarify clinical features of patients with false-negative QFT-GIT assay can lead to a better interpretation of its result in managing suspected pleural TB.
In this study, the false-negative result rate in QFT-GIT assay was 18.1% and the sensitivity of the blood QFT-GIT assay in pleural TB was 81.9%. There may be several reasons why our study showed higher sensitivity than the recent meta-analysis. First, the prevalence of TB in Korea is higher than in those countries included in the meta-analysis (9). In 2019, the prevalence of TB in Korea was 59.0 per 100,000 people (2). Additionally, the current study included probable TB cases in the category of pleural TB.
In the multivariate regression analysis, ESRD, pneumoconiosis, pleural pH >7.6 and hypoproteinaemia were associated with false-negativity in QFT-GIT assay in patients with pleural TB. A recent systematic review in patients with TB noted that the two factors related to false-negative IGRA assay were advanced age and low peripheral lymphocyte count (14). In a recent study of extra-pulmonary TB, pleural involvement in TB and possible diagnosed TB, not probable or proven cases, were the factors associated with false-negative QFT-GIT results (15). Although serum lymphocyte count and age were not statistically significant in our study, we also observed that the QFT-GIT-negative group tended to have lower serum lymphocyte count and be older than the QFT-GIT-positive group. Interestingly, the current study revealed that some other comorbidities such as ESRD, haematological malignancy and pneumoconiosis were significantly associated with a false-negative QFT-GIT result. Regarding ESRD, in a recent meta-analysis, the false-negative rate of QFT-GIT for the diagnosis of latent TB infection in ESRD with dialysis patients was 47% (16). In the present study, the false-negative QFT-GIT results in ESRD patients with pleural TB was 40% (adjusted OR =3.99; 95% CI: 1.01–14.70; P=0.039). In patients with ESRD or chronic renal failure, immune system dysfunction including cellular dysfunction occurred due to defects in antigen-presenting cells and a decrease in T-cell response (17). Since the IGRA assay relies on the measurement of IFN-γ produced by T-cells sensitized to M. tuberculosis antigen, ESRD patients could have false-negative QFT-GIT assay results despite the presence of active TB. Regarding pneumoconiosis, to our knowledge, this is the first report to note that underlying pneumoconiosis could affect the false negativity of IGRA in pleural TB (adjusted OR =8.96; 95% CI: 1.48–49.56; P=0.012). Of 8 pneumoconiosis patients enrolled in our study, 6 patients had coal workers pneumoconiosis and 2 patients had silicosis. Pneumoconiosis, especially silicosis is well known to be vulnerable to pulmonary TB because silica particles in the lungs induce macrophage dysfunction and decrease cell-mediated immunity (18,19). Also, coal workers pneumoconiosis is known to be vulnerable to pulmonary TB because coal dust is not a pure substance and usually contains 1–2% free silica (20). While it might be a plausible hypothesis that the aberrant immunity in the respiratory system in pneumoconiosis patients partly induces an abnormal immune response and consequently a false-negative result of QFT-GIT in pleural TB, further large-scale studies are needed to confirm this hypothesis.
We observed that high pH in pleural fluid or hypoproteinaemia was significantly associated with false-negative results for QFT-GIT in pleural TB. With regard to pH in pleural fluid, cases with pleural TB usually have pH >7.30, but in some cases the levels can be lower (21). The decrease in pleural pH in pleural infection is induced by CO2 increase in the pleural fluid arising from bacterial metabolism, activation of neutrophils and phagocytes by immune response, and the efflux of CO2 and hydrogen ions in the pleural space. Therefore, the high pH of the pleural effusion despite the presence of tuberculous pleurisy, showing as the IGRA-negative group in our study, may suggest a lack of cellular defence against M. tuberculosis pathogens. However, because the pleural pH is labile due to various conditions, such as exposure to air, lidocaine injection via thoracentesis, patient status, or the period between sampling and test, it is not clinically useful to interpret pleural pH by itself as a decisive parameter. On the other hand, the group with hypoproteinaemia <6.0 g/dL had a false-negative probability of QFT-GIT of more than seven times compared with the group with normal protein (P<0.001). Several studies have reported that hypoproteinaemia is associated with false-negative IGRA assay results (18,22,23). As hypoproteinaemia is an indicator of malnutrition, causing a decreased immune response, so too the protein status may lead to false-negative results of IGRA despite the presence of TB infection.
A previous study (24) reported that the IFN-γ response to M. tuberculosis antigens was higher in active TB than in latent TB. Focusing on patients with extra-pulmonary TB, Kim et al. (15) found that the quantitative value of QFT-GIT was higher in proven TB with microbiological evidence than in possible TB without a microbiological result. We also observed a similar pattern in that patients categorized as confirmed TB with the existence of M. tuberculosis had a higher titre of QFT-GIT than patients with probable TB without the microbiological clue. This finding may provide evidence that the higher the titre of IGRA, the more likely it is that pleural TB can be diagnosed if clinically suspected. Further investigation is needed to confirm this hypothesis and determine a cut-off value of QFT-GIT assay titre for differentiation of pleural TB.
Intriguingly, the current study showed that the QFT-GIT-negative group with pleural TB had a significantly higher unfavourable outcome including all-cause mortality than the QFT-GIT-positive group. Nguyen et al. (25) also reported that in culture-confirmed TB, false-negative IGRA results were related to poor survival, especially in T-spot TB assay. They noted that the false negativity led to delayed initiation of treatment and contributed to higher mortality. Another explanation is that a lack of systemic immune response to TB infection, inducing false-negative IGRA results, might imply more chance of concurrent conditions such as ESRD, and those conditions by themselves not only induced poor impact on the treatment response for TB but also enhanced vulnerability to other diseases, causing death.
There are some limitations to be addressed in the present study. First, as it was conducted retrospectively in a single centre, an inherent weakness of the results is the relatively small sample size. Nevertheless, interesting and informative results in our study should help clinicians give a useful guide in managing patients with suspected pleural TB. Second, the definition of pleural TB was included with the clinical diagnosis of probable TB as well as confirmative TB with microbiologic evidence. Although this might weaken the reliability of the findings, we believe that this approach reflects the real-world practice to approach pleural TB. Lastly, few patients (3 out of 199 cases) were tested with QFT-Gold Plus instead of QFT-GIT. However, the two tests are known to have similar diagnostic efficacy (26), so it seems little effect on our final conclusion.
Conclusions
In summary, in patients with pleural TB, the false-negative rate in the QFT-GIT assay was 18.1% in the TB intermediate incidence country. False-negative QFT-GIT in pleural TB was associated not only with factors such as ESRD, pneumoconiosis and hypoproteinaemia, but also with poor outcome. Therefore, in approaching patients with pleural TB, physicians should take into consideration the occurrence of false negatives of IGRA tests and their impact on clinical outcome.
Supplementary
The article’s supplementary files as
Acknowledgments
Funding: None.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). This study was conducted in accordance with the relevant legislation and approved by the Ethics Committee of Seoul St. Mary’s Hospital (KC21RISI0062). The need for informed consent from the subjects was waived by the Committee.
Footnotes
Reporting Checklist: The authors have completed the STROBE reporting checklist. Available at https://jtd.amegroups.com/article/view/10.21037/jtd-21-1723/rc
Data Sharing Statement: Available at https://jtd.amegroups.com/article/view/10.21037/jtd-21-1723/dss
Peer Review File: Available at https://jtd.amegroups.com/article/view/10.21037/jtd-21-1723/prf
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://jtd.amegroups.com/article/view/10.21037/jtd-21-1723/coif). The authors have no conflicts of interest to declare.
References
- 1.WHO. Global tuberculosis report 2020. Geneva: World Health Organization, 2020. [Google Scholar]
- 2.KDCA. Annual report on the notified tuberculosis in Korea, 2020. Cheongju: Korea Centers for Disease Control and Prevention, 2021. [Google Scholar]
- 3.Shaw JA, Diacon AH, Koegelenberg CFN. Tuberculous pleural effusion. Respirology 2019;24:962-71. 10.1111/resp.13673 [DOI] [PubMed] [Google Scholar]
- 4.Christopher DJ, Dinakaran S, Gupta R, et al. Thoracoscopic pleural biopsy improves yield of Xpert MTB/RIF for diagnosis of pleural tuberculosis. Respirology 2018;23:714-7. 10.1111/resp.13275 [DOI] [PubMed] [Google Scholar]
- 5.WHO. Report of the 10th Meeting of the Strategic and Technical Advisory Group for TB (STAG-TB). World Health Organization Report of the 10th meeting. Geneva: WHO, 2010. [Google Scholar]
- 6.Diel R, Loddenkemper R, Nienhaus A. Evidence-based comparison of commercial interferon-gamma release assays for detecting active TB: a metaanalysis. Chest 2010;137:952-68. 10.1378/chest.09-2350 [DOI] [PubMed] [Google Scholar]
- 7.Jia H, Pan L, Qin S, et al. Evaluation of interferon-γ release assay in the diagnosis of osteoarticular tuberculosis. Diagn Microbiol Infect Dis 2013;76:309-13. 10.1016/j.diagmicrobio.2013.03.030 [DOI] [PubMed] [Google Scholar]
- 8.Feng Y, Diao N, Shao L, et al. Interferon-gamma release assay performance in pulmonary and extrapulmonary tuberculosis. PLoS One 2012;7:e32652. 10.1371/journal.pone.0032652 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Aggarwal AN, Agarwal R, Gupta D, et al. Interferon Gamma Release Assays for Diagnosis of Pleural Tuberculosis: a Systematic Review and Meta-Analysis. J Clin Microbiol 2015;53:2451-9. 10.1128/JCM.00823-15 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Zhang R, Tian P, Zhao S, et al. Development and validation of novel diagnostic nomogram for tuberculous pleurisy based on TB-IGRA results. Int J Tuberc Lung Dis 2020;24:1178-85. 10.5588/ijtld.20.0001 [DOI] [PubMed] [Google Scholar]
- 11.Yang X, Zhang J, Liang Q, et al. Use of T-SPOT.TB for the diagnosis of unconventional pleural tuberculosis is superior to ADA in high prevalence areas: a prospective analysis of 601 cases. BMC Infect Dis 2021;21:4. 10.1186/s12879-020-05676-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kang HS, Lee HY, Jung JI, et al. Clinical significance of Glasgow Prognostic Score in patients with tuberculous pleurisy. J Thorac Dis 2018;10:6077-87. 10.21037/jtd.2018.10.02 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Aggarwal AN, Agarwal R, Sehgal IS, et al. Adenosine deaminase for diagnosis of tuberculous pleural effusion: A systematic review and meta-analysis. PloS One 2019;14:e0213728. 10.1371/journal.pone.0213728 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Yamasue M, Komiya K, Usagawa Y, et al. Factors associated with false negative interferon-γ release assay results in patients with tuberculosis: A systematic review with meta-analysis. Sci Rep 2020;10:1607. 10.1038/s41598-020-58459-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kim YJ, Kang JY, Kim SI, et al. Predictors for false-negative QuantiFERON-TB Gold assay results in patients with extrapulmonary tuberculosis. BMC Infect Dis 2018;18:457. 10.1186/s12879-018-3344-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ferguson TW, Tangri N, Macdonald K, et al. The diagnostic accuracy of tests for latent tuberculosis infection in hemodialysis patients: a systematic review and meta-analysis. Transplantation 2015;99:1084-91. 10.1097/TP.0000000000000451 [DOI] [PubMed] [Google Scholar]
- 17.Girndt M, Sester U, Sester M, et al. Impaired cellular immune function in patients with end-stage renal failure. Nephrol Dial Transplant 1999;14:2807-10. 10.1093/ndt/14.12.2807 [DOI] [PubMed] [Google Scholar]
- 18.Wang MS, Liu XJ. Risk Factors for False-Negative Interferon-γ Release Assay Results in Culture-Confirmed Childhood TB. Am J Trop Med Hyg 2019;101:1303-7. 10.4269/ajtmh.18-0684 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Allison AC, Hart PD. Potentiation by silica of the growth of Mycobacterium tuberculosis in macrophage cultures. Br J Exp Pathol 1968;49:465-76. [PMC free article] [PubMed] [Google Scholar]
- 20.COCHRANE AL . Tuberculosis and coalworkers’ pneumoconiosis. Br J Tuberc Dis Chest 1954;48:274-85. 10.1016/S0366-0869(54)80127-4 [DOI] [PubMed] [Google Scholar]
- 21.Light RW. Update on tuberculous pleural effusion. Respirology 2010;15:451-8. 10.1111/j.1440-1843.2010.01723.x [DOI] [PubMed] [Google Scholar]
- 22.Anuradha R, Munisankar S, Bhootra Y, et al. Malnutrition is associated with diminished baseline and mycobacterial antigen – stimulated chemokine responses in latent tuberculosis infection. J Infect 2018;77:410-6. 10.1016/j.jinf.2018.05.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Mandalakas AM, van Wyk S, Kirchner HL, et al. Detecting tuberculosis infection in HIV-infected children: a study of diagnostic accuracy, confounding and interaction. Pediatr Infect Dis J 2013;32:e111-8. 10.1097/INF.0b013e31827d77b7 [DOI] [PubMed] [Google Scholar]
- 24.Kobashi Y, Shimizu H, Ohue Y, et al. Comparison of T-cell interferon-gamma release assays for Mycobacterium tuberculosis-specific antigens in patients with active and latent tuberculosis. Lung 2010;188:283-7. 10.1007/s00408-010-9238-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Nguyen DT, Teeter LD, Graves J, et al. Characteristics Associated with Negative Interferon-γ Release Assay Results in Culture-Confirmed Tuberculosis Patients, Texas, USA, 2013-2015. Emerg Infect Dis 2018;24:534-40. 10.3201/eid2403.171633 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Takeda K, Nagai H, Suzukawa M, et al. Comparison of QuantiFERON-TB Gold Plus, QuantiFERON-TB Gold In-Tube, and T-SPOT.TB among patients with tuberculosis. J Infect Chemother 2020;26:1205-12. 10.1016/j.jiac.2020.06.019 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
The article’s supplementary files as