Abstract
Background
To evaluate whether homoharringtonine (HHT) combined with venetoclax could produce a synergistic anti-acute myeloid leukemia (AML) effect and determine the underlying mechanisms.
Methods
The effect of HHT and venetoclax combination on cell viability, apoptosis, and mitochondrial membrane potential was investigated in vitro using AML cell lines and primary cells. High-throughput mRNA sequencing was used to analyze mRNA level changes after the application of HHT and venetoclax on OCI-AML3 cells. Western blotting was used to verify the changes in protein expression within the mitogen-activated protein kinases/extracellular signal-regulated kinase (MAPK/ERK), phosphatidylinositiol 3-kinase (PI3K)/AKT and p53 pathway. The efficacy of HHT and venetoclax in vivo and their effects on survival time were evaluated in a xenograft model established in severe immunodeficiency (NOD/SCID) mice.
Results
Venetoclax and HHT synergistically inhibited the proliferation of AML cells, decreased the mitochondrial membrane potential, and promoted AML cell apoptosis in a time- and concentration-dependent manner. Venetoclax combined with HHT increased the expression of the caspase-3, Poly (ADP-ribose) polymerase (PARP), and γH2AX proteins. HHT enhanced the proapoptotic effect of venetoclax by reducing the expression of myeloid cell leukemia sequence 1 (Mcl-1). HHT arrested AML cells in G1 phase of the cell cycle. HHT enhanced the proapoptotic effect of venetoclax by inhibiting the activation of the MAPK/ERK and PI3K/AKT pathways and activating the p53 pathway. In vivo experiments confirmed that the combination of HHT and venetoclax could inhibit the growth of tumors in AML xenotransplanted mice and prolong the survival time of tumor-bearing mice.
Conclusions
HHT combined with venetoclax synergistically promoted apoptosis in AML cell lines and primary cells by inhibiting the activation of the MAPK/ERK and PI3K/AKT pathways and activating the p53 pathway.
Keywords: Homoharringtonine (HHT), venetoclax, acute myeloid leukemia (AML), apoptosis, mechanism
Introduction
Acute myeloid leukemia (AML) is a hematopoietic malignancy characterized by the rapid growth of myeloid clonal cells. It accounts for approximately 70% of acute leukemias. The incidence of AML in China is approximately 3.4/100,000, and it has been increasing in recent years. With advances in treatment, approximately 60% to 80% of adult AML patients can achieve complete remission (CR) through induction chemotherapy, while most AML patients will show resistance to chemotherapy at some point during the course of the disease. Approximately 20% of AML patients exhibit primary refractory disease, and more than 50% of young AML patients and up to 90% of elderly AML patients will eventually relapse (1-3). The survival of AML cells depends on their expression of Bcl-2 (4). Overexpression of Bcl-2 protein protects cells from apoptosis and is related to chemotherapy resistance (5-10). Therefore, inhibiting antiapoptotic Bcl-2 family members is promising in the treatment of AML.
Venetoclax is a selective Bcl-2 inhibitor, and its addition to Bcl-2-overexpressing cancer cells effectively triggers apoptosis in vitro (11). It is well tolerated and greatly reduces treatment risk compared with conventional chemotherapy, but its application as a single agent has low efficiency in AML patients. Therefore, it is necessary to explore combination regimens with venetoclax in AML. Mcl-1 has been demonstrated to play a role in both inherent and acquired resistance to venetoclax (12,13). The mechanism by which Mcl-1 blocks apoptosis is by binding to and sequestering the pro-apoptotic BH3-only proteins Bim, Puma, Noxa, Bak, and Bax (14), causing the formation of pores on the mitochondrial membrane is prevented and cytochrome c is released into the membrane cytoplasm (15). Combination regimens of venetoclax with hypomethylating agents (HMAs) or low-dose cytarabine (LDAC) have received accelerated approval from the US Food and Drug Administration (FDA) for the treatment of patients aged 75 or older or in newly diagnosed AML patients who are not candidates for strong chemotherapy. Several centers have explored venetoclax in combination with other drugs to synergistically promote apoptosis in AML cells, and experimental studies have shown that erythromycin, cytarabine, histone deacetylase inhibitors (HDACIs), and the Mcl-1 inhibitor A-1210477 could synergistically induce apoptosis in AML cells (16,17). Homoharringtonine (HHT), an indigenous plant-derived antitumor drug in China, has been used for the treatment of hematologic malignancies for nearly 30 years, and experimental studies (18-21) have shown that HHT can promote apoptosis in a variety of AML cells and can inhibit the protein translation process and downregulate Mcl-1 expression. HHT has presented a synergistic effect with venetoclax in inhibition of Mcl-1 and killing of lymphoma (22). Yu et al. found a combination of HHT with venetoclax and azacitidine exerts better treatment response in R/R-AML (23). This study aimed to use AML cell lines and primary cells to study the effects of venetoclax and HHT combination therapy on AML cell apoptosis, establishing an AML mouse model to examine the efficacy and survival time of combination therapy in vivo, and analyze the drug effects with multi-omics sequencing technology. In our study, we focused on the mechanism of HHT and venetoclax acting on AML, it was found by transcriptome sequencing and verified by Western blot and quantitative reverse transcription-PCR (RT-qPCR) that HHT combined with venetoclax synergistically promoted apoptosis in AML cell lines and primary cells by inhibiting the activation of the MAPK/ERK and PI3K/AKT pathways and activating the p53 pathway. The results of this study provide a theoretical basis for exploring combination treatment strategies including venetoclax in AML. We present the following article in accordance with the ARRIVE reporting checklist (available at https://atm.amegroups.com/article/view/10.21037/atm-22-1459/rc).
Methods
Materials
Phosphorylated and total MEK, ERK, AKT, CDK2, c-Myc, p21, CyclinD1, p53, caspase-3, PARP, Bax, Bcl-2, Bcl-xL, Bim, Mcl-1, and β-actin antibodies were purchased from Cell Signaling Technology (Beverly, MA, USA). Human CD45 antibody was purchased from Abcam (Cambridge, MA, USA). Venetoclax was purchased from Selleck Company (Houston, TX, USA). HHT was purchased from Solarbio Science & Technology Co., Ltd.
Cell lines and primary cells
OCI-AML3, THP-1, MV4-11, and MOLM13 cell lines were purchased from the American Type Culture Collection (Manassas, VA, USA) and cultured in RPMI 1640 medium supplemented with 10% fetal bovine serum (FBS). Bone marrow samples were obtained from AML patients attended in the Affiliated Cancer hospital of Zhengzhou University. Mononuclear cells were isolated by Ficoll-Hypaque (Sigma Aldrich) density gradient centrifugation. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). The study was approved by the Ethics Committee of the Affiliated Cancer Hospital of Zhengzhou University, China (approval No. 2019-KY-0017-001) and informed consent was taken from all the patients.
Cell proliferation assay
Cells were seeded in 96-well plates (5×103–1×104 cells/well) in triplicate and treated with different drugs (venetoclax and HHT) for 24, 48, or 72 h. Subsequently, 10 µL of Cell-Counting Kit-8 (CCK-8) proliferation assay reagent (Tongren Institute of Chemistry, Japan) was added to each well, followed by incubation for an additional 2 h at 37 °C. Cell numbers were assessed based on the production of formazan, which was quantified by determining the absorbance at 450 nm using a microplate reader (Biotech, NY, USA). The concentration of HHT is 0.5–400 nM, and the concentration of venetoclax is 0.5 nM–10 μM.
Apoptosis assay
Induction of apoptosis was assessed using an apoptosis detection kit (China Nanjing Kaiji Company). The concentration of HHT is 1 nM–100 nM, and the concentration of venetoclax is 0.1 nM–10 μM. After treatment with venetoclax and HHT alone or in combination for 8 or 24 h, the cells were washed twice with phosphate-buffered saline (PBS), resuspended in binding buffer, and incubated with Annexin V-FITC and propidium iodide (PI) for 15 min. Apoptotic cells were analyzed by flow cytometry using a Navios flow cytometer (Beckman Coulter, Brea CA, USA). The results were expressed as a percentage of Annexin V positive cells to the total number of cells. Combination indices (CI) were calculated using CompuSyn software to determine interactions (synergy, CI <l; additive, CI =1; or antagonism, CI >1).
Mitochondrial membrane potential analysis
After treatment with venetoclax and HHT alone or in combination for 24 h, cells were harvested, and JC-1, a cationic lipid fluorescent dye, was used to stain the cells. During apoptosis, the mitochondrial transmembrane potential decreases, JC-1 exists in the cytoplasm in the form of monomers, and the number of polymers decreases. Flow cytometry was used to determine whether JC-1 existed in the form of monomers or polymers to detect changes in mitochondrial transmembrane potential. Green fluorescence represents JC-1 monomers, whereas red fluorescence represents JC-1 aggregates. The concentration of HHT is 2–30 nM, and the concentration of venetoclax is 0.5 nM–1 μM.
RNA sequencing analysis
OCI-AML3 cells were lysed in TRIzol after treatment with HHT, venetoclax, or their combination for 24 h, and then RNA was isolated. The sequencing reaction was performed using Illumina HiSeq. Differentially expressed gene analysis, cluster analysis, Gene Ontology (GO) enrichment analysis, pathway enrichment analysis, and other functional annotations were performed at Nanjing Personal Aksomics Company Limited (Nanjing, China). The concentration of HHT is 20 nM, and the concentration of venetoclax is 1 μM.
Cell cycle analysis
After treatment with venetoclax and HHT alone or in combination for 24 h, cells were harvested and fixed overnight with 75% ethanol at 4 °C, followed by washing twice with PBS and incubation in buffer containing 50 μg/mL PI and 100 μg/mL RNase A for 15 min at room temperature. Cell cycle analysis was conducted using a Navios flow cytometer (Beckman Coulter, Brea, CA, USA). The DNA content was analyzed by flow cytometry. ModFit software (Verity Software House, Inc., Topsham, ME, USA) was used for data analysis. The concentration of HHT is 2 nM, and the concentration of venetoclax is 0.5 nM–1 μM.
Quantitative reverse transcription-polymerase chain reaction (qRT-PCR)
Total RNA was extracted using TRIzol (Invitrogen Life Technologies, Carlsbad, CA, USA) and immediately transcribed into cDNA by a Reverse Transcription Kit (TaKaRa, Dalian, China). Fast Start Universal SYBR Green Master Mix (ROX) (Roche, Germany) was used following the instructions of the supplier. Amplification was performed in 3 stages: the holding stage (94 °C for 10 min), the cycling stage (40 cycles of 94 °C for 10 s, 60 °C for 30 s), and the melting stage (72 °C for 3 min). Relative gene expression levels were calculated using the 2-ΔΔCt method with β-actin as an internal control. The concentration of HHT is 1–20 nM, and the concentration of venetoclax is 0.5 nM–10 μM.The sequences of the primers used for qRT-PCR are shown in Table 1.
Table 1. Primer sequences for qRT-PCR.
| AKT forward: 5'-GTGGCTATTGTGAAGGAGGGTTGG-3' |
| AKT reverse: 5'-GCAGGCAGCGGATGATGAAGG-3' |
| CDK2 forward: 5'-GGCCATCAAGCTAGCAGACT-3' |
| CDK2 reverse: 5'-GAATCTCCAGGGAATAGGGC-3' |
| ERK forward: 5'-TACACCAACCTCTCGTACATCG-3' |
| ERK reverse: 5'-CATGTCTGAAGCGCAGTAAGATT-3' |
| MEK forward: 5'-CCACGTCATTGCCGTTAAGC-3' |
| MEK reverse: 5'-GCACGATGTAGGGGCAGTC-3' |
| Myc forward: 5'-CCACAGCAAACCTCCTCACAG-3' |
| Myc reverse: 5'-GCAGGATAGTCCTTCCGAGTG-3' |
| p21 forward: 5'-CCTGTCACTGTCTTGTACCCT-3' |
| p21 reverse: 5'-GCGTTTGGAGTGGTAGAAATCT-3' |
| p53 forward: 5'-GAGGTTGGCTCTGACTGTACC-3' |
| p53 reverse: 5'-TCCGTCCCAGTAGATTACCAC-3' |
| β-actin forward: 5'-CGCTGCGCTGGTCGTCGACA-3' |
| β-actin reverse: 5'-GTCACGCACGATTTCCCGCT-3' |
qRT-PCR, quantitative reverse transcription-polymerase chain reaction.
Western blot analysis
After treating cells with different drugs for 24 h, cultured cells were harvested, washed with PBS, and then lysed with ice-cold lysis buffer. The cell lysate was centrifuged at 14,000 ×g for 15 min at 4 °C, and then the protein concentration of the cellular supernatant was determined using the bicinchoninic acid (BCA) reagent. Western blotting was performed after sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE), with the cellular proteins transferred onto a preactivated polyvinylidene fluoride (PVDF) membrane (Millipore, Billerica, MA, USA). The membranes were blocked with 5% nonfat milk for 1 h and incubated with primary antibodies overnight at 4 °C. After incubation with the primary antibody, the blots were washed 3 times with TBST buffer, and membranes were incubated with secondary antibodies for 1 h at room temperature. The target protein bands were visualized using enhanced chemiluminescence (ECL) and exposed to X-ray film. Immunoreactive proteins were visualized using the Odyssey Infrared Imaging System (Li-Cor, Lincoln, NE), as per the manufacturer’s instructions. Densitometry measurements were made using Odyssey V3.0 (Li-Cor). β-actin was used as a loading control. The concentration of HHT is 1–20 nM, and the concentration of venetoclax is 0.5 nM–10 μM.
In vivo experiment
Protocol of construction of firefly luciferase-expressing MOLM13 cells was defined in reference (24). NOD/SCID mice (5–6 weeks, female) were purchased from GemPharmatech Co., Ltd. MOLM13 cells (5×106) transfected with luciferase were injected into mice through the tail vein, and live imaging was performed on the 4th day to confirm that the AML xenograft model was successfully established. Mice were anesthetized with isoflurane gas, and fluorescein sodium salt substrate (concentration: 15 mg/mL) was injected intraperitoneally. Intravital imaging of mice using a Small Animal Intravital Imager. The average fluorescence intensity of three sites in each mouse was used as the final fluorescence intensity, which indirectly reflected the tumor burden of the mice. The mice were randomly divided into 4 groups of 9 mice each, namely, the control group, venetoclax group (100 mg/kg, intragastric administration, QD), HHT group (1 mg/kg, intraperitoneal injection, QD), and combination group. The weight of the mice in each group was monitored, and the tumor load changes were observed by in vivo imaging. The extramedullary infiltration of AML cells was detected by immunohistochemistry. The hCD45+ cell ratio of the bone marrow was detected by flow cytometry. The survival time of mice was observed and recorded, and survival curves were drawn. Animal experiments were performed under a project license (No. 20200322) granted by the Ethics Review Committee of life sciences, Zhengzhou University, in compliance with Zhengzhou University’s guidelines for the care and use of animals.
Statistical analysis
Two-group comparisons were made with Student’s t-test. The significance threshold was set at P<0.05. Error bars represent the SD. All statistical analyses were carried out with SPSS version 18.0.
Results
Inhibitory effect of HHT and venetoclax on proliferation
The effect of HHT and venetoclax monotherapy on the viability of several AML cell lines, including THP-1, OCI-AML3, MV4-11, and MOLM13, was investigated by the CCK-8 assay. The results are shown in Figure 1A,1B. Both HHT and venetoclax inhibited the viability of the 4 cell lines in a dose- and time-dependent manner. The half-maximal inhibitory concentrations (IC50) of venetoclax and HHT for the THP-1, OCI-AML3, MV4-11, and MOLM13 cell lines are shown in Figure 1C. According to the IC50 values, OCI-AML3 and THP-1 cells were relatively resistant to venetoclax, whereas MV4-11 and MOLM13 cells were relatively sensitive.
Figure 1.
The proliferation-inhibiting effects of HHT and venetoclax on AML cell lines. (A) Proliferation inhibition curves of THP1, OCI-AML3, MV4-11, and MOLM13 cell lines treated with different concentrations of venetoclax at 24, 48, and 72 h. (B) Proliferation inhibition curve of THP1, OCI-AML3, MV4-11, and MOLM13 cell lines treated with different concentrations of HHT at 24, 48, and 72 h. (C) The IC50 values of venetoclax and HHT in AML cell lines (THP1, OCI-AML3, MV4-11, MOLM13) at 24, 48, and 72 h. The color bar on the right indicates the range of IC50 value. HHT, homoharringtonine; AML, acute myeloid leukemia.
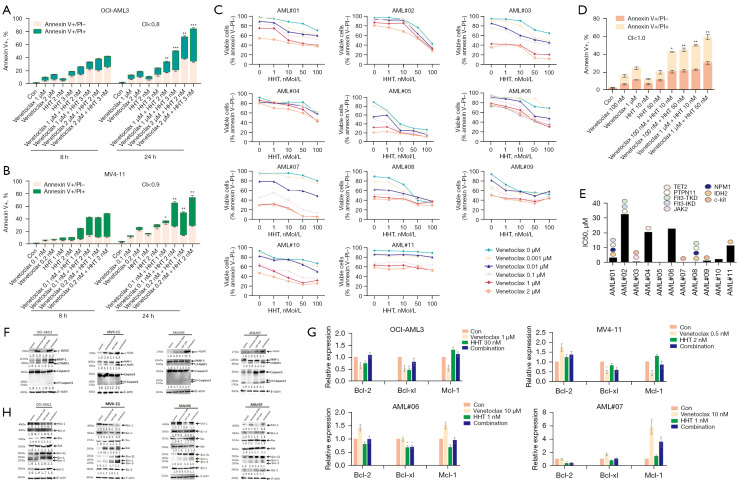
Venetoclax and HHT synergistically promoted apoptosis in AML cells
According to above results, both venetoclax and HHT could inhibit the growth of AML cells. We measured the apoptosis rate in response to monotherapy and combination treatment with flow cytometry. Figure 2A,2B show that venetoclax and HHT induced apoptosis in AML cell lines in a concentration- and time-dependent manner, and the combination of venetoclax and HHT had a synergistic effect on apoptosis (CI <1). The bone marrow of 11 newly diagnosed AML patients (AML#01-AML#11) was extracted, and mononuclear cells were isolated. The clinical characteristics of AML patients are shown in Table 2. Both venetoclax and HHT induced apoptosis in primary AML cells in a concentration-dependent manner, and the 2 drugs had a synergistic proapoptotic effect (CI <1; Figure 2C,2D). The IC50 values of AML#01-AML#11 primary cells treated with venetoclax were calculated based on the viable cell rate and are shown in Figure 2E.
Figure 2.
Apoptosis rates and gene and protein expression of apoptosis-related molecules in AML cells treated with venetoclax and HHT. (A,B) The apoptosis rates of AML cell lines treated with venetoclax and HHT alone or in combination for 8 h and 24 h [(A) OCI-AML3; (B) MV4-11]. (C) The viable cell ratio of primary AML samples after treatment with different concentrations of venetoclax, HHT, or their combination. (D) The IC50 value of venetoclax based on the viable cell ratio and the second-generation sequencing results of patient bone marrow samples. (E) The apoptosis rate of AML#01 primary cells treated with venetoclax and HHT alone or in combination for 24 h. (F) Changes in apoptosis-related proteins detected by WB. (G) Changes in Bcl-2 family apoptotic regulatory molecules detected by qRT-PCR. (H) Changes in Bcl-2 family apoptotic regulatory proteins detected by WB. *, P<0.05; **, P<0.01; ***, P<0.001, compared with the control group. HHT, homoharringtonine; AML, acute myeloid leukemia; WB, Western blotting; qRT-PCR, quantitative reverse transcription-polymerase chain reaction; CI, combination indices.
Table 2. Clinical characteristics of AML patients.
| Number | Sex | Age (years) | Fab type | Blasts (%) | Fusion gene | Mutation | Karyotype |
|---|---|---|---|---|---|---|---|
| AML#01 | Female | 52 | M1 | 98.4 | (−) | FLT3-ITD, NPM1, IDH2, TET2 | 46,XX |
| AML#02 | Male | 46 | M5 | 74.0 | (−) | FLT3-ITD, FLT3-TKD, PTPN11 | No cleavage |
| AML#03 | Female | 70 | M2 | 74.4 | (−) | c-KIT, JAK2 | 45,X,-Y,t(8,21)(q22;q22) |
| AML#04 | Female | 60 | M2 | 59.2 | AML1-ETO | TET2 | 46,XX,t(8,21)(q22;q22) |
| AML#05 | Female | 74 | M2 | 52.0 | (−) | (−) | 46,XX |
| AML#06 | Female | 44 | M5 | 51.6 | (−) | (−) | 46,XX,t(2;2;21)(q12;q37;q21) |
| AML#07 | Male | 21 | M2 | 86.8 | (−) | c-KIT | 45,X,-Y,t(8,21)(q22;q22) |
| AML#08 | Female | 67 | M4 | 71.6 | (−) | FLT3-TKD, NPM1, IDH2, PTPN11 | 46,XX |
| AML#09 | Male | 46 | M2 | 78.0 | AML-ETO | KIT | 46,X,-7,+4,t(8,21) |
| AML#10 | Male | 70 | M2 | 54.8 | (−) | (−) | 45,X,-Y,t(8;21)(q22;q22) |
| AML#11 | Female | 60 | M2 | 59.0 | (−) | IDH2 | 46,XX,t(4;14)(q22;q31),add(6)(p24) |
AML, acute myeloid leukemia.
In addition, we also examined apoptosis-related proteins in the mitochondrial pathway and Bcl-2 family apoptosis regulatory proteins by Western blotting. As shown in Figure 2F, venetoclax increased the protein expression of cf-Caspase3, cf-PARP, and γH2AX in AML cells, and these effects were further enhanced by combination with HHT. Caspase is the key executor of apoptosis, and the apoptotic process is actually the process of caspase activation and cascade reactions. Caspase-3 exists in cells in the form of inactive zymogen and is activated to become cf-caspase-3 upon apoptosis. cf-Caspase-3 degrades PARP to generate an 89 kDa fragment called cf-PARP, a post-translational modifying enzyme that maintains the structural integrity of chromosomes, participates in DNA replication and transcription, and plays an important role in maintaining cell stability and survival. γH2AX is formed by phosphorylation of Ser-139 residues of the histone variant H2AX, an early cellular response that induces DNA double-strand breaks. Therefore, detection of the γH2AX protein can reflect the extent of DNA damage (25).
Next, we detected changes in apoptosis-related genes at the mRNA level with qRT-PCR. As shown in Figure 2G, venetoclax and HHT monotherapy had no significant effect on Bcl-2 expression, and HHT monotherapy had no significant effect on Mcl-1 expression (P>0.05). Figure 2F,2H shows that venetoclax increased Mcl-1 protein expression, HHT decreased Mcl-1 protein expression, and the Mcl-1 protein expression in the combination group was decreased compared to that in the venetoclax monotherapy group. Expression of the proapoptotic protein Bax was increased in the HHT group and further increased in the combination group. The level of the proapoptotic protein Bak was increased in the venetoclax and HHT monotherapy groups and the combination group. The expression of the proapoptotic protein Bim was increased in the venetoclax and HHT monotherapy groups and further increased in the combination group.
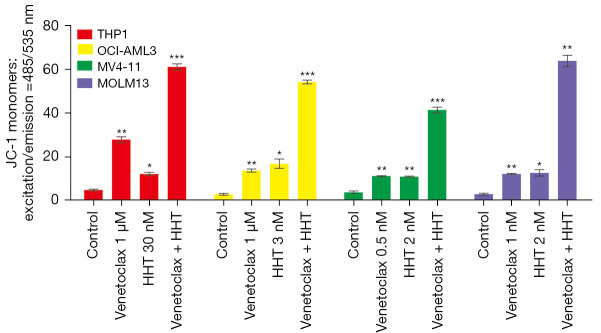
Venetoclax and HHT synergistically decreased mitochondrial membrane potential
The loss of mitochondrial transmembrane potential occurs in early apoptosis and is associated with the release of cytochrome c and regulated by Bcl-2 family proteins (26). JC-1, a membrane-permeable lipophilic cationic fluorochrome that accumulates in mitochondria in live cells, was used as a probe of mitochondrial transmembrane potential. Figure 3 shows that venetoclax and HHT monotherapy induced a loss of mitochondrial transmembrane potential at 24 h, and the combination of venetoclax and HHT significantly enhanced this effect.
Figure 3.
The mitochondrial membrane potential in AML cell lines treated with venetoclax and HHT. HHT, homoharringtonine; AML, acute myeloid leukemia. *, P<0.05; **, P<0.01; ***, P<0.001, compared with the control group.
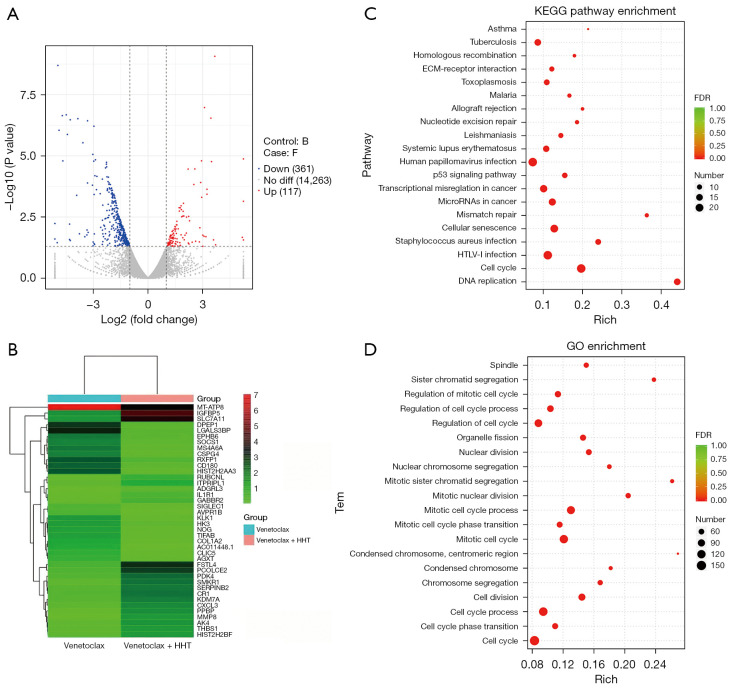
Transcriptome sequencing and enrichment analysis showed that differentially expressed genes were enriched in the PI3K-AKT, p53, and MAPK/ERK pathways and mainly participated in the cell cycle process
The previous results showed that HHT can cooperate with venetoclax to promote AML cell apoptosis. To explore the mechanism underlying the synergistic effects induced by venetoclax and HHT, we treated OCI-AML3 cells with venetoclax, HHT, and the combination of the 2 agents for 24 h and then performed transcriptome sequencing. Figure 4A shows that compared to the venetoclax group, the combination group had 117 upregulated genes and 361 downregulated genes. Figure 4B shows the top 20 upregulated and downregulated genes. Kyoto Encyclopedia of Genes and Genomes (KEGG) analysis was performed based on the KEGG database. Figure 4C shows that the differentially expressed genes were mainly enriched in the PI3K-AKT, p53, and MAPK/ERK pathways. GO enrichment analysis (according to molecular functions, biological processes, and cell components) was performed to determine the main biological functions of the differentially expressed genes. Figure 4D shows that the differentially expressed genes were primarily enriched in the cytoplasm and mainly participated in the biological process of the cell cycle.
Figure 4.
Differential gene expression analysis and enrichment results of transcriptome sequencing samples. (A) Volcano plot of differentially expressed genes in OCI-AML3 cells in the combination group and the venetoclax group (blue dots indicate downregulated genes, red dots indicate upregulated genes; B represents the venetoclax group, and F represents the combination group). (B) The top 20 upregulated and downregulated genes between the combination group and the venetoclax group in OCI-AML3 cells. The color bar on the right in red and green indicates the fold change from high to low. (C) KEGG enrichment analysis of differentially expressed genes. (D) GO enrichment analysis of differentially expressed genes. HHT, homoharringtonine; FDR, false discovery rate; KEGG, Kyoto encyclopedia of genes and genomes; GO, gene ontology.
HHT blocked the AML cell cycle in G1 phase by increasing the expression of p21 and decreasing the expression of c-Myc, CDK2, and CyclinD1
Cell cycle histogram, cell cycle-related genes, and protein changes were measured in AML cells treated with venetoclax and HHT. Figure 5A shows that HHT could induce G1 phase arrest in AML cell lines. The combination of HHT and venetoclax further enhanced this effect. Additionally, qRT-PCR was used to detect the expression of p21, CDK2, and c-Myc at the mRNA level. HHT upregulated the expression of p21 and downregulated the expression of CDK2 and c-Myc at the mRNA level (Figure 5B). Western blotting was used to detect the expression of cell cycle-related proteins. Venetoclax and HHT both upregulated the expression of p21 protein, while HHT also downregulated the expression of CDK2, CyclinD1, and c-Myc proteins. This effect was further enhanced when combined with venetoclax (Figure 5C).
Figure 5.
The expression of cell cycle-related proteins and molecules related to cell cycle regulation at the gene and protein level in cells treated with venetoclax and HHT. (A) Cell cycle analysis of AML cell lines (OCI-AML3 and MV4-11) after treatment with venetoclax and HHT monotherapy or combination therapy for 24 h. (B) The expression of p21, CDK2, and c-Myc at the mRNA level in AML cell lines after treatment with venetoclax and HHT for 24 h. (C) The expression of p21, CDK2, CyclinD1, and c-Myc at the protein level in AML cell lines and primary AML cells after treatment with venetoclax and HHT for 24 h. *, P<0.05; **, P<0.01; ***, P<0.001, compared with the control group. #, P<0.05; ##, P<0.01; ###, P<0.001. HHT, homoharringtonine; AML, acute myeloid leukemia; con, control group.
The combination of venetoclax and HHT inhibited the PI3K/AKT and MAPK/ERK pathways and activated the p53 pathway
Through transcriptome sequencing, we found that the differentially expressed genes were mainly enriched in the PI3K-AKT, p53, and MAPK/ERK pathways. We used qRT-PCR and Western blotting to detect the expression of the key molecules in the above pathways at the mRNA and protein levels for verification. The results are shown in Figure 6. In 2 AML cell lines (OCI-AML3 and MV4-11) and 2 AML primary cell lines (AML#06 and AML#07), venetoclax increased the expression of the AKT gene and decreased the expression of the p53 gene, while HHT increased the expression of p53 and decreased the expression of the ERK gene. Compared with that in the venetoclax group, the expression of AKT and ERK genes in the combination group was decreased, while the expression of the p53 gene was increased, and the difference was statistically significant (P<0.05; Figure 6A). According to the results of Western blotting in Figure 6B, venetoclax and HHT monotherapy each decreased the expression of p-AKT, and the expression of p-AKT further decreased in the combination group, indicating that the activation of the PI3K-AKT pathway was inhibited. HHT monotherapy increased p53 protein expression, and in combination with venetoclax, the expression of p53 protein was further increased, indicating that the p53 pathway was activated. The combination of venetoclax and HHT decreased the expression of p-MEK and p-ERK proteins, which meant that the activation of the MAPK/ERK signaling pathway was inhibited. MAPK/ERK activation promoted cell proliferation and increased Mcl-1 protein expression.
Figure 6.
The expression of the key molecules in the PI3K/AKT, MAPK/ERK, and p53 pathways at the mRNA and protein levels as determined by qRT-PCR and Western blotting. (A) The effects of venetoclax and HHT on gene expression in AML cells. (B) Effects of venetoclax and HHT on protein expression in AML cells. *, P<0.05; **, P<0.01; ***, P<0.001, compared with the control group. #, P<0.05; ##,P<0.01. HHT, homoharringtonine; AML, acute myeloid leukemia; qRT-PCR, quantitative reverse transcription-polymerase chain reaction; con, control group.
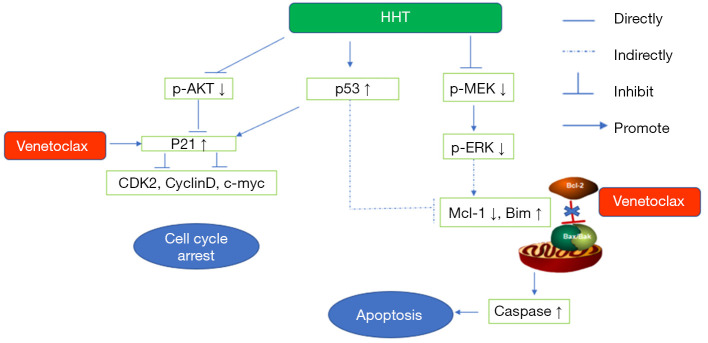
The signaling pathways of venetoclax combined with HHT in AML cells
To summarize the above conclusions, the signaling pathways of HHT and venetoclax in AML cells are condensed in Figure 7.
Figure 7.
The signaling pathways impacted by combination treatment with venetoclax and HHT in AML cells. HHT, homoharringtonine; AML, acute myeloid leukemia.
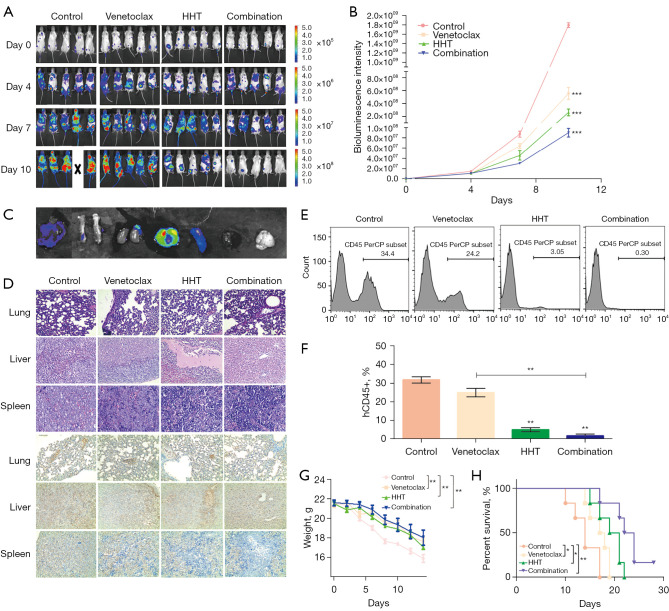
Synergistic antileukemia effect of venetoclax plus HHT in vivo
Compared with that in the control group, the fluorescence intensity was significantly reduced in the venetoclax group and the HHT group (P<0.001) and was lowest in the combination group (P<0.001; Figure 8A,8B). At d10, 3 mice from each group were sacrificed, and in vivo imaging showed lung, liver, spleen, kidney, and femur AML cell infiltration in the mice (Figure 8C). The spleen was immunohistochemically stained with hematoxylin and eosin (HE) and hCD45 antibodies. The expression of hCD45 was the lowest in the combination group (Figure 8D). Flow cytometry showed that compared with that in the control group, the proportion of hCD45+ cells in the bone marrow of the venetoclax group was reduced, but this difference was not statistically significant (P>0.05). The proportions of hCD45+ cells in the bone marrow of the HHT group and the combined treatment group were significantly reduced, and the combination treatment group had the lowest proportion (P<0.01). Compared with that in the venetoclax group, the proportion of hCD45+ cells in the bone marrow decreased in the combination group (P<0.05; Figure 8E,8F). Body weight was significantly lower in the control group than in the venetoclax group, HHT group, and combination group, and the differences were statistically significant (P<0.01; Figure 8G). Survival time was prolonged in the venetoclax group, HHT group, and combination group compared with the control group (P<0.05), and combination treatment had the most significant effect on survival time (P<0.01; Figure 8H).
Figure 8.
The effect of venetoclax and HHT on AML xenograft mice. (A) Tumorigenesis of AML xenograft mice treated with venetoclax and HHT alone or in combination. (B) Fluorescence intensity values in AML xenograft mice. (C) Imaging of sacrificed mice. (D) HE and hCD45+ immunohistochemical staining results of the liver, spleen, and lungs in AML xenograft mice at d10 (magnification, ×200 for liver and lung, ×400 for spleen). (E,F) hCD45+ expression in bone marrow cells from AML xenograft mice at d10. (G) The weight curve of AML xenograft mice. (H) Survival curve of AML xenograft mice. Statistical analysis of data was performed by the t-test (*, P<0.05, **, P<0.01, ***, P<0.001). HHT, homoharringtonine; AML, acute myeloid leukemia.
Discussion
Apoptosis is a major mechanism by which multicellular organisms remove senescent and incompetent cells and maintain their stability. It is especially important in preventing cancer development, and it occurs in millions of cells in the human body every minute. Mitochondria play a key role in apoptosis, and caspase protease activity is essential for apoptosis. Once activated, caspases cleave hundreds of different proteins, leading to rapid cell death with unique biochemical and morphological characteristics (27). Caspase proteases can be activated through cell surface death receptors (extrinsic pathway) (28) or through mitochondria (intrinsic pathway) (29). Most stimuli lead to mitochondrial outer membrane permeabilization, and cytochrome C is released into the cytoplasm which binds to apoptosis-associated factor 1 (Apaf-1), forming a multimer that binds caspase-9 and activates it. Then, activated caspase-9 activates other caspases, such as caspase-3, thereby inducing apoptosis.
Mitochondrial outer membrane permeability (MOMP) is mainly regulated by members of the Bcl-2 protein family. This family can be subdivided into proapoptotic effector proteins (Bax and Bak), proapoptotic BH3-only proteins (Bid Bim, Puma, Noxa, Hrk, Bik, Bmf, and Bad), and antiapoptotic Bcl-2 proteins (Bcl-2, Bcl-xL, Mcl-1, A1, Bcl-B, and Bcl-w). In most cases, Bax or Bak activation requires their direct interaction with members of the BH3-only protein family, which causes conformational changes in Bax and Bak to form oligomers that permeate the outer mitochondrial membrane and induce the opening of mitochondrial membrane lipid pores. The BH3-only protein family can be subdivided into activators (capable of directly activating Bax/Bak) and sensitizers (neutralizing the inhibition of apoptosis by antiapoptotic Bcl-2 proteins). Most BH3-only protein mimetic drugs (called BH3 mimetics) are sensitizers, and Bax and Bak activity are counteracted by antiapoptotic Bcl-2 proteins that prevent MOMP and apoptosis by binding to BH3-only proteins (preventing them from interacting with Bax and Bak) or by binding to activated Bax or Bak.
Venetoclax is a highly targeted therapeutic agent that binds almost exclusively to Bcl-2 when used at clinically achievable concentrations (11). In cells with consistently high expression of Bcl-2 and relatively low expression of other pro-survival proteins, termed Bcl-2-dependent disease, inhibition of Bcl-2 is sufficient to trigger apoptosis in a high proportion of cells, in which case venetoclax can hit the Bcl-2 “bull’s eye” and monotherapy can produce high rates of CR, as in chronic lymphocytic leukemia (CLL) (11,30). More often, Bcl-2 is only the target, and direct inhibition of Bcl-2 is not sufficient to “hit the bull’s eye”. The killing of cells by venetoclax depends on a series of secondary events, including secondary inhibition of other pro-survival proteins by displaced BH3-only proteins (31,32). These cells are not strictly dependent on Bcl-2. In most diseases where cells are affected in this manner, additional stimulation may be required to maximize apoptosis and achieve high response rates. The expression of Bcl-2 in AML is heterogeneous, with only a few patient samples showing significant sensitivity in vitro (4). In line with this, in a phase II clinical trial of venetoclax monotherapy for relapsed AML, only 19% of patients achieved CR/Cri with a short median response (4). These data suggest that Bcl-2 inhibition by venetoclax in AML has a “hammer” effect and that Bcl-2 is an important target but not a “bull’s eye”. Therefore, it is imperative to explore combination therapeutic strategies for venetoclax.
The PI3K/AKT pathway plays a key role in the control of cell growth, survival, transcription, protein translation, differentiation, migration, apoptosis, and autophagy (33). Abnormal activation of the PI3K/AKT/mTOR pathway is present in approximately 30% of human tumors, and analysis of AML primary cells has shown that up to 80% of patients exhibit structural activation of the PI3K/AKT/mTOR pathway (34). The AKT proto-oncogene encodes a serine-threonine protein kinase, and activated AKT moves to the cytoplasm or nucleus, where phosphorylated AKT acts on GSK3 and Bcl-2 family proteins, thereby affecting metabolism, survival, and proliferation (35). AKT induces the binding of phosphorylated Bad and 14-3-3 proteins, thereby allowing Bcl-2 and Bcl-xL to exert antiapoptotic functions (36) or block the intrinsic protease activity of caspase-9 (37). AKT also phosphorylates p21 on Thr-145 (near the carboxyl terminus of p21), which activates cytosolic CyclinD1, leading to cell cycle progression to G1/S phase for mitosis (38). AKT upregulates the cell cycle promoter c-Myc by upregulating transcript levels and protein expression.
The cell cycle is the process underlying cell replication. The cell cycle transition from G1 to S and G2 to mitosis is regulated by the sequential activation and inactivation of CDK protein family members (serine/threonine protein kinases). The activity of CDKs (CDK2, CDK4, and CDK6 in G1 phase, CDK2 in S phase, and CDK1 in G2 and M phases) remains stable during the cell cycle. Excessive activation of CDKs can lead to unregulated cell division and tumor development.
P21 is a well-known cell cycle inhibitor, and p53 is the main transcriptional regulator of p21. Various stresses, including DNA damage and oxidative stress, can upregulate p53 activity and subsequently lead to p21 expression (39). P21 binds to the cell cycle protein E/CDK2 and cell cycle protein D/CDK4 complexes, leading to G1 phase cell cycle arrest. It has been shown that Bcl-2 inhibition can overcome resistance to p53-activated apoptosis by shifting the cellular response from G1 phase arrest to apoptosis, achieving impressive antileukemic efficacy in vitro and in vivo (40). In this study, we performed GO enrichment analysis after transcriptome sequencing and found that the differentially expressed genes after the addition of HHT compared to the venetoclax monotherapy group were mainly involved in cell cycle regulation. We further verified that HHT blocked the AML cell cycle in G1 phase using cell cycle experiments. Further validation by qRT-PCR and Western blot showed that HHT alone and in combination with venetoclax significantly upregulated the expression of p21 and downregulated the expression of CDK2 and Myc at the gene and protein level, causing cell cycle arrest.
In this study, transcriptome sequencing revealed that PI3K-AKT pathway-related genes were differentially expressed after treatment with HHT combined with venetoclax. Further validation by qRT-PCR and Western blot showed that HHT alone downregulated AKT gene and p-AKT protein expression, and upon combination treatment with venetoclax, p-AKT levels further decreased, thereby inhibiting the activation of the PI3K-AKT pathway, upregulating the expression of its downstream factor p21, and downregulating CDK2, CyclinD1, and Myc proteins. CyclinD1 and Myc protein expression causes cell cycle arrest in G1 phase. HHT inhibits the activation of the PI3K/AKT signaling pathway to achieve the synergistic anti-AML effect of venetoclax.
There are 2 distinct but ultimately convergent apoptotic pathways in mammalian cells (41): the Bcl-2 regulatory pathway (also known as the endogenous, mitochondrial, or stress pathway), which is activated by stress conditions (e.g., cytokine deprivation, endoplasmic reticulum stress, or DNA damage), and the death receptor pathway, which is activated by linking members of the tumor necrosis factor receptor (TNFR) family with intracellular death structural domains (Bcl-2 and p53 represent 2 important nodes in the apoptosis signaling pathway). In the Bcl-2-regulated apoptotic pathway, cell death is caused by the transcriptional and/or post-transcriptional upregulation of the proapoptotic BH3-only member of the Bcl-2 protein family. BH3-only proteins bind to and repress pro-survival Bcl-2 proteins, resulting in the release of the cell death effectors Bax and Bak (42-44). Activation of Bax/Bak leads to MOMP, an irreversible event in apoptotic signaling, and subsequent activation of the caspase cascade reaction, which dismantles the cell (43). γH2AX proteins can reflect the extent of DNA damage and are a marker of DNA double-strand breaks (25). The results of the present study showed that venetoclax alone increased the expression of caspase-3, γH2AX, and PARP proteins in AML cells, and their expression was further increased by combination treatment with HHT, indicating that HHT can enhance the proapoptotic effect of venetoclax.
Overexpression of antiapoptotic Bcl-2 family members has been shown to be associated with chemoresistance in leukemia cell line models and poor clinical outcomes. Antiapoptotic Bcl-2 family members, such as Bcl-2, Bcl-xL, and Mcl-1, as well as sequester proapoptotic BH3-only proteins, such as Bim, were shown to block the activation of the proapoptotic proteins Bax and Bak and ultimately prevent MOMP, cytochrome C release, and apoptosis (45). Venetoclax releases Bim from Bcl-2, reducing the association of Bim with Bcl-2, but this was offset by an increased correlation of Bim with Mcl-1, stabilizing Mcl-1 and leading to venetoclax resistance. The results of this study showed that venetoclax alone increased Mcl-1 protein expression in AML cells, HHT alone decreased Mcl-1 protein expression, and combination treatment decreased Mcl-1 protein expression levels compared to that in the venetoclax-alone group, thus allowing Bim to bind more to Bcl-2 to activate Bax/Bak, which may be a result of HHT synergistically promoting one of the mechanisms of the proapoptotic effect of venetoclax.
TP53 is one of the most important genes in human cancer, and p53 is a key factor in the killing or silencing of tumor-initiating cancer cells (often considered stem/progenitor cells). In unstressed cells, p53 protein levels are very low, as it is a target for degradation by the E3 ubiquitin ligase MDM2 proteasome (46). Meanwhile, p53 is activated in response to many stress stimuli, including oncogene activation and DNA damage. Upon activation, p53 directly regulates the transcription of approximately 500 genes and indirectly regulates many others, thereby controlling a variety of cellular processes, including cell cycle arrest, cellular senescence, DNA repair, metabolic adaptation, and cell death (47,48). Furthermore, p53 induces apoptosis in untransformed cells primarily through direct transcriptional activation of the proapoptotic BH3-only proteins Puma and Noxa. Activated p53 acts on BH3-only proteins such as Puma and Noxa to induce apoptosis through the Bcl-2 regulatory pathway (49,50). Post-translational modifications such as phosphorylation significantly affect the functions of different Bcl-2 family proteins. Bad, which antagonizes Bcl-2, Bcl-xL, and Bcl-w, must combine inhibition of MAPK and PI3K signaling by dephosphorylation at 2 different serine residues to exert its proapoptotic function (51). The mouse double minute 2 (MDM2) inhibitor downregulates Mcl-1 by increasing ERK2 phosphorylation stabilization and upregulation, negatively regulates the Ras/Raf/MEK/ERK pathway, and activates glycogen synthase kinase-3 (GSK3) to regulate Mcl-1 phosphorylation and promote its degradation, thereby overcoming AML resistance to Bcl-2 inhibitors (52). MEK inhibitors were shown to effectively downregulate Mcl-1 in AML cell lines and primary AML cells, including CD34+38-123+ leukemia stem cells, and to synergize with ABT-737 (which induces Mcl-1 through ERK activation) in vivo assays (53). Our study showed that HHT upregulates p53 gene and protein expression, activating the p53 pathway and downregulating p-MEK and p-ERK protein expression, thereby inhibiting the MEK/ERK pathway and subsequently causing increased degradation of the Mcl-1 protein. In this way, HHT increases the proapoptotic effect of venetoclax.
In this study, transcriptome sequencing revealed that p53 and MAPK/ERK pathway-related genes were differentially expressed when HHT was combined with venetoclax. Further validation by qRT-PCR and Western blot showed that venetoclax alone downregulated p53 gene expression, while HHT alone upregulated p53 expression, downregulated p-MEK protein expression, and downregulated ERK gene and p-ERK protein expression, and the combination of the 2 upregulated p53 gene and protein expression and downregulated p-MEK and p-ERK protein expression. HHT can synergize with the anti-AML effect of venetoclax by activating the p53 pathway and inhibiting the MAPK/ERK signaling pathway.
To investigate how HHT reduces Mcl-1 expression, we first examined the expression of the Mcl-1 gene by qRT-PCR after 24 h of drug treatment. The qRT-PCR results showed that HHT did not downregulate Mcl-1 at the transcriptional level, while the Western blot assay showed that HHT downregulated Mcl-1 protein levels in AML cell lines and primary cells, and the Mcl-1 protein levels in the combination group were decreased compared with the venetoclax-alone group. These results suggest that HHT decreases Mcl-1 expression, reduces Mcl-1/Bim binding, and increases Bim release, mainly at the level of protein translation, thereby promoting apoptosis in concert with venetoclax. Although the precise molecular mechanism remains to be determined, we hypothesize that HHT induces cell cycle arrest by inhibiting the PI3K/AKT pathway, activating the p53 pathway to upregulate p21 expression, followed by downregulating c-Myc, CyclinD1, and CDK2 expression. Activating the p53 pathway also inhibits the MAPK/ERK pathway to enhance the downregulation of Mcl-1 and promote Bim release, exerting a synergistic proapoptotic effect.
Conclusions
The aim of this study was to investigate the mechanism of the synergistic antileukemic effect of HHT and venetoclax. Our study generated the following 4 conclusions. First, HHT could exert a synergistic anti-AML effect with venetoclax by promoting apoptosis and blocking cell cycle progression in AML cells. Second, HHT exerts a synergistic proapoptotic effect with venetoclax by reducing the expression of the Mcl-1 protein. Third, transcriptome sequencing, qRT-PCR, and Western blotting showed that HHT played a synergistic antileukemic role by inhibiting the PI3K-AKT and MAPK/ERK pathways and activating the p53 pathway. Finally, HHT combined with venetoclax significantly inhibited AML progression and significantly prolonged survival time in xenograft mice. The results of this study provide a theoretical basis for exploring combination treatment strategies including venetoclax in AML.
Supplementary
The article’s supplementary files as
Acknowledgments
The work was supported by the Department of Hematopathy, Henan Institute of Hematology, The Affiliated Cancer Hospital of Zhengzhou University and we would like to sincerely thank all staff involved.
Funding: This study was supported by the Henan Province Science and Technology Research Project (Nos. 202102310053 and LHGJ20200168) and the Henan Medical Science and Technology Project (No. SBGJ202003012).
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). The study was approved by the Medical Ethics Committee of the Affiliated Cancer Hospital of Zhengzhou University (approval No. 2019-KY-0017-001) and informed consent was taken from all the patients. Animal experiments were performed under a project license (No. 20200322) granted by Ethics Review Committee of life sciences, Zhengzhou University, in compliance with Zhengzhou University’s guidelines for the care and use of animals.
Footnotes
Reporting Checklist: The authors have completed the STROBE reporting checklist. Available at https://atm.amegroups.com/article/view/10.21037/atm-22-1459/rc
Data Sharing Statement: Available at https://atm.amegroups.com/article/view/10.21037/atm-22-1459/dss
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://atm.amegroups.com/article/view/10.21037/atm-22-1459/coif). The authors have no conflicts of interest to declare.
References
- 1.Döhner H, Estey E, Grimwade D, et al. Diagnosis and management of AML in adults: 2017 ELN recommendations from an international expert panel. Blood 2017;129:424-47. 10.1182/blood-2016-08-733196 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Walter RB, Othus M, Burnett AK, et al. Resistance prediction in AML: analysis of 4601 patients from MRC/NCRI, HOVON/SAKK, SWOG and MD Anderson Cancer Center. Leukemia 2015;29:312-20. 10.1038/leu.2014.242 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Schlenk RF, Frech P, Weber D, et al. Impact of pretreatment characteristics and salvage strategy on outcome in patients with relapsed acute myeloid leukemia. Leukemia 2017;31:1217-20. 10.1038/leu.2017.22 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Konopleva M, Pollyea DA, Potluri J, et al. Efficacy and Biological Correlates of Response in a Phase II Study of Venetoclax Monotherapy in Patients with Acute Myelogenous Leukemia. Cancer Discov 2016;6:1106-17. 10.1158/2159-8290.CD-16-0313 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Vaux DL, Cory S, Adams JM. Bcl-2 gene promotes haemopoietic cell survival and cooperates with c-myc to immortalize pre-B cells. Nature 1988;335:440-2. 10.1038/335440a0 [DOI] [PubMed] [Google Scholar]
- 6.McDonnell TJ, Deane N, Platt FM, et al. bcl-2-immunoglobulin transgenic mice demonstrate extended B cell survival and follicular lymphoproliferation. Cell 1989;57:79-88. 10.1016/0092-8674(89)90174-8 [DOI] [PubMed] [Google Scholar]
- 7.Strasser A, Harris AW, Bath ML, et al. Novel primitive lymphoid tumours induced in transgenic mice by cooperation between myc and bcl-2. Nature 1990;348:331-3. 10.1038/348331a0 [DOI] [PubMed] [Google Scholar]
- 8.Lauria F, Raspadori D, Rondelli D, et al. High bcl-2 expression in acute myeloid leukemia cells correlates with CD34 positivity and complete remission rate. Leukemia 1997;11:2075-8. 10.1038/sj.leu.2400854 [DOI] [PubMed] [Google Scholar]
- 9.Schimmer AD, O'Brien S, Kantarjian H, et al. A phase I study of the pan bcl-2 family inhibitor obatoclax mesylate in patients with advanced hematologic malignancies. Clin Cancer Res 2008;14:8295-301. 10.1158/1078-0432.CCR-08-0999 [DOI] [PubMed] [Google Scholar]
- 10.Davids MS, Letai A. Targeting the B-cell lymphoma/leukemia 2 family in cancer. J Clin Oncol 2012;30:3127-35. 10.1200/JCO.2011.37.0981 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Souers AJ, Leverson JD, Boghaert ER, et al. ABT-199, a potent and selective BCL-2 inhibitor, achieves antitumor activity while sparing platelets. Nat Med 2013;19:202-8. 10.1038/nm.3048 [DOI] [PubMed] [Google Scholar]
- 12.Tahir SK, Smith ML, Hessler P, et al. Potential mechanisms of resistance to venetoclax and strategies to circumvent it. BMC Cancer 2017;17:399. 10.1186/s12885-017-3383-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lin KH, Winter PS, Xie A, et al. Targeting MCL-1/BCL-XL Forestalls the Acquisition of Resistance to ABT-199 in Acute Myeloid Leukemia. Sci Rep 2016;6:27696. 10.1038/srep27696 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Vela L, Gonzalo O, Naval J, et al. Direct interaction of Bax and Bak proteins with Bcl-2 homology domain 3 (BH3)-only proteins in living cells revealed by fluorescence complementation. J Biol Chem 2013;288:4935-46. 10.1074/jbc.M112.422204 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Wang M, Wu D, Liu P, et al. Silence of MCL-1 upstream signaling by shRNA abrogates multiple myeloma growth. Exp Hematol Oncol 2014;3:27. 10.1186/2162-3619-3-27 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Niu X, Zhao J, Ma J, et al. Binding of Released Bim to Mcl-1 is a Mechanism of Intrinsic Resistance to ABT-199 which can be Overcome by Combination with Daunorubicin or Cytarabine in AML Cells. Clin Cancer Res 2016;22:4440-51. 10.1158/1078-0432.CCR-15-3057 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Schwartz J, Niu X, Walton E, et al. Synergistic anti-leukemic interactions between ABT-199 and panobinostat in acute myeloid leukemia ex vivo. Am J Transl Res 2016;8:3893-902. [PMC free article] [PubMed] [Google Scholar]
- 18.Zhang W, Lu Y, Zhen T, et al. Homoharringtonine synergy with oridonin in treatment of t(8; 21) acute myeloid leukemia. Front Med 2019;13:388-97. 10.1007/s11684-018-0624-1 [DOI] [PubMed] [Google Scholar]
- 19.Zhang T, Shen S, Zhu Z, et al. Homoharringtonine binds to and increases myosin-9 in myeloid leukaemia. Br J Pharmacol 2016;173:212-21. 10.1111/bph.13359 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Shen JP, Yang H, Ni WM, et al. Cytotoxicity of homoharringtonine on leukemic stem-like cells in AML cell line KG-1. Zhejiang Da Xue Xue Bao Yi Xue Ban 2012;41:485-90. [PubMed] [Google Scholar]
- 21.Xie C, Tang AP. Combined Effect of Bortezomib and Homoharringtonine on K562 Cells and their Mechanisms. Zhongguo Shi Yan Xue Ye Xue Za Zhi 2018;26:395-400. [DOI] [PubMed] [Google Scholar]
- 22.Klanova M, Andera L, Brazina J, et al. Targeting of BCL2 Family Proteins with ABT-199 and Homoharringtonine Reveals BCL2- and MCL1-Dependent Subgroups of Diffuse Large B-Cell Lymphoma. Clin Cancer Res 2016;22:1138-49. 10.1158/1078-0432.CCR-15-1191 [DOI] [PubMed] [Google Scholar]
- 23.Yu G, Xu N, Huang F, et al. Combination of Homoharringtonine with Venetoclax and Azacitidine Excerts Better Treatment Response in Relapsed /Refractory Acute Myeloid Leukemia. Blood 2020;136:26-7. 10.1182/blood-2020-138676 [DOI] [Google Scholar]
- 24.Li G, Li D, Yuan F, et al. Synergistic effect of chidamide and venetoclax on apoptosis in acute myeloid leukemia cells and its mechanism. Ann Transl Med 2021;9:1575. 10.21037/atm-21-5066 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Mah LJ, El-Osta A, Karagiannis TC. gammaH2AX: a sensitive molecular marker of DNA damage and repair. Leukemia 2010;24:679-86. 10.1038/leu.2010.6 [DOI] [PubMed] [Google Scholar]
- 26.Shimizu S, Narita M, Tsujimoto Y. Bcl-2 family proteins regulate the release of apoptogenic cytochrome c by the mitochondrial channel VDAC. Nature 1999;399:483-7. 10.1038/20959 [DOI] [PubMed] [Google Scholar]
- 27.Taylor RC, Cullen SP, Martin SJ. Apoptosis: controlled demolition at the cellular level. Nat Rev Mol Cell Biol 2008;9:231-41. 10.1038/nrm2312 [DOI] [PubMed] [Google Scholar]
- 28.Dickens LS, Powley IR, Hughes MA, et al. The 'complexities' of life and death: death receptor signalling platforms. Exp Cell Res 2012;318:1269-77. 10.1016/j.yexcr.2012.04.005 [DOI] [PubMed] [Google Scholar]
- 29.Tait SW, Green DR. Mitochondria and cell death: outer membrane permeabilization and beyond. Nat Rev Mol Cell Biol 2010;11:621-32. 10.1038/nrm2952 [DOI] [PubMed] [Google Scholar]
- 30.Roberts AW, Davids MS, Pagel JM, et al. Targeting BCL2 with Venetoclax in Relapsed Chronic Lymphocytic Leukemia. N Engl J Med 2016;374:311-22. 10.1056/NEJMoa1513257 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Roberts AW, Huang D. Targeting BCL2 With BH3 Mimetics: Basic Science and Clinical Application of Venetoclax in Chronic Lymphocytic Leukemia and Related B Cell Malignancies. Clin Pharmacol Ther 2017;101:89-98. 10.1002/cpt.553 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Gong JN, Khong T, Segal D, et al. Hierarchy for targeting prosurvival BCL2 family proteins in multiple myeloma: pivotal role of MCL1. Blood 2016;128:1834-44. 10.1182/blood-2016-03-704908 [DOI] [PubMed] [Google Scholar]
- 33.Altomare DA, Khaled AR. Homeostasis and the importance for a balance between AKT/mTOR activity and intracellular signaling. Curr Med Chem 2012;19:3748-62. 10.2174/092986712801661130 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Xu Q, Simpson SE, Scialla TJ, et al. Survival of acute myeloid leukemia cells requires PI3 kinase activation. Blood 2003;102:972-80. 10.1182/blood-2002-11-3429 [DOI] [PubMed] [Google Scholar]
- 35.Hers I, Vincent EE, Tavaré JM. Akt signalling in health and disease. Cell Signal 2011;23:1515-27. 10.1016/j.cellsig.2011.05.004 [DOI] [PubMed] [Google Scholar]
- 36.Datta SR, Dudek H, Tao X, et al. Akt phosphorylation of BAD couples survival signals to the cell-intrinsic death machinery. Cell 1997;91:231-41. 10.1016/S0092-8674(00)80405-5 [DOI] [PubMed] [Google Scholar]
- 37.Zhou H, Li XM, Meinkoth J, et al. Akt regulates cell survival and apoptosis at a postmitochondrial level. J Cell Biol 2000;151:483-94. 10.1083/jcb.151.3.483 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Zhou BP, Liao Y, Xia W, et al. Cytoplasmic localization of p21Cip1/WAF1 by Akt-induced phosphorylation in HER-2/neu-overexpressing cells. Nat Cell Biol 2001;3:245-52. 10.1038/35060032 [DOI] [PubMed] [Google Scholar]
- 39.Jung YS, Qian Y, Chen X. Examination of the expanding pathways for the regulation of p21 expression and activity. Cell Signal 2010;22:1003-12. 10.1016/j.cellsig.2010.01.013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Pan R, Ruvolo V, Mu H, et al. Synthetic Lethality of Combined Bcl-2 Inhibition and p53 Activation in AML: Mechanisms and Superior Antileukemic Efficacy. Cancer Cell 2017;32:748-760.e6. 10.1016/j.ccell.2017.11.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Strasser A, O'Connor L, Dixit VM. Apoptosis signaling. Annu Rev Biochem 2000;69:217-45. 10.1146/annurev.biochem.69.1.217 [DOI] [PubMed] [Google Scholar]
- 42.Strasser A, Harris AW, Huang DC, et al. Bcl-2 and Fas/APO-1 regulate distinct pathways to lymphocyte apoptosis. EMBO J 1995;14:6136-47. 10.1002/j.1460-2075.1995.tb00304.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Green DR. Apoptotic pathways: ten minutes to dead. Cell 2005;121:671-4. 10.1016/j.cell.2005.05.019 [DOI] [PubMed] [Google Scholar]
- 44.Czabotar PE, Lessene G, Strasser A, et al. Control of apoptosis by the BCL-2 protein family: implications for physiology and therapy. Nat Rev Mol Cell Biol 2014;15:49-63. 10.1038/nrm3722 [DOI] [PubMed] [Google Scholar]
- 45.Juin P, Geneste O, Gautier F, et al. Decoding and unlocking the BCL-2 dependency of cancer cells. Nat Rev Cancer 2013;13:455-65. 10.1038/nrc3538 [DOI] [PubMed] [Google Scholar]
- 46.Reich NC, Levine AJ. Growth regulation of a cellular tumour antigen, p53, in nontransformed cells. Nature 1984;308:199-201. 10.1038/308199a0 [DOI] [PubMed] [Google Scholar]
- 47.Vousden KH, Lane DP. p53 in health and disease. Nat Rev Mol Cell Biol 2007;8:275-83. 10.1038/nrm2147 [DOI] [PubMed] [Google Scholar]
- 48.Freed-Pastor WA, Prives C. Mutant p53: one name, many proteins. Genes Dev 2012;26:1268-86. 10.1101/gad.190678.112 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Shibue T, Takeda K, Oda E, et al. Integral role of Noxa in p53-mediated apoptotic response. Genes Dev 2003;17:2233-8. 10.1101/gad.1103603 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Jeffers JR, Parganas E, Lee Y, et al. Puma is an essential mediator of p53-dependent and -independent apoptotic pathways. Cancer Cell 2003;4:321-8. 10.1016/S1535-6108(03)00244-7 [DOI] [PubMed] [Google Scholar]
- 51.She QB, Solit DB, Ye Q, et al. The BAD protein integrates survival signaling by EGFR/MAPK and PI3K/Akt kinase pathways in PTEN-deficient tumor cells. Cancer Cell 2005;8:287-97. 10.1016/j.ccr.2005.09.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Pan R, Ruvolo V, Hong M, et al. BCL-2 Inhibition By ABT-199 (Venetoclax/GDC-0199) and p53 Activation By RG7388 (Idasanutlin) Reciprocally Overcome Leukemia Apoptosis Resistance to Either Strategy Alone: Efficacy and Mechanisms. American Society of Hematology Conference; 2015. [Google Scholar]
- 53.Konopleva M, Milella M, Ruvolo P, et al. MEK inhibition enhances ABT-737-induced leukemia cell apoptosis via prevention of ERK-activated MCL-1 induction and modulation of MCL-1/BIM complex. Leukemia 2012;26:778-87. 10.1038/leu.2011.287 [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
The article’s supplementary files as